Abstract
Background
Survivors of childhood cancer develop early and severe chronic health conditions (CHCs). A quantitative landscape of morbidity among survivors, however, has not been described.
Methods
Among 5,522 patients treated for childhood cancer at St. Jude Children’s Research Hospital who survived ≥10 years and were ≥18 years old, 3,010 underwent prospective clinical assessment and retrospective medical validation of health records as part of the St. Jude Lifetime Cohort Study. Age- and sex-frequency-matched community-controls (n=272) were used for comparison. 168 CHCs for all participants were graded for severity using a modified Common Terminology Criteria of Adverse Events. Multiple imputation with predictive mean matching was used for missing occurrences and grades of CHCs among the 2512 survivors not clinically evaluated. Mean cumulative count and marked-point-process regression were used for descriptive and inferential cumulative burden analyses, respectively.
Findings
The cumulative incidence of any grade CHC at age 50 was 99·9%; 96·0% (95·3%–96·8%) for severe/disabling, life-threatening or fatal CHCs. By age 50, a survivor experienced, on average, 17·1 (16·2–18·0) CHCs including 4·7 (4·6–4·9) graded as severe/disabling, life-threatening or fatal. The cumulative burden among survivors was nearly 2-fold greater than matched community-controls (p<0·001). Second neoplasms, spinal disorders and pulmonary disease were major contributors to the excess total cumulative burden. Significant heterogeneity in CHCs among survivors with differing primary cancer diagnoses was observed. Multivariable analyses demonstrated that age at diagnosis, treatment era and higher doses of brain and chest radiation are significantly associated with a greater cumulative burden and severity of CHCs.
Interpretation
The burden of surviving childhood cancer is substantial and highly variable. The total cumulative burden experienced by survivors of pediatric cancer, in conjunction with detailed characterization of long-term CHCs, provide data to better inform future clinical guidelines, research investigations and health services planning for this vulnerable, medically-complex population.
Introduction
With current 10-year survival rates greater than 80% for pediatric cancer patients and reduced late mortality among recent long-term survivors, there exists an ever-increasing population of pediatric cancer survivors.1–4 Incidence and prevalence data, largely generated by cohort studies, have documented that survivors experience a life-long increased risk of morbidity associated with their curative therapies.5–10 However, the true price of cure is reflected by the cumulative burden of disease, or total disease morbidity experienced, after taking into account the occurrences and severities of multiple medical conditions and recurrent events.
Comprehensive ascertainment and characterization of the excess cumulative burden of morbidity associated with childhood cancer survivorship is a lacking but necessary piece of evidence for addressing clinical and health policy interventions in this population. Previous research has focused on reporting relative risk, cumulative incidence (i.e., time to first occurrence) or prevalence of chronic health conditions (CHCs). Moreover, other cohort studies have often been limited by patient-reported morbidities without medical validation, absence of a control population, and/or lack of detailed treatment exposure data. By addressing each of these limitations and utilizing new analytic methods, the St. Jude Lifetime Cohort Study (SJLIFE) provides an opportunity to detail and visualize, for the first time, overall and excess cumulative burden of curative cancer therapy among a clinically-assessed aging population of long-term survivors.
Methods
Study design and participants
All data were obtained through two ongoing cohort studies approved by the St. Jude Children’s Research Hospital (SJCRH) institutional review board (IRB): the SJLIFE and the St. Jude Long-term Follow-up Study (SJLTFU).11,12 The SJLIFE is a retrospective cohort study initiated in April 2007 with prospective follow-up and ongoing accrual (Supplement, Page 6). All patients treated at SJCRH for an oncologic disease, who are ≥18 years of age and ≥10 years post-diagnosis from their malignancy, are eligible for the cohort with the first survivor in our analysis diagnosed on October, 1961 and meeting eligibility on October 15, 1971.11 A population of 272 SJLIFE community-control participants matched on 5-year age blocks within each sex were included for comparison. Exclusion criteria for community-controls were being a first-degree relative of a SJLIFE participant, having a history of childhood cancer, or being pregnant. Procedures for how community-controls were recruited are available in the Supplement Page 5.
Written informed consent was obtained from all SJLIFE participants. Demographic, mortality and therapy-related exposure data for the 2512 survivors who died prior to recruitment into SJLIFE, refused participation or had not completed a SJLIFE clinical assessment visit were obtained from medical records using an IRB-approved waiver from SJLTFU. As these individuals did not return to campus for prospective medical follow-up, they were non-clinically evaluable and their chronic health condition outcomes were not directly assessed and graded.
Procedures
Twenty-one treatment exposure variables were included in the analysis, with selection and categorization of specific treatment-related exposures based on long-term follow-up guidelines.13 Cumulative doses of chemotherapeutic agents were abstracted by trained research staff from medical records using a structured protocol.11 Radiation dosimetry was performed or estimated from primary radiation prescription records.
One hundred and sixty-eight CHCs were classified using the SJCRH-modified National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) 4·03 [mild (grade 1), moderate (grade 2), severe/disabling (grade 3), life-threatening (grade 4) or death (grade 5)].14 To better accommodate grading of CHCs among long-term survivors, modifications were made to the CTCAE to: (1) define how clinical data (e.g., medical or surgical interventions) were used in severity grading; (2) define more conservative diagnostic ranges with the objective of avoiding over-diagnosis of specific conditions; and (3) conform to diagnostic practice at SJCRH. To describe components of total disease burden, the 168 CHCs were grouped into 48 condition-specific categories (Supplement, Page 7).
All SJLIFE participants and community-control participants completed at least one comprehensive clinical assessment at SJCRH including medical outcome surveys, a complete medical history and physical exam, a standardized laboratory battery, a formal evaluation of neuromuscular function and additional risk-directed diagnostic imaging and testing as previously described.11 CHCs identified by After Completion of Therapy Clinic evaluations were identified by retrospective medical record review. Survivor-reported clinical events were validated by diagnostic reports obtained from community providers (Supplement, Page 6). Medical conditions were clinically assessed in the same fashion for SJLIFE survivors and community-controls11 with the exception of five conditions: hearing loss, glaucoma, cataracts and retinopathy, each self-reported by community-controls, and decreased bone mineral density, evaluated only among survivors using dual-energy X-ray absorptiometry. Decreased bone mineral density was not directly assessed for community-controls but incorporated into analyses using multiple imputation utilizing robust population-based normative data from the National Health and Nutrition Examination Survey (NHANES), which employed the same device used at SJCRH.15,16
Fatal health-related events (grade 5) were ascertained using a combination of data from (1) a National Death Index (NDI) search of survivors in the SJLTFU conducted through December 31, 2011 and (2) continuous annual follow-up from the SJCRH Cancer Registry. Cause of death was identified using ICD 9 and 10 codes from the NDI or direct review of death certificates, medical records or next-of-kin interviews conducted by SJLIFE staff and/or the SJCRH Cancer Registry.
Statistical analysis
Survivors entered the cohort at attained age 18 years or 10 years from primary cancer diagnosis, whichever occurred later. At-risk status ended on June 30, 2015 (censoring), or on the date of death. Community-control participants entered the analysis cohort at age 18 and were censored one day after the completion of clinical assessment. Demographic and treatment differences between campus-visit and non-campus-visit SJLIFE eligible survivors were compared using Chi-square and t-tests. Occurrences and CTCAE grades of CHCs for SJLIFE eligible survivors who were not clinically assessed (non-campus-visit survivors) were handled by the predictive-mean-matching method of multiple imputation in order to minimize potential bias by the missing CHC data.16,17 This approach makes the Missing-at-random assumption, a weaker, more tenable assumption than assuming complete randomness of non-campus visits in the whole cohort. Specifically, it assumes that, after considering the demographic and treatment-exposure variables, non-campus visit occurs at random within each subgroup, but with potentially different rates across subgroups, of survivors formed by these variables. In the first step of predictive-mean-matching, a piecewise exponential model for each of the 48 grouped CHC outcomes was built using the demographic and 21 treatment variables that are available for all survivors regardless of clinical assessment status (Table 1; Supplement, Pages 12–13). Then, for each non-campus-visit survivor, 50 “closest” matched campus-visit survivors were identified based on the sum of the squared distances of standardized predicted rates of the 48 grouped CHC outcomes, of whom one was eventually selected using Applied Bayesian Bootstrap to “donate” his/her CHC data to the non-campus-visit survivor.16 This multivariate imputation of CHC data was repeated 10 times to generate 10 complete datasets of observed plus imputed CHC data for all 5522 survivors in our cohort, reflecting the uncertainty for missing CHCs of each non-campus-visit survivor with 10 possible sets of CHCs. Complete imputation methods are described with greater technical details in the Supplement, Pages 2–4.
Table 1.
Characteristics of the SJLIFE Eligible Survivors and Community Controls
| SJLIFE Eligible Survivors | |||||
|---|---|---|---|---|---|
|
|
|||||
| Total Study Population (n=5522) |
Clinically Evaluable (n=3010) |
Non-Clinically Evaluable (n=2512) |
P value | Community- Controls (n=272)***** |
|
| Gender | <0·001 | ||||
|
| |||||
| Female | 2456 (44%) | 1442 (48%) | 1014 (40%) | 142 (52%) | |
|
| |||||
| Male | 3066 (56%) | 1568 (52%) | 1498 (60%) | 130 (48%) | |
|
| |||||
| Age at Diagnosis (Years) | 0·07 | ||||
|
| |||||
| Mean | 8·4 | 8·3 | 8·6 | - | |
|
| |||||
| Median (Interquartile range) | 7·6 (3·4 – 13·2) | 7·3 (3·3 – 13·1) | 7·9 (3·6 – 13·3) | - | |
|
| |||||
| Range | 0·0 – 28·6 | 0·0 – 24·8 | 0·0 – 28·6 | - | |
|
| |||||
| Age at Censor (Years) | <0·001 | ||||
|
| |||||
| Mean | 34·8 | 36·1 | 33·3 | 35·1 | |
|
| |||||
| Median (Interquartile range) | 33·8 (27·4 – 41·3) | 35·1 (29·2 – 42·3) | 32·3 (25·0 – 40·1) | 34·7 (28·0 – 42·3) | |
|
| |||||
| Range | 18·1 – 70·4 | 18·9 – 68·3 | 18·1 – 70·4 | 18·3 – 70·2 | |
|
| |||||
| Race | 0·005 | ||||
|
| |||||
| White | 4550 (82%) | 2520 (84%) | 2030 (81%) | 238 (87%) | |
|
| |||||
| Other | 972 (18%) | 490 (16%) | 482 (19%) | 34 (13%) | |
|
| |||||
| Treatment Era | <0·001 | ||||
|
| |||||
| <1980 | 1200 (22%) | 649 (22%) | 551 (22%) | - | |
|
| |||||
| 1980–1994 | 2775 (50%) | 1632 (54%) | 1143 (46%) | - | |
|
| |||||
| 1995 or Later | 1547 (28%) | 729 (24%) | 818 (33%) | - | |
|
| |||||
| Any Death (Any Grade 5 Event) | <0·001 | ||||
|
| |||||
| Rate per 10,000 Person Years | 70·3 | 17·7 | 148·2 | - | |
|
| |||||
| Competing Death* | <0·001 | ||||
|
| |||||
| Rate per 10,000 Person Years | 22·6 | 6·6 | 46·2 | - | |
|
| |||||
| Primary Cancer Diagnosis | <0·001 | ||||
|
| |||||
| Acute Lymphoblastic Leukemia | 1685 (31%) | 1007 (33%) | 678 (27%) | - | |
|
| |||||
| Acute Myeloid Leukemia | 214 (4%) | 104 (3%) | 110 (4%) | - | |
|
| |||||
| Hodgkin Lymphoma | 667 (12%) | 368 (12%) | 299 (12%) | - | |
|
| |||||
| Non-Hodgkin Lymphoma | 440 (8%) | 223 (7%) | 217 (9%) | - | |
|
| |||||
| Central Nervous System Tumor | 673 (12%) | 312 (10%) | 361 (14%) | - | |
|
| |||||
| Bone Sarcoma** | 375 (7%) | 208 (7%) | 167 (7%) | - | |
|
| |||||
| Soft Tissue Sarcoma | 354 (6%) | 188 (6%) | 166 (7%) | - | |
|
| |||||
| Wilms Tumor | 358 (6%) | 200 (7%) | 158 (6%) | - | |
|
| |||||
| Neuroblastoma | 239 (4%) | 137 (5%) | 102 (4%) | - | |
|
| |||||
| Retinoblastoma | 153 (3%) | 90 (3%) | 63 (3%) | - | |
|
| |||||
| Germ Cell Tumor | 141 (3%) | 69 (2%) | 72 (3%) | - | |
|
| |||||
| Other*** | 223 (4%) | 104 (3%) | 119 (5%) | - | |
|
| |||||
| Treatment Characteristics**** | 0·01 | ||||
|
| |||||
| Chemotherapy Only | 713 (13%) | 392 (13%) | 321 (13%) | - | |
|
| |||||
| Radiation Only | 28 (1%) | 16 (1%) | 12 (0%) | - | |
|
| |||||
| Surgery Only | 463 (8%) | 217 (7%) | 246 (10%) | - | |
|
| |||||
| Chemotherapy + Radiation | 866 (16%) | 491 (16%) | 375 (15%) | - | |
|
| |||||
| Chemotherapy + Surgery | 1151 (21%) | 638 (21%) | 513 (20%) | - | |
|
| |||||
| Radiation + Surgery | 397 (7%) | 204 (7%) | 193 (8%) | - | |
|
| |||||
| Chemotherapy + Radiation + Surgery | 1884 (34%) | 1045 (35%) | 839 (33%) | - | |
Competing deaths included any grade 5 event that were not categorized among the 168 graded conditions (i.e. accidents, suicide).
Bone Sarcoma: Osteosarcoma and Ewing’s Sarcoma.
Other: Chronic myeloid leukemia (n=46), bi-phenotypic leukemia (n=5), colon carcinoma (n=15), nasopharyngeal carcinoma (n=38), carcinoma NOS (n=38), liver malignancies (n=46), melanomas (n=35).
Full treatment characteristics provided in the supplement, pages 10–11.
P values comparing the the total study population and our community controls are: gender p=0.012, age at censor is 0.632 and race is 0.0301.
CHC data were processed using previously-described cumulative burden subtypes based on clinical definitions of chronicity and recurrence.12,14 Three event subtypes were clinically assigned to each of the 168 graded CHCs: (1) single, recurrent events that can occur multiple times at any grade; (2) chronic, not recurrent events that were counted only once at the time of onset; and (3) chronic, recurrent events, which represent a hybrid of the prior two subtypes. Full classification of conditions and their assigned subtype are presented in the Supplement, pages 7–11. Cumulative burden was calculated using the method of mean cumulative count, which estimates the mean number of recurrent/multiple health events a cohort member experiences by a given time point in the presence of competing risk events.18 The cumulative burden for each of the 168 CHCs was individually calculated and then summed to generate the grouped condition and organ-system categories. The bootstrap percentile method was used to estimate 95% confidence intervals (CI) for individual and organ-system categories. Since survivors entered the cohort at different ages, our calculation of cumulative burden by age accounted for left-truncation.19 All curves and analyses were continued through age 50, as beyond this timepoint our overall and primary-diagnosis-specific cumulative burden estimates were less stable due to limited numbers of survivors older than 50 years.
Marked-point-process regression was performed to assess associations of treatment exposures with cumulative burden (Supplement, Pages 2–4).12,20 This method separates the associations into two stages while evaluating and adjusting for demographic and treatment variables for both: (1) the overall rate of developing any of the 168 grade 1–5 CHCs (associations with the variables expressed as rate ratios) and (2) the propensity for a CHC to be a worse grade given that a condition has developed (associations with the variables expressed as odds ratios for a condition to be grade 2 or 3–5, rather than grade 1). Variables were selected in each model based on backward selection, removing a variable by likelihood-ratio-test based p-values, stopping at p=0.05. Complete marked-point-process regression methods with greater technical detail are further described in the Supplement, Page 4. All statistical analyses were conducted for each of the 10 complete datasets and then 10 sets of the results are summarized by the standard multiple imputation methods.15,16 SAS (version 9·4), R (version 3·2·3) and STATA (version 14·1) were used for statistical analyses.
Role of funding source
None.
Results
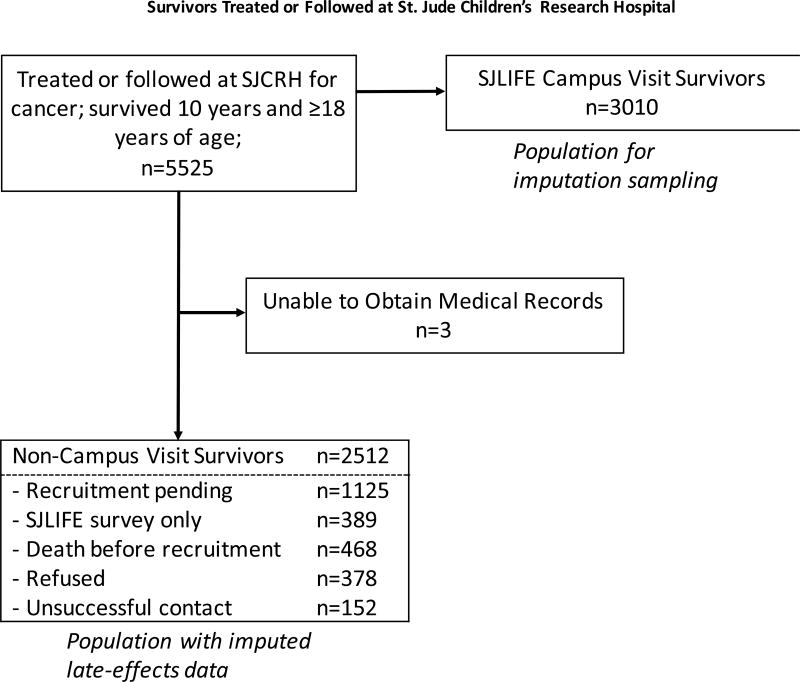
Of 5525 eligible survivors who survived 10 years and became 18 years of age, 5522 had complete records and were included in the analysis (Figure 1). As of the cut-off date for this analysis, 3399 (67·3%) of 5054 patients known to be alive had actively enrolled in SJLIFE, of whom 3010 (88·6%) had completed their initial clinical assessment. Demographic characteristics of the two SJLIFE-eligible groups and community-controls are presented in Table 1. Radiation and chemotherapy differences between the campus-visit and non-campus-visit SJLIFE eligible survivors are further detailed in the supplement, pages 12–13. Using NHANES data, age-sex-race-standardized prevalence rates for CHCs obtained in an analogous manner to SJLIFE showed the prevalence among SJLIFE community-controls is similar to the general US population (Supplement, Page 14).
Figure 1.
Consort Diagram of Survivors Treated or Followed at St. Jude Children’s Research Hospital
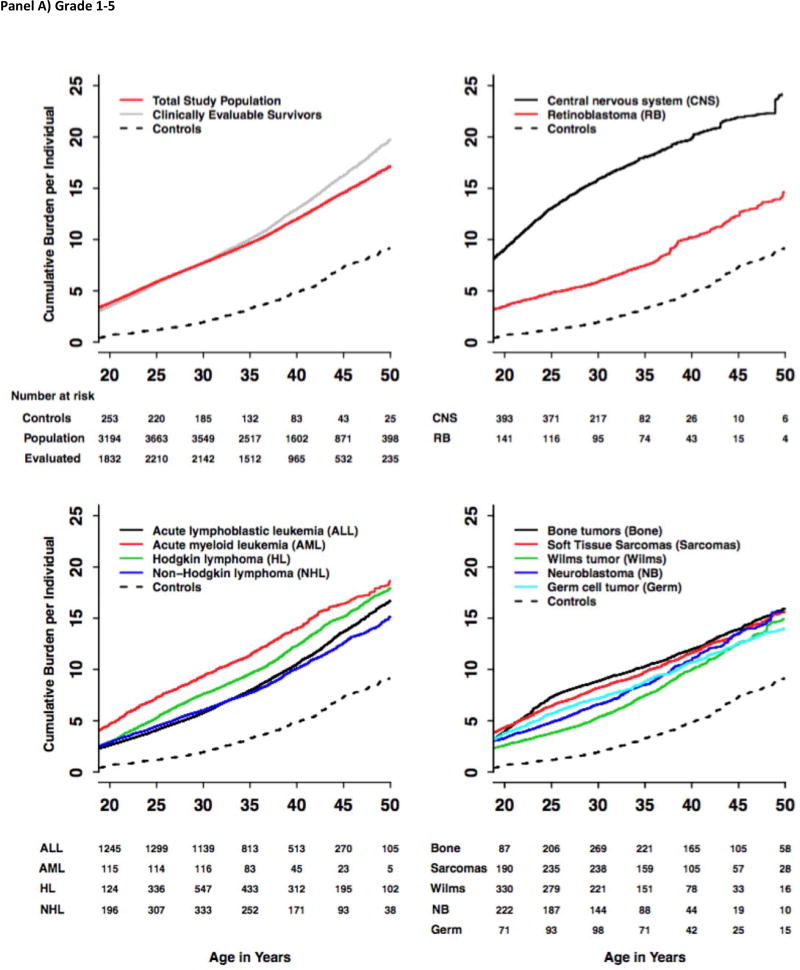
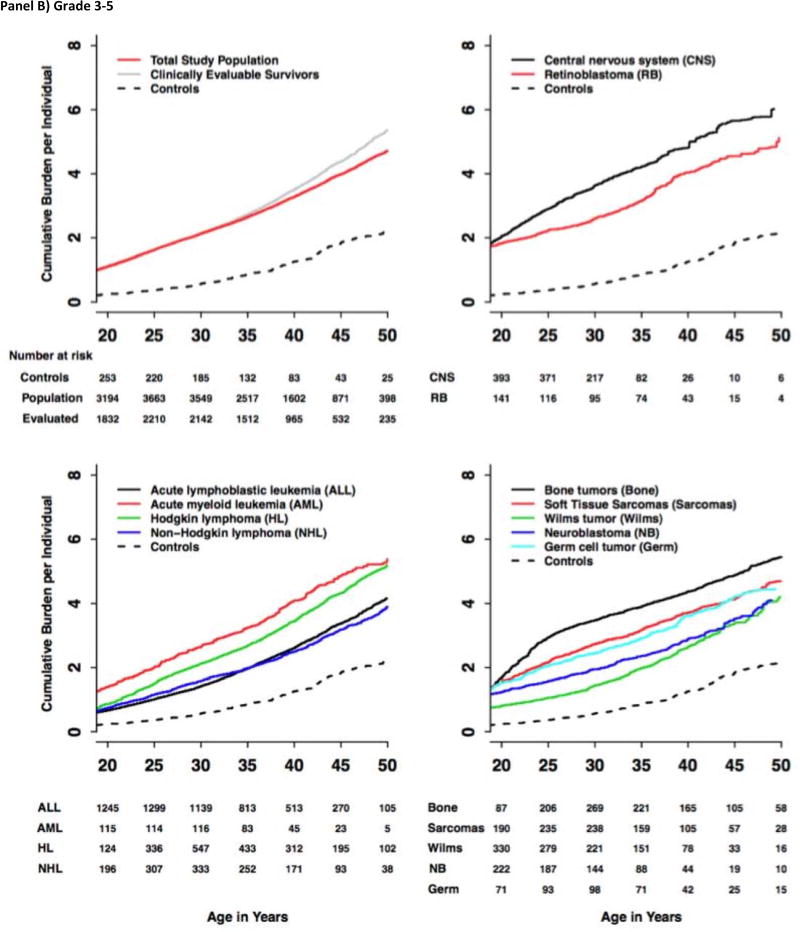
The grade 1–5 and 3–5 cumulative incidence and cumulative burden for the total study population, clinically evaluable survivors, community-controls and each of the primary cancer-specific diagnoses from the total study population were calculated (Figure 2; Supplement, pages 15–27). For the total study population, the cumulative burden was slightly lower compared to the clinically evaluable survivors alone starting after age 35. At age 50, the cumulative incidence of grade 1–5 and grade 3–5 CHCs among the survivors was 99·9% (95% CI 99·9%–99·9%) and 96·0% (95·3%–96·8%), respectively. The cumulative burden at age 50 was, on average, 17·1 (16·2–18·0) grade 1–5 conditions per individual, including 4·7 (4·6–4·9) grade 3–5 conditions. This is in contrast to community controls who had grade 1–5 and grade 3–5 CHC cumulative incidence of 96·0% (93·6%–98·5%) and 84·9% (77·1%–90·0%) (both P-values <0·001 compared to survivors) and cumulative burden of 9·2 (7·9–10·6) and 2·3 (1·9–2·7), respectively (P-values <0·001). The grade 1–5 cumulative incidence at age 50 among the survivors was highest for the cardiovascular [93·2% (92·4%–94·0%)], endocrine [91·6% (90·6%–92·5%)] and musculoskeletal [83·6% (82·3%–85·0%)] systems, with corresponding cumulative burden of 4·0 (3·9–4·2), 2·6 (2·0–3·2), and 1·7 (1·5–2·0), respectively. The cumulative incidence of subsequent malignant neoplasms (SMNs) was 37·3% (34·4%–40·2%) by age 50 with corresponding cumulative burden of 0·9 (0·8–1·1), highlighting that multiple SMNs are an important late-effect in the survivor cohort.
Figure 2.
Cumulative Burden of Grade 1–5 and Grade 3–5 Chronic Health Conditions among SJLIFE Childhood Cancer Survivors and Community-Controls by Diagnosis Groups and Age.
The cumulative burden at age 30 and rate of cumulative burden growth were variable across cancer subtypes and organ systems. For Hodgkin lymphoma survivors, the average number of grade 1–5 cardiovascular CHCs per survivor nearly quadrupled from 1·20 (0·92–1·49) at age 30 to 4·38 (3·99–4·77) by age 50, while the average number of grade 1–5 SMNs increased nearly six fold from 0·17 (0·06–0·28) at age 30 to 1·00 (0·76–1·24) by age 50. In contrast, the CHC burden for other organ systems started high and only slowly increased with age. For example, on average, 67 per 100 CNS tumor survivors experienced grade 1–5 hearing loss (0·60–0·73) at age 30, a number that increased to 83 per 100 (0·70–0·96) at age 50. Neurologic outcomes were similar, increasing slowly from high baseline of 3·70 (3·38–4·03) at age 30 to 4·68 (4·19–5·17) by age 50.
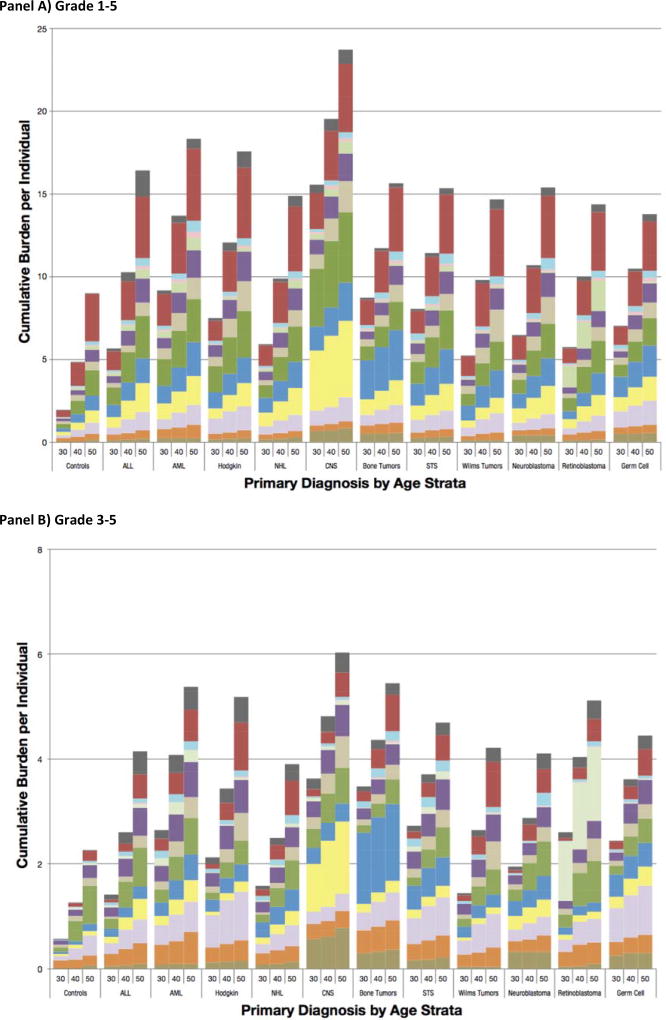
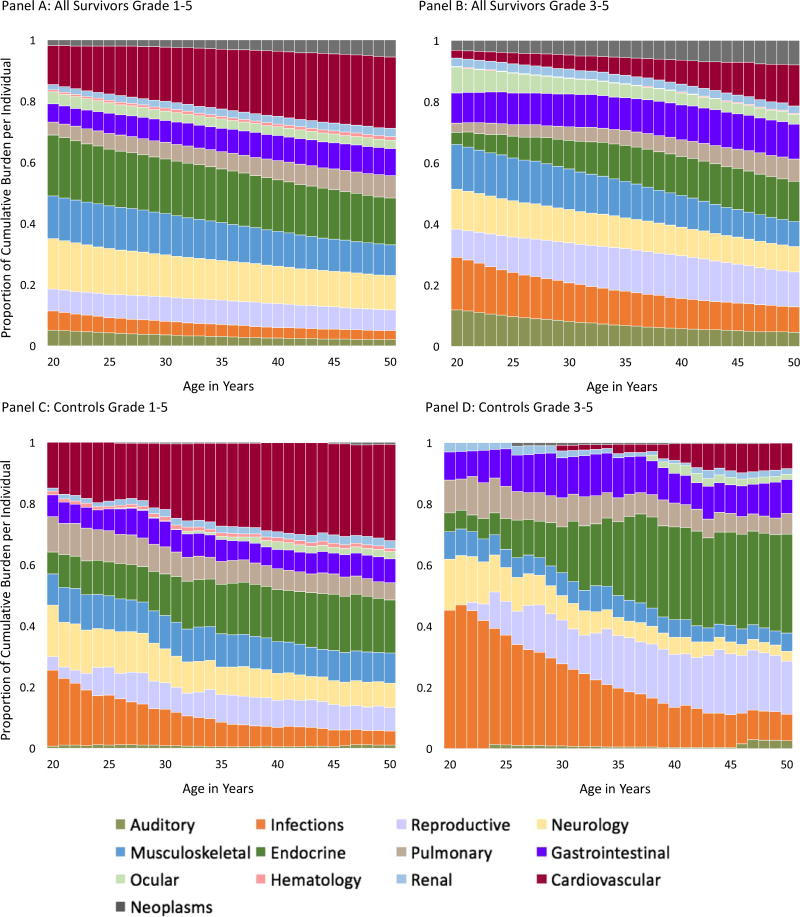
Provided in Figure 3 are distributions of overall and outcome-specific cumulative burden for community-controls and survivors. The cumulative burden of grade 1–5 CHCs at age 50 was highest in survivors of CNS malignancies [24·2 (20·9–27·5)] and lowest in survivors of germ cell tumors [14·0 (11·5–16·6)]. The cumulative burden of grade 3–5 CHCs ranged from 3.9 for survivors of non-Hodgkin lymphoma to 6·0 for survivors of CNS malignancies. Proportional contributions of outcome-specific categories by age (survivors and community-controls) are provided in Figure 4. A pairwise analysis of proportional differences in grade 1–5 cumulative burden among community-controls and cancer-diagnosis subgroups revealed that two pairs of primary cancers: germ cell tumors and soft-tissue sarcomas and germ cell tumors and bone tumors exhibited a similar paired pattern of outcome-specific morbidity, with distributions of morbidity being significantly different across all other paired groups (Supplement, Page 34).
Figure 3.
Distribution of Cumulative Burden among SJLIFE Childhood Cancer Survivors and Community-Controls by Diagnosis Group and Age. Panels A and B present the grade 1–5 and grade 3–5 cumulative burden, respectively.
Numbers on the x-axis represent age in years. ALL: Acute Lymphoblastic Leukemia; AML: Acute Myeloid Leukemia; NHL: Non-Hodgkin Lymphoma; CNS: Central Nervous System Malignancies; Bone-Tumor: Osteosarcoma and Ewing Sarcoma; Soft-Tissue: Soft-Tissue Sarcomas. All data, with 95% confidence intervals, are provided in the supplement, pages 14–23.
Figure 4.
Stacked Bar-plots Representing the Proportional Contribution of Organ System Cumulative Burden to the Total Cumulative Burden Among Controls and Each of the Primary Cancer Sub-groups
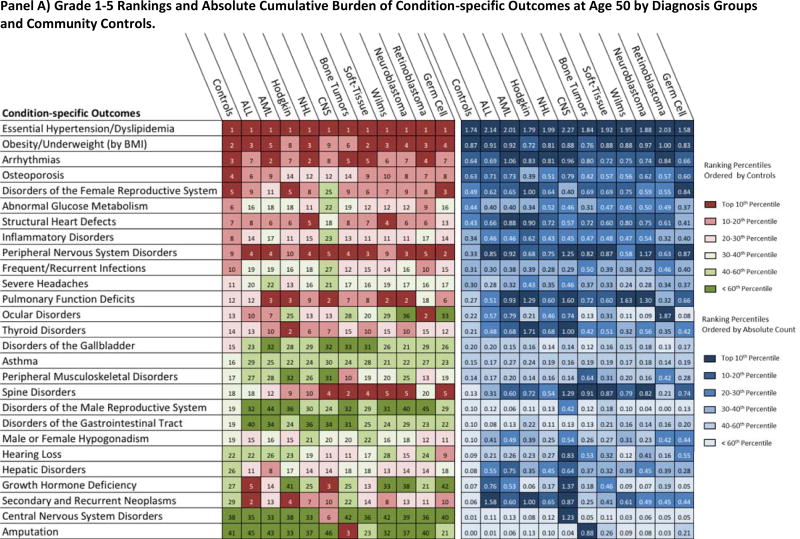
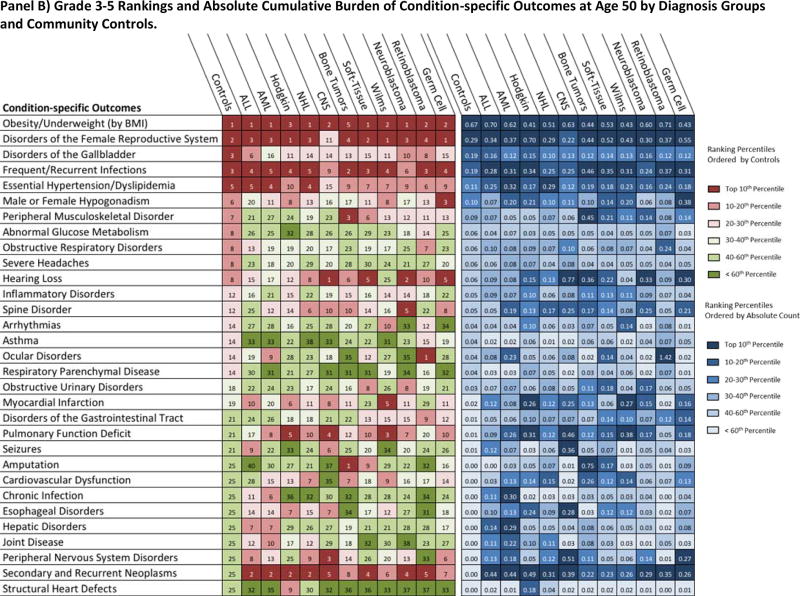
Provided in Figure 5 is both the ranked and absolute cumulative burden at age 50 by outcome and all cohort subgroups. Conditions contributing to metabolic syndrome (essential hypertension, dyslipidemia, abnormal glucose metabolism and obesity) were highly ranked among both survivors and community-controls, with each group exhibiting similar absolute cumulative burdens. Among grade 1–5 outcome-specific categories, arrhythmias and structural heart defects ranked highly in both community-controls and survivors, while secondary and recurrent neoplasms, spinal disorders and pulmonary function deficits were ranked highly among survivors only. For the grade 3–5 categories, secondary and recurrent neoplasms and pulmonary function deficits were ranked below the top 10 for community-controls but were ranked among the top five for two or more primary-diagnosis subgroups.
Figure 5.
Rank and Contribution to Cumulative Burden of Condition-specific Outcomes among SJLIFE Childhood Cancer Survivors and Community-Controls by Diagnosis Group at 50 Years of Age. Panels A and B present the ranking and absolute cumulative burden for the grade 1–5 and grade 3–5 condition-specific outcomes, respectively.
Condition-specific outcomes (detailed composition in the supplement, pages 5–9) are rank ordered in the first portion of figure (reds and greens) according to the top 20 community-control cumulative burden. All condition-specific outcomes ranked below the top 20 among community-controls but within the top 10 among any primary cancer subgroup were also included. In the second portion of the figure (blues), each box corresponds to absolute cumulative burden count per person for each condition-specific outcome and cohort subgroup. For example, ocular disorders rank 14th in terms of absolute grade 3–5 cumulative burden per individual among controls with, on average, 1 occurrence of a severe or life-threatening ocular condition among 25 persons (0.04 cumulative burden per individual). Among AML survivors, ocular disorders rank as the 9th largest absolute cumulative burden with, on average, 1 occurrence of a grade 3–5 condition among 4 survivors (0.23 cumulative burden per survivor). Colors represent overall percentiles per the legend on right. ALL: Acute Lymphoblastic Leukemia; AML: Acute Myeloid Leukemia; NHL: Non-Hodgkin Lymphoma; CNS: Central Nervous System Malignancies; Bone-Tumor: Osteosarcoma and Ewing Sarcoma; Soft-Tissue: Soft-Tissue Sarcomas.
Table 2 shows results of multivariable regression analyses. Two models are provided that separate associations into an overall rate of developing a condition (Model 1) and, if a condition had developed, the propensity for it being a more severe grade (Model 2). After adjusting for all significant demographics and treatment exposures, age at diagnosis, treatment era, and higher brain and chest radiation doses were associated with increased cumulative burden and more severe CHCs. Plant alkaloid and methotrexate exposure appeared protective.
Table 2.
Results of Regression Analysis of Cumulative Burden by Demographic and Treatment Exposures
| Model 1*: Overall Rate | Model 2*: Propensity for a Higher Grade Condition | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Grade 2 vs. 1 | Grade 3–5 vs. 1 | |||||
| RR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Gender | ||||||
|
| ||||||
| Male | - | - | Ref | Ref | ||
|
| ||||||
| Female | - | - | 1·26 (1·18 – 1·35) | <0·001 | 1·31 (1·22 – 1·41) | <0·001 |
|
| ||||||
| Race | ||||||
|
| ||||||
| White | - | - | Ref | Ref | ||
|
| ||||||
| Other | - | - | 1·00 (0·92 – 1·09) | 0·93 | 1·15 (1·05 – 1·27) | 0·003 |
|
| ||||||
| Age at Diagnosis | ||||||
|
| ||||||
| 0–4 | Ref | Ref | Ref | |||
|
| ||||||
| 5–9 | 0·71 (0·66 – 0·76) | <0·001 | 1·04 (0·95 – 1·13) | 0·39 | 1·12 (1·01 – 1·24) | 0·029 |
|
| ||||||
| 10–14 | 0·46 (0·43 – 0·50) | <0·001 | 1·06 (0·93 – 1·19) | 0·38 | 1·26 (1·10 – 1·44) | <0·001 |
|
| ||||||
| 15+ | 0·33 (0·30 – 0·35) | <0·001 | 1·06 (0·90 – 1·26) | 0·47 | 1·51 (1·28 – 1·78) | <0·001 |
|
| ||||||
| Year of Diagnosis | ||||||
|
| ||||||
| <1980 | 0·48 (0·44 – 0·51) | <0·001 | 1·21 (1·08 – 1·35) | 0·001 | 1·57 (1·36 – 1·80) | <0·001 |
|
| ||||||
| 1980–1994 | Ref | Ref | Ref | |||
|
| ||||||
| 1995 or Later | 2·54 (2·37 – 2·72) | <0·001 | 0·94 (0·82 – 1·07) | 0·34 | 0·59 (0·51 – 0·68) | <0·001 |
|
| ||||||
| Anthracycline Dose | ||||||
|
| ||||||
| None | Ref | Ref | Ref | |||
|
| ||||||
| 1–249 mg/m2 | 1·18 (1·10 – 1·27) | <0·001 | 0·89 (0·81 – 0·98) | 0·015 | 0·78 (0·71 – 0·85) | <0·001 |
|
| ||||||
| ≥250 mg/m2 | 1·22 (1·12 – 1·33) | <0·001 | 0·94 (0·84 – 1·05) | 0·28 | 0·95 (0·85 – 1·07) | 0·40 |
|
| ||||||
| Methotrexate | ||||||
|
| ||||||
| No | Ref | - | - | - | - | |
|
| ||||||
| Yes | 0·83 (0·75 – 0·91) | <0·001 | - | - | - | - |
|
| ||||||
| CED Dose** | ||||||
|
| ||||||
| None | - | - | Ref | Ref | ||
|
| ||||||
| 1–6,300 mg/m2 | - | - | 1·04 (0·94 – 1·14) | 0·48 | 1·04 (0·94 – 1·16) | 0·42 |
|
| ||||||
| 6,301–10,893 mg/m2 | - | - | 0·98 (0·89 – 1·08) | 0·62 | 0·99 (0·90 – 1·09) | 0·86 |
|
| ||||||
| ≥10 ,893 mg/m2 | - | - | 1·10 (0·99 – 1·21) | 0·06 | 1·12 (1·01 – 1·25) | 0·023 |
|
| ||||||
| Bleomycin | ||||||
|
| ||||||
| No | - | - | Ref | Ref | ||
|
| ||||||
| Yes | - | - | 1·20 (1·04 – 1·38) | 0·010 | 1·25 (1·06 – 1·47) | 0·006 |
|
| ||||||
| Cytarabine | ||||||
|
| ||||||
| No | Ref | - | - | - | - | |
|
| ||||||
| Yes | 1·23 (1·13 – 1·34) | <0·001 | - | - | - | - |
|
| ||||||
| Plant Alkaloids | ||||||
|
| ||||||
| No | Ref | - | - | - | - | |
|
| ||||||
| Yes | 0·88 (0·82 – 0·96) | 0·001 | - | - | - | - |
|
| ||||||
| Platinum Agents | ||||||
|
| ||||||
| No | Ref | Ref | Ref | |||
|
| ||||||
| Yes | 1·29 (1·16 – 1·43) | <0·001 | 1·12 (0·98 – 1·29) | 0·09 | 1·07 (0·92 – 1·23) | 0·38 |
|
| ||||||
| Steroids | ||||||
|
| ||||||
| No | Ref | Ref | Ref | |||
|
| ||||||
| Yes | 1·13 (1·02 – 1·26) | 0·016 | 0·97 (0·87 – 1·07) | 0·50 | 0·92 (0·84 – 1·01) | 0·08 |
|
| ||||||
| Brain Radiation Dose*** | ||||||
|
| ||||||
| None | Ref | Ref | Ref | |||
|
| ||||||
| <18Gy | 1·08 (0·97 – 1·20) | 0·17 | 1·12 (0·95 – 1·31) | 0·16 | 1·07 (0·92 – 1·24) | 0·37 |
|
| ||||||
| 18–<30Gy | 1·04 (0·97 – 1·12) | 0·28 | 1·11 (1·00 – 1·24) | 0·05 | 1·14 (1·03 – 1·28) | 0·014 |
|
| ||||||
| 30–<40Gy | 1·19 (0·93 – 1·52) | 0·15 | 1·09 (0·76 – 1·56) | 0·63 | 1·10 (0·78 – 1·55) | 0·56 |
|
| ||||||
| 40+Gy | 1·57 (1·43 – 1·72) | <0·001 | 1·27 (1·09 – 1·46) | 0·001 | 1·24 (1·09 – 1·42) | 0·001 |
|
| ||||||
| Chest Radiation Dose*** | ||||||
|
| ||||||
| None | Ref | Ref | Ref | |||
|
| ||||||
| <10Gy | 0·95 (0·87 – 1·05) | 0·33 | 1·20 (1·03 – 1·40) | 0·015 | 1·08 (0·92 – 1·28) | 0·34 |
|
| ||||||
| 10+Gy | 1·41 (1·32 – 1·50) | <0·001 | 1·31 (1·18 – 1·46) | <0·001 | 1·14 (1·01 – 1·28) | 0·030 |
|
| ||||||
| Pelvic Radiation | ||||||
|
| ||||||
| No | - | - | Ref | Ref | ||
|
| ||||||
| Yes | - | - | 1·12 (1·01 – 1·24) | 0·029 | 1·05 (0·94 – 1·18) | 0·35 |
For both models, backward selection was conducted from a set of exposure variables.
CED Dose: Cyclophosphamide equivalent dose with category cutoffs based on tertiles.
Assigned dose is the maximum dose received within the region.
Discussion
Using the SJLIFE cohort, we present the most extensive assessment and comprehensive characterization to date of the long-term health-related morbidity experienced by survivors of childhood cancer. Our current analysis goes beyond previously published results in two important ways. First, many cohort studies have limitations by either relying solely upon self-reported outcomes without concurrent medical validation of CHCs,9 absence of an appropriate control population, and/or lack of detailed treatment exposure data. The SJLIFE cohort was designed to overcome these limitations through prospective clinical assessment and retrospective medical record validation of 168 graded CHCs, recruitment of a comparably assessed community-control population, and abstraction of medical records with detailed survivor-specific demographic and treatment exposure data.7,11,12,18 Second, the traditional methods used to characterize long-term morbidity in survivor populations, such as cumulative incidence and prevalence of health conditions,6–8,21 only describe the first occurrence of an outcome and do not adequately expose the magnitude of the multiple morbidities present in the survivorship population. By applying the cumulative burden, a method of disease burden measurement that incorporates multiple health conditions and recurrent events into a single metric, to the SJLIFE cohort, we define the landscape of disease burden by providing a clinically informative description of the long-term pattern of morbidity survivors of childhood cancer face.12,22 Using cumulative prevalence, we previously reported that by age 45 years, 95·2% of survivors within the SJLIFE cohort experienced at least one CHC and 80% had at least one serious/disabling or life-threatening CHC.5 Now, within the same cohort, we report more specifically that survivors have over twice the burden of disease compared to the general population at age 45, represented by an excess of 7 more CHCs, 2 of which will be serious/disabling, life-threatening or fatal.
Our findings have wide-ranging implications for healthcare delivery, clinical research and health policy. For clinicians, the complex patterns of CHCs contributing to cumulative burden among different subgroups of survivors highlights the healthcare needs of this population that surpass those commonly provided in routine practice. Based on changes in cumulative burden over time (Supplement, Pages 15–27), survivors appear to experience two classes of morbidities: those that are late-occurring, increasing as the cohort ages and at a faster rate compared to community-controls, and early-onset conditions associated with acute effects of cancer therapy. For example, among survivors of hematological malignancies, the contribution of cardiovascular disease and secondary and recurrent neoplasms to overall cumulative burden grows at a faster relative-rate over time compared to other health conditions, contributing a greater proportion as survivors age. Alternatively, the cumulative burden of neurologic and auditory outcomes among survivors of CNS malignancies remain mostly static over our period of follow-up and are primarily represented by irreversible early toxicity such as hearing loss and neuropathies. At any time point, these static conditions may be either controlled or inadequately managed, adding another complex, time-consuming task for healthcare practitioners who must not only tailor and implement survivorship management guidelines for their patients but also conduct consistent and vigilant monitoring of potentially numerous previously diagnosed conditions.
By ranking and quantifying condition-specific outcomes, we provide clinical investigators a more comprehensive knowledge base upon which to draw when designing cancer therapy trials for newly diagnosed patients or intervention approaches for early detection, prevention, or amelioration of treatment-related late effects among long-term survivors of childhood cancer. Currently, an increasing number of clinical trials are being designed to minimize risk of selected treatment-related morbidity. Design of these therapeutic trials is largely based on the results of previous trials, and/or disease-risk measures (incidence, prevalence).23 The additional information provided by the cumulative burden metric allows investigators to delve beyond associations with individual late effects, and characterize sub-populations of survivors with multiple co-morbidities who may benefit from more precise therapeutic interventions.
From a health policy perspective, the heterogeneity of CHCs that comprise cumulative burden between survivor sub-groups emphasizes that this is not a homogenous population. Our data shows the complexity of their medical needs, which vary based on primary cancer diagnosis, treatment and era of exposure. These results, when combined with the early onset and increased severity of medical conditions relative to the general population, help justify why specialized health delivery services could benefit childhood cancer survivors. Previously, others have argued the added value of a community-based shared-care model provided through specialized clinics.24 In the US, these efforts have been complicated as survivors have historically had limited access to health services and insurance due to higher rates of disability and unemployment compared to the general population. Additionally, recent work from SJLIFE found that protocol-based screening and clinical assessment identified undiagnosed conditions, presumably in part due to the unfamiliarity general practitioners have with recommended screening guidelines.7,25 These findings align with studies conducted in Europe where access to primary care services is not insurance dependent yet childhood cancer survivors still have higher rates of hospitalization and poorer health outcomes compared to the general population.21,26,27 This combination of limited access to health services combined with the severe excess morbidity we present in our analysis confirm the vulnerability of this population and question whether consultant long-term follow-up in which primary responsibility remains with community physicians is sufficient. An alternative option, currently being broadly tested in the US among other vulnerable populations such as HIV-infected individuals28 is the patient-centered medical home (PCMH) model, which addresses unique medical and psychosocial needs through coordinated multidisciplinary services.29 Despite rapid changes occurring in insurance coverage and reimbursement due to ongoing debate surrounding the US Patient Protection and Affordable Care Act, American Cancer Centers that already provide survivorship services have a unique opportunity to provide global leadership in survivorship healthcare delivery while benefitting both themselves and the population they serve by accepting increased liability and piloting the feasibility of multi-disciplinary survivorship PCHMs as a model for comprehensive and consolidated services (i.e., primary care, cardiology, pulmonary, endocrine).
Although our application of the cumulative burden metric has quantified morbidity in a new way which complements other approaches/metrics for measuring disease occurrence, there are several limitations and biases we consider important when interpreting our results.22 Although the methods for cumulative burden have been previously vetted, our results should be considered in the context of several limitations.12 First, some of the treatments used to treat childhood cancer do not reflect modern standards of care as older cohort members potentially received more radiation, different doses of chemotherapy and delayed access to screening for late effect conditions.23 Second, since screening guidelines have evolved over time, we anticipate our cohort underestimates lower-grade conditions (CTCAE grades 1–2) from earlier treatment eras since they would remain latently expressed without active screening.7 To address both of these concerns, treatment era was incorporated as a variable in our regression models. Furthermore, while we recognize the observed descriptive data are not easily generalized across treatment eras, they are still clinically relevant for older survivors who will still benefit from improved characterization of their health deficits. Third, while we report the cumulative burden of 168 CTCAE conditions, we restricted these outcomes to non-psychiatric diagnoses. Inclusion of other medical outcomes such as neurocognitive/psychiatric disorders could have resulted in different estimates and explained the protective effects seen with methotrexate.30 In exploring potential trade-offs between treatment exposures affecting cumulative burden, careful and transparent consideration of measured outcomes included in multivariable analyses will be important. Fourth, as previously described the cumulative burden itself is an egalitarian metric that does not incorporate the impact of clinically relevant factors such as social function and health-related quality of life. Although we rank-order our results in Figure 5 based on the quantity of burden, clinical discretion of patient perspectives when making decisions based on these data is still important as survivors would likely estimate their burden based not on quantity but quality.
In order to report results that represent a complete cohort and are broadly generalizable, we used imputation methods and assumed that the missing data among the non-clinically evaluable survivors was missing-at-random after considering demographic and treatment exposures. While we incorporated all available data, potentially important characteristics such as lifestyle factors and socioeconomic status were not known for our non-clinically evaluable survivors and could potentially bias our imputation process and analytic results. Yet, without knowing the true CHC status among the non-clinically evaluated SJLIFE eligible survivors, it is impossible to know whether the combined estimate that we report or the higher estimate from the clinically-evaluated survivors only is closer to the truth. However, in order to provide generalizable data for a clearly-defined cohort of survivors, which we have previously shown7,11 is representative of childhood cancer survivors in the United States (US), we elected to include all long-term survivors eligible for SJLIFE, while acknowledging that our estimates likely represent a conservative lower bound of disease burden in this population. To further examine the generalizability of our results with respect to potential bias due to differences in diagnosis mix between SJLIFE and the general population, we additionally estimated the cumulative burden at age 50 in the 10-year survivors of childhood cancer in the population of the Surveillance, Epidemiology and End Results Program (SEER), a population-based cancer registry of the US covering approximately 10% of the US population. This was accomplished by taking a weighted average of diagnosis-specific cumulative burden values from SJLIFE at age 50, with weights being the diagnosis-specific numbers of SEER childhood-cancer survivors who are age 50 or older and alive on December 31st, 2016. The grade 1–5 and 3–5 estimated cumulative burden at age 50 in the SEER population, compared to the overall SJLIFE cohort, was not different, 17·4 (15·5–19·3) compared to 17·1 (16·2–18·1) and 4·9 (4·0–5·8) compared to 4·7 (4·5–4·8), respectively.
Finally, there are several potential biases to consider when interpreting our results. First, there are racial and gender differences between our total study population and our community-controls. Moreover, our community-control population is relatively small such that, while we demonstrate significant differences between survivor and controls groups, high precision is not achieved. Although we do adjust for demographic variation in our marked-point-process regression models and have shown the CHCs in our community-controls are representative of the general population of the US, these are two important limitations of this initial report and we anticipate continued recruitment and matching of our community-controls will both reduce potential bias and improve precision in subsequent analyses. Second, for five chronic health conditions, ascertainment differed between survivors and community-controls. Although the controls did not have formal audiology or ophthalmologic evaluations, all controls had a complete history and physical exam that would have identified higher grade 2–4 conditions. For grade 1 (asymptomatic or mild) hearing loss or vision CHCs, we anticipate community-controls may have been underdiagnosed since low grade conditions are unlikely to be identified on physical exam alone. As a final point, we acknowledge there is a potential surveillance bias between our survivor population and community-controls, especially for grade 1 conditions. Due to more frequent recommended screenings, it is likely survivors were more closely assessed over time. Thus, we anticipate the onset date for survivors’ CHCs is likely closer to the physiologic date of onset compared to the community-controls’, especially for asymptomatic conditions that are unlikely to be identified without active screening.
In summary, survivors of childhood cancer experience an excess burden of disease associated with their curative therapies. Within this vulnerable population, this is the first study to comprehensively measure and report the landscape of absolute and excess morbidity. These findings reinforce the importance and complexity of successfully providing active clinical management for these high-risk patients.
Supplementary Material
Research in context.
Evidence before this study
Due to their curative treatment-related exposures, survivors of childhood cancer are at increased risk for a broad range of chronic health conditions. We searched PubMed from inception to October 13, 2016, using the terms “childhood cancer survivor” and “childhood or adolescent” and “burden or chronic health conditions or morbidity or long-term outcome” for English language publications describing the burden of chronic health conditions in this population. Prior efforts to describe disease burden among childhood cancer survivors have all used traditional statistics such as relative risk and cumulative incidence, largely relied on either patient reported data without concurrent medical validation of chronic health conditions, lacked a control cohort, and/or were missing the detailed radiation and chemotherapy exposure data we have abstracted in our cohort.
Added value of the study
To our knowledge, this is the first study to provide a comprehensive medical account of the disease burden landscape for a clinically assessed cohort of childhood cancer survivors, with comparison of survivor morbidity to a community-control population. Earlier studies have examined limited aspects of this narrative, generally within selected subsets of the survivor population and relying upon self-reported outcomes. None have explored, in a clinically assessed cohort, how a large and diverse series of chronic health conditions representing all major organ systems relate to one another to form unique patterns of illness between survivor subgroups that, when summed, result in a cumulative burden of disease that is substantially larger than and distinct from that observed in the general population.
Implications of all the available evidence
By the addition of a new statistical method (topic of our 2016 manuscript in Lancet Oncology), which provides greater resolution of disease burden than ever before, and addressing longstanding cohort limitations in survivorship research, we present and visualize a detailed condition by condition assessment of morbidity among the growing high-risk population of childhood cancer survivors. Previous work has shown, in a less global manner, that survivors of childhood cancer suffer more chronic health conditions compared to the general population. Our data go much further and provide a comprehensive landscape of morbidity, while presenting context on the inter-relationships among the various components of disease burden. In clinical and research settings, general health practitioners and clinical investigators can use the information we provide to: (1) address risks as part of patient care, (2) assess trade-offs between exposures and different chronic health conditions to aid the design of future clinical trials, and (3) inform the development of follow-up guidelines. Moreover, from a policy perspective, our data offer the most extensive documentation to date that survivors of childhood cancer are not a monolithic population but are instead comprised of heterogeneous subgroups with complex medical needs and a substantially higher overall disease burden. Although adjunctive survivorship care clinics and close adherence to survivorship guidelines in primary healthcare settings are the current global standard, the numerous morbidity profiles that we describe suggest that survivors may benefit from specialized healthcare delivery, similar to that being advocated for other high-risk populations.
Acknowledgments
Funding: The U.S. National Cancer Institute (U01 CA-195547, P30 CA-21765), St. Baldrick’s Foundation and the American Lebanese Syrian Associated Charities all partially funded this study and had no role in the study design, data collection, data analysis, data interpretation, the writing of the report, or the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ Contributions: NB, MMH, YY and LLR designed the study. NB, FY, MB, HE, WC, MJE, JB, MWB, KS, LL, SH, ZL, CH and KKN prepared the data. NB, QL, YY analyzed the data and prepared the manuscript. NB, QL, KKN, MB, HE, FY, WC, MJE, JB, MWB, EC, JL, TF, VJ, DMG, DAM, GA, KK, TB, RK, DKS, MH, YY and LLR discussed and revised the manuscript. NB, MMH, YY and LLR supervised the study. NB, QL, KKN, MMH, YY and LLR had access to the raw data. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
Declaration of interest: The other authors declared no conflicts of interest.
References
- 1.Armstrong GT, Chen Y, Yasui Y, et al. Reduction in Late Mortality among 5-Year Survivors of Childhood Cancer. N Engl J Med. 2016;374(9):833–42. doi: 10.1056/NEJMoa1510795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howlader NNA, Krapcho M, et al. SEER Cancer Statistics Review (CSR), 1975–2012. [accessed August 1, 2016];2015 http://seer.cancer.gov/csr/1975_2012/
- 3.Winther JF, Kenborg L, Byrne J, et al. Childhood cancer survivor cohorts in Europe. Acta Oncol. 2015;54(5):655–68. doi: 10.3109/0284186X.2015.1008648. [DOI] [PubMed] [Google Scholar]
- 4.Fidler MM, Reulen RC, Winter DL, et al. Long term cause specific mortality among 34 489 five year survivors of childhood cancer in Great Britain: population based cohort study. BMJ. 2016;354:i4351. doi: 10.1136/bmj.i4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer. 2014;14(1):61–70. doi: 10.1038/nrc3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–82. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 7.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309(22):2371–81. doi: 10.1001/jama.2013.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geenen MM, Cardous-Ubbink MC, Kremer LC, et al. Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA. 2007;297(24):2705–15. doi: 10.1001/jama.297.24.2705. [DOI] [PubMed] [Google Scholar]
- 9.Bhatia S, Armenian SH, Armstrong GT, et al. Collaborative Research in Childhood Cancer Survivorship: The Current Landscape. J Clin Oncol. 2015;33(27):3055–64. doi: 10.1200/JCO.2014.59.8052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reulen RC, Frobisher C, Winter DL, et al. Long-term risks of subsequent primary neoplasms among survivors of childhood cancer. JAMA. 2011;305(22):2311–9. doi: 10.1001/jama.2011.747. [DOI] [PubMed] [Google Scholar]
- 11.Hudson MM, Ness KK, Nolan VG, et al. Prospective medical assessment of adults surviving childhood cancer: study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort study. Pediatr Blood Cancer. 2011;56(5):825–36. doi: 10.1002/pbc.22875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhakta N, Liu Q, Yeo F, et al. Cumulative burden of cardiovascular morbidity in paediatric, adolescent, and young adult survivors of Hodgkin's lymphoma: an analysis from the St Jude Lifetime Cohort Study. Lancet Oncol. 2016;17(9):1325–34. doi: 10.1016/S1470-2045(16)30215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landier W, Bhatia S, Eshelman DA, et al. Development of risk-based guidelines for pediatric cancer survivors: the Children's Oncology Group Long-Term Follow-Up Guidelines from the Children's Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol. 2004;22(24):4979–90. doi: 10.1200/JCO.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 14.Hudson MM, Ehrhardt MJ, Bhakta N, et al. Approach for Classification and Severity-grading of Long-term and Late-onset Health Events among Childhood Cancer Survivors in the St. Jude Lifetime Cohort. Cancer Epidemiol Biomarkers Prev. 2016 doi: 10.1158/1055-9965.EPI-16-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelly TL, Wilson KE, Heymsfield SB. Dual energy X-Ray absorptiometry body composition reference values from NHANES. PLoS One. 2009;4(9):e7038. doi: 10.1371/journal.pone.0007038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molenberghs G, Fitzmaurice GM, Kenward MG, Tsiatis AA, Verbeke G. Handbook of missing data methodology. Boca Raton: CRC Press, Taylor & Francis Group; 2015. [Google Scholar]
- 17.Little RJA, Rubin DB. Statistical analysis with missing data. 2. New York, N.Y.: Wiley; 2014. [Google Scholar]
- 18.Dong H, Robison LL, Leisenring WM, Martin LJ, Armstrong GT, Yasui Y. Estimating the burden of recurrent events in the presence of competing risks: the method of mean cumulative count. Am J Epidemiol. 2015;181(7):532–40. doi: 10.1093/aje/kwu289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geskus RB. Cause-Specific Cumulative Incidence Estimation and the Fine and Gray Model Under Both Left Truncation and Right Censoring. Biometrics. 2011;67(1):39–49. doi: 10.1111/j.1541-0420.2010.01420.x. [DOI] [PubMed] [Google Scholar]
- 20.Berridge DM. An application of a marked point process in pre-clinical medicine. Stat Med. 1996;15(24):2751–62. doi: 10.1002/(SICI)1097-0258(19961230)15:24<2751::AID-SIM497>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 21.Rebholz CE, Reulen RC, Toogood AA, et al. Health care use of long-term survivors of childhood cancer: the British Childhood Cancer Survivor Study. J Clin Oncol. 2011;29(31):4181–8. doi: 10.1200/JCO.2011.36.5619. [DOI] [PubMed] [Google Scholar]
- 22.Aznar MC, Darby S, Collins GP, Cutter D. Cumulative burden of disease: a relevant measure of the late side-effects of cancer treatment. Lancet Oncol. 2016;17(9):1189–90. doi: 10.1016/S1470-2045(16)30283-2. [DOI] [PubMed] [Google Scholar]
- 23.Hudson MM, Neglia JP, Woods WG, et al. Lessons from the past: opportunities to improve childhood cancer survivor care through outcomes investigations of historical therapeutic approaches for pediatric hematological malignancies. Pediatr Blood Cancer. 2012;58(3):334–43. doi: 10.1002/pbc.23385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oeffinger KC, McCabe MS. Models for delivering survivorship care. J Clin Oncol. 2006;24(32):5117–24. doi: 10.1200/JCO.2006.07.0474. [DOI] [PubMed] [Google Scholar]
- 25.Suh E, Daugherty CK, Wroblewski K, et al. General internists' preferences and knowledge about the care of adult survivors of childhood cancer: a cross-sectional survey. Ann Intern Med. 2014;160(1):11–7. doi: 10.7326/M13-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sieswerda E, Font-Gonzalez A, Reitsma JB, et al. High Hospitalization Rates in Survivors of Childhood Cancer: A Longitudinal Follow-Up Study Using Medical Record Linkage. PLoS One. 2016;11(7):e0159518. doi: 10.1371/journal.pone.0159518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brewster DH, Clark D, Hopkins L, et al. Subsequent hospitalisation experience of 5-year survivors of childhood, adolescent, and young adult cancer in Scotland: a population based, retrospective cohort study. Br J Cancer. 2014;110(5):1342–50. doi: 10.1038/bjc.2013.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pappas G, Yujiang J, Seiler N, et al. Perspectives on the role of patient-centered medical homes in HIV Care. Am J Public Health. 2014;104(7):e49–53. doi: 10.2105/AJPH.2014.302022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dale SB, Ghosh A, Peikes DN, et al. Two-Year Costs and Quality in the Comprehensive Primary Care Initiative. N Engl J Med. 2016;374(24):2345–56. doi: 10.1056/NEJMsa1414953. [DOI] [PubMed] [Google Scholar]
- 30.Krull KR, Cheung YT, Liu W, et al. Chemotherapy Pharmacodynamics and Neuroimaging and Neurocognitive Outcomes in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia. J Clin Oncol. 2016;34(22):2644–53. doi: 10.1200/JCO.2015.65.4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.