Abstract
Background
Despite potential adverse-events in a pediatric population, corticosteroids (CS) are used to induce remission in pediatric Crohn’s disease. Exclusive enteral nutrition (EEN) also induces remission, but is infrequently used in the United States because CS is considered the superior therapy. New data have become available since the publication of the most recent meta-analysis in 2007.
Methods
All studies with comparator arms of EEN and an exclusive CS, with remission clearly defined were identified. Online bibliographic databases including MEDLINE, EMBASE, Web of Science, Cochrane Databases, Open Grey, Grey Lit, Clinicaltrials.gov, and the WHO lists of clinical trials were searched.
Results
Of 2795 identified sources, nine studies met our inclusion criteria. Eight of these (n=451), had data that could be abstracted into our meta-analysis. EEN was as effective as CS in inducing remission (OR=1.26 [95% CI 0.77, 2.05] in pediatric Crohn’s disease. There was no difference between EEN and CS efficacy when comparing newly diagnosed Crohn’s (OR = 1.61 [ 95% CI 0.87, 2.98]) or relapsed (OR = 0.76 [95% CI 0.29–1.98]). Intestinal healing was significantly more likely among patients receiving EEN compared to CS (OR=4.5 [95% CI 1.64, 12.32]). There was no difference in the frequency of biomarker normalization including CRP (OR=.85 [.44, 1.67]) and fecal calprotectin (OR 2.79 [95% CI 0.79–10.90]).
Conclusion
There is no difference in efficacy between EEN and CS in induction of remission in Crohn’s disease in a pediatric population. Exploratory analyses suggest that a greater proportion of patients treated with EEN achieved mucosal healing.
INTRODUCTION
Crohn’s Disease (CD) is a chronic illness characterized by destructive transmural inflammation of the gastrointestinal tract with periods of flares and remission.1 While CD is most commonly diagnosed among 15–35 year olds, a quarter of patients are diagnosed as children under the age of 18.2,3
Corticosteroids are the most commonly used medication to induce remission in the United States,4 but can pose particular risks in a pediatric population including: growth retardation, low bone mineral density, adrenal suppression, and body image dissatisfaction.5 As an alternative, gastroenterologists sometimes elect to use exclusive enteral nutrition (EEN) as induction therapy in these patients6 because adverse effects of enteral nutrition are generally limited to gastrointestinal tolerability (e.g. nausea, vomiting, diarrhea) though rare reports of refeeding syndrome exist.7 This therapy is frequently used in Europe, but fewer than 4% of pediatric gastroenterologists use it in North America, though emerging data shows it is gaining more prominence in Canada.6,8
The benefits of enteral nutrition in patients with Crohn’s disease were auspiciously identified in the surgical literature when patients administered enteral nutrition to optimize their nutritional status preoperatively, improved unexpectedly, with some even avoiding the intended surgery.9 The mechanism of the beneficial effects of enteral nutrition was hypothesized to be the avoidance of enteral antigens resulting in improvements in intestinal permeability, though recent studies have elucidated other possibilities10, including down regulation of pro-inflammatory cytokines.11 Others have found beneficial changes to the gut microbiome among Crohn’s patients treated with enteral nutrition.12,13
There are three major categories of enteral nutrition formulas, and they are differentiated by the structure of their protein content. Elemental diets contain no intact protein, only amino acids. Unfortunately, elemental diets are distinctly distasteful and often require a feeding tube to administer. Even semi-elemental diets (i.e. peptides of varying length) were found to be intolerable with 57% of patients withdrawing from a major study mostly due to poor palatability.14 Polymeric diets (i.e. intact protein), which are more palatable and do not always require feeding tube placement for administration, have been shown to perform as well as elemental and semi-elemental diets in the induction of remission of CD.15
Previous comparisons regarding the effectiveness of EEN versus CS in remission induction have yielded mixed results. Five out of seven meta-analyses published over the last 20 years have found CS to be superior to EEN at inducing remission.15–19 Of the seven meta-analyses, two have mixed populations of adults and children, three included only adult studies and two focused only on children. EEN is hypothesized to work more effectively in children than adults, though the reasons for this are unclear.6 The two pediatric meta-analyses focused only on induction of remission,20,21 found no difference in remission induction comparing CS to EEN.
In the ten years that have elapsed since the most recent meta-analysis on this subject, several more studies22–25,35 have been published, which have included new data on biomarkers and mucosal healing, two previously unexplored end points. Therefore, we performed an updated systematic review and meta-analysis on the efficacy of CS versus EEN in the pediatric population. Our primary outcome was the induction of remission. We distinguished the treatment effects between newly diagnosed and relapsed patients. Because the field has moved towards recognizing the importance of more objective criteria to identify improvement and monitor disease status,26 we conducted three hypothesis-generating analyses to examine the effect of EEN vs. CS on durability of remission, mucosal healing and biomarkers.
METHODS
Inclusion criteria for studies
Types of studies
All randomized and observational studies with at least two comparator arms including at least one dietary intervention administered by any route and one non-dietary intervention in English were considered for inclusion. Abstracts and conference proceedings were excluded, as data suggests that abstracts may be inconsistent with the final published article27 and they have not had the benefit of peer-review.
Types of participants
Patients under the age of 18 with newly diagnosed or relapsed active Crohn’s disease.
Types of interventions
Administration of EEN in the following formulations: polymeric formula, semi-elemental formula, and elemental formula to one group of patients and CS to the comparator arm in this systematic review and meta-analysis. Patients in the EEN arm must not be receiving any other medication, and those receiving CS must only be treated with CS. Though there has been no convincing evidence that there is any difference in benefit between the types of formulas used, and the Zachos et al.15 Cochrane analysis combined all exclusive enteral nutrition analyses, we conducted both combined and stratified analyses.
Exclusion Criteria
Trials without an EEN arm and trials without at least one clearly defined CS comparator arm were excluded from this analysis. Additionally, authors who did not define remission within the text of their manuscript were excluded.
Types of outcome measures
All of the potential studies that met inclusion criteria (Figure 1) measured disease activity with either the Lloyd-Still Index (LSI) or Pediatric Crohn’s Disease Activity Index (PCDAI). Because the cut off points for each scale is subjective, we defined our outcome variable as the percentage of patients who remained in remission, as defined by the study authors. In cases where this information was not provided in the published manuscript, the additional information was sought directly from the authors.
Figure 1.
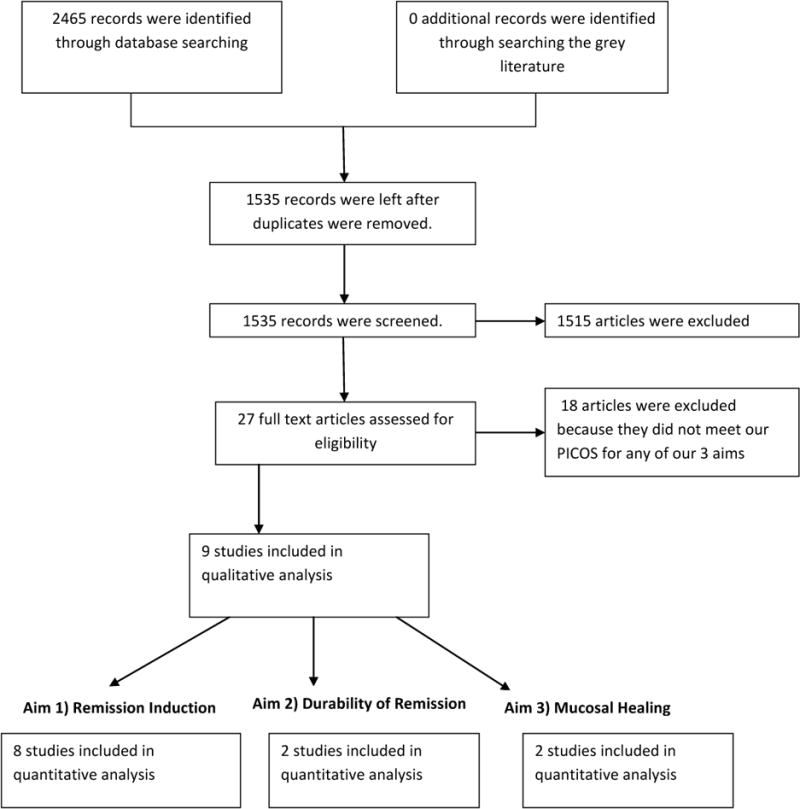
Search methods for identification of studies
We searched MEDLINE, EMBASE, Web of Science, The Cochrane Library, Open Grey, Grey Literature Report, Clinicaltrials.gov and the WHO Clinical Trials Registry Platform from inception to September of 2016. All relevant subject headings and free text terms were used to represent Crohn’s Disease and enteral nutrition, including exp Inflammatory Bowel Diseases/OR (Crohn$ adj (disease or enteritis)).tw AND Enteral Nutrition/ OR((enteral or enteric or intragastric or intraintestinal or intestinal or tube) adj (nutrition or feeding)).tw. (see Appendix 1 for full text of search strategies). These terms were adapted for the other databases.
Data collection and analysis
Study selection
A research librarian (LF) designed and conducted the complete search strategy. Articles were screened independently by three reviewers (SLF, AF, and AS). Any disagreement at this stage of screening was remediated by acquiring the full text of the article, and discussion until consensus was achieved. Of the 2795 papers identified through our search strategy, 43 articles potentially meeting inclusion criteria were identified and agreed upon by all three reviewers and the full text was reviewed. Nine articles met inclusion criteria, but three articles (Sanderson 198728, Papadopoulou 199529, and Levine 201425) did not provide the necessary data for inclusion in the meta-analysis. These authors were contacted for more complete data. Two authors (Levine 201425 and Sanderson 198728) provided additional data, leaving a total of eight papers which were included in the meta-analysis (Table 1, Figure 1). Justifications for excluded articles are included in Table 2.
Table 1.
Characteristics of Included studies
| Authors | Biases | Comparator Arms | Number of Participants | New and/or Relapsed Disease | Disease location | Outcome measurement |
|---|---|---|---|---|---|---|
| Randomized Controlled Trials | ||||||
| Sanderson et al. 1987 | Randomized, not blinded | Sulfasalazine + EEN vs. Elemental Diet vs. CS+ sulfasalazine | 15 | Relapsed | Small Bowel Only | LSI |
| Borrelli et al. 2006 | Randomized, blinded, ITT analysis | Oral Polymeric Formula vs. CS | 37 | New | All | Absence of symptoms related to Crohn’s Disease and a PCDAI of 10 or less; colonoscopy with CDEIS |
| Retrospective Cohort Studies | ||||||
| Papadopoulou et al. 1995 | Not randomized, not blinded | EEN vs. CS | 36 | Relapsed | All | Biomarkers & LSI |
| Berni Canani et al. 2005 | Not randomized, not blinded | EEN vs. CS | 47 | New and relapsed | All | Endoscopic & histologic remission; PCDAI <10 |
| Lambert et al. 2012 | Not randomized, not blinded, EN was preferentially used | EEN vs. CS | 57 | New | All | PCDAI<15 |
| Hojsak et al. 2014 | Not randomized, not blinded | EEN vs. CS | 74 | Relapsed | All | PCDAI <10 |
| Prospective Cohort Studies | ||||||
| Kierkus et al 2013 | Not randomized, not blinded | EEN vs CS | 44 | Relapsed | All | PCDAI <10 |
| Levine et al. 2014* | Not randomized, not blinded | EEN vs. CS vs. Mesalamine vs. EEN+Mesalamine | 201 | New | All | CRP & PCDAI <10 |
| Lee et al. 2015 | Not randomized, not blinded | PEN vs. EEN vs. Anti-TNF Therapy | 90 | New and relapsed | All | Reduction of PCDAI by >=15 points OR PCDAI < 10 |
RCT, Randomized controlled trial; ITT, Intent to treat analysis; PCDAI, Pediatric Crohn’s Disease Activity Index; LSI, Lloyd Still Disease Activity Index Score
Table 2.
Characteristics of excluded studies
| Study | Year | Reasons for exclusion |
|---|---|---|
| Seidman et al. | 1991 | abstract only |
| Seidman et al. | 1993 | abstract only |
| Thomas et al. | 1992 | wrong outcome |
| Thomas et al. | 1993 | no patient level data |
| Breese et al. | 1995 | poor classification of steroid exposre |
| Terrin et al. | 2002 | poor drug classification, no information on disease distribution |
| Ruuska et al. | 1994 | wrong outcome, no discussion of randomization |
| Soo et al. | 2013 | 80% of EEN group on AZA and 64% on 5-ASA, not a clean comparison of EEN vs. CS |
| Saadah et al. | 2012 | combines data from EEN and CS arm, not a true comparator two armed study |
| Gorard et al. | 2003 | adult population |
| Mesker et al. | 2009 | no EN only comparator arm |
| Zhu et al. | 2013 | adult population |
| Hartmann et al. | 2008 | no EN only comparator arm |
| Sigall-Boneh et al. | 2014 | no non-diet treatment arm |
| Workman et al. | 1984 | no non-diet treatment arm |
| Harries et al. | 1983 | adult population |
| Riordan et al. | 1993 | adult population |
| Matsumoto et al. | 2005 | no EN only comparator arm, adult population |
AZA, azathioprine; 5-ASA, mesalamine; EEN, exclusive enteral nutrition; EN, Enteral Nutrition; CS, corticosteroids
Levine 201425 had 4 arms: receipt of only EEN, only CS, only. Mesalamine and last a combined EN and mesalamine arm. In our meta-analysis we only included the EEN and CS arm. Sanderson et al. 198728 used corticosteroids and sulfasalazine in one arm, and an elemental diet only in the other arm. Since sulfasalazine has not shown any benefit in inducing remission in small bowel Crohn’s disease,30 we included this study in the meta-analysis with the assumption that CS + sulfasalazine (in small bowel Crohn’s disease) is equivalent to CS only treatment.
Quality assessment
Three authors (SLF, AF, AS) independently reviewed the quality of the studies. We evaluated the bias in observational studies using the New Castle-Ottawa scale31 and randomized controlled trials using the Cochrane RoB tool32 (Table 1, Table 3). Observational studies, less rigorous than randomized controlled trials (RCT), inherently introduced uncertainty and heterogeneity into our meta-analysis. As such, we conducted two sensitivity analyses. First, we removed the study where the author admitted that the study team preferentially assigned patients to the enteral nutrition arm of the trial (Lambert et al. 201224). Second, we analyzed the results for the RCTs, prospective cohort studies, and retrospective cohort studies separately—to determine if the results from the non-RCTs affected our heterogeneity calculation or produced a different result from the more robust RCTs. Because there was not a heterogenous effect across strata when we examined each study type separately for our main outcome, we felt it was appropriate to combine study types as hypothesis generating rather than confirmatory analyses.
Table 3.
Newcastle–Ottawa Scale for assessing quality of cohort studies.
| Quality assessment Scale | Accepted Criteria | Papadopoulou et al. 1995 | Berni Canani et al. 2006 | Lambert et al. 2012 | Hojsak et al. 2014 | Lou et al. 2015 | Kierkus et al. 2013 | Levine et al. 2014 |
|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort | Representative of average pediatric IBD patient | * | * | * | * | * | * | * |
| Ascertainment of exposure | Secure records, structured interview | * | * | * | * | * | * | * |
| Demonstration that outcome of interest was not present at start of study | No prior exposure to EEN/Steroids | * | – | * | * | * | – | * |
| Comparability of cohorts on the basis of design or analysis | Comparability of medical treatment arm | – | * | * | * | * | * | – |
| Assessment of outcome | Utilization of predefined activity score or clinical evaluation | * | * | * | * | * | * | * |
| Enough follow-up for outcome to occur | At least 4 weeks | * | * | * | * | * | * | * |
| Adequacy of follow-up of cohorts | Follow-up of complete cohort or less than 15% loss to follow-up | * | * | * | – | – | – | * |
| Total (max=7) | 6 | 6 | 7 | 6 | 6 | 5 | 6 |
Data extraction
Data extraction forms were developed by two of the authors (AF, AS) and confirmed by a third (SLF). Data were extracted jointly by two authors (AF, AS) and then a third author (SLF) independently extracted the data. Disagreements were reconciled by discussion. Extracted data included: participant age and disease characteristics, number of patients in each treatment arm, outcome measurement (PCDAI vs. LSI vs. “biochemical remission”), study design, study inclusion criteria, details of enteral nutrition formulation and intervention, details of medical intervention, length of follow-up, and remission rates at the follow-up visit.
Statistical analyses
The percentages of patients in remission at the end of the first follow-up period from each study were pooled. Cross-study variation due either to heterogeneity or chance was quantified using both I2 and Chi2 where P < 0.10 or I² > 50% indicates substantial heterogeneity. Fixed-effect models were used. If heterogeneity was present, a random effects model was used to calculate the most conservative confidence intervals. Each study’s weight was determined using Mantel-Haenszel methods. All analyses were conducted using RevMan5 (version 5.3.5 Copenhagen, Denmark).
RESULTS
Risk of Bias in included studies
We evaluated bias in randomized controlled trials according to the methods described in the Cochrane Handbook32 which includes consideration of the following: blinding, randomization, completeness of outcome data and method of reporting data (Table 1). Observational studies were evaluated for bias using the Newcastle-Ottawa tool32,33 (Table 3). Data have reported the data according to MOOSE guidelines.34
Characteristics of Included Studies
Of the nine trials that met our inclusion criteria (Table 1, Figure 1), eight study authors either included or provided the proportion of patients who entered remission in each arm which could be pooled in the meta-analyses.
Of the eight trials included in the quantitative meta-analysis, seven compared EEN to CS (Borreli et al. 200635, Berni Canani et al. 200636, Hojsak et al. 201422, Kierkus et al. 201323, Lambert et al. 201224 Levine et al. 201425, Luo et al. 201537). Sanderson et al.28 compared EEN to sulfasalazine + CS.
Five studies used a polymeric diet. Sanderson et al.28 used an elemental diet, and Berni Canani et al.36 had separate arms for elemental, semi elemental, and polymeric diets that were pooled together for our meta-analysis. Luo et al.37 did not specify the type of enteral nutrition that they used.
Three studies provided specific details about the steroid dosing arms. Berni Canani et al.36 used methylprednisolone (1–2mg/kg) up to max of 40mg/d for 4 weeks, and then tapered over the following 4 weeks. Levine et al.25 used prednisone 1–2mg/kg up to max of 60mg/d for 8 weeks. Sanderson et al.28 used adrenocorticotropic hormone (ACTH) 2 IU/kg administered intramuscularly for 5 days followed by oral prednisone 2mg/kg/d up to maximum of 30mg/d with a taper starting after 3 weeks.
The study participants in all trials included in the meta-analysis were children, and the diets were administered via nasogastric tube (elemental diets) or orally. Of the eight studies, four studies (Borrelli 200635, Lambert 201224, Levine 201425, Luo 201537) included only newly diagnosed patients, whereas the others included both newly diagnosed and relapsed patients, or relapsed only. Disease activity and remission were based on PCDAI scores, though the cutoff for remission varied and some studies did include supplemental information from biomarkers or endoscopic scores (CDEIS). Sanderson et al.28 used the LSI along with biomarkers (CRP, ESR, albumin concentration) to determine remission. Correspondence with the author was required in order to obtain patient level Lloyd Still scores levels at multiple time points. Outcomes were assessed at 6–8 weeks except Borrelli et al.35 assessed outcomes after 10 weeks of treatment.
One other paper met inclusion criteria, but was not included in the meta-analysis because data could not be abstracted from the paper and could not be obtained from the corresponding author. Papadopoulous et al.29 used an elemental diet arm (n=19) and oral prednisone (2mg/kg/d, max 60mg/d; n=17) with taper over 8 weeks, but did not provide individual patient status at end of induction. Instead they describe successful treatment of flare episodes (each patient could have more than one episode of flare). They found remission in 83% of episodes treated with EEN and 64% treated with prednisone.
Effects of Interventions
A. Enteral Nutrition Therapy for Induction of Remission
This meta-analysis of eight trials included 226 pediatric Crohn’s patients treated with an exclusive elemental diet and 225 pediatric Crohn’s patients treated with corticosteroids for newly diagnosed or relapsed disease demonstrated no statistically significant difference between the treatment arms (OR 1.26; [95% CI 0.77–2.05]; Figure 2. Comparison of Remission induction for EEN vs. CS). Heterogeneity was not demonstrated (I2 = 0).
Figure 2.
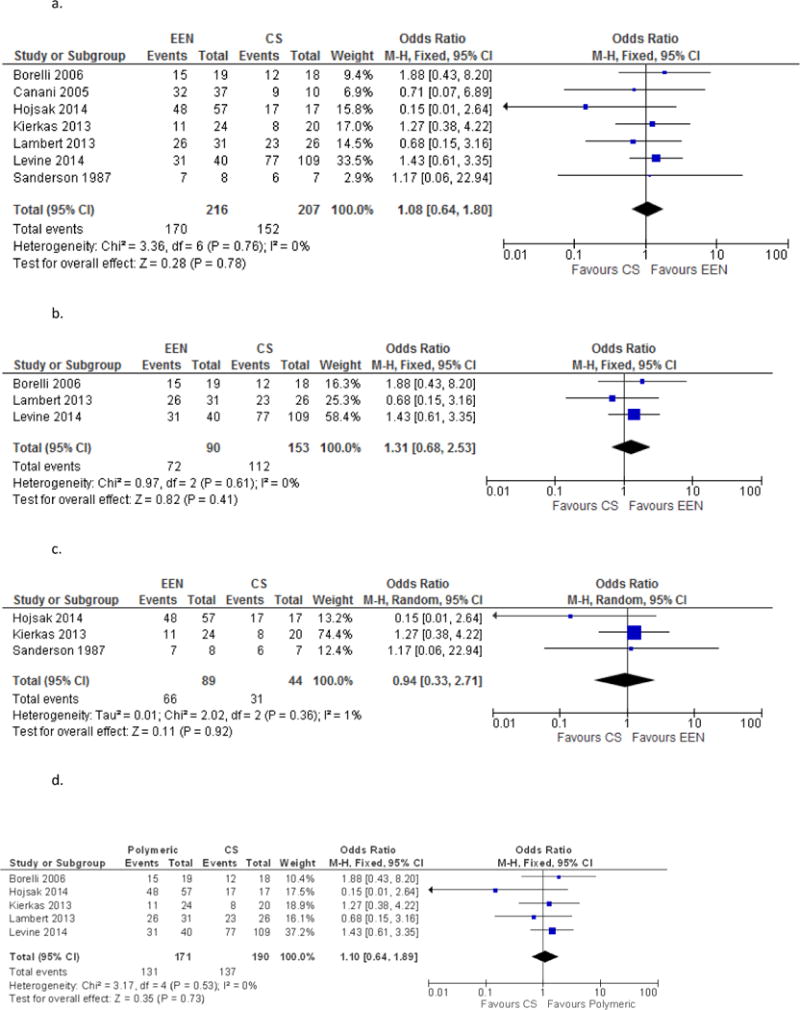
a. Comparison of Remission induction for EEN vs. CS
b. Comparison of EEN vs. CS in Newly Diagnosed Patients Only
c. Comparison of EEN vs. CS in Relapsed Patients Only
d. Polymeric for Remission Induction
e. Elemental Diet for Remission Induction
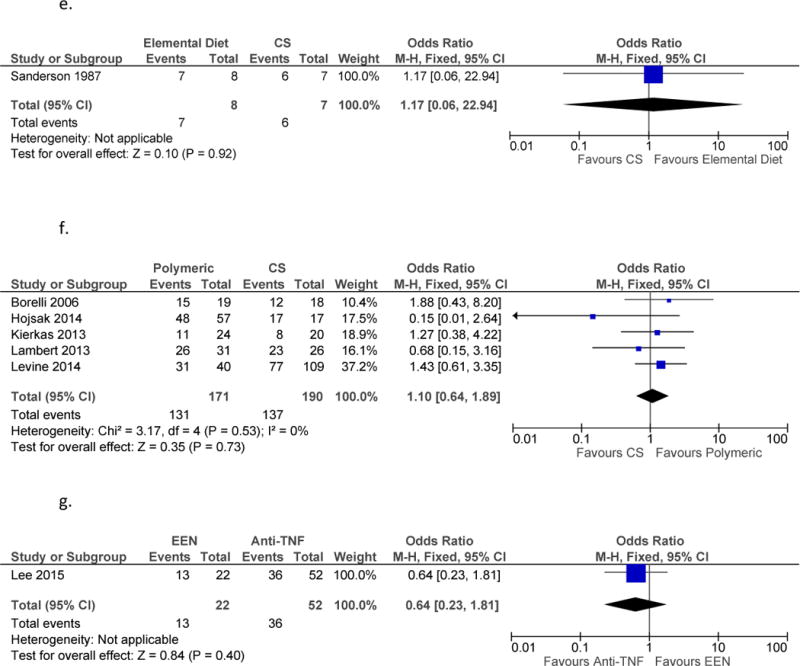
g. Anti-TNF vs. EEN for Remission Induction
f. EEN vs. Mesalamine for Remission Induction
g. Anti-TNF vs. EEN for Remission Induction
Given the interest in possible treatment differences between newly diagnosed CD patients and those with relapsed disease, the studies were separately analyzed by type of patient. Borrelli et al.35, Lambert et al.24, Lou et al.37 and Levine et al.25 included only newly diagnosed patients. Though these studies were an RCT, 2 retrospective cohort studies and a prospective cohort study respectively, we combined them to do an exploratory analysis. The sub analysis of these 4 trials included 100 patients treated with enteral nutrition and 171 patients treated with corticosteroids. No statistically significant difference was demonstrated (OR 1.61, 95% CI 0.87–2.98, Figure 3a. Comparison of EEN vs. CS in Newly Diagnosed Patients Only). A subanalysis of the 3 studies including only relapsed patients (Sanderson et al.28, Kierkus et al.23, Hojsak et al.22) included 89 patients treated with enteral nutrition and 44 treated with steroids found no statistically identifiable differences between induction of remission between the groups (OR 0.76, 95% CI 0.29–1.98, Figure 3b. Comparison of EEN vs. CS in Relapsed Patients Only). There was no statistically significant difference in remission odds distinguishing newly diagnosed patients and relapsed patients, though the trend was towards EEN being more effective in those who were newly diagnosed as opposed to relapsed patients. Heterogeneity was not demonstrated (I2=1%).
Figure 3.
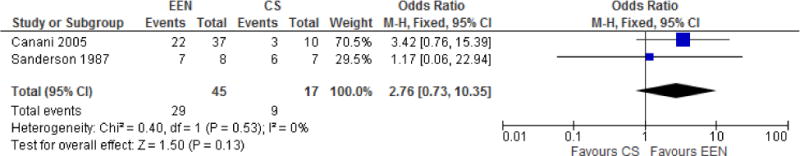
Post Induction Benefits of EEN vs. CS
B. Benefit of enteral nutrition therapy beyond end of induction
An exploratory subanalysis of 2 studies (Sanderson et al.28, Berni Canani et al.36) was performed to ascertain if being induced into remission with EEN vs. CS had led to a more durable remission. Although Sanderson et al.28 was an RCT and Berni Canani et al.36 was a retrospective cohort study, we combined their results in an effort to generate a hypothesis regarding the potential durability of remission under the two treatment regimes. Sanderson et al.28 provided data at 12wks (6 weeks after end of induction). Patients who had achieved remission in the steroid group were maintained on prednisone 10mg daily. Those who had achieved remission in the EN group were in a prespecified food reintroduction program with tapering doses of EN. At 12 weeks, 6 of 7 patients in the steroid induction group remained in remission whereas, 7of 8 patients in the EN induction group remained in remission.
Berni Canani et al.36 provided data at 12 months of follow-up (10 months after the end of induction). All patients in both EN and CS groups who achieved remission were treated with mesalamine 50–75mg/kg daily. At 12 months, 22 of 37 patients in the EEN induction group remained in remission while only 3 of 10 patients in the steroid induction group remained in remission.
Although there was no statically significant difference between the groups (OR 2.75, [95% CI 0.72–10.53], Figure 4, Durability of Remission, EEN vs. CS), the trend was toward benefit with EEN. Heterogeneity was not demonstrated (I2=0%).
Figure 4.
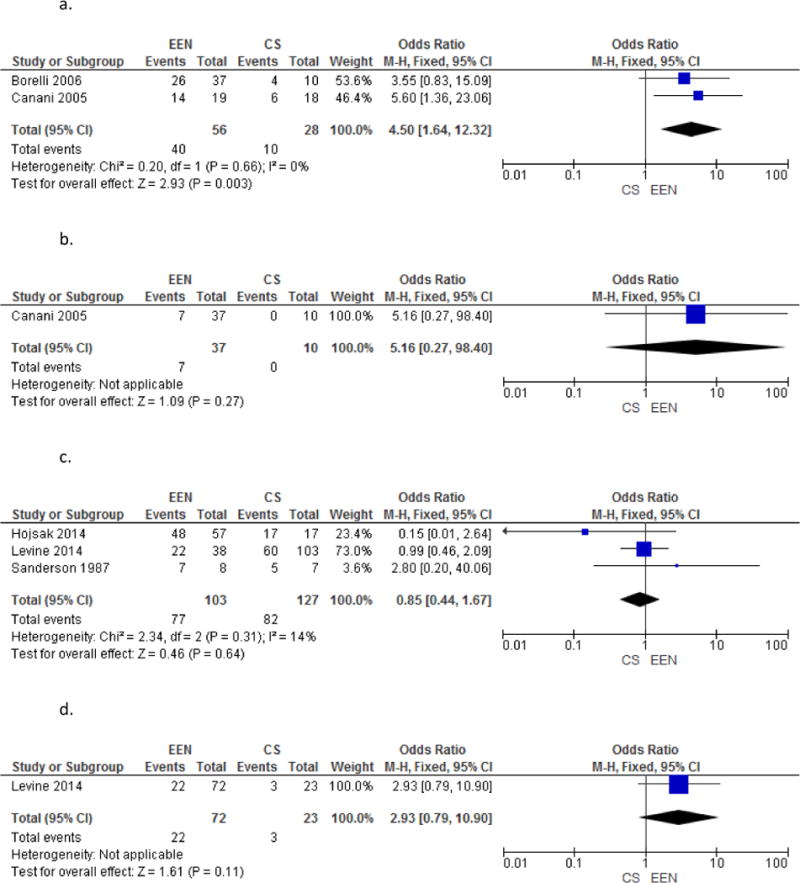
a. Comparison of Mucosal Healing in EEN vs. CS
b. Comparison of Complete Endoscopic and Histologic Healing in EEN vs. CS
c. Comparison of CRP normalization in EEN vs. CS
d. Comparison of Fecal Calprotectin in EEN vs. CS
C. Polymeric formula and Elemental formula for remission induction
The majority of the included studies (Borrelli et al.35, Hojsak et al.22, Kierkas et al.23, Lambert et al.24, and Levine et al.25), used a polymeric formula in their enteral nutrition arm. When we compared only these five studies for remission induction, there was no difference between CS and the polymeric formula of enteral nutrition (OR 1.10, [95% CI 0.64–1.89], Figure 5. Polymeric formula only for Remission Induction, EEN vs. CS). Sanderson et al.28 was the only study which used an elemental diet only arm and Lou et al37 did not specify the type of formula used in their study.
Figure 5.
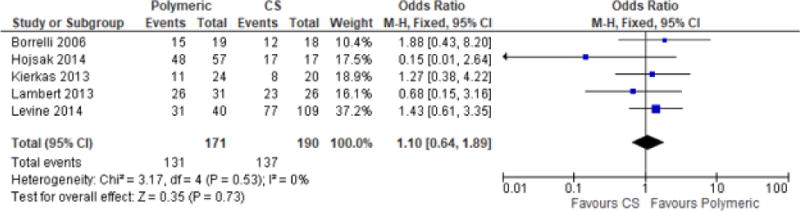
Polymeric formula only for Remission Induction, EEN vs. CS
D. Mucosal healing
Borrelli et al.35 and Berni Canani et al.36 provided mucosal healing data at the end of induction. Though these were two different types of studies—an RCT and a retrospective cohort study—we conducted a hypothesis generating analysis to determine whether mucosal healing rates were higher among patients who received EEN compared to those who received CS.
Borrelli found 26 of 37 patients in the EEN group achieved mucosal healing where only 4 of 10 in the steroid group achieved the same endpoint. Berni Canani found 14 of 19 patients induced with EEN achieved mucosal healing whereas only 6 of 18 patients treated with CS achieved the same endpoint. Overall, patients who received EEN were 4.5 times more likely to demonstrate mucosal healing (OR 4.50, [95% Cl 1.64, 12.32]; Figure 6a. Comparison of Mucosal Healing in EEN vs. CS) compared to those who received CS. This result was statistically significant. Heterogeneity was not demonstrated (I2=0%).
Figure 6.
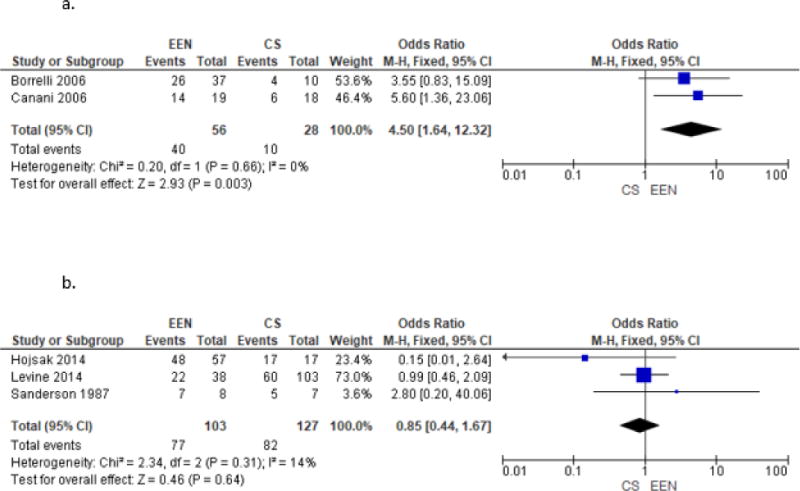
a. Comparison of Mucosal Healing in EEN vs. CS
b. Comparison of CRP normalization in EEN vs. CS
E. Effect of EEN vs. CS on CRP normalization
Hojsak et al.22, Levine et al.25, and Sanderson et al.28 measured CRP at baseline and at the end of induction in patients receiving EEN and CS. Again, though these were three different types of studies—a retrospective cohort study, a prospective study, and an RCT—we conducted a hypothesis generating analysis to ascertain whether patients who received EEN vs. CS had differed in rates of CRP normalization. Correspondence with Hojsak and Sanderson provided data that was not included in their original published papers.
Hojsak found CRP normalized in 48 of 57 patients treated with EEN and all 17 patients treated with CS. Levine found 22 of 38 patients treated with EEN had normalization of CRP compared to 60 of 103 patients treated with CS. Sanderson found 7 of 8 patients treated with EEN and 5 of 7 patients treated with CS had normalization of CRP. Overall, no difference was detected in CRP normalization between patients who received CRP or EEN (OR 0.85, [CI 95% 0.44, 1.67]; Figure 6b. Comparison of CRP normalization in EEN vs. CS). Again, heterogeneity was not demonstrated (I2=0%).
DISCUSSION
Our systematic review and meta-analysis of induction regimens in children with Crohn’s disease, did not find a treatment benefit of corticosteroids over exclusive enteral nutrition. This finding held true in the sub-analysis of newly diagnosed patients. In our hypothesis generating analysis EEN also had superior mucosal healing odds (p<.05), suggesting that this is an end point to be explored in future trials. There is also a possibility that the benefits of induction with enteral nutrition are more durable than induction with corticosteroids, but this finding is driven by one study, Berni Canani et al.36 In fact, the two studies combined to study this question may not reflect current practice, namely using steroids or mesalamines as post-induction maintenance regimen in Crohn’s disease.28,36
Our findings are in contrast with the most recent Cochrane review on the subject15 which found corticosteroids superior to enteral nutrition (OR 0.33). This is potentially explained by the fact that we only used high-quality studies. When the Cochrane authors restricted their analysis to only high-quality studies, they also found no difference between CS and EEN. Another potential explanation for their contrary findings is that they combined data from studies of both adults and children. Of the seven studies (n=352) they pooled, only one study (n=37, Borrelli et al.35) was composed of pediatric patients. Given the heterogeneity of the results when stratifying by population age, it would seem reasonable to avoid combining pediatric and adult data in future analyses as it may result in underestimating the benefit of enteral nutrition in the pediatric population. Some physicians believe that the discordance in efficacy between adults and children is overstated. In clinical situations where tolerability of the EN product is high, enteral nutrition can induce remission in adult populations, suggesting that improved tolerability should be an area of further research.
Two previous meta-analyses and two systematic reviews on the subject of treatments for induction of remission in children with Crohn’s disease have been performed, and our results confirmed their findings. Heuschkel et al.21 (n=194), concluded that there was no difference in efficacy between enteral nutrition and corticosteroids to treat active Crohn’s disease. However, this study was underpowered (n=144) to identify differences less than/equal 20% between the steroid and EEN groups.6 Of the seven studies combined in their meta-analysis four did not meet our inclusion criteria. Two studies38,39 have only ever been published as abstracts, and have not undergone detailed peer-review. It is not clear whether patients overlapped between the two studies by the same author and the decision to avoid including in their meta-analysis was shared by the Cochrane review group.15 Thomas et al.199340, was excluded from our analysis because their outcome was “improvement”, rather than remission. Another, Breese et al. 199541, included 8 different steroid dosing strategies (prednisone 5mg/d – 40mg/d) among 10 patients treated with corticosteroids. The heterogeneous patient populations and an inability to evaluate details of trial mechanics, made outcomes for more than 50% of the included population impossible to assess.
An earlier meta-analysis, Dziechciarz et al.20 (n=204), also suggested similar efficacy between EN and CS for treating active Crohn’s disease in children. Their meta-analysis combined 4 studies. Three of these studies did not meet our inclusion criteria. Like Heuschkel et al.21, Dziechciarz et al.20 also included the same two abstracts from Seidman et al.38,39 as previously described. Terrin et al.2 was published in an Italian journal with an English abstract and non-English manuscript. Because the full manuscript was not in English, we excluded it from our meta-analysis.
Recently, Pegnani et al.42 conducted a large systematic review of pediatric nutrition and CD with several end points, including the efficacy of enteral nutrition on the induction of remission in a pediatric population. While they did not conduct a meta-analysis themselves, using evidence from Heuschkel et al.21 and Dziechciarz et al.20, they concluded that enteral nutrition in a pediatric population can effectively induce remission. However, they included Thomas et al.199340 and Terrin et al. 20022, which we excluded for the reasons listed in the previous paragraph. They also included Ruuska et al. 199443, Grover et al. 201544, and Soo et al. 201345, which we excluded because the former two articles did not use remission as their primary end point and the latter one did not have an enteral nutrition only arm.
Day et al.46 also conducted a broad-view systematic review without meta-analysis on enteral nutrition in the treatment of pediatric Crohn’s disease with several end points. While they also found that enteral nutrition can induce remission, they included the Seidman et al. abstracts38,39, the Thomas et al.199340 paper and the Terrin et al.2 papers that we excluded for previously explained reasons. While both of these systematic reviews contribute substantially to the understanding of pediatric CD and enteral nutrition, they were both broad in scope. Because we took a narrower view point and only looked at enteral nutrition and its’ ability to affect remission induction, we were able to more completely examine the topic.
The goal of every meta-analysis is to include the best possible data and pool data from comparable patient populations. Our rigorous inclusion criteria disallowed the majority of studies included in 2 previous meta-analyses. Our meta-analysis included data from randomized controlled trials, prospective cohorts, and retrospective cohort studies. Our systematic sensitivity analysis showed that when we stratified our results by study design, there was no difference across strata, meaning that the results from the prospective studies were similar to those from the retrospective studies and the RCTs.
By not including abstracts, we may have introduced publication bias into our study, but only including full manuscripts, we were able to more fully assess the limitations of each study and understand sources of bias in assignment of EEN vs. CS. We have detailed these biases in Table 1. Moreover, we had no heterogeneity in the majority of our analyses, making our findings robust. A limitation to our systematic review is that the primary outcome for most included studies was clinical remission, which is measured by PCDAI, a scale considered to be a fallible measure for evaluating response to therapy. As such, we included the previously unexplored end point of mucosal healing, a more robust measure of therapy response. Another limitation to any research involving exclusive enteral nutrition is, of course, the different dietary cheats allowed and the difficulty in measuring the amount of non-formula nutrition ingested by participants. However, we believe this error to be random, and therefore would bias our results towards the null. A final potential limitation to our paper is that we chose our statistical models based on heterogeneity, while current Cochrane guidelines warrant choosing a model a priori and then using an alternative test as a sensitivity analysis.
Current clinical guidelines, from both the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition and its European counterpart, consider enteral nutrition acceptable to use in all patients with Crohn’s disease.47,48 In fact, the European Society for Clinical Nutrition and Metabolism guidelines recommend EN as a first line therapy for children and adolescents with CD, with “strong consensus”.48 However, there are many persistent unanswered questions on this subject. Specifically, the mechanism of action of enteral nutrition remains vague and there is still little guidance on which types of patients would be most likely to benefit, though weak evidence suggests effectiveness is greater with small bowel involvement.7 With regard to disease duration, our analysis suggests that outcomes may differ between newly diagnosed and previously diagnosed patients. Future guidelines may need to make that distinction when it comes to benefits of induction with these therapies47. Challenges also remain in considering induction treatment duration and the best strategy to maintain remission as long-term exclusive enteral nutrition is unlikely to be acceptable to most patients.
In summary, our study suggests that exclusive enteral nutrition works equally as well as corticosteroids in inducing remission in pediatric Crohn’s disease based on clinical symptom scores, but EEN could potentially be superior when assessing improvement by mucosal healing endpoints. We believe these results suggest a need for further research in this area. EEN may have the added benefit of minimizing growth failure, avoiding undesirable and difficult to reverse changes to body habitus, and can potentially result in a deeper and longer duration of remission. As such, greater advocacy for this therapy among physicians from the United States may be warranted.
Supplementary Material
MOOSE Guidelines for Meta-Analyses and Systematic Reviews of Observational Studies34.
-
✓
Title identifies the study as a meta-analysis (or systematic review)
-
✓
Abstract uses the journal’s structured format
Introduction Presents:
-
✓
The clinical problem
-
✓
The hypothesis
-
✓
A statement of objectives that includes the study population, the condition of interest, the exposure or intervention, and the outcome(s) considered
Sources Describe:
-
✓
Qualifications of searchers (eg, librarians and investigators)
-
✓
Search strategy, including time period included in the synthesis and keywords
-
✓
Effort to include all available studies, including contact with authors
Databases and registries searched
-
✓
Search software used, name and version, including special features used (eg, explosion)
-
✓
Use of hand searching (eg, reference lists of obtained articles)
-
✓
List of citations located and those excluded, including justification
-
✓
Method of addressing articles published in languages other than English
-
✓
Method of handling abstracts and unpublished studies
-
✓
Description of any contact with authors
Study Selection Describes:
-
✓
Types of study designs considered
-
✓
Relevance or appropriateness of studies gathered for assessing the hypothesis to be tested
-
✓
Rationale for the selection and coding of data (eg, sound clinical principles or convenience)
-
✓
Documentation of how data were classified and coded (eg, multiple raters, blinding, and interrater reliability)
-
✓
Assessment of confounding (eg, comparability of cases and controls in studies where appropriate)
-
✓
Assessment of study quality, including blinding of quality assessors; stratification or regression on possible predictors of study results
-
✓
Assessment of heterogeneity
-
✓
Statistical methods (eg, complete description of fixed or random effects models, justification of whether the chosen models account for predictors of study results, dose-response models, or cumulative meta-analysis) in sufficient detail to be replicated
Results Present:
-
✓
A graph summarizing individual study estimates and the overall estimate
-
✓
A table giving descriptive information for each included study
-
✓
Results of sensitivity testing (eg, subgroup analysis)
-
✓
Indication of statistical uncertainty of findings
Discussion Discusses:
-
✓
Strengths and weaknesses
-
✓
Potential biases in the review process (eg, publication bias)
-
✓
Justification for exclusion (eg, exclusion of non–English-language citations)
-
✓
Assessment of quality of included studies
-
✓
Consideration of alternative explanations for observed results
-
✓
Generalization of the conclusions (ie, appropriate for the data presented and within the domain of the literature review)
-
✓
Guidelines for future research
-
✓
Disclosure of funding source
Acknowledgments
Arun Swaminath is the guarantor of this article. He was responsible for the study concept and design, analysis and interpretation of the data as well as the drafting and supervision of the manuscript. Alexandra Feathers completed the analysis and interpretation of data and assisted in the drafting of the manuscript. Ashwin Ananthakrishnan was responsible for critical revision of the manuscript for important intellectual content. Louise Falzon wrote the search terms for the review and acquired the data. Sophia Li Ferry assisted with analysis and interpretation of data as well as drafting of the manuscript. All authors have contributed to, and have read and approved submission of this manuscript. The manuscript represents an original, unpublished report and is not being considered for publication elsewhere, in whole or part, in any language.
SOURCES OF SUPPORT: None
Footnotes
CONFLICTS OF INTEREST TO DISCLOSE: None
References
- 1.Abraham C, Cho JH. Inflammatory bowel disease. The New England journal of medicine. 2009;361(21):2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Terrin G, Canani R, Ambrosini A, Viola F, Mosque MSC. The semielemental diet (Pregomin) the primary therapy for inducing remission in children with active Crohn’s disease. Ital J Pediatr. 2002;28:401–405. [Google Scholar]
- 3.Rogers BH, Clark LM, Kirsner JB. The epidemiologic and demographic characteristics of inflammatory bowel disease: an analysis of a computerized file of 1400 patients. Journal of chronic diseases. 1971;24(12):743–773. doi: 10.1016/0021-9681(71)90087-7. [DOI] [PubMed] [Google Scholar]
- 4.Siegel CA. What options do we have for induction therapy for Crohn’s disease? Digestive diseases. 2010;28(3):543–547. doi: 10.1159/000320414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruemmele FM, Veres G, Kolho KL, et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. Journal of Crohn’s & colitis. 2014;8(10):1179–1207. doi: 10.1016/j.crohns.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Griffiths AM. Enteral nutrition: the neglected primary therapy of active Crohn’s disease. Journal of pediatric gastroenterology and nutrition. 2000;31(1):3–5. doi: 10.1097/00005176-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Afzal NA, Addai S, Fagbemi A, Murch S, Thomson M, Heuschkel R. Refeeding syndrome with enteral nutrition in children: a case report, literature review and clinical guidelines. Clinical nutrition. 2002;21(6):515–520. doi: 10.1054/clnu.2002.0586. [DOI] [PubMed] [Google Scholar]
- 8.Levine A, Milo T, Buller H, Markowitz J. Consensus and controversy in the management of pediatric Crohn disease: an international survey. Journal of pediatric gastroenterology and nutrition. 2003;36(4):464–469. doi: 10.1097/00005176-200304000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Voitk AJ, Echave V, Feller JH, Brown RA, Gurd FN. Experience with elemental diet in the treatment of inflammatory bowel disease. Is this primary therapy? Archives of surgery. 1973;107(2):329–333. doi: 10.1001/archsurg.1973.01350200189039. [DOI] [PubMed] [Google Scholar]
- 10.Sanderson IR, Boulton P, Menzies I, Walker-Smith JA. Improvement of abnormal lactulose/rhamnose permeability in active Crohn’s disease of the small bowel by an elemental diet. Gut. 1987;28(9):1073–1076. doi: 10.1136/gut.28.9.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fell JM, Paintin M, Arnaud-Battandier F, et al. Mucosal healing and a fall in mucosal pro-inflammatory cytokine mRNA induced by a specific oral polymeric diet in paediatric Crohn’s disease. Alimentary pharmacology & therapeutics. 2000;14(3):281–289. doi: 10.1046/j.1365-2036.2000.00707.x. [DOI] [PubMed] [Google Scholar]
- 12.Leach ST, Mitchell HM, Eng WR, Zhang L, Day AS. Sustained modulation of intestinal bacteria by exclusive enteral nutrition used to treat children with Crohn’s disease. Alimentary pharmacology & therapeutics. 2008;28(6):724–733. doi: 10.1111/j.1365-2036.2008.03796.x. [DOI] [PubMed] [Google Scholar]
- 13.Lionetti P, Callegari ML, Ferrari S, et al. Enteral nutrition and microflora in pediatric Crohn’s disease. JPEN Journal of parenteral and enteral nutrition. 2005;29(4 Suppl):S173–175. doi: 10.1177/01486071050290S4S173. discussion S175–178, S184–178. [DOI] [PubMed] [Google Scholar]
- 14.Malchow H, Steinhardt HJ, Lorenz-Meyer H, et al. Feasibility and effectiveness of a defined-formula diet regimen in treating active Crohn’s disease. European Cooperative Crohn’s Disease Study III. Scandinavian journal of gastroenterology. 1990;25(3):235–244. [PubMed] [Google Scholar]
- 15.Zachos M, Tondeur M, Griffiths AM. Enteral nutritional therapy for induction of remission in Crohn’s disease. The Cochrane database of systematic reviews. 2007;1:CD000542. doi: 10.1002/14651858.CD000542.pub2. [DOI] [PubMed] [Google Scholar]
- 16.Griffiths AM, Ohlsson A, Sherman PM, Sutherland LR. Meta-analysis of enteral nutrition as a primary treatment of active Crohn’s disease. Gastroenterology. 1995;108(4):1056–1067. doi: 10.1016/0016-5085(95)90203-1. [DOI] [PubMed] [Google Scholar]
- 17.Messori A, Trallori G, D’Albasio G, Milla M, Vannozzi G, Pacini F. Defined-formula diets versus steroids in the treatment of active Crohn’s disease: a meta-analysis. Scandinavian journal of gastroenterology. 1996;31(3):267–272. doi: 10.3109/00365529609004877. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez-Banares F, Cabre E, Esteve-Comas M, Gassull MA. How effective is enteral nutrition in inducing clinical remission in active Crohn’s disease? A meta-analysis of the randomized clinical trials. JPEN Journal of parenteral and enteral nutrition. 1995;19(5):356–364. doi: 10.1177/0148607195019005356. [DOI] [PubMed] [Google Scholar]
- 19.Zachos M, Tondeur M, Griffiths AM. Enteral nutritional therapy for inducing remission of Crohn’s disease. The Cochrane database of systematic reviews. 2001;3:CD000542. doi: 10.1002/14651858.CD000542. [DOI] [PubMed] [Google Scholar]
- 20.Dziechciarz P, Horvath A, Shamir R, Szajewska H. Meta-analysis: enteral nutrition in active Crohn’s disease in children. Alimentary pharmacology & therapeutics. 2007;26(6):795–806. doi: 10.1111/j.1365-2036.2007.03431.x. [DOI] [PubMed] [Google Scholar]
- 21.Heuschkel RB, Menache CC, Megerian JT, Baird AE. Enteral nutrition and corticosteroids in the treatment of acute Crohn’s disease in children. Journal of pediatric gastroenterology and nutrition. 2000;31(1):8–15. doi: 10.1097/00005176-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Hojsak I, Pavic AM, Misak Z, Kolacek S. Risk factors for relapse and surgery rate in children with Crohn’s disease. European journal of pediatrics. 2014;173(5):617–621. doi: 10.1007/s00431-013-2230-1. [DOI] [PubMed] [Google Scholar]
- 23.Kierkus Jaroslaw SS, Szczepanski Michal, Wiernicka Anna, Szmanska Edyta, Matuszczyk Malgorzata, Ryzko Josef. The efficacy of total enteralion in inducing remission and improving nutritional status in children with moderate to severe Crohn’s disease. Przeglad Gastroenterologiczny. 2013;8(1):57–61. [Google Scholar]
- 24.Lambert B, Lemberg DA, Leach ST, Day AS. Longer-term outcomes of nutritional management of Crohn’s disease in children. Digestive diseases and sciences. 2012;57(8):2171–2177. doi: 10.1007/s10620-012-2232-2. [DOI] [PubMed] [Google Scholar]
- 25.Levine A, Turner D, Pfeffer Gik T, et al. Comparison of outcomes parameters for induction of remission in new onset pediatric Crohn’s disease: evaluation of the porto IBD group “growth relapse and outcomes with therapy” (GROWTH CD) study. Inflammatory bowel diseases. 2014;20(2):278–285. doi: 10.1097/01.MIB.0000437735.11953.68. [DOI] [PubMed] [Google Scholar]
- 26.Mosli MH, Zou G, Garg SK, et al. C-Reactive Protein, Fecal Calprotectin, and Stool Lactoferrin for Detection of Endoscopic Activity in Symptomatic Inflammatory Bowel Disease Patients: A Systematic Review and Meta-Analysis. The American journal of gastroenterology. 2015;110(6):802–819. doi: 10.1038/ajg.2015.120. quiz 820. [DOI] [PubMed] [Google Scholar]
- 27.Pitkin RM, Branagan MA, Burmeister LF. Accuracy of data in abstracts of published research articles. Jama. 1999;281(12):1110–1111. doi: 10.1001/jama.281.12.1110. [DOI] [PubMed] [Google Scholar]
- 28.Sanderson IR, Udeen S, Davies PS, Savage MO, Walker-Smith JA. Remission induced by an elemental diet in small bowel Crohn’s disease. Archives of disease in childhood. 1987;62(2):123–127. doi: 10.1136/adc.62.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papadopoulou A, Rawashdeh MO, Brown GA, McNeish AS, Booth IW. Remission following an elemental diet or prednisolone in Crohn’s disease. Acta paediatrica (Oslo, Norway : 1992) 1995;84(1):79–83. doi: 10.1111/j.1651-2227.1995.tb13490.x. [DOI] [PubMed] [Google Scholar]
- 30.Lim WC, Hanauer S. Aminosalicylates for induction of remission or response in Crohn’s disease. The Cochrane database of systematic reviews. 2010;12:CD008870. doi: 10.1002/14651858.CD008870. [DOI] [PubMed] [Google Scholar]
- 31.Wells GSB, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2017 [Google Scholar]
- 32.Collaboration TC. Cochrane Handbook for Systematic Reviews and Interventions. 5.1.0: http://handbook.cochrane.org/chapter_8/table_8_5_a_the_cochrane_collaborations_tool_for_assessing.htm.
- 33.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, L M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2017 http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 5/12/17.
- 34.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Jama. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 35.Borrelli O, Cordischi L, Cirulli M, et al. Polymeric diet alone versus corticosteroids in the treatment of active pediatric Crohn’s disease: a randomized controlled open-label trial. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2006;4(6):744–753. doi: 10.1016/j.cgh.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 36.Berni Canani R, Terrin G, Borrelli O, et al. Short- and long-term therapeutic efficacy of nutritional therapy and corticosteroids in paediatric Crohn’s disease. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2006;38(6):381–387. doi: 10.1016/j.dld.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 37.Luo Y, Yu J, Zhao H, et al. Short-Term Efficacy of Exclusive Enteral Nutrition in Pediatric Crohn’s Disease: Practice in China. Gastroenterol Res Pract. 2015;2015:428354. doi: 10.1155/2015/428354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seidman EGGA, Jones A, Issenman R. Gastroenterology Semi-elemental diet versus prednisone in pediatric Crohn’s disease. 1993;104:A778. [Google Scholar]
- 39.Seidman EGLM, Turgeon J, Bouthillier L, Morin CL. Elemental diet versus prednisone as initial therapy in Crohn’s disease: early and long-term results. Gastroenterology. 1991;100:150A. [Google Scholar]
- 40.Thomas AG, Taylor F, Miller V. Dietary intake and nutritional treatment in childhood Crohn’s disease. Journal of pediatric gastroenterology and nutrition. 1993;17(1):75–81. doi: 10.1097/00005176-199307000-00011. [DOI] [PubMed] [Google Scholar]
- 41.Breese EJMC, Nicholls SW. The effect f treatment on lymphokine-secreting cells in the intestinal mucosa of children with Crhn’s disease. Alimentary pharmacology & therapeutics. 1995;9:547–552. doi: 10.1111/j.1365-2036.1995.tb00419.x. [DOI] [PubMed] [Google Scholar]
- 42.Penagini F, Dilillo D, Borsani B, et al. Nutrition in Pediatric Inflammatory Bowel Disease: From Etiology to Treatment. A Systematic Review. Nutrients. 2016;8(8) doi: 10.3390/nu8060334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruuska T, Savilahti E, Maki M, Ormala T, Visakorpi JK. Exclusive whole protein enteral diet versus prednisolone in the treatment of acute Crohn’s disease in children. Journal of pediatric gastroenterology and nutrition. 1994;19(2):175–180. doi: 10.1097/00005176-199408000-00006. [DOI] [PubMed] [Google Scholar]
- 44.Grover ZPL. Two-Year Outcomes After Exclusive Enteral Nutrition Induction Are Superior to Corticosteroids in Pediatric Crohn’s Disease Treated Early with Thiopurines. Digestive diseases and sciences. 2015;60(10):3069–3074. doi: 10.1007/s10620-015-3722-9. [DOI] [PubMed] [Google Scholar]
- 45.Soo J, Malik BA, Turner JM, et al. Use of exclusive enteral nutrition is just as effective as corticosteroids in newly diagnosed pediatric Crohn’s disease. Digestive diseases and sciences. 2013;58(12):3584–3591. doi: 10.1007/s10620-013-2855-y. [DOI] [PubMed] [Google Scholar]
- 46.Day AS, Whitten KE, Sidler M, Lemberg DA. Systematic review: nutritional therapy in paediatric Crohn’s disease. Alimentary pharmacology & therapeutics. 2008;27(4):293–307. doi: 10.1111/j.1365-2036.2007.03578.x. [DOI] [PubMed] [Google Scholar]
- 47.Critch J, Day AS, Otley A, et al. Use of enteral nutrition for the control of intestinal inflammation in pediatric Crohn disease. Journal of pediatric gastroenterology and nutrition. 2012;54(2):298–305. doi: 10.1097/MPG.0b013e318235b397. [DOI] [PubMed] [Google Scholar]
- 48.Forbes AEJ, Hébuterne X, Kłęk S, Krznaric Z, Schneider S, Shamir R, Stardelova K, Wierdsma N, Wiskin AE, Bischoff SC. ESPEN guideline: Clinical nutrition in inflammatory bowel disease. Clin Nutr. 2017;36(2):321–347. doi: 10.1016/j.clnu.2016.12.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


