Abstract
Introduction
Antibodies to intravenous idursulfase enzyme replacement therapy (ERT) for patients with Hunter syndrome (mucopolysaccharidosis type II, MPS II) can have a harmful clinical impact, including both increasing risk of infusion reactions and inhibiting therapeutic activity. Thus, failure to monitor anti-idursulfase antibodies and neutralizing antibodies, and delays in reporting results, may postpone critical clinical decisions.
Hypothesis
Urinary glycosaminoglycan (GAG) levels may be used as a biomarker for anti-idursulfase antibodies and neutralizing antibodies to improve timeliness in monitoring and managing ERT.
Methods
This is a case report describing a patient with MPS II with high levels of neutralizing antibodies and worsened clinical status who was treated for five years with a non-immunosuppressive and non-cytotoxic immune tolerance (NICIT) regimen, consisting of intravenous immune globulin and frequent infusions of idursulfase. Neutralizing antibodies and total anti-idursulfase antibodies were measured by two different methods, the direct 1,9-dimethylmethylene blue (DMB) assay and cetylpyridinium chloride carbazole-borate (CPC) assay.
Results
Neutralizing antibodies, measured as percent inhibition of enzyme activity and also by total neutralizing antibody titer, were correlated with quantitative urinary GAG measured by DMB assay (p = 0.026, p = 0.0067), and quantitative urinary GAG by CPC assay with percent inhibition of enzyme activity by neutralizing antibodies (p = 0.0475). The NICIT regimen showed a sustained immune tolerance after five years and was well-tolerated.
Conclusions
Urinary GAG, measured by DMB assay, may be a biomarker for anti-idursulfase neutralizing antibodies and is useful for managing immune tolerance regimens for patients with MPS II who have high levels of anti-idursulfase neutralizing antibodies. This study highlights the importance of regular and frequent monitoring of urinary GAG in patients with MPS II who are receiving ERT. The NICIT regimen, with less drug toxicities, may be preferred in patients with MPS who have a high risk of infections and whose disease progresses less rapidly than some other lysosomal storage diseases, such as infantile Pompe disease.
Keywords: Lysosomal storage disease, Mucopolysaccharidosis II, Enzyme replacement therapy, Glycosaminoglycans, Idursulfase, Neutralizing antibodies
1. Introduction
Mucopolysaccharidosis type II (MPS II), also known as Hunter syndrome, is an X-linked lysosomal disease caused by a deficiency in the enzyme iduronate-2-sulfatase, which is encoded by the IDS gene [1]. This enzyme is a rate-limiting step in the catabolism of glycosaminoglycans (GAG), heparan sulfate and dermatan sulfate [1]. In patients with MPS II, GAG accumulates in tissues throughout the body, contributing to tissue damage and multi-organ dysfunction [1,2]. The prevalence of MPS II is estimated to be 1 in 170,000 live male births [1,2]. The age of onset, disease severity, and rate of progression varies [1–3]. An important distinction in the MPS II phenotype is the presence or absence of cognitive impairment [1]. Patients with the most severe MPS II phenotype show signs of gradual cognitive and neurodevelopmental decline within the first few years of life [1]. If untreated, patients with the more severe phenotype do not survive past the first 15 years of life [1]. Patients with the most attenuated MPS II phenotype exhibit normal intelligence and survive well into adulthood [1]. Clinical features common to MPS II, with or without cognitive impairment, include skeletal deformities with contractures, joint stiffness and decreased range of movement, facial dysmorphism, heart valve dysfunction, reduced endurance, and life-threatening respiratory failure [1]. Hepatosplenomegaly may be present. Patients may also develop hydrocephalus and/or spinal cord compression [1]. MPS II is diagnosed by a low iduronate-2-sulfatase enzyme activity measured in leukocytes, serum or tissue, and the diagnosis should be refined by identification of the IDS gene mutation. Abnormally elevated GAG i.e., GAG/creatinine ratio, is used as a screening tool for multiple MPS conditions, and all patients with MPS II have high levels at diagnosis [1–3].
At this time, intravenous enzyme replacement therapy (ERT) is the only widely recognized therapy for MPS II, i.e., idursulfase (Elaprase®) approved by the U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA); and idursulfase beta (Hunterase®) in seven countries. Clinical trials of idursulfase demonstrated improvement in endurance, reduction in liver and spleen size, and reduction in urinary GAG [4,5]. ERT does not cross the blood-brain barrier to any appreciable extent and therefore is not considered useful for treating the central nervous system (CNS) aspects of MPS II.
Anti-drug antibodies to idursulfase can develop in patients receiving idursulfase infusions [9]. Although the presence of anti-idursulfase antibodies does not always translate into a confirmed decrease in ERT efficacy, anti-idursulfase antibodies have been associated with reduced systemic exposure to idursulfase and less reduction of urinary GAG in some patients [4,6]. Clinical trials of idursulfase have shown that approximately half of the patients developed anti-idursulfase IgG antibodies [4,7]. Of patients developing antibodies, 21% to 35% developed neutralizing IgG antibodies to idursulfase [4,7]. Neutralizing antibodies to idursulfase were associated with decreased improvements in pulmonary function [4,6]. Patients with MPS II who have more severe mutations, such as large gene rearrangements or complete gene deletion, have a higher risk of developing anti-idursulfase antibodies and neutralizing antibodies [6].
The presence of neutralizing antibodies is determined by methods that inhibit the catalytic activity in vitro [8]. There are other potential ways in which antibodies can impact the activity of ERT, including reduction in ERT stability, degradation of ERT in the bloodstream, blockade of ERT cellular uptake, and misrouting of the ERT within the cell [9,10]. Laboratory methods to measure and differentiate these mechanisms are not readily available in clinical practice [9,10].
Life-threatening consequences of neutralizing antibodies on ERT efficacy have been well documented in another lysosomal disease, infantile Pompe disease [11]. In Pompe disease, high neutralizing antibody titers were associated with a shortened survival and worsened cardiac function [11]. Patients with infantile Pompe disease who are at the highest risk of developing neutralizing antibodies are given immune tolerance regimens to eradicate such antibodies. These regimens commonly include immunosuppressive and cytotoxic agents, such as rituximab and methotrexate [11,12].
In contrast to Pompe disease, immune tolerance regimens have not been well studied in patients with MPS II who have neutralizing antibodies to idursulfase. Kishnani et al. have reported achieving immune modulation in a patient with MPS II who had high levels of neutralizing antibodies to idursulfase by using a regimen similar to the regimens used for Pompe disease. The regimen included an anti-CD20 monoclonal antibody, methotrexate, intermittent corticosteroids, and intravenous immune globulin (IVIG) [12]. The patient experienced a significant decline in neutralizing antibodies over an 18-month period but not full eradication of neutralizing antibodies. Toxicities of this regimen included painful peripheral neuropathy (a common toxicity of bortezomib, which may have long-term effects), hypokalemia, immune suppression due to B-cell elimination by anti-CD20 monoclonal antibody, steroid use which required weekly oral antibiotic prophylaxis, and diarrhea from antibiotics [13,14]. At the time of the publication, due to incomplete eradication of neutralizing antibodies, the patient continued on a maintenance regimen, anticipated to last one year, which included weekly methotrexate, IVIG, and quarterly anti-CD20 monoclonal antibody. Long-term toxicities of methotrexate, even when given orally once weekly, can include hepatotoxicity, pulmonary damage, nephrotoxicity and myelosuppression [15,16].
That immunosuppressive-cytotoxic method for Pompe disease has been adapted to successfully treat a patient with MPS II. That patient, and preliminary results of the novel non-immunosuppressive and non-cytotoxic approach reported here (NICIT regimen), were discussed at a U.S. FDA and National Organization for Rare Disease (NORD) public workshop in June 2014 [12].
Although it is recognized that anti-idursulfase neutralizing antibodies can decrease the efficacy of idursulfase, clinical practice does not routinely include monitoring for neutralizing anti-idursulfase antibodies [12]. Even when total anti-idursulfase antibodies are measured, measuring neutralizing antibodies is seldom done. Current lab procedures require a special request and justification for measuring neutralizing antibodies. This procedure also requires special packaging and shipping of samples. Additionally, even when measured, anti-idursulfase neutralizing antibody results are available from only a single CLIA-certified laboratory and are not usually reported within a reasonable timeframe for making clinical decisions [8]. Total anti-idursulfase antibodies often take 4 to 8 weeks to be reported, and neutralizing antibody may take many months to be reported.
A more timely way to monitor total anti-idursulfase antibodies and neutralizing antibodies is needed for effective clinical management of ERT. Previous studies have used urinary GAG levels to assess the pharmacodynamic effect of idursulfase [4,5,7,17]. These studies have shown a higher urinary GAG in patients who have anti-idursulfase antibodies [6]. Urinary GAG measurements are reported within one to two weeks. To date, no studies have looked at whether urinary GAG can be used as a biomarker for detecting changes in total anti-idursulfase antibodies and neutralizing antibodies.
1.1. Hypothesis
This research was conducted with an operating hypothesis that urinary GAG is a potential biomarker for monitoring changes in anti-idursulfase neutralizing antibodies in patients with MPS II receiving idursulfase ERT. The relationship between urinary GAG, total anti-idursulfase antibodies and neutralizing antibodies was explored through a retrospective analysis of a patient with MPS II who had high levels of neutralizing antibodies to idursulfase ERT and was treated with an immune tolerance regimen.
2. Methods
This study was conducted at the University of Minnesota with Institutional Review Board (IRB) approval and IRB-approved parental consent.
2.1. Patient case
A male patient diagnosed with MPS II at 14 months of age began idursulfase ERT at 15 months of age. The idursulfase dose was 0.5 mg/kg, infused once weekly over 4 h via a Port-a-Cath. Pre-treatment/diagnostic quantitative urinary GAG, measured by the cetylpyridinium chloride-carbazole borate (CPC) assay was 109 mg GAG/mmol creatinine, or 9-fold the upper limit of normal (normal reference value of ≤12 mg GAG/mmol creatinine). The patient's initial response to ERT was represented by an approximate 80% reduction in the urinary GAG after 1 month. During the first year of idursulfase, the frequency of respiratory infections decreased and his range of motion and activity levels improved, per parent report. He tolerated the idursulfase infusions well and transferred his infusion care from the clinic to home infusion after 1 year. After receiving idursulfase for 26 months (at 41 months of age), his parents noted that he was having more respiratory infections, reduced range of motion and reduced endurance. He also began exhibiting more pronounced facial dysmorphism. At this time, his dose of idursulfase was increased to 1 mg/kg/week. Despite this dose increase, these symptoms persisted. Quantitative urinary GAG was measured and found to have increased to 94.2 mg GAG/mmol creatinine, or approximately 6-fold the upper limit of normal (normal reference value of ≤16 mg GAG/mmol creatinine, measured by direct 1,9-dimethylmethylene blue (DMB) assay). Total anti-idursulfase antibodies and neutralizing antibodies were requested. Neutralizing antibodies reported a 96% inhibition of idursulfase activity in vitro. Two additional measurements taken at five months and 8 months after the first measurement both reported 100% percent inhibition.
2.1.1. NICIT regimen
Due to concerns about immune suppression in the presence of frequently recurring respiratory infections, and concerns for acute toxicities of cytotoxic agents such as bortezomib and methotrexate and the potential long-term toxicities of methotrexate therapy, an alternative immune tolerance regimen was initiated that was non-immunosuppressive and also non-cytotoxic (NICIT). This regimen was described at the U.S. FDA and NORD public workshop in June 2014, as well as later in a publication that summarized the workshop [12]. The NICIT regimen design was based on principles used in immune tolerance protocols for patients with severe hemophilia A, who have inhibitors to factor VIII therapy. The NICIT regimen incorporated frequent exposures to antigen combined with IVIG therapy [18,19]. The initial NICIT dose of idursulfase was 0.07 mg/kg, with idursulfase infusions administered twice daily for a total weekly dose of 1 mg/kg/week. This was a higher dose and higher frequency than the FDA-recommended dose of 0.5 mg/kg once weekly, thus providing more frequent and more consistent antigen exposure over time. IVIG was initiated at 200 mg/kg per week. It was initially administered over 6 h, and if tolerated, reduced to a 4 hour infusion with subsequent infusions. Total and neutralizing anti-idursulfase antibodies and quantitative urinary GAG were measured once monthly.
Achievement of immune tolerance was defined as reduction of neutralizing antibody levels to zero or to values deemed negligible by the reference lab. Once immune tolerance was achieved with the NICIT regimen, the next step was to taper the frequency of idursulfase infusions, while keeping the total weekly dose of idursulfase at 1 mg/kg/week. Changes in idursulfase dosing frequency were planned to occur approximately every 3 to 6 months, as long as immune tolerance remained stable, with the eventual goal of returning the patient to once weekly dosing (e.g., from twice daily to once daily, then to 3 times weekly, then to twice weekly, then to once weekly). The frequency and dose of IVIG was to be decreased after immune tolerance was achieved, by slow titration steps, every 3 to 6 months, until eventual discontinuation of IVIG.
2.1.2. Patient's tapering of NICIT regimen
The tapering of both idursulfase and IVIG dosing schedules was prolonged due to delays in receiving neutralizing antibody results. Tapering of the idursulfase frequency did not occur until 18 months after starting the NICIT regimen, at which time the dose was changed from 0.07 mg/kg twice daily to 0.14 mg/kg once daily and eventually to once weekly infusions (1 mg/kg per week). Currently, the patient continues to receive idursulfase 1 mg/kg once weekly infused over 4 h.
The frequency and dosing of IVIG was gradually decreased from 200 mg/kg per week to 46 mg/kg every other week, with the titration steps occurring approximately every 6 to 12 months over a 60-month period. The patient continues to tolerate the regimen well and maintains immune tolerance.
2.2. Total anti-idursulfase antibodies and neutralizing antibodies
Total anti-idursulfase antibodies and neutralizing antibody tests were processed by two different labs during the course of this study. The pre-treatment values through month 25 of the NICIT regimen were processed by Shire Human Genetic Therapies (HGT). After month 25, Shire HGT stopped offering the anti-idursulfase antibodies test. Month 26 through month 59 specimens were processed by LabCorp. Total anti-idursulfase antibodies were reported as titers by Shire HGT and LabCorp, but the different laboratories had different reference ranges. Neutralizing antibody levels were reported in two ways. Shire HGT reported results as neutralizing antibodies as the percent inhibition of the idursulfase activity (i.e. NAb% inhibition). LabCorp reported results as neutralizing antibody titers.
The method used by Shire HGT and LabCorp are described elsewhere [20]. In brief, the blood samples were screened for detection of idursulfase IgG antibodies using the enzyme-linked immunosorbent (ELISA) assay or conformation-specific antibody (CSA) assay. Samples meeting pre-specified cutoff points were confirmed by radioimmunoprecipitation (RIP) assay. If the sample was confirmed by RIP assay, then it was reported as positive and the titer determined by ELISA or CSA assay. If the sample was negative by RIP assay, it was reported as antibody negative. Antibody positive samples were further analyzed for presence of neutralizing antibodies using an in vitro activity-neutralizing antibody assay [8].
2.3. Urinary GAG
Quantitative urinary GAG was measured using two assays: (1) the instant DMB dye-binding assay at the Mayo Medical Laboratories; and (2) the assay employing CPC precipitation followed by carbazole borate colorimetric quantification of uronic acid at the University of Illinois Biochemical Genetics Laboratory [21–24].
2.4. Statistical analysis
A Pearson's correlation coefficient was calculated to evaluate correlation between the following: (1) total anti-idursulfase antibody titers and neutralizing antibodies; (2) anti-idursulfase neutralizing antibodies and urinary GAG; (3) total anti-idursulfase antibody titers and urinary GAG. Comparisons were made only for samples obtained on the same day.
3. Results
Sampling times are indicated in months, with negative values for samples obtained prior to initiation of NICIT immune tolerance regimen. NICIT initiation is indicated at time zero and samples obtained after beginning NICIT are designated in positive values.
3.1. Total anti-idursulfase antibodies and neutralizing antibodies
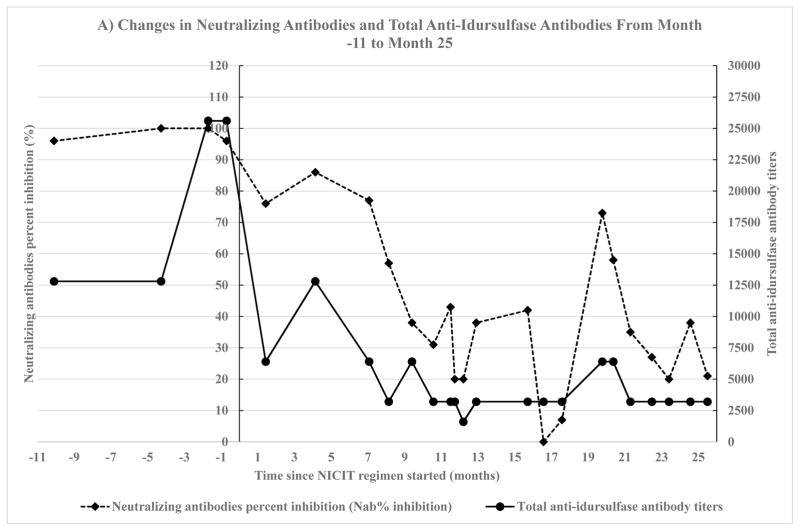
Two months after starting the NICIT regimen, the patient's parents reported less frequent respiratory infections and improved endurance. Nine months after starting the NICIT regimen, his neutralizing antibody levels decreased to approximately 40%. Sixteen months after starting the NICIT regimen, his neutralizing antibody levels reached zero (Fig. 1).
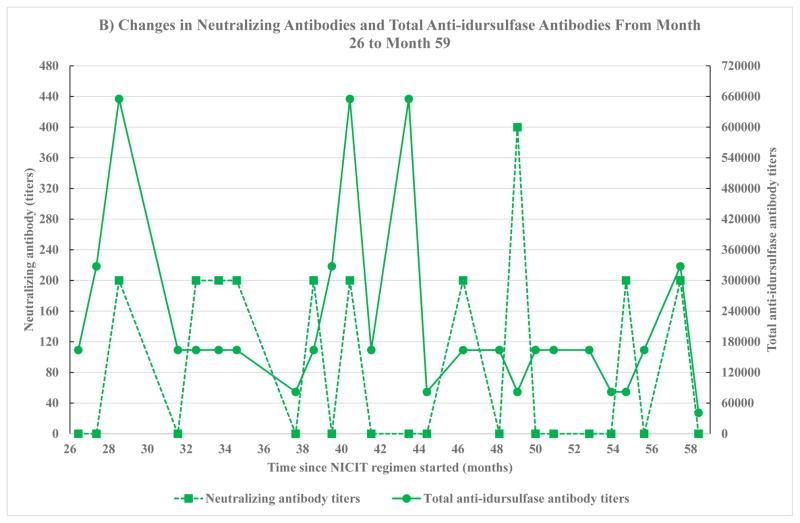
Fig. 1.
Changes in neutralizing antibodies and total anti-idursulfase antibodies. Neutralizing antibodies were measured by two methods, percent inhibition of enzyme activity by neutralizing antibodies (NAb% inhibition) and neutralizing antibody titers. Total anti-idursulfase antibody levels were measured by two labs, Shire HGT and LabCorp. The method used to measure neutralizing antibodies and the lab that measured total anti-idursulfase depended on the number of months since non-immunosuppressive and non-cytotoxic immune tolerance (NICIT) regimen was started. Month 0 is the month that NICIT regimen was started. A) Neutralizing antibodies measured as percent inhibition of enzyme activity by neutralizing antibodies (NAb % inhibition) on month −11 to month 25 since NICIT regimen was started. The left y-axis depicts neutralizing antibodies. The right y-axis depicts total anti-idursulfase antibodies. Total anti-idursulfase antibodies were measured by Shire HGT. B) Neutralizing antibodies measured as neutralizing antibody titers on month 26 to month 59 since NICIT regimen was started. The left y-axis depicts neutralizing antibodies. The right y-axis depicts total anti-idursulfase antibodies. Total anti-idursulfase antibodies were measured by LabCorp.
Changes in neutralizing antibodies and total anti-idursulfase antibodies from month −11 to month 25, measured by Shire HGT lab, showed significant correlation between NAb% inhibition and total anti-idursulfase antibody titers (Fig. 1A): no transformation, r = 0.78, p < 0.0001, 95% CI (0.55, 0.90).
Changes in neutralizing antibodies titers and total anti-idursulfase antibodies from month 26 to month 59, by LabCorp, showed no significant correlation (Fig. 1B): 1/x and 1/y transformation, r = −0.57, p = 0.083, 95% CI (−0.88, 0.089).
3.2. Neutralizing antibodies and urinary GAG
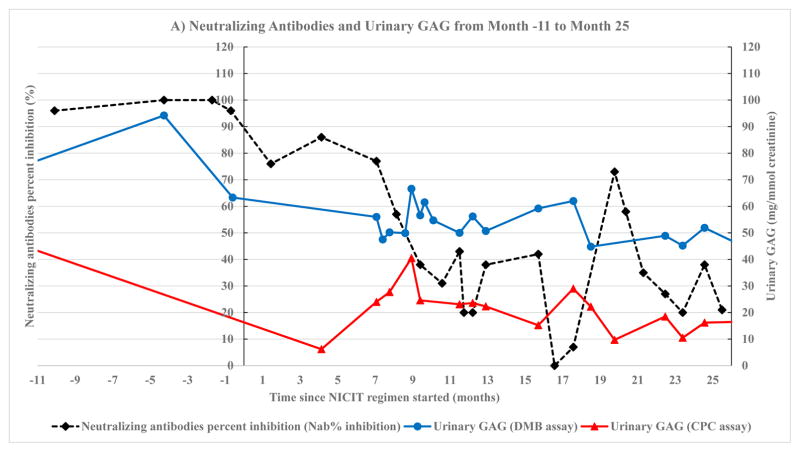
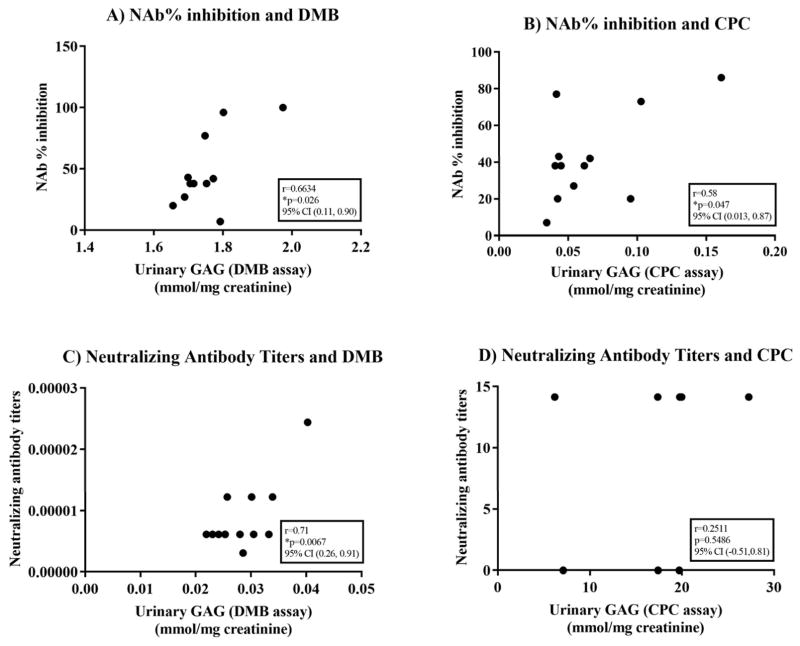
Significant correlation was found between neutralizing antibodies and urinary GAG by DMB assay from month −11 to month 25 (Fig. 2A). This includes correlation between NAb% inhibition and urinary GAG by DMB assay (Fig. 3A): log(x) transformation, r = 0.66, p = 0.026, 95% CI (0.11, 0.9). The correlation between NAb% inhibition and urinary GAG by CPC assay (Fig. 3B) was: 1/x transformation, r = 0.58, p = 0.047, 95% CI (0.013, 0.87).
Fig. 2.
Neutralizing antibodies and urinary GAG. Neutralizing antibodies were measured by two methods, percent inhibition of enzyme activity by neutralizing antibodies (NAb% inhibition) and neutralizing antibody titers. The method used to measure neutralizing antibodies depended on the number of months since non-immunosuppressive and non-cytotoxic immune tolerance (NICIT) regimen was started. Month 0 is the month that NICIT regimen was started. Quantitative urinary GAG was measured using DMB assay and CPC assay throughout the study. A) Neutralizing antibodies measured as percent inhibition of enzyme activity by neutralizing antibodies (NAb % inhibition) on month −11 to month 25 since NICIT regimen was started. The left y-axis depicts neutralizing antibodies. The right y-axis depicts quantitative urinary GAG. B) Neutralizing antibodies measured as neutralizing antibody titers on month 26 to month 59 since NICIT regimen was started. The left y-axis depicts neutralizing antibodies. The right y-axis depicts quantitative urinary GAG.
Fig. 3.
Correlation between neutralizing antibodies and urinary GAG. Neutralizing antibody was measured using two methods, percent inhibition of enzyme activity (NAb% inhibition) and neutralizing antibody titers. Quantitative urinary GAG was measured using two methods, DMB and CPC assay. p-Values <0.05 are starred. A) Correlation between neutralizing antibody measured as percent inhibition of enzyme activity and quantitative urinary GAG measured by DMB assay. Log(x) transformation. B) Correlation between neutralizing antibody measured as percent inhibition of enzyme activity and quantitative urinary GAG measured by CPC assay. 1/x transformation. C) Correlation between neutralizing antibody measured as titers and quantitative urinary GAG measured by DMB assay. 1/x and 1/y transformation. D) Correlation between neutralizing antibody measured as titers and quantitative urinary GAG measured by CPC assay. Sqrt(y) transformation.
Neutralizing antibody titers and urinary GAG were measured by LabCorp from month 26 to month 59 (Fig. 2B). A significant correlation was found when the DMB assay was used (Fig. 3C): 1/x and 1/y transformation, r = 0.71, p = 0.0067, 95% CI (0.26, 0.91); but not when CPC assay was used (Fig. 3D): sqrt(y) transformation, r = 0.25, p = 0.55, 95% CI (−0.51, 0.81).
3.3. Total anti-idursulfase antibodies and urinary GAG
No significant correlation was found between total anti-idursulfase antibodies and urinary GAG by DMB assay (Shire HGT lab, month −11 through month 25): 1/x transformation, r = 0.26, p = 0.54, 95% CI (−0.55, 0.81). There was a significant correlation, however, when measured by CPC assay: 1/x transformation, r = 0.69, p = 0.04, 95% CI (0.039, 0.93).
Significant correlation was found between total anti-idursulfase antibodies and urinary GAG by DMB assay (LabCorp, month 25 through month 59): 1/x and 1/y transformation, r = 0.7, p = 0.0059, 95% CI (0.26, 0.89); but not when measured by CPC assay: no transformation, r = 0.44, p = 0.28, 95% CI (−0.39, 0.87).
4. Discussion
This is the first case report to evaluate urinary GAG as a biomarker for monitoring neutralizing antibodies to idursulfase ERT in patients with MPS II. There was a significant correlation between urinary GAG by DMB assay and both NAb% inhibition and neutralizing antibody titers. Currently, only LabCorp offers the total anti-idursulfase antibody and neutralizing antibody test.
The NICIT regimen and patient case described herein were previously reported by the corresponding author of this paper at the June 2014 U.S. FDA and NORD public workshop [12]. At that time, the author reported on the patient's response for two and a half years after the NICIT regimen was initiated [12]. The study now presented is the first long-term follow-up report on this same patient case, and it describes sustained immune tolerance after five years. Importantly, this regimen does not use immunosuppressive or cytotoxic agents, but still shows sustained efficacy. Such a non-immunosuppressive regimen may not be appropriate for patients who require rapid immune tolerance, such as patients with infantile Pompe disease who are cross-reactive immunologic material (CRIM) negative with high levels of neutralizing antibodies. However, the NICIT regimen may offer an advantage for patients in whom achieving an immune tolerance over a timeframe of 6 to 18 months is clinically acceptable and for whom short-term and long-term toxicities of cytotoxic regimens are of concern.
4.1. Delay in laboratory results
In this patient case, the results for total anti-ERT antibody were usually received several months after the samples were submitted. Neutralizing antibody results were usually reported longer than six months after samples were submitted. The delay in receiving these laboratory reports postponed important decisions for titrating doses of the ERT and IVIG. Difficulties with obtaining real-time antibody levels were also emphasized at the previously mentioned FDA and NORD public workshop [12]. Ultimately, difficulties with reporting these antibody results creates barriers to establishing effective protocols for monitoring anti-idursulfase antibodies and managing ERT outcomes.
In contrast, quantitative urinary GAG results are available within a short time-frame of 7 to 14 days. Moreover, measuring quantitative urinary GAG is painless, low-cost, and non-invasive.
The NICIT regimen was designed with the goal of achieving complete immune tolerance, defined as sustained negligible neutralizing antibodies after discontinuing the immune tolerance regimen. Complete immune tolerance for this patient case has not been determined yet, as the patient continues to receive IVIG. To date, the IVIG has been reduced by 88%, and the patient is receiving the ERT dose of 1 mg/kg infused once a week. The patient's neutralizing antibodies during the past 4.5 years have been stable in a low to negligible range, even while ERT and IVIG doses were being reduced, consistent with at least a partial immune tolerance.
4.2. Frequency of urinary GAG monitoring
While urinary GAG is used as a diagnostic test for MPS conditions, it is often not followed closely once the diagnosis has been confirmed. In this case report, urinary GAG was not only correlated with anti-idursulfase neutralizing antibody levels, but also coincided with worsened clinical status, such as more frequent respiratory infections, reduced range of motion, reduced endurance, and worsening facial dysmorphism. Consistent with this, a decrease in urinary GAG coincided with improved clinical status, specifically less frequent respiratory infections and improved endurance as reported by the parents.
This patient's clinical worsening did not initially cause the care team to question whether the patient had a decreased response to idursulfase. Instead, his clinical worsening was initially viewed as disease progression. But in fact, the patient had a greatly diminished response to idursulfase from the neutralizing antibodies. This clinical scenario may be more common than generally recognized. Frequent monitoring of urinary GAG, e.g., monthly, helps differentiate whether clinical worsening is from disease progression or from changes in idursulfase's efficacy. Loss of idursulfase's efficacy shows an increase in urinary GAG in association with worsening clinical status, whereas disease progression alone, in the absence of anti-idursulfase neutralizing antibodies, occurs in the setting of a stable urinary GAG [2–5].
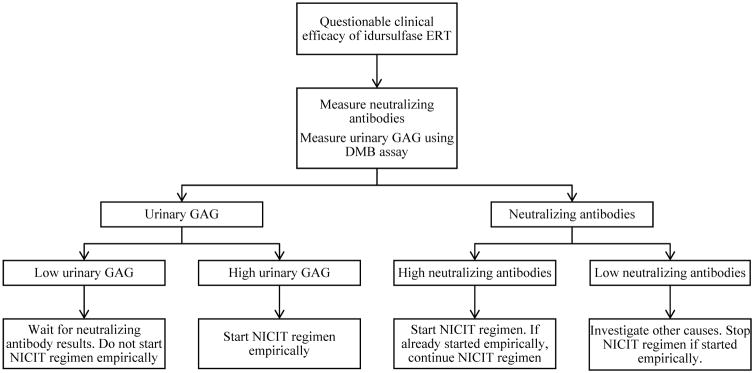
A proposed treatment algorithm using frequent urinary GAG monitoring as a biomarker for identifying and managing anti-idursulfase neutralizing antibody is shown in Fig. 4.
Fig. 4.
Treatment algorithm for monitoring idursulfase ERT efficacy. Abbreviations: DMB = direct 1,9-dimethylmethylene blue. ERT = enzyme replacement therapy. GAG = glycosaminoglycans. NICIT = non-immunosuppressive and non-cytotoxic immune tolerance.
4.3. Future considerations
This case report demonstrates how quantitative urinary GAG by DMB assay may be used as a biomarker for managing neutralizing antibodies that threaten idursulfase efficacy in patients with MPS II. Multi-patient studies evaluating urinary GAG as a screening tool for identifying neutralizing antibodies and monitoring response to immune tolerance regimens would further enhance understanding of how urinary GAG can be used as a clinical tool in management of idursulfase for patients with MPS II.
5. Conclusion
This study highlights the importance of regular and frequent monitoring of urinary GAG for patients with MPS II who are receiving ERT. To assure optimal monitoring, we recommend obtaining at least 3 urinary GAG/creatinine determinations at the time of diagnosis and before starting therapy, and to follow response to therapy by monitoring urinary GAG/creatinine levels monthly. Because urinary GAG/creatinine methods very greatly between laboratories, we recommend obtaining pretreatment and subsequent urinary GAG levels at a single source with highly reproducible results to obtain interpretable results. Also, it is important to recognize that urinary GAG/creatinine varies with age due to the normal increase in urinary creatinine corresponding with increasing muscle mass [22], Fig. 2.
The correlation between urinary GAG measured by DMB assay, and anti-idursulfase neutralizing antibodies suggests that urinary GAG measured using DMB assay may be a useful biomarker to monitor response to immune tolerance regimens. This may be especially applicable during periods when anti-idursulfase neutralizing antibody results are not readily available. Changes in urinary GAG coincide with changes in clinical status, as well as neutralizing antibody status, thereby allowing urinary GAG to be used to differentiate clinical worsening caused by loss of idursulfase efficacy, from clinical worsening caused by disease progression. The timeframe for achieving negligible neutralizing antibodies was similar at 18 months to that found in the regimen used by Kishnani et al. [12]. In contrast to the case report by Kishnani et al., the patient in this case report did not experience increased risk of infection from immune suppression, peripheral neuropathy, hypokalemia or diarrhea [12]. The patient did not have to use steroids or weekly prophylactic antibiotics. Finally, this study shows at least a partial and sustained immune tolerance after five years on a non-immunosuppressive and non-cytotoxic immune tolerance regimen, making this a promising regimen for clinical management of anti-ERT neutralizing antibodies in patients with MPS II.
Acknowledgments
Jeanine R. Jarnes Utz and Chester Whitley are supported by the Lysosomal Disease Network. The Lysosomal Disease Network (U54NS065768) is part of Rare Diseases Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), NCATS. The Lysosomal Disease Network is funded through collaboration between NCATS, the National Institute of Neurological Disorders and Stroke (NINDS), and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
Footnotes
The authors have no conflicts of interest pertaining to the research reported in this study. Color graphs are part of this article.
Acknowledgement: Jeanine R. Jarnes Utz and Chester B. Whitley are supported by the Lysosomal Disease Network. The Lysosomal Disease Network (U54NS065768) is part of Rare Diseases Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), NCATS. The Lysosomal Disease Network is funded through collaboration between NCATS, the National Institute of Neurological Disorders and Stroke (NINDS), and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
References
- 1.Sarafoglou K, Hoffmann GF, Roth KS. Pediatric Endocrinology and Inborn Errors of Metabolism. McGraw-Hill Company; New York: 2009. pp. 731–733. [Google Scholar]
- 2.Martin R, Beck M, Eng C, Giugliani R, Harmatz P, Munoz V, Muenzer J. Recognition and diagnosis of mucopolysaccharidosis II (Hunter syndrome) Pediatrics. 2008;121:e377–e386. doi: 10.1542/peds.2007-1350. [DOI] [PubMed] [Google Scholar]
- 3.Scarpa M. Mucopolysaccharidosis type II. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Ledbetter N, Mefford HC, Smith RJH, Stephens K, editors. GeneReviews®. Last Updated: March 26, 2015. (© 1993–2017 University of Washington, Seattle, WA.) [Google Scholar]
- 4.Muenzer J, Beck M, Eng CM, Giugliani R, Harmatz P, Martin R, Ramaswami U, Vellodi A, Wraith JE, Cleary M, Gucsavas-Calikoglu M, Puga AC, Shinawi M, Ulbrich B, Vijayaraghavan S, Wendt S, Conway AM, Rossi A, Whiteman DA, Kimura A. Long-term, open-labeled extension study of idursulfase in the treatment of Hunter syndrome. Genet Med. 2011;13:95–101. doi: 10.1097/GIM.0b013e3181fea459. [DOI] [PubMed] [Google Scholar]
- 5.Muenzer J, Wraith JE, Beck M, Giugliani R, Harmatz P, Eng CM, Vellodi A, Martin R, Ramaswami U, Gucsavas-Calikoglu M, Vijayaraghavan S, Wendt S, Puga AC, Ulbrich B, Shinawi M, Cleary M, Piper D, Conway AM, Kimura A. A phase II/III clinical study of enzyme replacement therapy with idursulfase in mucopolysaccharidosis II (Hunter syndrome) Genet Med. 2006;8:465–473. doi: 10.1097/01.gim.0000232477.37660.fb. [DOI] [PubMed] [Google Scholar]
- 6.Package insert, ELAPRASE® Idursulfase Solution, Concentrate. Shire US Manufacturing Inc; 2016. [Google Scholar]
- 7.Giugliani R, Harmatz P, Jones SA, Mendelsohn NJ, Vellodi A, Qiu Y, Hendriksz CJ, Vijayaraghavan S, Whiteman DA, Pano A. Evaluation of impact of anti-idursulfase antibodies during long-term idursulfase enzyme replacement therapy in mucopolysaccharidosis II patients. Mol Genet Metab Rep. 2017;12:2–7. doi: 10.1016/j.ymgmr.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sellos-Moura M, Barzegar S, Pan L, Shi P, Oommen S, Durant J, Ruiz JA. Development of a panel of highly sensitive, equivalent assays for detection of antibody responses to velaglucerase alfa or imiglucerase enzyme replacement therapy in patients with Gaucher disease. J Immunol Methods. 2011;373:45–53. doi: 10.1016/j.jim.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 9.Matzner U, Matthes F, Weigelt C, Andersson C, Eistrup C, Fogh J, Gieselmann V. Non-inhibitory antibodies impede lysosomal storage reduction during enzyme replacement therapy of a lysosomal storage disease. J Mol Med (Berl) 2008;86:433–442. doi: 10.1007/s00109-008-0309-3. [DOI] [PubMed] [Google Scholar]
- 10.Turner CT, Hopwood JJ, Bond CS, Brooks DA. Immune response to enzyme replacement therapy: 4-sulfatase epitope reactivity of plasma antibodies from MPS VI cats. Mol Genet Metab. 1999;67:194–205. doi: 10.1006/mgme.1999.2859. [DOI] [PubMed] [Google Scholar]
- 11.Banugaria SG, Prater SN, Ng YK, Kobori JA, Finkel RS, Ladda RL, Chen YT, Rosenberg AS, Kishnani PS. The impact of antibodies on clinical outcomes in diseases treated with therapeutic protein: lessons learned from infantile Pompe disease. Genet Med. 2011;13:729–736. doi: 10.1097/GIM.0b013e3182174703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kishnani PS, Dickson PI, Muldowney L, Lee JJ, Rosenberg A, Abichandani R, Bluestone JA, Burton BK, Dewey M, Freitas A, Gavin D, Griebel D, Hogan M, Holland S, Tanpaiboon P, Turka LA, Utz JJ, Wang YM, Whitley CB, Kazi ZB, Pariser AR. Immune response to enzyme replacement therapies in lysosomal storage diseases and the role of immune tolerance induction. Mol Genet Metab. 2016;117:66–83. doi: 10.1016/j.ymgme.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Campo C, da Silva Filho MI, Weinhold N, Mahmoudpour SH, Goldschmidt H, Hemminki K, Merz M, Forsti A. Bortezomib-induced peripheral neuropathy: a genome-wide association study on multiple myeloma patients. Hematol Oncol. 2017 doi: 10.1002/hon.2391. http://dx.doi.org/10.1002/hon.2391 ([Epub ahead of print]) [DOI] [PubMed]
- 14.Kerckhove N, Collin A, Conde S, Chaleteix C, Pezet D, Balayssac D. Long-term effects, pathophysiological mechanisms, and risk factors of chemotherapy-induced peripheral neuropathies: a comprehensive literature review. Front Pharmacol. 2017;8:86. doi: 10.3389/fphar.2017.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romao VC, Lima A, Bernardes M, Canhao H, Fonseca JE. Three decades of low-dose methotrexate in rheumatoid arthritis: can we predict toxicity? Immunol Res. 2014;60:289–310. doi: 10.1007/s12026-014-8564-6. [DOI] [PubMed] [Google Scholar]
- 16.Gilani ST, Khan DA, Khan FA, Ahmed M. Adverse effects of low dose methotrexate in rheumatoid arthritis patients. J Coll Physicians Surg Pak. 2012;22:101–104. [PubMed] [Google Scholar]
- 17.Kim KH, Messinger YH, Burton BK. Successful reduction of high-sustained anti-idursulfase antibody titers by immune modulation therapy in a patient with severe mucopolysaccharidosis type II. Mol Genet Metab Rep. 2015;2:20–24. doi: 10.1016/j.ymgmr.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benson G, Auerswald G, Elezovic I, Lambert T, Ljung R, Morfini M, Remor E, Salek SZ. Immune tolerance induction in patients with severe hemophilia with inhibitors: expert panel views and recommendations for clinical practice. Eur J Haematol. 2012;88:371–379. doi: 10.1111/j.1600-0609.2012.01754.x. [DOI] [PubMed] [Google Scholar]
- 19.Stiefel M, Pinkwart C, Haase R, Merkel N, Forsberg D, Mauz-Korholz C. Immune tolerance induction with high-dose FVIII and pulsed intravenous immunoglobulin. Hamostaseologie. 2010;30(Suppl 1):S119–S121. [PubMed] [Google Scholar]
- 20.Barbier AJ, Bielefeld B, Whiteman DA, Natarajan M, Pano A, Amato DA. The relationship between anti-idursulfase antibody status and safety and efficacy outcomes in attenuated mucopolysaccharidosis II patients aged 5 years and older treated with intravenous idursulfase. Mol Genet Metab. 2013;110:303–310. doi: 10.1016/j.ymgme.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Whitley CB, Ridnour MD, Draper KA, Dutton CM, Neglia JP. Diagnostic test for mucopolysaccharidosis. I. Direct method for quantifying excessive urinary glycosaminoglycan excretion. Clin Chem. 1989;35:374–379. [PubMed] [Google Scholar]
- 22.Whitley CB, Draper KA, Dutton CM, Brown PA, Severson SL, France LA. Diagnostic test for mucopolysaccharidosis. II. Rapid quantification of glycosaminoglycan in urine samples collected on a paper matrix. Clin Chem. 1989;35:2074–2081. [PubMed] [Google Scholar]
- 23.Bitter T, Muir HM. A modified uronic acid carbazole reaction. Anal Biochem. 1962;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- 24.Di Ferrante NM. The measurement of urinary mucopolysaccharides. Anal Biochem. 1967;21:98–106. doi: 10.1016/0003-2697(67)90087-5. [DOI] [PubMed] [Google Scholar]