Abstract
The protein-tyrosine phosphatase PTP1B is a negative regulator of insulin and leptin signaling and a highly validated therapeutic target for diabetes and obesity. Conventional approaches to drug development have produced potent and specific PTP1B inhibitors, but these inhibitors lack oral bioavailability, which limits their potential for drug development. Here, we report that DPM-1001, an analog of the specific PTP1B inhibitor trodusquemine (MSI-1436), is a potent, specific, and orally bioavailable inhibitor of PTP1B. DPM-1001 also chelates copper, which enhanced its potency as a PTP1B inhibitor. DPM-1001 displayed anti-diabetic properties that were associated with enhanced signaling through insulin and leptin receptors in animal models of diet-induced obesity. Therefore, DPM-1001 represents a proof of concept for a new approach to therapeutic intervention in diabetes and obesity. Although the PTPs have been considered undruggable, the findings of this study suggest that allosteric PTP inhibitors may help reinvigorate drug development efforts that focus on this important family of signal-transducing enzymes.
Keywords: drug discovery, obesity, phosphorylation, signal transduction, type 2 diabetes, tyrosine-protein phosphatase (tyrosine phosphatase), drug discovery
Introduction
The aberrant regulation of signal transduction pathways, and the accompanying disruption of the normal patterns of protein phosphorylation, has been implicated in the etiology of a variety of major human diseases, including diabetes and obesity (1, 2). The ability to target such signaling pathways selectively holds enormous therapeutic potential. In the last decade protein kinases have emerged as a major class of druggable targets. In particular, several drugs directed against protein-tyrosine kinases (PTKs)4 have had a considerable impact on the treatment of various cancers (3). Nevertheless, the focus on PTKs for drug development has encountered several challenges, including intrinsic and acquired resistance to such therapies. Consequently, additional targets and approaches are required. In this context, it is important to remember that protein phosphorylation is a reversible process, in which the coordinated and competing activities of kinases and phosphatases are important for determining signaling outcome. The protein-tyrosine phosphatases (PTPs), which represent a large family of proteins that work in coordination with the PTKs to control cell signaling, have also been implicated in the etiology of several human diseases (4). Nevertheless, PTPs remain underexploited as therapeutic targets.
The prototypic PTP, PTP1B, plays a well-established role in down-regulating insulin and leptin signaling and is a validated target for therapeutic intervention in diabetes and obesity (2, 5). Targeted deletion of the PTP1B gene produced healthy mice that displayed increased insulin sensitivity and resistance to obesity induced by a high-fat diet (6, 7). Considering its extensive validation as a therapeutic target, many programs were established, both in industry and academia, to generate small molecule, active site-directed inhibitors of PTP1B (8, 9). Several high-affinity, reversible, and selective inhibitors of PTP1B were generated that displayed efficacy in animal models (4, 8, 9). Nevertheless, these were often highly charged, which limited their potential for development as drugs. Consequently, novel approaches were needed to generate inhibitors of this highly validated target that exhibited greater drug development potential.
One such drug development strategy would be to avoid the catalytic center and identify allosteric inhibitors. We have characterized trodusquemine, also known as MSI-1436, which is a natural product, a spermine-cholesterol adduct, that inhibits PTP1B by a novel mechanism (10). Originally, PTP1B was purified from human placenta as a 37-kDa protein comprising predominantly the catalytic domain. This version of the protein has been widely used for biochemical analysis and drug-discovery purposes; however, in vivo, the enzyme exists as a 50-kDa protein with an extended C-terminal segment. We demonstrated that MSI-1436 is a specific, reversible, and non-competitive inhibitor of PTP1B. It targets preferentially the long form of PTP1B(1–405), which contains the extended C-terminal segment, 10-fold more potently than the truncated protein containing just the catalytic domain (residues 1–321). Furthermore, MSI-1436 targets PTP1B in vivo and attenuates its ability to promote HER2-dependent tumorigenesis (10). As a consequence, MSI-1436 is being tested in a Phase 1 clinical trial in metastatic breast cancer patients (ClinicalTrials.gov: NCT02524951). Nevertheless, MSI-1436 is also a charged molecule with limited oral bioavailability, which restricts the indications in which it can be applied therapeutically.
In our efforts to generate more potent analogs of MSI-1436, we identified DPM-1001. In this study, we present the characterization of DPM-1001. Furthermore, using an animal model of diet-induced obesity, we have demonstrated that the compound is an orally bioavailable inhibitor of PTP1B. Overall, this study illustrates a novel mechanism for inhibiting PTP1B that may reinvigorate interest in this phosphatase as a therapeutic target for diabetes and obesity.
Results
DPM-1001 was a non-competitive inhibitor of PTP1B
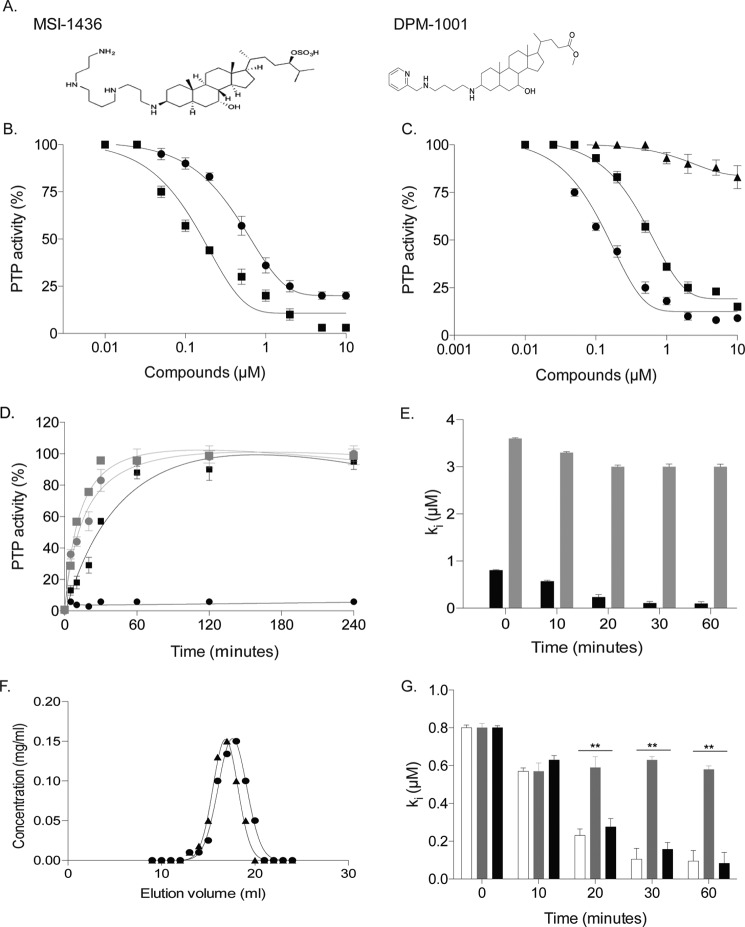
Previously, we demonstrated that MSI-1436 is a reversible, allosteric inhibitor of PTP1B (10). It inhibited preferentially PTP1B(1–405), a form of the enzyme that contains the non-catalytic C-terminal segment of the protein, over the catalytic domain, PTP1B(1–321). We identified an analog of MSI-1436, DPM-1001, in which a pyridin-2-ylmethyl-amino-butyl-amine group replaced the spermine tail in the C-3 position and a methyl ester replaced the sulfate group in the C-24 position (Fig. 1A). The two compounds were tested against PTP1B(1–405); DPM-1001 (IC50 100 nm) was found to be a more potent inhibitor of the phosphatase than MSI-1436 (IC50 600 nm) (Fig. 1B).
Figure 1.
DPM-1001 was a non-competitive inhibitor of PTP1B. A, chemical structures of MSI-1436 (left) and DPM-1001 (right). B, increasing concentrations of DPM-1001 (squares) and MSI-1436 (circles) tested for inhibition of the long form of the phosphatase, PTP1B(1–405), using 6,8-difluoro-4-methylumbelliferyl phosphate (DiFMUP) as substrate. C, effects of increasing concentrations of DPM-1001 (squares) and MSI-1436 (triangles) tested against the double mutant S372P/L192A PTP1B. D, PTP1B(1–405) (circles) or PTP1B(1–321) (squares) (100 nm) incubated with DPM-1001 (1 μm) for 15 min. The complex was diluted 100-fold, and the activity was monitored for 240 min. The results were compared with assays conducted with MSI-1436 against PTP1B(1–405) (circles) or PTP1B(1–321) (squares), as represented in gray. E, time dependence of the inhibition of the long (black bars) and short (gray bars) forms of PTP1B by DPM-1001. Statistical analysis was performed using one-way ANOVA (p = 0.08). F, elution profile of PTP1B(1–394) in the absence (circles) and presence (triangles) of DPM-1001. G, time dependence in the inhibition of PTP1B(1–405) by DPM-1001 tested in the absence (open bars) or presence of H2O2-degrading enzymes catalase (gray bars) and peroxiredoxin (black bars). Statistical analysis was performed using one-way ANOVA (**, p < 0.01). Unless otherwise indicated, data are representative of three or more independent experiments performed with technical replicates (triplicates) each time the experiment was conducted. Error bars represent mean ± S.E.
To investigate the mechanism of inhibition by DPM-1001, we tested it against PTP1B-L192A/S372P, an MSI-1436-resistant mutant form of the protein (10). Although PTP1B-L192A/S372P was insensitive to MSI-1436, the mutant enzyme was inhibited by DPM-1001 (IC50 of 1 μm) but with decreased sensitivity compared with the wildtype phosphatase (Fig. 1C). This suggested that there may be differences in the mechanism of inhibition of PTP1B by MSI-1436 compared with DPM-1001. We examined reversibility of inhibition by diluting the enzyme–inhibitor complex and monitoring the extent to which the phosphatase activity could be recovered. In contrast to MSI-1436, which was found to be a reversible inhibitor of both PTP1B(1–405) and PTP1B(1–321), DPM-1001 inhibited the short form of PTP1B reversibly, whereas PTP1B(1–405) remained inactive over an extended period of time (Fig. 1D). To examine the time dependence of inhibition by DPM-1001, we measured the activity of the long and short forms of PTP1B after incubation with DPM-1001 for varying times. We noticed that the inhibitory potency of DPM-1001 against PTP1B(1–405) improved with longer incubation of the enzyme and the compound. With no pre-incubation the IC50 value for PTP1B(1–405) was 600 nm, similar to that of MSI-1436; however, after a 30-min pre-incubation, the potency was improved to 100 nm. In contrast, there was no obvious time-dependent change in the IC50 value for PTP1B(1–321), suggesting that the C terminus of PTP1B was required for the difference in behavior of the two compounds (Fig. 1E).
In examining further the basis for time dependence, we demonstrated that DPM-1001 did not induce aggregation of PTP1B, thus eliminating this nuisance mechanism (Fig. 1F). It is now well established that the active-site cysteine in PTP1B is highly susceptible to oxidation (11), and there have been reports that small molecules can promote production of reactive oxygen species in phosphatase assays (12). Therefore, we tested whether DPM-1001 promoted preferential oxidation and inactivation of the long form of the enzyme. We examined DPM-1001-mediated inhibition of PTP1B(1–405) in the presence or absence of either peroxiredoxin 1 (PRX1) or catalase, because both of these enzymes degrade H2O2 to H2O, but by different mechanisms. We observed a time-dependent increase in potency for DPM-1001-mediated inhibition of PTP1B(1–405) in the presence or absence of PRX1 (Fig. 1G), suggesting that this effect was not due to oxidation and inactivation of the phosphatase mediated by the compound. Interestingly, we observed no obvious time dependence of inhibition in the presence of catalase (Fig. 1G). Unlike PRX1, catalase is a metal-dependent enzyme. With this in mind, we hypothesized that catalase may bind to metal ions present in the assay buffer as impurities and therefore prevent DPM-1001 from inhibiting PTP1B in a metal ion-dependent manner.
DPM-1001 formed a stable complex with copper(II)
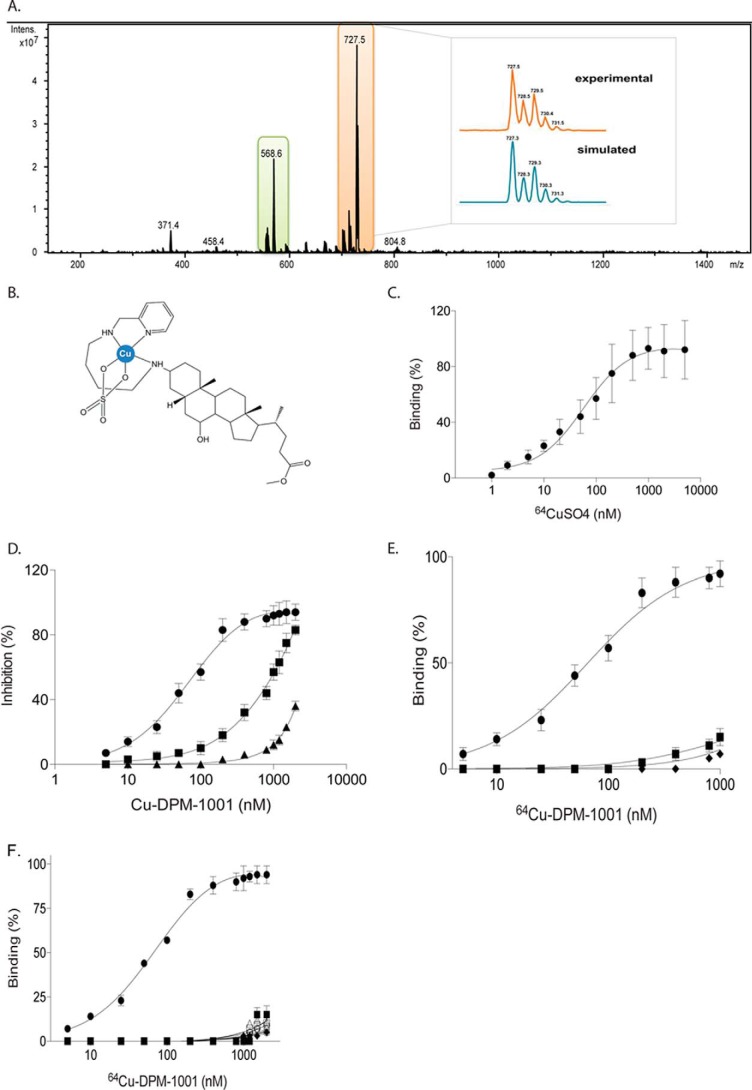
To test this hypothesis, DPM-1001 was reacted with an excess of CuSO4 or Cu(NO3)2 and subjected to ESI-MS analysis. The ESI-MS spectra of the DPM-1001/CuSO4 reaction mixture revealed two major peaks at m/z 568.6 and 727.5, consistent with the free DPM-1001 and a Cu(II) complex bound to both DPM-1001 and a sulfate anion, respectively. After analyzing the isotopic pattern, the latter was identified as [Cu(DPM-1001)(SO4) + H+]+ (Fig. 2A). In the presence of copper nitrate, we detected spectral peaks at m/z 692.5 and 755.5 (Fig. S1A), which correspond to the structures of [Cu(DPM-1001)(NO3)]+ and [Cu(DPM-1001)(NO3)2 + H+]+ (Fig. S1B). In the proposed models, DPM-1001 would act as a tridentate ligand forming one seven-membered and one five-membered chelate ring, whereas the environment around the Cu(II) is most likely to be five-coordinate displaying either a square pyramidal or a trigonal bipyramidal geometry (Fig. 2B and Fig. S1B). Square planar geometry, although rather typical for Cu(II), is not favored in this case due to the nature of the DPM-1001 ligand, whereas tetrahedral geometry is quite rare (the structure normally goes through solvent/counteranion coordination or ligand rearrangement affording thermodynamically more stable compounds with higher coordination numbers) (13).
Figure 2.
DPM-1001 formed a stable complex with copper and inhibited the long form of PTP1B selectively. A, DPM-1001 (1 mm) was reacted with CuSO4 (8 mm), and the reaction mixture was analyzed by ESI-MS. Isotopic pattern analysis of the peak at 727.5 m/z shown in the inset. Data are representative of three independent experiments. B, proposed structure of the complex [Cu(DPM-1001)SO4)]. C, radiolabeled copper (64Cu) at increasing concentrations was titrated against DPM-1001. D, effects of increasing concentrations of copper–DPM-1001 complex were tested on WT PTP1B (circles), H320A/H331A PTP1B(1–405) (squares), and H320A/H331A/S372P PTP1B(1–405) (triangles). E, increasing concentrations of the 64Cu–DPM-1001 complex were titrated against PTP1B(1–405), PTP1B(1–321), and H320A/H331A mutant PTP1B. F, increasing concentrations of 64Cu–DPM-1001 complex were titrated against a panel of PTPs (PTP1B(1–405) (circles); PTP1B(1–321) (squares); T-cell protein-tyrosine phosphatase (diamonds); SHP2 (open circles); leukocyte common antigen–related (LAR) family receptor protein-tyrosine phosphatase (open squares); PTPα (upward triangle); PTPσ (downward triangle); and phosphatase and tensin homolog (PTEN) (open diamonds)). Data are representative of three or more independent experiments performed with technical replicates (triplicates) each time the experiment was conducted. Error bars represent mean ± S.E.
To examine further the chelation properties of DPM-1001, we used radiolabeled copper (64Cu) to determine the affinity of DPM-1001 for the metal. By titrating DPM-1001 against increasing concentrations of 64Cu, we measured a Kd of 75 nm and a stoichiometry of 1 mol/mol (Fig. 2C). Other known Cu2+ chelators have been shown also to chelate Zn2+ ions; therefore, we reacted DPM-1001 with ZnSO4 or Zn(NO3)2 and subjected the solution to ESI-MS analysis. In all cases, we detected only a major peak at m/z 568.6 corresponding to DPM-1001; we did not observe any complex formation between DPM-1001 and Zn(II) (Fig. S2).
DPM-1001 bound to the C terminus of PTP1B and inhibited the enzyme
To define the mechanism by which a copper–DPM-1001 complex inhibited PTP1B, we generated truncation mutations in which we varied the lengths of the C-terminal segment of the phosphatase. We found that, similar to MSI-1436 (10), DPM-1001 was a more potent inhibitor of PTP1B in the presence of the extended C-terminal segment of the protein.
Considering the documented importance of His residues in copper-binding sites in proteins (14), we tested the effects of mutating His residues to Ala in the C-terminal segment of PTP1B on time-dependent inhibition mediated by the copper–DPM-1001 complex. In particular, we observed that mutation of the His residues His-320 and His-331 resulted in a marked reduction in the inhibitory potency of copper–DPM-1001 (Fig. 2D). Furthermore, combining the H320A/H331A mutations with S372P/L192A, which desensitized PTP1B to MSI-1436, produced a further rightward shift in the potency of copper–DPM-1001-mediated inhibition of PTP1B, from nanomolar to micromolar (Fig. 2D).
To examine the specificity of the interaction, we tested the copper–DPM-1001 complex against a panel of six PTPs and observed, again similar to MSI-1436, that there was preferential inhibition of the long form of PTP1B, containing the C-terminal segment, as compared with the other PTPs tested (Fig. S3A). Furthermore, we demonstrated a direct interaction between copper–DPM-1001 and PTP1B. Using radiolabeled copper (64Cu), we generated a complex of 64Cu and DPM-1001 and conducted binding assays with wildtype and mutant forms of PTP1B. First, we titrated free radiolabeled copper against the long and short forms of PTP1B and observed that copper, at a high concentration, could bind to PTP1B without a preference for the long and short form of the enzyme (Fig. S3B). All subsequent assays were conducted at copper concentrations below 0.1 mm to minimize direct interaction between copper and PTP1B. We tested wildtype PTP1B(1–405) against 64Cu–DPM-1001 complex, which revealed a Kd of 75 nm for the interaction (Fig. 2E). This was consistent with the observed IC50 value in our in vitro phosphatase activity assays. In contrast, we detected little binding of the 64Cu–DPM-1001 complex to the C-terminally truncated PTP1B(1–321). Similarly, the binding of the 64Cu–DPM-1001 complex to the H320A/H331A double mutant form of PTP1B was dramatically decreased relative to wildtype, even at high concentrations of the complex (Fig. 2E). Finally, as seen in activity assays (Fig. S3A), the specificity of the copper–DPM-1001 complex for PTP1B over other members of the PTP family was recapitulated in these binding assays (Fig. 2F). Overall, these data reveal a novel mechanism for inhibition of PTP1B that incorporates features of the allosteric site that is targeted by MSI-1436 but superimposes an additional feature of copper-dependent binding.
DPM-1001 inhibited diet-induced obesity in mice by improving insulin and leptin signaling
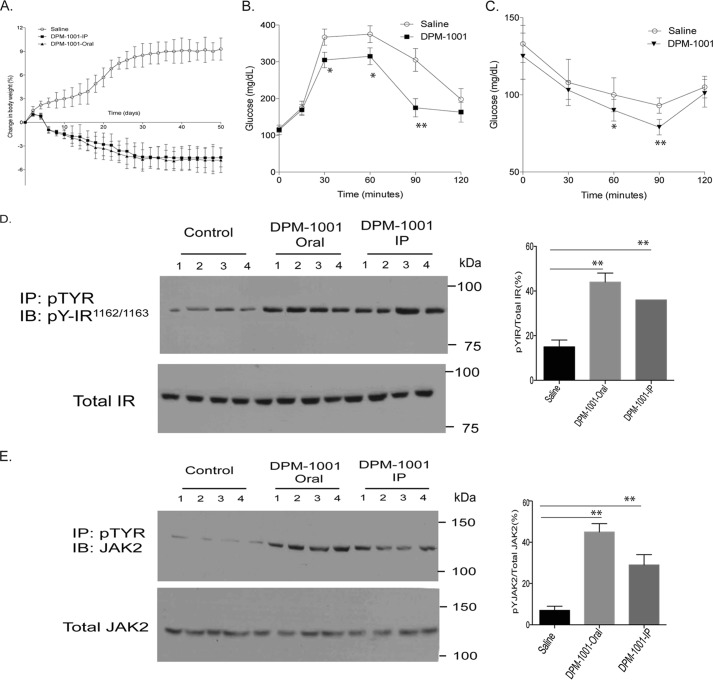
PTP1B is a negative regulator of insulin and leptin signaling; consequently, an inhibitor of PTP1B, such as DPM-1001, would be expected to promote signaling through insulin and leptin receptors (2, 6, 7, 15, 16). Therefore, we used high-fat diet-fed C57Bl6/J mice to examine the effects of DPM-1001 on obesity, metabolism, and signal transduction in animals. We treated high-fat diet- and chow diet-fed mice with DPM-1001. We compared the effect of the compound delivered orally or intraperitoneally to saline-treated mice. In contrast to saline, DPM-1001-treated, high-fat diet-fed mice started losing weight within 5 days of treatment. The weight loss continued for approximately 3 weeks, after which no further decrease in body weight was observed. Overall, treatment with DPM-1001 led to an ∼5% decrease in body weight (Fig. 3A). Importantly, we observed the same effects when the compound was delivered orally or intraperitoneally. Furthermore, no weight loss was noted with chow-fed mice, suggesting that the weight loss observed is a consequence of improved energy metabolism in the obese animals.
Figure 3.
DPM-1001 inhibited PTP1B and promoted insulin and leptin signaling in high-fat diet-fed mice. A, beginning at 10 weeks of age, until the study was terminated (18 weeks of age), high-fat diet (HFD)-fed obese male mice (C57bl6/J) were treated daily with DPM-1001 (5 mg/kg, either orally or intraperitoneally), and body weight was compared with saline-treated obese mice (mean ± S.E., n = 10 in each group). B, HFD-fed 14-week-old male mice treated with saline or DPM-1001 were administered d-glucose (2 mg/g body weight), and blood glucose was monitored (in each group, n = 10). Statistical analysis was performed using one-way ANOVA (*, p < 0.05; **, p < 0.01). C, HFD-fed 14-week-old male mice treated with saline or DPM-1001 were administered insulin (0.75 milliunits/g body weight), and blood glucose was monitored (in each group, n = 10). Statistical analysis was performed using one-way ANOVA (*, p < 0.05; **, p < 0.01). D, representative immunoblots showing insulin-induced tyrosine phosphorylation of IR-β and its total protein levels in liver tissue lysates from 14-week-old mice treated with saline or DPM-1001, either orally or intraperitoneally (IP). For insulin stimulation, animals were treated with insulin (0.75 milliunits/g, i.p.) for 15 min. E, representative immunoblots (IB) showing leptin-induced tyrosine phosphorylation of JAK2 and total JAK2 levels in hypothalamus tissue lysates from 14-week-old mice treated with saline or DPM-1001, either orally or intraperitoneally (IP). For leptin stimulation, animals were treated with leptin (1 mg/kg, s.c.) for 15 min. D and E, all immunoblots are representative of experiments performed independently three times. Immunoblots were quantified using ImageJ software. The quantitation includes the data from all three experiments, presented as the mean ± S.E. Statistical analysis was performed using one-way ANOVA (**, p < 0.01).
In contrast to administration of saline, treatment with DPM-1001, either orally or intraperitoneally, improved glucose tolerance and insulin sensitivity in glucose tolerance (Fig. 3B) and insulin tolerance (Fig. 3C) tests. These data suggest that the compound led to enhanced insulin signaling in the high-fat diet-fed mice. To examine this further, we measured the effect of the compound on tyrosine phosphorylation of the insulin receptor β subunit. We observed a marked increase in β-subunit phosphorylation following administration of the compound either orally or intraperitoneally, which was not observed with saline, indicating improved insulin signaling in response to the compound (Fig. 3D).
Considering also the important role of leptin in the control of glucose homeostasis and obesity, we tested the effects of the compound on leptin signaling in the hypothalamus. Similar to the improvement in insulin signaling, we observed that treatment with DPM-1001, but not saline, led to enhanced phosphorylation of JAK2 (Fig. 3E), consistent with enhanced leptin signaling.
Discussion
Obesity and type 2 diabetes together represent a huge, worldwide medical challenge. The American Diabetes Association highlights that 86 million Americans over the age of 20 are in a prediabetic state, with almost 10% of the United States population already diabetic. Worldwide, it is anticipated that 600 million people will be afflicted with diabetes by 2035 (17, 18). Although there are treatment options, clearly, they are not addressing this challenge effectively. Consequently, novel therapeutic approaches are required. The hormones insulin and leptin play critical roles in the control of glucose and energy homeostasis. Insulin controls the uptake of glucose from the bloodstream into peripheral tissues, such as skeletal muscle, and regulates glycogen synthesis. Leptin influences multiple pathways that control appetite and energy expenditure. An ideal drug target would be one that addresses both the insulin- and leptin-resistant states that characterize diabetes and obesity. PTP1B is such a target (2, 5).
Since it was first purified to homogeneity, PTP1B has been implicated as a regulator of insulin signaling (19, 20). The PTPN1 gene, which encodes PTP1B, is located on chromosome 20q13, which has been broadly linked to insulin resistance and obesity (21). Furthermore, the generation of PTP1B knock-out mice helped to cement the regulatory link between PTP1B and signaling through insulin and leptin receptors. These mice displayed enhanced glucose tolerance with increased insulin sensitivity, and they were lean, resistant to diet-induced obesity, and displayed hypersensitivity to leptin (15, 22). It is now established that PTP1B plays a critical role in down-regulating insulin signaling, by dephosphorylating the insulin receptor and IRS1, and down-regulating leptin signaling, by dephosphorylating the leptin receptor-associated PTK, JAK2 (16). Consequently, considerable effort has been expended on generating small molecule inhibitors of PTP1B to promote signaling through these pathways in resistant states (9, 23). By following the conventional path of producing small molecules that target the enzyme-active site, several potent, specific, and reversible inhibitors of PTP1B were generated. However, this was a challenging path for the PTP family of enzymes, and these small molecule inhibitors, which were often substrate mimetics, were charged, lacked oral bioavailability, and were limited in their drug development potential (2, 9). Therefore, new approaches are required to generate orally bioavailable inhibitors of PTP1B.
To meet this challenge, we focused on developing allosteric inhibitors (10). Subsequently, a similar approach was taken for generating inhibitors of other members of the PTP family, including an allosteric inhibitor of SHP2 that decreases tumor growth through suppression of RAS-MAP kinase signaling (24). In particular, we searched for inhibitors that recognized preferentially the “long form” of PTP1B, which contained the regulatory C-terminal segment not found in the catalytic domain construct used predominantly in small molecule screens. We identified a small molecule, aminosterol natural product, MSI-1436, as a reversible, selective, and non-competitive inhibitor of PTP1B, which inhibited preferentially the full-length form of PTP1B (10). We identified the binding sites for MSI-1436 in PTP1B, including a primary site in the C-terminal segment, and we demonstrated that the compound targeted PTP1B in vivo. Although MSI-1436 is an exciting therapeutic candidate, currently being tested in a Phase 1 clinical trial in metastatic breast cancer patients (ClinicalTrials.gov: NCT02524951), it is also charged and limited in oral bioavailability. Now, as presented here, we have identified DPM-1001, an analog of MSI-1436 that was an orally bioavailable inhibitor of PTP1B with anti-diabetic effects.
Like MSI-1436, DPM-1001 is a sterol; however, unlike MSI-1436, it is not a charged molecule, which likely contributes to its oral bioavailability. This and the increased potency relative to MSI-1436 are major steps forward in the quest for druggable inhibitors of PTP1B. As expected, there were some similarities in their mechanisms of inhibition. In fact, in the absence of metals, MSI-1436 and DPM-1001 displayed similar potency. Furthermore, a S372P/L192A double-point mutant form of PTP1B that is insensitive to MSI-1436 also displayed impaired inhibition by DPM-1001. Nevertheless, this study reveals a novel and unique aspect of the inhibition of PTP1B. DPM-1001 was an efficient chelator of copper. In fact, copper-bound DPM-1001 was a more potent inhibitor of PTP1B than the compound alone, and this improved potency was abrogated by mutation of two critical His residues in the C-terminal segment of PTP1B, suggesting that coordination with these residues may facilitate the stabilization of PTP1B in an inactive conformation.
Various metal ions have been shown to inhibit PTPs. Before their original purification and characterization, sensitivity to zinc ions (Zn2+) was used as a way to distinguish PTPs from the known phosphatases that target Ser/Thr residues in proteins (25, 26). Cr3+ supplementation has been shown to improve glucose metabolism and insulin signaling by inhibiting the activity of the phosphatase PTP1B (27), and several other metals have been shown to inactivate PTPs, including Cd2+, Fe3+, and Cu2+ (28). Many of these metal ions can react with molecular O2 to produce reactive oxygen species. Hence, it is not clear whether these metal ions inhibit PTPs directly or indirectly, by promoting oxidation of the active-site cysteine. In this study, it is the complex of copper and DPM-1001 that was inhibitory rather than the metal itself.
Metal complexes or metal ions have been used in the treatment of various human diseases. For example, cisplatin is a chemotherapeutic agent used in the treatment of a wide variety of cancers (29). Auranofin, which is a gold complex, is used as a therapeutic agent to improve symptoms of rheumatoid arthritis (29). Lanthanum carbonate (Fosrenol) is an approved drug to reduce serum phosphate levels in patients with end-stage renal disease (30). Sodium stibogluconate, which is used in the treatment of leishmaniasis, has been proposed to act by targeting the PTP SHP-1 (29). Thus, there is the potential for clinical application of metal-based drugs that target specific proteins. In the PTP field, peroxovanadium complexes were generated to target PTP1B (28, 31). Vanadium complexes inhibited PTP1B in vitro and in cells, where they led to improved insulin signaling (32, 33). Nevertheless, these complexes were found to be toxic, possibly due to their reactivity against other members of the PTP family. There are also examples of copper, gold, and ruthenium complexes that can selectively inhibit certain PTPs by targeting the active site (28, 34, 35); however, the mechanism for such selectivity is unclear.
In this study, we have identified and characterized a small molecule inhibitor of PTP1B, the potency of which was enhanced in the presence of copper. The compound displayed oral bioavailability and exhibited anti-diabetic properties in an animal model of diet-induced obesity that coincided with enhanced signaling through insulin and leptin receptors. One of the major challenges faced by PTP1B-based drug discovery efforts has been to generate orally bioavailable inhibitors of the phosphatase. In fact, the lack of oral bioavailability of active site-directed PTP inhibitors was a major factor in industry describing these enzymes as “undruggable.” Our efforts with this class of allosteric inhibitors now provide proof of concept that these challenges associated with active site-directed inhibitors of PTP1B can be overcome. Furthermore, it is interesting to note that there have been reports of elevated copper levels in patients with diabetes (36), with proposals that chelation of copper may offer a new strategy for treating the disease and its complications (37, 38). The combination of PTP1B inhibition and copper chelation presented in DPM-1001 reflects a new approach to the treatment of diabetes and obesity that we hope will re-invigorate drug development efforts in this area.
Experimental procedures
All materials and “Experimental procedures” are described in the supporting data.
Author contributions
N. K. is responsible for all of the data in Figs. 1, 2, C–F, and 3. K. F. K. and G. G. are responsible for the data in Fig. 2, A and B. N. K. and N. K. T. designed the study and wrote the paper with input from all of the authors. N. K. T. directed the study.
Supplementary Material
Acknowledgment
DPM-1001 was provided by DepYmed Inc. (New York).
This work was supported in part by National Institutes of Health Grants CA53840 and GM55989 (to N. K. T.), Cold Spring Harbor Laboratory Cancer Centre Support Grant CA45508 from the National Institutes of Health, the CSHL-Northwell Alliance, and the “Investissements d'Avenir” Program launched by the French Government and implemented by the ANR with reference ANR-10-IDEX-0001-02 PSL (to G. G.). N. K. and N. K. T. serve on the Scientific Advisory Board of DepYmed Inc., a company that focuses on inhibitors of PTP1B in various disease indications. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Figs. S1–S3 and supporting Methods.
- PTK
- protein-tyrosine kinase
- PTP
- protein-tyrosine phosphatase
- ANOVA
- analysis of variance
- ESI-MS
- electrospray ionization-mass spectrometry
- PRX1
- peroxiredoxin 1.
References
- 1. Cohen P. (2001) The role of protein phosphorylation in human health and disease. The Sir Hans Krebs Medal Lecture. Eur. J. Biochem. 268, 5001–5010 10.1046/j.0014-2956.2001.02473.x [DOI] [PubMed] [Google Scholar]
- 2. Tonks N. K. (2013) Protein-tyrosine phosphatases–from housekeeping enzymes to master regulators of signal transduction. FEBS J. 280, 346–378 10.1111/febs.12077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gross S., Rahal R., Stransky N., Lengauer C., and Hoeflich K. P. (2015) Targeting cancer with kinase inhibitors. J. Clin. Invest. 125, 1780–1789 10.1172/JCI76094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stanford S. M., and Bottini N. (2017) Targeting tyrosine phosphatases: time to end the stigma. Trends Pharmacol. Sci. 38, 524–540 10.1016/j.tips.2017.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Feldhammer M., Uetani N., Miranda-Saavedra D., and Tremblay M. L. (2013) PTP1B: a simple enzyme for a complex world. Crit. Rev. Biochem. Mol. Biol. 48, 430–445 10.3109/10409238.2013.819830 [DOI] [PubMed] [Google Scholar]
- 6. Elchebly M., Payette P., Michaliszyn E., Cromlish W., Collins S., Loy A. L., Normandin D., Cheng A., Himms-Hagen J., Chan C. C., Ramachandran C., Gresser M. J., Tremblay M. L., and Kennedy B. P. (1999) Increased insulin sensitivity and obesity resistance in mice lacking the protein-tyrosine phosphatase-1B gene. Science 283, 1544–1548 10.1126/science.283.5407.1544 [DOI] [PubMed] [Google Scholar]
- 7. Klaman L. D., Boss O., Peroni O. D., Kim J. K., Martino J. L., Zabolotny J. M., Moghal N., Lubkin M., Kim Y. B., Sharpe A. H., Stricker-Krongrad A., Shulman G. I., Neel B. G., and Kahn B. B. (2000) Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol. Cell. Biol. 20, 5479–5489 10.1128/MCB.20.15.5479-5489.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. He R. J., Yu Z. H., Zhang R. Y., and Zhang Z. Y. (2014) Protein-tyrosine phosphatases as potential therapeutic targets. Acta Pharmacol. Sin. 35, 1227–1246 10.1038/aps.2014.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maheswari N., Karthikeyan C., Trivedi P., and Moorthy N. S. (2017) Recent advances in protein tyrosine phosphatase 1B targeted drug discovery for Type II diabetes and obesity. Curr. Drug Targets. 10.2174/1389450118666170222143739 [DOI] [PubMed] [Google Scholar]
- 10. Krishnan N., Koveal D., Miller D. H., Xue B., Akshinthala S. D., Kragelj J., Jensen M. R., Gauss C. M., Page R., Blackledge M., Muthuswamy S. K., Peti W., and Tonks N. K. (2014) Targeting the disordered C terminus of PTP1B with an allosteric inhibitor. Nat. Chem. Biol. 10, 558–566 10.1038/nchembio.1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haque A., Andersen J. N., Salmeen A., Barford D., and Tonks N. K. (2011) Conformation-sensing antibodies stabilize the oxidized form of PTP1B and inhibit its phosphatase activity. Cell 147, 185–198 10.1016/j.cell.2011.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martin K. R., Narang P., Medina-Franco J. L., Meurice N., and MacKeigan J. P. (2014) Integrating virtual and biochemical screening for protein-tyrosine phosphatase inhibitor discovery. Methods 65, 219–228 10.1016/j.ymeth.2013.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haas K. L., and Franz K. J. (2009) Application of metal coordination chemistry to explore and manipulate cell biology. Chem. Rev. 109, 4921–4960 10.1021/cr900134a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rubino J. T., and Franz K. J. (2012) Coordination chemistry of copper proteins: how nature handles a toxic cargo for essential function. J. Inorg. Biochem. 107, 129–143 10.1016/j.jinorgbio.2011.11.024 [DOI] [PubMed] [Google Scholar]
- 15. Zabolotny J. M., Bence-Hanulec K. K., Stricker-Krongrad A., Haj F., Wang Y., Minokoshi Y., Kim Y. B., Elmquist J. K., Tartaglia L. A., Kahn B. B., and Neel B. G. (2002) PTP1B regulates leptin signal transduction in vivo. Dev. Cell 2, 489–495 10.1016/S1534-5807(02)00148-X [DOI] [PubMed] [Google Scholar]
- 16. Myers M. P., Andersen J. N., Cheng A., Tremblay M. L., Horvath C. M., Parisien J. P., Salmeen A., Barford D., and Tonks N. K. (2001) TYK2 and JAK2 are substrates of protein-tyrosine phosphatase 1B. J. Biol. Chem. 276, 47771–47774 10.1074/jbc.C100583200 [DOI] [PubMed] [Google Scholar]
- 17. Das A. (2015) Diabetic retinopathy: a global epidemic. Middle East Afr. J. Ophthalmol. 22, 133–134 10.4103/0974-9233.154385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marn-Peñalver J. J., Martín-Timón I., Sevillano-Collantes C., and Del Cañizo-Gómez F. J. (2016) Update on the treatment of type 2 diabetes mellitus. World J. Diabetes 7, 354–395 10.4239/wjd.v7.i17.354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tonks N. K., Cicirelli M. F., Diltz C. D., Krebs E. G., and Fischer E. H. (1990) Effect of microinjection of a low-Mr human placenta protein tyrosine phosphatase on induction of meiotic cell division in Xenopus oocytes. Mol. Cell. Biol. 10, 458–463 10.1128/MCB.10.2.458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cicirelli M. F., Tonks N. K., Diltz C. D., Weiel J. E., Fischer E. H., and Krebs E. G. (1990) Microinjection of a protein-tyrosine-phosphatase inhibits insulin action in Xenopus oocytes. Proc. Natl. Acad. Sci. U.S.A. 87, 5514–5518 10.1073/pnas.87.14.5514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tsou R. C., and Bence K. K. (2012) The genetics of PTPN1 and obesity: insights from mouse models of tissue-specific PTP1B deficiency. J. Obes. 2012, 926857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cheng A., Uetani N., Simoncic P. D., Chaubey V. P., Lee-Loy A., McGlade C. J., Kennedy B. P., and Tremblay M. L. (2002) Attenuation of leptin action and regulation of obesity by protein-tyrosine phosphatase 1B. Dev. Cell 2, 497–503 10.1016/S1534-5807(02)00149-1 [DOI] [PubMed] [Google Scholar]
- 23. Johnson T. O., Ermolieff J., and Jirousek M. R. (2002) Protein-tyrosine phosphatase 1B inhibitors for diabetes. Nat. Rev. Drug Discov. 1, 696–709 10.1038/nrd895 [DOI] [PubMed] [Google Scholar]
- 24. Chen Y. N., LaMarche M. J., Chan H. M., Fekkes P., Garcia-Fortanet J., Acker M. G., Antonakos B., Chen C. H., Chen Z., Cooke V. G., Dobson J. R., Deng Z., Fei F., Firestone B., Fodor M., et al. (2016) Allosteric inhibition of SHP2 phosphatase inhibits cancers driven by receptor tyrosine kinases. Nature 535, 148–152 10.1038/nature18621 [DOI] [PubMed] [Google Scholar]
- 25. Brautigan D. L., Bornstein P., and Gallis B. (1981) Phosphotyrosyl-protein phosphatase: Specific inhibition by Zn. J Biol Chem. 256, 6519–6522. [PubMed] [Google Scholar]
- 26. Hörlein D., Gallis B., Brautigan D. L., and Bornstein P. (1982) Partial purification and characterization of phosphotyrosyl-protein phosphatse from Ehrlich ascites tumor cells. Biochemistry 21, 5577–5584. 10.1021/bi00265a030 [DOI] [PubMed] [Google Scholar]
- 27. Levina A., and Lay P. A. (2011) Metal-based anti-diabetic drugs: advances and challenges. Dalton Trans. 40, 11675–11686 10.1039/c1dt10380f [DOI] [PubMed] [Google Scholar]
- 28. Lu L., and Zhu M. (2014) Protein-tyrosine phosphatase inhibition by metals and metal complexes. Antioxid. Redox Signal. 20, 2210–2224 10.1089/ars.2013.5720 [DOI] [PubMed] [Google Scholar]
- 29. Mjos K. D., and Orvig C. (2014) Metallodrugs in medicinal inorganic chemistry. Chem. Rev. 114, 4540–4563 10.1021/cr400460s [DOI] [PubMed] [Google Scholar]
- 30. Fricker S. P. (2007) Metal based drugs: from serendipity to design. Dalton Trans. 43, 4903–4917 [DOI] [PubMed] [Google Scholar]
- 31. Meggers E. (2009) Targeting proteins with metal complexes. Chem. Commun. 9, 1001–1010 [DOI] [PubMed] [Google Scholar]
- 32. McNeill J. H., Yuen V. G., Hoveyda H. R., and Orvig C. (1992) Bis(maltolato)oxovanadium(IV) is a potent insulin mimic. J. Med. Chem. 35, 1489–1491 10.1021/jm00086a020 [DOI] [PubMed] [Google Scholar]
- 33. Heyliger C. E., Tahiliani A. G., and McNeill J. H. (1985) Effect of vanadate on elevated blood glucose and depressed cardiac performance of diabetic rats. Science 227, 1474–1477 10.1126/science.3156405 [DOI] [PubMed] [Google Scholar]
- 34. Chatterjee D., Mitra A., Levina A., and Lay P. A. (2008) A potential role for protein-tyrosine phosphatase inhibition by a RuIII-edta complex (edta = ethylenediaminetetraacetate) in its biological activity. Chem. Commun. 25, 2864–2866 [DOI] [PubMed] [Google Scholar]
- 35. Krishnamurthy D., Karver M. R., Fiorillo E., Orrú V., Stanford S. M., Bottini N., and Barrios A. M. (2008) Gold(I)-mediated inhibition of protein-tyrosine phosphatases: a detailed in vitro and cellular study. J. Med. Chem. 51, 4790–4795 10.1021/jm800101w [DOI] [PubMed] [Google Scholar]
- 36. Squitti R., Mendez A. J., Simonelli I., and Ricordi C. (2017) Diabetes and Alzheimer's disease: can elevated free copper predict the risk of the disease? J. Alzheimers Dis. 56, 1055–1064 10.3233/JAD-161033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tanaka A., Kaneto H., Miyatsuka T., Yamamoto K., Yoshiuchi K., Yamasaki Y., Shimomura I., Matsuoka T. A., and Matsuhisa M. (2009) Role of copper ion in the pathogenesis of type 2 diabetes. Endocr. J. 56, 699–706 10.1507/endocrj.K09E-051 [DOI] [PubMed] [Google Scholar]
- 38. Cooper G. J. (2012) Selective divalent copper chelation for the treatment of diabetes mellitus. Curr. Med. Chem. 19, 2828–2860 10.2174/092986712800609715 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.