Abstract
Phosphoinositides (PIs) are phospholipids that perform crucial cell functions, ranging from cell migration and signaling to membrane trafficking, by serving as signposts of compartmental membrane identity. Although phosphatidylinositol 4,5-bisphosphate, 3-phosphate, and 3,5-bisphosphate are commonly considered as hallmarks of the plasma membrane, endosomes, and lysosomes, these compartments contain other functionally important PIs. Here, we review the roles of PIs in different compartments of the endolysosomal system in mammalian cells and discuss the mechanisms that spatiotemporally control PI conversion in endocytosis and endolysosomal membrane dynamics during endosome maturation and sorting. As defective PI conversion underlies human genetic diseases, including inherited myopathies, neurological disorders, and cancer, PI-converting enzymes represent potential targets for drug-based therapies.
Keywords: phosphatidylinositol, phosphatidylinositol kinase (PI Kinase), phosphatidylinositol phosphatase, phosphatidylinositol signaling, autophagy, endocytosis, endosome, lysosome, mTOR complex (mTORC)
Introduction
PIs2 are a minor class of comparably short-lived membrane phospholipids that mediate crucial cellular and organismal functions, including signaling, gating of ion channels, cytoskeleton regulation and motility, development, as well as the regulation of intracellular membrane traffic (1, 2). The seven PI species found in mammalian cells differ with respect to the phosphorylation status of the 3-, 4-, and 5-OH group in the inositol ring of phosphatidylinositol and can be interconverted by specific PI kinases and phosphatases (Fig. 1) (1, 2). Different PI species display distinct subcellular distributions with PI 4-phosphates such as phosphatidylinositol 4-phosphate (PI(4)P) being concentrated in the exocytic pathway, in particular in the Golgi complex, the trans-Golgi network (TGN), and at the plasma membrane. In contrast, PI 3-phosphates such as PI(3)P and PI(3,5)P2 are found predominantly within early and late endosomes or lysosomes. As PIs are essential for key functions of the organelles or membrane domains where they reside, they have been postulated to serve as spatiotemporally controlled signposts of membrane identity (1–3). While organelles or membrane transport intermediates (e.g. vesicles and tubules) mature, change their functional status, or fuse with each other, their PI content changes. We are only now beginning to understand how PI conversion is spatiotemporally controlled at the molecular level. In this minireview, we highlight recent advances in our understanding of PI conversion with a focus on endocytosis and the endolysosomal system.
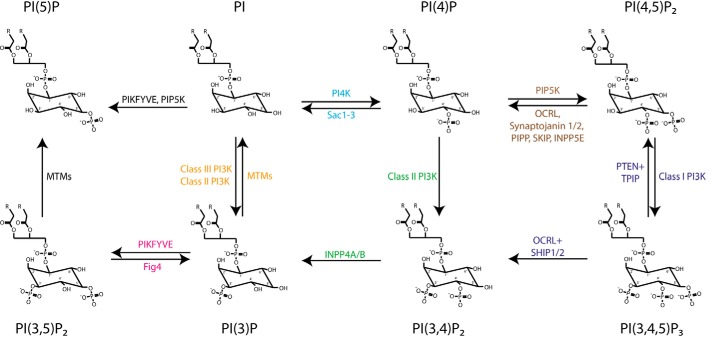
Figure 1.
Interconversion of PIs by kinases and phosphatases. Phosphatidylinositol can be phosphorylated by PI4K to yield PI(4)P), which can be further phosphorylated by PIP5K to PI(4,5)P2, which serves as a substrate for class I PI 3-kinases (Class I PI3K) to produce PI(3,4,5)P3. Phosphorylation of PI at the 3-OH position by class I PI 3-kinase (termed Vps34) (Class III PI3K) yields PI(3)P that can be further phosphorylated by PIKFYVE to produce PI(3,5)P2. PIKFYVE may also synthesize PI(5)P from PI. Class II PI 3-kinases (Class II PI3K) synthesize PI(3,4)P2 from PI(4)P and PI(3)P from the PI. Myotubularins (MTMs) are 3-phosphatases that hydrolyze PI(3)P and PI(3,5)P2. OCRL, synaptojanin 1/2, PIPP, SKIP, and INPP5E are PI(4,5)P2 5-phosphatases, Fig4 is a 5-phosphatase for PI(3,5)P2. PI(3,4,5)P3 can be dephosphorylated by the 3-phosphatases PTEN and TPIP to PI(4,5)P2 or by the 5-phosphatases OCRL and SHIP1/2 to produce PI(3,4)P2. The 4-phosphatases Sac1–3 and INPP4A/B dephosphorylate PI(4)P and PI(3,4)P2, respectively.
PI(4,5)P2 to PI(3,4)P2 conversion controls maturation of endocytic coated pits
Plasma membrane function, including endocytosis, is intimately linked to the presence of PI(4,5)P2, which is generated by consecutive phosphorylation of PI to PI(4)P and PI(4,5)P2 by PI 4-kinase IIIα (4) and type I PI 4-phosphate 5-kinases (PIPKIs) (1). Among other clathrin adaptors, including FCHo, CALM/AP180, epsins, etc., PI(4,5)P2 recruits the heterotetrameric AP-2 complex, which associates with and activates PIPKIs (5). This results in a feed-forward loop of PIPKI activation creating a local PI(4,5)P2 pool required for the initial stages of clathrin-coated pit (CCP) formation. Assembly of clathrin then displaces PIPKIs from AP-2 (6), consistent with the absence of PIP5KIs from clathrin-coated vesicles (7) and from maturing CCPs (5, 8). Feedback inhibition by clathrin thus restricts PI(4,5)P2 synthesis to the early stages of clathrin-mediated endocytosis (CME). Maturation of CCPs is accompanied by the recruitment of the PI(3,4,5)P3 and PI(4,5)P2 5-phosphatases synaptojanin, oculocerebrorenal syndrome of Lowe protein (OCRL) and Src-homology 2 containing 5-phosphatase 2 (SHIP2). Loss of these enzymes impairs CME due to defective PI(4,5)P2 hydrolysis, resulting in the accumulation of clathrin-coated vesicles in various biological systems (9–14). The exact timing of PI(4,5)P2 turnover by synaptojanin, OCRL, and possibly SHIP2 remains to be determined but may depend on the cell type and tissue.
Recent data show that loss of PI(4,5)P2 late in CME is accompanied by synthesis of phosphatidylinositol 3,4-bisphosphate (PI(3,4)P2) mediated by phosphatidylinositol 3-kinase C2α (PI3KC2α) (15), suggesting a local conversion from PI(4,5)P2 to PI(3,4)P2 as CCPs mature. In support of this model, depletion of plasma membrane PI(3,4)P2 by expression of a membrane-targeted variant of the PI(3,4)P2 4-phosphatase INPP4B stalls CCP dynamics similar to depletion of PI3KC2α. In contrast, plasma membrane CCPs are lost upon PI(4,5)P2 depletion (16). Thus, PI(4,5)P2 and PI(3,4)P2 exhibit distinct regulatory roles during the early and late stages of CME (Fig. 2). How the formation and turnover of these PIs is controlled in time and space remains incompletely understood. Clathrin appears to play a dual role in this process as it not only restricts PI(4,5)P2 production to early stages but also is essential for the recruitment and activation of PI3KC2α (15, 17). It is likely that additional factors, such as plasma membrane PIs and/or small GTPases, control the nanoscale localization and activity of PI3KC2α. Moreover, it is possible that the activity of PI3KC2α is physically and functionally linked to hydrolysis of PI(4,5)P2 by PI 5-phosphatases, which generate the PI3KC2α substrate PI(4)P. Dissecting these mechanisms in detail remains a fruitful area for future studies.
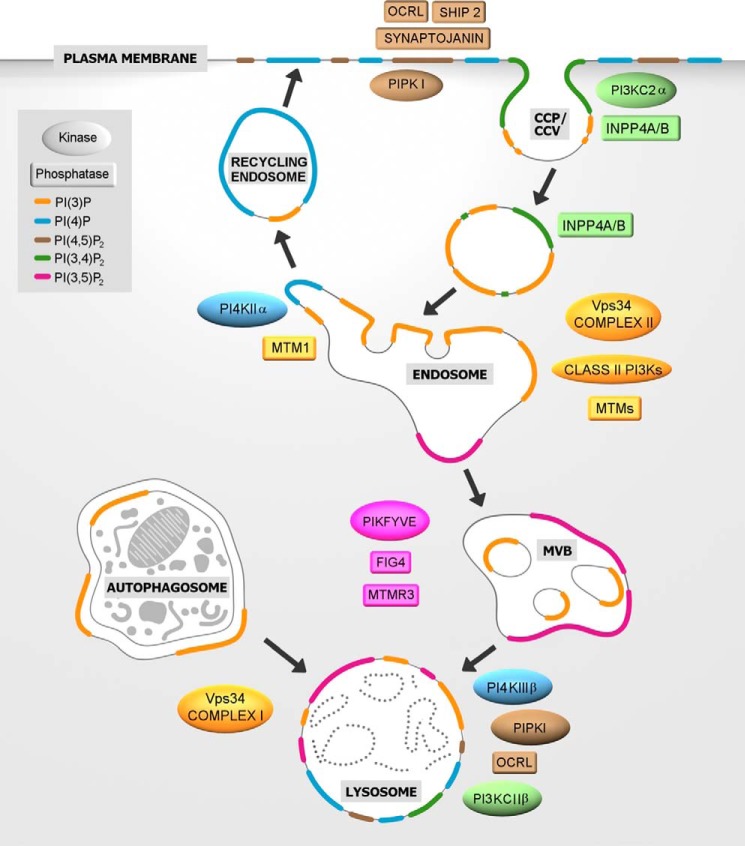
Figure 2.
PI conversion in CME and in the endolysosomal system. Clathrin-mediated endocytosis requires plasma membrane PI(4,5)P2, which is a substrate for the PI 5-phosphatases OCRL, synaptojanin, and SHIP1/2. Class II PI3Kα generates a plasma membrane pool of PI(3,4)P2 necessary for CCP maturation and formation of free clathrin-coated vesicles (CCV). PI(3)P, an essential feature of early endosomes, is generated primarily by the class III PI3K Vps34 complex II with a possible contribution of class II PI3Ks (encircled by dashed line), either by direct PI(3)P synthesis or indirectly via PI(3,4)P2 hydrolysis by the PI 4-phosphatases INPP4A/B. Endosomal recycling to the cell surface requires PI(3)P hydrolysis by myotubularin phosphatases (MTMs) such as MTM1 and the concomitant generation of PI(4)P by PI4KIIα to enable exocytosis. As endosomes mature into late endosomes/MVBs, the PI(3)P 5-kinase PIKFYVE converts PI(3)P into PI(3,5)P2. PI(3,5)P2 turnover at MVBs and/or lysosomes is mediated by MTMs together with the PI(3,5)P2 5-phosphatase Fig4. Lysosomal membranes contain several PIs such as PI(3)P, PI(4)P, and PI(4,5)P2. PI(3)P can be produced by class III PI3K/Vps34 directly at the lysosome or is obtained by fusion with autophagosomes, where PI(3)P is produced by VPS34 complex I. PI(4)P is generated by PI4KIIIβ, and PI(4,5)P2 is hydrolyzed by OCRL. PI(4)P can be converted to PI(3,4)P2 by the class II PI3KC2β.
Recent computational modeling and super-resolution imaging data show that local PI(3,4)P2 synthesis by PI3KC2α at CCPs triggers the selective recruitment of the PX-BAR domain protein sorting nexin 9 (SNX9; and its close homolog SNX18) (18) to the invagination neck where its self-assembly regulates membrane constriction (19). Interestingly, the membrane-deforming activity of SNX9 is controlled by an allosteric structural switch involving coincident detection of the clathrin adaptor AP-2 and PI(3,4)P2 at endocytic sites (20). This mechanism thus allows the spatiotemporal coupling of SNX9/18-mediated membrane constriction to the progression of the endocytic pathway (21).
As SNX9 not only interacts with PIs but also with actin-regulatory factors (18), it is conceivable that its action in CME may couple membrane deformation to changes in actin dynamics that–given the non-essential role of actin in CME in mammals–likely are regulatory in nature (22, 23). Consistent with this idea, it has been recently proposed based on in vitro studies using liposomes that the concomitant presence of PI(3)P and PI(4,5)P2 can facilitate F-actin assembly by SNX9 (24). According to this model, PI(3,4)P2 produced by PI3KC2α is rapidly converted to PI(3)P by the 4-phosphatase INPP4A. This PI(3)P pool serves to recruit SNX9 to late-stage CCPs. Whether PI(3,4)P2 to PI(3)P conversion can occur at the plasma membrane in vivo is uncertain. INPP4A has been localized to endosomes (25) rather than CCPs. Moreover, a re-engineered class III-like PI3KC2α mutant with wild-type PI(3)P-synthesizing activity in vivo (26), but unable to make PI(3,4)P2, fails to rescue defective CME in the absence of the endogenous enzyme (15). These data suggest that PI(3,4)P2 rather than PI(3)P is required for CCP maturation and SNX9-mediated membrane constriction. Future studies are needed to provide a better understanding of the mechanisms that control PI(3,4)P2 hydrolysis by INPP4A/B and possibly other enzymes in time and space within the early endocytic pathway.
PI(3)P defines endosomal membrane identity and is key to endosome function
PI(3)P is a hallmark of the endosomal system and is of key importance for endosome function. Within the endosomal system, cargo may be recycled to the cell surface, trafficked retrogradely to the TGN, or sorted to multivesicular bodies (MVBs)/late endosomes for lysosomal degradation. Although conversion of plasma membrane-derived PI(3,4)P2 to PI(3)P by INPP4A/B and possibly other PI phosphatases contributes to endosomal PI(3)P levels, the majority of PI(3)P at endosomes is generated by class III PI3K Vps34 and to a minor extent class II PI3Ks (e.g. PI3KC2α) (Fig. 2) (26, 27). Class III PI3K forms two distinct complexes that function in autophagy (complex I) and endosomal sorting (complex II). The endosomal complex II consisting of Vps34, p150/Vps15, Beclin 1/Vps30, and UVRAG/Vps38 (28) assembles at early endosomes via association of Vps15/p150 with Rab5-GTP to generate PI(3)P from phosphatidylinositol. Endosomal PI(3)P recruits various downstream effectors, including early endosome autoantigen 1 (EEA1), the ESCRT component hepatocyte growth factor-regulated tyrosine kinase substrate (Hrs, termed Vps27 in yeast), and endosomal SNXs. These proteins directly bind to PI(3)P, e.g. via FYVE or PX domains, to regulate endosomal fusion, intralumenal vesicle formation, tubulation, and maturation (29–31).
PI(3)P production at endosomes by class III PI3K/Vps34 is counteracted by the myotubularin family PI 3-phosphatases MTM1, MTMR1, and MTMR2. MTM1 and MTMR2 can associate with the WD40 domain of the Vps15 subunit of the class III PI3K complex II on endosomes. Interestingly, the association of the class III PI3K complex with MTM1 or with endosomal Rabs is mutually exclusive (32, 33). How PI(3)P production by class III PI3K/Vps34 and hydrolysis by MTM1/MTMRs are reciprocally regulated is unknown, but regulation of Rab GTPases, possibly in conjunction with PI signals, is likely of crucial importance. For example, it has recently been shown that Sbf, the Drosophila ortholog of the PI 3-phosphatase MTMR13, recruits active MTM to promote PI(3)P turnover at endosomes and functions as a GEF to activate the Rab5 family member Rab21 (34), a regulator of integrin trafficking (35). These results show that PI(3)P levels and endosomal sorting are controlled by a complex interplay between PI kinases, phosphatases, and Rab GTPases. Similar modules may control PI turnover and conversion at other subcellular locations.
PI(3)P to PI(4)P conversion is required for endosomal exocytosis
The crucial importance of PI(3)P for endosomal identity raises the question how cargo can exit the endosomal system, for example during endosomal recycling, which delivers integrins and other receptors to the cell surface. Recent data suggest that surface delivery of endosomal cargo requires loss of endosomal identity by hydrolysis of PI(3)P mediated by MTM1, a PI 3-phosphatase mutated in X-linked centronuclear myopathy patients in humans (Table 1). PI(3)P turnover during endosomal exocytosis is accompanied by PI 4-kinase IIα (PI4KIIα)-dependent generation of PI(4)P (Fig. 2) (36, 37). MTM1 and PI4KIIα are part of a PI(3)P-to-PI(4)P conversion module that involves complex formation of PI4KIIα with MTM1 and with the PI- binding exocyst tethering complex that mediates fusion with the plasma membrane enriched in PI(4)P and PI(4,5)P2 (36). Membrane recruitment of MTM1 depends on PI4KIIα and is associated with Rab conversion from Rab5 to Rab11, a GTPase switch required for endosomal recycling (38). These data link defective PI conversion at endosomes to muscle and non-muscle defects in patients suffering from X-linked centronuclear myopathy due to mutations in the MTM1 gene (36). Importantly, defects caused by mutations in MTM1 can be partially reversed by pharmacological inhibition of PI(3)P production by Vps34 (36) or genetic ablation of the class II PI3K PI3KC2β in zebrafish or mouse models (39). These exciting observations may thus pave a way for treatment of this otherwise fatal human disease.
Table 1.
Examples of PI-metabolizing enzymes implicated in human genetic diseases
| Gene | Catalytic activity | Kinase–phosphatase complexes | Disease |
|---|---|---|---|
| FIG4 | PI (3,5)P2 5-phosphatase | PIKFYFE | Charcot-Marie-Tooth type 4J |
| Yunis-Varon syndrome | |||
| MTM1 | PI (3)P, PI (3,5)P2 3-phosphatase | PI4KIIα, VPS34 complex II | Centronuclear X-linked myopathy |
| MTMR2 | PI (3)P, PI (3,5)P2 3-phosphatase | VPS34 complex II | Charcot-Marie-Tooth type 4B1 |
| MTMR5 | Pseudophosphatase | Phosphatase-active MTMs | Charcot-Marie-Tooth type 4B3 |
| MTMR13 | Pseudophosphatase | Phosphatase-active MTMs, PI3KCIIα | Charcot-Marie-Tooth type 4B2 |
| MTMR14 | PI (3)P, PI (3,5)P2 3-phosphatase | Centronuclear X-linked myopathy | |
| OCRL | PI (4,5)P2 5-phosphatase | Lowe syndrome Dent-2 disease | |
| PIKFYVE | PI, PI (3)P 5-kinase | with Fig4 | Fleck corneal dystrophy |
| PIP5K1C | PI (4)P 5-kinase | Lethal congenital contracture syndrome type 3 | |
| INPPL1 (SHIP2) | PI (3,4,5)P3, PI (4,5)P2 5-phosphatase | Opsismodysplasia | |
| INPP5E | PI (4,5)P2 5-phosphatase | Joubert syndrome | |
| MORM syndrome | |||
| SYNJ1 | PI (3,4,5)P3, PI (4,5)P2 5-phosphatase | Parkinson's disease |
PI(3)P to PI(3,5)P2 conversion promotes endosomal maturation and degradative sorting
Endosomal maturation is accompanied by Rab conversion from early endosomal Rab5 to late endosomal Rab7 (40, 41) and active cargo sorting into intraluminal vesicles by the ESCRT complex, two processes that critically depend on PI(3)P. PI(3)P activates the Rab7 GEF Mon1-Ccz1 (40–42) and facilitates recruitment of Hrs/Vps27, a subunit of ESCRT-0, and of ESCRT-II, a complex that mediates inward vesicle budding (43). Furthermore, PI(3)P promotes the microtubule-dependent translocation of late endosomes and lysosomes to the cell periphery. This pathway involves membrane contacts between late endosomes/lysosomes and the endoplasmic reticulum (ER) via the kinesin adaptors protrudin and FYCO1 (44, 45). These findings reveal an unexpected connection between endolysosomal PI 3-phosphates and contact site formation with the ER to regulate membrane dynamics and cell signaling (as discussed below).
Endosomal maturation along the late endosome/lysosome pathway is accompanied by conversion of PI(3)P to PI(3,5)P2 at the limiting membrane of late endosomes (Fig. 2). This process depends on PIKFYVE, a phosphatidylinositol and PI(3)P 5-kinase, which binds to PI(3)P via its FYVE domain and uses the same lipid as a substrate to form PI(3,5)P2. PI(3,5)P2 serves several important functions at late endosomes/lysosomes. It is crucial for the sorting of degradative cargo into MVBs (46) and may aid intralumenal vesicle formation by associating with the ESCRT-III component Vps24 (47). Consistently, acute inhibition of PIKFYVE has been shown to block late endosomal protein sorting or turnover as well as retroviral budding, a process that depends on the cellular ESCRT machinery (48). Furthermore, lysosomal PI(3,5)P2 regulates Ca2+ release from the lysosome lumen and is required for acidification by the v-ATPase (49, 50). How precisely PI(3,5)P2 acts in these pathways and which effector proteins directly bind to PI(3,5)P2 remain to be elucidated.
Given the multiple functions of PI(3,5)P2 at late endosomes/lysosomes, the activity of PIKFYVE must be tightly regulated. The PIKFYVE kinase is in fact part of a larger protein complex, including the PI 5-phosphatase Fig4, that is held together by the scaffold protein Vac14. Loss of either PIKFYVE, Fig4, or Vac14 causes a similar depletion of PI(3,5)P2 in models that range from yeast to mammals (51–53). Active cell signaling along the PI3K-Akt pathway promotes PIKFYVE activity via its direct phosphorylation by Akt (54). A similar activation of PIKFYVE by AMPK-mediated phosphorylation is observed during muscle contraction (55). Much remains to be learned about the physiological roles of PI(3,5)P2, the regulation of its synthesis and turnover by extracellular signals, and its interplay with other PI lipids.
PI 3-phosphates are required for autophagosome formation and maturation
Autophagy is a major pathway for the degradation of cytoplasmic material that eventually is targeted to the lysosome lumen for proteolysis. It is initiated by the engulfment of bulk cytosol (containing protein aggregates) and/or organelles by a cup-shaped double-membrane sheet known as the phagophore. The phagophore closes on itself to form the autophagosome, which delivers its contents to the lysosome for degradation by lysosomal hydrolases (56). Autophagy is reciprocally regulated by class I and class III PI3Ks (57). Receptor-mediated class I PI3K signaling activates the mechanistic target of rapamycin complex 1 (mTORC1) during times of nutrient abundance. Active mTORC1 phosphorylates and thereby inactivates the serine/threonine kinase ULK1 (termed Atg1 in yeast) (57) and the acetyltransferase p300 (58), resulting in suppression of starvation-induced autophagy. The serine/threonine kinase ULK1 together with ATG13, FIP200, and ATG101 form part of a multiprotein complex, which plays a pivotal role in the earliest steps of the autophagy process (56). ULK1 stimulates the class III PI3K–Vps34 complex I to generate a local pool of PI(3)P on autophagic membranes (59). This pool of PI(3)P then serves to recruit various proteins needed for the formation of the phagophore (60). A prominent example are WIPIs, PI(3)P-binding proteins with β-propeller folds, that mediate the recruitment of the ATG12–ATG5–ATG16L1 E3 ligase complex to the phagophore membrane to promote LC3 conjugation and autophagosome formation (61, 62).
In addition to PI(3)P, which is essential for early steps of autophagosome formation, PI(3,5)P2 may regulate later stages of the autophagy/lysosomal pathway (Fig. 2). Defective autophagy/lysosome-mediated protein turnover has been observed under conditions of either impaired PI(3,5)P2 synthesis via PIKFYVE (63) or defective PI(3,5)P2 turnover in the absence of MTMR8/9 (64) and related MTMRs (65) or the 5-phosphatase INPP5E, mutations in which cause Joubert syndrome (Table 1), a human ciliopathy (66). Hence, cytoplasmic turnover via the autophagy/lysosomal pathway require spatiotemporally controlled formation and conversion of PI 3-phosphates, the mechanisms of which remain poorly understood.
PI 3-phosphates regulate nutrient signaling
The lysosome acts as a central metabolic regulator by directing the cell into either an anabolic or catabolic state. A central element in this metabolic control hub is mTORC1 (Fig. 3), which integrates extracellular growth factor signals with the cellular nutrient and energy status to elicit downstream signals that directly impinge on autophagy, protein and lipid synthesis, and cell growth (67, 68). Disruptions in mTORC1-mediated lysosomal signaling are implicated in diseases such as diabetes, cancer, and neurological disorders (69, 70). Recruitment of mTORC1 to the lysosome depends on the cellular and lysosomal amino acid status and is mediated by the Rag small GTPases (71–73). The activation status of lysosomal mTORC1 is under further control by growth factor signals, most notably class I PI3K-mediated synthesis of plasma membrane PI(3,4,5)P3, which activates mTORC1 via its effector Akt (Fig. 3) (74, 75).
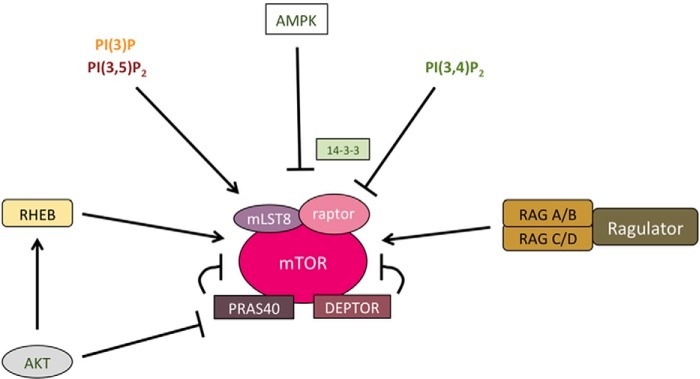
Figure 3.
Subunit composition and regulation of mTORC1. mTORC1 consists of the mTOR kinase, the complex defining subunit raptor mLST8, and the two negative regulators PRAS40 and DEPTOR. Activation of mTORC1 depends on recruiting the complex to its place of activation–the lysosomal surface–by Ras-related GTP-binding protein (Rag) GTPases. mTORC1 is fully activated by the RHEB GTPase downstream of AKT-dependent pathways. Furthermore, lysosomal PI(3)P and PI(3,5)P2 play roles in mTORC1 activation. AMPK and PI(3,4)P2 can inhibit mTORC1 activity by regulating the binding of raptor to inhibitory 14-3-3 proteins.
Recent data have identified additional PIs other than PI(3,4,5)P3 as important regulators of mTORC1-mediated nutrient signaling. For example, amino acids stimulate class III PI3K/Vps34-mediated PI(3)P synthesis (76). PI(3)P facilitates lysosomal recruitment of phospholipase D1 (PLD1) via its PX domain, resulting in the formation of phosphatidic acid, which then triggers dissociation of the inhibitory DEPTOR subunit from mTORC1 (77). Similarly, PI3KC2α and PIKFYVE have been implicated in mTORC1 activation via the sequential phosphorylation of phosphatidylinositol to generate PI(3,5)P2, which activates mTORC1 by associating with the WD40 domain of its Raptor subunit (Fig. 3) (78). In yeast, an additional role of PI(3,5)P2 in nutrient signaling via recruitment of the mTORC1 substrate Sch9 (the homolog of mammalian ribosomal S6 kinase, S6K) has been proposed (79).
In addition to affecting nutrient signaling via mTORC1 directly, PI(3,5)P2 may regulate nutrient signaling indirectly via activating lysosomal TRPML Ca2+ channels (49). Blockade of lysosomal Ca2+ release has been suggested to inhibit mTORC1 signaling (80). Moreover, it has been shown that lysosomal Ca2+ release via TRPMLs triggers activation of calcineurin, resulting in the activation and nuclear translocation of transcription factor EB (TFEB) to up-regulate the expression of autophagy/lysosomal genes (81, 82). These data suggest a possible role for PI(3,5)P2 in both anabolic (mTORC1 activation) and catabolic pathways (induction of autophagy via TFEB). Future studies will need to address how PI lipids, including PI(3,5)P2, couple mTORC1 regulation to lysosomal ion homeostasis and protein turnover via the autophagy/lysosome pathway.
PI(3)P and PI(3,4)P2 play opposing roles in the coupling of nutrient signaling to lysosome position
Recent studies have revealed a surprising but mechanistically poorly understood link between lysosomal position and function and nutrient signaling via mTORC1. Peripheral lysosomes display a more alkaline pH due to reduced v-ATPase function and elevated mTORC1 activity compared with perinuclear lysosomes (83, 84). Lysosomal distribution is largely controlled by the small GTPases Rab7 and Arl8A/B (83). Rab7 links lysosomes via its effector RILP to retrograde dynein motors (85), whereas Arl8A/B directly (in the case of kinesin 3) or indirectly (via the kinesin 1 adaptor SKIP/PLEKHM2 (86)) couples lysosomes to different kinesins 1 and 3 (87), which promote their peripheral dispersion (Fig. 4). Rab7 can also interact with and recruit the late endosomal/lysosomal kinesin 1 adaptor FYCO1, a protein associated with PI(3)P (45), and with PLEKHM1, a negative regulator of Arl8/SKIP/PLEKHM2-mediated lysosomal dispersion (88). Thus, lysosome position and dynamics are determined by a complex interplay between competing GTPases and their associated motors that likely are regulated by multiple factors, including lysosomal Ca2+ signals and PIs.
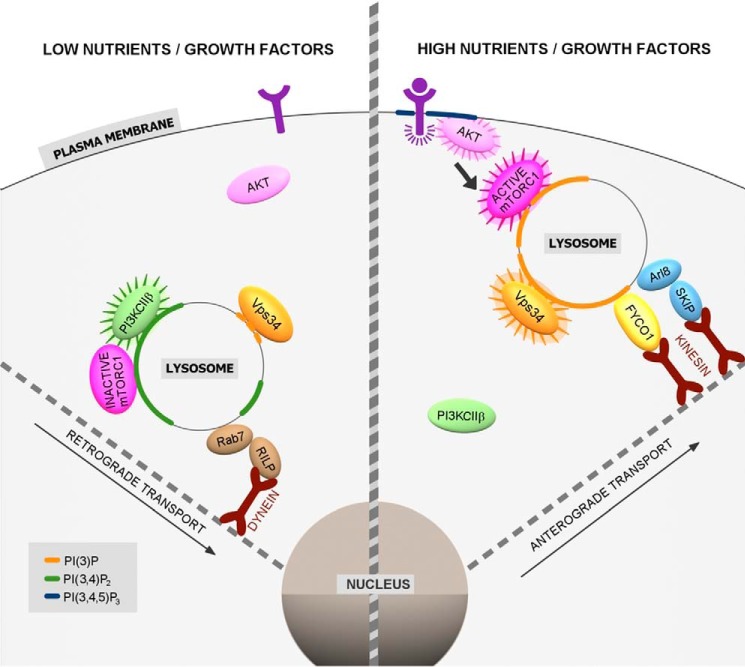
Figure 4.
Interplay between lysosome position and function and nutrient signals regulated by PIs. In the absence of nutrients and growth factors, lysosomes are transported retrogradely via dynein motors linked via Rab7/RILP. Growth factor deprivation also triggers PI3KC2β recruitment to lysosomes, where it generates PI(3,4)P2 to suppress mTORC1 activity, and facilitates perinuclear clustering of lysosomes. High nutrient and growth factor conditions cause activation of the class III PI3K Vps34. Vps34-mediated synthesis of PI(3)P results in lysosomal recruitment of the kinesin 1 adaptor FYCO1, which may supply kinesin motors to the Arl8-associated adaptor SKIP/PLEKHM2. Lysosomes undergo anterograde transport resulting in their dispersion to the cell periphery and in activation of mTORC1, e.g. by growth factor-derived Akt signaling.
Under conditions of ample nutrient supply, activation of Vps34-mediated PI(3)P synthesis facilitates the recruitment of the PI(3)P-associated kinesin-1 adaptor FYCO1 to late endosome/lysosome contact sites with the ER. There the motor protein kinesin-1 is transferred from the PI(3)P-binding integral ER membrane protein protrudin to lysosome-associated FYCO1 (45). FYCO1-kinesin–mediated anterograde traffic of lysosomes to the cell periphery causes the concomitant activation of mTORC1 (44). Conversely, growth factor deprivation has recently been shown to cause the late endosomal/lysosomal recruitment of class II PI3Kβ (PI3KC2β), which locally produces a lysosomal pool of PI(3,4)P2 that facilitates perinuclear clustering of lysosomes and suppresses mTORC1 activity (89). These data argue that lysosomal PI content is subject to nutrient regulation; conversely, lysosomal PIs, including PI(3)P and PI(3,4)P2, couple lysosome position to the regulation of nutrient signaling via mTORC1 (Fig. 4). How local pools of PI(3)P or PI(3,4)P2 couple lysosome position to the activity status of mTORC1 is essentially unknown. Late endosomal/lysosomal PI(3,4)P2 has been shown to facilitate recruitment of 14-3-3 proteins to the mTORC1 subunit Raptor (89), but whether 14-3-3 proteins regulate lysosome position is unknown. Therefore, it is conceivable, if not likely, that some of the factors that control lysosome position are regulated by the local PI content. Conceivably, PIs may also modulate the association of mTORC1 components with the lysosomal transport machinery, such as the recently described interaction between the Arl8-activating BLOC1-related complex and the mTORC1-associated ragulator–LAMTOR complex (90).
So far, little is known about the PI phosphatases that control lysosome position and nutrient signaling. The PI(3)P 3-phosphatase MTMR3 has recently been found to interact with mTORC1, and overexpression of this enzyme inhibits mTORC1 activity (91), further supporting the view that PI(3)P facilitates nutrient signaling via mTORC1. Moreover, as lysosomes contain a variety of other PI lipids, including PI(4)P (92) and PI(4,5)P2 (93), the question arises whether and how these lipid pools may be subject to nutrient regulation or, conversely, contribute to the coupling of lysosome function and position to nutrient signals and, possibly, to membrane contact site formation with other organelles (94). Given the key functions that lysosomes execute in cell physiology, the answers to these questions may impact our understanding of lysosome-related disease and be of relevance for the treatment of diseases related to lysosome function.
Conclusions and perspectives
Although the role of PIs as signposts of membrane identity and as important signaling factors is well established (1–3), we are only now beginning to appreciate the complex pathways that mediate the spatiotemporally regulated turnover of PI identity that underlies membrane dynamics and signaling in eukaryotic cells and tissues. Recent years have also witnessed the identification of new functions of PIs, for example in the formation of membrane contact sites between various organelles, including the plasma membrane, the ER, and the endolysosomal system, that likely contribute to cellular lipid homeostasis and couple membrane dynamics to signaling pathways (95). Among the major obstacles to resolve these mechanisms are the limited tools to visualize and manipulate PIs in live cells and with nanoscale resolution in real time. As novel techniques ranging from chemical genetic and optogenetic systems to pharmacological inhibitors of PI kinase and phosphatase function, as well as chemical tools (e.g. photocaged PI derivatives) (96), and new sensors are being developed (97), we should soon be able to obtain a more complete picture of the mechanisms that govern PI conversion in endocytosis and the endolysosomal system. Such knowledge may turn out to be crucial when it comes to developing new treatment avenues for the growing list of human genetic disorders of PI metabolism (98).
This work was supported by Deutsche Forschungsgemeinschaft Grants SFB 740/TP C08 and TRR 186/TP A08. The authors declare that they have no conflicts of interest with the contents of this article.
- PI
- phosphoinositide
- PI(4)P
- phosphatidylinositol 4-phosphate
- TGN
- trans-Golgi network
- PI(3)P
- phosphatidylinositol 3-phosphate
- PI(3,5)P2
- phosphatidylinositol 3,5-bisphosphate
- PI(3,4)P2
- phosphatidylinositol 3,4-bisphosphate
- PI(4,5)P2
- phosphatidylinositol 4,5-bisphosphate
- PI(3,4,5)P3
- phosphatidylinositol 3,4,5-trisphosphate
- CME
- clathrin-mediated endocytosis
- CCP
- clathrin-coated pit
- PIPKI
- PI 4-phosphate 5-kinase
- MVB
- multivesicular body
- GEF
- guanine nucleotide exchange factor
- ER
- endoplasmic reticulum
- MTMR
- myotubularin-related protein
- TRPML
- transient receptor potential mucolipin
- TFEB
- transcription factor EB
- SNX
- sorting nexin
- AMPK
- AMP kinase
- OCRL
- oculocerebrorenal syndrome of Lowe
- PI3K
- phosphoinositide 3-kinase.
References
- 1. Di Paolo G., and De Camilli P. (2006) Phosphoinositides in cell regulation and membrane dynamics. Nature 443, 651–657 10.1038/nature05185 [DOI] [PubMed] [Google Scholar]
- 2. Balla T. (2013) Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiol. Rev. 93, 1019–1137 10.1152/physrev.00028.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Behnia R., and Munro S. (2005) Organelle identity and the signposts for membrane traffic. Nature 438, 597–604 10.1038/nature04397 [DOI] [PubMed] [Google Scholar]
- 4. Baskin J. M., Wu X., Christiano R., Oh M. S., Schauder C. M., Gazzerro E., Messa M., Baldassari S., Assereto S., Biancheri R., Zara F., Minetti C., Raimondi A., Simons M., Walther T. C., et al. (2016) The leukodystrophy protein FAM126A (hyccin) regulates PtdIns(4)P synthesis at the plasma membrane. Nat. Cell Biol. 18, 132–138 10.1038/ncb3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Krauss M., Kukhtina V., Pechstein A., and Haucke V. (2006) Stimulation of phosphatidylinositol kinase type I-mediated phosphatidylinositol (4,5)-bisphosphate synthesis by AP-2μ-cargo complexes. Proc. Natl. Acad. Sci. U.S.A. 103, 11934–11939 10.1073/pnas.0510306103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thieman J. R., Mishra S. K., Ling K., Doray B., Anderson R. A., and Traub L. M. (2009) Clathrin regulates the association of PIPKIγ661 with the AP-2 adaptor β2 appendage. J. Biol. Chem. 284, 13924–13939 10.1074/jbc.M901017200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Borner G. H., Harbour M., Hester S., Lilley K. S., and Robinson M. S. (2006) Comparative proteomics of clathrin-coated vesicles. J. Cell Biol. 175, 571–578 10.1083/jcb.200607164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Antonescu C. N., Aguet F., Danuser G., and Schmid S. L. (2011) Phosphatidylinositol-(4,5)-bisphosphate regulates clathrin-coated pit initiation, stabilization, and size. Mol. Biol. Cell 22, 2588–2600 10.1091/mbc.E11-04-0362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nakatsu F., Perera R. M., Lucast L., Zoncu R., Domin J., Gertler F. B., Toomre D., and De Camilli P. (2010) The inositol 5-phosphatase SHIP2 regulates endocytic clathrin-coated pit dynamics. J. Cell Biol. 190, 307–315 10.1083/jcb.201005018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Erdmann K. S., Mao Y., McCrea H. J., Zoncu R., Lee S., Paradise S., Modregger J., Biemesderfer D., Toomre D., and De Camilli P. (2007) A role of the Lowe syndrome protein OCRL in early steps of the endocytic pathway. Dev. Cell 13, 377–390 10.1016/j.devcel.2007.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schuske K. R., Richmond J. E., Matthies D. S., Davis W. S., Runz S., Rube D. A., van der Bliek A. M., and Jorgensen E. M. (2003) Endophilin is required for synaptic vesicle endocytosis by localizing synaptojanin. Neuron 40, 749–762 10.1016/S0896-6273(03)00667-6 [DOI] [PubMed] [Google Scholar]
- 12. Verstreken P., Koh T. W., Schulze K. L., Zhai R. G., Hiesinger P. R., Zhou Y., Mehta S. Q., Cao Y., Roos J., and Bellen H. J. (2003) Synaptojanin is recruited by endophilin to promote synaptic vesicle uncoating. Neuron 40, 733–748 10.1016/S0896-6273(03)00644-5 [DOI] [PubMed] [Google Scholar]
- 13. Milosevic I., Giovedi S., Lou X., Raimondi A., Collesi C., Shen H., Paradise S., O'Toole E., Ferguson S., Cremona O., and De Camilli P. (2011) Recruitment of endophilin to clathrin-coated pit necks is required for efficient vesicle uncoating after fission. Neuron 72, 587–601 10.1016/j.neuron.2011.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nández R., Balkin D. M., Messa M., Liang L., Paradise S., Czapla H., Hein M. Y., Duncan J. S., Mann M., and De Camilli P. (2014) A role of OCRL in clathrin-coated pit dynamics and uncoating revealed by studies of Lowe syndrome cells. eLife 3, e02975 10.7554/eLife.02975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Posor Y., Eichhorn-Gruenig M., Puchkov D., Schöneberg J., Ullrich A., Lampe A., Müller R., Zarbakhsh S., Gulluni F., Hirsch E., Krauss M., Schultz C., Schmoranzer J., Noé F., and Haucke V. (2013) Spatiotemporal control of endocytosis by phosphatidylinositol-3,4-bisphosphate. Nature 499, 233–237 10.1038/nature12360 [DOI] [PubMed] [Google Scholar]
- 16. Zoncu R., Perera R. M., Sebastian R., Nakatsu F., Chen H., Balla T., Ayala G., Toomre D., and De Camilli P. V. (2007) Loss of endocytic clathrin-coated pits upon acute depletion of phosphatidylinositol 4,5-bisphosphate. Proc. Natl. Acad. Sci. U.S.A. 104, 3793–3798 10.1073/pnas.0611733104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gaidarov I., Smith M. E., Domin J., and Keen J. H. (2001) The class II phosphoinositide 3-kinase C2α is activated by clathrin and regulates clathrin-mediated membrane trafficking. Mol. Cell 7, 443–449 10.1016/S1097-2765(01)00191-5 [DOI] [PubMed] [Google Scholar]
- 18. Bendris N., and Schmid S. L. (2017) Endocytosis, metastasis and beyond: multiple facets of SNX9. Trends Cell Biol. 27, 189–200 10.1016/j.tcb.2016.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schöneberg J., Lehmann M., Ullrich A., Posor Y., Lo W. T., Lichtner G., Schmoranzer J., Haucke V., and Noé F. (2017) Lipid-mediated PX-BAR domain recruitment couples local membrane constriction to endocytic vesicle fission. Nat. Commun. 8, 15873 10.1038/ncomms15873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lo W. T., Vujičić Žagar A., Gerth F., Lehmann M., Puchkov D., Krylova O., Freund C., Scapozza L., Vadas O., and Haucke V. (2017) A coincidence detection mechanism controls PX-BAR domain mediated endocytic membrane remodeling via an allosteric structural switch. Dev. Cell 43, 522–529 10.1016/j.devcel.2017.10.019 [DOI] [PubMed] [Google Scholar]
- 21. Daumke O., Roux A., and Haucke V. (2014) BAR domain scaffolds in dynamin-mediated membrane fission. Cell 156, 882–892 10.1016/j.cell.2014.02.017 [DOI] [PubMed] [Google Scholar]
- 22. Boulant S., Kural C., Zeeh J. C., Ubelmann F., and Kirchhausen T. (2011) Actin dynamics counteract membrane tension during clathrin-mediated endocytosis. Nat. Cell Biol. 13, 1124–1131 10.1038/ncb2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fujimoto L. M., Roth R., Heuser J. E., and Schmid S. L. (2000) Actin assembly plays a variable, but not obligatory role in receptor-mediated endocytosis in mammalian cells. Traffic 1, 161–171 10.1034/j.1600-0854.2000.010208.x [DOI] [PubMed] [Google Scholar]
- 24. Daste F., Walrant A., Holst M. R., Gadsby J. R., Mason J., Lee J. E., Brook D., Mettlen M., Larsson E., Lee S. F., Lundmark R., and Gallop J. L. (2017) Control of actin polymerization via the coincidence of phosphoinositides and high membrane curvature. J. Cell Biol. 216, 3745–3765 10.1083/jcb.201704061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shin H. W., Hayashi M., Christoforidis S., Lacas-Gervais S., Hoepfner S., Wenk M. R., Modregger J., Uttenweiler-Joseph S., Wilm M., Nystuen A., Frankel W. N., Solimena M., De Camilli P., and Zerial M. (2005) An enzymatic cascade of Rab5 effectors regulates phosphoinositide turnover in the endocytic pathway. J. Cell Biol. 170, 607–618 10.1083/jcb.200505128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Franco I., Gulluni F., Campa C. C., Costa C., Margaria J. P., Ciraolo E., Martini M., Monteyne D., De Luca E., Germena G., Posor Y., Maffucci T., Marengo S., Haucke V., Falasca M., et al. (2014) PI3K class IIα controls spatially restricted endosomal PtdIns3P and Rab11 activation to promote primary cilium function. Dev. Cell 28, 647–658 10.1016/j.devcel.2014.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Devereaux K., Dall'Armi C., Alcazar-Roman A., Ogasawara Y., Zhou X., Wang F., Yamamoto A., De Camilli P., and Di Paolo G. (2013) Regulation of mammalian autophagy by class II and III PI 3-kinases through PI3P synthesis. PLoS One 8, e76405 10.1371/journal.pone.0076405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rostislavleva K., Soler N., Ohashi Y., Zhang L., Pardon E., Burke J. E., Masson G. R., Johnson C., Steyaert J., Ktistakis N. T., and Williams R. L. (2015) Structure and flexibility of the endosomal Vps34 complex reveals the basis of its function on membranes. Science 350, aac7365 10.1126/science.aac7365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Raiborg C., and Stenmark H. (2009) The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature 458, 445–452 10.1038/nature07961 [DOI] [PubMed] [Google Scholar]
- 30. Schöneberg J., Lee I. H., Iwasa J. H., and Hurley J. H. (2017) Reverse-topology membrane scission by the ESCRT proteins. Nat. Rev. Mol. Cell Biol. 18, 5–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zerial M., and McBride H. (2001) Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2, 107–117 10.1038/35052055 [DOI] [PubMed] [Google Scholar]
- 32. Cao C., Laporte J., Backer J. M., Wandinger-Ness A., and Stein M. P. (2007) Myotubularin lipid phosphatase binds the hVPS15/hVPS34 lipid kinase complex on endosomes. Traffic 8, 1052–1067 10.1111/j.1600-0854.2007.00586.x [DOI] [PubMed] [Google Scholar]
- 33. Cao C., Backer J. M., Laporte J., Bedrick E. J., and Wandinger-Ness A. (2008) Sequential actions of myotubularin lipid phosphatases regulate endosomal PI(3)P and growth factor receptor trafficking. Mol. Biol. Cell 19, 3334–3346 10.1091/mbc.E08-04-0367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jean S., Cox S., Schmidt E. J., Robinson F. L., and Kiger A. (2012) Sbf/MTMR13 coordinates PI(3)P and Rab21 regulation in endocytic control of cellular remodeling. Mol. Biol. Cell 23, 2723–2740 10.1091/mbc.E12-05-0375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pellinen T., Tuomi S., Arjonen A., Wolf M., Edgren H., Meyer H., Grosse R., Kitzing T., Rantala J. K., Kallioniemi O., Fässler R., Kallio M., and Ivaska J. (2008) Integrin trafficking regulated by Rab21 is necessary for cytokinesis. Dev. Cell 15, 371–385 10.1016/j.devcel.2008.08.001 [DOI] [PubMed] [Google Scholar]
- 36. Ketel K., Krauss M., Nicot A. S., Puchkov D., Wieffer M., Müller R., Subramanian D., Schultz C., Laporte J., and Haucke V. (2016) A phosphoinositide conversion mechanism for exit from endosomes. Nature 529, 408–412 10.1038/nature16516 [DOI] [PubMed] [Google Scholar]
- 37. Henmi Y., Morikawa Y., Oe N., Ikeda N., Fujita A., Takei K., Minogue S., and Tanabe K. (2016) PtdIns4KIIα generates endosomal PtdIns(4)P and is required for receptor sorting at early endosomes. Mol. Biol. Cell 27, 990–1001 10.1091/mbc.E15-08-0564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Takahashi S., Kubo K., Waguri S., Yabashi A., Shin H. W., Katoh Y., and Nakayama K. (2012) Rab11 regulates exocytosis of recycling vesicles at the plasma membrane. J. Cell Sci. 125, 4049–4057 10.1242/jcs.102913 [DOI] [PubMed] [Google Scholar]
- 39. Sabha N., Volpatti J. R., Gonorazky H., Reifler A., Davidson A. E., Li X., Eltayeb N. M., Dall'Armi C., Di Paolo G., Brooks S. V., Buj-Bello A., Feldman E. L., and Dowling J. J. (2016) PIK3C2B inhibition improves function and prolongs survival in myotubular myopathy animal models. J. Clin. Invest. 126, 3613–3625 10.1172/JCI86841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nordmann M., Cabrera M., Perz A., Bröcker C., Ostrowicz C., Engelbrecht-Vandré S., and Ungermann C. (2010) The Mon1-Ccz1 complex is the GEF of the late endosomal Rab7 homolog Ypt7. Curr. Biol. 20, 1654–1659 10.1016/j.cub.2010.08.002 [DOI] [PubMed] [Google Scholar]
- 41. Poteryaev D., Datta S., Ackema K., Zerial M., and Spang A. (2010) Identification of the switch in early-to-late endosome transition. Cell 141, 497–508 10.1016/j.cell.2010.03.011 [DOI] [PubMed] [Google Scholar]
- 42. Cabrera M., Nordmann M., Perz A., Schmedt D., Gerondopoulos A., Barr F., Piehler J., Engelbrecht-Vandré S., and Ungermann C. (2014) The Mon1-Ccz1 GEF activates the Rab7 GTPase Ypt7 via a longin-fold-Rab interface and association with PI3P-positive membranes. J. Cell Sci. 127, 1043–1051 10.1242/jcs.140921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Slagsvold T., Aasland R., Hirano S., Bache K. G., Raiborg C., Trambaiolo D., Wakatsuki S., and Stenmark H. (2005) Eap45 in mammalian ESCRT-II binds ubiquitin via a phosphoinositide-interacting GLUE domain. J. Biol. Chem. 280, 19600–19606 10.1074/jbc.M501510200 [DOI] [PubMed] [Google Scholar]
- 44. Hong Z., Pedersen N. M., Wang L., Torgersen M. L., Stenmark H., and Raiborg C. (2017) PtdIns3P controls mTORC1 signaling through lysosomal positioning. J. Cell Biol. 216, 4217–4233 10.1083/jcb.201611073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Raiborg C., Wenzel E. M., Pedersen N. M., Olsvik H., Schink K. O., Schultz S. W., Vietri M., Nisi V., Bucci C., Brech A., Johansen T., and Stenmark H. (2015) Repeated ER-endosome contacts promote endosome translocation and neurite outgrowth. Nature 520, 234–238 10.1038/nature14359 [DOI] [PubMed] [Google Scholar]
- 46. Odorizzi G., Babst M., and Emr S. D. (1998) Fab1p PtdIns(3)P 5-kinase function essential for protein sorting in the multivesicular body. Cell 95, 847–858 10.1016/S0092-8674(00)81707-9 [DOI] [PubMed] [Google Scholar]
- 47. Whitley P., Reaves B. J., Hashimoto M., Riley A. M., Potter B. V., and Holman G. D. (2003) Identification of mammalian Vps24p as an effector of phosphatidylinositol 3,5-bisphosphate-dependent endosome compartmentalization. J. Biol. Chem. 278, 38786–38795 10.1074/jbc.M306864200 [DOI] [PubMed] [Google Scholar]
- 48. Jefferies H. B., Cooke F. T., Jat P., Boucheron C., Koizumi T., Hayakawa M., Kaizawa H., Ohishi T., Workman P., Waterfield M. D., and Parker P. J. (2008) A selective PIKfyve inhibitor blocks PtdIns(3,5)P(2) production and disrupts endomembrane transport and retroviral budding. EMBO Rep. 9, 164–170 10.1038/sj.embor.7401155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dong X. P., Shen D., Wang X., Dawson T., Li X., Zhang Q., Cheng X., Zhang Y., Weisman L. S., Delling M., and Xu H. (2010) PI(3,5)P(2) controls membrane trafficking by direct activation of mucolipin Ca2+ release channels in the endolysosome. Nat. Commun. 1, 38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Li S. C., Diakov T. T., Xu T., Tarsio M., Zhu W., Couoh-Cardel S., Weisman L. S., and Kane P. M. (2014) The signaling lipid PI(3,5)P(2) stabilizes V1-Vo sector interactions and activates the V-ATPase. Mol. Biol. Cell 25, 1251–1262 10.1091/mbc.E13-10-0563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Duex J. E., Tang F., and Weisman L. S. (2006) The Vac14p-Fig4p complex acts independently of Vac7p and couples PI3,5P2 synthesis and turnover. J. Cell Biol. 172, 693–704 10.1083/jcb.200512105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ikonomov O. C., Sbrissa D., Delvecchio K., Xie Y., Jin J. P., Rappolee D., and Shisheva A. (2011) The phosphoinositide kinase PIKfyve is vital in early embryonic development: preimplantation lethality of PIKfyve−/− embryos but normality of PIKfyve+/− mice. J. Biol. Chem. 286, 13404–13413 10.1074/jbc.M111.222364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jin N., Chow C. Y., Liu L., Zolov S. N., Bronson R., Davisson M., Petersen J. L., Zhang Y., Park S., Duex J. E., Goldowitz D., Meisler M. H., and Weisman L. S. (2008) VAC14 nucleates a protein complex essential for the acute interconversion of PI3P and PI(3,5)P(2) in yeast and mouse. EMBO J. 27, 3221–3234 10.1038/emboj.2008.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Er E. E., Mendoza M. C., Mackey A. M., Rameh L. E., and Blenis J. (2013) AKT facilitates EGFR trafficking and degradation by phosphorylating and activating PIKfyve. Sci. Signal. 6, ra45 10.1126/scisignal.2004015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Liu Y., Lai Y. C., Hill E. V., Tyteca D., Carpentier S., Ingvaldsen A., Vertommen D., Lantier L., Foretz M., Dequiedt F., Courtoy P. J., Erneux C., Viollet B., Shepherd P. R., Tavaré J. M., et al. (2013) Phosphatidylinositol 3-phosphate 5-kinase (PIKfyve) is an AMPK target participating in contraction-stimulated glucose uptake in skeletal muscle. Biochem. J. 455, 195–206 10.1042/BJ20130644 [DOI] [PubMed] [Google Scholar]
- 56. Stanley R. E., Ragusa M. J., and Hurley J. H. (2014) The beginning of the end: how scaffolds nucleate autophagosome biogenesis. Trends Cell Biol. 24, 73–81 10.1016/j.tcb.2013.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. O Farrell F., Rusten T. E., and Stenmark H. (2013) Phosphoinositide 3-kinases as accelerators and brakes of autophagy. FEBS J. 280, 6322–6337 10.1111/febs.12486 [DOI] [PubMed] [Google Scholar]
- 58. Wan W., You Z., Xu Y., Zhou L., Guan Z., Peng C., Wong C. C. L., Su H., Zhou T., Xia H., and Liu W. (2017) mTORC1 Phosphorylates acetyltransferase p300 to regulate autophagy and lipogenesis. Mol. Cell 68, 323–335 10.1016/j.molcel.2017.09.020 [DOI] [PubMed] [Google Scholar]
- 59. Russell R. C., Tian Y., Yuan H., Park H. W., Chang Y.-Y., Kim J., Kim H., Neufeld T. P., Dillin A., and Guan K.-L. (2013) ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat. Cell Biol. 15, 741–750 10.1038/ncb2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Marat A. L., and Haucke V. (2016) Phosphatidylinositol 3-phosphates-at the interface between cell signalling and membrane traffic. EMBO J. 35, 561–579 10.15252/embj.201593564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bakula D., Müller A. J., Zuleger T., Takacs Z., Franz-Wachtel M., Thost A. K., Brigger D., Tschan M. P., Frickey T., Robenek H., Macek B., and Proikas-Cezanne T. (2017) WIPI3 and WIPI4 β-propellers are scaffolds for LKB1-AMPK-TSC signalling circuits in the control of autophagy. Nat. Commun. 8, 15637 10.1038/ncomms15637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dooley H. C., Razi M., Polson H. E., Girardin S. E., Wilson M. I., and Tooze S. A. (2014) WIPI2 links LC3 conjugation with PI3P, autophagosome formation, and pathogen clearance by recruiting Atg12–5-16L1. Mol. Cell 55, 238–252 10.1016/j.molcel.2014.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ferguson C. J., Lenk G. M., and Meisler M. H. (2009) Defective autophagy in neurons and astrocytes from mice deficient in PI(3,5)P2. Hum. Mol. Genet. 18, 4868–4878 10.1093/hmg/ddp460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zou J., Zhang C., Marjanovic J., Kisseleva M. V., Majerus P. W., and Wilson M. P. (2012) Myotubularin-related protein (MTMR) 9 determines the enzymatic activity, substrate specificity, and role in autophagy of MTMR8. Proc. Natl. Acad. Sci. U.S.A. 109, 9539–9544 10.1073/pnas.1207021109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Amoasii L., Hnia K., and Laporte J. (2012) Myotubularin phosphoinositide phosphatases in human diseases. Curr. Top. Microbiol. Immunol. 362, 209–233 [DOI] [PubMed] [Google Scholar]
- 66. Hasegawa J., Iwamoto R., Otomo T., Nezu A., Hamasaki M., and Yoshimori T. (2016) Autophagosome-lysosome fusion in neurons requires INPP5E, a protein associated with Joubert syndrome. EMBO J. 35, 1853–1867 10.15252/embj.201593148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hardie D. G. (2014) AMPK–sensing energy while talking to other signaling pathways. Cell Metab. 20, 939–952 10.1016/j.cmet.2014.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhang C. S., Jiang B., Li M., Zhu M., Peng Y., Zhang Y. L., Wu Y. Q., Li T. Y., Liang Y., Lu Z., Lian G., Liu Q., Guo H., Yin Z., Ye Z., et al. (2014) The lysosomal v-ATPase-ragulator complex is a common activator for AMPK and mTORC1, acting as a switch between catabolism and anabolism. Cell Metab. 20, 526–540 10.1016/j.cmet.2014.06.014 [DOI] [PubMed] [Google Scholar]
- 69. Zoncu R., Efeyan A., and Sabatini D. M. (2011) mTOR: from growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 12, 21–35 10.1038/nrm3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ferguson S. M. (2015) Beyond indigestion: emerging roles for lysosome-based signaling in human disease. Curr. Opin. Cell Biol. 35, 59–68 10.1016/j.ceb.2015.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sancak Y., Peterson T. R., Shaul Y. D., Lindquist R. A., Thoreen C. C., Bar-Peled L., and Sabatini D. M. (2008) The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320, 1496–1501 10.1126/science.1157535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zoncu R., Bar-Peled L., Efeyan A., Wang S., Sancak Y., and Sabatini D. M. (2011) mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H+-ATPase. Science 334, 678–683 10.1126/science.1207056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bar-Peled L., Chantranupong L., Cherniack A. D., Chen W. W., Ottina K. A., Grabiner B. C., Spear E. D., Carter S. L., Meyerson M., and Sabatini D. M. (2013) A tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1. Science 340, 1100–1106 10.1126/science.1232044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Inoki K., Li Y., Zhu T., Wu J., and Guan K. L. (2002) TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol. 4, 648–657 10.1038/ncb839 [DOI] [PubMed] [Google Scholar]
- 75. Vander Haar E., Lee S. I., Bandhakavi S., Griffin T. J., and Kim D. H. (2007) Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat. Cell Biol. 9, 316–323 10.1038/ncb1547 [DOI] [PubMed] [Google Scholar]
- 76. Byfield M. P., Murray J. T., and Backer J. M. (2005) hVps34 is a nutrient-regulated lipid kinase required for activation of p70 S6 kinase. J. Biol. Chem. 280, 33076–33082 10.1074/jbc.M507201200 [DOI] [PubMed] [Google Scholar]
- 77. Yoon M. S., Rosenberger C. L., Wu C., Truong N., Sweedler J. V., and Chen J. (2015) Rapid mitogenic regulation of the mTORC1 inhibitor, DEPTOR, by phosphatidic acid. Mol. Cell 58, 549–556 10.1016/j.molcel.2015.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bridges D., Ma J. T., Park S., Inoki K., Weisman L. S., and Saltiel A. R. (2012) Phosphatidylinositol 3,5-bisphosphate plays a role in the activation and subcellular localization of mechanistic target of rapamycin 1. Mol. Biol. Cell 23, 2955–2962 10.1091/mbc.E11-12-1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Jin N., Mao K., Jin Y., Tevzadze G., Kauffman E. J., Park S., Bridges D., Loewith R., Saltiel A. R., Klionsky D. J., and Weisman L. S. (2014) Roles for PI(3,5)P2 in nutrient sensing through TORC1. Mol. Biol. Cell 25, 1171–1185 10.1091/mbc.E14-01-0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Li X., Rydzewski N., Hider A., Zhang X., Yang J., Wang W., Gao Q., Cheng X., and Xu H. (2016) A molecular mechanism to regulate lysosome motility for lysosome positioning and tubulation. Nat. Cell Biol. 18, 404–417 10.1038/ncb3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sardiello M., Palmieri M., di Ronza A., Medina D. L., Valenza M., Gennarino V. A., Di Malta C., Donaudy F., Embrione V., Polishchuk R. S., Banfi S., Parenti G., Cattaneo E., and Ballabio A. (2009) A gene network regulating lysosomal biogenesis and function. Science 325, 473–477 [DOI] [PubMed] [Google Scholar]
- 82. Medina D. L., Di Paola S., Peluso I., Armani A., De Stefani D., Venditti R., Montefusco S., Scotto-Rosato A., Prezioso C., Forrester A., Settembre C., Wang W., Gao Q., Xu H., Sandri M., et al. (2015) Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat. Cell Biol. 17, 288–299 10.1038/ncb3114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Johnson D. E., Ostrowski P., Jaumouillé V., and Grinstein S. (2016) The position of lysosomes within the cell determines their luminal pH. J. Cell Biol. 212, 677–692 10.1083/jcb.201507112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Korolchuk V. I., Saiki S., Lichtenberg M., Siddiqi F. H., Roberts E. A., Imarisio S., Jahreiss L., Sarkar S., Futter M., Menzies F. M., O'Kane C. J., Deretic V., and Rubinsztein D. C. (2011) Lysosomal positioning coordinates cellular nutrient responses. Nat. Cell Biol. 13, 453–460 10.1038/ncb2204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Jordens I., Fernandez-Borja M., Marsman M., Dusseljee S., Janssen L., Calafat J., Janssen H., Wubbolts R., and Neefjes J. (2001) The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein-dynactin motors. Curr. Biol. 11, 1680–1685 10.1016/S0960-9822(01)00531-0 [DOI] [PubMed] [Google Scholar]
- 86. Rosa-Ferreira C., and Munro S. (2011) Arl8 and SKIP act together to link lysosomes to kinesin-1. Dev. Cell 21, 1171–1178 10.1016/j.devcel.2011.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Guardia C. M., Farías G. G., Jia R., Pu J., and Bonifacino J. S. (2016) BORC functions Upstream of kinesins 1 and 3 to coordinate regional movement of lysosomes along different microtubule tracks. Cell Rep. 17, 1950–1961 10.1016/j.celrep.2016.10.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Marwaha R., Arya S. B., Jagga D., Kaur H., Tuli A., and Sharma M. (2017) The Rab7 effector PLEKHM1 binds Arl8b to promote cargo traffic to lysosomes. J. Cell Biol. 216, 1051–1070 10.1083/jcb.201607085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Marat A. L., Wallroth A., Lo W. T., Müller R., Norata G. D., Falasca M., Schultz C., and Haucke V. (2017) mTORC1 activity repression by late endosomal phosphatidylinositol 3,4-bisphosphate. Science 356, 968–972 10.1126/science.aaf8310 [DOI] [PubMed] [Google Scholar]
- 90. Pu J., Keren-Kaplan T., and Bonifacino J. S. (2017) A ragulator-BORC interaction controls lysosome positioning in response to amino acid availability. J. Cell Biol. 216, 4183–4197 10.1083/jcb.201703094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Hao F., Itoh T., Morita E., Shirahama-Noda K., Yoshimori T., and Noda T. (2016) The PtdIns3-phosphatase MTMR3 interacts with mTORC1 and suppresses its activity. FEBS Lett. 590, 161–173 10.1002/1873-3468.12048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sridhar S., Patel B., Aphkhazava D., Macian F., Santambrogio L., Shields D., and Cuervo A. M. (2013) The lipid kinase PI4KIIIβ preserves lysosomal identity. EMBO J. 32, 324–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. De Leo M. G., Staiano L., Vicinanza M., Luciani A., Carissimo A., Mutarelli M., Di Campli A., Polishchuk E., Di Tullio G., Morra V., Levtchenko E., Oltrabella F., Starborg T., Santoro M., Di Bernardo D., et al. (2016) Autophagosome-lysosome fusion triggers a lysosomal response mediated by TLR9 and controlled by OCRL. Nat. Cell Biol. 18, 839–850 10.1038/ncb3386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Raiborg C., Wenzel E. M., Pedersen N. M., and Stenmark H. (2016) Phosphoinositides in membrane contact sites. Biochem. Soc. Trans. 44, 425–430 10.1042/BST20150190 [DOI] [PubMed] [Google Scholar]
- 95. Phillips M. J., and Voeltz G. K. (2016) Structure and function of ER membrane contact sites with other organelles. Nat. Rev. Mol. Cell Biol. 17, 69–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Höglinger D., Nadler A., and Schultz C. (2014) Caged lipids as tools for investigating cellular signaling. Biochim. Biophys. Acta 1841, 1085–1096 10.1016/j.bbalip.2014.03.012 [DOI] [PubMed] [Google Scholar]
- 97. Idevall-Hagren O., and De Camilli P. (2015) Detection and manipulation of phosphoinositides. Biochim. Biophys. Acta 1851, 736–745 10.1016/j.bbalip.2014.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Staiano L., De Leo M. G., Persico M., and De Matteis M. A. (2015) Mendelian disorders of PI metabolizing enzymes. Biochim. Biophys. Acta 1851, 867–881 10.1016/j.bbalip.2014.12.001 [DOI] [PubMed] [Google Scholar]