Abstract
It has been suggested that voltage-dependent anion channels (VDACs) control the release of superoxide from mitochondria. We have previously shown that reactive oxygen species (ROS) such as superoxide (O2˙̄) and hydrogen peroxide (H2O2) stimulate epithelial sodium channels (ENaCs) in sodium-transporting epithelial tissue, including cortical collecting duct (CCD) principal cells. Therefore, we hypothesized that VDACs could regulate ENaC by modulating cytosolic ROS levels. Herein, we find that VDAC3-knockout(KO) mice can maintain normal salt and water balance on low-salt and high-salt diets. However, on a high-salt diet for 2 weeks, VDAC3-KO mice had significantly higher systolic blood pressure than wildtype mice. Consistent with this observation, after a high-salt diet for 2 weeks, ENaC activity in VDAC3-KO mice was significantly higher than wildtype mice. EM analysis disclosed a significant morphological change of mitochondria in the CCD cells of VDAC3-KO mice compared with wildtype mice, which may have been caused by mitochondrial superoxide overload. Of note, compared with wildtype animals, ROS levels in VDAC3-KO animals fed a normal or high-salt diet were consistently and significantly increased in renal tubules. Both the ROS scavenger 1-oxyl-2,2,6,6-tetramethyl-4-hydroxypiperidine (TEMPOL) and the mitochondrial ROS scavenger (2-(2,2,6,6-tetramethylpiperidin-1-oxyl-4-ylamino)-2-oxoethyl)triphenylphosphonium chloride (mito-TEMPO) could reverse the effect of high-salt on ENaC activity and systolic blood pressure in the VDAC3-KO mice. Mito-TEMPO partially correct the morphological changes in VDAC3-KO mice. Our results suggest that knocking out mitochondrial VDAC3 increases ROS, alters renal sodium transport, and leads to hypertension.
Keywords: epithelial sodium channel (ENaC), hypertension, mitochondria, reactive oxygen species (ROS), voltage-dependent anion channel (VDAC), VDAC3-knockout mice, dietary salt
Introduction
Epithelial sodium channels (ENaCs)4 are expressed in the apical membrane of polarized epithelial cells mainly in the distal nephron, lung, and distal colon. In the distal nephron, ENaC activity is the rate-limiting step for Na+ reabsorption. Working together with the basolateral Na+/K+-ATPase, this channel regulates salt resorption and plays a major role in the control of total body salt and water balance and blood pressure (1, 2). There are several physiological causes of hypertension, but one significant cause is constitutively elevated reabsorption of sodium through ENaC by principal cells in the distal nephron. ENaC gain-of-function mutations cause retention of active ENaC channels at the cell surface and lead to an autosomal dominant form of hypertension, Liddle's syndrome (3, 4). Essential hypertension is an important public health challenge, and because ENaC can be its cause, a thorough understanding of the regulation of ENaC may lead to the prevention and treatment of this disease.
Voltage-dependent anion channels (VDACs) are located at the outer membrane of mitochondria where they serve as a major pathway for Ca2+ uptake, but also as a pathway across the mitochondrial outer membrane for the movement of a wide variety of molecules including NAD+, ATP, and superoxide (5–7). There are three isoforms in mammals, VDAC1–3, which are almost ubiquitously expressed and thought to all transport Ca2+ in their natural state (7). Knockout of VDAC2 is embryonic lethal and double knockout VDAC1/3 has a severe phenotype (8), thus most studies have focused on knockout only of VDAC1 or VDAC3.
VDAC3 is the least well known isoform of mammalian VDACs in the outer mitochondrial membrane. However, VDAC3 can sense the oxidative state of the intermembrane space of mitochondria through specific cysteine residue modifications, and can buffer the ROS load in the intermembrane space of mitochondria (9). We have shown that reactive oxygen species, like superoxide (O2˙̄) and hydrogen peroxide (H2O2) stimulate ENaCs in sodium-transporting epithelial tissue including cortical collecting duct (CCD) principal cells (10). ROS is also related to ENaC function and hypertension (11). In this paper, we focused on whether knockout of mitochondrial VDAC3 alters renal sodium transport and whether ROS is involved in this regulation.
Results
VDAC3-KO mice can maintain salt and water balance
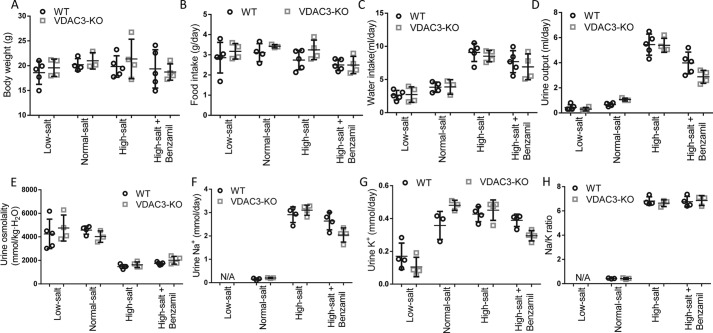
We measured the metabolic responses of wildtype and VDAC3-KO animals to low-salt and high-salt diet challenges. We measured food intake, water intake, body weight, urine output, urine osmolality, urine sodium and potassium, and urine sodium/potassium ratio initially and after 2 weeks on the diets (Fig. 1 and Table 1). The results were unremarkable. There was no significant change in body weight for any animal and no significant difference in food intake. As might be expected, animals fed a high-salt diet drank more and had higher amounts of sodium in their urine. They drank enough to increase their urine volumes and to decrease their urine osmolality. There were, however, no significant differences in any of the values between wildtype and knockout animals: both responded the same to the different diets. We also measured hematocrit and urine protein and found no significant differences under any condition in urine protein or hematocrit (data not shown).
Figure 1.
Comparison of metabolic parameters of wildtype and VDAC3-KO mice. Both wildtype (WT) and VDAC3-KO mice were divided into 4 groups, low-salt diet, normal-salt diet, high-salt diet, and high-salt diet plus benzamil (ENaC channel blocker) for 2 weeks. Metabolic parameters were measured initially and after 2 weeks on the diets (values are mean ± S.D., specific values are given in Table 1). After 2 weeks on the diets, metabolic parameters from wildtype and VDAC3-KO animals were shown as above. A, body weight. B, food intake. C, water intake. D, urine output. E, urine osmolality. F, urine Na+. G, urine K+. H, Na/K ratio.
Table 1.
Metabolic parameters of wildtype and VDAC3-KO mice
Week 0 are baseline measurements before special diet; Week 2 is after 2 weeks on normal-salt, low-salt, high-salt diet, or high-salt diet plus benzamil (see “Experimental procedures”). Values are mean ± S.D. Values in parentheses are number of mice.
| Parameter | Week | Wildtype |
VDAC3-KO |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Normal diet | Low-salt | High-salt | High-salt plus benzamil | Normal diet | Low-salt | High-salt | High-salt plus benzamil | ||
| Food intake | 0 | 2.18 ± 0.450 (4) | 2.23 ± 0.450 (5) | 2.82 ± 1.220 (5) | 2.56 ± 0.593 (5) | 2.80 ± 1.031 (3) | 2.38 ± 0.453 (4) | 2.68 ± 0.993 (4) | 2.52 ± 0.697 (4) |
| (g/day) | 2 | 3.11 ± 0.444 (4) | 2.86 ± 0.760 (5) | 2.74 ± 0.481 (5) | 2.50 ± 0.279 (5) | 3.43 ± 0.083 (3) | 3.18 ± 0.385 (4) | 3.25 ± 0.487 (4) | 2.50 ± 0.429 (4) |
| Water intake | 0 | 3.24 ± 0.250 (4) | 3.46 ± 0.788 (5) | 3.92 ± 1.588 (5) | 3.48 ± 0.648 (5) | 3.86 ± 0.605 (3) | 3.33 ± 0.393 (4) | 3.67 ± 1.198 (4) | 4.64 ± 1.877 (4) |
| (ml/day) | 2 | 3.84 ± 0.783 (4) | 2.64 ± 0.714 (5) | 9.09 ± 1.373 (5) | 7.69 ± 1.660 (5) | 3.88 ± 1.093 (3) | 2.73 ± 1.172 (4) | 8.52 ± 0.857 (4) | 6.91 ± 0.971 (4) |
| Body weight | 0 | 19.2 ± 0.206 (4) | 18.5 ± 3.243 (5) | 20.5 ± 2.098 (5) | 19.8 ± 3.935 (5) | 20.7 ± 2.803 (3) | 19.3 ± 1.865 (4) | 21.3 ± 3.629 (4) | 18.8 ± 2.051 (4) |
| (g) | 2 | 20.2 ± 1.251 (4) | 18.6 ± 2.348 (5) | 19.8 ± 2.166 (5) | 19.4 ± 3.871 (5) | 21.0 ± 1.665 (3) | 19.6 ± 1.746 (4) | 21.4 ± 3.981 (4) | 18.7 ± 1.652 (4) |
| Urine output | 0 | 0.59 ± 0.278 (4) | 1.01 ± 0.358 (5) | 0.85 ± 0.48 (4) | 0.76 ± 0.411 (5) | 1.10 ± 0.377 (3) | 0.58 ± 0.222 (4) | 1.11 ± 0.539 (4) | 0.90 ± 0.337 (4) |
| (ml/day) | 2 | 0.66 ± 0.138 (4) | 0.44 ± 0.219 (5) | 5.44 ± 0.841 (5) | 3.98 ± 0.87 (5) | 1.07 ± 0.115 (3) | 0.30 ± 0.135 (4) | 5.39 ± 0.557 (4) | 2.88 ± 0.512 (4) |
| Urine osmolality | 0 | 4153 ± 741.9 (4) | 3804 ± 361.5 (5) | 4746 ± 1015 (5) | 4370 ± 548.9 (5) | 4053 ± 507.2 (3) | 5425 ± 984.3 (4) | 4250 ± 394 (4) | 5010 ± 730.8 (4) |
| (mmol/kg·H2O) | 2 | 4575 ± 346.7 (4) | 4250 ± 1255 (5) | 1471 ± 169.9 (5) | 1739 ± 123.7 (5) | 4023 ± 486.4 (3) | 4748 ± 1107 (4) | 1628 ± 239.5 (4) | 2014 ± 330.3 (4) |
| Urine Na+ | 0 | 0.08 ± 0.023 (3) | 0.16 ± 0.08 (4) | 0.17 ± 0.142 (3) | 0.16 ± 0.114 (4) | 0.18 ± 0.097 (3) | 0.12 ± 0.063 (4) | 0.21 ± 0.106 (4) | 0.18 ± 0.079 (4) |
| (mmol/day) | 2 | 0.16 ± 0.047 (3) | 2.91 ± 0.336 (4) | 2.64 ± 0.376 (4) | 0.20 ± 0.019 (3) | 3.10 ± 0.216 (4) | 2.04 ± 0.303 (4) | ||
| Urine K+ | 0 | 0.18 ± 0.020 (3) | 0.39 ± 0.123 (4) | 0.36 ± 0.271 (3) | 0.36 ± 0.210 (4) | 0.43 ± 0.148 (3) | 0.23 ± 0.086 (4) | 0.50 ± 0.218 (4) | 0.43 ± 0.155 (4) |
| (mmol/day) | 2 | 0.36 ± 0.085 (3) | 0.17 ± 0.080 (4) | 0.43 ± 0.046 (4) | 0.39 ± 0.038 (4) | 0.48 ± 0.031 (3) | 0.11 ± 0.059 (4) | 0.45 ± 0.062 (4) | 0.30 ± 0.032 (4) |
| Na/K ratio | 0 | 0.46 ± 0.123 (3) | 0.41 ± 0.089 (4) | 0.43 ± 0.063 (3) | 0.44 ± 0.079 (4) | 0.41 ± 0.086 (3) | 0.51 ± 0.118 (4) | 0.42 ± 0.057 (4) | 0.40 ± 0.068 (4) |
| 2 | 0.43 ± 0.034 (3) | 6.82 ± 0.347 (4) | 6.77 ± 0.433 (4) | 0.42 ± 0.064 (3) | — | 6.64 ± 0.280 (3) | 6.86 ± 0.405 (3) | ||
VDAC3-KO mice have an abnormal blood pressure response to changes in dietary salt
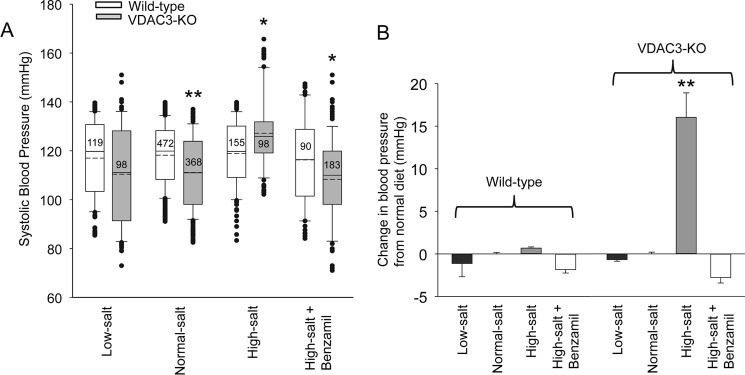
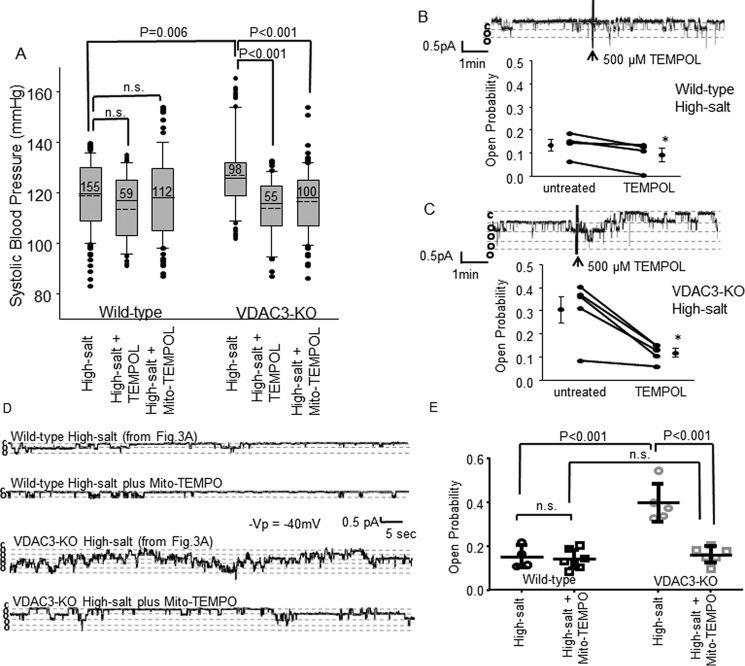
We measured the blood pressure of wildtype and knockout animals fed low-salt, normal-salt, high-salt, or high-salt plus benzamil diet (see methods). After 2 weeks, the blood pressure of wildtype animals was not significantly different regardless of diet or treatment (low-salt: 117 ± 15.3, n = 119; normal-salt: 118 ± 12.6, n = 472; high-salt: 119 ± 13.2, n = 155; high-salt + benzamil: 116 ± 18.1, n = 90), but the blood pressure of VDAC3-KO animals fed the normal diet or high-salt plus benzamil diet was significantly less than wildtype animals on the same diet (normal-salt: 111 ± 14.8, n = 368, p < 0.001; high-salt + benzamil: 108 ± 18.2, n = 183, p < 0.05), and there was no significant difference in blood pressure between wildtype and VDAC3-KO mice on a low-salt diet (VDAC3-KO low-salt, 110 ± 20.2, n = 98). In contrast, knockout animals on a high-salt diet had elevated blood pressure compared with wildtype (high-salt, 127 ± 14.8, n = 98, p < 0.05) (Fig. 2A). The difference between wildtype and knockout blood pressure can best be appreciated by examining the change in blood pressure compared with normal diet (Fig. 2B). Exposure to a high-salt diet does not significantly change the blood pressure of wildtype animals by 0.71 ± 0.11 mm Hg (mean ± S.E., n = 155) above normal diet. In contrast, a high-salt diet increased the blood pressure of VDAC3-KO animals significantly by 16.1 ± 2.84 mm Hg (mean ± S.E., n = 98; p < 0.001). Treatment with benzamil completely reverses the salt-induced increase in blood pressure. Because benzamil is a highly specific inhibitor of ENaCs that is not known to inhibit any other transporters, we conclude that the increase in blood pressure is mediated by increased ENaC activity. These results suggest that knocking out VDAC3 causes sodium imbalance and hypertension by increasing ENaC activity.
Figure 2.
VDAC3-KO mice have an abnormal blood pressure response to changes in salt in their diet. We measured the blood pressure of wildtype and knockout animals fed low-salt, normal-salt, high-salt, or high-salt plus benzamil diets. A, systolic blood pressure of wildtype and VDAC3-KO mice on diets after 2 weeks. The boundary of the box plots closest to zero indicates the 25th percentile, a solid line within the box marks the median and the dashed line is the mean, and the boundary of the box farthest from zero indicates the 75th percentile. Whiskers (error bars) above and below the box indicate the 90th and 10th percentiles. Outlying points are represented by filled circles. Numbers within boxes are total number of measurements for each condition. B, the difference between wildtype and knock-out blood pressure can best be appreciated by examining the change in blood pressure compared with normal diet. **, p < 0.001; *, p < 0.05, Kruskal-Wallis ANOVA on ranks with Dunn's post test.
VDAC3-KO mice fail to regulate ENaC channel activity in response to dietary salt challenge
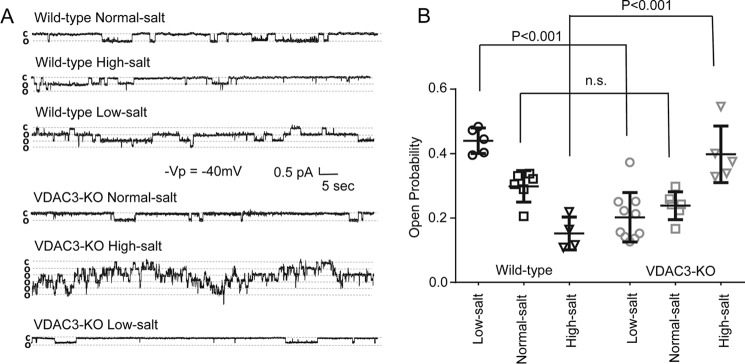
Ordinarily, as blood pressure increases, ENaC activity decreases, presumably to excrete more sodium and correct blood pressure. This decrease occurs in the face of increased tubular sodium, which would normally be expected to increase sodium reabsorption. Wild-type and VDAC3-KO mice were fed with low-salt, normal-salt, or high-salt diet. After 2 weeks, mice were sacrificed by cervical dislocation and renal tubules were manually dissected. ENaC activity was recorded from cell-attached patches on principal cells (Fig. 3). As expected, for wildtype animals, ENaC activity decreases as salt increases in the diet (low-salt, 0.440 ± 0.0397, n = 5; normal-salt, 0.298 ± 0.0489, n = 6; high-salt, 0.152 ± 0.0510, n = 4), but for VDAC3-KO animals, ENaC activity was not well regulated in response to dietary salt change (low-salt, 0.202 ± 0.0767, n = 10; normal-salt, 0.238 ± 0.0432, n = 6; high-salt, 0.398 ± 0.0879, n = 5). In particular, VDAC3-KO mice on a high-salt diet had increased ENaC activity compared with wildtype (VDAC3-KO high-salt, 0.398 ± 0.0879, n = 5; wildtype high-salt, 0.152 ± 0.0510, n = 4, p < 0.001), VDAC3-KO mice on a low-salt diet had decreased ENaC activity compared with wildtype (VDAC3-KO low-salt, 0.202 ± 0.0767, n = 10; wildtype low-salt, 0.440 ± 0.0397, n = 5, p < 0.001), but ENaC activity of VDAC3-KO and wildtype mice on normal-salt diet was not significantly different. A difference in number of channels was not observed in our study. These results indicate that knockout mice have lost the ability to normally regulate sodium reabsorption, which would help to explain the elevated blood pressure of knockout mice on a high-salt diet.
Figure 3.
VDAC3-KO mice fail to regulate ENaC channel activity in response to dietary salt challenge. Wildtype and VDAC3-KO mice were fed with low-salt, normal-salt, or high-salt diets. After 2 weeks, mice were sacrificed by cervical dislocation and renal tubules were manually dissected. ENaC activity was recorded from cell-attached patches on principal cells as previously described (3, 12). A, representative traces from wildtype and VDAC3-KO mice on different diets. B, summary of the open probability from wildtype and VDAC3-KO mice on different diets, presented as scatter plot with standard deviation. p values were marked as above, one-way ANOVA with Holm-Sidak post test.
VDAC3-KO mitochondria differ in morphology from wildtype mitochondria
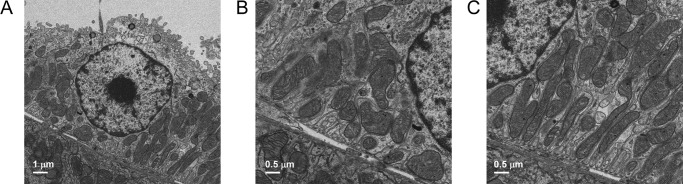
VDAC3-KO is a defect in a mitochondrial anion/calcium channel that manifests as alteration in cellular function. We wondered whether this defect might also produce detectable changes in mitochondrial morphology. Principal cells from wildtype and VDAC3-KO on a normal-salt diet were imaged by transmission electron microscopy. Fig. 4 shows that there are obvious differences between knockout and wildtype mitochondria. Mitochondria in knockout cells are less well organized that the densely packed, parallel structure in wildtype cells (Fig. 4, A and B). In addition, mitochondria in wildtype cells are longer and wider than mitochondria from VDAC3-KO mice. The mean length and width of wildtype mitochondria are 1.36 ± 0.797 and 0.532 ± 0.164 μm (n = 97). The mean length and width of knockout mitochondria are 0.760 ± 0.335 and 0.458 ± 0.186 μm (n = 89). Both the length and width of the wildtype mitochondria are significantly larger than the knockout mitochondria (p < 0.001). The calculated volume of the knockout mitochondria (0.680 ± 1.12, n = 89) is also significantly smaller than wildtype mitochondria (1.30 ± 1.02, n = 97) (p < 0.001; determined by the Mann-Whitney rank sum test). Mitochondria in knockout animals also have electron translucent areas that are not seen in wildtype mitochondria (Fig. 4, C and D, see expanded image of the area outlined by the white box). 26% of 89 knockout mitochondria had void spaces, whereas no voids were observed in 97 wildtype mitochondria (significantly different by z-test, p < 0.001).
Figure 4.

VDAC3-KO mitochondria differ in morphology from wildtype mitochondria. Principal cells from wildtype and VDAC3-KO on normal-salt diets were imaged by transmission electron microscopy (note primary cilium on apical surface of cells). A, mitochondria in wildtype cells at 2 μm. B, mitochondria in wildtype cells at 0.5 μm. C, mitochondria in VDAC3-KO cells at 1 μm. D, mitochondria in VDAC3-KO cells at 0.2 μm (expended image of the area outlined by white box in Fig. 4C).
Increases in ROS are associated with sodium imbalance in VDAC3-KO animals
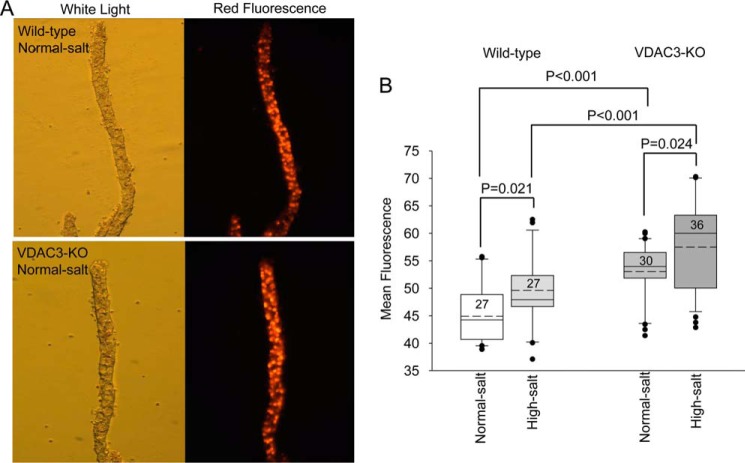
As mentioned above, VDAC3-KO is expected to compromise mitochondrial function. Knockout could do this in several ways. VDAC3 as an ion channel helps determine the mitochondrial outer membrane potential so knockout would compromise this potential and compromise ATP production; VDAC3 also acts to buffer the ROS load in the mitochondrial intramembrane space by acting as a pathway for the movement of superoxide out of mitochondria the production of which is increased when ATP production is compromised. Especially because of this role in regulating superoxide, we hypothesized that VDAC3-KO mitochondria might have elevated ROS and that the void spaces we observed in mitochondria of VDAC3-KO animals could be due to ROS overload. Therefore, we fed wildtype and VDAC3-KO mice with normal-salt and high-salt diets. Renal tubules were dissected manually and CCD were identified by morphology (white light picture, Fig. 5A). We used dihydroethidium (DHE), which by virtue of its ability to freely permeate cell membranes can report superoxide production. DHE upon reaction with superoxide anions forms a red fluorescent product (ethidium) that intercalates with DNA. A high-salt diet by itself increases ROS fluorescence in both wildtype and VDAC3-KO animals (wildtype normal-salt, 44.9 ± 5.32, n = 27; wildtype high-salt, 49.6 ± 6.61, n = 27, p < 0.05; VDAC3-KO normal-salt, 53.1 ± 5.24, n = 30; VDAC3-KO high-salt, 57.5 ± 8.38, n = 36, p < 0.05). ROS in VDAC3-KO animals is significantly increased compared with wildtype regardless of diet (p < 0.001) (Fig. 5B). These results suggest that a high-salt diet can increase the in vivo ROS level and VDAC3-KO animals do have higher ROS levels than wildtype animals.
Figure 5.
ROS levels are elevated in VDAC3-KO CCD. Wildtype and VDAC3-KO mice were fed normal and high-salt diets for 2 weeks. A, representative tubules from wildtype and VDAC3-KO mice on a normal diet in white light and red fluorescence forms. B, mean fluorescence in wildtype and VDAC3-KO animals on normal and high-salt diets. Box plot with whiskers as explained in Fig. 2A. p values were marked as above, one-way ANOVA with Holm-Sidak post test.
ROS scavengers can reverse the effect of a high-salt diet on blood pressure in VDAC3-KO animals
If the elevated ROS is causing elevated blood pressure in knockout mice then correcting the ROS levels should correct the increase in blood pressure. To investigate this, we fed wildtype and knockout animals a high-salt diet, high-salt diet plus the ROS scavenger, TEMPOL (1 mmol/liter) in their drinking water and high-salt diet plus intraperitoneal injection of the mitochondrial ROS scavenger mito-TEMPO (0.7 mg/kg/day) for 2 weeks and, as expected the blood pressure of the knockout animals was significantly higher than wildtype animals on the same diet (specific blood pressure values on a high-salt diet as shown before, p = 0.006). The blood pressure of VDAC3-KO animals fed high-salt plus TEMPOL in their drinking water and high-salt plus mito-TEMPO by intraperitoneal injection was significantly lower than those fed with high-salt diet only (high-salt plus TEMPOL, 114 ± 11.6, n = 55; high-salt plus mito-TEMPO, 117 ± 12.8, n = 100, p < 0.001), but the blood pressure was not significantly different in wildtype groups (high-salt plus TEMPOL, 114 ± 13.4, n = 59; high-salt plus mito-TEMPO, 118 ± 16.4, n = 112); ROS scavengers had the largest effect on the VDAC3-KO animals to the extent that the blood pressure of TEMPOL- or mito-TEMPO-treated wildtype and knockout animals was not significantly different (Fig. 6A).
Figure 6.
ROS scavengers reverse sodium imbalance in VDAC3-KO animals. A, systolic blood pressure form wildtype and VDAC3-KO mice were fed with high-salt diet (to show a more direct compare, take high-salt blood pressure from Fig. 2A), high-salt diet plus 1 mmol/liter of TEMPOL in drinking water, and a high-salt diet plus intraperitoneal injection of 0.7 mg/kg/day of mito-TEMPO for 2 weeks. p values were generated by Kruskal-Wallis ANOVA on ranks with a Dunn's post test. B and C, TEMPOL effects on ENaC activity of wildtype and VDAC3-KO mice on a high-salt diet for 2 weeks. Representative traces and open probability summary are shown, *, p < 0.05, paired t test. D and E, representative traces and ENaC channel open probability summary from wildtype and VDAC3-KO mice on a high-salt diet plus mito-TEMPO injection, p values were generated by one-way ANOVA with Holm-Sidak post test (to see a more direct comparison, examine high-salt representative traces and data from Fig. 3, A and B).
ROS scavengers reverse the effect of high-salt diet on ENaC activity in VDAC3-KO animals
The high-salt-induced increase in blood pressure in knockout mice is associated with an increase in ENaC open probability (Fig. 3), but ROS scavengers reversed the effect of high-salt on blood pressure. Could it also reduce the high-salt-induced increase in ENaC activity? Wild-type and VDAC3-KO mice were fed with a high-salt diet or high-salt diet plus intraperitoneal injection of mito-TEMPO (0.7 mg/kg/day) for 2 weeks. After 2 weeks, mice were sacrificed and kidneys were removed and dissected (see “Experimental procedures”). ENaC activity was recorded from cell-attached patches on principal cells.
For TEMPOL experiments, first, baseline was recorded, then 500 μmmol/liter of TEMPOL was added directly into the bath solution in contrast to the whole animals when TEMPOL was given in the drinking water. Once tubules are removed from the animals and are no longer in contact with the systemic circulation, bath application is more direct. For the wildtype animals, TEMPOL reduces ENaC activity (untreated, 0.135 ± 0.0506; after TEMPOL, 0.0930 ± 0.0594, p = 0.033, n = 4, paired t test); and also reduces ENaC activity in the VDAC3-KO animals (before, 0.304 ± 0.128; after, 0.118 ± 0.0392, p = 0.012, n = 5, paired t test) to a level not significantly different from wildtype activity after TEMPOL (p = 0.556, Mann-Whitney rank sum test) (Fig. 6, B and C).
For mito-TEMPO experiments, ENaC activity was recorded directly on injected animals. Mito-TEMPO is a mitochondrial ROS scavenger, it has to cross the cell membrane, mitochondrial outer and inner membranes, we are not sure about how long it takes to get into the mitochondria, so chronic intraperitoneal treatment is better for this specific experiment. On a high-salt diet for 2 weeks, ENaC activity was significantly higher in VDAC3-KO animals than wildtype animals (specific values as shown before, p < 0.001). On a high-salt diet plus mito-TEMPO for 2 weeks, ENaC activity was significantly lower in VDAC3-KO animals than on a high-salt diet only (0.161 ± 0.0374, n = 6, p < 0.001); but in wildtype animals, ENaC activity was not different between the high-salt diet and high-salt diet plus mito-TEMPO (0.140 ± 0.0431, n = 7). With high-salt plus mito-TEMPO, ENaC activity was not different between wildtype and VDAC3-KO animals (Fig. 6, D and E).
A mitochondrial ROS scavenger can partially correct the morphology changes in VDAC3-KO animals
There were obvious morphological differences between VDAC3-KO and wildtype mitochondria (as shown in Fig. 4) and we hypothesized that the changes were due to mitochondrial superoxide overload. We speculated that we could correct the morphology if we corrected the mitochondrial superoxide. We examined by EM, mitochondria of CCD cells from VDAC3-KO mice on a high-salt diet plus intraperitoneal injection of mito-TEMPO (0.7 mg/kg/day) for 2 weeks, the length, width, and volume of mitochondria were not significantly different from those in wildtype cells (length, 1.13 ± 0.580; width, 0.502 ± 0.167; volume, 1.28 ± 1.07, n = 98). Both the length and volume of the mito-TEMPO-treated mitochondria are significantly larger than the knock-out mitochondria (p < 0.001, Kruskal-Willis one-way analysis on ranks with Dunn's post test). Only 1 out of 98 had a void in the mitochondria, and the mitochondria are more organized than those from VDAC3-KO cells, but still not as organized as those from wildtype cells (Fig. 7, A–C).
Figure 7.
Mito-TEMPO partially correct the morphological changes in mitochondria from VDAC3-KO cells. VDAC3-KO mice were treated with a high-salt diet plus mito-TEMPO injection for 2 weeks. After 2 weeks, mice were sacrificed and CCD cells were imaged by EM. A, mitochondria from CCD cell at 1 μm. B and C, mitochondria from CCD cell at 0.5 μm.
Discussion
Function of mitochondria and VDACs
Mitochondria produce cellular energy in the form of ATP. The mechanism for the production was first described in the early 1960s by Mitchell (12, 13). However, mitochondria are involved in several other important cellular functions. These functions include the exchange of proteins, lipids, or ions between the internal mitochondrial matrix and the cytosol, the regulated initiation of apoptotic cascades, control of signaling pathways that regulate respiration and metabolic functions, the regulation of the levels of reactive oxygen species produced by the respiratory chain, and the control of mitochondrial morphogenesis. Mitochondria contain two aqueous compartments: the matrix and the intermembrane space (14). Many of the functions of mitochondria depend upon the selective permeability of the outer membrane and the composition of the intermembrane space. The permeability of the membrane and the ionic composition depend upon several transporters including the so-called VDACs, voltage-dependent anion channels. There are 3 isoforms of VDAC, 1–3. The three VDAC isoforms are highly conserved, particularly with respect to their 3D structure. All VDAC isoforms are ubiquitously expressed and VDACs are involved in cell metabolism by transporting ATP, Ca2+, and superoxide across the outer mitochondrial membrane. VDACs form a wide β-barrel structure, inside of which the N-terminal is located and controls the permeability of the pore. The sequence of the VDAC3 isoform contains an abundance of cysteines, which are sensitive to reactive oxygen species and affect the flexibility and selectivity of the β-barrel (15).
VDAC3 phenotype
We would expect that VDAC3-KO mice would have a phenotype associated with defects in mitochondrial function and in the ability of mitochondria to regulate ROS in the mitochondria and cytosol. Surprisingly to us, when we examined several different parameters of renal function that could depend upon functional mitochondria, we could find no significant difference between wildtype and VDAC3-KO animals in any of the metabolic parameters we measured (Fig. 1). There were, however, other measures of mitochondrial deficits. Blood pressure in VDAC3-KO mice was abnormal. When animals were stressed by placing them on a high-salt diet, blood pressure was different from wildtype animals (Fig. 2A). Specifically, normal diet KO animals had blood pressure lower than their wildtype counterparts. The difference in blood pressure between normal diet and high-salt diet was also profound: for wildtype animals, there was little change in blood pressure, but knockout animals had a significant increase in blood pressure. The likely source of these differences in blood pressure was suggested by the fact that the ENaC-selective inhibitor, benzamil, normalized the blood pressure of the KO animals. This involvement of ENaC is supported by our observation of single ENaC channels in principal cells after animals were treated with different diets. In wildtype animals, open probability of ENaC decreases as salt in the diet increases (Fig. 3). This is an expected response of the channels to protect against increased ENaC activity that could lead to increased blood pressure. Studies have shown that this decrease is due to an increase in intracellular Ca2+ that reduces ENaC activity (16–18). In contrast, for VDAC3-KO animals, ENaC activity was not appropriately controlled: ENaC activity was very high in principal cells from animals on a high-salt diet. The response to high-salt is consistent with significantly elevated blood pressure in knockout animals on high-salt diet.
The role of ROS
The reduced blood pressure in animals on normal diet is likely due to mildly elevated intracellular Ca2+ because Ca2+ is not taken up by VDAC3-KO mitochondria. This hypothesis is consistent with the normal diet animals and the function of VDAC3 to sequester Ca2+ in mitochondria. This model is not consistent with the elevated blood pressure in high-salt diet animals. Elevated blood pressure and high ENaC single channel activity would require that apical Ca2+ was substantially reduced. This is inconsistent with VDAC3 loss-of-function. An explanation of the high blood pressure is suggested by an examination of the electron micrographs of knockout principal cells (Fig. 4). The void spaces are similar to those in mitochondria that have been exposed to excess ROS produced by the mitochondria (19–21). It is also known that high-salt diets can lead to excess mitochondrial production of superoxide and other ROS (22–24). We speculated that in animals on a high-salt diet ROS was strongly elevated. In fact, when we used dihydroethidium to measure ROS, we found that ROS in knockout animals on the normal diet was higher than wildtype animals. Feeding animals a high-salt diet modestly increased ROS in wildtype animals, but dramatically increased ROS in knock-out animals (Fig. 5). In other words, the levels of ROS correlated with blood pressure. We examined whether we could correct the blood pressure with an ROS scavenger, TEMPOL. In Fig. 6A, TEMPOL decreased blood pressure in wildtype and knockout animals on a high-salt diet but only the decrease was significant in knockout animals. Addition of TEMPOL to drinking water corrected the blood pressure and made wildtype and knockout animals appear the same. Examination of the open probability of single ENaC channels shows that TEMPOL reduces activity in animals on both types of diet, but the decrease is most dramatic in knockout animals (Fig. 6, B and C). We have previously shown that ENaC activity is dependent on levels of ROS, particularly superoxide (25–28). We felt that our observed results were due to excess mitochondrial ROS production, but TEMPOL reduces ROS throughout the cell. Therefore, we also used a TEMPOL derivative that specifically targets mitochondria, mito-TEMPO. This agent must be administered by i.p. injection. Nonetheless, like TEMPOL, it reverses the effect of a high-salt diet in VDAC3-KO animals on both blood pressure and ENaC activity (Fig. 6). It also reverses almost all of the morphological changes seen in the KO animals (Fig. 7) reversing the reduction in size and producing mitochondrial organization similar to wildtype.
Extra-renal effects of VDAC3
VDAC3-KO is a global knockout and it has been reported to produce effects in other tissues especially those with very high energy requirements like skeletal and cardiac muscle (29), sperm (30), and CNS cells (31). There is also a small effect of VDAC3-KO on mouse growth (32). On the other hand, the phenotype we observe involves abnormal activity of ENaC so that an extra-renal effect would somehow have to activate ENaC. There is only one major systemic agent that increases ENaC, the steroid hormone aldosterone. It is produced by the adrenal cortex in response to elevated angiotensin II or to elevated potassium. We measured urinary potassium and found no difference between KO and wildtype animals implying that systemic potassium was not increased and would not stimulate aldosterone.
An increase in angiotensin II seems even more unlikely. Angiotensin II is stimulated by low blood pressure and hypovolemia, neither of which are likely to apply under conditions of the high blood pressure associated with a high-salt diet in the KO animals. Thus, we conclude that the increase in ROS in renal principal cells is the primary mechanism underlying the increase in ENaC. Even if ROS is generally produced, the lifetime of ROS is such that only very locally produced ROS could affect ENaC.
In summary, knockout of one protein responsible for superoxide regulation across the outer membrane of mitochondria produce abnormalities in blood pressure. We have shown that this is due to an inability of the mitochondria to regulate mitochondrial ROS in response to high-salt that leads to mitochondrial damage and a substantial increase in ENaC activity.
Experimental procedures
Animals
Heterozygous VDAC3 mice were obtained from Dr. William Craigen at Baylor College of Medicine and maintained in house. All animal experiments were performed on male and female VDAC3 homozygous knockout mice and their wildtype littermate controls (an inbred 129SvEv background) ages 2–6 months. The genotypes of the mice were determined by PCR, as previously described by Dr. William Craigen (30). Mice were kept on a 12:12-h light-dark cycle and given free access to food and water. Mice were generally divided into 4 groups and fed normal-salt (LabDiet 5001, 0.39% Na+), low-salt diet (ENVIGO TD.08290, 0.01–0.02% Na+), high-salt diet (ENVIGO TD.92012, 3.2% Na+), or high-salt diet with a daily intraperitoneal injection of 1.4 mg/kg body weight of benzamil (ENaC channel blocker) for 2 weeks. To test whether ROS is involved in ENaC regulation, wildtype mice and VDAC3-KO mice were fed with a high-salt diet for 2 weeks. For blood pressure measurements, 1 mmol/liter of TEMPOL was added in drinking water, and water was changed once a week. For single channel recording, 500 μmol/liter of TEMPOL was added to the bath solution directly. To test whether mitochondria are the source of ROS, wildtype and VDAC3-KO mice were fed with high-salt diets plus intraperitoneal injection of 0.7 mg/kg/day of mito-TEMPO (mitochondria-targeted antioxidant) for 2 weeks, we performed both blood pressure and patch-clamp measurements. All of our animal protocols and procedures in this paper were approved by the Emory Institutional Animal Care and Use Committee (IACUC).
Metabolic parameters
Mice were placed in metabolic cages (Techniplast) for 3 days: on the first day mice were put into cages to acclimate, on the second day water and food were measured, and on the third day water intake, food intake, body weight, and urine volume measurements were taken. Baseline measurements began on day 0, whereas animals were on normal diet and then the same measurements were made after the animals were on a test diet for 2 weeks. Urine collected was used for osmolality and electrolyte measurements.
Urine analysis
Urine was centrifuged at 15,000 rpm for 15 min to separate any insoluble substance before analysis. Urine osmolality was measured by a vapor pressure osmometer (Wescor). Urine electrolytes (sodium and potassium) levels were measured from urine samples taken before and after 2 weeks of treatment. Urine was diluted 1:10 with urine diluent (EasyLyte Medica) and analyzed with a EasyLyte analyzer (Medica Corporation).
Blood pressure measurements
Systolic blood pressure was measured by tail cuff as previously described (33). Briefly, blood pressures were measured for 3–5 consecutive days before and then after 2 weeks of treatments. Data from the first 1 or 2 days of each cycle were discarded as this was considered a transition period in which the mice become accustomed to the procedure. Between measurement times, mice were allowed to rest for 2–4 days to avoid extraordinarily high stress levels. Blood pressures were measured on a warmed platform (BP-2000, Visitech Systems), and mice were put on the platform to rest for 10 min before measurement. Five preliminary measurements were made and discarded to accustom mice to the procedure. Daily blood pressures are an average of 10 measurements each day on 4 to 6 mice (up to 8 mice for wildtype high-salt diet).
Transmission electron microscopy
Wildtype control mice, VDAC3-KO mice, and VDAC3-KO mice fed with a high-salt diet plus intraperitoneal injection of 0.7 mg/kg/day of mito-TEMPO (mitochondria-targeted antioxidant) for 2 weeks were anesthetized with a intraperitoneal injection mixture of ketamine:xylazine. Toe pinch was used to check the depth of the anesthesia. The abdominal cavity was opened to the diaphragm, and a butterfly needle was inserted into the abdominal aorta at the bifurcation of the iliac arteries. The aorta was tied above the level of the renal arteries, and the left renal vein was cut to allow exit of perfusate. Mice were perfused with PBS to remove blood followed by a fixative (2.5% glutaraldehyde in 0.1 m cacodylate buffer, pH 7.4, provided by the Robert P. Apkarian Electron Microscopy Facility at Emory). Kidneys were removed and fixed in the same fixative, subsequently processed and imaged by the Robert P. Apkarian Electron Microscopy Facility.
Single channel recording in isolated, split-opened kidney tubules
Renal tubules were manually dissected in cold Hanks' balanced salt solution, and the CCD was identified by morphology. Tubules were placed in extracellular bathing solution (150 mm NaCl, 5 mm KCl, 1 mm CaCl2, 2 mm MgCl2, 5 mm glucose, and 10 mm HEPES adjusted pH to 7.4) in a plastic dish before being split open to reveal the apical surface of the cells before single-channel patch-clamp as previously described (33, 34). Briefly, a microelectrode was filled with physiological buffer solution in which lithium was substituted for sodium (140 mm LiCl, 2 mm MgCl2, and 10 mm HEPES, pH 7.4) and lowered to a single cell before application of a small amount of suction to achieve a >1 G seal. ENaC were identified by characteristic channel kinetics (long mean open and closed times >0.5 s) and the current-voltage relationship of the channel (unit conductance close to 6 pS and a very positive, >40 mv, reversal potential).
In vivo detection of ROS
Detection of ROS in single kidney tubules is modified from the previous protocol of Owusu-Ahsah et al. (35). Briefly, renal tubules were dissected in Hanks' balance solution at room temperature, and CCDs were identified by morphology. Single CCD was adhered to the bottom of a plastic dish coated with adhesive (Corning Cell-Tak) in Hanks' balance solution. After dissection and immediately before use, we made a DHE stock solution (30 mm in anhydrous DMSO). We added 3 μl of the stock solution in 3 ml of Hanks' balanced salt solution to give a final concentration of ∼30 μm and vortexed to evenly disperse the dye. Tubules were incubated with the dye three times for 3 min in a dark chamber, on an orbital shaker at room temperature. Images of the tubules were captured immediately using a florescence microscope (Nikon ECLIPSE TE200). ImageJ was used to analyze the mean fluorescence of the ROS in kidney tubule by outlining the tubule and quantifying the average fluorescence within the tubule.
Chemicals
Most chemicals were purchased from Sigma. Benzamil was purchased from Tocris Bioscience (catalog number 3380) and dissolved in 1× PBS with gentle sonication. DHE was purchased from ThermoFisher Scientific (Molecular Probes, catalog number D11347). Mito-TEMPO was purchased from Enzo Life Sciences (catalog number ALX-430-150-M005).
Data analysis and statistics
GraphPad Prism6 was used to generate the scatter plot graphs. SigmaPlot 13 (SigmaStat) was used to perform all other data analysis and generate graphs. One-way ANOVA with Holm-Sidak post test was used to compare differences between treatments when values where normally distributed; e.g. between genotype and diets. For data that was not normally distributed; e.g. blood pressure measurements and mito-TEMPO, we used a Kruskal-Wallis ANOVA on ranks with Dunn's post test to determine significant differences between treatments. Except for scatter plot figures and the difference in means in Fig. 2B, data are presented as box plots in which the boundary of the box closest to zero indicates the 25th percentile, a solid line within the box marks the median, and the dashed line is the mean, and the boundary of the box farthest from zero indicates the 75th percentile. Whiskers (error bars) above and below the box indicate the 90th and 10th percentiles. Outlying points are shown individually in the graph. Morphology changes in VDAC3-KO animals compared with wildtype were determined with a Mann-Whitney rank sum test. The difference in the percentage of voids in mitochondria of VDAC3-KO and wildtype mice was assessed with z-test. The difference before and after TEMPOL treatment was assessed with a paired t test. Specific statistical methods for each experiment was marked in the figure legends and under “Results.” Data are presented as mean ± S.D. except where noted. p values less than 0.05 were considered significant.
Author contributions
Conception and design: L. Z., T. L. T., J. J., and D. C. E.; executing experiments: L. Z., V. L., Y. Z., L. G., L. L., T. L. T., Q. Y., O. A., H. B., and D. C. E.; analysis and interpretation: L. Z., V. L., Y. Z., T. L. T., Q. Y., J. J., H. M., and D. C. E.; drafting manuscript: L. Z., J. J., T. L. T., and D. C. E.
Acknowledgments
Heterozygous VDAC3 mice were generously provided by Dr. William Craigen. Blood pressure and metabolic measuring equipment were shared with Drs. Jeff M. Sands, Janet D. Klein, Susan M. Wall, and Clintoria Williams in the Renal Division of the Department of Medicine, Emory University. We also thank Dr. Brandi Wynne for helpful discussions about our experiments.
This work was supported by National Institutes of Health Grants R37-DK037963 (to D. C. E.) and R01-DK100582 (to H.-P. M.), in part by the Robert P. Apkarian Integrated Electron Microscopy Core (RPAIEMC), which is subsidized by the Emory College of Arts and Sciences and the Emory University School of Medicine and is one of the Emory Integrated Core Facilities, and National Center for Advancing Translational Sciences, National Institutes of Health Grants UL1TR000454 and S10 RR025679. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institutes of Health.
- ENaC
- epithelial sodium channel
- VDAC
- voltage-dependent anion channel
- TEMPOL
- 1-oxyl-2,2,6,6-tetramethyl-4-hydroxypiperidine
- ROS
- reactive oxygen species
- CCD
- cortical collecting duct
- DHE
- dihydroethidium
- mito-TEMPO
- (2-(2,2,6,6-tetramethylpiperidin-1-oxyl-4-ylamino)-2-oxoethyl)triphenylphosphonium chloride
- ANOVA
- analysis of variance.
References
- 1. Bhalla V., and Hallows K. R. (2008) Mechanisms of ENaC regulation and clinical implications. J. Am. Soc. Nephrol. 19, 1845–1854 10.1681/ASN.2008020225 [DOI] [PubMed] [Google Scholar]
- 2. Garty H., and Palmer L. G. (1997) Epithelial sodium channels: function, structure, and regulation. Physiol. Rev. 77, 359–396 [DOI] [PubMed] [Google Scholar]
- 3. Hansson J. H., Nelson-Williams C., Suzuki H., Schild L., Shimkets R., Lu Y., Canessa C., Iwasaki T., Rossier B., and Lifton R. P. (1995) Hypertension caused by a truncated epithelial sodium channel γ subunit: genetic heterogeneity of Liddle syndrome. Nat. Genet. 11, 76–82 10.1038/ng0995-76 [DOI] [PubMed] [Google Scholar]
- 4. Shimkets R. A., Warnock D. G., Bositis C. M., Nelson-Williams C., Hansson J. H., Schambelan M., Gill J. R. Jr., Ulick S., Milora R. V., Findling J. W., et al. (1994) Liddle's syndrome: heritable human hypertension caused by mutations in the beta subunit of the epithelial sodium channel. Cell 79, 407–414 10.1016/0092-8674(94)90250-X [DOI] [PubMed] [Google Scholar]
- 5. Rostovtseva T., and Colombini M. (1997) VDAC channels mediate and gate the flow of ATP: implications for the regulation of mitochondrial function. Biophys. J. 72, 1954–1962 10.1016/S0006-3495(97)78841-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Han D., Antunes F., Canali R., Rettori D., and Cadenas E. (2003) Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J. Biol. Chem. 278, 5557–5563 10.1074/jbc.M210269200 [DOI] [PubMed] [Google Scholar]
- 7. Messina A., Reina S., Guarino F., and De Pinto V. (2012) VDAC isoforms in mammals. Biochim. Biophys. Acta 1818, 1466–1476 10.1016/j.bbamem.2011.10.005 [DOI] [PubMed] [Google Scholar]
- 8. Anflous-Pharayra K., Cai Z. J., and Craigen W. J. (2007) VDAC1 serves as a mitochondrial binding site for hexokinase in oxidative muscles. Biochim. Biophys. Acta 1767, 136–142 10.1016/j.bbabio.2006.11.013 [DOI] [PubMed] [Google Scholar]
- 9. Reina S., Checchetto V., Saletti R., Gupta A., Chaturvedi D., Guardiani C., Guarino F., Scorciapino M. A., Magrí A., Foti S., Ceccarelli M., Messina A. A., Mahalakshmi R., Szabo I., and De Pinto V. (2016) VDAC3 as a sensor of oxidative state of the intermembrane space of mitochondria: the putative role of cysteine residue modifications. Oncotarget 7, 2249–2268 10.18632/oncotarget.6850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ma H. P. (2011) Hydrogen peroxide stimulates the epithelial sodium channel through a phosphatidylinositide 3-kinase-dependent pathway. J. Biol. Chem. 286, 32444–32453 10.1074/jbc.M111.254102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Loperena R., and Harrison D. G. (2017) Oxidative stress and hypertensive diseases. Med. Clin. North Am. 101, 169–193 10.1016/j.mcna.2016.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mitchell P. (1961) Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature 191, 144–148 10.1038/191144a0 [DOI] [PubMed] [Google Scholar]
- 13. Mitchell P. (1976) Possible molecular mechanisms of the protonmotive function of cytochrome systems. J. Theor. Biol. 62, 327–367 10.1016/0022-5193(76)90124-7 [DOI] [PubMed] [Google Scholar]
- 14. Herrmann J. M., and Riemer J. (2010) The intermembrane space of mitochondria. Antioxid. Redox Signal. 13, 1341–1358 10.1089/ars.2009.3063 [DOI] [PubMed] [Google Scholar]
- 15. Amodeo G. F., Scorciapino M. A., Messina A., De Pinto V., and Ceccarelli M. (2014) Charged residues distribution modulates selectivity of the open state of human isoforms of the voltage dependent anion-selective channel. PloS One 9, e103879 10.1371/journal.pone.0103879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thai T. L., Yu L., Galarza-Paez L., Wu M. M., Lam H. Y., Bao H. F., Duke B. J., Al-Khalili O., Ma H. P., Liu B., and Eaton D. C. (2015) The polarized effect of intracellular calcium on the renal epithelial sodium channel occurs as a result of subcellular calcium signaling domains maintained by mitochondria. J. Biol. Chem. 290, 28805–28811 10.1074/jbc.M115.668293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alli A. A., Bao H. F., Liu B. C., Yu L., Aldrugh S., Montgomery D. S., Ma H. P., and Eaton D. C. (2015) Calmodulin and CaMKII modulate ENaC activity by regulating the association of MARCKS and the cytoskeleton with the apical membrane. Am. J. Physiol. Renal Physiol. 309, F456–F463 10.1152/ajprenal.00631.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ling B. N., Zuckerman J. B., Lin C., Harte B. J., McNulty K. A., Smith P. R., Gomez L. M., Worrell R. T., Eaton D. C., and Kleyman T. R. (1997) Expression of the cystic fibrosis phenotype in a renal amphibian epithelial cell line. J. Biol. Chem. 272, 594–600 10.1074/jbc.272.1.594 [DOI] [PubMed] [Google Scholar]
- 19. Guo C., Sun L., Chen X., and Zhang D. (2013) Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regener. Res. 8, 2003–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pagano A., Donati Y., Métrailler I., and Barazzone Argiroffo C. (2004) Mitochondrial cytochrome c release is a key event in hyperoxia-induced lung injury: protection by cyclosporin A. Am. J. Physiol. Lung Cell. Mol. Physiol. 286, L275–L283 10.1152/ajplung.00181.2003 [DOI] [PubMed] [Google Scholar]
- 21. Zorov D. B., Juhaszova M., and Sollott S. J. (2014) Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 94, 909–950 10.1152/physrev.00026.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Imaizumi Y., Eguchi K., Murakami T., Arakawa K., Tsuchihashi T., and Kario K. (2016) High salt intake is independently associated with hypertensive target organ damage. J. Clin. Hypertens. 18, 315–321 10.1111/jch.12668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nistala R., Whaley-Connell A., and Sowers J. R. (2008) Redox control of renal function and hypertension. Antioxid. Redox Signal. 10, 2047–2089 10.1089/ars.2008.2034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Uetake Y., Ikeda H., Irie R., Tejima K., Matsui H., Ogura S., Wang H., Mu S., Hirohama D., Ando K., Sawamura T., Yatomi Y., Fujita T., and Shimosawa T. (2015) High-salt in addition to high-fat diet may enhance inflammation and fibrosis in liver steatosis induced by oxidative stress and dyslipidemia in mice. Lipids Health Dis. 14, 6 10.1186/s12944-015-0002-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Downs C. A., and Helms M. N. (2013) Regulation of ion transport by oxidants. Am. J. Physiol. Lung Cell Mol. Physiol. 305, L595–L603 10.1152/ajplung.00212.2013 [DOI] [PubMed] [Google Scholar]
- 26. Downs C. A., Kreiner L., Zhao X. M., Trac P., Johnson N. M., Hansen J. M., Brown L. A., and Helms M. N. (2015) Oxidized glutathione (GSSG) inhibits epithelial sodium channel activity in primary alveolar epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 308, L943–L952 10.1152/ajplung.00213.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Downs C. A., Kumar A., Kreiner L. H., Johnson N. M., and Helms M. N. (2013) H2O2 regulates lung epithelial sodium channel (ENaC) via ubiquitin-like protein Nedd8. J. Biol. Chem. 288, 8136–8145 10.1074/jbc.M112.389536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Helms M. N., Jain L., Self J. L., and Eaton D. C. (2008) Redox regulation of epithelial sodium channels examined in alveolar type 1 and 2 cells patch-clamped in lung slice tissue. J. Biol. Chem. 283, 22875–22883 10.1074/jbc.M801363200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Anflous-Pharayra K., Lee N., Armstrong D. L., and Craigen W. J. (2011) VDAC3 has differing mitochondrial functions in two types of striated muscles. Biochim. Biophys. Acta 1807, 150–156 10.1016/j.bbabio.2010.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sampson M. J., Decker W. K., Beaudet A. L., Ruitenbeek W., Armstrong D., Hicks M. J., and Craigen W. J. (2001) Immotile sperm and infertility in mice lacking mitochondrial voltage-dependent anion channel type 3. J. Biol. Chem. 276, 39206–39212 10.1074/jbc.M104724200 [DOI] [PubMed] [Google Scholar]
- 31. Levy M., Faas G. C., Saggau P., Craigen W. J., and Sweatt J. D. (2003) Mitochondrial regulation of synaptic plasticity in the hippocampus. J. Biol. Chem. 278, 17727–17734 10.1074/jbc.M212878200 [DOI] [PubMed] [Google Scholar]
- 32. Craigen W. J., and Graham B. H. (2008) Genetic strategies for dissecting mammalian and Drosophila voltage-dependent anion channel functions. J. Bioenerg. Biomembr. 40, 207–212 10.1007/s10863-008-9146-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bao H. F., Thai T. L., Yue Q., Ma H. P., Eaton A. F., Cai H., Klein J. D., Sands J. M., and Eaton D. C. (2014) ENaC activity is increased in isolated, split-open cortical collecting ducts from protein kinase Calpha knockout mice. Am. J. Physiol. Renal Physiol. 306, F309–F320 10.1152/ajprenal.00519.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mironova E., Bugay V., Pochynyuk O., Staruschenko A., and Stockand J. D. (2013) Recording ion channels in isolated, split-opened tubules. Methods Mol. Biol. 998, 341–353 10.1007/978-1-62703-351-0_27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Owusu-Ansah E., Yavari A., and Banerjee U. (2008) A protocol for in vivo detection of reactive oxygen species. Protoc. Exch. 10.1038/nprot.2008.23 [DOI] [Google Scholar]