Significance
Emotion-related responses, such as fear and anxiety, are important behavioral phenomena in most animal species, as well as in humans. However, the underlying mechanisms of fear and anxiety in animals and in humans are still largely unknown, and anxiety disorders continue to represent a large unmet medical need in the human clinic. Animal models may speed up discovery of these mechanisms and may also lead to betterment of human health. Herein, we report the identification of a chemokine-like gene family, samdori (sam), and present functional characterization of sam2. We observed increased anxiety-related responses in both zebrafish and mouse knockout models. Taken together, these results support a crucial and evolutionarily conserved role of sam2 in regulating anxiety-like behavior.
Keywords: chemokine-like, anxiety, fear, knockout, zebrafish
Abstract
Emotional responses, such as fear and anxiety, are fundamentally important behavioral phenomena with strong fitness components in most animal species. Anxiety-related disorders continue to represent a major unmet medical need in our society, mostly because we still do not fully understand the mechanisms of these diseases. Animal models may speed up discovery of these mechanisms. The zebrafish is a highly promising model organism in this field. Here, we report the identification of a chemokine-like gene family, samdori (sam), and present functional characterization of one of its members, sam2. We show exclusive mRNA expression of sam2 in the CNS, predominantly in the dorsal habenula, telencephalon, and hypothalamus. We found knockout (KO) zebrafish to exhibit altered anxiety-related responses in the tank, scototaxis and shoaling assays, and increased crh mRNA expression in their hypothalamus compared with wild-type fish. To investigate generalizability of our findings to mammals, we developed a Sam2 KO mouse and compared it to wild-type littermates. Consistent with zebrafish findings, homozygous KO mice exhibited signs of elevated anxiety. We also found bath application of purified SAM2 protein to increase inhibitory postsynaptic transmission onto CRH neurons of the paraventricular nucleus. Finally, we identified a human homolog of SAM2, and were able to refine a candidate gene region encompassing SAM2, among 21 annotated genes, which is associated with intellectual disability and autism spectrum disorder in the 12q14.1 deletion syndrome. Taken together, these results suggest a crucial and evolutionarily conserved role of sam2 in regulating mechanisms associated with anxiety.
Emotional responses, such as fear (responses to the appearance of well-defined and clearly present aversive stimuli) and anxiety (responses seen under aversive contexts in which the clear presence of fear inducing stimuli cannot be ascertained or these stimuli are continuously present and diffuse), are essential behavioral phenomena that have strong fitness components in all species (1). Anxiety-related disorders continue to represent a major unmet medical need in our society. Despite concerted efforts to develop anxiolytic and antidepressant pharmacotherapies, a large proportion of patients remain unresponsive to medication (2). This is due to the fact that we still do not fully understand the mechanisms of these diseases. To facilitate mechanistic analysis and to speed up the process of target identification and psychopharmacological characterization of compounds, animal models have been proposed.
Emotional responses, such as fear and anxiety, are regulated by various neuromodulators (3). Recently, studies have raised the possibility that chemokines may also possess neuromodulatory functions. Chemokines are small, secreted signaling proteins that commonly act as chemo-attractants to guide the migration of cells of the immune system. Importantly, chemokines are also expressed in neurons and glial cells of the central nervous system (CNS); while their role in the brain has mainly been associated with neuro-inflammatory responses, it is becoming more evident that chemokines in the brain may hold other functions (4–6).
Emotion dysregulation can lead to serious behavioral problems and impaired social interaction associated with a variety of disease conditions, including attention deficit hyperactivity disorder, bipolar disorder, and posttraumatic stress disorder (7, 8). Despite the paucity of proper treatment options for such disorders, the exploration of possible underlying genetic and biological mechanisms has been slow. The identification of novel genes associated with emotional behavior and understanding neural mechanisms of higher brain function remains a major goal of biology.
The use of animal models has greatly aided this research. The zebrafish (Danio rerio) has emerged as a promising model organism for the study of complex neuropsychiatric diseases because of the well-defined behavioral phenotypes of this species (9), and because of its evolutionarily conserved brain structures and functions. One example of such a brain structure is the habenula (Hb), which is connected with the subpallium and hypothalamus, and is implicated in fear, anxiety, addiction, and mood disorders (10, 11).
The zebrafish is useful for large-scale forward genetic screens to discover novel genes involved in CNS development, an approach we have utilized (12, 13). Over 10 y ago we discovered a chemokine-like gene family, called samdori (sam), using genetic screens (i.e., insertional mutagenesis with a unique dual reporter system) (SI Appendix, Fig. S1). We present herein the body of work that has extended from initial gene discovery and encompasses various analyses of the physiological functions of this family.
A member of this family, sam2 exhibits exclusive brain-specific expression, predominantly in neurons of the dorsal Hb (dHb), as well as the telencephalic area and hypothalamus. Here, we report the development of a zebrafish null mutant (KO) in which this gene is silenced. We investigate the effect of this null mutation using behavioral assays developed to induce and measure fear and anxiety responses. To investigate the generalizability of our findings obtained with zebrafish, we also report herein the development and analysis of a mouse null mutant in which the mammalian homolog of the zebrafish sam2 gene is silenced. We found phenotypical alterations in the Sam2 KO mice that are consistent with those we observed in the zebrafish null mutants, suggesting that Sam2 is critical for regulating anxiety across such evolutionarily distant species as mammals and fish.
In addition, we also analyzed a variety of markers in the zebrafish sam2 KO (colocalization studies), which uncovered changes in mRNA expression of the gene encoding corticotropin-releasing hormone (CRH), a hormone involved in stress and anxiety responses in mammals (14). We followed up this investigation with whole-cell patch-clamp electrophysiological recordings from CRH neurons in the mouse hypothalamus, and found that Sam2 modulated GABAergic synaptic transmission on CRH neurons.
Finally, we investigated whether mutations in the human genome potentially affecting SAM2 function may have functional, behavioral consequences. We identified patients with microdeletions/microduplications in 12q14.1, including SAM2, who suffered from a variety of behavioral and intellectual problems. Using six copy-number variations (CNVs), we were able to refine the candidate gene region encompassing SAM2 among 21 annotated genes at 12q14.1.
In summary, we describe the discovery of a chemokine-like protein family and provide initial functional characterization of one of its members, sam2. Taken together, our multidisciplinary results demonstrate a potentially crucial role of this candidate gene in the regulation of emotion-related behaviors across evolutionarily distant species, and delineate a target that may allow the development of effective pharmacotherapy for human CNS disorders associated with altered fear, anxiety, and stress.
Results
Identification of samdori2.
Using insertional mutagenesis to identify novel functional genes, we isolated a chemokine-like gene, sam [also called TAFA (15) and FAM19A in the National Center for Biotechnology Information database] (SI Appendix, Fig. S1). Subsequently, we also isolated other members of sam family based upon the sequences in the database. We have identified eight members in the zebrafish sam gene family, and found them highly conserved in mouse and human at the amino acid sequence level (SI Appendix, Fig. S2 A–E). All Sam proteins exhibited 10 regularly spaced cysteine residues with a pattern of CX7CCX13CXCX14CX11CX4CX5CX10C, a sequence similar to that of CC-type chemokines (15).
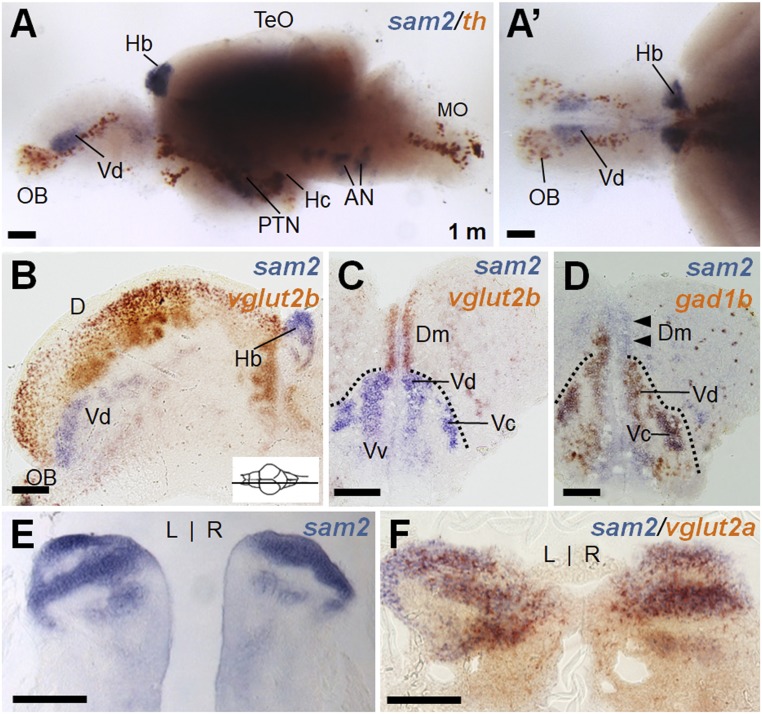
Using whole-mount in situ hybridization in zebrafish, we found all sam genes to be exclusively expressed in the CNS at larval stages (SI Appendix, Fig. S2 F–O). Among them, we focused on the functional analysis of the sam2 gene, because we found sam2 to be predominantly expressed in the dHb of adult fish (Fig. 1). To further characterize sam2-expressing cells, we performed whole-mount in situ hybridization in larval and adult fish brains using various neuronal markers. In addition to dHb, sam2-expressing cells were also detected in the telencephalon and otic neurons in larvae (SI Appendix, Fig. S3A). In situ hybridization of adult brain and serial sectioning revealed the detailed expressions of sam2 in the medial zone of the dorsal telencephalic area (Dm), the dorsal nucleus of the ventral telencephalon (Vd), preoptic area of hypothalamus (PPa), and other hypothalamic regions (ATN, anterior tuberal nucleus; Hc, caudal zone of periventricular hypothalamus; Hd, dorsal zone of periventricular hypothalamus; and PTN, posterior tuberal nucleus) (Fig. 1 and SI Appendix, Fig. S3 and Table S1). To assess the distribution of sam2 within excitatory and inhibitory circuits, we performed double in situ hybridization with GABAergic markers (16, 17) and glutamatergic markers (16, 17). We found sam2+ cells in GAD67/gad1b-expressing cells in the Vd, central nucleus of the ventral region (Vc), and PPa (Fig. 1D and SI Appendix, Fig. S4). In the diencephalon, sam2-expressing cells were observed adjacent to GAD65/gad2+ cells in the hypothalamic area, such as the ATN and PTN, and periventricular hypothalamus (SI Appendix, Fig. S4). With the glutamatergic markers, sam2 mRNA was partially overlapped with vglut2b in the Dm but not in other forebrain regions (Fig. 1 B and C). In addition, sam2 was found to overlap with vglut2a in the dorsal region of the Hb (Fig. 1F). Expression of sam2 in the Hb was further examined by costaining with dHb-specific kctd12.1 (lov) (18, 19) or ventral Hb (vHb)-specific marker, aoc1 or dHb/vHb marker fam84b (20) (SI Appendix, Fig. S5 A–E). sam2 expression was found not to colocalize with known markers of dopaminergic (21), oxytocinergic (22), or hypocretinergic (23) neurons (Fig. 1 and SI Appendix, Fig. S5 F–H′). The Hb has been implicated in the regulation of emotion, including anxiety, fear, depression, and reward (7, 24). The fish telencephalic regions are homologous to the mammalian amygdala (Dm) and striatum (Vd), structures that are known to play fundamental roles in emotion-related behaviors in mammals (25). Thus, expression of the sam2 gene detected in the dHb, telencephalic areas (Dm, Vd), and hypothalamic regions prompted us to investigate its function in the regulation of emotion.
Fig. 1.
Characterization of sam2-expressing cells in the adult zebrafish brain. (A and A′) Whole-mount two color in situ hybridization of a dissected zebrafish brain with sam2 and a dopaminergic neuron marker, th. Anterior is to the left; lateral (A) and dorsal view (A′). The sam2 expression region did not overlap with th+ neurons. Prominent sam2 expression is seen in the Vd and Hb as well as hypothalamic regions. (B–D) Section images of brain hybridized with sam2/vglut2b (B, sagittal section; C, cross-section) or sam2/gad1b (D). (E and F) Cross-section of Hb region stained with sam2 alone (E) or sam2/vglut2a (F) probes. AN, auditory nerve; D, area dorsalis telencephlali; Dc, central zone of area dorsalis telencephali; MO, medulla oblongata; OB, olfactory bulb; TeO, optic tectum; Vv, ventral nucleus of area ventral telencephali. (Scale bars, 100 µm.)
Normal Development of Neuronal Cells in sam2 KO Fish.
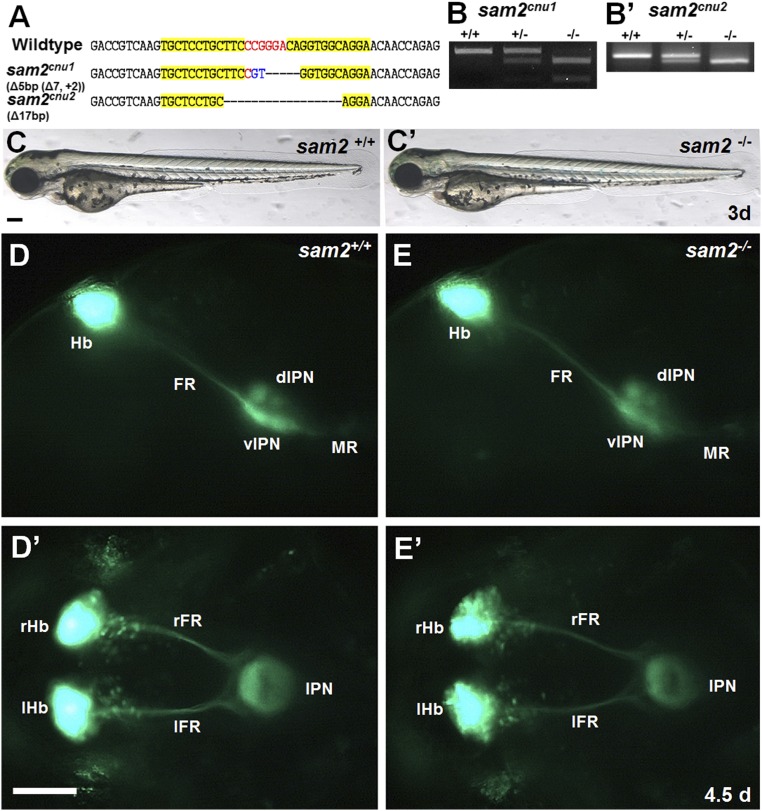
To investigate the function of sam2, we created two sam2 KO zebrafish lines, taking advantage of targeted mutagenesis utilizing zinc-finger nucleases (ZFNs) (26, 27). The 5-bp deletion (sam2cnu1) and 17-bp deletion (sam2cnu2) of the two alleles induced frame-shift mutations (Fig. 2 A–B′), which lead to alteration of the protein-coding sequence (SI Appendix, Fig. S6A). Both sam2 KO lines showed normal morphology during embryonic development (Fig. 2 C and C′), survived to adulthood, and were fertile. To test whether sam2 is necessary for the development of specific neuronal cell-types, we examined the expression of various molecular markers in sam2 KO zebrafish (SI Appendix, Fig. S6 B–G′). Compared with wild-type fish, we could not detect any significant differences in the expression patterns of dopaminergic neuronal markers [tyrosine hydroxylase (th) (21), dopamine transporter (dat) (28), and nuclear receptor related 1 protein (nurr-1) (28)], serotonergic neuronal markers [tryptophan hydroxylase raphe (tphR) and 5-hydroxytryptophan (5-HT)] (29), oxytocinergic neuronal marker oxytocin (oxt) (22), and interpeduncular nucleus (IPN) marker somatostatin1.1 (sst1.1) (30). Moreover, sam2 KO embryos showed normal expression of neurohormonal genes (SI Appendix, Fig. S6 H–M′), including hypocretin/orexin (hcrt) (23), melanin concentrating hormone (mch) (31), agouti related protein homolog (agrp) (32), neuropeptide Y (npy) (33), and pet-1 (fev; ETS oncogene family) (29). In addition, we observed normal hypothalamus–locus coeruleus projections in progenies of the Tg(hcrt:mEGFP) (23) transgenic line crossed to the sam2cnu1 mutant (SI Appendix, Fig. S6 N–O′). Moreover, the targeting of Hb efferent axons innervating the IPN appeared to be largely unaffected in the Et(-1.0otpa:mmGFP)hd1 (34) transgenic embryos crossed to sam2cnu1 KO fish (Fig. 2 D and E′). Therefore, we conclude that sam2 is not directly involved in the generation, migration, and axonal growth of neurons.
Fig. 2.
Generation of sam2 KO fish using ZFNs. (A) DNA sequencing analysis of two sam2 KO alleles (sam2cnu1 and sam2cnu2) with 5-bp and 17-bp deletion, respectively. Yellow mark, Left and Right ZFN-binding regions; red, spacer. (B and B′) Allele-specific genotyping of sam2cnu1 and sam2cnu2 KO fish by genomic PCR and digestion for BtgI site (CCGTGG) newly created in sam2cnu1. (C and C′) Normal morphology of sam2cnu1 KO embryo at 3 d. (D–E′) Projections of Hb efferent axons targeting the IPN in the Et(-1.0otpa:mmGFP)hd1 transgenic sam2cnu1 KO fish were not visibly affected. Lateral views (D and E) and dorsal views (D′ and E′). dIPN, dorsal IPN; FR, fasciculus retroflexus; lFR, Left FR; lHb, Left Hb; MR, median raphe; rFR, Right FR; rHb, Right HbvIPN, ventral IPN. (Scale bar, 100 µm.)
Increase of Anxiety-Like Responses in sam2 Mutant Fish.
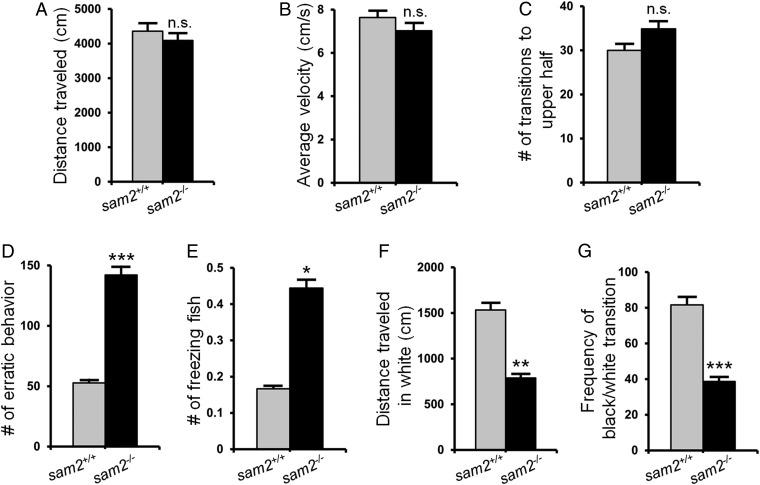
We next examined whether the loss of sam2 affects behaviors associated with anxiety using the open (novel) tank (35, 36) and dark/light preference (scototaxis) (37) tests. Anxiety and fear are related phenomena, but can be distinguished. Responses induced by a present and clearly detectable aversive stimulus are regarded as fear responses (behavior aimed at the avoidance of the stimulus), whereas anxiety-like behavior is found under diffuse aversive conditions: that is, without the appearance or clear presence of such stimuli. Novelty is an aversive context without the presence of specific fear-inducing stimuli, and thus novel test situations are often used to quantify anxiety. In the novel tank, when placed individually, sam2cnu1 KO fish showed normal locomotor activity, as indicated by no changes in total distance traveled (Fig. 3A) (sam2+/+, 4,359.29 ± 218.68 cm, n = 18; sam2−/−, 4,088.71 ± 249.83 cm, n = 28) and in average velocity (Fig. 3B) (sam2+/+, 7.63 ± 0.37 cm/s, n = 18; sam2−/−, 7.02 ± 0.41 cm/s, n = 28), compared with control sibling fish. Moreover, there were no significant differences in the frequency of transition to the upper half of the test tank between sam2cnu1 KO and control fish (Fig. 3C) (sam2+/+, 30 ± 6.93, n = 18; sam2−/−, 34.86 ± 4.19, n = 28), a measure of vertical exploration. However, sam2cnu1 KO fish exhibited behavioral characteristics indicative of elevated levels of anxiety, including an increased number of erratic movements (1, 38) (Fig. 3D) (sam2+/+, 52.67 ± 9.19, n = 18; sam2−/−, 142.14 ± 12.50, n = 28) and number of freezing events (35, 36) (Fig. 3E) (sam2+/+, 0.17 ± 0.09, n = 18; sam2−/−, 0.44 ± 0.10, n = 27). Freezing is a typical response seen in aversive contexts and can be used to quantify the level of anxiety in a variety of species, including zebrafish and rodents (36). Erratic movement has also been shown to be a characteristic sign of anxiety or fear in zebrafish (36, 39). Thus, our results indicate that sam2 may be involved in the regulation of anxiety-like responses in zebrafish. To further investigate anxiety-like behavior in sam2cnu1 KO fish, we also measured thigmotaxis (avoidance of open areas) in the novel tank. Increased thigmotaxis in novel contexts has been interpreted as a sign of anxiety (36). sam2cnu1 KO fish showed higher preference for the corner over the center of the test tank (SI Appendix, Fig. S7A) (sam2+/+ at the corner, 21.22 ± 0.56 s, at the center 34.15 ± 0.70 s, n = 12; sam2−/− at the corner, 26.83 ± 0.95 s, at the center, 29.14 ± 0.89 s, n = 12). The light/dark paradigm is another frequently employed test of fear and anxiety in zebrafish and rodents (37), and zebrafish have been shown to exhibit scototaxis (i.e., dark preference). Consistent with the results obtained in the novel tank task, in the light/dark paradigm, adult sam2cnu1 KO fish traveled a shorter distance (Fig. 3F) (sam2+/+, 1,533.59 ± 191.18 cm, n = 18; sam2−/−, 788.88 ± 116.09 cm, n = 24), spent less time in the white arena (SI Appendix, Fig. S7 B and C) (sam2+/+ in the black, 733.125 ± 80.66 s, in the white 168.38 ± 80.42 s, n = 18; sam2−/− in the black, 811.5 ± 64.81 s, in the white 104.08 ± 87.67 s, n = 24), and exhibited reduced frequency of black/white transitions compared with wild-type (Fig. 3G) (sam2+/+, 81.67 ± 8.74, n = 18; sam2−/−, 38.69 ± 4.36, n = 24), alterations that further corroborate the notion that the null mutant fish suffered from elevated levels of anxiety. These results suggest that sam2 may regulate emotion-related behaviors.
Fig. 3.
Increase of anxiety-related behaviors in sam2cnu1 KO zebrafish. (A–E) Novel tank tests of individually placed fish. The distance traveled (A) (sam2+/+, n = 18; sam2−/−, n = 28; Cohen’s d = 0.24; Mann–Whitney U = 181, P = 0.11), average velocity (B) (sam2+/+, n = 18; sam2−/−, n = 28; Cohen’s d = 0.32; Mann–Whitney U = 171, P = 0.07), and transition to upper half (C) (sam2+/+, n = 18; sam2−/−, n = 28; Cohen’s d = 0.19; Mann–Whitney U = 202, P = 0.27) were not significantly changed in sam2 KO fish. However, the frequency of erratic behavior (D) (sam2+/+, n = 18; sam2−/−, n = 28; Cohen’s d = 1.65; Mann–Whitney U = 41, P < 0.00001) and the number of freezing fish (E) (sam2+/+, n = 18; sam2−/−, n = 27; Cohen’s d = 0.62; P = 0.042, Student’s t test) were increased in sam2 KO fish. (F and G) Black/white preference (scototaxis) test. Distance traveled in white arena (F) (sam2+/+, n = 18; sam2−/−, n = 24; Cohen’s d = 1.06; Mann–Whitney U = 85, P = 0.0032) and frequency of transitions (G) (sam2+/+, n = 18; sam2−/−, n = 24; Cohen’s d = 1.42; Mann–Whitney U = 61.5, P = 0.00016) were measured. *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant (P > 0.05).
Increase of Social Cohesion in sam2 KO Fish.
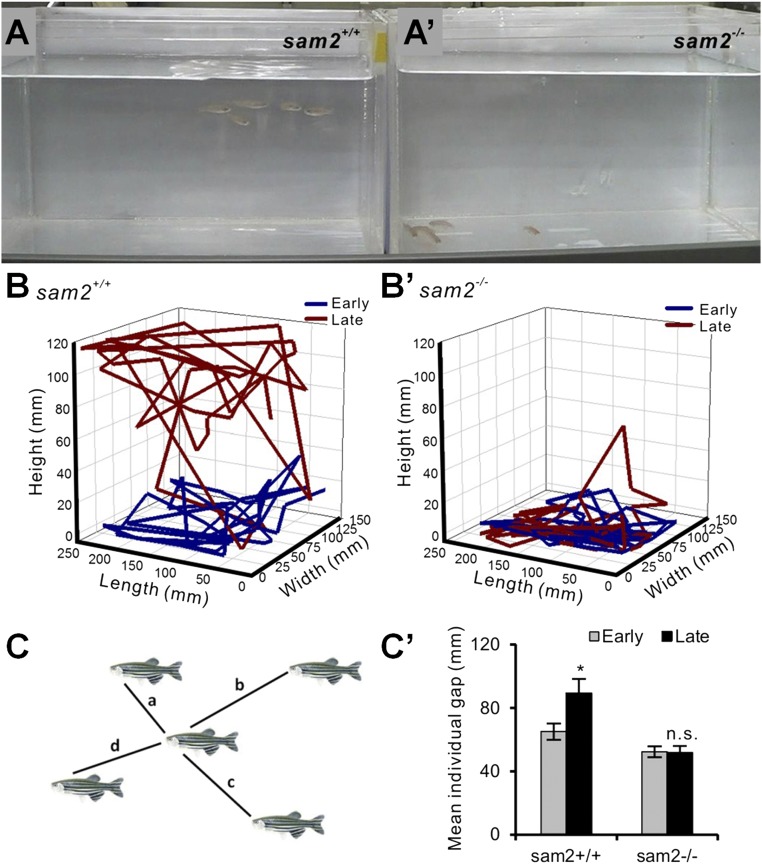
Zebrafish form groups, a behavior called shoaling. One of the adaptive functions of this social behavior has been shown to be predator-avoidance. Measures of shoal cohesion have been successfully utilized in the analysis and modeling of anxiety and fear in vertebrates, including zebrafish (1, 36). To test whether sam2 KO fish exhibit altered shoaling behavior, a group of five sam2 KO fish were placed in a novel tank at a time and monitored by 3D video tracking. We recorded the swim paths of the fish in the group for 30 min (Fig. 4 and SI Appendix, S8 A–B′) (40). Because wild-type zebrafish typically take 10 min to habituate to a novel tank (41), we analyzed the behavior of fish at two time points: early phase (before habituation, 5–8 min) and late phase (after habituation, 13–16 min) of the session. During the early phase, both sam2 KO and control fish showed typical anxiety-like responses by staying close to the bottom of the tank. However, at the late phase control sibling fish started to swim in the middle and the top layers of the water (Fig. 4 A and B), a response interpreted as reduction of anxiety. In sharp contrast, sam2 KO fish remained near the bottom of the tank (Fig. 4 A′ and B′ and Movie S1), suggesting impaired habituation to the novel environment (i.e., maintenance of elevated levels of anxiety) in sam2 KO fish. We next examined social cohesion in sam2 KO zebrafish by measuring the distance between a focal fish and each of its shoal members (interindividual distance) (41–43). Control fish significantly increased their mean interindividual distance from the early phase to the late phase, suggesting the reduction of novelty-induced anxiety (Fig. 4 C and C′). However, sam2 KO fish did not show such changes in mean individual distance, and maintained the same strong shoal cohesion (Fig. 4C′ and SI Appendix, Fig. S8). The effect size (Cohen’s d) for cohesion of the control and sam2cnu1 KO fish was d = −0.063 at the early phase (5–8 min) and d = −0.80 at the late phase (13–16 min). These results confirm that sam2 KO mutants exhibit elevated levels of anxiety compared with wild-type fish.
Fig. 4.
Anxiety-like behavior and increased social cohesion in sam2cnu1 KO fish. Five fish (3-mo-old male siblings) were placed as a group in a novel tank. (A and A′) A snapshot of video tracking after 20-min novel tank test. (B and B′) Temporal 3D reconstructions of video tracking before habituation (early, 5–8 min, blue; Cohen’s d = −0.063) and after habituation (late, 13–16 min, red; Cohen’s d = −0.80) to the novel environment. (C and C′) Measurement of social cohesion as the mean individual gap. We measured the distance between the focal fish and one of its shoal members (lines a, b, c, and d; interindividual distances). P = 0.02 for control and P = 0.88 for sam2 KO fish (sam2+/+, n = 5; sam2−/−, n = 5). *P < 0.05; ns, not significant (P > 0.05).
Excessive Anxiety/Fear Behavior in sam2 KO Fish in Response to Alarm Substance.
The above behavioral paradigms are considered to induce anxiety-like responses, because they do not use specific clearly present aversive stimuli. To test fear responses, we conducted another test in which an aversive stimulus was specifically provided and was clearly present during the test. A clearly present aversive stimulus that may be delivered in a well-defined manner to the zebrafish is the alarm substance (38). Among other behavioral responses, the alarm substance has been shown to induce a reliable alteration in shoaling that may be used to measure fear in zebrafish (38). In our study, we examined changes in shoal cohesion in response to administration of the alarm substance (SI Appendix, Fig. S9). Both control and sam2 KO fish exhibited increased shoal cohesion (reduced distance among shoal members) in response to the delivery of alarm substance. However, the relative decrease of interindividual distance in sam2 KO fish was more robust compared with that of wild-type control siblings. The effect size between before and after alarm treatment was d = −1.09 for control and d = −1.22 for sam2cnu1 KO fish, respectively. The effect size between control and sam2cnu1 KO fish was d = −0.96 before and d = −1.57 after alarm treatment. This was despite that the former had already displayed small interindividual distance even before the delivery of the alarm substance (SI Appendix, Fig. S9E). Thus, taken together these results demonstrate elevated anxiety and fear in sam2 KO fish.
Increase of mRNA Expression of Stress-Related crhb in sam2 KO Fish.
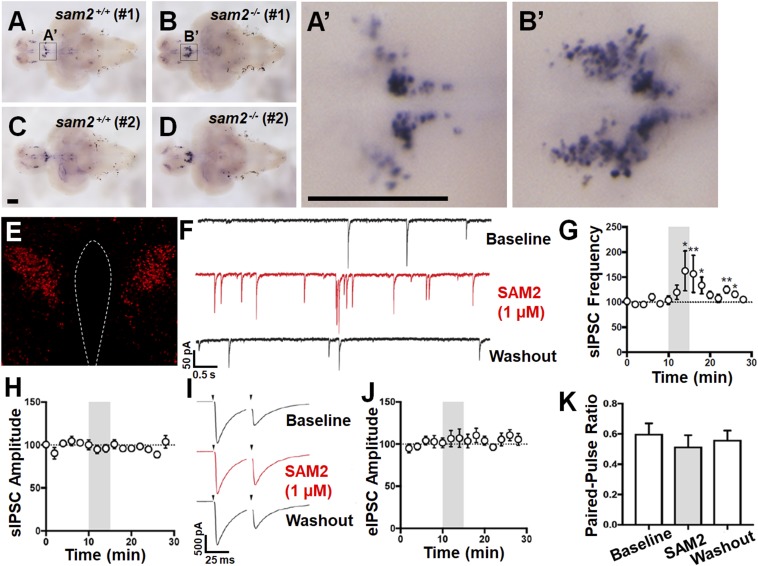
Aversive stimuli that induce fear and anxiety are expected to lead to physiological changes: for example, neurohormonal stress responses mediated by the hypothalamic–pituitary–adrenal (HPA) axis (14, 44). A previous study demonstrated that disruption of glucocorticoid-negative feedback of the HPA axis resulted in elevated cortisol levels and anxiety-like behavior in zebrafish (14). We hypothesized that the increased level of anxiety in sam2 KO fish may also be associated with dysregulation of the HPA axis. Using in situ hybridization, we found sam2cnu1 KO fish to exhibit elevated crhb expression, an important HPA axis marker, in the PPa compared with wild-type (see, for example, Fig. 6 A–D). Increased crhb mRNA levels in the PPa, a region homologous to the mammalian paraventricular nucleus (PVN), is consistent with previous reports of dysregulated HPA axis function in zebrafish and mice (14, 45).
Fig. 6.
Increase of mRNA expression of stress-related crhb in sam2cnu1 KO fish and spontaneous inhibitory postsynaptic currents onto CRH neurons by SAM2. (A–D) Increase of crhb mRNA expression in sam2cnu1 KO fish (sam2+/+, n = 6; sam2−/−, n = 6). Ventral views of the whole brain of control sam2+/+ (A and C) and sam2−/− KO fish (B and D). (A′ and B′) Higher magnifications of the boxed regions in A and B are the PPa in zebrafish, homologous to the mouse PVN. (Scale bars, 200 µm.) (E) Representative photomicrograph of the Cre-dependent TdTomato in the PVN CRH neurons. (Magnification: E, 10×.) (F) Representative voltage-clamp traces of sIPSCs in response to SAM2 application in PVN CRH neurons. (G) SAM2 application significantly increased sIPSC frequency. (H) SAM2 did not affect sIPSC amplitude. (I) Representative voltage-clamp trace of eIPSC onto CRH neurons in response to SAM2 application. (J) SAM2 did not affect the amplitude of eIPSCs. (K) SAM2 did not change the paired-pulse ratio. *P < 0.05; **P < 0.01.
Sam2 KO Mice also Exhibit Anxiety-Like and Fear-Related Behaviors.
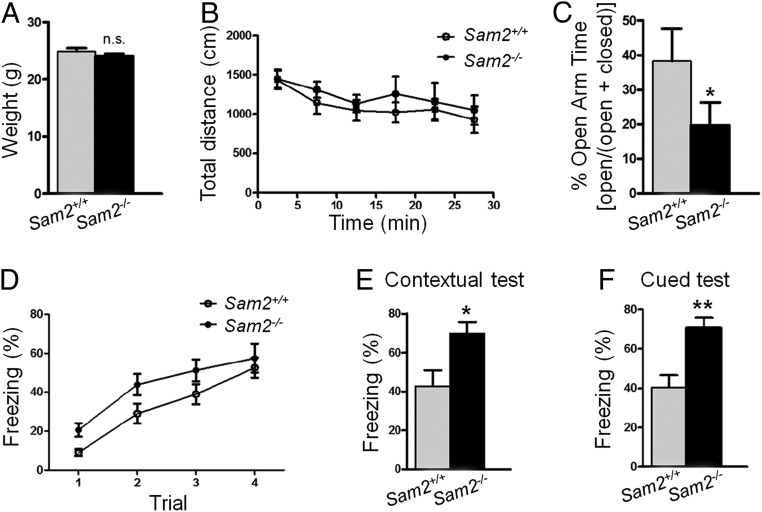
To investigate whether the role of sam2 in anxiety and fear is evolutionarily conserved or specific only to the zebrafish, we generated a Sam2 KO mouse line (SI Appendix, Fig. S10) and compared the behavior of these mutants to wild-type littermates. Sam2 showed brain-specific expression in mouse similar to that of zebrafish, having a higher expression level in the hippocampus and Hb but not in the PVN (SI Appendix, Fig. S11). We found Sam2 KO mice to spend significantly less time in the open arms on an elevated plus maze (Fig. 5C) (Sam2+/+ mice, 38.3 ± 9.16%, n = 11; Sam2−/− mice, 19.66 ± 6.66%, n = 8) and to show increased freezing in both the contextual (Fig. 5E) (Sam2+/+ mice, 42.86 ± 8.08%, n = 11; Sam2−/− mice, 69.74 ± 6.12%, n = 7) and cued fear-conditioning tests (Fig. 5F) (Sam2+/+ mice, 40.33 ± 6.32%, n = 11; Sam2−/− mice, 70.95 ± 4.97%, n = 7) compared with wild-type littermates. The elevated freezing in the null mutant mice was not due to a motor function-related change, as both mutant and wild-type animals exhibited similar levels of freezing on training day (Fig. 5D) [two-way repeated-measures ANOVA, Time × Group interaction: F(3, 48) = 0.48, P = 0.69, Sam2+/+ mice, n = 11; Sam2−/− mice, n = 7]. Body weight (Fig. 5A) (Sam2+/+ mice, 24.86 ± 0.59 g, n = 25; Sam2−/− mice, 24.07 ± 0.39 g, n = 10) and total locomotion on an open-field test (Fig. 5B) [two-way repeated-measures ANOVA, Time × Group interaction: F(5, 75) = 0.32, P = 0.89, Sam2+/+ mice, n =11; Sam2−/− mice, n = 6] were not significantly different between Sam2 KO and wild-type control mice. Collectively, the Sam2 KO mice exhibited phenotypical alterations reminiscent of the KO zebrafish, suggesting that Sam2 plays a potentially critical and evolutionally conserved role in regulating anxiety- and fear-related behavioral responses.
Fig. 5.
Increase of anxiety-related behaviors in Sam2 KO mice. (A) Sam2 KO mice have normal body weight (Sam2+/+, n = 25; Sam2−/−, n = 10; Cohen’s d = 0.31; unpaired two-tailed, Mann–Whitney U = 124, P = 0.98). (B) Sam2 KO mice show normal total locomotion in an open-field test [Sam2+/+, n = 11; Sam2−/−, n = 6; two-way repeated-measures ANOVA, Time × Group interaction: F(5, 75) = 0.32, P = 0.89]. (C) Sam2 KO mice spent significantly less time in the open arms on an elevated plus maze (Sam2+/+, n = 11; Sam2−/−, n = 8; Cohen’s d = 0.74; unpaired one-tailed, Mann–Whitney U = 23, P = 0.045). (D) Sam2 KO mice show similar levels of freezing response during training days [Sam2+/+, n = 11; Sam2−/−, n = 7; two-way repeated-measures ANOVA, Time × Group interaction: F(3, 48) = 0.48, P = 0.69]. (E) Sam2 KO mice show higher freezing behavior in contextual test 24 h after training (Sam2+/+, n = 11; Sam2−/−, n = 7; Cohen’s d = 1.21; unpaired two-tailed, Mann–Whitney U = 15, P = 0.035). (F) Sam2 KO mice show higher freezing behavior in cued test 24 h after training (Sam2+/+, n = 11; Sam2−/−, n = 7; Cohen’s d = 1.75; unpaired two-tailed, Mann–Whitney U = 7, P = 0.0028). *P < 0.05; **P < 0.01; ns, not significant (P > 0.05).
Increase in the Frequency of Spontaneous Inhibitory Postsynaptic Currents by SAM2.
Increased activity in the stress axis is one of the most enduring findings in both animal models and human patients with anxiety. Elevated fear and anxiety responses in mice are accompanied by both Crh mRNA overexpression and increased excitability of the PVN neurons (14, 45). Similarly, selective knockout of Crh in the PVN results in reduced anxiety (46). Therefore, we hypothesized that Sam2 could regulate CRH neuron excitability in the PVN. To investigate this hypothesis, we exposed PVN CRH neurons to a 5-min-long bath application of SAM2 (1 µM) (SI Appendix, Fig. S12). In response to this treatment, we found a significant increase in the frequency of spontaneous inhibitory postsynaptic currents (sIPSC); that is, 162.5 ± 39.76% of baseline (n = 11, P < 0.01, Wilcoxon signed-rank matched-pairs test) (Fig. 6 F and G). We found no significant change in the sIPSC amplitude, however (100.9 ± 5.3% of baseline, n = 11, P = 0.15, Wilcoxon signed-rank matched-pairs test) (Fig. 6H). We also observed no significant differences in evoked IPSC (eIPSC) amplitude and paired-pulse ratio (Fig. 6 I–J, and K). Thus, we conclude that SAM2 is able to significantly increase the frequency, but not the amplitude, of spontaneous GABAergic currents onto CRH neurons, rather than the amplitude of evoked GABAergic currents. These observations indicate that SAM2 may control tonic GABAergic inputs onto CRH neurons.
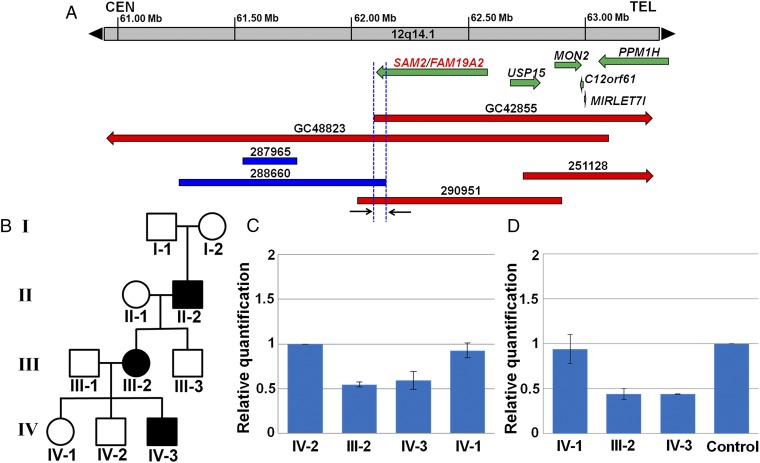
Comparative Genomic Mapping Using Six CNVs at 12q14.1.
Mutations (i.e., microdeletions or duplications) arising at the 12q14.1 region are characterized by a previously unreported feature set including craniofacial anomalies, cryptorchidism, intellectual disability, autism, and behavioral problems. Using six informative CNVs, we were able to refine the candidate gene region encompassing SAM2 at 12q14.1 (Fig. 7). We found deletions in two females, GC42855 and GC48823, sized 6.2 Mb (chr12: 62,097,144 to 68,266,543/hg19) and 5.1 Mb (chr12: 58,011,515 to 63,115,073/hg19), respectively (Fig. 7). GC48823 is 4-y-old with autism and seizures, whereas GC42855 is 6-y-old with developmental delay. A 1-Mb minimal overlapping region of these two deletions at 12q14.1 contains four genes including SAM2. Furthermore, we identified two more small CNVs in the DECIPHER (database of chromosomal imbalance and phenotype in humans using Ensembl resources) human genome variants database (47) overlapping the gene. Among them, patient 288660 with an 887-kb duplication (chr12: 61,271,591 to 62,158,216/hg19) has attention deficit hyperactivity disorder, autism spectrum disorder, and generalized joint laxity. However, this duplication overlaps only the 3′ end of the gene and may not disrupt its function. More importantly, patient 290951 has inherited an 880-kb deletion (chr12: 62,038,772 to 62,918,916/hg19) encompassing SAM2 and USP15 in a three-generation pedigree from the maternal grandfather through his mother with significant intellectual disability. Patient 290951 had a clinical diagnosis of autism spectrum disorder, behavioral difficulties, and severe speech and language delay. Overall he performed in the mild range of intellectual disability, as did his mother and maternal grandfather, who all had the same deletion. His unaffected brother, sister, and uncle did not inherit the deletion (Fig. 7 B–D). In mice, Usp15 is ubiquitously expressed in various tissues. Usp15 KO mice had normal survival rate and did not show abnormalities in development, although its deficiency enhanced T cell response against bacterial infection and tumor (48). A 61-kb minimal overlapping region of these four CNVs flanked by two blue vertical dotted lines encompasses the 56-kb 3′ end of SAM2. These data suggest that SAM2 contributes to the neurological phenotype of these patients. DECIPHER also has two additional small CNVs in this region, not including SAM2: child 251128 with behavioral problems, low-set ears, intellectual disability, and speech delay, has inherited an 886-kb deletion (chr12: 62,799,199 to 63,684,842/hg19) from a parent with similar phenotypes, and patient 287965 with a 224-kb duplication (chr12: 61,544,595 to 61,768,510/hg19) has global developmental delay.
Fig. 7.
CNV mapping at 12q14.1 with six human patients, implicating SAM2 as a candidate gene. Deletions and duplications are depicted in red and blue, respectively. (A) Alignment of three microdeletions and one microduplication at 12q14.1 has refined the candidate gene region to a 61 kb (arrows), which contains 56-kb 3′ end of SAM2. Notably, the microdeletion in a 6-y-old boy 290951 with autism spectrum disorder and intellectual disability contains SAM2, as well as USP15 and part of MON2. (B) Pedigree showing three family members affected by intellectual disability in three generations. We have confirmed the deletion in each family member; II-2 (del/+), III-2 (del/+), III-3 (+/+), IV-1 (+/+), IV-2 (+/+), and IV-3 (del/+). (C) SAM2 copy number in the family as determined by qPCR. The proband IV-3 has inherited the deletion from the maternal grandfather II-2 through his mother III-2 and affected members have only one SAM2 copy. The patient’s siblings are normal having two SAM2 copies. (D) Transcript levels of SAM2 in patient, his mother, sister, and white male control as revealed by qRT-qPCR. SAM2 transcripts are reduced approximately by half in both the patient and his mother relative to control.
Discussion
The functions of chemokines are well documented in the immune system. However, the potential role of CNS-expressed chemokines and chemokine-like proteins as neuromodulators is still largely unknown. In this study, we identified a chemokine-like sam gene family, showing CNS-specific expression. We provide empirical evidence for the involvement of a chemokine-like protein, SAM2, in vertebrate brain function and behavior associated with the regulation of anxiety and fear responses.
The targeted knockout of a single gene, sam2, in zebrafish produced an anxiogenic phenotype reminiscent of changes induced by Hb ablation. For example, ablation of the dHb in zebrafish has been shown to increase freezing and other behavioral signs of anxiety (25, 49–51). The anxiety-like and fear-related behavioral changes we detected in our zebrafish null mutants were also observed in our mouse KO model, highlighting the evolutionarily conserved role of Sam2 in the regulation of anxiety and fear in vertebrates.
Our in situ hybridization results showed the presence of sam2 transcripts in the telencephalic areas (Dm, Vd) and hypothalamic regions of the zebrafish brain. The Dm and Vd are known as the homologous regions for the mammalian amygdala and striatum, respectively. The involvement of these regions in anxiety has been demonstrated by numerous behavioral and molecular studies using both mammalian species and zebrafish (25, 52). In addition to traditional measures of anxiety and fear—such as freezing, thigmotaxis, or erratic movement—we also found evidence of increased anxiety in our zebrafish null mutants by measuring their shoaling responses. Shoaling is an adaptive and natural antipredatory behavior utilized in the analysis and modeling of vertebrate anxiety and fear (1). Furthermore, sam2 KO fish, when exposed to the alarm substance, exhibited significantly increased shoaling (reduction of interindividual distance) compared with wild-type. Also notably, novelty-induced increase of shoaling remained persistently elevated in the null mutants, whereas it showed the expected normal habituation (time-dependent reduction) in wild-type control fish (Fig. 4). Consistent with these findings, our Sam2 KO mice also exhibited increased anxiety-like responses, for example, on the elevated plus maze (Fig. 5C).
In addition to the elevated anxiety-like and fear responses in our null mutant zebrafish, we found an increase in crhb mRNA expression in the hypothalamus. CRH neurons are under constant GABAergic suppression in vivo (53), but importantly, our experimental manipulation (i.e., bath application of SAM2) increased sIPSC frequency. These data indicate that SAM2 plays a role in tonic GABAergic suppression of CRH excitability, which may, at least in part, be responsible for the elevated anxiety-like responses observed in both KO models. It is also worth considering that the downstream effects of CRH and the resulting release of glucocorticoids may also contribute to the anxiety phenotype (14, 54).
Identification of the specific neural circuits mediating the effects of SAM2 first requires the determination of neuronal cell types or neurotransmitter systems involved. In case of glucocorticoid receptors, their actions on fear and anxiety behaviors have recently been shown to be mediated via glutamatergic but not GABAergic neural circuits in the mouse forebrain (45). We performed in situ hybridization analysis with GABAergic, glutamatergic, and sam2 markers in the adult fish brain to determine colocalization. sam2 expression within the Vd, Vc, Hc, and Hd was found to be predominantly confined to GAD65/67+ GABAergic neurons, whereas expression in the Dm revealed colocalization with vglut2b+ glutamatergic neurons. Whether sam2-expressing neurons employ GABA or glutamate as a second transmitter remains to be determined (55). Also, whether sam2 regulates the neuronal activity of GABAergic or glutamatergic neurons by neuromodulatory action will need to be ascertained in the future. Nevertheless, the expression and colocalization patterns reported in our current study open the possibility of a neuromodulatory role of sam2 in both excitatory and inhibitory neurotransmitter systems comprising anxiety circuitry.
The specific molecular mechanisms underlying increased anxiety-like behavioral responses observed in sam2 KO fish will require further characterization. Nevertheless, the behavioral changes we found appeared to be unrelated to the serotonergic neurotransmitter system, as the expression of indicative markers, such as tphR and slc6a4a, were not affected by the loss of sam2 (SI Appendix, Fig. S6 P–Q′) (29, 56). However, after the novel tank assays, sam2 KO fish did show higher level of c-fos expression in the entire brain compared with wild-type (SI Appendix, Fig. S6 S and S′), confirming their abnormal response to novelty but also implying potential involvement of neurotransmitters other than the glutamatergic and GABAergic systems.
Finally, comparative genomic mapping with microdeletions and microduplications in human patients with intellectual disability, autism, and behavioral tantrums has identified SAM2 as a candidate gene for these phenotypes in individuals with 12q14.1 CNVs (Fig. 7). Although the latter results do not prove the role of SAM2 in these diseases, these results, together with our findings with zebrafish and mice, present an interesting possibility: evolutionarily distant species, such as fish, rodents, and humans, may possess similar mechanisms underlying anxiety and emotion, and these mechanisms may include sam2. We thus propose that the discovery of the samdori gene family and the functional information of one of its member sam2, may have important translational relevance and therapeutic potential.
Materials and Methods
Full methods and any associated references are available in SI Appendix, SI Materials and Methods.
Animals.
All experimental protocols and procedures were approved and conducted according to the approved guidelines and regulations of the Animal Ethics Committee of Chungnam National University (CNU-00866).
Isolation of samdori Gene Family.
We established a unique insertional mutagenesis system based on the Sleeping Beauty transposon, called the golden fish project (SI Appendix, Fig. S1). In addition to the GFP gene, we used a melanin-concentrating hormone gene as a transgene reporter for visual screening of mutants. During the course of mutagenesis, we mapped the insertion site in a chemokine gene, named samdori (sam). The full-length cDNAs of zebrafish sam-1a, 1b, 2, 3a, 3b, 4, 5a, and 5b genes were isolated using the RACE technique. The sam2 KO zebrafish lines (sam2cnu1 and sam2cnu2) and Sam2 KO mice were generated by the targeted mutagenesis utilizing ZFNs (26, 27) and transcription activator-like endonucleases (57), respectively.
Behavior Tests in Zebrafish and Mice.
Male zebrafish siblings of identical age (3-mo-old) and size were used for the following behavioral tests: open novel tank (35, 36, 41), scototaxis (37), and shoaling behavior (1, 42). The open-field test, elevated plus maze, and fear conditioning were employed in mice. All values represent mean ± SEM.
Human Genetics Data.
Patients GC42855 and GC48823 were identified among a cohort of ∼32,700 patients undergoing clinical, oligonucleotide-based array comparative genomic hybridization (aCGH) (58, 59) at Signature Genomic Laboratories. The DECIPHER database is a publicly accessible web-based resource of human genome variants with associated phenotypes, which facilitates the identification and interpretation of pathogenic genetic variation in thousands of patients with rare disorders worldwide (47). The description of case 290951 was ascertained from the DECIPHER database. We tested the deletion in 290951 family members; II-2 (affected, has deletion), III-2 (mother, affected, has deletion), III-3 (uncle, unaffected, does not have deletion), IV-1 (elder sister, unaffected, no deletion), IV-2 (elder brother, unaffected, no deletion), IV-3 (the proband, affected, has the deletion). This study was approved by the Institutional Review Board of Augusta University, Augusta, Georgia (HAC 0904264) for collection of deidentified phenotypic and genotypic data of human subjects or, with informed consent, for additional studies completed on blood or DNA. Informed consent, blood, and cells, were obtained from the family of 290951, while deidentified data were used for the rest of the analyses.
Supplementary Material
Acknowledgments
We thank Ted Inpyo Hong, Soojin Ryu, and Cecilia Hillard for proofreading and critical review of the manuscript. This study makes use of data generated by the DECIPHER community. A full list of centers who contributed to the generation of the data is available from https://decipher.sanger.ac.uk and via email from decipher@sanger.ac.uk. This work was supported by Ministry of Trade, Industry and Energy, Korea Grant 10063396; the National Research Foundation of Korea Grants 2017R1A2B2010696 and 2017M3A9A5051180; Institute for Basic Science Grants IBS-R001-D1 and IBS-R021; and a Natural Sciences and Engineering Research Council grant (to R.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. A.S. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1707663115/-/DCSupplemental.
References
- 1.Gerlai R. Zebrafish antipredatory responses: A future for translational research? Behav Brain Res. 2010;207:223–231. doi: 10.1016/j.bbr.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patterson B, Van Ameringen M. Augmentationt strategies for treatment-resistant anxiety disorders: A systematic review and meta-analysis. Depress Anxiety. 2016;33:728–736. doi: 10.1002/da.22525. [DOI] [PubMed] [Google Scholar]
- 3.Okamoto H, Aizawa H. Fear and anxiety regulation by conserved affective circuits. Neuron. 2013;78:411–413. doi: 10.1016/j.neuron.2013.04.031. [DOI] [PubMed] [Google Scholar]
- 4.Deverman BE, Patterson PH. Cytokines and CNS development. Neuron. 2009;64:61–78. doi: 10.1016/j.neuron.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Rostène W, Kitabgi P, Parsadaniantz SM. Chemokines: A new class of neuromodulator? Nat Rev Neurosci. 2007;8:895–903. doi: 10.1038/nrn2255. [DOI] [PubMed] [Google Scholar]
- 6.Réaux-Le Goazigo A, Van Steenwinckel J, Rostène W, Mélik Parsadaniantz S. Current status of chemokines in the adult CNS. Prog Neurobiol. 2013;104:67–92. doi: 10.1016/j.pneurobio.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Hikosaka O. The habenula: From stress evasion to value-based decision-making. Nat Rev Neurosci. 2010;11:503–513. doi: 10.1038/nrn2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amo R, et al. Identification of the zebrafish ventral habenula as a homolog of the mammalian lateral habenula. J Neurosci. 2010;30:1566–1574. doi: 10.1523/JNEUROSCI.3690-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalueff AV, Stewart AM, Gerlai R. Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol Sci. 2014;35:63–75. doi: 10.1016/j.tips.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turner KJ, et al. Afferent connectivity of the zebrafish habenulae. Front Neural Circuits. 2016;10:30. doi: 10.3389/fncir.2016.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang BB, Yao YY, Zhang HF, Kawakami K, Du JL. Left habenula mediates light-preference behavior in zebrafish via an asymmetrical visual pathway. Neuron. 2017;93:914–928.e4. doi: 10.1016/j.neuron.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Kim C-H, et al. Repressor activity of Headless/Tcf3 is essential for vertebrate head formation. Nature. 2000;407:913–916. doi: 10.1038/35038097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Itoh M, et al. Mind bomb is a ubiquitin ligase that is essential for efficient activation of Notch signaling by Delta. Dev Cell. 2003;4:67–82. doi: 10.1016/s1534-5807(02)00409-4. [DOI] [PubMed] [Google Scholar]
- 14.Ziv L, et al. An affective disorder in zebrafish with mutation of the glucocorticoid receptor. Mol Psychiatry. 2013;18:681–691. doi: 10.1038/mp.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tom Tang Y, et al. TAFA: A novel secreted family with conserved cysteine residues and restricted expression in the brain. Genomics. 2004;83:727–734. doi: 10.1016/j.ygeno.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Higashijima S, Mandel G, Fetcho JR. Distribution of prospective glutamatergic, glycinergic, and GABAergic neurons in embryonic and larval zebrafish. J Comp Neurol. 2004;480:1–18. doi: 10.1002/cne.20278. [DOI] [PubMed] [Google Scholar]
- 17.Bae YK, et al. Anatomy of zebrafish cerebellum and screen for mutations affecting its development. Dev Biol. 2009;330:406–426. doi: 10.1016/j.ydbio.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 18.Gamse JT, et al. Directional asymmetry of the zebrafish epithalamus guides dorsoventral innervation of the midbrain target. Development. 2005;132:4869–4881. doi: 10.1242/dev.02046. [DOI] [PubMed] [Google Scholar]
- 19.deCarvalho TN, et al. Neurotransmitter map of the asymmetric dorsal habenular nuclei of zebrafish. Genesis. 2014;52:636–655. doi: 10.1002/dvg.22785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.deCarvalho TN, Akitake CM, Thisse C, Thisse B, Halpern ME. Aversive cues fail to activate fos expression in the asymmetric olfactory-habenula pathway of zebrafish. Front Neural Circuits. 2013;7:98. doi: 10.3389/fncir.2013.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryu S, Holzschuh J, Mahler J, Driever W. Genetic analysis of dopaminergic system development in zebrafish. J Neural Transm Suppl. 2006;70:61–66. doi: 10.1007/978-3-211-45295-0_11. [DOI] [PubMed] [Google Scholar]
- 22.Unger JL, Glasgow E. Expression of isotocin-neurophysin mRNA in developing zebrafish. Gene Expr Patterns. 2003;3:105–108. doi: 10.1016/s1567-133x(02)00064-9. [DOI] [PubMed] [Google Scholar]
- 23.Faraco JH, et al. Regulation of hypocretin (orexin) expression in embryonic zebrafish. J Biol Chem. 2006;281:29753–29761. doi: 10.1074/jbc.M605811200. [DOI] [PubMed] [Google Scholar]
- 24.Jesuthasan S. Fear, anxiety, and control in the zebrafish. Dev Neurobiol. 2012;72:395–403. doi: 10.1002/dneu.20873. [DOI] [PubMed] [Google Scholar]
- 25.Lau BYB, Mathur P, Gould GG, Guo S. Identification of a brain center whose activity discriminates a choice behavior in zebrafish. Proc Natl Acad Sci USA. 2011;108:2581–2586. doi: 10.1073/pnas.1018275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim J-S, Lee HJ, Carroll D. Genome editing with modularly assembled zinc-finger nucleases. Nat Methods. 2010;7:91, author reply 91–92. doi: 10.1038/nmeth0210-91b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foley JE, et al. Targeted mutagenesis in zebrafish using customized zinc-finger nucleases. Nat Protoc. 2009;4:1855–1867. doi: 10.1038/nprot.2009.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Filippi A, et al. Expression and function of nr4a2, lmx1b, and pitx3 in zebrafish dopaminergic and noradrenergic neuronal development. BMC Dev Biol. 2007;7:135. doi: 10.1186/1471-213X-7-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lillesaar C. The serotonergic system in fish. J Chem Neuroanat. 2011;41:294–308. doi: 10.1016/j.jchemneu.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 30.Doll CA, Burkart JT, Hope KD, Halpern ME, Gamse JT. Subnuclear development of the zebrafish habenular nuclei requires ER translocon function. Dev Biol. 2011;360:44–57. doi: 10.1016/j.ydbio.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berman JR, Skariah G, Maro GS, Mignot E, Mourrain P. Characterization of two melanin-concentrating hormone genes in zebrafish reveals evolutionary and physiological links with the mammalian MCH system. J Comp Neurol. 2009;517:695–710. doi: 10.1002/cne.22171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song Y, Golling G, Thacker TL, Cone RD. Agouti-related protein (AGRP) is conserved and regulated by metabolic state in the zebrafish, Danio rerio. Endocrine. 2003;22:257–265. doi: 10.1385/ENDO:22:3:257. [DOI] [PubMed] [Google Scholar]
- 33.Löhr H, Hammerschmidt M. Zebrafish in endocrine systems: Recent advances and implications for human disease. Annu Rev Physiol. 2011;73:183–211. doi: 10.1146/annurev-physiol-012110-142320. [DOI] [PubMed] [Google Scholar]
- 34.Beretta CA, Dross N, Guiterrez-Triana JA, Ryu S, Carl M. Habenula circuit development: Past, present, and future. Front Neurosci. 2012;6:51. doi: 10.3389/fnins.2012.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cachat J, et al. Measuring behavioral and endocrine responses to novelty stress in adult zebrafish. Nat Protoc. 2010;5:1786–1799. doi: 10.1038/nprot.2010.140. [DOI] [PubMed] [Google Scholar]
- 36.Stewart A, et al. Modeling anxiety using adult zebrafish: A conceptual review. Neuropharmacology. 2012;62:135–143. doi: 10.1016/j.neuropharm.2011.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maximino C, Marques de Brito T, Dias CA, Gouveia A, Jr, Morato S. Scototaxis as anxiety-like behavior in fish. Nat Protoc. 2010;5:209–216. doi: 10.1038/nprot.2009.225. [DOI] [PubMed] [Google Scholar]
- 38.Speedie N, Gerlai R. Alarm substance induced behavioral responses in zebrafish (Danio rerio) Behav Brain Res. 2008;188:168–177. doi: 10.1016/j.bbr.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Egan RJ, et al. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav Brain Res. 2009;205:38–44. doi: 10.1016/j.bbr.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Partridge BL, Ptcher T, Cullen JM, Wilson J. The three-dimensional structure fo fish schools. Behav Ecol Sociobiol. 1980;6:277–288. [Google Scholar]
- 41.Wong K, et al. Analyzing habituation responses to novelty in zebrafish (Danio rerio) Behav Brain Res. 2010;208:450–457. doi: 10.1016/j.bbr.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 42.Mahabir S, Chatterjee D, Buske C, Gerlai R. Maturation of shoaling in two zebrafish strains: A behavioral and neurochemical analysis. Behav Brain Res. 2013;247:1–8. doi: 10.1016/j.bbr.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Major P, Dill L. The three-dimensional structure of airborne bird flocks. Behav Ecol Sociobiol. 1978;4:111–122. [Google Scholar]
- 44.Stewart AM, Braubach O, Spitsbergen J, Gerlai R, Kalueff AV. Zebrafish models for translational neuroscience research: From tank to bedside. Trends Neurosci. 2014;37:264–278. doi: 10.1016/j.tins.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hartmann J, et al. Forebrain glutamatergic, but not GABAergic, neurons mediate anxiogenic effects of the glucocorticoid receptor. Mol Psychiatry. 2017;22:466–475. doi: 10.1038/mp.2016.87. [DOI] [PubMed] [Google Scholar]
- 46.Zhang R, et al. Loss of hypothalamic corticotropin-releasing hormone markedly reduces anxiety behaviors in mice. Mol Psychiatry. 2017;22:733–744. doi: 10.1038/mp.2016.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Firth HV, et al. DECIPHER: Database of chromosomal imbalance and phenotype in humans using Ensembl resources. Am J Hum Genet. 2009;84:524–533. doi: 10.1016/j.ajhg.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zou Q, et al. USP15 stabilizes MDM2 to mediate cancer-cell survival and inhibit antitumor T cell responses. Nat Immunol. 2014;15:562–570. doi: 10.1038/ni.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Agetsuma M, et al. The habenula is crucial for experience-dependent modification of fear responses in zebrafish. Nat Neurosci. 2010;13:1354–1356. doi: 10.1038/nn.2654. [DOI] [PubMed] [Google Scholar]
- 50.Lee A, et al. The habenula prevents helpless behavior in larval zebrafish. Curr Biol. 2010;20:2211–2216. doi: 10.1016/j.cub.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 51.Okamoto H, Agetsuma M, Aizawa H. Genetic dissection of the zebrafish habenula, a possible switching board for selection of behavioral strategy to cope with fear and anxiety. Dev Neurobiol. 2012;72:386–394. doi: 10.1002/dneu.20913. [DOI] [PubMed] [Google Scholar]
- 52.Ganz J, et al. Subdivisions of the adult zebrafish pallium based on molecular marker analysis. F1000Res. 2014;3:308. doi: 10.12688/f1000research.5595.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stratton MS, Searcy BT, Tobet SA. GABA regulates corticotropin releasing hormone levels in the paraventricular nucleus of the hypothalamus in newborn mice. Physiol Behav. 2011;104:327–333. doi: 10.1016/j.physbeh.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Griffiths BB, et al. A zebrafish model of glucocorticoid resistance shows serotonergic modulation of the stress response. Front Behav Neurosci. 2012;6:68. doi: 10.3389/fnbeh.2012.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Filippi A, Mueller T, Driever W. vglut2 and gad expression reveal distinct patterns of dual GABAergic versus glutamatergic cotransmitter phenotypes of dopaminergic and noradrenergic neurons in the zebrafish brain. J Comp Neurol. 2014;522:2019–2037. doi: 10.1002/cne.23524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lillesaar C, Stigloher C, Tannhäuser B, Wullimann MF, Bally-Cuif L. Axonal projections originating from raphe serotonergic neurons in the developing and adult zebrafish, Danio rerio, using transgenics to visualize raphe-specific pet1 expression. J Comp Neurol. 2009;512:158–182. doi: 10.1002/cne.21887. [DOI] [PubMed] [Google Scholar]
- 57.Sung YH, et al. Knockout mice created by TALEN-mediated gene targeting. Nat Biotechnol. 2013;31:23–24. doi: 10.1038/nbt.2477. [DOI] [PubMed] [Google Scholar]
- 58.Ballif BC, et al. Identification of a previously unrecognized microdeletion syndrome of 16q11.2q12.2. Clin Genet. 2008;74:469–475. doi: 10.1111/j.1399-0004.2008.01094.x. [DOI] [PubMed] [Google Scholar]
- 59.Duker AL, et al. Paternally inherited microdeletion at 15q11.2 confirms a significant role for the SNORD116 C/D box snoRNA cluster in Prader-Willi syndrome. Eur J Hum Genet. 2010;18:1196–1201. doi: 10.1038/ejhg.2010.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.