Significance
We report a disease-causing mutation in the β-cell–enriched MAFA transcription factor. Strikingly, the missense p.Ser64Phe MAFA mutation was associated with either of two distinct phenotypes, multiple insulin-producing neuroendocrine tumors of the pancreas—a condition known as insulinomatosis—or diabetes mellitus, recapitulating the physiological properties of MAFA both as an oncogene and as a key islet β-cell transcription factor. The implication of MAFA in these human phenotypes will provide insights into how this transcription factor regulates human β-cell activity as well as into the mechanisms of Maf-induced tumorigenesis.
Keywords: MAFA, insulinoma, insulinomatosis, diabetes, MODY
Abstract
The β-cell–enriched MAFA transcription factor plays a central role in regulating glucose-stimulated insulin secretion while also demonstrating oncogenic transformation potential in vitro. No disease-causing MAFA variants have been previously described. We investigated a large pedigree with autosomal dominant inheritance of diabetes mellitus or insulinomatosis, an adult-onset condition of recurrent hyperinsulinemic hypoglycemia caused by multiple insulin-secreting neuroendocrine tumors of the pancreas. Using exome sequencing, we identified a missense MAFA mutation (p.Ser64Phe, c.191C>T) segregating with both phenotypes of insulinomatosis and diabetes. This mutation was also found in a second unrelated family with the same clinical phenotype, while no germline or somatic MAFA mutations were identified in nine patients with sporadic insulinomatosis. In the two families, insulinomatosis presented more frequently in females (eight females/two males) and diabetes more often in males (12 males/four females). Four patients from the index family, including two homozygotes, had a history of congenital cataract and/or glaucoma. The p.Ser64Phe mutation was found to impair phosphorylation within the transactivation domain of MAFA and profoundly increased MAFA protein stability under both high and low glucose concentrations in β-cell lines. In addition, the transactivation potential of p.Ser64Phe MAFA in β-cell lines was enhanced compared with wild-type MAFA. In summary, the p.Ser64Phe missense MAFA mutation leads to familial insulinomatosis or diabetes by impacting MAFA protein stability and transactivation ability. The human phenotypes associated with the p.Ser64Phe MAFA missense mutation reflect both the oncogenic capacity of MAFA and its key role in islet β-cell activity.
The V-Maf avian musculoaponeurotic fibrosarcoma oncogene homolog A (MAFA) basic leucine zipper-containing protein is unique among the many distinct islet-enriched transcription factors, as it plays a pivotal role in the regulation of insulin secretion in vivo, while at the same time displaying oncogenic transformation potential in vitro (1–3). MAFA belongs to the family of large Maf transcription factors, also including MAFB, MAF, and NRL. MAFA and MAFB are both expressed in islet β-cells, but only MAFA is required for postnatal function of murine β-cells (4–6), acting as transactivator of insulin and several genes involved with glucose-stimulated insulin secretion (1, 7–9). The transformation potential of MAFA was shown by its ability to induce proliferation of quail neuroretina cells (2) and chicken embryo fibroblasts (3) when overexpressed in vitro. Notably, the MAF gene is up-regulated in 50% of human multiple myelomas and 60% of angioimmunoblastic T cell lymphomas (10, 11). In addition, recurrent translocations involving MAF, MAFB, and MAFA are identified in 5–10% of multiple myelomas (12–14), highlighting the significant role of these oncogenes in hematological malignancies.
In this study, we aimed to determine the genetic etiology of insulinomatosis, a condition characterized by the occurrence of multicentric insulinomas, pancreatic neuroendocrine tumors with β-cell–like features causing hyperinsulinemic hypoglycemia. Insulinomatosis usually occurs sporadically (15), although it had also been described to occur in a familial setting in one single kindred where hyperinsulinemic hypoglycemia was paradoxically associated with a strong family history of diabetes mellitus (16). Due to the multicentric nature of the disease, patients with insulinomatosis have a significantly higher chance of persistent or recurrent disease following conservative surgery compared with patients with a single sporadic insulinoma, and their management is often challenging (15). By sequencing the exomes of multiple affected individuals from a large autosomal dominant pedigree with insulinomatosis and diabetes, we identified a missense p.Ser64Phe (c.191C>T) mutation in the MAFA gene segregating with both phenotypes. Targeted sequencing in a second independent family with an identical clinical phenotype revealed the same MAFA mutation, while no pathogenic variants were found in a series of patients with insulinomatosis with sporadic clinical presentation. Functional analysis demonstrated that the p.Ser64Phe mutation not only significantly increased the stability of MAFA, whose levels were unaffected by variable glucose concentrations in β-cell lines, but also enhanced its transactivation activity.
Results
Exome and Targeted Sequencing Identify the p.Ser64Phe MAFA Mutation.
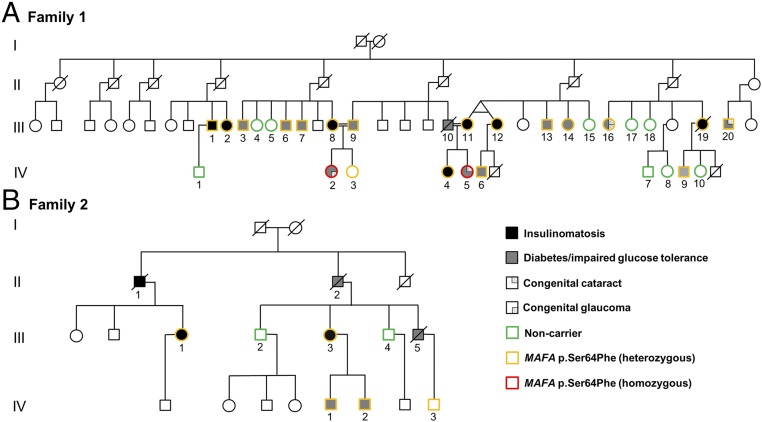
The study population consisted of an index family with autosomal dominant insulinomatosis and diabetes (family 1; Fig. 1A; 29 subjects, 17 females), a second family with the same phenotype, whose case was previously clinically described (16) (family 2; Fig. 1B; 7 subjects, 2 females), and nine cases of sporadic insulinomatosis (8 females). All subjects were of white Caucasian ethnic background.
Fig. 1.
Family trees of the two families (A and B) with insulinomatosis and diabetes mellitus. Different colors mark the MAFA genotypes. Unmarked subjects were not tested. A simplified version of the family tree was used for family 1 to improve readability.
Exome sequencing of subjects III/1, III/2, III/8, and IV/4 from family 1 identified 59, 85, 80, and 84 previously not reported heterozygous variants, respectively, annotated as missense, nonsense, frameshift, or splice site variants. Only one of these, MAFA p.Ser64Phe (c.191C>T; NM_201589.3), was shared by all four affected individuals (SI Appendix, Tables S1 and S2). This variant affects a highly conserved amino acid within the transactivation domain of MAFA, and has not been reported before (ExAC, GnomAD, ESP, dbSNP, and 1,000 Genomes databases). In silico prediction supported a pathogenic role (SI Appendix, Table S3). Testing for this MAFA missense variant in 25 additional members from family 1 identified 14 further heterozygous individuals (18 in total) and two homozygotes (IV/2 and IV/5). Nine unaffected family members did not inherit the variant (Fig. 1A). Seven of the 18 heterozygotes had insulinomatosis, 10 had diabetes, and one was clinically unaffected (IV/3, age 35). No DNA was available from patient III/10, who was an obligate carrier of the MAFA variant (Fig. 1A) and known to have impaired fasting glucose.
Targeted sequencing of MAFA in family 2 identified the same heterozygous p.Ser64Phe MAFA mutation in the proband (III/1, with insulinomatosis) and in four additional family members, one currently affected with insulinomatosis (III/3), one with diabetes (IV/1), and two who were not known to be affected (IV/2, age 47 and IV/3, age 41). The three deceased affected subjects in family 2—two with diabetes (II/2 and III/5) and one with insulinomatosis (II/1)—were obligate carriers (Fig. 1B). Disease penetrance for both phenotypes (insulinomatosis or diabetes) was 90%. Haplotype analysis within the two families suggested that the mutations had arisen on separate alleles (SI Appendix, Fig. S1), although a recombination event within a 364-kb region encompassing MAFA could not be excluded. DNA sequence analysis of the nine sporadic insulinomatosis cases did not detect germline or somatic MAFA pathogenic variants.
Individuals with the p.Ser64Phe MAFA Mutation Develop Either Hyperinsulinemic Hypoglycemia or Diabetes Mellitus.
In the two families we report, 10 subjects had hyperinsulinemic hypoglycemia secondary to insulinomatosis (SI Appendix, Table S4), while 15 patients were diagnosed with diabetes mellitus (SI Appendix, Table S5). Most subjects with hyperinsulinemic hypoglycemia were females (male-to-female patient ratio was 1:4), and the mean age at diagnosis was 39.4 ± 13.1 y. There was no history of early-onset hypoglycemia suggestive of congenital hyperinsulinism. In four of the six patients that underwent imaging investigations, multicentric pancreatic neuroendocrine tumors (ranging in size between 0.4 and 1.1 cm) were shown, while local or distant metastases were not observed. In 1 patient with hyperinsulinemic hypoglycemia from family 2 (III/1) who was diagnosed before cross-sectional imaging investigations became available, a 5-mm insulinoma was found in the resected sample following pancreatic surgery (16). Overall, 6 patients underwent surgery, with persistent or recurrent disease in all cases, and 4 patients underwent more than one operation. The subjects with persistent or recurrent disease, and those who did not undergo pancreatic surgery, were managed with medical treatment with generally poor results and recurrent symptomatic hypoglycemia.
Most patients diagnosed with diabetes or impaired fasting glucose were males (male-to-female ratio was 3:1), and the mean age at diagnosis was 38.4 ± 16.5 y. The mean body mass index (BMI) of patients with diabetes with available data was 25 ± 3 kg/m2. There were no other clinical features of insulin resistance, no history of diabetic ketoacidosis, and islet autoantibodies were negative, configuring a phenotype resembling maturity-onset diabetes of the young (MODY) (17). Diabetes was managed with diet or oral medications (i.e., metformin and/or sulphonylureas) in most cases, with current HbA1c levels ranging between 37 and 74 mmol/mol (5.5–8.9%). There was no history of clinically significant micro- or macrovascular complications. Among the subjects with diabetes, two homozygous patients from family 1 born to consanguineous parents presented with congenital glaucoma (IV/2) and congenital cataract (IV/5), while two heterozygous subjects (III/16 and III/20) from the same family had congenital cataract associated, in one of these (III/16), with congenital glaucoma. There was no history of congenital eye disorders in family 2.
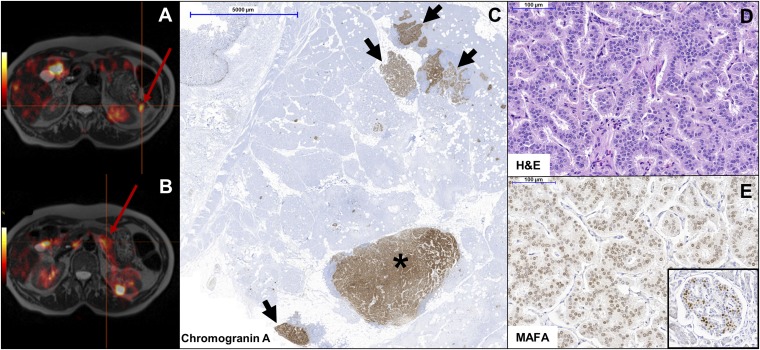
Insulinomatosis and diabetes seemed to be mutually exclusive diagnoses in most patients. However, one subject from family 2 (III/3) might have developed the two phenotypes in a sequential manner. This subject was diagnosed with gestational diabetes at the age of 27. After giving birth, she had impaired glucose tolerance and was treated with sulphonylureas between ages 33 and 35. An oral glucose tolerance test while off treatment at the age of 39 y was reported normal. This patient started to show symptoms of hypoglycemia at the age of 55, and was later diagnosed with hyperinsulinemic hypoglycemia and multiple pancreatic neuroendocrine tumors on 18F-DOPA PET imaging (Fig. 2 A and B).
Fig. 2.
Features of MAFA mutation-positive insulinomatosis. (A and B) 18F-DOPA PET in a patient with MAFA mutation-positive insulinomatosis (family 2, subject III/3) showing two pancreatic neuroendocrine tumors (red arrow) (A, tail; B, body of the pancreas). (C) Chromogranin A immunohistochemistry in subject III/19 (family 1) shows a macrotumor (>5 mm) (asterisk) and multiple small (microadenomas, <5 mm) neuroendocrine tumors (black arrows). (D) H&E staining showing the trabecular pattern of MAFA mutation-positive insulinomas. (E) Immunostaining shows diffuse MAFA expression in the tumor, at lower levels compared with the neighboring normal islets strongly expressing MAFA (Inset).
The three unaffected heterozygotes had normal HbA1c and fasting glucose levels in the absence of clinical symptoms of hypoglycemia. Subject IV/2 (family 2) was prospectively diagnosed with impaired glucose tolerance following an oral glucose tolerance test (oGTT) (SI Appendix, Fig. S2). The insulinogenic index calculated for this patient on the basis of baseline and 30-min glucose and insulin levels was 37.6 pmol/mmol, with a normal homeostatic model assessment of insulin resistance (HOMA-IR) of 1.7, in keeping with impaired insulin secretion. An oGTT in one of the unaffected heterozygotes from family 1 (IV/3) showed normal glucose tolerance, with 120-min glucose levels of 5.4 mmol/L.
Hyperinsulinemic Hypoglycemia in Patients with the p.Ser64Phe MAFA Mutation is Due to Multiple Insulinomas.
In the subjects with hyperinsulinemic hypoglycemia that underwent surgery, histopathology showed the presence of small (microadenomas, <5 mm) and larger (macrotumors, >5 mm) multifocal well-differentiated neuroendocrine tumors (Ki-67 <2%) with a trabecular tissue architecture (Fig. 2 C and D). The number of lesions was variable, depending on the type of surgery and extension of sampling. Over 100 lesions were identified in a patient from family 2 (III/1) whose surgical specimen was fully sampled (15). Islets with β-cell hyperplasia transforming into microadenomas were not observed. None of the tumors exceeded 2 cm in size. All tumors expressed insulin, while immunostaining for the other pancreatic hormones was negative. MAFA immunostaining revealed a diffuse positivity in one case from family 1 (III/19), which was less intense compared with the neighboring normal islets (Fig. 2E), and a patchy positivity in the index case from family 2 (III/1). Notwithstanding the limitations due to the small number of tissue samples available for further analysis, the MAFA staining intensity did not appear to be different in MAFA mutation-positive tumor cells compared with MAFA mutation-negative sporadic insulinomatosis or sporadic insulinomas (SI Appendix, Table S6).
The p.Ser64Phe MAFA Mutation Affects MAFA Protein Stability and Transactivation Activity.
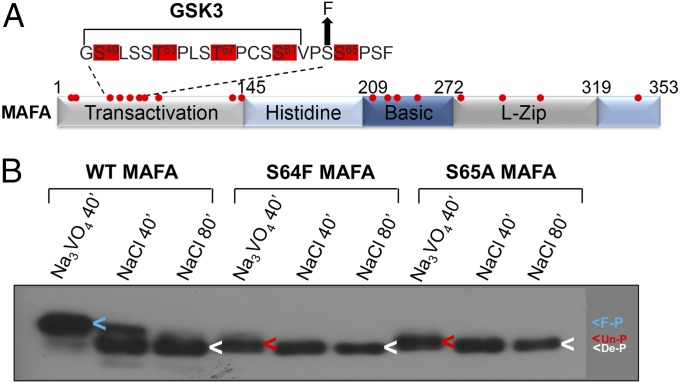
Ser64 is found within the N-terminal transactivation domain of MAFA (Fig. 3A). The neighboring Ser65 residue was previously shown to act as a priming phosphorylation site, as phosphorylation at Ser65 enables glycogen synthase kinase 3 (GSK3) to phosphorylate Ser61, Thr57, Thr53, and Ser49 in a sequential manner (Fig. 3A) (18, 19). We found the mobility of the p.Ser64Phe mutant protein to be indistinguishable from the kinase priming defective mutant, p.Ser65Ala (Fig. 3B), suggesting that the substitution of serine with a phenylalanine at residue 64 prevents phosphorylation at the priming Ser65 site, and the subsequent GSK3-mediated phosphorylation within the transactivation domain of MAFA. However, both p.Ser65Ala and p.Ser64Phe MAFA were still heavily phosphorylated proteins at the many other phosphorylation sites (Fig. 3A), as shown by the ability of an endogenous phosphatase(s) to alter protein mobility when incubated in the presence of NaCl, but not the phosphatase inhibitor, sodium orthovanadate (Na3VO4) (Fig. 3B) (20).
Fig. 3.
The mobility of p.Ser64Phe (S64F) MAFA is indistinguishable from the p.Ser65Ala (S65A) kinase mutant. (A) Schematic of MAFA showing sites of phosphorylation (red dots) within the transactivation, DNA-binding (basic), and dimerization region (leucine zipper, L-zip). (B) Wild type (WT) and mutant MAFA transfected HeLa nuclear extracts were incubated at 37 °C for 40 or 80 min (40′ or 80′) in the presence of the phosphatase inhibitor, sodium orthovanadate (Na3VO4, 10 mM), or NaCl (10 mM). The arrowheads denote the location of fully phosphorylated MAFA (F-P, blue), the form lacking Ser65 and GSK3-mediated phosphorylation (Un-P, red), and the completely dephosphorylated protein produced by incubating in the presence of NaCl (De-P, white).
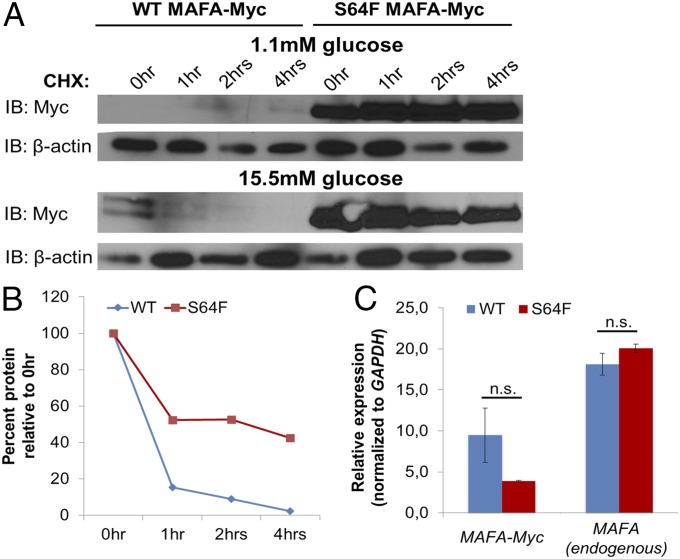
The p.Ser64Phe mutation was found to have a profound effect on MAFA turnover. The stability of the mutant protein was dramatically increased in both the human EndoC-βH1 β-cell line (Fig. 4) and MIN6 cells (SI Appendix, Fig. S3) in the presence of cycloheximide, a protein synthesis inhibitor. Normally, MAFA is highly unstable in β-cells at low, nonstimulating glucose concentrations, while its stability is enhanced in the presence of high glucose concentrations (19). However, the p.Ser64Phe mutant was stable and abundant regardless of glucose levels (Fig. 4 A and B and SI Appendix, Fig. S3). No significant difference was observed between transfected wild-type (WT) and mutant MAFA mRNA levels (Fig. 4C), confirming the posttranscriptional nature of the effect observed on protein turnover.
Fig. 4.
The p.Ser64Phe (S64F) mutation greatly stabilizes MAFA in human EndoC-βH1 cells grown in 1.1 or 15.5 mM glucose. (A) EndoC-βH1 cells were transfected with wild type (WT) and p.Ser64Phe (S64F) MAFA-Myc and, after 48 h, incubated with medium containing 1.1 mM or 15.5 mM glucose for an additional 12 h. The transfected cells were then incubated with 25 μg/mL cycloheximide (CHX) for the indicated time. Transfected MAFA-Myc and endogenous β-actin protein levels were determined by immunoblotting (IB) using anti-Myc and anti–β-actin antibodies, respectively. (B) The Myc protein band intensity was measured in the 15.5 mM glucose sample, normalized to β-actin, and plotted as a percentage of the initial band intensity. (C) No significant difference was found between WT and p.Ser64Phe (S64F) MAFA mRNA levels in transfected cells grown in 1.1 mM glucose. Endogenous MAFA mRNA levels also did not change under these conditions. Student’s two-tailed t test. n.s., not significant. n = 3. Error bars represent SEM.
We next tested whether the p.Ser64Phe mutation affected stimulation of an insulin enhancer/promoter-driven reporter. There appeared to be no difference in the transactivation capacity in HeLa cells, as no predictable change in the specific activity pattern was observed between constructs (SI Appendix, Fig. S4). Notably, there was a nonlinear relationship in WT or mutant construct activity in relation to increasing amounts of protein, presumably due to the inability to properly phosphorylate the protein at its many phosphorylation sites (21) under these conditions. To focus on the impact of the p.Ser64Phe mutation on transactivation activity, chimeric proteins containing the N-terminal transactivation domain fused to the yeast Gal4 DNA-binding domain were produced. When analyzed in Gal4-binding site-driven reporter assays, the Gal4-Ser64Phe MAFA chimera was found to be more active than the WT chimera in INS-1 β-cells compared with HeLa cells (SI Appendix, Fig. S5A). Importantly, the chimeric WT and p.Ser64Phe mutant proteins were expressed at equal levels (SI Appendix, Fig. S5B), as they both lack the lysine residues targeted for ubiquitination in the C-terminal DNA-binding/dimerization region (20). Collectively, these results suggest that the activity of p.Ser64Phe MAFA would be enhanced due to both increased transactivation capacity and increased protein stability.
Discussion
We report a disease-causing mutation in the β-cell–enriched MAFA transcription factor. A p.Ser64Phe MAFA missense mutation was identified in 25 individuals from two unrelated families who were affected with either insulinomatosis or noninsulin-dependent diabetes resembling MODY. Our results are in keeping with previous evidence highlighting the role of MAFA in glucose-stimulated insulin secretion, and at the same time suggest that the p.Ser64Phe missense mutation can allow the oncogenic potential of MAFA—previously described in different cell contexts —to be manifested in the β-cell.
MAFA regulates the expression of insulin and several genes involved in glucose-stimulated insulin secretion (1, 7–9), and serves as a glucose “barometer,” since its stability and activity in β-cells are increased under high glucose-stimulating conditions and repressed in the presence of low glucose (19). The p.Ser64Phe mutation affects a highly conserved residue within the N-terminal transactivation domain of MAFA, neighboring the priming kinase Ser65 phosphorylation site. Significantly, no missense variants have been reported in publicly available databases at any of the N-terminal residues in MAFA subjected to sequential phosphorylation (Ser49, Thr53, Thr57, Ser61, or Ser65) or at immediately neighboring residues, including Ser64. The identical mobility of the p.Ser64Phe and p.Ser65Ala mutants strongly suggests that the p.Ser64Phe mutation impairs phosphorylation at Ser65, and the consequent GSK3-mediated phosphorylation within the transactivation domain of MAFA. These phosphorylation events in the N-terminal transactivation domain of MAFA induce both transactivation capacity (22) and protein degradation (18–20), the latter resulting from ubiquitination in the C-terminal domain. Consistently with the impaired phosphorylation within the transactivation domain, the p.Ser64Phe MAFA protein was strikingly more stable compared with WT MAFA, and its turnover was unaffected by different glucose concentrations in β-cell lines. Moreover, the activity of the Gal4-Ser64Phe chimeric protein was found to be greater than Gal4-WT MAFA in INS-1 β-cells compared with non-β HeLa cells. Previous studies have shown that the transactivation activity of the Gal4-Ser65Ala protein was reduced in non-β cells (20), while the activity of chimeric proteins lacking the priming phosphorylation and the GSK3 phosphorylation sites was found to be enhanced in an insulinoma cell line (19). This suggests that phosphorylation within the transactivation domain may affect MAFA function in a cell context-dependent way, likely through interactions with other β-cell–specific transcription factors and/or coregulators. Together, our results suggest that the p.Ser64Phe mutation increases the activity of endogenous MAFA in β-cells by impacting both protein stability and transactivation potential.
The family of Maf transcription factors derives its name from v-maf, which is transduced as a viral oncogene capable of inducing muscoloaponeurotic fibrosarcoma in chickens (23, 24). MAF, MAFB, and MAFA all display oncogenic activity (25), with MAFA having the greatest transformation potential in vitro (3). Notably, only high copy number Maf expressing transgenic mice develop T cell lymphomas (10), and the translocations occurring in human multiple myelomas (12, 13) determine the ectopic overexpression of large Maf proteins, suggesting that cell transformation is dependent on the overexpression of these transcription factors. Both the higher protein levels and the increased activity of the p.Ser64Phe mutant are predicted to induce the expression of genes involved with cell cycle regulation, including CCND2, a known target of MAFA (6) and key regulator of β-cell proliferation (26), presumably causing β-cell transformation and occurrence of insulinomatosis. Our data also suggest that the p.Ser64Phe mutation alters the tight regulation of MAFA stability in response to changes in glucose concentration. The lack of up-regulation of MAFA in response to hyperglycemia is expected to impair glucose-stimulated insulin secretion, consistent with the results of the oGTT in one of the prospectively identified mutation carriers, and this mechanism presumably underlies the diabetes phenotype.
The mechanisms explaining how the same gene mutation can lead to diabetes or insulinomatosis remains to be fully elucidated, and in vivo models will have to be developed to further investigate the effects of the p.Ser64Phe mutation. A similarly paradoxical phenotype has been described for mutations in the transcription factor HNF4A (27–29) and the potassium channel gene ABCC8 (30), where diabetes can be preceded, in some patients, by transient congenital hyperinsulinism. Insulinomatosis is, however, a very different disease, as it only manifests in adults, and is a neoplastic condition defined by the occurrence of multicentric insulin-producing neuroendocrine tumors, as opposed to congenital hyperinsulinism, characterized by islet cell hypertrophy in the absence of neoplastic changes (31). Although we cannot exclude the possibility that patients with insulinomatosis had diabetes before developing symptoms of hyperinsulinemic hypoglycemia, in most cases the two phenotypes seemed mutually exclusive, and interindividual factors might determine the development of either insulinomatosis or diabetes. Interestingly, in our two families, patients with insulinomatosis were mostly females and those with diabetes were more frequently males. The reasons for this gender difference are not known, although sporadic insulinomas also occur more frequently in females, with a male-to-female ratio of 1:1.4 (32). Treatment with estrogens has been shown to promote proliferation (33) and increase insulin release in human β-cells and human insulinomas in vitro (34, 35). Moreover, the expansion of β-cell mass observed during pregnancy is thought to be induced by prolactin and placental lactogen signaling, mediated by the prolactin receptor (PRLR) (36–38). Notably, Prlr was significantly down-regulated in Mafa knockout islets and in MIN6 β-cells following siRNA-mediated knockdown of Mafa (39), and, in the same study, the Prlr promoter was shown to be directly activated by MAFA in luciferase reporter assays. Estrogens and prolactin could potentially promote β-cell proliferation, predisposing female carriers of the p.Ser64Phe MAFA mutation to develop insulinomatosis—remarkably all insulinomatosis female patients manifested symptoms of the disease after puberty and most of them displayed the first hypoglycemic symptoms either during (16) or after pregnancy—although we cannot exclude the possibility that additional factors might influence the development of either phenotype.
Four subjects, including the only two homozygotes, presented with congenital cataract and/or glaucoma. MAFA is expressed in the developing lens (40), and mutations in the MAF gene have been previously linked with congenital cataract and disorders of the anterior segment (41), supporting a role for the p.Ser64Phe MAFA mutation in the pathogenesis of the ocular phenotype. Moreover, no MAFA mutations, either at the germline or somatic level, were detected in patients with insulinomatosis with sporadic clinical presentation, implying that MAFA-independent mechanisms are involved in the pathogenesis of sporadic insulinomatosis. Similarly, no MAFA pathogenic variants were previously identified in a series of patients with genetically undetermined MODY (42), indicating that MAFA mutations are specifically linked to the association of diabetes and familial insulinomatosis.
In conclusion, we identified a MAFA missense mutation as the cause of a dual familial condition of diabetes mellitus or hyperinsulinemic hypoglycemia secondary to insulinomatosis. Our data show that the p.Ser64Phe mutation impairs phosphorylation in the transactivation domain of MAFA, leading to significantly enhanced protein stability and activity in β-cell lines. The implication of a MAFA mutation in human disease is expected to provide further insights on the role of this transcription factor in the β-cell.
Materials and Methods
Patient Samples.
We recruited two families with autosomal dominant insulinomatosis and diabetes mellitus (36 subjects, 19 females), and nine patients with sporadic insulinomatosis (eight females; clinical features are summarized in SI Appendix, Table S7). All patients and family members agreed to take part in our multicenter study approved by the National Research Ethics Service Committee East of England–Cambridge East by providing signed informed consent.
Genetic Analyses.
Genomic DNA was extracted from peripheral blood leukocytes, saliva, or formalin-fixed archival tissue using commercially available kits (further details are provided in SI Appendix). Exome sequencing was performed in four individuals affected with insulinomatosis from family 1 (III/1, III/2, III/8, and IV/4) using the SureSelect Human All Exon Kit (v5) (Agilent) with sequencing on an HiSeq2500 system (Illumina). Sequencing metrics for the four samples are reported in SI Appendix, Table S8. We assumed a rare autosomal dominant model of inheritance to filter heterozygous variants (not previously reported in the ExAC, ESP, dbSNP, and 1,000 Genomes databases) annotated as missense, nonsense, frameshift, or splice site variants. The effect of the identified MAFA missense variant was investigated in silico using SIFT (sift.jcvi.org/), PolyPhen-2 (genetics.bwh.harvard.edu/pph2/), and Align GVGD (agvgd.hci.utah.edu/) prediction tools. Sanger sequencing was used for validation and cosegregation studies in family 1, and for the sequencing of the whole coding sequence of MAFA in family 2 and in patients with sporadic insulinomatosis. Primer sequences are provided in SI Appendix, Table S9. Methods for haplotype analysis are reported in SI Appendix.
Pathological Assessment and MAFA Immunohistochemistry.
Immunohistochemistry on archival pancreatic tissue for neuroendocrine markers, Ki-67, and pancreatic hormones (insulin, gastrin, glucagon, and pancreatic polypeptide) was performed as previously described (43). MAFA expression was assessed using immunohistochemistry in two familial insulinomatosis samples, eight sporadic insulinomatosis, and six sporadic insulinoma controls and classified as negative, weak, moderate, strong, or patchy. All cases were reviewed by an experienced endocrine pathologist (G.K.). Further details are reported in SI Appendix.
Protein Mobility Analysis.
Details for plasmid preparation are reported in SI Appendix. Nuclear extracts of WT, p.Ser64Phe, and p.Ser65Ala MAFA-transfected HeLa cells were incubated at 37 °C for 40 or 80 min in the presence of sodium orthovanadate (Na3VO4, 10 mM) or NaCl (10 mM). The samples were analyzed by SDS polyacrylamide gel electrophoresis and immunoblotting with an anti-MAFA antibody (A300-611A, Bethyl Laboratories).
Luciferase Assays.
The rat insulin II enhancer/promoter driven −238 firefly luciferase plasmid (Promega) was transfected in HeLa cells along with pCMV4-MAFA and phRL-TK (Promega) using the Lipofectamine protocol (Life Technologies). Gal4-MAFA(1–167) was transfected in HeLa and INS-1 832/13 cells along with (Gal4)5E1bLuc and phRL-TK. Cellular extracts were collected 48 h posttransfection, and the Dual-Luciferase Reporter Assay (Promega) was performed according to the manufacturer’s directions. MAFA protein levels were normalized to endogenous β-actin by immunoblotting with anti-MAFA (A300-611A, Bethyl Laboratories) and anti–β-actin (4967S, Cell Signaling) antibodies.
Cycloheximide Chase Experiments.
WT and p.Ser64Phe MAFA-Myc were introduced into EndoC-βH1 cells (44) using the Amaxa Nucleofector 2 (program G-016, Lonza). The medium was changed 48 h following nucleofection to either 1.1 or 15.5 mM glucose for 12 h, and cycloheximide (Sigma) was then added at a concentration of 25 μg/mL for the time indicated. Nuclear extracts were prepared for immunoblotting and probed with anti-Myc (clone 9E10, Roche) and anti–β-actin (4967S, Cell Signaling) antibodies. RNA from EndoC-βH1 cells was collected 72 h postnucleofection using the TRIzol reagent (Life Technologies), and the iScript cDNA synthesis kit (Bio-Rad) was used for cDNA synthesis. The qPCR reactions were performed with MAFA-Myc, MAFA (endogenous), and GAPDH gene primers on a LightCycler 480 II (Roche) and analyzed by the ∆∆CT method. Cycloheximide chase experiments were also performed in MIN6 cells transfected with WT and p.Ser64Phe MAFA-Myc using the Lipofectamine protocol. Each experiment was repeated at least three times.
Statistical Analysis.
Parametric data are presented as mean ± SD or SEM in the figures. Normal distribution was assessed using the Shapiro–Wilk test. Experimental data (luciferase and qPCR experiments) were analyzed through the Student’s t test using the software Prism v5 (GraphPad Software). Cycloheximide chase experiments were analyzed using a one-phase decay equation, and the degradation rate constant (k) was compared between the mutant and the WT protein using the extra sum-of-squares F test. Significance was set for P values < 0.05.
Supplementary Material
Acknowledgments
We thank Prof. Andrew Hattersley (University of Exeter) for his assistance and advice in the study setup, Dr. Joachim Müller (Kantonsspital St. Gallen) for reviewing the imaging investigations of one of the patients, Prof. Carmen Georgescu (Iuliu Haţieganu University of Medicine and Pharmacy Cluj-Napoca) for providing sporadic insulinoma control samples, and Dr. Karin Jung (Zentrum für Labormedizin St. Gallen) for undertaking the biochemistry investigations for one of the patients. Grant support was provided by Diabetes UK, the UK National Institute of Health Research, and NIH Grants DK-090750 (to R.S.) and DK-109577 (to E.W.). D.I. is supported by a George Alberti Research Training Fellowship funded by Diabetes UK (16/0005395). S.E.F. is supported by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (105636/Z/14/Z). S.E. holds Wellcome Trust Senior Investigator Award 098395/Z/12/A.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1712262115/-/DCSupplemental.
References
- 1.Zhang C, et al. MafA is a key regulator of glucose-stimulated insulin secretion. Mol Cell Biol. 2005;25:4969–4976. doi: 10.1128/MCB.25.12.4969-4976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benkhelifa S, et al. mafA, a novel member of the maf proto-oncogene family, displays developmental regulation and mitogenic capacity in avian neuroretina cells. Oncogene. 1998;17:247–254. doi: 10.1038/sj.onc.1201898. [DOI] [PubMed] [Google Scholar]
- 3.Nishizawa M, Kataoka K, Vogt PK. MafA has strong cell transforming ability but is a weak transactivator. Oncogene. 2003;22:7882–7890. doi: 10.1038/sj.onc.1206526. [DOI] [PubMed] [Google Scholar]
- 4.Nishimura W, et al. A switch from MafB to MafA expression accompanies differentiation to pancreatic beta-cells. Dev Biol. 2006;293:526–539. doi: 10.1016/j.ydbio.2006.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Artner I, et al. MafA and MafB regulate genes critical to beta-cells in a unique temporal manner. Diabetes. 2010;59:2530–2539. doi: 10.2337/db10-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hang Y, et al. The MafA transcription factor becomes essential to islet β-cells soon after birth. Diabetes. 2014;63:1994–2005. doi: 10.2337/db13-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kataoka K, et al. MafA is a glucose-regulated and pancreatic beta-cell-specific transcriptional activator for the insulin gene. J Biol Chem. 2002;277:49903–49910. doi: 10.1074/jbc.M206796200. [DOI] [PubMed] [Google Scholar]
- 8.Matsuoka TA, et al. The MafA transcription factor appears to be responsible for tissue-specific expression of insulin. Proc Natl Acad Sci USA. 2004;101:2930–2933. doi: 10.1073/pnas.0306233101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao L, et al. The islet beta cell-enriched MafA activator is a key regulator of insulin gene transcription. J Biol Chem. 2005;280:11887–11894. doi: 10.1074/jbc.M409475200. [DOI] [PubMed] [Google Scholar]
- 10.Morito N, et al. Overexpression of c-Maf contributes to T-cell lymphoma in both mice and human. Cancer Res. 2006;66:812–819. doi: 10.1158/0008-5472.CAN-05-2154. [DOI] [PubMed] [Google Scholar]
- 11.Hurt EM, et al. Overexpression of c-maf is a frequent oncogenic event in multiple myeloma that promotes proliferation and pathological interactions with bone marrow stroma. Cancer Cell. 2004;5:191–199. doi: 10.1016/s1535-6108(04)00019-4. [DOI] [PubMed] [Google Scholar]
- 12.Chesi M, et al. Frequent dysregulation of the c-maf proto-oncogene at 16q23 by translocation to an Ig locus in multiple myeloma. Blood. 1998;91:4457–4463. [PubMed] [Google Scholar]
- 13.Hanamura I, et al. Ectopic expression of MAFB gene in human myeloma cells carrying (14;20)(q32;q11) chromosomal translocations. Jpn J Cancer Res. 2001;92:638–644. doi: 10.1111/j.1349-7006.2001.tb01142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanamura I, et al. Identification of three novel chromosomal translocation partners involving the immunoglobulin loci in newly diagnosed myeloma and human myeloma cell line. Blood. 2005;106:1552. [Google Scholar]
- 15.Anlauf M, et al. Insulinomatosis: A multicentric insulinoma disease that frequently causes early recurrent hyperinsulinemic hypoglycemia. Am J Surg Pathol. 2009;33:339–346. doi: 10.1097/PAS.0b013e3181874eca. [DOI] [PubMed] [Google Scholar]
- 16.Tragl KH, Mayr WR. Familial islet-cell adenomatosis. Lancet. 1977;2:426–428. doi: 10.1016/s0140-6736(77)90609-2. [DOI] [PubMed] [Google Scholar]
- 17.Ellard S, Bellanné-Chantelot C, Hattersley AT. European Molecular Genetics Quality Network (EMQN) MODY group Best practice guidelines for the molecular genetic diagnosis of maturity-onset diabetes of the young. Diabetologia. 2008;51:546–553. doi: 10.1007/s00125-008-0942-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rocques N, et al. GSK-3-mediated phosphorylation enhances Maf-transforming activity. Mol Cell. 2007;28:584–597. doi: 10.1016/j.molcel.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Han SI, Aramata S, Yasuda K, Kataoka K. MafA stability in pancreatic beta cells is regulated by glucose and is dependent on its constitutive phosphorylation at multiple sites by glycogen synthase kinase 3. Mol Cell Biol. 2007;27:6593–6605. doi: 10.1128/MCB.01573-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo S, et al. The stability and transactivation potential of the mammalian MafA transcription factor are regulated by serine 65 phosphorylation. J Biol Chem. 2009;284:759–765. doi: 10.1074/jbc.M806314200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo S, Vanderford NL, Stein R. Phosphorylation within the MafA N terminus regulates C-terminal dimerization and DNA binding. J Biol Chem. 2010;285:12655–12661. doi: 10.1074/jbc.M110.105759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benkhelifa S, et al. Phosphorylation of MafA is essential for its transcriptional and biological properties. Mol Cell Biol. 2001;21:4441–4452. doi: 10.1128/MCB.21.14.4441-4452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishizawa M, Kataoka K, Goto N, Fujiwara KT, Kawai S. v-maf, a viral oncogene that encodes a “leucine zipper” motif. Proc Natl Acad Sci USA. 1989;86:7711–7715. doi: 10.1073/pnas.86.20.7711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawai S, et al. Isolation of the avian transforming retrovirus, AS42, carrying the v-maf oncogene and initial characterization of its gene product. Virology. 1992;188:778–784. doi: 10.1016/0042-6822(92)90532-t. [DOI] [PubMed] [Google Scholar]
- 25.Eychène A, Rocques N, Pouponnot C. A new MAFia in cancer. Nat Rev Cancer. 2008;8:683–693. doi: 10.1038/nrc2460. [DOI] [PubMed] [Google Scholar]
- 26.Fatrai S, et al. Akt induces beta-cell proliferation by regulating cyclin D1, cyclin D2, and p21 levels and cyclin-dependent kinase-4 activity. Diabetes. 2006;55:318–325. doi: 10.2337/diabetes.55.02.06.db05-0757. [DOI] [PubMed] [Google Scholar]
- 27.Flanagan SE, et al. Diazoxide-responsive hyperinsulinemic hypoglycemia caused by HNF4A gene mutations. Eur J Endocrinol. 2010;162:987–992. doi: 10.1530/EJE-09-0861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kapoor RR, et al. Persistent hyperinsulinemic hypoglycemia and maturity-onset diabetes of the young due to heterozygous HNF4A mutations. Diabetes. 2008;57:1659–1663. doi: 10.2337/db07-1657. [DOI] [PubMed] [Google Scholar]
- 29.Pearson ER, et al. Macrosomia and hyperinsulinaemic hypoglycaemia in patients with heterozygous mutations in the HNF4A gene. PLoS Med. 2007;4:e118. doi: 10.1371/journal.pmed.0040118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huopio H, et al. A new subtype of autosomal dominant diabetes attributable to a mutation in the gene for sulfonylurea receptor 1. Lancet. 2003;361:301–307. doi: 10.1016/S0140-6736(03)12325-2. [DOI] [PubMed] [Google Scholar]
- 31.Suchi M, et al. Histopathology of congenital hyperinsulinism: Retrospective study with genotype correlations. Pediatr Dev Pathol. 2003;6:322–333. doi: 10.1007/s10024-002-0026-9. [DOI] [PubMed] [Google Scholar]
- 32.Service FJ, McMahon MM, O’Brien PC, Ballard DJ. Functioning insulinoma: Incidence, recurrence, and long-term survival of patients: A 60-year study. Mayo Clin Proc. 1991;66:711–719. doi: 10.1016/s0025-6196(12)62083-7. [DOI] [PubMed] [Google Scholar]
- 33.Yuchi Y, et al. Estrogen receptor alpha regulates beta-cell formation during pancreas development and following injury. Diabetes. 2015;64:3218–3228. doi: 10.2337/db14-1798. [DOI] [PubMed] [Google Scholar]
- 34.Al-Majed HT, et al. Effect of 17beta-estradiol on insulin secretion and cytosolic calcium in Min6 mouse insulinoma cells and human islets of Langerhans. Pancreas. 2005;30:307–313. doi: 10.1097/01.mpa.0000161886.17492.22. [DOI] [PubMed] [Google Scholar]
- 35.Alabraba EB, et al. Expression and functional consequences of oestrogen and progesterone receptors in human insulinomas. Endocr Relat Cancer. 2007;14:1081–1088. doi: 10.1677/ERC-07-0093. [DOI] [PubMed] [Google Scholar]
- 36.Vasavada RC, et al. Targeted expression of placental lactogen in the beta cells of transgenic mice results in beta cell proliferation, islet mass augmentation, and hypoglycemia. J Biol Chem. 2000;275:15399–15406. doi: 10.1074/jbc.275.20.15399. [DOI] [PubMed] [Google Scholar]
- 37.Freemark M, et al. Targeted deletion of the PRL receptor: Effects on islet development, insulin production, and glucose tolerance. Endocrinology. 2002;143:1378–1385. doi: 10.1210/endo.143.4.8722. [DOI] [PubMed] [Google Scholar]
- 38.Huang C, Snider F, Cross JC. Prolactin receptor is required for normal glucose homeostasis and modulation of beta-cell mass during pregnancy. Endocrinology. 2009;150:1618–1626. doi: 10.1210/en.2008-1003. [DOI] [PubMed] [Google Scholar]
- 39.Eto K, et al. MafA is required for postnatal proliferation of pancreatic β-cells. PLoS One. 2014;9:e104184. doi: 10.1371/journal.pone.0104184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takeuchi T, et al. Neither MafA/L-Maf nor MafB is essential for lens development in mice. Genes Cells. 2009;14:941–947. doi: 10.1111/j.1365-2443.2009.01321.x. [DOI] [PubMed] [Google Scholar]
- 41.Jamieson RV, et al. Domain disruption and mutation of the bZIP transcription factor, MAF, associated with cataract, ocular anterior segment dysgenesis and coloboma. Hum Mol Genet. 2002;11:33–42. doi: 10.1093/hmg/11.1.33. [DOI] [PubMed] [Google Scholar]
- 42.Garin I, et al. Spanish GEDIMO Group Mutations in MAFA and IAPP are not a common cause of monogenic diabetes. Diabet Med. 2009;26:746–748. doi: 10.1111/j.1464-5491.2009.02758.x. [DOI] [PubMed] [Google Scholar]
- 43.Anlauf M, et al. Microadenomatosis of the endocrine pancreas in patients with and without the multiple endocrine neoplasia type 1 syndrome. Am J Surg Pathol. 2006;30:560–574. doi: 10.1097/01.pas.0000194044.01104.25. [DOI] [PubMed] [Google Scholar]
- 44.Ravassard P, et al. A genetically engineered human pancreatic β cell line exhibiting glucose-inducible insulin secretion. J Clin Invest. 2011;121:3589–3597. doi: 10.1172/JCI58447. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.