Significance
Here, we identify a role for D2HG in the progression of colitis to colon cancer. In a mouse model of colitis-associated colon cancer (CAC), urine D2HG level during colitis correlates positively with severity of tumorigenesis. Elevated D2HG enhances CAC in mice and confers a hyperproliferative, nonapoptotic phenotype in cultured intestinal epithelial cells prone to neoplastic progression in the setting of chronic inflammation. Hif-1α is a key regulator of D2HGDH transcription, an enzyme that eliminates D2HG. Mucosal biopsies from ulcerative colitis (UC) patients who progressed to CAC exhibit decreased expression of D2HGDH compared with UC patients remaining dysplasia-free. These results reveal a critical function of D2HG in the progression of CAC and identify D2HG/D2HGDH as potential biomarkers and therapeutic targets for the treatment of CAC.
Keywords: inflammatory bowel disease, colitis-associated cancer, dysplasia, metabolites, Hif-1α
Abstract
d-2-hydroxyglutarate (D2HG) is produced in the tricarboxylic acid cycle and is quickly converted to α-ketoglutarate by d-2-hydroxyglutarate dehydrogenase (D2HGDH). In a mouse model of colitis-associated colon cancer (CAC), urine level of D2HG during colitis correlates positively with subsequent polyp counts and severity of dysplasia. The i.p. injection of D2HG results in delayed recovery from colitis and severe tumorigenesis. The colonic expression of D2HGDH is decreased in ulcerative colitis (UC) patients at baseline who progress to cancer. Hypoxia-inducible factor (Hif)-1α is a key regulator of D2HGDH transcription. Our study identifies urine D2HG and tissue D2HGDH expression as biomarkers to identify patients at risk for progressing from colitis to cancer. The D2HG/D2HGDH pathway provides potential therapeutic targets for the treatment of CAC.
It is widely recognized that chronic inflammation can promote neoplasia (1). Much of our understanding of the association between chronic inflammation and cancer is illustrated through inflammatory bowel diseases (IBD) and colon carcinogenesis. Patients with chronic IBD, the most common forms being ulcerative colitis (UC) and Crohn’s disease, have an increased risk of developing colorectal cancer (CRC) dependent upon the extent of disease at diagnosis, disease severity and duration, and efficacy of IBD management (2). However, the molecular mechanisms initiating and driving progression from colitis to cancer are not completely understood. Emerging evidence suggests a crucial role for altered expression of enzymes with functions in cellular metabolism in the pathogenesis of inflammation and cancer (3).
The two enantiomers of 2-hydroxglutarate (2HG), D2HG and L2HG, are normal endogenous metabolites found in all human body fluids. D2HG is produced by the enzyme hydroxyacid-oxoacid transhydrogenase (HOT) in the tricarboxylic acid cycle with γ-hydroxybutyric acid and α-ketoglutarate (α-KG) as substrates (4). Levels of D2HG are normally low since D2HG is readily converted back to α-KG by the mitochondrial enzyme d-2-hydroxyglutarate dehydrogenase (D2HGDH). D2HGDH is most active in colon, liver, kidney, and brain (4). Mouse and human intestines have very high levels of γ-hydroxybutyric acid, which could cause a high production of D2HG by HOT (5). Therefore, it is thought that high activity of D2HGDH in the intestine could have an important role in preventing an increase in D2HG concentration. Mutations in the gene encoding D2HGDH are present in d-2-hydroxyglutaric aciduria, a rare recessive neurometabolic disorder resulting in elevated D2HG levels. Accumulation of D2HG has also been linked to oncogenesis in glioma, acute myeloid leukemia, and cartilaginous neoplasms, including chondrosarcoma, in which gain-of-function mutations in isocitrate dehydrogenase (IDH)1 or IDH2 convert α-KG to D2HG (6, 7). Accumulation of D2HG has recently been noted in breast cancer (8–10), and IDH1 mutation occurs in a small proportion of intestinal adenocarcinomas associated with IBD or CpG island methylator phenotype (CIMP), BRAF mutant, microsatellite-stable colorectal cancers (11–13). In the present study, we identify a role of D2HG in the progression of colitis to colon cancer. We demonstrate that Hif-1α regulates D2HGDH transcription and that D2HGDH expression at baseline is decreased in UC patients who progress to cancer.
Results
Urine D2HG Correlates Positively with the Severity of Tumorigenesis in the Azoxymethane-Dextran Sodium Sulfate Model of Colitis-Associated Colon Cancer.
To identify the mechanisms involving cellular metabolism that drive progression from colitis to cancer, we performed quantitative metabolic profiling. We adopted a mouse model of colitis-associated colon cancer (CAC) in which wild-type mice were injected with azoxymethane (AOM) and then were exposed to dextran sodium sulfate (DSS) in their drinking water for 7 d, followed by 14 d of recovery with water alone; a second cycle of DSS was repeated with 3 wk of recovery. To identify organic acids altered during the progression of colitis to colon cancer, urine was serially collected from individual mice at baseline before AOM injection, after the first cycle of DSS (colitis stage), and the day before mice were killed (advanced neoplasia stage) for targeted metabolomics analysis. Nine organic acids were significantly altered [P < 10−6; false-discovery rate (FDR) < 10−5] in urine during colitis or after advanced neoplasia formation (Table S1), and included metabolites of lysine (2-oxoadipic, 2-hydroxyadipic, and glutaric), carbohydrate metabolism (glyceric), the tricarboxylic acid cycle (citric and 2HG), and microbiota (phenyllactic and 4-hydroxyphenyllactic). Of these, 2HG, specifically the enantiomer D2HG, has an emerging role in oncogenesis (14, 15). To measure levels of both 2HG enantiomers, we then differentiated D2HG and L2HG by derivatization with methyl chloroformate to form methyl lactones, which were separated by 2D chiral column GS and quantified by TOF MS. Urine D2HG levels during colitis, but not after advanced neoplasia formation, in individual mice positively correlated with the number of colon polyps quantitated macroscopically after the mice were killed and with the severity of histological dysplasia/adenoma scoring (Table S2).
D2HG Impedes Recovery from DSS Colitis.
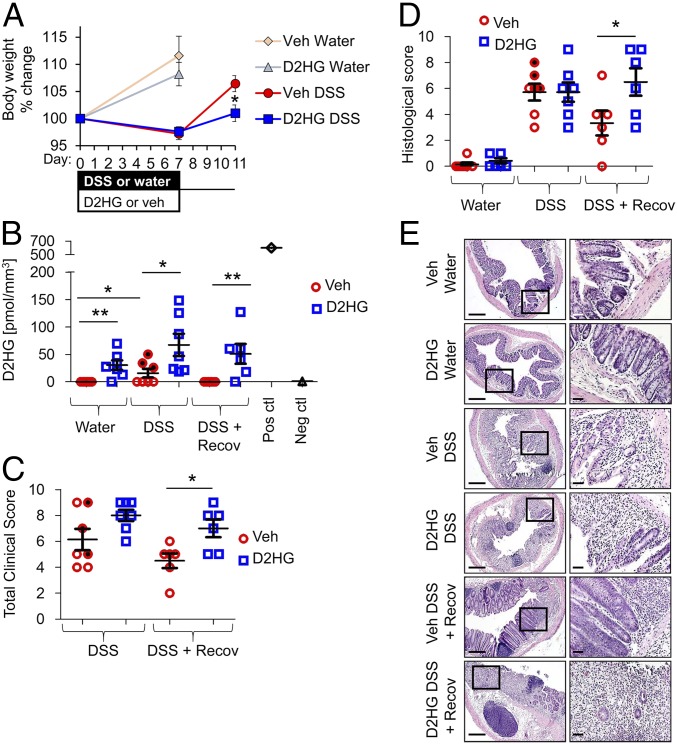
Since the severity of tumorigenesis in the AOM-DSS model is dependent on the severity of inflammation (16), we determined the effect of elevated D2HG on DSS-induced colitis and on recovery of inflammation. Mice were i.p. injected with 25 mg/kg D2HG or vehicle once daily during 7 d of DSS administration. A subset of mice was allowed to recover for four additional days, during which time DSS was removed from their drinking water (Fig. 1A). In vehicle-injected mice, colonic D2HG levels were elevated at day 7 of DSS colitis compared with water control mice and returned to baseline following recovery (Fig. 1B). Vehicle-treated mice with the highest D2HG levels during DSS (shaded data points in Fig. 1B) also exhibited high total clinical scores (Fig. 1C) and histological scores (Fig. 1D). In D2HG-injected mice, colonic D2HG levels were increased in control mice given water (water + D2HG vs. those given water + vehicle), increased further during DSS (DSS + D2HG vs. DSS + vehicle), and remained elevated during recovery compared with vehicle-treated mice (DSS recovery + D2HG vs. DSS recovery + vehicle) (Fig. 1B). Compared with DSS + vehicle-treated mice, DSS + D2HG mice exhibited similar overt measures of colitis such as loss in body weight (Fig. 1A), clinical score (Fig. 1C), and severity of histological inflammation (Fig. 1 D and E). However, D2HG injection prolonged DSS-induced colitis, as indicated by slower recovery of lost body weight (Fig. 1A), more severe clinical score (Fig. 1C), and more severe histological inflammation after 4 d recovery (Fig. 1 D and E).
Fig. 1.
D2HG impedes recovery from DSS colitis. (A–D) Percent body weight change (A), colonic D2HG levels measured by enzymatic assay (B), clinical scores (C), and histological score (D) of mice i.p. injected with vehicle (red trace) or 25 mg/kg D2HG (blue trace) daily during DSS-induced colitis. Glioma tissue with IDH1 mutation was used as a positive control, and glioma with wild-type IDH1 was used as a negative control (B). Shaded data points indicate vehicle-treated mice with highest D2HG levels during DSS. Results are presented as means ± SEM of pooled data (A) or individual data points ± SEM of six to seven mice per group (B–D). Before completion of the experiment, one DSS + D2HG-treated mouse and one DSS recovery + D2HG-treated mouse did not survive and are not included in the sample size. *P < 0.05, **P < 0.01 relative to vehicle, by one-way ANOVA followed by Bonferroni’s test. (E, Left) Photomicrographs of histological sections of H&E-stained distal colon. (Scale bars: 200 μm.) (Right) Boxes denote areas shown at higher magnification. (Scale bars: 100 μm.)
We next sought to determine whether colonic epithelial cells exposed to D2HG produce cytokines/chemokines that enhance immune cell migration. Conditioned medium from Caco2-BBE cells exposed to D2HG (CM-D2HG) for 16 h exhibited increased levels of RANTES, IFN-γ–induced protein 10 (IP-10), and IL-8 out of the 42 cytokines/chemokines measured (Fig. S1A). CM-D2HG increased the migration of cultured T cells and macrophages compared with conditioned medium from vehicle-treated control Caco2-BBE cells (Fig. S1B). The addition of RANTES-, IP-10–, or IL-8–neutralizing antibodies to the CM-D2HG prevented the increased immune cell migration by CM-D2HG (Fig. S1B).
D2HG Enhances Tumorigenesis in the AOM-DSS Mouse Model of CAC.
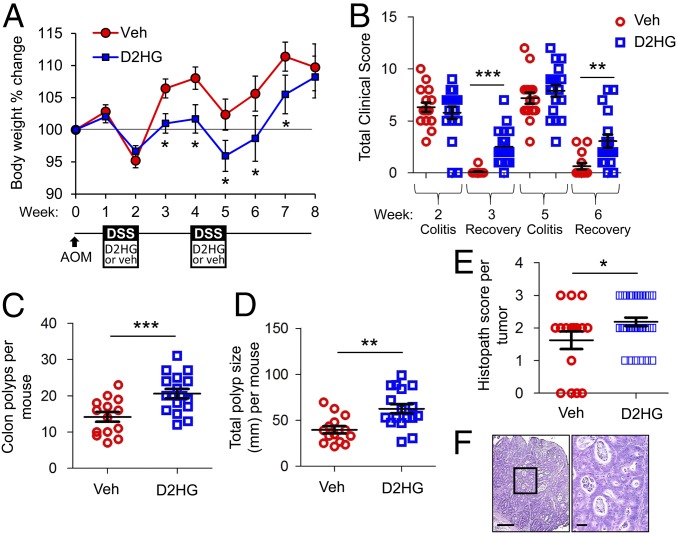
Since prolonged inflammation promotes tumorigenesis (1), we next determined the effect of D2HG during colitis on tumorigenesis in the AOM-DSS model. Mice were i.p. injected with 25 mg/kg D2HG or vehicle once daily during both periods of DSS administration (Fig. 2A). Again, D2HG did not affect overt measures of colitis, such as loss in body weight (Fig. 2A) or clinical score (Fig. 2B), at week 2 but impeded recovery from inflammation as indicated by slower recovery of lost body weight (Fig. 2A) and more severe clinical score 1 wk after DSS administration (Fig. 2B). D2HG-treated mice developed significantly more (Fig. 2C) and larger (Fig. 2D) polyps than vehicle-treated mice. Additionally, D2HG-treated mice had a higher occurrence of high-grade dysplasia (histopath score of 2) and intramucosal adenocarcinoma (histopath score of 3) (Fig. 2 E and F).
Fig. 2.
D2HG enhances tumorigenesis in the AOM-DSS mouse model of CAC. (A) Percent body weight change of mice i.p. injected with vehicle (red trace) or 25 mg/kg D2HG (blue trace) during DSS administration. (B) Clinical scores after DSS treatment and 1 wk recovery. (C and D) Number (C) and size distribution (D) of macroscopic polyps as determined using a dissecting microscope. (E) H&E-stained sections of Swiss-rolled colons were scored in a blind fashion for neoplasia. Four vehicle-treated mice did not have evidence of tumors when histopath scoring was performed. Results are presented as mean ± SEM of pooled data (A) or individual data points ± SEM (B–E) of 16 or 17 mice per group from three pooled, independent experiments. Two vehicle-treated mice and one D2HG-treated mouse did not survive until week 8 and are not included in sample size. *P < 0.05, **P < 0.01, ***P < 0.01 relative to vehicle, by one-way ANOVA followed by Bonferroni’s test (A and B) or unpaired, two-tailed Student’s t test (C–E). (F, Left) Photomicrograph of an intramucosal adenocarcinoma from a D2HG-treated mouse. Note the nuclear atypia, loss of basement membrane of glands, glandular crowding, and necrotic debris. (Scale bar: 200 μm.) (Right) Boxed area shown at higher magnification. (Scale bar: 100 μm.)
Elevated D2HG Enhances Cell Survival, Proliferation, and Migration in Colonic Epithelial Cells.
To determine epithelial responses in the setting of high levels of D2HG, we used a representative polarized colonic epithelial cell line (Caco2-BBE) and a nontransformed normal intestinal epithelial cell line (IEC-6). These cell lines were transfected with RNAi against D2HGDH (siD2HGDH) or RNAi negative control (siNC). The efficiency of D2HGDH knockdown using two independent siRNAs is shown in Fig. S2A. As expected, knockdown of D2HGDH increased D2HG levels in both cell lines (Fig. S2B). Cells transfected with siD2HGDH exhibited increased proliferating cell nuclear antigen (PCNA) protein expression, a marker of cell proliferation (Fig. S2A), decreased cell cytotoxicity as measured by lactate dehydrogenase (LDH) release (Fig. S2C), decreased apoptosis (Fig. S2D), and enhanced cell migration (Fig. S2E). Caco2-BBE cells, IEC-6 cells, and enteroids derived from colonic crypts (colonoids) of wild-type mice treated with exogenous D2HG demonstrated alterations similar to those seen with transfection with siD2HGDH with decreased cell cytotoxicity (Figs. S3A and S4B), increased PCNA expression (Figs. S3B and S4C), decreased apoptosis (Figs. S3C and S4C), and increased cell migration (Fig. S3D). These results suggest that D2HG governs intestinal epithelial cell fate, enhancing survival, proliferation, and migration and inhibiting apoptosis.
Hif-1α Modulates D2HGDH Expression.
To determine the mechanism whereby D2HG is elevated during colitis, IDH1 and IDH2 were sequenced for the common Arg100/Arg132 or Arg140/Arg172 gene mutations, respectively, that can drive elevated D2HG levels (6, 7). No mutations in IDH1 or IDH2 genes were demonstrated during colitis or after polyp formation in the AOM-DSS model.
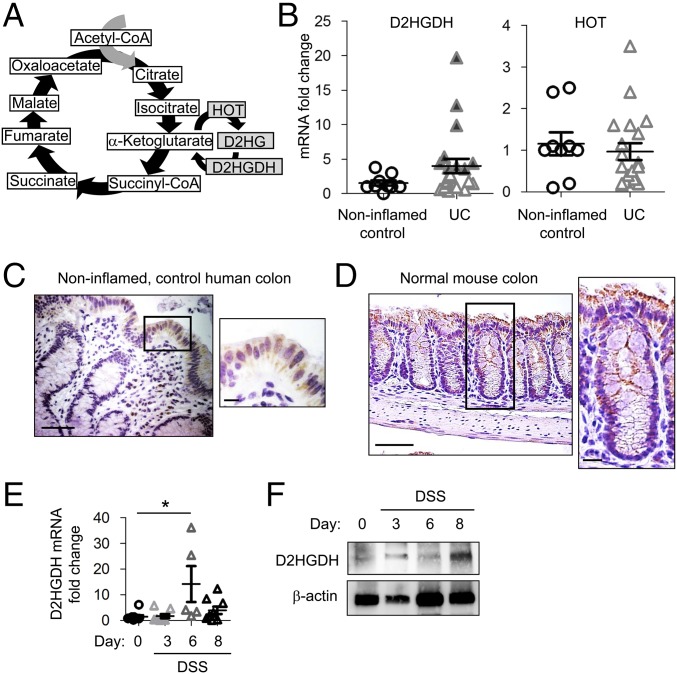
We next assessed colonic expression of enzymes involved in the D2HG pathway, HOT and D2HGDH (Fig. 3A), in human UC or noninflamed control mucosal biopsies. D2HGDH mRNA expression was increased in some UC mucosal biopsies (Fig. 3B; shaded triangles indicate a greater-than-threefold increase), but across all patients this did not reach statistical significance (P = 0.1369). HOT mRNA expression was not altered in UC mucosal biopsies compared with noninflamed normal specimens (Fig. 3B). Since mucosal biopsies contain many cell types, such as epithelial cells, lamina propria immune cells, and fibroblasts, we next assessed tissue localization of D2HGDH expression by immunohistochemical staining. Noninflamed, control human colon exhibited D2HGDH expression primarily in the epithelium with minimal expression observed in lamina propria immune cells (Fig. 3C). Similar to expression in human colonic biopsies, D2HGDH was expressed primarily in the colonic epithelium of control mice (Fig. 3D). To assess whether any alteration of epithelial D2HGDH occurs over the course of acute colitis, mRNA and protein from isolated colonic epithelial cells of DSS-treated mice were assessed for D2HGDH expression. Epithelial D2HGDH mRNA expression was increased on day 6 of DSS-induced colitis (Fig. 3E) followed by increased epithelial D2HGDH protein expression on day 8 (Fig. 3F).
Fig. 3.
D2HGDH is predominantly expressed in the colonic epithelium and is increased during colitis. (A) D2HG formation in the tricarboxylic acid cycle. (B) Colonic D2HGDH and HOT mRNA expression measured by qPCR in human mucosal biopsies. Shaded triangles indicate a greater-than-threefold increase. (C and D) Immunohistochemistry staining of D2HGDH (brown stain) in human colonic mucosal biopsies (C) or mouse colon (D). (Scale bars: 50 μm.) Boxed areas are shown at higher magnification at right. (Scale bars for higher magnification images are 10 μm.) (E and F) D2HGDH mRNA expression measured by qPCR (E) and representative Western blot of D2HGDH protein expression (F) in isolated colonic epithelial cells from DSS-treated mice. Results in B and E are presented as individual data points ± SEM of nine normal patients or 21 UC patients (B) or five to nine mice per time point (E). *P < 0.05 by one-way ANOVA followed by Bonferroni’s test (E).
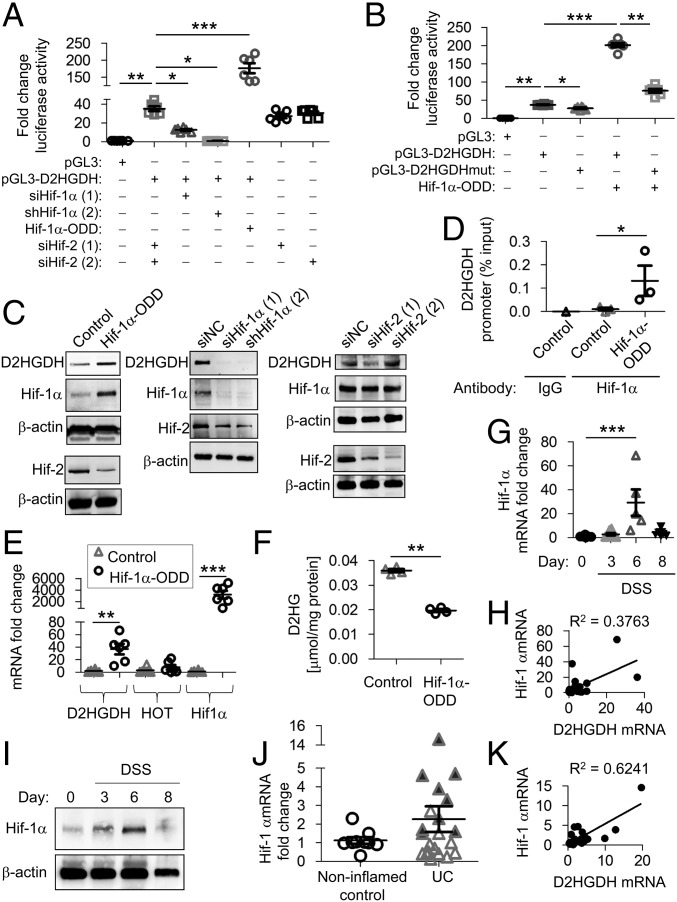
The transcriptional regulation of D2HGDH has not been previously reported. We cloned and characterized the D2HDGH promoter (−1,286 to −17 from the transcriptional start site) from human chromosome 2 and identified a putative Hif-1α–binding site (5′-RCGTG-3′) at −127 to −131 from the transcriptional start site. Promoter activity was determined by measuring luciferase reporter expression (pGL3 vector) in Caco2-BBE cells. D2HGDH promoter activity was significantly decreased during RNAi-mediated knockdown of Hif-1α expression driven by siRNA or shRNA, but not during Hif-2α knockdown, and was significantly increased during constitutive Hif-1α–oxygen-dependent degradation domain (ODD)–phosphorylated internal ribosome entry site (pIRES) overexpression (Fig. 4A). To determine if the putative Hif-1α–binding site is necessary for D2HGDH transcriptional activation, a mutation was introduced into the Hif-1α–binding site by site-directed mutagenesis (pGL3-D2HGDHmut). Mutation of the Hif-1α–binding site decreased basal D2HGDH promoter activity and significantly decreased promoter activation by Hif-1α overexpression (Fig. 4B). D2HGDH protein expression was increased in cells overexpressing Hif-1α–ODD–pIRES and decreased in cells following knockdown of Hif-1α but not Hif-2α (Fig. 4C), suggesting that relative Hif-1α levels modulate the expression of endogenous D2HGDH. Hif-1α binding to the putative Hif-1α–binding site in the D2HGDH promoter was increased in cells overexpressing Hif-1α–ODD–pIRES (Fig. 4D). Constitutive Hif-1α overexpression also increased D2HGDH mRNA expression, with no effect on HOT (Fig. 4E), and decreased D2HG levels (Fig. 4F). Cells exposed to hypoxia exhibited transient increased Hif-1α protein levels with a concomitant increase in D2HGDH protein (Fig. S5A) and decreased D2HG levels (Fig. S5B), demonstrating that endogenous Hif-1α regulates D2HGDH. Together, these results suggest that Hif-1α regulates D2HG via transcriptional control of D2HGDH expression.
Fig. 4.
Hif-1α modulates D2HGDH expression. (A and B) Caco2-BBE cells were transfected with Hif-1α–ODD–pIRES (Hif-1α-ODD), a constitutively active Hif-1α expression plasmid, empty vector (Control), two independent RNAi constructs against Hif-1α, two independent RNAi constructs against Hif-2α, or RNAi negative control (siNC) for 48 h. D2HGDH promoter activation was measured by luciferase reporter expression (A) and compared with D2HGDH promoter harboring mutation of the putative Hif-1α–binding site (pGL3-D2HGDHmut) (B). (C) Representative Western blots of D2HGDH. Hif-1α and Hif-2 protein expression is shown to demonstrate overexpression or knockdown efficiency. (D) An anti–Hif-1α antibody or IgG negative control antibody was used for ChIP in extracts from Caco2-BBE cells overexpressing Hif-1α–ODD–pIRES or empty vector (control). Immunoprecipitates were analyzed by qPCR using D2HGDH promoter-specific primers targeting the putative Hif-1α–binding site. The relative enrichment of Hif-1α binding is shown as the percentage of total input DNA. (E) mRNA expression by qPCR in Caco2-BBE cells overexpressing Hif-1α–ODD–pIRES or empty vector (control). (F) D2HG levels measured by enzymatic assay. (G) Mouse colonic epithelial Hif-1α mRNA expression measured by qPCR. (H) Regression analysis of mouse colonic epithelial mRNA expression during DSS-induced colitis. (I) Representative Western blots of Hif-1α protein expression in colonic epithelial cells. (J) Colonic Hif-1α mRNA expression measured by qPCR in human mucosal biopsies. Shaded triangles represent patients with a greater-than-threefold increase in DH2GDH mRNA in Fig. 3B. (K) Regression analysis of human colonic mucosal mRNA expression. Results are presented as six Caco2-BBE samples representative of two independent experiments (A, B, and E) or three (C and D) or four (F) Caco2-BBE samples per group. Results are presented as individual data points ± SEM of five to nine mice per time point (G) or nine normal or 21 UC patients (J) or as representative blots of three mice per group (I). *P < 0.05, **P < 0.01, and ***P < 0.005 by one-way ANOVA followed by Bonferroni’s test (A, B, E, and G) or by unpaired, two-tailed Student’s t test (D, F, and J).
To determine whether D2HGDH expression correlated with Hif-1α expression in vivo, Hif-1α expression was measured in isolated colonic epithelial cells in DSS-treated mice and human mucosal biopsies. Hif-1α mRNA was significantly increased on day 6 of DSS-induced colitis and returned to baseline by day 8 (Fig. 4G). Hif-1α mRNA expression directly correlated (R2 = 0.3763, P < 0.0005) (Fig. 4H) with D2HGDH mRNA (Fig. 3E) in mouse colonic epithelial cells. During the initial stage of DSS-induced colitis (day 3), epithelial Hif-1α protein was not consistently changed (Fig. 4I), as expected due to the limited role of hypoxia in the early stages of colitis (17–19). On day 6 of DSS-induced colitis, epithelial Hif-1α protein expression was transiently increased, as previously shown (Fig. 4I) (19), a time point immediately before increased epithelial D2HGDH protein is demonstrated (Fig. 3F). Hif-1α mRNA expression was increased in some UC mucosal biopsies, but across all patients this did not reach statistical significance; patients with a greater-than-threefold increase in D2HGDH mRNA expression (as shown in Fig. 3B) demonstrated higher HIF-1α mRNA expression (shaded triangles in Fig. 4J). Hif-1α mRNA expression directly correlated with D2HGDH mRNA in mucosal biopsies from UC patients (R2 = 0.6241, P < 0.0001) (Fig. 4K). These results suggest that Hif-1α expression is associated with increased D2HGDH transcription in vivo.
D2HGDH Expression Is Increased in Advanced Neoplasia.
Following AOM-DSS, advanced neoplasia and normal epithelium were isolated from mouse colon. D2HGDH and Hif-1α mRNA and protein expression were increased in advanced neoplasia, whereas HOT expression was unchanged (Fig. S6). These results suggest that, in addition to during colitis, increased Hif-1α expression in advanced neoplasia, which occurs when the tumor microenvironment becomes hypoxic (20), is associated with increased D2HGDH expression.
D2HGDH Expression Is Decreased at Baseline in UC Patients Who Progress to Cancer.
We obtained retrospective, paraffin-embedded colon mucosa samples of involved UC (baseline). From the same patients, we obtained retrospective paraffin-embedded colon mucosa samples at follow-up, 1–8 y later, at which time 11 patients had progressed to high-grade dysplasia/adenocarcinoma (progressors) compared with 13 UC patients who remained dysplasia-free (nonprogressors) (Table S3). We attempted to measure D2HG levels using methods previously published for paraffin-embedded tissue samples (21–24), but we were unable to obtain sensitive D2HG measurement using N-(p-toluenesulfonyl)-l-phenylalanyl chloride (TSPC) or diacetyl-l-tartaric anhydride (DATAN) chiral derivatization methods combined with LC-electrospray ionization (ESI)-MS/MS in our human colonic biopsy samples. This is likely due to the small size of these biopsies compared with samples cited in other publications.
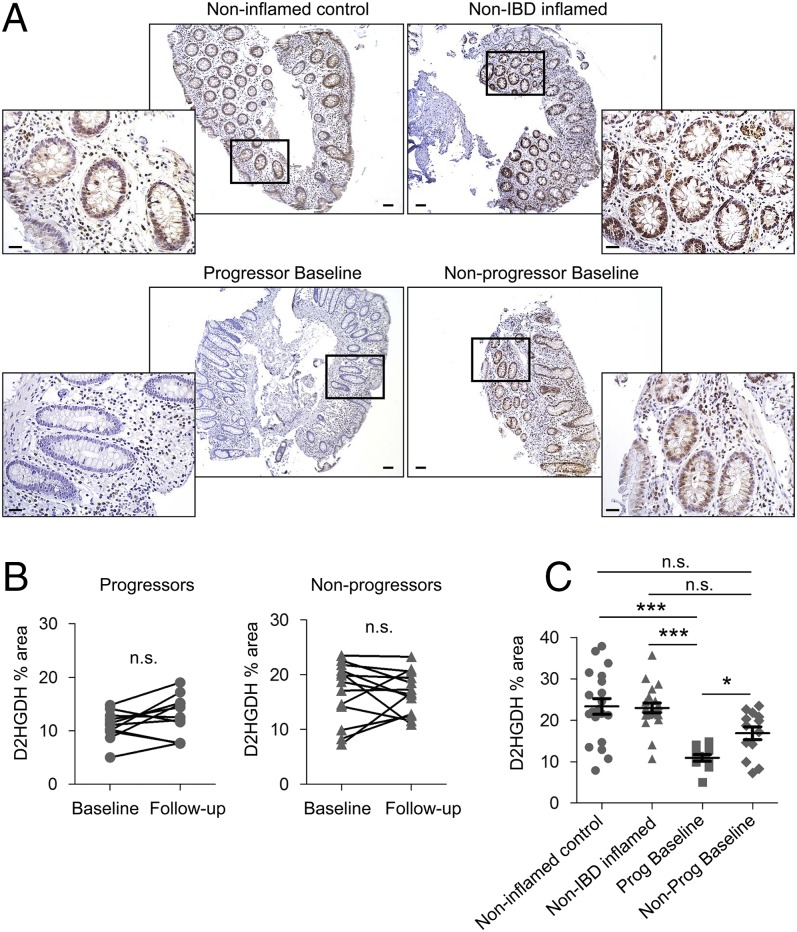
Due to this limitation, we opted to assess D2HGDH, since its expression was altered during DSS-induced colitis. Immunohistochemistry staining demonstrated that D2HGDH was expressed in lamina propria immune cells and in the epithelium of the normal colon mucosa from noninflamed control patients, non-IBD inflamed patients, and nonprogressors, but epithelial D2HGDH expression was decreased in progressors (Fig. 5A). To quantify epithelial D2HGDH expression, digital images of sections stained for D2HGDH by immunohistochemistry were captured, the epithelium was defined as the region of interest in the digital images, and 3,3′-diaminobenzidine-tetrahydrochloride (DAB) staining was quantified and expressed as the percentage of the whole region of interest. Epithelial D2HGDH expression did not change between baseline and follow-up in either progressors (P = 0.1146) or nonprogressors (P = 0.9049) (Fig. 5B). Progressors expressed significantly lower epithelial D2HGDH at baseline than nonprogressors (Fig. 5 A and C). Progressors also expressed significantly lower D2HGDH in the epithelium compared with noninflamed control biopsies or inflamed colonic biopsies from non-IBD patients (Fig. 5 A and C).
Fig. 5.
D2HGDH expression at baseline is decreased in UC patients who progress to cancer. (A) Immunohistochemistry staining of D2HGDH (brown stain) in colonic mucosa. (Scale bars: 100 μm.) The boxed areas are shown at higher magnification below. (Scale bars: 50 μm.) (B) Percentage of the area of immunostaining of epithelial D2HGDH in human colonic mucosa from progressors and nonprogressors at baseline and follow-up. N.s., not significant by paired t test. (C) Percentage of the area of immunostaining of epithelial D2HGDH in human colonic mucosa from progressors and nonprogressors at baseline compared with noninflamed control and non-IBD inflamed mucosa. Results are presented as individual data points ± SEM of 11 progressors, 13 nonprogressors, 20 noninflamed controls, and 20 non-IBD inflamed patients. *P < 0.05, ***P < 0.001 by Kruskal–Wallis test followed by Dunn’s test.
Discussion
Here, we identify a mechanism by which elevated D2HG during colitis precedes and promotes tumor progression in the colon. Hif-1α plays an important role in regulating D2HG via transcriptional control of D2HGDH. These conclusions are supported by metabolomics, biochemical and expression data, functional studies in a mouse model of CAC, and translational studies in mucosal biopsies from UC patients who progressed to colon cancer.
D2HG is normally a low-abundance metabolite. D2HG elevation due to mutations in IDH1 or IDH2 is frequent in glioma and acute myeloid leukemia and is emerging in some solid tumors (6–13). A recent mathematical analysis of CRC gene expression predicted that IDH1 and IDH2 expression is imbalanced in CRC and is associated with worse patient prognosis (25). Here, we show that IDH1 and IDH2 did not harbor common gene mutations that can drive elevated D2HG levels (6, 7) during colitis or after advanced neoplasia formation in the AOM-DSS mouse model of CAC. Although decreasing D2HG has been proposed as one mechanism for the well-established role of aspirin in preventing CRC (26), levels of D2HG or L2HG or the enzymes controlling their expression during CRC or colitis were not previously reported. Here, using the AOM-DSS model, we demonstrate that D2HG, but not L2HG, was elevated in the colon during colitis and enhanced tumorigenesis. Importantly, elevated D2HG was also detected in the urine during colitis, and these levels in individual mice, but not those after advanced neoplasia formation, directly correlated with the subsequent severity of tumorigenesis. These results suggest that the D2HG level in the urine may provide a biomarker to predict an IBD patient’s risk of progressing to cancer.
The colonic expression of D2HGDH, an enzyme that eliminates D2HG, was decreased in baseline mucosal biopsies from UC patients who upon follow-up had progressed to high-grade dysplasia/adenocarcinoma compared with those from UC patients who remained dysplasia-free, from noninflamed controls, and from inflamed colonic biopsies from non-IBD patients. Importantly, these results further establish the potential of the D2HG/D2HGDH pathway as a biomarker to identify CRC risk in human colitis patients. We speculate that D2HG levels would be elevated in progressors, given that D2HGDH expression was decreased. However, we were unable to obtain sensitive D2HG measurement using published methods (21–24) in our human colonic biopsy samples, likely due to the limited amount of tissue. Future prospective studies are necessary to assess D2HG tissue and/or urine expression in IBD patients who progress to CRC to fully elucidate the potential of D2HG as a biomarker for patients at risk for developing cancer.
Our in vitro findings using polarized epithelial cells (Caco2-BBE and IEC-6) and colonoids derived from wild-type mice extend the function of D2HG to inhibit apoptosis and enhance survival, proliferation, and migration. Sustained inflammatory physiological changes such as oxidative stress are known to cause genotoxic effects, promoting cancer-initiating mutations (27). In this setting, epithelial cells that are proliferative and show no evidence of apoptosis are prone to neoplastic progression. These results suggest that D2HG is an indicator of prolonged inflammation that is not recovering and at risk for progressing to cancer. Additionally, D2HG increased epithelial secretion of the proinflammatory chemokines RANTES, IP-10, and IL-8, which recruit T cells, macrophages, dendritic cells, and neutrophils. Blockade of RANTES, IP-10, or IL-8 with neutralizing antibodies prevented in vitro T cell or macrophage recruitment by D2HG conditioned medium from Caco2-BBE cells. These results suggest that D2HG promotes neoplastic progression in the colon by directly altering epithelial cell-fate decisions and secondly by perpetuating epithelial cell-driven recruitment of immune cells, resulting in prolonged inflammation whereby the inflammatory milieu further drives neoplasia (1). Indeed, our in vivo findings demonstrate that increased D2HG impeded recovery from DSS colitis.
Hif-1α is a transcription factor responsive to hypoxia and beneficial in reducing intestinal inflammation and regulating epithelial barrier function, in contrast to Hif-2α, which has been shown to promote colitis (28). Our results suggest that Hif-1α expression is directly proportional to the mitochondrial enzyme D2HGDH that eliminates D2HG. We identified a Hif-1α–binding site located in the D2HGDH promoter using ChIP and luciferase reporter assays and demonstrated that relative levels of Hif-1α, but not Hif-2α, regulated D2HGDH expression via transcriptional activation. Furthermore, constitutive overexpression of Hif-1α in cultured colonic epithelial cells decreased the level of D2HG. Therefore, our study reveals a role of Hif-1α in modulating D2HG levels via D2HGDH expression. However, it is not the only transcription factor involved in regulating the D2HGDH promoter, since some transcriptional activation remains in the D2HGDH promoter with the mutated putative Hif-1α–binding site. Our in silico analysis of D2HGDH promoter identified other putative transcription factor-binding sites including Snail, Oct1, Fox, and Klf1, which require further investigation into their roles, if any, during colitis.
Hif-1α is generally considered an oncoprotein increased by intratumoral hypoxia, which, in turn, has been shown to drive tumor growth and promote the survival of tumor cells residing in a low-oxygen environment (29). Thus, in established tumors Hif-1α plays a pathological, detrimental role. Indeed, recent studies have confirmed that Hif-1α overexpression is associated with poor prognosis in CRC patients (30). On the contrary, during colitis, Hif-1α activation plays a protective role in the inflamed mucosa by enhancing epithelial barrier function and eliciting protective innate immune and antimicrobial responses (18, 31). Hif-1α activation in mouse models of colitis and IBD patients is prevalent in areas of inflammatory hypoxia (31, 32). Our study extends the beneficial functions of Hif-1α during colitis to include the activation of epithelial D2HGDH expression. Cells exposed to hypoxia exhibited increased Hif-1α protein with a concomitant increase in D2HGDH protein and decreased D2HG levels, demonstrating that endogenous Hif-1α regulates D2HGDH. Our human data using colonic biopsies from UC patients support the mechanism of D2HGDH regulation identified in the DSS mouse model of colitis, with a direct correlation of HIF-1α mRNA with D2HGDH mRNA in human UC mucosal biopsies. In the DSS-colitis model, D2HG levels were elevated in the urine on day 7. Our in vitro data suggest that D2HG helps recruit immune cells, which is a normal response to intestinal injury to remove the insult and bacterial penetration. We found that during DSS-induced colitis, Hif-1α was transiently activated and D2HGDH expression was up-regulated to contribute to decreasing D2HG levels. This may be one mechanism to initiate dampening of the inflammatory cascade, since sustained, elevated D2HG prolonged recovery from DSS-induced colitis. Prolonged recovery from inflammation is known to cause genotoxic, cancer-promoting effects (27). Since Hif-1α up-regulation in colonic epithelial cells was transient, as evidenced in the DSS model and in vitro exposure to hypoxia, an agent that increases Hif-1α activation to sustain high levels of D2HGDH in IBD patients in the early stages of colitis may have therapeutic potential to prevent the progression to CAC. The use of Hif-1α–stabilizing agents is currently being explored as therapy for inflammatory mucosal diseases, such as IBD (33).
Together, our results suggest that persistent mild-to-moderate colitis in which the epithelium is not destroyed, with minimal hypoxic inflammation and therefore minimal Hif-1α activation, may present the scenario in which epithelial D2HGDH expression is deficient, D2HG levels are elevated, and hallmarks of transformation such as increased epithelial cell proliferation, decreased apoptosis, and increased migration are enhanced, thereby promoting progression to tumorigenesis. Elevated D2HG in the urine and/or low D2HGDH expression in the colon may provide biomarkers to predict a UC patient’s risk of progressing to cancer. D2HG-linked mechanisms may provide functional therapeutic targets to prevent the progression from colitis to cancer.
Materials and Methods
All human experimental protocols were approved by the Baylor Scott & White Research Institute Institutional Review Board, and all participants provided written, informed consent. All experiments were approved by the Baylor Scott & White Research Institute Institutional Animal Care and Use Committee.
Information on AOM-DSS administration and sample collection, metabolomic analysis, human tissue samples, histological dysplasia/adenoma and inflammatory scoring, enzymatic D2HG assay, IDH1 and IDH2 sequencing, immunohistochemistry, cell lines, colonoid culture, cloning and mutation of the 5′ flanking region of the human D2HGDH gene, ChIP, LDH release, cell migration, Luminex multiplex assays, TUNEL staining, RNA isolation and real-time qPCR analysis, Western blot analysis, and statistics is provided in Supporting Information.
Supplementary Material
Acknowledgments
We thank Dr. Richard Bruick and Hanzhi Wang (University of Texas Southwestern Medical Center) for assistance with the hypoxic incubator, Arwa S. Kathiria and Dr. Teodoro Bottiglieri for technical assistance, and Dr. Stuart J. Spechler (Baylor Scott & White Research Institute) for thoughtful suggestions during the preparation of this manuscript.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. KX227379).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1712625115/-/DCSupplemental.
References
- 1.Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–2114.e5. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 2.Grivennikov SI. Inflammation and colorectal cancer: Colitis-associated neoplasia. Semin Immunopathol. 2013;35:229–244. doi: 10.1007/s00281-012-0352-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jang M, Kim SS, Lee J. Cancer cell metabolism: Implications for therapeutic targets. Exp Mol Med. 2013;45:e45. doi: 10.1038/emm.2013.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Struys EA, et al. Kinetic characterization of human hydroxyacid-oxoacid transhydrogenase: Relevance to D-2-hydroxyglutaric and gamma-hydroxybutyric acidurias. J Inherit Metab Dis. 2005;28:921–930. doi: 10.1007/s10545-005-0114-x. [DOI] [PubMed] [Google Scholar]
- 5.Tedeschi L, et al. Endogenous gamma-hydroxybutyric acid is in the rat, mouse and human gastrointestinal tract. Life Sci. 2003;72:2481–2488. doi: 10.1016/s0024-3205(03)00143-7. [DOI] [PubMed] [Google Scholar]
- 6.Amary MF, et al. IDH1 and IDH2 mutations are frequent events in central chondrosarcoma and central and periosteal chondromas but not in other mesenchymal tumours. J Pathol. 2011;224:334–343. doi: 10.1002/path.2913. [DOI] [PubMed] [Google Scholar]
- 7.Molenaar RJ, Radivoyevitch T, Maciejewski JP, van Noorden CJ, Bleeker FE. The driver and passenger effects of isocitrate dehydrogenase 1 and 2 mutations in oncogenesis and survival prolongation. Biochim Biophys Acta. 2014;1846:326–341. doi: 10.1016/j.bbcan.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Fathi AT, et al. Isocitrate dehydrogenase 1 (IDH1) mutation in breast adenocarcinoma is associated with elevated levels of serum and urine 2-hydroxyglutarate. Oncologist. 2014;19:602–607. doi: 10.1634/theoncologist.2013-0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smolková K, Dvořák A, Zelenka J, Vítek L, Ježek P. Reductive carboxylation and 2-hydroxyglutarate formation by wild-type IDH2 in breast carcinoma cells. Int J Biochem Cell Biol. 2015;65:125–133. doi: 10.1016/j.biocel.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Terunuma A, et al. MYC-driven accumulation of 2-hydroxyglutarate is associated with breast cancer prognosis. J Clin Invest. 2014;124:398–412. doi: 10.1172/JCI71180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartman DJ, et al. Isocitrate dehydrogenase-1 is mutated in inflammatory bowel disease-associated intestinal adenocarcinoma with low-grade tubuloglandular histology but not in sporadic intestinal adenocarcinoma. Am J Surg Pathol. 2014;38:1147–1156. doi: 10.1097/PAS.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 12.Tougeron D, Guilloteau K, Karayan-Tapon L. Absence of IDH mutation in colorectal cancers with microsatellite instability. Dig Liver Dis. 2016;48:681–683. doi: 10.1016/j.dld.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 13.Whitehall VL, et al. Isocitrate dehydrogenase 1 R132C mutation occurs exclusively in microsatellite stable colorectal cancers with the CpG island methylator phenotype. Epigenetics. 2014;9:1454–1460. doi: 10.4161/15592294.2014.971624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koivunen P, et al. Transformation by the (R)-enantiomer of 2-hydroxyglutarate linked to EGLN activation. Nature. 2012;483:484–488. doi: 10.1038/nature10898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Losman JA, et al. (R)-2-hydroxyglutarate is sufficient to promote leukemogenesis and its effects are reversible. Science. 2013;339:1621–1625. doi: 10.1126/science.1231677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Robertis M, et al. The AOM/DSS murine model for the study of colon carcinogenesis: From pathways to diagnosis and therapy studies. J Carcinog. 2011;10:9. doi: 10.4103/1477-3163.78279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giles RH, et al. Interplay between VHL/HIF1alpha and Wnt/beta-catenin pathways during colorectal tumorigenesis. Oncogene. 2006;25:3065–3070. doi: 10.1038/sj.onc.1209330. [DOI] [PubMed] [Google Scholar]
- 18.Shah YM. The role of hypoxia in intestinal inflammation. Mol Cell Pediatr. 2016;3:1. doi: 10.1186/s40348-016-0030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah YM, et al. Hypoxia-inducible factor augments experimental colitis through an MIF-dependent inflammatory signaling cascade. Gastroenterology. 2008;134:2036–2048. doi: 10.1053/j.gastro.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simiantonaki N, Taxeidis M, Jayasinghe C, Kurzik-Dumke U, Kirkpatrick CJ. Hypoxia-inducible factor 1 alpha expression increases during colorectal carcinogenesis and tumor progression. BMC Cancer. 2008;8:320. doi: 10.1186/1471-2407-8-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ng ES, Kangarloo SB, Konno M, Paterson A, Magliocco AM. Extraction of tamoxifen and its metabolites from formalin-fixed, paraffin-embedded tissues: An innovative quantitation method using liquid chromatography and tandem mass spectrometry. Cancer Chemother Pharmacol. 2014;73:475–484. doi: 10.1007/s00280-013-2346-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sahm F, et al. Detection of 2-hydroxyglutarate in formalin-fixed paraffin-embedded glioma specimens by gas chromatography/mass spectrometry. Brain Pathol. 2012;22:26–31. doi: 10.1111/j.1750-3639.2011.00506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wojakowska A, et al. An optimized method of metabolite extraction from formalin-fixed paraffin-embedded tissue for GC/MS analysis. PLoS One. 2015;10:e0136902. doi: 10.1371/journal.pone.0136902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan M, Breitkopf SB, Yang X, Asara JM. A positive/negative ion-switching, targeted mass spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nat Protoc. 2012;7:872–881. doi: 10.1038/nprot.2012.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koseki J, et al. Mathematical analysis predicts imbalanced IDH1/2 expression associates with 2-HG-inactivating β-oxygenation pathway in colorectal cancer. Int J Oncol. 2015;46:1181–1191. doi: 10.3892/ijo.2015.2833. [DOI] [PubMed] [Google Scholar]
- 26.Liesenfeld DB, et al. Aspirin reduces plasma concentrations of the oncometabolite 2-hydroxyglutarate: Results of a randomized, double-blind, crossover trial. Cancer Epidemiol Biomarkers Prev. 2016;25:180–187. doi: 10.1158/1055-9965.EPI-15-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scarpa M, et al. Inflammatory colonic carcinogenesis: A review on pathogenesis and immunosurveillance mechanisms in ulcerative colitis. World J Gastroenterol. 2014;20:6774–6785. doi: 10.3748/wjg.v20.i22.6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramakrishnan SK, Shah YM. Role of intestinal HIF-2α in health and disease. Annu Rev Physiol. 2016;78:301–325. doi: 10.1146/annurev-physiol-021115-105202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pawlus MR, Hu CJ. Enhanceosomes as integrators of hypoxia inducible factor (HIF) and other transcription factors in the hypoxic transcriptional response. Cell Signal. 2013;25:1895–1903. doi: 10.1016/j.cellsig.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshimura H, et al. Prognostic impact of hypoxia-inducible factors 1alpha and 2alpha in colorectal cancer patients: Correlation with tumor angiogenesis and cyclooxygenase-2 expression. Clin Cancer Res. 2004;10:8554–8560. doi: 10.1158/1078-0432.CCR-0946-03. [DOI] [PubMed] [Google Scholar]
- 31.Karhausen J, et al. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest. 2004;114:1098–1106. doi: 10.1172/JCI21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giatromanolaki A, et al. Hypoxia inducible factor 1alpha and 2alpha overexpression in inflammatory bowel disease. J Clin Pathol. 2003;56:209–213. doi: 10.1136/jcp.56.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colgan SP, Campbell EL, Kominsky DJ. Hypoxia and mucosal inflammation. Annu Rev Pathol. 2016;11:77–100. doi: 10.1146/annurev-pathol-012615-044231. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.