Significance
The emission of light generated in a process referred to as bioluminescence can be used for imaging of living cells over long timespans without phototoxicity or bleaching. The amounts of light produced in the bioluminescence process are very low, and exogenous substrate molecules are often required. We improved the brightness of bacterial bioluminescence, a system that features the advantage that all of the required molecular components are genetically encoded within a single operon. Consequently, we have engineered an improved operon ilux, which enables long-term visualization of single bacterial cells while simultaneously providing information about cellular viability.
Keywords: bioluminescence, luciferase, antibiotics, bacteria, microscopy
Abstract
Bioluminescence imaging of single cells is often complicated by the requirement of exogenous luciferins that can be poorly cell-permeable or produce high background signal. Bacterial bioluminescence is unique in that it uses reduced flavin mononucleotide as a luciferin, which is abundant in all cells, making this system purely genetically encodable by the lux operon. Unfortunately, the use of bacterial bioluminescence has been limited by its low brightness compared with other luciferases. Here, we report the generation of an improved lux operon named ilux with an approximately sevenfold increased brightness when expressed in Escherichia coli; ilux can be used to image single E. coli cells with enhanced spatiotemporal resolution over several days. In addition, since only metabolically active cells produce bioluminescent signal, we show that ilux can be used to observe the effect of different antibiotics on cell viability on the single-cell level.
Bioluminescent cells generate light by a chemical reaction. The bioluminescence reaction is catalyzed by an enzyme called luciferase, with a luciferin required as substrate. Molecules of luciferin are converted into a product in an electronically excited state and emit a photon on return to the ground state, with visible light emitted in the process. There are many different luciferases and corresponding luciferins found in nature, indicating that bioluminescence has evolved more than 40 times independently during evolution (1), although in several cases, its biological function remains not fully understood. Most luciferins are only produced by organisms that express the corresponding luciferase, with the exception of the bacterial luciferin FMNH2, reduced flavin mononucleotide (FMN), which is abundant in all cells.
The bacterial bioluminescence reaction is catalyzed by an αβ-heterodimeric luciferase coded by the genes luxA and luxB. In addition to FMNH2, the luciferase binds molecular oxygen and a long-chain fatty aldehyde. The fatty aldehyde is oxidized to the corresponding fatty acid, and FMNH2 is oxidized to FMN, thereby emitting a blue photon with a wavelength around the spectral emission maximum λmax of ∼490 nm:
To keep this reaction ongoing, the fatty aldehyde must be continuously regenerated. This is performed by the fatty acid reductase complex, which consists of a fatty acid reductase, transferase, and synthetase coded by luxC, luxD, and luxE, respectively. Since an FMN reductase that generates FMNH2 is present in Escherichia coli, introduction of the luxCDABE operon is sufficient to produce a bioluminescence output in these cells.
Due to its very low light levels compared with fluorescence, bioluminescence imaging is not routinely applied so far. However, bioluminescence provides several benefits compared with fluorescence measurements. First, there is virtually no background because of the lack of autofluorescence. Bioluminescence background levels in living cells are extremely low, making bioluminescence up to 50 times more sensitive than fluorescence (ref. 2 and references therein). Second, no excitation light source and filters are required, making the setup very simple. In addition, it is possible to study processes where the intense excitation light required for fluorescence measurements would be disturbing, such as circadian rhythms or Ca2+ activity in the retina (3, 4). Third, no phototoxicity or bleaching occurs, allowing image acquisition over arbitrary timespans. Furthermore, bioluminescence is dependent on metabolic energy, and hence, only metabolically active cells are visible, preventing artifacts due to the observation of severely damaged or dead cells.
In addition to the limitation by their low brightness, the luciferases that are most commonly used exhibit several drawbacks, as the luciferin must be externally supplied. Limited solubility, stability, or cell permeability of the luciferin may, in some cases, hamper its usability (5–7). Administering of excess amounts of the luciferin is readily done for standard single-layered cell cultures, but luciferin consumption within larger collections of cells, such as tumors, is more rapid. In these situations, the luciferin concentration is not constant over time, and the signal decays sometimes within minutes (8, 9). Therefore, the luciferin has to be applied repeatedly for long-term imaging, which complicates quantification of the signal. Moreover, autooxidation of coelenterazine, the substrate of commonly used Renilla and Gaussia luciferase, can produce luminescence background signal (6, 10). Bacterial luciferase is the only luciferase to circumvent all of these problems, since FMN is present in all cell types and can be converted into free FMNH2 by additional expression of an FMN reductase. Its main limitation is the poor brightness that is several orders of magnitude lower than that of other luciferases (11). Several attempts have been made to improve the brightness of bacterial bioluminescence, including splitting the lux operon for enhanced expression, codon optimization and additional expression of an FMN reductase in mammalian cells, and exogenous addition of the fatty aldehyde (12–15). However, to our knowledge, introduction of mutations in the luxCDABE operon to increase the brightness has so far been unsuccessful. Here, we show that bioluminescence from the lux operon from Photorhabdus luminescens expressed in E. coli can be substantially enhanced by coexpression of an additional FMN reductase and subsequent error-prone mutagenesis of the complete lux operon. The improved lux operon dubbed ilux can be used to image single E. coli cells for extended time periods and to assay cell viability in the presence of different antibiotics.
Results
Engineering and Characterization of the ilux Operon.
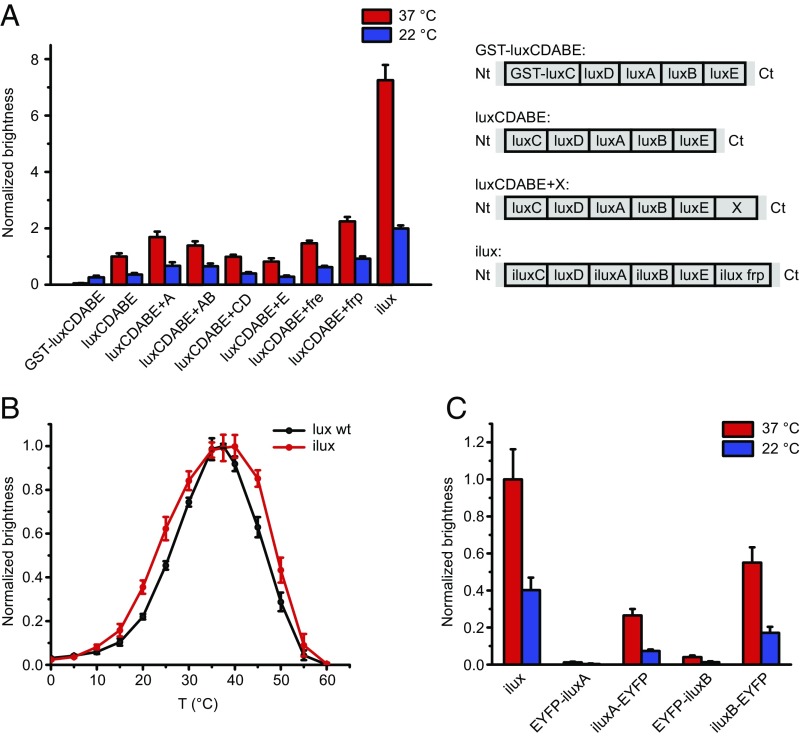
To engineer a bacterial bioluminescence system with improved brightness at 37 °C, we chose the luxCDABE operon from P. luminescens, as its luciferase has been reported to be more thermostable than Vibrio harveyi luciferase (ref. 16 and references therein). The P. luminescens luxCDABE operon was cloned into the vector pGEX-6P-1. Details of all primers used for cloning and error-prone PCR are contained in Table S1. Expression in E. coli DH5α cells resulted in only weakly luminescent colonies. Since the function of the fatty acid reductase coded by the luxC gene may be affected by the N-terminal GST tag contained in pGEX-6P-1, we expressed the luxCDABE operon from a GST-deleted version of this vector, dubbed pGEX(−). This increased the brightness by ∼40% at room temperature and ∼20-fold at 37 °C (Fig. 1A), suggesting that the activity of the fatty acid reductase is strongly inhibited by the relatively large GST tag at elevated temperature, possibly by inhibiting assembly of the fatty acid reductase complex. In addition, we observed that the bioluminescence signal increases with temperature, yielding two to three times more signal at 37 °C compared with room temperature (22 °C) (Fig. 1A).
Fig. 1.
Generation and comparison of lux variants with increased brightness. (A) Brightness of different lux variants. The lux operons schematically shown on the Right were expressed from the vector pGEX(−) in DH5α cells on LB agar plates at 37 °C. Plates were imaged at 37 °C and 22 °C. Error bars represent SDs of six different clones. Nt, N terminus; Ct, C terminus. (B) Temperature curves of luxCDABE WT and ilux. The bioluminescence signal from DH5α cells expressing luxCDABE WT or ilux was measured in suspension at various temperatures. Error bars represent SDs of six independent measurements. (C) Brightness of ilux with EYFP-tagged versions of luxA and luxB. EYFP was introduced into ilux pGEX(−) at the N and C termini of luxA and luxB separated by a glycine-serine linker. Brightness was measured in DH5α cells on LB agar plates and normalized to the brightness of unlabeled ilux at 37 °C. Error bars represent SDs of four different clones.
To further enhance the bioluminescence intensity, we sought to identify the rate-limiting enzymes of the bioluminescence reaction by cloning a second copy of luxAB, luxCD, and luxE as well as two different FMN reductases downstream of the luxCDABE operon and comparing the brightness with the original construct. The FMN reductase from E. coli coded by the fre gene and the NADPH-flavin oxidoreductase from Vibrio campbellii coded by the frp gene resulted in 1.5- and 2.3-fold increases in brightness, respectively (Fig. 1A). This suggested that the endogenous FMN reductase in E. coli does not regenerate sufficient amounts of FMNH2 for maximum levels of bioluminescence. We chose luxCDABE+frp pGEX(−) for mutagenesis and performed multiple rounds of error-prone PCR in the luxAB, luxCD, luxE, and frp genes. The resulting clones were screened for enhanced luminescence in DH5α cells on LB agar plates at 37 °C. We identified several mutations in luxA, luxB, luxC, and frp that resulted in higher bioluminescence signal, whereas no beneficial mutations in luxD and luxE were found. The final improved operon called ilux contains the mutations listed in Table 1 (complete amino acid sequences of the ilux proteins are in Fig. S1). E. coli cells expressing the WT lux operon (luxCDABE WT) and ilux exhibited identical bioluminescence emission spectra (Fig. S2). The brightness of ilux compared with luxCDABE WT was increased not only at 37 °C but also at room temperature (Fig. 1A). Maximum levels of bioluminescence from E. coli cells were observed at ∼37 °C for both luxCDABE WT and ilux, with a slightly broader temperature curve for ilux (Fig. 1B). Since we were not able to further improve the cellular signal intensity enabled by the ilux operon (“brightness of ilux”) by error-prone mutagenesis, we attempted to increase its brightness by bioluminescence resonance energy transfer from the luciferase to an acceptor with high fluorescence quantum yield. For this purpose, we chose the fluorescent protein EYFP [Φfl = 0.61 (17)] and fused it N- and C-terminally to both the luciferase α and β subunit. However, this did not improve the brightness (Fig. 1C).
Table 1.
Mutations contained in ilux
| Gene | Mutations |
| luxA | K22E, T119A, S178A |
| luxB | S13P, V121A, N259D |
| luxC | N10T, N59D, E74D, S256P, M355T, N360D |
| luxD | — |
| luxE | — |
| frp | M213L, R242L, K256R |
The listed mutations were introduced by error-prone PCR into the luxCDABE operon from P. luminescens supplemented with the frp gene from V. campbellii, resulting in the improved operon ilux.
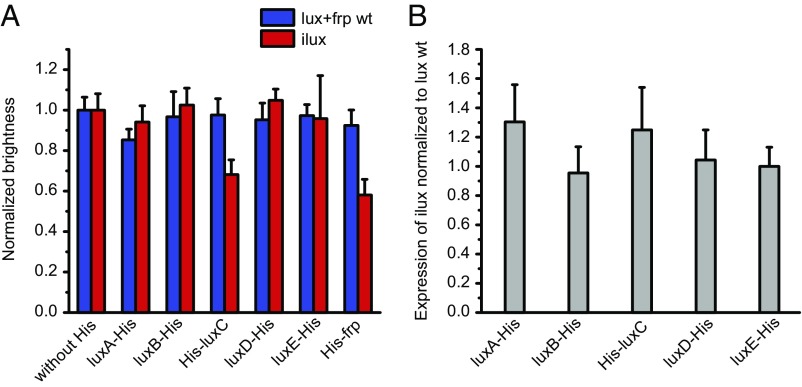
To investigate whether the increase in brightness of ilux is due to enhanced enzymatic activity or elevated expression of the lux proteins, we quantified protein levels by Western blot analysis of cell lysates (Fig. S3). Since antibodies for the lux proteins from P. luminescens were not available, single proteins in the luxCDABE WT and ilux operon were expressed with a C-terminal His tag and detected with an anti-His antibody. To control whether the His tag itself affects protein expression, the brightness of cells expressing the His-tagged lux operons was compared with the lux operon without His tag. The brightness of ilux luxC-His and frp-His was strongly reduced (Fig. S4), whereas for the other His-tagged proteins the brightness remained comparable with the nontagged operon (Fig. 2A). Therefore, luxC and frp were instead expressed with an N-terminal His tag, which influenced the brightness less strongly (Fig. 2A). However, the His antibody failed to detect His-frp (Fig. S3). Expression of the detectable functional fusion proteins luxA-His, luxB-His, His-luxC, luxD-His, and luxE-His in both luxCDABE+frp and ilux was quantified by Western blot (Fig. 2B). Whereas expression of luxA-His and His-luxC was increased by 30 and 25%, respectively, expression of luxB-His, luxD-His, and luxE-His remained unaffected. Therefore, the increased brightness of ilux seems to be partly due to enhanced expression and partly due to increased activity of the lux proteins.
Fig. 2.
Brightness and expression of His-tagged proteins in luxCDABE+frp and ilux. (A) Brightness of luxCDABE+frp and ilux expressed from pGEX(−) in DH5α cells. A His tag with a glycine-serine linker was introduced into the indicated proteins in the lux operons. Brightness was measured on LB agar plates and normalized to the corresponding nontagged lux operon. Error bars represent SDs of 10 different clones. (B) Expression of His-tagged lux proteins. Whole-cell lysates of the same clones as in A were analyzed by Western blot. The His signal of each clone was normalized to the housekeeping protein DnaK. Subsequently, the ilux signal was normalized to the signal of the same protein in the luxCDABE+frp WT operon. Error bars represent SD.
Imaging of Single E. coli Cells.
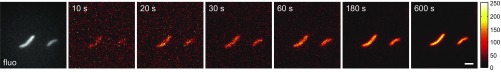
To obtain a higher brightness for imaging of single E. coli cells, the ilux operon was cloned into the vector pQE(−) and expressed in E. coli Top10 cells. pQE(−) was generated from pQE30 by deletion of the His tag. Expression of ilux from pQE(−) in Top10 cells resulted in a 2.0-fold higher signal at room temperature compared with ilux pGEX(−) in DH5α on LB agar plates (Fig. S5). Although bioluminescence emission was two- to threefold higher at 37 °C, the imaging was performed at room temperature for technical simplicity. Single Top10 cells could already be discriminated after exposure times of only 10–20 s (Fig. 3). Longer exposure times resulted in significantly improved signal-to-noise ratio. A calibration of the camera indicated that 100–200 photons per pixel were detected during a 10-min exposure time, corresponding to 103–104 detected photons per cell per minute.
Fig. 3.
E. coli Top10 cells expressing ilux imaged for different exposure times. Bioluminescence images of E. coli Top10 cells expressing ilux were taken with the indicated exposure times. A fluorescence (fluo) image excited with a 405-nm laser is shown in gray. For the 600-s image, the color bar represents the number of detected photons per pixel. For the other images, the color map was scaled to the minimum and maximum pixel values. (Scale bar: 2 µm.)
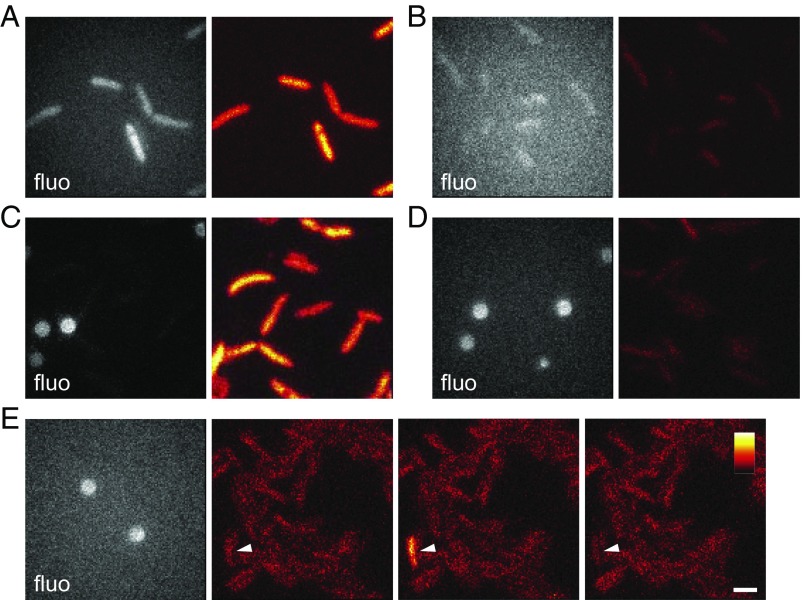
We compared the brightness of single Top10 cells expressing ilux and luxCDABE WT. Whereas cells expressing ilux could clearly be discriminated after exposure times of 10 min (Fig. 4A), luxCDABE WT provided only little signal above background both in the fluorescence and in the bioluminescence images (Fig. 4B). In addition, we compared the brightness of ilux with the widely used firefly luciferase (FLuc) (Fig. 4 C and D). High concentrations of d-Luciferin of 1 mg/mL were required to visualize the cells, presumably because of low penetration through the bacterial cell wall and membrane. Still, the brightness of FLuc was six to eight times lower than that of ilux. In addition, the brightness of FLuc was not constant over time, but single cells sometimes became suddenly much brighter (Fig. 4E). The reason for this is not clear but may be due to increased uptake of the luciferin, since a similar effect was not observed for ilux. Together, this shows that ilux outperforms both luxCDABE WT and FLuc for imaging of single E. coli cells due to its superior brightness and stability of the signal.
Fig. 4.
Comparison of ilux with luxCDABE WT and FLuc. (A and B) Comparison of brightness of E. coli Top10 cells expressing (A) ilux or (B) luxCDABE WT. The same color map was used for both bioluminescence images. (C and D) Comparison of brightness of E. coli Top10 cells expressing (C) ilux or (D) FLuc. The same color map was used for both bioluminescence images. (E) E. coli Top10 cells expressing FLuc. A cell with a sudden increase and decrease of brightness between three consecutive images is indicated. The same color map was used for all three bioluminescence images. All bioluminescence images were taken with an exposure time of 10 min. Fluorescence (fluo) images excited with a 405-nm laser are shown in gray. For FLuc, fluorescent beads were used for focusing. In the bioluminescence images, the color map was scaled to the minimum and maximum pixel values of A and C and the third column in E. Fig. S6 shows B and D scaled to the minimum and maximum pixel values. (Scale bar: 2 µm.)
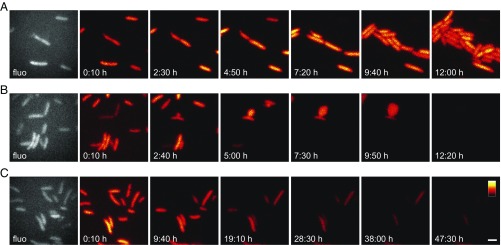
Next, we aimed at investigating the utility of ilux for the observation of single bacteria for extended periods of time. Most cells remained viable over the whole recording time of 12 h and divided several times while maintaining an almost constant bioluminescence signal (Fig. 5A). Subsequently, we investigated the effect of different antibiotics on cell viability. Since continuous supply of ATP and NADPH is required for the regeneration of fatty aldehyde and FMNH2 to keep the bioluminescence reaction ongoing, the signal is expected to disappear on cell death. We imaged Top10 cells in the presence of timentin, a mixture of the β-lactame antibiotic ticarcillin and clavulanic acid. Since pQE(−) contains a β-lactamase resistance marker, cells expressing ilux are expected to be resistant to ticarcillin. However, since the β-lactamase is inhibited by clavulanic acid, the cells become susceptible for the cell wall-disrupting effects of ticarcillin and ampicillin. On cell division, this leads to the formation of small holes in the cell wall. As a result, the inner membrane occasionally forms large protrusions due to osmotic pressure (Fig. 5B). This finally leads to cell lysis. After 12 h, all cells in the field of view had died.
Fig. 5.
E. coli Top10 cells expressing ilux in the presence of different antibiotics. E. coli Top10 cells expressing ilux were imaged under an LB agar pad containing 50 µg/mL ampicillin and (A) no additional antibiotics, (B) 100 µg/mL timentin, or (C) 100 µg/mL kanamycin. Single images were taken with 10-min exposure time. Fluorescence (fluo) images excited with a 405-nm laser are shown in gray. The same color map was used for all bioluminescence images in each row. Complete time series are shown in Movies S1–S3. (Scale bar: 2 µm.)
The second antibiotic that we examined was kanamycin, an inhibitor of protein synthesis (Fig. 5C). The brightness decreased continuously, consistent with a reduction of protein levels of the lux enzymes. Most cells died within the 48-h observation time; nevertheless, bioluminescence was still detectable from a few cells. This shows that, even at high kanamycin concentrations of 100 µg/mL, cellular metabolism continues for relatively long timespans, although cell division is prevented immediately.
Interestingly, we often observed “blinking” of cells before cell death. The signal from cells that had already disappeared often recovered, sometimes even between two 10- or 3-min frames (Fig. 6A, Fig. S7, and Movies S1–S4). This effect was most pronounced in kanamycin-treated cells, where blinking often occurred repeatedly (Movies S3 and S4), but it was also occasionally observed in dying cells without additional antibiotics (Movie S1). Blinking was not affected by the presence of ampicillin and was not observed on kanamycin treatment of kanamycin-resistant cells (Movie S5). Blinking cells often continued living for many hours. To determine if blinking results from altered levels of the ilux proteins or variations in substrate concentrations, we imaged cells expressing ilux with an EYFP-tagged version of luxB in the presence of kanamycin. Fluorescence images of EYFP were taken between the bioluminescence images to determine possible alterations of the luxB-EYFP protein concentration. Some of the cells that irreversibly lost their bioluminescence signal also exhibited a loss of EYFP fluorescence, whereas other cells retained EYFP fluorescence (Fig. S8A). Blinking cells always retained EYFP fluorescence (Fig. S8B), showing that the protein concentration remains constant. Therefore, we conclude that blinking is caused by rapid fluctuations in metabolite concentrations, most likely ATP or NADPH. To test this hypothesis, we coexpressed the fluorescent ATP biosensor QUEEN-2m with the ilux proteins (18). QUEEN-2m fluorescence excited at 405-nm increases with ATP concentration, whereas its fluorescence excited at 490-nm decreases (18). Although QUEEN-2m was designed as a ratiometric ATP sensor, we used its indicator properties at just one excitation wavelength of 491 nm. This allowed us to disentangle the ATP signal from the fluorescence from ilux-expressing cells on excitation at 405 nm, while additionally reducing phototoxicity and bioluminescence bleaching. Bioluminescence and fluorescence images were recorded alternately (Fig. 6B). When the bioluminescence signal declined during blinking, the fluorescence signal simultaneously increased. This indicates that the loss of bioluminescence signal during kanamycin-induced cell death in blinking cells is accompanied by a decrease in ATP concentration.
Fig. 6.
Blinking of E. coli Top10 cells during kanamycin-induced cell death. E. coli Top10 cells were imaged under an LB agar pad containing 50 µg/mL ampicillin and 100 µg/mL kanamycin. (A) Bioluminescence of ilux-expressing cells was imaged with 10-min exposure time. A fluorescence (fluo) image excited with a 405-nm laser is shown in gray. The same color map was used for all bioluminescence images. For the indicated cells, the normalized signal is plotted over time. The complete time series is shown in Movie S4. (B) Bioluminescence (BL) images of Top10 cells expressing ilux and the ATP sensor QUEEN-2m were taken with 3-min exposure time. Fluo of QUEEN-2m was excited at 491 nm and recorded between the bioluminescence images. The first fluo and BL images are displayed. For the indicated cell, the normalized signal after subtraction of the background signal outside the cell area is plotted over time. (Scale bars: 2 µm.)
Discussion and Conclusions
Our results show that bacterial bioluminescence from E. coli cells can be enhanced by mutagenesis of the luxCDABE genes in combination with introduction of an additional FMN reductase. This allows imaging of single E. coli cells with improved spatiotemporal resolution in comparison with previous approaches of single-cell imaging using bacterial bioluminescence (3, 15, 19) without the need of exogenous aldehyde supply. Since the brightness of ilux is increased two- to threefold at 37 °C compared with room temperature, heating of the sample during imaging is expected to reduce the necessary recording times further. We have shown that ilux can be used to observe processes, such as division and death of single E. coli cells, extending the range of applications of bacterial bioluminescence imaging.
The spreading of bacterial antibiotic resistances is becoming an increasing problem for the treatment of human diseases. Therefore, the development of improved and new antibiotics as well as methods to investigate their influence on bacterial viability are urgently required. Taking individual differences between bacteria in the response to antibiotics into account, these methods should preferentially examine bacteria on the single-cell level. A commonly used test for the viability of individual bacteria is the staining with membrane-impermeable dyes, such as propidium iodide (PI), assuming that the dye only enters dead cells with impaired membrane integrity. However, this does not necessarily reflect their metabolic state. Using a FRET-based ATP biosensor, it has been found that not all PI-negative cells of Mycobacterium smegmatis exhibit high ATP levels (20), indicating that the membrane may remain intact after cell death. In addition, the authors also observed PI-negative cells with high ATP levels that did not resume growth after antibiotic washout, showing the need to distinguish between membrane integrity, metabolic activity, and the ability of bacteria to divide; ilux provides a means for continuous long-term imaging of single bacteria that simultaneously provides information about the metabolic state of the cell.
Unexpectedly, we observed that kanamycin-treated dying cells that lost their bioluminescence can temporarily recover. Given that the protein concentration of luxB remains constant during this process and that kanamycin would inhibit the synthesis of new functional proteins, it seems likely that this blinking is not caused by changes in cellular protein levels but rather by altered substrate concentrations. Fluorescence imaging with the ATP sensor QUEEN-2m showed that the loss of bioluminescence during blinking is correlated with a decrease in the cellular ATP concentration. A possible explanation for this observation is a breakdown of the proton gradient due to the transient formation of membrane defects, which might inhibit ATP synthesis. Indeed, it has been described that aminoglycoside antibiotics can increase cellular permeability by the incorporation of mistranslated membrane proteins (21, 22). Therefore, cell death in kanamycin-treated cells might be the final result of pronounced membrane damage. This would also explain the rapid loss of EYFP fluorescence observed in some kanamycin-treated cells expressing luxB-EYFP, as membrane defects might lead to leakage of cellular proteins. Although different explanations for the blinking cannot be excluded, this effect points at interesting applications of ilux by providing information about the metabolic activity of the cell.
The independence from exogenous luciferin makes the lux system particularly interesting for long-term imaging studies, although its utility has so far been limited by its low brightness compared with other luciferases. Codon-optimized versions of the lux proteins have been shown to be functional in eukaryotic cells (13, 14), facilitating observation of bacterial bioluminescence from cell types other than bacteria. Therefore, ilux holds promise as a valuable future tool for the observation of mammalian cells as well. In addition, it might be possible to image cellular structures by fusing the luciferase to a protein of interest, allowing its usage in a similar way as fluorescent proteins.
Materials and Methods
Details of the cloning and mutagenesis of the lux constructs, measurement of temperature curves, Western blot analysis, and the imaging are described in SI Materials and Methods. Briefly, bioluminescence imaging was performed on a custom microscopy setup (Fig. S9) equipped with an oil immersion objective (1.4 N.A.) and an electron multiplying charge-coupled device (EMCCD) camera. The setup additionally contained laser sources for wide-field fluorescence excitation at 405 and 491 nm and a focus lock system for long-term imaging.
Supplementary Material
Acknowledgments
We thank Prof. Hiromi Imamura (Kyoto University) for donation of the QUEEN-2m sensor. We thank Dr. Grazvydas Lukinavicius, Torsten Hartmann, and Dr. Waja Wegner for helpful discussions and critical reading of the manuscript and Jasmin Pape for assistance with microscope building. K.C.G. received funding through a graduate scholarship by the Cusanuswerk.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1715946115/-/DCSupplemental.
References
- 1.Haddock SH, Moline MA, Case JF. Bioluminescence in the sea. Annu Rev Mar Sci. 2010;2:443–493. doi: 10.1146/annurev-marine-120308-081028. [DOI] [PubMed] [Google Scholar]
- 2.Welsh DK, Noguchi T. Cellular bioluminescence imaging. Cold Spring Harb Protoc. 2012 doi: 10.1101/pdb.top070607. [DOI] [PubMed] [Google Scholar]
- 3.Mihalcescu I, Hsing W, Leibler S. Resilient circadian oscillator revealed in individual cyanobacteria. Nature. 2004;430:81–85. doi: 10.1038/nature02533. [DOI] [PubMed] [Google Scholar]
- 4.Agulhon C, et al. Bioluminescent imaging of Ca2+ activity reveals spatiotemporal dynamics in glial networks of dark-adapted mouse retina. J Physiol. 2007;583:945–958. doi: 10.1113/jphysiol.2007.135715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teranishi K, Shimomura O. Solubilizing coelenterazine in water with hydroxypropyl-β-cyclodextrin. Biosci Biotechnol Biochem. 1997;61:1219–1220. [Google Scholar]
- 6.Shimomura O, Kishi Y, Inouye S. The relative rate of aequorin regeneration from apoaequorin and coelenterazine analogues. Biochem J. 1993;296:549–551. doi: 10.1042/bj2960549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Wet JR, Wood KV, DeLuca M, Helinski DR, Subramani S. Firefly luciferase gene: Structure and expression in mammalian cells. Mol Cell Biol. 1987;7:725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inoue Y, et al. Gaussia luciferase for bioluminescence tumor monitoring in comparison with firefly luciferase. Mol Imaging. 2011;10:377–385. doi: 10.2310/7290.2010.00057. [DOI] [PubMed] [Google Scholar]
- 9.Stacer AC, et al. NanoLuc reporter for dual luciferase imaging in living animals. Mol Imaging. 2013;12:1–13. [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao H, et al. Characterization of coelenterazine analogs for measurements of Renilla luciferase activity in live cells and living animals. Mol Imaging. 2004;3:43–54. doi: 10.1162/15353500200403181. [DOI] [PubMed] [Google Scholar]
- 11.Close DM, et al. Comparison of human optimized bacterial luciferase, firefly luciferase, and green fluorescent protein for continuous imaging of cell culture and animal models. J Biomed Opt. 2011;16:047003. doi: 10.1117/1.3564910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yagur-Kroll S, Belkin S. Upgrading bioluminescent bacterial bioreporter performance by splitting the lux operon. Anal Bioanal Chem. 2011;400:1071–1082. doi: 10.1007/s00216-010-4266-7. [DOI] [PubMed] [Google Scholar]
- 13.Close DM, et al. Autonomous bioluminescent expression of the bacterial luciferase gene cassette (lux) in a mammalian cell line. PLoS One. 2010;5:e12441. doi: 10.1371/journal.pone.0012441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu T, Ripp S, Sayler GS, Close DM. Expression of a humanized viral 2A-mediated lux operon efficiently generates autonomous bioluminescence in human cells. PLoS One. 2014;9:e96347. doi: 10.1371/journal.pone.0096347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sternberg C, Eberl L, Poulsen LK, Molin S. Detection of bioluminescence from individual bacterial cells: A comparison of two different low-light imaging systems. J Biolumin Chemilumin. 1997;12:7–13. doi: 10.1002/(SICI)1099-1271(199701/02)12:1<7::AID-BIO427>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 16.Westerlund-Karlsson A, Saviranta P, Karp M. Generation of thermostable monomeric luciferases from Photorhabdus luminescens. Biochem Biophys Res Commun. 2002;296:1072–1076. doi: 10.1016/s0006-291x(02)02052-1. [DOI] [PubMed] [Google Scholar]
- 17.Patterson G, Day RN, Piston D. Fluorescent protein spectra. J Cell Sci. 2001;114:837–838. doi: 10.1242/jcs.114.5.837. [DOI] [PubMed] [Google Scholar]
- 18.Yaginuma H, et al. Diversity in ATP concentrations in a single bacterial cell population revealed by quantitative single-cell imaging. Sci Rep. 2014;4:6522. doi: 10.1038/srep06522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phiefer CB, Palmer RJJ, Jr, White DC. Comparison of relative photon flux from single cells of the bioluminescent marine bacteria Vibrio fischeri and Vibrio harveyi using photon-counting microscopy. Luminescence. 1999;14:147–151. doi: 10.1002/(SICI)1522-7243(199905/06)14:3<147::AID-BIO532>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 20.Maglica Ž, Özdemir E, McKinney JD. Single-cell tracking reveals antibiotic-induced changes in mycobacterial energy metabolism. MBio. 2015;6:e02236–14. doi: 10.1128/mBio.02236-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis BD, Chen LL, Tai PC. Misread protein creates membrane channels: An essential step in the bactericidal action of aminoglycosides. Proc Natl Acad Sci USA. 1986;83:6164–6168. doi: 10.1073/pnas.83.16.6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kohanski MA, Dwyer DJ, Collins JJ. How antibiotics kill bacteria: From targets to networks. Nat Rev Microbiol. 2010;8:423–435. doi: 10.1038/nrmicro2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.