Significance
To survive, an organism must adjust its behavior based upon past experiences. In Drosophila, aggression affects fitness as it ensures access to food and mating resources. Here, we show that upon repeated aggressive encounters, males adopt a winner or loser state that shows the qualities of persistence and generalization. Winning is perceived as rewarding, while losing is aversive. We also demonstrate that the activity of specific dopamine neurons is needed for males to avoid an odor previously paired with losing. Although the effects of losing and winning have been extensively studied in other species, our work advances the use of Drosophila as a model for circuit dissection of internal states that promote behavioral changes associated with winning or losing fights.
Keywords: Drosophila, aggression, valence, generalization, persistence
Abstract
Multiple studies have investigated the mechanisms of aggressive behavior in Drosophila; however, little is known about the effects of chronic fighting experience. Here, we investigated if repeated fighting encounters would induce an internal state that could affect the expression of subsequent behavior. We trained wild-type males to become winners or losers by repeatedly pairing them with hypoaggressive or hyperaggressive opponents, respectively. As described previously, we observed that chronic losers tend to lose subsequent fights, while chronic winners tend to win them. Olfactory conditioning experiments showed that winning is perceived as rewarding, while losing is perceived as aversive. Moreover, the effect of chronic fighting experience generalized to other behaviors, such as gap-crossing and courtship. We propose that in response to repeatedly winning or losing aggressive encounters, male flies form an internal state that displays persistence and generalization; fight outcomes can also have positive or negative valence. Furthermore, we show that the activities of the PPL1-γ1pedc dopaminergic neuron and the MBON-γ1pedc>α/β mushroom body output neuron are required for aversion to an olfactory cue associated with losing fights.
Although the study of emotions has been of great interest to neuroscience and psychology, there is still not a clear agreement on a definition of emotion. However, it is generally accepted that emotions, or emotion states, are associated with profound changes in behavior (1). In higher order animals, the emotion state of an organism can be inferred based upon its behavioral reactions, such as vocalizations and facial expressions. Recently, Anderson and Adolphs (2) proposed that emotions can be viewed as expressions of the internal state of an organism, and that this state can be studied by its characteristics of persistence, generalization, valence, and scalability. The availability of a vast number of neurogenetic tools in Drosophila melanogaster make it a suitable model organism in which to study the causal relationship between internal states and observable behavior.
In their natural environment, male fruit flies compete with each other for the acquisition and defense of food resources, and potential mates (3, 4). In Drosophila, these fights include a series of complex and stereotyped aggressive behaviors, such as fencing, wing threat, chasing, lunging, holding, boxing, and tussling (5). In addition, the experience of winning or losing affects the outcome of subsequent fights: Winners are more likely to win, while losers are more likely to lose (6, 7). Different neuronal groups, such as octopamine (8–10), serotonin (11), dopamine (12), neuropeptide F (13), tachykinin (14), and P1 neurons (15), have been implicated in regulating aggressive behavior. Interestingly, activation of P1 neurons not only induces aggression, but also triggers an internal state where flies remain hyperaggressive even after the activation of the neurons has ceased (15).
While multiple studies have focused on the mechanisms of aggressive behaviors in Drosophila (8–15), less is known about the effects of repeated fighting experience and the underlying neural circuits. Here, we show that the experience of repeatedly winning or losing fights induces an internal state with the properties of persistence and generalization. Winning and losing also have associated positive and negative valence, respectively. Moreover, we show that the activities of the protocerebral posterior lateral 1 (PPL1)-γ1pedc dopaminergic neuron and the MBON-γ1pedc>α/β mushroom body output neuron are required for the formation of an aversive memory associated with losing.
Results
Generation of Chronic Winners and Losers.
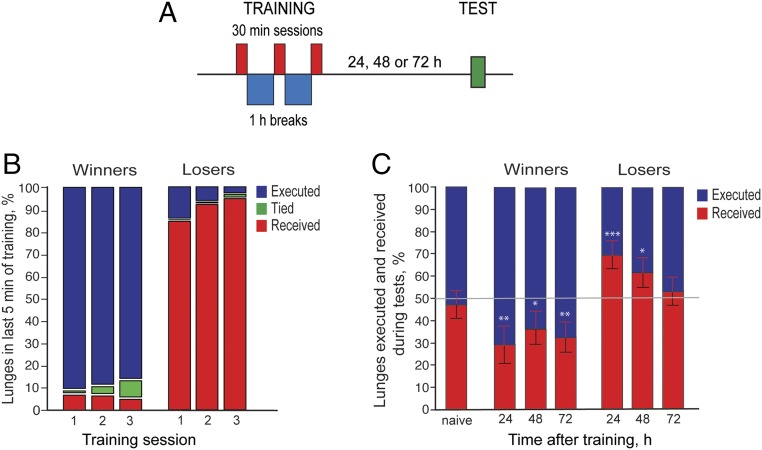
To investigate the effects of fighting experience on behavior, we aimed to generate chronic winners and losers by pairing socially naive wild-type Canton S (CS) males with hypoaggressive or hyperaggressive males, respectively. We found that, compared with genetic controls, males in which the receptor 1 for the neuropeptide diuretic hormone 44 (DH44; ref. 16) is down-regulated in DH44R1 neurons (DH44R1 > DH44R1RNAi) are highly aggressive after having been singly housed (SH), executing ∼210 total lunges in a 30-min period toward SH naive CS males. The same males are hypoaggressive (executing ∼10 lunges in 30 min) after having been group housed (GH) in the presence of virgin females (Fig. S1A). The number of lunges, which is a good proxy for aggression, was quantified using the CADABRA software (17). Winners were generated by pairing SH naive wild-type CS males with GH DH44R1 > DH44R1RNAi males for three 30-min periods separated by an hour of rest; losers were generated in an analogous manner by paring SH CS males with SH DH44R1 > DH44R1RNAi males (Fig. 1A). Social hierarchies were quickly established during the first training session with winners lunging at hypoaggressive males and losers trying to escape from hyperaggressive males; a new hypoaggressive or hyperaggressive DH44R1 > DH44R1RNAi male was added for each training session. We found that the fraction of winners (paired with hypoaggressive males) receiving lunges in the last 5 min of each training session (when dominance relationships are already established) decreased throughout the training sessions, although a few naive males still executed lunges. The converse was seen with losers paired with hyperaggressive males (Fig. 1B). In a small fraction of fights, flies appeared to not have established dominance relationships as manifested by nearly equal number of lunges being executed and received. To select flies for further experiments, we scored the average number of lunges in the three 30-min training sessions; we chose as losers those males that in fights with hyperaggressive flies had received an average of 100–200 lunges in the three training sessions, and as winners those that had executed on average 50–125 lunges against hypoaggressive males (Fig. S1B).
Fig. 1.
Generation of a persistent winner and loser effect. (A) A single SH CS male was paired with a single opponent fly (hypoaggressive or hyperaggressive male) in three 30-min sessions, with a 1 h of rest in between sessions. Unfamiliar opponents were used in each fighting encounter. The winner or loser effect was tested 24, 48, or 72 h afterward. (B) Proportion of lunges received or executed during the last 5 min of training sessions of winners (n = 851) or losers (n = 897). A small proportion of fights in each group showed no dominance (tied). These males were used for all experiments described in this paper. (C) Trained flies were individually paired with naive SH CS males 24, 48, or 72 h after the last training session and the proportion of lunges executed or received quantified. The winner effect persisted for at least 72 h (one sample t test against 50%; nwinners = 19, 25, 35 at each respective time point; 24 h: **P < 0.01; 48 h: *P < 0.05; 72 h: **P < 0.01), while the loser effect persisted for 48 h (one sample t test against 50%; nlosers = 34, 37, 35 at each respective time point; 24 h: ***P < 0.001; 48 h: *P < 0.05; 72 h: P > 0.05). On average, the proportion of executed and received lunges for two naive SH CS males (left bar) was near 50% during the test period (one-sample t test, P > 0.05).
Because of the vigor of the three successive aggressive encounters, we were concerned that winners and/or losers may be adversely affected more generally by their fighting experience. Flies were allowed to rest for 24 h, which is the shortest time interval used for subsequent behavioral assays, before being tested for their startle-induced climbing ability and locomotor activity in the Drosophila Activity Monitor. Both measures of activity were found to be the same among winners, losers, and naive CS males (Fig. S2).
The Experiences of Winning and Losing Generate a Long-Lasting Internal State.
To determine if successive rounds of winning or losing induce the formation of a long-lasting internal state, we paired either winners or losers with age-matched naive SH CS males 24, 48, and 72 h after training (Fig. 1C). In fights between two naive CS males, they each executed and received on average the same number of lunges. Winners and losers executed and received significantly more lunges than their naive opponents, respectively, when tested 24 h after training. A significant difference was still observed 48 h after training for both winners and losers. After 72 h, the losers had returned to their naive levels of lunging, while the winners still lunged more than their naive opponents (Fig. 1C). Therefore, the effect of winning and losing persisted for substantial periods of time in our paradigm. These results differ somewhat from a previous report in which repeated aggressive encounters produced long-lasting loser but not winner effects (7); this is most likely due to differences in training protocols.
The Experiences of Winning or Losing Have Opposite Valence.
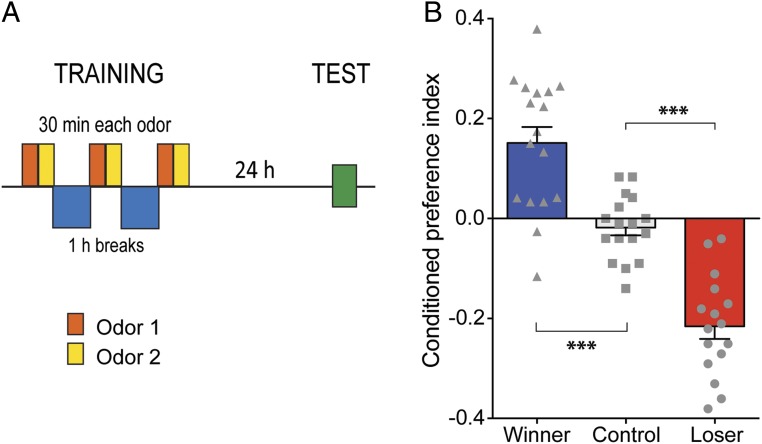
To test if different fighting experiences have an associated valence, we trained flies to associate an olfactory cue with the experience of repeatedly winning (pairing with a hypoaggressive fly) or with the experience of repeatedly losing (pairing with a hyperaggressive fly). Single naive CS males were trained with three sessions separated by 1 h, with each session consisting of a 30-min exposure to odor 1 (without opponent) followed by a 30-min exposure to odor 2 paired with either a single hypoaggressive or a single hyperaggressive opponent for generating winners or losers, respectively (Fig. 2A). When the memory was tested 24 h after training, we found that winners showed a conditioned preference, while losers showed a conditioned aversion for the odor previously paired with the fighting experience (Fig. 2B). As expected, control flies (exposed to odors alone) did not show a conditioned response (Fig. 2B). To determine if potential pheromonal differences between hypoaggressive and hyperaggressive males, experienced by winners and losers during training, maybe be responsible for the conditioned responses, we trained SH CS males with decapitated opponents. We observed no conditioned responses under these conditions indicating that the pheromonal signature of the hypoaggressive and hyperaggressive opponents was not sufficient for memory formation in our assay (Fig. S3). In conclusion, winning and losing have opposite valences, with winning being perceived as rewarding and losing being perceived as aversive.
Fig. 2.
Valence of the winning and losing. (A) Naive SH CS males were trained to associate a particular fighting experience with an olfactory cue in three sessions, each consisting of a 30-min exposure to odor 1 (without opponent) followed by a 30-min exposure to odor 2 paired with the presence of an opponent fly: hypoaggressive male opponents were used to generate winners and hyperaggressive opponents were used to generate losers. New opponents were used in each training session. Flies were given a 1-h rest between sessions, and the memory tested 24 h after the end of training. Control flies were exposed to the two olfactory cues in the absence of opponents. (B) Compared with control flies (n = 17 experiments with groups of 24 males each), winners showed a conditioned preference (n = 17; ***P < 0.001), whereas losers showed a conditioned aversion (n = 16; ***P < 0.001) for the odor previously associated with the respective fighting experience. One-way ANOVA followed by Tukey’s multiple comparisons test.
The Fighting-Induced Internal State Generalizes to Other Behaviors.
We next asked if the internal state generated by either losing or winning fights had the quality of generalization and, therefore, impacted the expression of other behaviors. To assess this, we tested the winners and losers in three behavioral paradigms.
Risk-taking behavior.
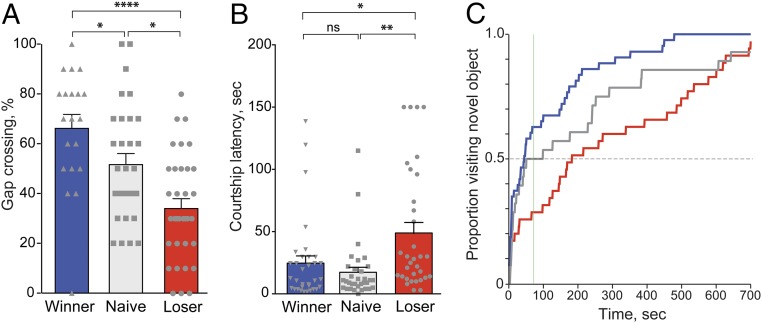
The Gap-crossing assay measures the willingness/ability of a fly (with its wings removed) to cross a gap in its trajectory, a task that is believed to depend on the capacity of the flies to integrate multiple sensory cues (18, 19). In our case, individual winners, losers, and naive males were given 10 trials to cross a 3-mm gap, a gap breadth where naive CS flies showed an ∼50% success rate, and the number of trials in which flies successfully crossed the gap was quantified. As shown in Fig. 3A (and Fig. S4), winners showed a higher rate of successful crossings than losers. This increased crossing by winners was mirrored by a significant decrease in returns (aborted attempts) and also falls during attempted crossings (Fig. S4). Both winners and losers showed statistically different behavior from naive control males in their crossing success rate. Thus, prior fighting experience affected the flies’ central sensory processing such that winners were more willing/able to cross a challenging gap than losers.
Fig. 3.
Generalization of the loser and winner effect. Testing loser and winner flies in three different behavioral paradigms showed that the effect of persistently losing or wining fights extended to other behaviors. All behaviors were assayed 24 h after training. (A) Gap crossing. Winners and losers showed increased and decreased success, respectively, in crossing a 3-mm gap compared with naive CS males. Steel–Dwass multiple comparison test after nonparametric ANOVA, *P < 0.05, ****P < 0.0001; nloser = 34, nwinner = 20, nnaive = 31. (B) Courtship. Losers exhibited significantly prolonged courtship latency compared with naive and winner flies, when paired with wild-type virgin females. Tukey’s multiple comparisons test after one-way ANOVA: *P < 0.05; **P < 0.01, n = 33 for all groups. (C) Object exploration. A greater number of flies with repeat winning experience (blue; n = 35) had moved toward and climbed the post by 76 s into the observations (green vertical line; t50, based on concurrently run naive SH CS males, gray; n = 28), than those that had chronically lost (red; n = 43), χ2, P = 0.0026. Prior fighting experience appeared to affect the exploration of males in comparison with naive males; however, the trends failed to be statistically significant: At t50 (the time at which 50% of flies had reached the post), 63% of flies with repeat winning experience had found the post (winners vs. naive; χ2, P = 0.2863) and yet only 29% of the chronic losers (losers vs. naive; χ2, P = 0.0818). ns, nonsignificant.
Courtship.
Next, we investigated the effects of winning or losing fights on courtship behavior. Individual winners or losers were paired with a 4-d-old virgin female in a courtship arena. Aspects of courtship behavior were quantified by measuring the time for the male to display the first wing extension (courtship latency), the total amount of time the male spends performing courtship behavior over a 10-min period (courtship index), and copulation latency (the cumulative time to copulation). Compared with naive controls, losers had significantly increased courtship latency (Fig. 3B) and reduced courtship index (Fig. S5A). Winners showed significantly higher total copulation (in the 10 min of the assay) than naive males (Fig. S5B). We conclude that the experience of winning and losing fights affects the propensity for courtship and is consistent with a previous report (20) suggesting that losers have a lowered drive to court females than winners.
Object exploration.
The tendency of walking flies to turn toward vertical shapes (21) and their preference for tall objects (22) led us to design an assay to study the exploration of flies within an arena that included a tall, novel object (Fig. S6 A–C). We observed that winners were significantly faster than losers in reaching and ascending the post (Fig. 3C). Naive males showed an intermediate phenotype that was not significantly different from either winners or losers. Winners moved toward and climbed the post nearly 200 s faster than losers (Fig. S6D) and also traveled along a shorter path before climbing the post (Fig. S5E). All groups, however, showed similar levels of general activity (total distance traveled during the assay) (Fig. S6F), consistent with our observations described previously (Fig. S2). We conclude that winners exhibit enhanced directed movement toward attractive objects relative to males who have repeatedly lost, and this difference is not due to changes in the general activity of the flies.
Dopaminergic Neurons in PPL1 Cluster Are Required for Aversive Memory.
Multiple studies have shown that dopaminergic neurons in the PPL1 cluster that innervate mushroom body (MB) lobes are required for aversive memory formation (23–25). To investigate if these neurons are also required for odor memory associated with losing fights, we used the MB438B-GAL4 driver (26), to express Shibirets1 (Shits1) (27) in the PPL1-γ1pedc dopaminergic neuron. Shits1 is a dominant-negative and temperature-sensitive variant of dynamin that blocks neuronal activity at temperatures at or above 29 °C, thus allowing for the conditional silencing of neurons.
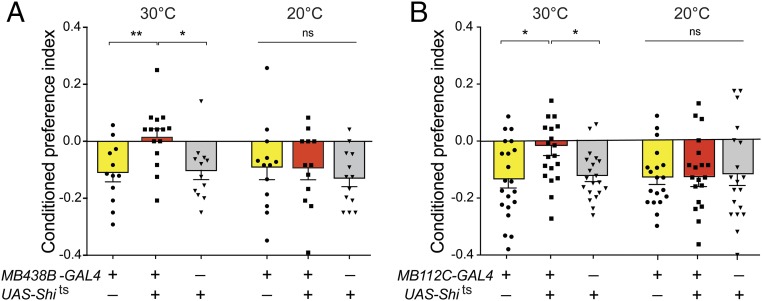
MB438B-GAL4 > shits1 and genetic control males were SH for 6 d at 20 °C (Shits1 inactive), after which they were trained as losers (three 30-min pairings with a hyperaggressive male) while also being exposed to an olfactory cue. Training was performed at either 30 °C or 20 °C, and the memory was tested 24 h later at 20 °C. When trained at the restrictive temperature (30 °C), MB438B-GAL4 > shits1 males showed a reduced conditioned aversion compared with genetic control males (Fig. 4A). This effect was not observed when the training was carried out at 20 °C (Fig. 4A).
Fig. 4.
The activities of PPL1- γ1pedc dopamine neuron and MBON-γ1pedc>α/β mushroom body output neurons are required for the memory of a cue associated with losing. (A) When trained at the restrictive temperature (30 °C), but not the permissive temperature (20 °C), MB438B-GAL4 > shits (PPL1-γ1pedc blocked) showed a reduced conditioned aversion for an olfactory cue associated with the experience of losing. *P < 0.05, **P < 0.01; n = 12–16. (B) Similar results were obtained with MB112C-GAL4 > shits1 (MBON-γ1pedc>α/β blocked). *P < 0.05; n = 20–21. One-way ANOVA was followed by the Tukey’s multiple comparisons test. Tests were carried out 24 h after training. ns, nonsignificant.
Each lobe of the MB is divided into compartments that are innervated by specific dopamine neurons and output neurons (MBONs) (26). To determine if the MBON-γ1pedc>α/β neuron, the output neuron for the γ1 compartment, is required for the association of an odor with losing, we used the MB112C-GAL4 driver (26) to express Shits1 in this neuron. MB112C-GAL4 > shits1 males trained at the restrictive temperature (30 °C) showed a reduced conditioned aversion compared with genetic control males (Fig. 4B). No difference among groups was observed when the training was performed at the permissive temperature (20 °C) (Fig. 4B). Overall, we conclude that both the PPL1-γ1pedc dopamine neuron and the MBON-γ1pedc>α/β output neuron are required during the formation of the aversive memory of an odor paired with losing aggressive encounters.
Discussion
In this study, we use criteria outlined by Anderson and Adolphs (2) (persistence and generalization) to show that repeated aggressive encounters induce the formation of an internal state with long-term behavioral consequences in the fruit fly D. melanogaster. We also show that losing and winning have opposite valences, with winning being perceived as rewarding and losing as aversive. This work extends previous accounts (7, 20) of the formation of winner and loser states in the fly and begins a description of the underlying neuroanatomy.
The Winner and Loser Internal State.
The winner and loser effect has been documented in several species (reviewed in ref. 28): Winning individuals are more likely to win, and losing individuals are more likely to lose subsequent aggressive encounters. Long-lasting effects of losing fights have been described in rodents using the resident-intruder paradigm (reviewed in ref. 29). In this paradigm, social defeat has been shown to produce social avoidance, reduced exploration, and anxiety-like behavior (29). The evolutionary advantage of this effect is unclear, although it is thought to play a role in the establishment of social hierarchies (30) and in the process by which individuals learn their own fighting abilities (31). Consistent with the idea of learning from fighting encounters, our experiments show not only the formation of persistent winner and loser states (Fig. 1), but also that males learn to associate value or valence with these states (Fig. 2). To determine whether the winner and/or loser states generalize to other behaviors, we tested winners and losers in three different behavioral assays: gap-crossing [thought to measure risk-taking behavior; refs. 18 and 19)], courtship, and object exploration (Fig. 3 and Figs. S4–S6). While winners and losers showed statistically significant differences in behavior in all of three assays, the differences between naive males and winners or losers were smaller and more variable. Regardless, we found that both the winner and loser states generalized to risk-taking behavior: Compared with naive males, the winners were more able/willing to cross a challenging gap and the losers were less able/willing to do so. Results with various measures of courtship behavior were more complex and dependent on the parameter measured. The loser, but not the winner, effect generalized to courtship latency and courtship index, with losers showing increased latency to court and reduced total courtship compared with naive males. Only the winner effect generalized to total copulation, with winners achieving higher copulation rates than naive males and losers in a 10-min period; this suggests a fitness consequence to winning fights. Neither the winner nor loser states generalized to object exploration, although winners were significantly faster than losers in their first approach to the novel object.
Together, these data show that winner and loser internal states can generalize to other behaviors. Many different chemosensory cues have been shown to regulate how pairs of flies interact with each other, for instance, in the context of aggression and courtship (32). The fact that winning and losing led to alterations in single-fly behavior (gap crossing and exploration) suggests that the fighting-induced persistent internal state is intrinsic to the winners and losers, as opposed to a response of their paired opponent to some physical, chemical, or behavioral alteration. It also suggests that central information processing is involved in the generation of the winner and loser states.
Dopamine Neurons as Mediators of Internal States.
Integration of multiple sensory cues and the activity of multiple groups of neurons has been implicated in the regulation of aggression in Drosophila (32). Thus, the experience of repeated fighting encounters is likely due to a complex activity pattern of neuronal groups. Our olfactory conditioning experiments (Fig. 4) showed that activity of the PPL1 dopamine neuron is required for the formation of the aversive memory for an odor paired with losing. In Drosophila, dopaminergic neurons in the protocerebral anterior medial (PAM) (33, 34) and PPL1 (23–25) clusters mediate the processes of appetitive and aversive learning and memory, respectively. Given that the acquisition of the aversive memory of an odor associated with losing is dependent on the activity of a PPL1 dopamine neuron, it would be interesting to determine whether the appetitive memory associated with the winner state depends on the activity of subsets of PAM dopamine neurons.
The utility of using Drosophila as a model organism to study internal states resides on the feasibility of performing neuronal activation/inactivation manipulations so as to study the causal relationship between the activity of specific neural circuits and a particular internal state. In flies, the artificial activation of the PAM (33, 34) and PPL1 (23–25) dopamine neurons is able to substitute for an unconditioned stimulus during the formation of an appetitive or aversive memory, respectively. Given that the activity of PPL1-γ1pedc and its cognate output neuron, MBON-γ1pedc>α/β, are required for the formation of the aversive memory of an odor associated with losing, it will be interesting to ask if the formation of a loser and/or winner states can be achieved by activating these neurons.
Further studies should determine whether the winner and loser states are associated with different levels of activity of the fly’s central reward system. While not directly related to aggressive behaviors, Shohat-Ophir et al. (35) showed that the experience of sexual rejection in Drosophila males generates what could be argued to be an internal state that leads to males consuming more alcohol. This state could be mimicked by changing the activity levels of neuropeptide F signaling, which has been proposed to represent the state of the flies’ central reward system.
Materials and Methods
Fly Breeding.
Unless otherwise specified, experimental flies were raised on cornmeal/yeast/molasses/agar medium at 25 °C and at 60% humidity. The following strains were used in the present study: wild-type Canton-S (CS) originally from Martin Heisenberg’s laboratory, University of Würzburg, Würzburg, Germany, DH44R1-GAL4 (Michael Texada and James W. Truman, Janelia Research Campus), UAS-DH44R1RNAi (Vienna Drosophila Resource Center), MB438B-GAL4 and MB112C-GAL4 (Yoshinori Aso, Janelia Research Campus).
Social Isolation and Group Housing.
Newly eclosed CS or DH44R1-GAL4 > UAS-DH44R1RNAi males were isolated for 6 d and kept at 25 °C and at 60% humidity. To suppress aggressiveness of the hyperaggressive DH44R1-GAL4 > UAS-DH44R1RNAi males, 15 CS virgin females were paired with 15 DH44R1-GAL4 > UAS-DH44R1RNAi virgin males for 4 d. On the fifth day, males and females were separated, and males were housed in groups of 30 individuals until the next day (day of training).
Aggression Assay.
All aggression assays were performed as previously described (15). Briefly, a pair of flies was introduced into each arena of a 12-cell chamber, and the aggressive behavior was recorded for a period of 30 min at 25 °C and at 45% humidity. The bottom of the chamber was covered with a thin layer of 4% apple juice agar. Walls were covered with Fluon (BioQuip) to prevent the flies from climbing onto the walls. Quantification was carried out using the CADABRA (Caltech Automated Drosophila Aggression-Courtship Behavioral Repertoire Analysis) software, which detects several aggressive behaviors displayed by flies (17). Since CADABRA does not differentiate between the fly carrying out and receiving the lunges, a semiautomated system that allowed us to assign lunges to individual males was developed; one fly, alternating between experiments, had one wing clipped for identification. Wing clipping had no effect on aggression and was ascertained by manually scoring fights between SH CS males and between DH44R1-GAL4 > UAS-DH44R1RNAi males.
Generation of Winners and Losers.
Single-housed CS males were trained with three consecutive sessions of aggressive encounters with an opponent fly. For identification, we alternated clipping the wing of one of the two flies. We confirmed that wing clipping did not affect aggressive behavior of the flies. For generating winners, the opponent fly was a hypoaggressive male (DH44R1-GAL4 > UAS-DH44R1RNAi, GH). For generating losers, the opponent fly was a hyperaggressive male (DH44R1-GAL4 > UAS-DH44R1RNAi, SH). During each session, the aggressive behavior between males was recorded for 30 min, after which the flies were separated and the CS flies kept individually for 1 h. For each session, a new hypoaggressive or hyperaggressive opponent was used. After the three training sessions were completed, the CS males were retrieved and kept individually for 24, 48, or 72 h at 25 °C and 60% humidity for subsequent behavioral experiments.
Climbing Assay and General Locomotion.
Using an aspirator, individual males were introduced to an 8-dram plastic vial. Vials were gently tapped and the time for the winner, loser, or naive male to reach to the top of the vial was recorded. Each male was tested twice, and the final score was calculated as the average of the two attempts. We also measured the fly’s general locomotion using the Drosophila Activity Monitoring system (Trikinetics) (36). Individual males were placed in 65 mm × 5 mm transparent plastic tubes with standard molasses agar media and placed in the activity monitoring system. Locomotor activity data were collected in 1-min bins using flies kept in a 12 h:12 h light:dark regimen at 25 °C and 65% humidity. Sleep data were extracted from the locomotor data as described previously (37), with sleep being defined as a period of 5 min or longer of inactivity.
Olfactory Conditioning.
To train the flies to associate an olfactory cue with fighting experience, the 12-well chamber used in the aggression assay was modified by perforating the coverlid, thus allowing for flies to be exposed to different odors. Individual SH CS males were paired with a single hypoaggressive or hyperaggressive opponent in each well. For odor exposure, the adapted aggression chambers were introduced into odor delivery boxes as used by Kaun et al. (38). The odors used were ethyl acetate (EA; Sigma-Aldrich) and isoamyl acetate (IAA; Sigma-Aldrich) diluted 1:36 in mineral oil (Sigma-Aldrich). Flies were trained with spaced-training protocol consisting of three sessions separated by 1 h. In each session, the SH CS male was first exposed to odor 1 for 30 min without opponent, after which an opponent fly (either a hypoaggressive male for winners or a hyperaggressive male for losers) was introduced, and the pair was exposed to odor 2 for 30 min. The opponent male was then removed, and the CS male was kept until the next training session when a new opponent was added. The reciprocal training group was performed so as to rule out any inherent bias for either olfactory cue. For the control group, SH CS males underwent the same protocol but without pairing with an opponent. After the training was complete, the CS males were retrieved and kept individually at 25 °C and 60% humidity. Memory was tested 24 h later in a T-maze apparatus (39). Groups of 12 winners or losers were loaded into the middle portion of the T-maze chamber and allowed to choose between EA or IAA for 4 min. For each group, a preference index (PI) was calculated as PI = [(number of flies in paired odor arm) − (number of flies in unpaired odor arm)]/total number of flies. A single conditioned preference index value was obtained by taking the average of the PIs from the reciprocal training groups. Therefore, 24 winners or losers are used for n = 1.
Gap Crossing.
This assay was performed as previously described (18, 19). Briefly, a black plastic object (10 mm height × 30 mm length × 4.6 mm width) having a 3.0-mm gap of 6.0-mm depth in the middle was placed on the lid of a small Petri dish (54-mm diameter), and this gap-crossing plate was again placed on another large Petri dish (150-mm diameter) filled in water to avoid escape. Twenty-four hours before testing, flies were briefly cold-shocked at 4 °C and both wings cut using a sharp scissor. Single flies were gently dropped onto the gap-crossing plate and observed. Ten trials were given to each fly, and the percentage of flies that successfully crossed the gap were counted. Returns and rare occasions when the fly walked through or around the gap, or fell were also quantified (Fig. S4).
Courtship Behavior.
For this assay, a two-layered chamber, similar to ones used during the aggression assay, was used. Single 4-d-old CS virgin females were loaded into each well, and the entire chamber was covered with a transparent film. A second chamber was put on top of the first one, and single trained males were introduced into the wells. After the plastic film was removed, the fly’s courtship behavior was recorded for 10 min. For each pair of flies, the courtship latency, courtship index, and copulation latency were quantified.
Exploration Assay.
A free-walking arena containing an attractive post (4-mm diameter by 8-mm tall) projecting from the center of the arena floor (Fig. S6A) was developed. The 0.5-mm thin walls of the post never obscured the location of the fly from a top view (Fig. S6B). Each arena consisted of a transparent floor covered by translucent filter paper surrounded by a cylindrical wall 5 cm in diameter and 1.2 cm tall covered by a lid. To prevent a fly from climbing, both the wall and lid of the arena were coated with Sigmacote (Sigma-Aldrich). A 3-mm diameter hole in the arena lid allowed the gentle introduction of a fly by aspiration (Fig. S6A). The entire arena sat within a 10-cm-diameter by 15-cm-tall cylindrical panorama of varying light and dark patterns to provide background visual stimuli. To ensure a fly could navigate by sight, a constant low level of light was provided by an array of daylight white LEDs (LED6MR16/NFL/50K; NaturaLED). The arena was backlit from underneath by a panel array of IR LEDs (Advanced Illumination). The movement of a fly was recorded using a digital camera (Basler A622f; Edmund Optics, Inc.) with a 720-nm high pass optical filter (R-72; Edmund Optics, Inc.). For each trial, a single fly was filmed for 12 min starting within 2 s after its introduction to the arena. The fly’s translational movements (Fig. S6C) were determined using Ctrax (40); the various measures and classification of behaviors, including the exact coordinates of the post for each trial, were generated using custom software written in Matlab (MathWorks).
Blocking Neuronal Activity During Olfactory Conditioning.
Flies were raised at 18 °C until eclosion, after which they were isolated for 6 d. MB438B-GAL4 > shits or MB112C-GAL4 > shits flies, and their respective genetic controls, were trained with the olfactory conditioning protocol described above, with the exception that the training was performed at either the restrictive (30 °C) or permissive (20 °C) temperature for Shits (27). After the training was completed, the CS males were retrieved and kept individually at 20 °C and 60% humidity. The memory was tested 24 h later.
Statistical Analysis.
The statistical significance of the data was analyzed using JMP (11th version; SAS Institute Inc.) and Prim7 (GraphPad Software). Matlab was used for the statistical analysis of the data in Fig. 3A and Fig. S3 (41). Unless specified, we used parametric or nonparametric ANOVAs, followed by post hoc tests of significance. When required, we log-transformed data to improve normality. If the transformed data did not distribute normally (Shapiro–Wilk test), we performed Mann–Whitney U, Wilcoxon, or Steel–Dwass tests of significance.
Supplementary Material
Acknowledgments
We thank Eric Hoopfer, Frank Midgley, and Tanya Tabachnik for their invaluable help; Yoshi Aso, Daniel Bath, Ron Tanimoto, Vivek Jayaraman, Sung Soo Kim, Joshua Mast, and Tillman Triphan for providing stocks and equipment for this project; and Carmen Robinett for her critical comments on the earlier version of this manuscript. This work was funded by HHMI.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1716612115/-/DCSupplemental.
References
- 1.Dolan RJ. Emotion, cognition, and behavior. Science. 2002;298:1191–1194. doi: 10.1126/science.1076358. [DOI] [PubMed] [Google Scholar]
- 2.Anderson DJ, Adolphs R. A framework for studying emotions across species. Cell. 2014;157:187–200. doi: 10.1016/j.cell.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffmann AA. A laboratory study of male territoriality in the sibling species Drosophila melanogaster and D. simulans. Anim Behav. 1987;35:807–818. [Google Scholar]
- 4.Hoffmann AA, Cacoyianni Z. Territoriality in Drosophila melanogaster as a conditional strategy. Anim Behav. 1990;40:526–537. [Google Scholar]
- 5.Chen S, Lee AY, Bowens NM, Huber R, Kravitz EA. Fighting fruit flies: A model system for the study of aggression. Proc Natl Acad Sci USA. 2002;99:5664–5668. doi: 10.1073/pnas.082102599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vrontou E, Nilsen SP, Demir E, Kravitz EA, Dickson BJ. Fruitless regulates aggression and dominance in Drosophila. Nat Neurosci. 2006;9:1469–1471. doi: 10.1038/nn1809. [DOI] [PubMed] [Google Scholar]
- 7.Trannoy S, Penn J, Lucey K, Popovic D, Kravitz EA. Short and long-lasting behavioral consequences of agonistic encounters between male Drosophila melanogaster. Proc Natl Acad Sci USA. 2016;113:4818–4823. doi: 10.1073/pnas.1520953113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou C, Rao Y, Rao Y. A subset of octopaminergic neurons are important for Drosophila aggression. Nat Neurosci. 2008;11:1059–1067. doi: 10.1038/nn.2164. [DOI] [PubMed] [Google Scholar]
- 9.Hoyer SC, et al. Octopamine in male aggression of Drosophila. Curr Biol. 2008;18:159–167. doi: 10.1016/j.cub.2007.12.052. [DOI] [PubMed] [Google Scholar]
- 10.Andrews JC, et al. Octopamine neuromodulation regulates Gr32a-linked aggression and courtship pathways in Drosophila males. PLoS Genet. 2014;10:e1004356. doi: 10.1371/journal.pgen.1004356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alekseyenko OV, Lee C, Kravitz EA. Targeted manipulation of serotonergic neurotransmission affects the escalation of aggression in adult male Drosophila melanogaster. PLoS One. 2010;5:e10806. doi: 10.1371/journal.pone.0010806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alekseyenko OV, Chan YB, Li R, Kravitz EA. Single dopaminergic neurons that modulate aggression in Drosophila. Proc Natl Acad Sci USA. 2013;110:6151–6156. doi: 10.1073/pnas.1303446110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dierick HA, Greenspan RJ. Serotonin and neuropeptide F have opposite modulatory effects on fly aggression. Nat Genet. 2007;39:678–682. doi: 10.1038/ng2029. [DOI] [PubMed] [Google Scholar]
- 14.Asahina K, et al. Tachykinin-expressing neurons control male-specific aggressive arousal in Drosophila. Cell. 2014;156:221–235. doi: 10.1016/j.cell.2013.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoopfer ED, Jung Y, Inagaki HK, Rubin GM, Anderson DJ. P1 interneurons promote a persistent internal state that enhances inter-male aggression in Drosophila. Elife. 2015;4:e11346. doi: 10.7554/eLife.11346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nässel DR, Winther AM. Drosophila neuropeptides in regulation of physiology and behavior. Prog Neurobiol. 2010;92:42–104. doi: 10.1016/j.pneurobio.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Dankert H, Wang L, Hoopfer ED, Anderson DJ, Perona P. Automated monitoring and analysis of social behavior in Drosophila. Nat Methods. 2009;6:297–303. doi: 10.1038/nmeth.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pick S, Strauss R. Goal-driven behavioral adaptations in gap-climbing Drosophila. Curr Biol. 2005;15:1473–1478. doi: 10.1016/j.cub.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 19.Triphan T, Poeck B, Neuser K, Strauss R. Visual targeting of motor actions in climbing Drosophila. Curr Biol. 2010;20:663–668. doi: 10.1016/j.cub.2010.02.055. [DOI] [PubMed] [Google Scholar]
- 20.Teseo S, Veerus L, Mery F. Fighting experience affects fruit fly behavior in a mating context. Naturwissenschaften. 2016;103:38. doi: 10.1007/s00114-016-1368-x. [DOI] [PubMed] [Google Scholar]
- 21.Horn E, Wehher R. Mechanism of visual-pattern fixation in walking fly, Drosophila melanogaster. J Comp Physiol. 1975;101:39–56. [Google Scholar]
- 22.Robie AA, Straw AD, Dickinson MH. Object preference by walking fruit flies, Drosophila melanogaster, is mediated by vision and graviperception. J Exp Biol. 2010;213:2494–2506. doi: 10.1242/jeb.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Claridge-Chang A, et al. Writing memories with light-addressable reinforcement circuitry. Cell. 2009;139:405–415. doi: 10.1016/j.cell.2009.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aso Y, et al. Specific dopaminergic neurons for the formation of labile aversive memory. Curr Biol. 2010;20:1445–1451. doi: 10.1016/j.cub.2010.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aso Y, et al. Three dopamine pathways induce aversive odor memories with different stability. PLoS Genet. 2012;8:e1002768. doi: 10.1371/journal.pgen.1002768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aso Y, et al. The neuronal architecture of the mushroom body provides a logic for associative learning. Elife. 2014;3:e04577. doi: 10.7554/eLife.04577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitamoto T. Targeted expression of temperature-sensitive dynamin to study neural mechanisms of complex behavior in Drosophila. J Neurogenet. 2002;16:205–228. doi: 10.1080/01677060216295. [DOI] [PubMed] [Google Scholar]
- 28.Hsu Y, Earley RL, Wolf LL. Modulation of aggressive behaviour by fighting experience: Mechanisms and contest outcomes. Biol Rev Camb Philos Soc. 2006;81:33–74. doi: 10.1017/S146479310500686X. [DOI] [PubMed] [Google Scholar]
- 29.Hammels C, et al. Defeat stress in rodents: From behavior to molecules. Neurosci Biobehav Rev. 2015;59:111–140. doi: 10.1016/j.neubiorev.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 30.Dugatkin LA, Druen M. The social implications of winner and loser effects. Proc Biol Sci. 2004;271:S488–S489. doi: 10.1098/rsbl.2004.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rutte C, Taborsky M, Brinkhof MW. What sets the odds of winning and losing? Trends Ecol Evol. 2006;21:16–21. doi: 10.1016/j.tree.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 32.Hoopfer ED. Neural control of aggression in Drosophila. Curr Opin Neurobiol. 2016;38:109–118. doi: 10.1016/j.conb.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 33.Liu C, et al. A subset of dopamine neurons signals reward for odour memory in Drosophila. Nature. 2012;488:512–516. doi: 10.1038/nature11304. [DOI] [PubMed] [Google Scholar]
- 34.Burke CJ, et al. Layered reward signalling through octopamine and dopamine in Drosophila. Nature. 2012;492:433–437. doi: 10.1038/nature11614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shohat-Ophir G, Kaun KR, Azanchi R, Mohammed H, Heberlein U. Sexual deprivation increases ethanol intake in Drosophila. Science. 2012;335:1351–1355, and erratum (2012) 337:799. doi: 10.1126/science.1215932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosato E, Kyriacou CP. Analysis of locomotor activity rhythms in Drosophila. Nat Protoc. 2006;1:559–568. doi: 10.1038/nprot.2006.79. [DOI] [PubMed] [Google Scholar]
- 37.Donelson NC, et al. High-resolution positional tracking for long-term analysis of Drosophila sleep and locomotion using the “tracker” program. PLoS One. 2012;7:e37250, and erratum (2012) 7, 10.1371/journal.pone.0037250. doi: 10.1371/journal.pone.0037250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaun KR, Azanchi R, Maung Z, Hirsh J, Heberlein U. A Drosophila model for alcohol reward. Nat Neurosci. 2011;14:612–619. doi: 10.1038/nn.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ichinose T, et al. Reward signal in a recurrent circuit drives appetitive long-term memory formation. Elife. 2015;4:e10719. doi: 10.7554/eLife.10719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Branson K, Robie AA, Bender J, Perona P, Dickinson MH. High-throughput ethomics in large groups of Drosophila. Nat Methods. 2009;6:451–457. doi: 10.1038/nmeth.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moulos P. 2008 Chi-square test for 2x2 contingency tables using chisquarecont. Available at https://www.mathworks.com/matlabcentral/fileexchange/18705. Accessed June 8, 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.