Significance
Some have speculated that the rising prevalence of obesity may explain why the rate of mortality improvement in the United States has declined relative to other wealthy countries. This paper estimates that rising body mass index (BMI) has reduced the annual rate of improvement in US death rates between 1988 and 2011 by more than half a percentage point—equivalent to a 23% relative reduction in the rate of mortality decline—a large amount by international standards. The increase in BMI has reduced life expectancy at age 40 by 0.9 years in 2011 and accounted for 186,000 excess deaths that year. Rising BMI has prevented the United States from enjoying the full benefits of factors working to improve mortality.
Keywords: obesity, mortality, life expectancy, population health
Abstract
Recent studies have described a reduction in the rate of improvement in American mortality. The pace of improvement is also slow by international standards. This paper attempts to identify the extent to which rising body mass index (BMI) is responsible for reductions in the rate of mortality improvement in the United States. The data for this study were obtained from subsequent cohorts of the National Health and Nutrition Examination Survey (NHANES III, 1988–1994; NHANES continuous, 1999–2010) and from the NHANES linked mortality files, which include follow-up into death records through December 2011. The role of BMI was estimated using Cox models comparing mortality trends in the presence and absence of adjustment for maximum lifetime BMI (Max BMI). Introducing Max BMI into a Cox model controlling for age and sex raised the annual rate of mortality decline by 0.54% (95% confidence interval 0.45–0.64%). Results were robust to the inclusion of other variables in the model, to differences in how Max BMI was measured, and to how trends were evaluated. The effect of rising Max BMI is large relative to international mortality trends and to alternative mortality futures simulated by the Social Security Administration. The increase in Max BMI over the period 1988–2011 is estimated to have reduced life expectancy at age 40 by 0.9 years in 2011 (95% confidence interval 0.7–1.1 years) and accounted for 186,000 excess deaths that year. Rising levels of BMI have prevented the United States from enjoying the full benefits of factors working to improve mortality.
Two recent studies by the National Academy of Sciences documented a slowdown in rates of mortality improvement in the United States and an increasing US mortality disadvantage relative to other wealthy countries (1, 2). Middle-aged white populations, especially white women, have actually experienced rising mortality over much of the past several decades (3–5). Rising mortality rates occurred earlier and more dramatically in many US counties (6, 7).
Causes of death implicated in the poor performance of mortality indicators among US whites include accidental poisoning—linked to the epidemic of prescription opioids—suicide, and chronic liver disease (3, 5, 8, 9). The adverse trends in mortality persist, however, even after removing the effects of these so-called “deaths of despair,” suggesting that these causes do not provide a complete explanation for the slowdown (4). Consistent with this possibility, the steady declines in death rates from cardiovascular disease that have been observed for nearly 40 y have decelerated or halted altogether (4, 8, 10). Death rates from heart disease at ages 50–54 in the United States declined at a slower rate than in any of 13 comparison countries between 1999 and 2015 (8). Rates of decline in cancer mortality were also comparatively slow in the United States (8).
A leading candidate to account for the poor performance of mortality indicators in the United States is a rise in the prevalence of obesity. The age-standardized prevalence of obesity in US adults based on examination-measured height and weight increased from 15% in 1976–1980 to 38% in 2013–2014 (11, 12). Obesity is associated with a variety of outcomes, including diabetes, cardiovascular disease, cancer, and all-cause mortality (13–17). A growing body of evidence indicates that these associations may be causal (18–21). Ma et al. (10) suggest that the recent attenuation in declining death rates for heart disease, stroke, and diabetes may reflect the lagged consequences of increased obesity prevalence. Other analysts have endorsed this suggestion (6, 22, 23), including Tom Frieden, then Director of the Centers for Disease Control and Prevention (24). One suggestive association is that, among rich countries, the prevalence of adult obesity is highest in the United States (25). The increase in mean BMI in the United States in recent decades was also larger than in comparable European countries (26, 27).
In this paper, we used nationally representative data on the US adult population to estimate the contribution of changes in body mass index (BMI) to trends in US mortality at ages 40–84 during the period 1988–2011.
Data and Methods
To assess the role of BMI in recent national mortality trends, we combined data from the National Health and Nutrition Examination Survey (NHANES) III pertaining to the period 1988–1994 and data from the NHANES for the period 1999–2011 (28, 29). NHANES is a nationally representative survey of the noninstitutionalized US population and includes a questionnaire as well as clinical and laboratory components. The latest year to which NHANES surveys have been linked to death records from the National Death Index is 2011. We restricted the sample to medically examined adults eligible for mortality follow-up aged 40–79 without missing data on maximum BMI (n = 931) or missing data on sex, race/ethnicity, education, and smoking status (n = 71). We also excluded 19 individuals considered underweight, i.e., having values of max BMI below 18.5, and 17 individuals with max BMI above 75. The final sample consists of 25,269 individuals, experiencing 4,620 deaths over 230,728 person-years of exposure.
Our indicator of BMI combined an individual’s reported maximum weight (exclusive of pregnancy) with measured height at survey to calculate maximum body mass index (Max BMI). Using model selection criteria, this variable has repeatedly been shown to be superior to BMI at baseline in predicting mortality (15, 17). One suggested reason for its superior performance is that it is less susceptible to weight loss associated with illness, which biases estimates of mortality associated with baseline BMI. Another reason is that it conveys important elements of weight history which may have enduring effects on health (30). In the present analysis, we transformed Max BMI to reflect the number of BMI units above 25 kg/m2, with values between 18.5–25 kg/m2 assigned to zero. A BMI value of 25 represents the beginning of the “Overweight” range as defined by the World Health Organization.
We used a Cox proportional hazards model to estimate the relation between Max BMI and mortality, using time since survey as exposure time. Individuals remained exposed to the risk of death until they were censored by death, by reaching January 1, 2012, or by reaching age 85. A key variable is “calendar year,” a numeric value that corresponds to a particular year of exposure to the risk of death. After initial assignment to a particular year at survey, the “calendar year” variable increases by 1 y for each year of follow-up. Since the Cox model predicts the natural log of the death rate, the coefficient of “calendar year” is the estimated annual rate of mortality decline during the period under study. The focus of this paper is on how the rate of mortality change is altered when Max BMI is introduced into the model.
The effect of Max BMI on the mortality trend was first established using a model that controls only age and sex. We do not employ cohort identifiers in the models so we do not encounter the age-period-cohort identification problem. We subsequently introduced three other sets of variables into the model: race/ethnicity (white non-Hispanic, black non-Hispanic, Hispanic, other); educational attainment (<9 y, 9–11 y, 12 y, some college or an associate’s degree, 4-y college graduate); and smoking status (never, former, current). These variables are correlated with BMI and with the risk of death and, therefore, potentially confound the estimated impact of BMI as well as its effects on mortality trends (30). In preliminary analyses, we evaluated interactions between Max BMI and calendar year and between Max BMI and smoking. The interactions, shown in Table S3, were insignificant and not retained in subsequent analyses. A second-degree term in Max BMI was also insignificant and was dropped.
Using national vital statistics from the Human Mortality Database (31), we calculated trends in age-standardized death rates over the same range of ages and years included in the NHANES analysis. We did this for the United States and 15 comparison countries chosen for their social and economic similarity to the United States. Rates were standardized using the age distribution of the United States in 2000. The rate of mortality change was estimated by an ordinary least squares linear regression of ln DR(t) on t, where DR(t) is the death rate in year t, t = 1988…2011. To avoid any influence from changing sex composition, DR(t) is the mean of male and female death rates.
We estimated the effect of changing Max BMI on national life expectancy over the period. To do so, we applied two sets of NHANES-based mortality changes to the US age-specific death rates in the official life table of 1988, one with and one without Max BMI controlled, and compared the projected life expectancies in 2011. Since our analysis did not extend beyond age 85, we used the actual mortality level in the official US life table for 2011 above age 85 to derive our projected life expectancies (32).
We explored the sensitivity of results to the following changes in procedure:
-
i)
We treated time as a dichotomous variable rather than as a continuous variable by constructing observations for two baseline periods, 1988–1994 and 1999–2006. We censored individuals after 5 y of exposure so that data structures in the two periods were identical.
-
ii)
We treated max BMI as a categorical variable using the World Health Organization-recommended BMI categories of <18.5, 18.5–24.9, 25–29.9, 30–34.9, 35–39.9, and ≥40. This model added 19 observations to the dataset, those with max BMI < 18.5.
-
iii)
We investigated the effect of introducing an interaction between max BMI and age in its effect on mortality trends.
-
iv)
We investigated the effect on estimated mortality trends of measuring Max BMI from a value of 18.5, the start of the “normal” BMI category recommended by the World Health Organization, rather than 25.0, the start of the overweight category.
Data analyses were performed using Stata version 15 (StataCorp), and all estimates were adjusted for the complex survey design of the NHANES. Variances were estimated with the SVY routine, which uses Taylor series linearization. Bootstrapping was performed to generate 95% confidence intervals on the estimate of the change in the coefficient of calendar year when Max BMI is introduced into a model including age, sex, and date of observation, as well as on the estimate of the subsequent change in life expectancy (33). We used the simulate command in Stata to perform 1,000 simulations, resampling with replacement.
Results
Table 1 shows some of the major characteristics of the cohorts under study. Between 1988 and 1994 and 2005 and 2010, the proportion of the US adult population aged 40–79 that had been obese at some point in their lives increased from 40 to 52% while the proportion reporting a history of smoking declined from 60 to 50%.
Table 1.
Descriptive statistics of the sample of adults aged 40–79 at baseline in NHANES, 1988–1994 and 1999–2010
| Characteristics | Survey years [SE] | ||
| 1988–1994 | 1999–2004 | 2005–2010 | |
| Obesity | |||
| Ever obese, % | 39.81 [0.96] | 49.07 [1.05] | 52.00 [0.93] |
| Max BMI units above | 5.00 [0.12] | 6.25 [0.14] | 6.93 [0.12] |
| 25 kg/m2 (mean) | |||
| Age (mean) | |||
| At baseline | 56.28 [0.32] | 55.37 [0.17] | 55.75 [0.24] |
| At follow-up | 72.46 [0.29] | 64.58 [0.17] | 59.57 [0.23] |
| Male, % | 47.41 [0.66] | 47.81 [0.53] | 48.33 [0.53] |
| Race/ethnicity, % | |||
| Non-Hispanic white | 80.42 [1.29] | 76.21 [1.61] | 74.32 [1.79] |
| Non-Hispanic black | 9.67 [0.59] | 9.93 [0.97] | 10.80 [0.98] |
| Hispanic | 7.07 [0.81] | 9.53 [1.40] | 9.48 [1.09] |
| Other | 2.85 [0.54] | 4.32 [0.46] | 5.40 [0.55] |
| Education, % | |||
| <ninth grade | 14.21 [0.89] | 7.65 [0.46] | 6.66 [0.55] |
| <High school degree | 14.03 [0.86] | 12.95 [0.64] | 11.52 [0.60] |
| High school degree | 33.03 [0.82] | 26.07 [0.79] | 25.34 [0.78] |
| Some college/AA degree | 17.1 [0.70] | 28.18 [0.77] | 28.20 [0.79] |
| BA or more | 21.62 [1.26] | 25.16 [1.21] | 28.28 [1.21] |
| Smoking status, % | |||
| Never | 40.25 [0.91] | 46.00 [0.88] | 49.61 [0.95] |
| Former | 35.52 [0.82] | 32.35 [0.79] | 30.06 [0.80] |
| Current | 24.23 [0.90] | 21.66 [0.71] | 20.33 [0.81] |
| N | 8,395 | 7,593 | 9,281 |
| Deaths | 3,018 | 1,175 | 427 |
| Person-years | 127,788 | 69,024 | 33,917 |
All results reflect sample weighting, except N, deaths, and person-years. AA, associate of arts; BA, bachelor of arts.
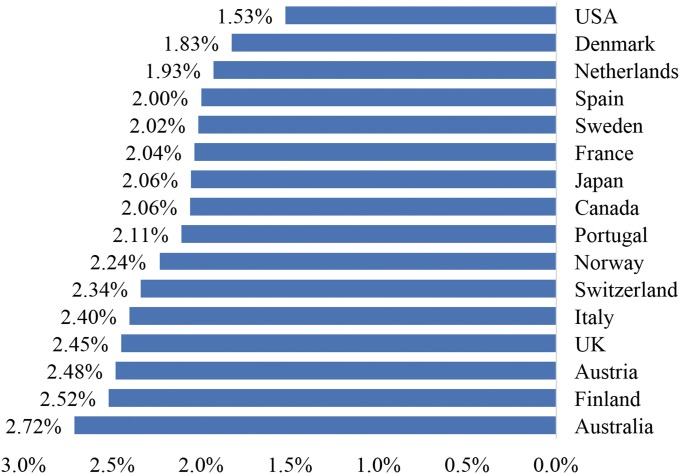
Fig. 1 shows annual rates of mortality decline at ages 40–84 in 16 countries between 1988 and 2011. At 1.53%/y, the United States had the slowest decline in mortality. The mean rate of decline for the remaining 15 countries was 2.21%/y.
Fig. 1.
Annual rates of mortality decline among adults ages 40–84 in 16 OECD countries, 1988–2011. Mortality decline is shown for adults ages 40–85 in the United States and 15 comparable OECD countries between 1988 and 2011, if all countries had the same age distribution of 40–84 y olds as in the 2000 US Census. Data from the Human Mortality Database.
Table 2 presents the rate of mortality decline in different models before and after the introduction of Max BMI. The models themselves are presented in Tables S1 and S2. The rate of mortality decline in a model including only age, sex, and date of observation was 0.0181, which is within one SD of the 0.0153 rate observed using national vital statistics. When Max BMI was introduced into the model including age, sex, and date of observation, the increase in the rate of mortality decline was 0.0054, with a 95% confidence interval on the change of 0.0045–0.0064. Thus, rising Max BMI is estimated to have slowed the annual rate of mortality decline during this period by 0.54%. This is equivalent to a 23% [(0.0054/0.0235) × 100] relative reduction in the rate of mortality decline as a result of rising obesity.
Table 2.
Results from Cox regressions with and without control for maximum BMI in NHANES, 1988–2011
| Model | BMI coefficient [SE] | Annual % decline without BMI* [SE] | Annual % decline with BMI* [SE] | Change in % decline from introducing BMI |
| Age + Sex | 0.0433 [0.0034] | 1.81 [0.46] | 2.35 [0.44] | 0.54 |
| Age + Sex + Race | 0.0412 [0.0035] | 1.83 [0.44] | 2.35 [0.43] | 0.52 |
| Age + Sex + Race + Smoking | 0.0455 [0.0035] | 1.37 [0.42] | 1.92 [0.40] | 0.55 |
| Age + Sex + Race + Education | 0.0368 [0.0036] | 1.18 [0.43] | 1.70 [0.42] | 0.52 |
| Age + Sex + Race + Smoking + Education | 0.0425 [0.0036] | 0.90 [0.41] | 1.47 [0.40] | 0.57 |
All results reflect sample weighting.
Annual percent decline calculated as calendar year coefficient multiplied by −100.
Across the five models shown in Table 2, the change in the coefficient of calendar year when Max BMI was introduced ranged only from 0.52 to 0.57%. Introducing smoking into a model that included all other variables changed the implied rate of decline from 1.70 to 1.47%. So it appears that rising Max BMI had a negative effect on mortality trends that was roughly double the positive effect of reduced smoking.
Table 3 presents results equivalent to those in Table 2 for several alternative models, all of which included age, sex, race/ethnicity, educational attainment, and smoking. Alternative A uses a dichotomous time variable and limits mortality follow-up to 5 y for each survey wave. The annual rate of mortality change was estimated as [ln B]/11.57, where B is the coefficient of the variable representing the later period and 11.57 is the mean number of years separating the two sets of observations. The rate of mortality decline in the dichotomous-time specification before the addition of max BMI was 0.95%, compared with 0.90% in the comparable preferred model. The equivalent values after the introduction of Max BMI are 1.43% and 1.47%, respectively.
Table 3.
Effects of alternative procedures on estimated impact of maximum BMI on mortality trends in NHANES, 1988–2011
| Model | Annual % decline without BMI* [SE] | Annual % decline with BMI* [SE] | Change in % decline from introducing BMI |
| Preferred | 0.90 [0.41] | 1.47 [0.40] | 0.57 |
| A. Discrete time† | 0.95 [0.66] | 1.43 [0.66] | 0.48 |
| B. Categorical BMI‡ | 0.90 [0.41] | 1.45 [0.40] | 0.55 |
| C. Age interaction§ | 0.90 [0.41] | 1.42 [0.40] | 0.52 |
| D. BMI beginning at 18.5 | 0.90 [0.41] | 1.46 [0.40] | 0.56 |
All results from Cox regressions reflect sample weighting and include covariates for age, sex, race/ethnicity, educational attainment, and smoking. Exclusion criteria identical across alternative analyses, except where indicated.
Annual percent decline calculated as calendar year coefficient multiplied by −100.
Annual percent decline for discrete time models calculated as calendar year coefficient divided by 11.57, the mean number of years separating the two periods, multiplied by −100. Sample limited to years 1988–1994 and 1999–2006. n = 18,429; Deaths = 1,457; Person-years = 88,877.
Categorical BMI classified using World Health Organization guidelines. Underweight: <18.5; Normal weight: 18.5–24.9; Overweight: 25.0–29.9; Class I obesity: 30.0–34.9; Class II obesity: 35.0–39.9; Class III obesity: ≥40.0. n = 25,288; Deaths = 4,625; Person-years = 230,870.
Model with BMI includes an interaction between maximum BMI and age at baseline.
The remaining alternatives also induced little change in the estimated impact of including Max BMI. Alternative B in Table 3 used categorical values of Max BMI rather than continuous values. The change in the rate of mortality decline when the categorical values were introduced was 0.55%/y, compared with 0.57%/y in the preferred model. Alternative C added an interaction term between Max BMI and age. The interaction term was significant but its inclusion had only a small effect on the impact of Max BMI. In alternative D, we measured Max BMI from 18.5 rather than 25.0 with little effect.
To estimate the effect of rising BMI on life expectancy, we compared results using the rates of mortality reduction in the model controlling only age and sex to those in the model controlling age, sex, and Max BMI. If age-specific death rates had fallen at the BMI-uncontrolled rate of 1.81%/y, life expectancy at age 40 would have risen from 37.6 y in 1988 (34) to 41.4 y in 2011. If death rates had fallen at the BMI-controlled rate of 2.35%/y, life expectancy at age 40 in 2011 would have risen to 42.3 y. This comparison suggests that rising BMI reduced gains in life expectancy at age 40 by 0.9 y over this period, with a confidence interval on the effect of introducing Max BMI of 0.7–1.1 y. Note that actual US life expectancy in 2011 at age 40 was 40.6 y (32), so the actual gain in life expectancy was 3.0 y vs. our modeled gain of 3.8 y. This discrepancy is consistent with slightly faster declines noted earlier in age-standardized death rates in NHANES than in national vital statistics.
As a final check on the plausibility of our estimates, we combined directly observed changes in the Max BMI variable with the coefficient representing the estimated effect of Max BMI on mortality. As shown in Table 1, the mean value of Max BMI (measured from a starting value of 25) rose from 5.00 in 1988–94 to 6.93 in 2005–10, or by 1.93 units. The coefficient of Max BMI in the regression that also includes age, sex, and the mortality trend is 0.0433 (Table 2). Combining these two values gives a predicted change in the natural log of the death rate of 0.0433 × 1.93 = 0.0836. The mean length of time between the two sets of observations is 16.51 y, so the estimated effect on the annual rate of mortality change is 0.0836/16.51 = 0.51%. This value is closely aligned with the estimates shown in Tables 2 and 3, which range from 0.48 to 0.57%. Those latter estimates utilize substantially more detail on the exact timing of exposure and death during the 1988–2011 period.
Discussion
It has been widely speculated that a rising prevalence of obesity is responsible for slowing gains in mortality and life expectancy in the United States. Such a relationship was predicted by Olshansky et al. (35) and has been hypothesized by several other analysts. The present paper is an effort to quantify the impact of rising obesity in recent decades on national mortality trends.
We estimate that rising Max BMI during the period 1988–2011 has lowered the rate of decline in US age-standardized death rates by ∼0.5–0.6%/y. The estimate is robust to the inclusion of other major drivers of national mortality levels, including smoking, educational attainment, and racial composition. It is also robust to alternative ways of operationalizing BMI and identifying trends.
Is a difference in rates of mortality decline of 0.5–0.6%/y large or small? One useful metric is provided by mortality projections done by the US Social Security Administration. In their most recent projections, the intermediate rate of mortality decline projected between 2013 and 2040 for all ages combined is 0.91%, whereas the low-cost alternative rate of decline is 0.45% (36). This difference of 0.46% is slightly smaller than the estimated effect of rising BMI on recent trends in mortality. At ages 65+, the difference between intermediate and low-cost projections is 0.42%, also below the estimated impact of rising BMI. So the rise in BMI appears to be a powerful factor in mortality relative to the amount of uncertainty embedded in Social Security projections.
A second metric is international. The SD of national rates of mortality decline shown in Fig. 1 is 0.30%. So rising BMI in the United States has generated an effect on mortality decline (0.50–0.60%) that is close to two international SD units. Relative to observed international variation in rates of mortality improvement, the effect of increasing body mass indices in the United States is large.
A third metric is the number of excess deaths. If death rates had declined since 1988 at the Max BMI-controlled rate of 2.35% rather than the uncontrolled rate of 1.81%, there would have been ∼88.3% {e−[0.0054(23)]} as many deaths at ages 40–84 in 2011 as actually occurred that year (37). So in the age interval 40–84, we estimate that rising BMI is responsible for 11.7% of the 1,590,254 deaths, or a total of 186,000 excess deaths, in 2011 alone. The total number of deaths in 2011 at all ages combined was 2,515,458, so the excess deaths attributable to rising Max BMI at ages 40–84 represent 7.4% of the total deaths in the United States in 2011 (37).
The finding that rising BMI has translated into population-level effects is notable given the increased adoption of pharmacological treatments for several obesity-related chronic conditions, including hypertension, hyperglycemia, and dyslipidemia, over the period of investigation. It is possible that the increased uptake of these medications has blunted the impact of rising obesity (38). However, we did not find any evidence of secular declines in the individual-level associations between Max BMI and mortality.
The present study has several limitations. First, we treated BMI as a causal variable in its relation to mortality. BMI itself is an amalgam of genes, diet, and physical activity (13). In this paper, we do not attempt to identify the factors that help to produce high levels of BMI, factors which may have their own impact on mortality. Our results are based on observational data rather than on randomized trials. Many unmeasured factors, such as childhood poverty, may be positively correlated with BMI and, independently, with mortality. Their absence from the regression model is likely to bias upwards the estimated effect of rising BMI on mortality trends. The fact that introducing key socio-economic variables—educational attainment and race/ethnicity—into the model had almost no effect on the estimated impact of Max BMI on mortality trends is one indication that omitted socioeconomic variables may also have relatively little effect, but there is no assurance that the effect would be small.
The single most important risk factor in US mortality, cigarette smoking, is negatively correlated with Max BMI (39). Smoking is a complex exposure with multiple dimensions of duration and intensity and is difficult to measure precisely in self-reported data. Because of smoking’s negative correlation with BMI, measurement error in our smoking variable is likely to bias downward the estimated impact of BMI on mortality and mortality trends (40). The net effect of these several biases is difficult to predict (30).
A second limitation is that our estimated annual rate of mortality decline among US adults using NHANES was somewhat faster than that estimated using data from vital statistics, although the latter value is within one SE of the former. That national vital statistics include the institutionalized population and NHANES does not may account for some of the discrepancy. Nonresponse in NHANES surveys may also play a role (41). Finally, our estimated impact of rising BMI on the number of deaths at ages 40–84 and on life expectancy at age 40 assumes that its impact is constant across these ages, an assumption that may or may not be accurate.
Conclusion
During the past several decades, rising levels of BMI have reduced rates of improvement in age-specific death rates and in life expectancy in the United States. The effect of rising BMI is quite large relative to international mortality trends and to alternative mortality futures simulated by the Social Security Administration. We estimate that rising Max BMI over the period 1988–2011 has reduced life expectancy at age 40 by 0.9 y in 2011 and accounted for roughly 186,000 excess deaths that year. The headwinds posed by increasing levels of BMI have prevented the United States from enjoying the full benefits of factors working to improve mortality, including reductions in smoking and advances in medical technology. Continued increases in BMI would threaten future gains in life expectancy as well (42).
Supplementary Material
Acknowledgments
We thank Paul Allison, Dana Glei, and reviewers for valuable suggestions. This research was funded by National Institute on Aging Grants R01AG040212 and R03AG05572401, National Center for Health Statistics Grant R03SH000037, Robert Wood Johnson Foundation Grant 544026, and Population Research Training Grant T32 HD007242 from the National Institutes of Health’s Eunice Kennedy Shriver National Institute of Child Health and Human Development. The study sponsors had no role in the design or implementation of this research.
Footnotes
Conflict of interest statement: A.S. has received research funding from Johnson & Johnson, Inc.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1716802115/-/DCSupplemental.
References
- 1.Institute of Medicine and National Research Council . U.S. Health in International Perspective: Shorter Lives, Poorer Health. The National Academies Press; Washington, DC: 2013. [PubMed] [Google Scholar]
- 2.National Research Council . Explaining Divergent Levels of Longevity in High-Income Countries. The National Academies Press; Washington, DC: 2011. [PubMed] [Google Scholar]
- 3.Kochanek KD, Arias E, Bastian BA. 2016. The effect of changes in selected age-specific causes of death on non-Hispanic white life expectancy between 2000 and 2014. NCHS Data Brief, 1–8. [PubMed]
- 4.Squires D, Blumenthal D. Mortality trends among working-age whites: The untold story. Issue Brief (Commonw Fund) 2016;3:1–11. [PubMed] [Google Scholar]
- 5.Case A, Deaton A. Rising morbidity and mortality in midlife among white non-Hispanic Americans in the 21st century. Proc Natl Acad Sci USA. 2015;112:15078–15083. doi: 10.1073/pnas.1518393112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ezzati M, Friedman AB, Kulkarni SC, Murray CJL. The reversal of fortunes: Trends in county mortality and cross-county mortality disparities in the United States. PLoS Med. 2008;5:e66. doi: 10.1371/journal.pmed.0050066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dwyer-Lindgren L, et al. US county-level trends in mortality rates for major causes of death, 1980-2014. JAMA. 2016;316:2385–2401. doi: 10.1001/jama.2016.13645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Case A, Deaton A. Mortality and Morbidity in the 21st Century. Brookings Inst Work Pap; Washington, DC: 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Astone NM, Martin S, Aron L. 2015. Death rates for US women ages 15 to 54 some unexpected trends (Urban Inst, Washington, DC)
- 10.Ma J, Ward EM, Siegel RL, Jemal A. Temporal trends in mortality in the United States, 1969-2013. JAMA. 2015;314:1731–1739. doi: 10.1001/jama.2015.12319. [DOI] [PubMed] [Google Scholar]
- 11.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 12.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315:2284–2291. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu F. Obesity Epidemiology. Oxford Univ Press; New York: 2008. [Google Scholar]
- 14.Stokes A. Using maximum weight to redefine body mass index categories in studies of the mortality risks of obesity. Popul Health Metr. 2014;12:6. doi: 10.1186/1478-7954-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stokes A, Preston SH. Revealing the burden of obesity using weight histories. Proc Natl Acad Sci USA. 2016;113:572–577. doi: 10.1073/pnas.1515472113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Angelantonio E, et al. Global BMI Mortality Collaboration Body-mass index and all-cause mortality: Individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388:776–786. doi: 10.1016/S0140-6736(16)30175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu E, et al. Weight history and all-cause and cause-specific mortality in three prospective cohort studies. Ann Intern Med. 2017;166:613–620. doi: 10.7326/M16-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fall T, et al. European Network for Genetic and Genomic Epidemiology (ENGAGE) consortium The role of adiposity in cardiometabolic traits: A Mendelian randomization analysis. PLoS Med. 2013;10:e1001474. doi: 10.1371/journal.pmed.1001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carreras-Torres R, Johansson M, Gaborieau V, et al. The role of obesity, type 2 diabetes, and metabolic factors in pancreatic cancer: A Mendelian randomization study. J Natl Cancer Inst. 2017;109:1–9. doi: 10.1093/jnci/djx012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thrift AP, et al. Mendelian randomization study of body mass index and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2015;24:1024–1031. doi: 10.1158/1055-9965.EPI-14-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nordestgaard BG, et al. The effect of elevated body mass index on ischemic heart disease risk: Causal estimates from a Mendelian randomisation approach. PLoS Med. 2012;9:e1001212. doi: 10.1371/journal.pmed.1001212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lloyd-Jones DM. Slowing progress in cardiovascular mortality rates: You reap what you sow. JAMA Cardiol. 2016;1:599–600. doi: 10.1001/jamacardio.2016.1348. [DOI] [PubMed] [Google Scholar]
- 23.Masters RK, Tilstra AM, Simon DH. Explaining recent mortality trends among younger and middle-aged White Americans. Int J Epidemiol. 2017 doi: 10.1093/ije/dyx127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernstein L. December 8, 2016 US life expectancy declines for the first time since 1993. Washington Post. Available at https://www.washingtonpost.com/national/health-science/us-life-expectancy-declines-for-the-first-time-since-1993/2016/12/07/7dcdc7b4-bc93-11e6-91ee-1adddfe36cbe_story.html?utm_term=.aa672f8a26ff. Accessed June 29, 2017.
- 25.Preston SH, Stokes A. Contribution of obesity to international differences in life expectancy. Am J Public Health. 2011;101:2137–2143. doi: 10.2105/AJPH.2011.300219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Afshin A. The GBD 2015 Obesity Collaborators Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377:13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. World Health Organization Global Health Observatory Data. Available at www.who.int/gho/ncd/risk_factors/overweight/en/. Accessed July 10, 2017.
- 28.National Center for Health Statistics 1994. Plan and Operation of the Third National Health and Nutrition Examination Survey, 1988-94, Vital and Health Statistics Series 1 (Natl Cent Health Stat, Hyattsville, MD)
- 29.National Center for Health Statistics National health and nutrition examination survey: Plan and operations, 1999–2010. Vital Heal Stat 1. 2010:1–37. [PubMed] [Google Scholar]
- 30.Stokes A, Preston SH. How dangerous is obesity? Issues in measurement and interpretation. Popul Dev Rev. 2016;42:595–614. doi: 10.1111/padr.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilmoth JR, Shkolnikov V. The Human Mortality Database. (University of California at Berkeley and Max Planck Institute for Demogr Res). Available at www.mortality.org. Accessed June 29, 2017.
- 32.Arias E. United States life tables, 2011. Natl Vital Stat Rep. 2015;64:1–63. [PubMed] [Google Scholar]
- 33.Efron B, Tibshirani R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat Sci. 1986;1:54–75. [Google Scholar]
- 34.National Center for Health Statistics 1991. Vital Statistics of the United States, 1988 (Natl Cent Health Stat, Hyattsville, MD)
- 35.Olshansky SJ, et al. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med. 2005;352:1138–1145. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- 36.Office of the Chief Actuary - Social Security Administration 2016. The long-range demographic assumptions for the 2016 trustees report (Social Security Administration, Washington, DC), p 41.
- 37.Kochanek KD, Murphy SL, Xu J. National vital statistics reports deaths: Final data for 2011. Natl Vital. 2015;63:1–119135. [PubMed] [Google Scholar]
- 38.NCD Risk Factor Collaboration (NCD-RisC) Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. 2016;387:1377–1396. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stokes A, Preston SH. How smoking affects the proportion of deaths attributable to obesity: Assessing the role of relative risks and weight distributions. BMJ Open. 2016;6:e009232. doi: 10.1136/bmjopen-2015-009232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Renehan AG, Leitzmann MF, Zwahlen M. Re: Body mass index and risk of lung cancer among never, former, and current smokers. J Natl Cancer Inst. 2012;104:1680–1681; author reply 1681. doi: 10.1093/jnci/djs381. [DOI] [PubMed] [Google Scholar]
- 41.National Center for Health Statistics 2013 National Health and Nutrition Examination Survey: Analytic Guidelines, 1999-2010. Available at https://wwwn.cdc.gov/nchs/data/series/sr02_161.pdf. Accessed July 10, 2017.
- 42.Preston SH, Stokes A, Mehta NK, Cao B. Projecting the effect of changes in smoking and obesity on future life expectancy in the United States. Demography. 2014;51:27–49. doi: 10.1007/s13524-013-0246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.