Significance
A famous American entomologist, Andrew D. Hopkins, estimated in 1920 the progressive delay in tree leaf-out with increasing latitude, longitude, and elevation, a phenomenon referred to as “Hopkins’ bioclimatic law.” Here, based on massive ground observations in the European Alps, we show that global warming has altered this law. In the early 1960s, the elevation-induced phenological shift (EPS) was approximately 34 days’ delay for every 1,000-m increase in elevation, conforming to Hopkins’ bioclimatic law, whereas, nowadays, this shift has reduced by 35% to 22 d⋅1,000 m−1. Winter warming is likely to be responsible for this strong reduction in the EPS and future climate warming may strengthen this trend. Important consequences for the functioning of mountain ecosystems are thus anticipated.
Keywords: Hopkins’ law, European Alps, elevation-induced phenological shift, leaf-out, mountain ecosystems
Abstract
One hundred years ago, Andrew D. Hopkins estimated the progressive delay in tree leaf-out with increasing latitude, longitude, and elevation, referred to as “Hopkins’ bioclimatic law.” What if global warming is altering this well-known law? Here, based on ∼20,000 observations of the leaf-out date of four common temperate tree species located in 128 sites at various elevations in the European Alps, we found that the elevation-induced phenological shift (EPS) has significantly declined from 34 d⋅1,000 m−1 conforming to Hopkins’ bioclimatic law in 1960, to 22 d⋅1,000 m−1 in 2016, i.e., −35%. The stronger phenological advance at higher elevations, responsible for the reduction in EPS, is most likely to be connected to stronger warming during late spring as well as to warmer winter temperatures. Indeed, under similar spring temperatures, we found that the EPS was substantially reduced in years when the previous winter was warmer. Our results provide empirical evidence for a declining EPS over the last six decades. Future climate warming may further reduce the EPS with consequences for the structure and function of mountain forest ecosystems, in particular through changes in plant–animal interactions, but the actual impact of such ongoing change is today largely unknown.
In mountain regions, the beauty of the progressive leaf appearance in spring along elevational gradients has always fascinated humans beyond any scientific scope. However, this phenomenon is not only fascinating but is an essential driver of ecosystem structure and functioning. In particular it regulates carbon, water, and energy exchanges between the biosphere and the atmosphere, influencing the entire Earth’s climate system (1) and is a major determinant of plant fitness (2). An attempt to generalize how phenology of plants and animals varies across geographical space covering climatic gradients (latitude, longitude, and altitude) was conducted almost one century ago by the American entomologist, Andrew Delmar Hopkins, and is summarized in the so-called “Hopkins’ bioclimatic law” (3–5). For instance, all other conditions being equal, this law claims that in North America, plant spring phenology shifts by 4 d for each degree of latitude northward, and for each 400-foot increase in elevation (∼122 m, i.e., ∼33 d⋅1,000 m−1). This general law suffers from obvious limitations when used at regional or local scales, due to substantial differences among species (6) or populations (7). However, at larger scales, this law provides an estimation of the shift in vegetation greening in temperate latitudes, matching well with ground and remote sensing observations (8, 9).
Global warming is dramatically altering the phenology of plants and animals and their interactions (10–12), so there has been growing interest during the last decade in investigating phenological events over large geographical scales and in conducting manipulative warming experiments. With this purpose, phenological observations across countries or states have been compiled into large datasets, especially focusing on key tree phenological events such as the time of leaf-out, flowering, and leaf coloration (e.g., the Pan European Phenology Project, PEP725 or the USA National Phenology Network). Most of the studies that have used these large datasets have focused on quantifying temporal changes in phenological events over the last decades (13–16). By contrast, little attention has been paid to how climate warming has altered biogeographical patterns of phenology (10) and the applicability of the Hopkins’ bioclimatic law. However, there are several reasons why we may not expect warming to lead to uniform advancement along a bioclimatic gradient, such as across elevations. First, warming trend may vary across the bioclimatic gradient, for example being stronger at higher elevations (17). Second, warming trend may vary across seasons, for example being stronger in late rather than early spring, possibly leading to larger phenological advance at higher elevations (18). Third, global warming may change the duration of chilling exposure responsible for bud dormancy release with two nonexclusive subhypotheses: (i) warmer winters are reducing the chilling exposure at lower elevations leading to increasing forcing requirement and/or later dormancy break (19), and so, to a smaller shift in response to spring warming advance than at higher elevations; and (ii) warmer winters are increasing the chilling exposure at higher elevations, usually assumed to range between 0 and 8–10 °C (20), making these populations sensitive to spring warming earlier in the season.
Here, we assessed how elevation-induced phenological shift (EPS) in the time of leaf-out has changed over the period 1960–2016 for four dominant European tree species [the common hazel, Corylus avellana L.; the European larch, Larix decidua Mill.; the European beech, Fagus sylvatica L.; and the Norway spruce, Picea abies (L.) Karst], using a unique dataset gathered by MeteoSwiss [Swiss Phenological Network (SPN)], including ∼20,000 observations collected from 128 sites, covering large elevational gradients [from 200 m above sea level (a.s.l.) to 1,800 m a.s.l.] over short geographical distance (see Materials and Methods and the location of the sites in SI Appendix, Fig. S1). We then examine each of the above-mentioned hypotheses for explaining this change.
Results
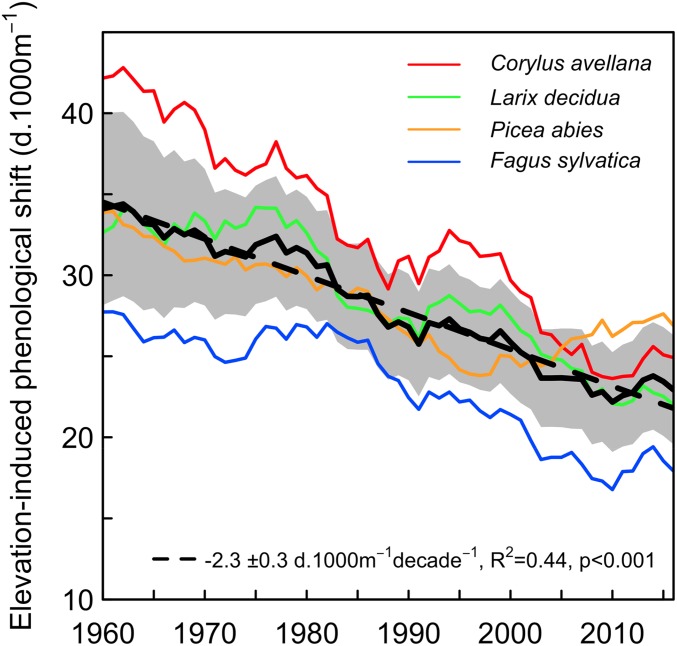
In line with previous studies, we found that the leaf-out dates significantly advanced over the period 1960–2016 with an average rate of −1.0 ± 0.4 d·decade−1 across all species and sites (−2.2 ± 0.7 d·decade−1 since 1980; see SI Appendix, Fig. S2A). The leaf-out dates showed a slight delay trend during ∼1960–1980, which is consistent with the temperature anomalies in spring (SI Appendix, Fig. S3). Interestingly, we have found that the EPS has been significantly reduced by about 35% across the four species over the last six decades, i.e., from 34-d delay every 1,000 m increase in elevation in 1960 to 22 d⋅1,000 m−1 in 2016 (Fig. 1). Similar patterns were found in all species but with differences in amplitudes (Fig. 1 and SI Appendix, Fig. S4). The largest reduction was found for C. avellana with −19 d⋅1,000 m−1 (−45%) over the study period, whereas the smallest reduction was found for P. abies with −9 d⋅1,000 m−1 (−27%).
Fig. 1.
Changes of the elevation-induced phenological shift (EPS) for four tree species over the period 1960–2016 in Switzerland. Eleven-years moving averages are represented (black line) and slope of the linear regression (dashed line) and SD (gray area) across species are also shown. SI Appendix, Tables S2–S5 provide data necessary for this figure.
To test the robustness of this intriguing pattern, we recalculated the EPS using the same method but with a selected phenology subset, i.e., using only phenological stations including leaf-out observations for at least 50 y for each species (instead of 30 y) within the study period. We found similar results with even stronger shifts in the EPS (3.3 ± 0.4 d⋅1,000 m−1⋅decade−1 across species, see SI Appendix, Fig. S5). We further explored the phenological shifts between high and low elevations and found that the decreasing EPS over time is mainly due to a larger phenological advance at high elevations rather than at low elevations. Specifically, irrespective of species, the advancement of leaf-out was approximately −1.9 d⋅decade−1 across species at high elevations, but only ∼ −0.4 d⋅decade−1 at low elevations (SI Appendix, Fig. S2B).
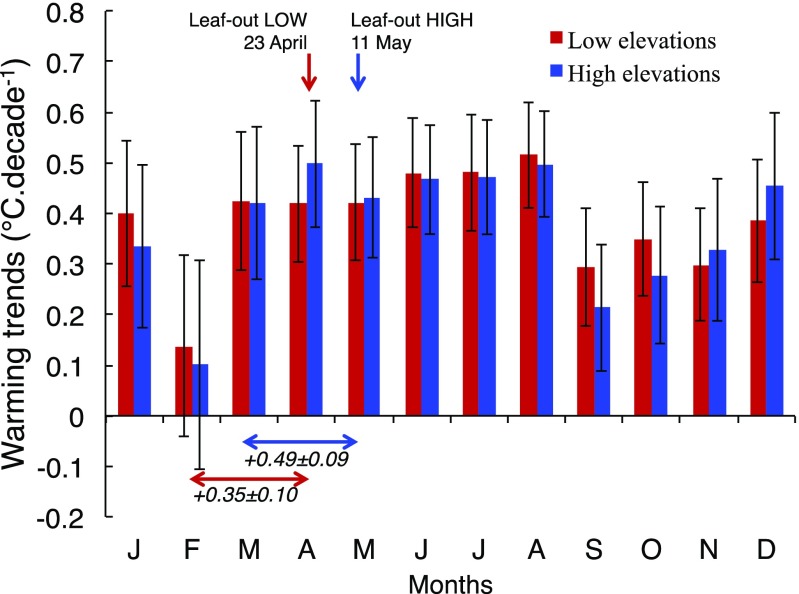
We then tested the following hypotheses: First, warming trends might have been stronger at higher elevations. We analyzed 24 long-term series of homogenized air temperature from weather stations covering the same range of elevations as the phenological stations all over Switzerland during the study period (Materials and Methods and SI Appendix, Fig. S1). This analysis showed no significant difference in warming trends between low and high elevations for any month (Fig. 2) and no elevation-dependent relationships when focusing on the warming trends during spring [March–May (MAM), r = 0.28, P = 0.18; SI Appendix, Fig. S6) or during winter (December–February (DJF), r = 0.18, P = 0.39; SI Appendix, Fig. S6], suggesting that the warming trends between low and high elevations were homogeneous and did not change over time, and so are therefore not responsible for the reduced EPS.
Fig. 2.
Warming trends per month at low- and high-elevation sites during the study period 1960–2016. Long-term series of homogenized temperatures from stations above 808 m (n = 11, mean elevation 1,360 m) were used to calculate mean monthly temperature for high elevations, whereas weather stations below 522 m (n = 9, mean elevation 414 m) were used to calculate mean monthly temperature for low elevations. These two thresholds correspond to the 33th and 66th percentile of the elevational range of the phenological stations across species. Warming trends correspond to the slope of the linear regressions between mean temperature and years over the entire study period with the associated SEs. Warming trends during the preseason of low- and high-elevation sites are reported below the corresponding arrows along with the mean leaf-out dates across species during the study period. Months appear by their first initials.
Second, warming trends could be stronger in late rather than in early spring causing larger advancement of leaf-out at higher elevations compared with lower elevations. The analysis of a long-term homogeneous series of temperature reveals quite homogeneous trends of strong warming during the study period from March to June (from 0.42 to 0.48 °C⋅decade−1), no significant warming trend in February, and intermediate warming trends during late autumn and winter (from 0.31 °C⋅decade−1 in November to 0.42 °C⋅decade−1 in December, respectively, see Fig. 2). As the mean leaf-out date across species is on April 23 [day of year (DOY) 113] and May 11 (DOY 131) at the low- and high-elevation sites, respectively (Fig. 2), and the optimal preseason length showing the strongest correlation between temperature and leaf-out dates is 63 and 60 d for low- and high-elevation sites, respectively (from ∼50 to ∼80 d, depending on species, see SI Appendix, Fig. S7), the second half of February may thus have influenced the leaf-out dates of trees growing at lower elevations. However, the daily mean temperature across low-elevation sites over the entire study period never exceeded 5 °C in February (SI Appendix, Fig. S8), a temperature threshold, often used in the literature, above which trees accumulate heat units leading to budburst. This suggests that the reduction of the EPS over the period 1960–2016 could not be explained solely by the heterogeneity of warming trends during the late winter and spring season.
Third, the EPS decline observed in the last six decades may be related to variation in chilling duration during winter. We found a significant negative correlation (r = −0.63; P = 0.003) between mean air temperature during the winter period (from November to January) and the EPS when selecting the 20 y having the warmest spring only (based on the mean species-specific preseason, see Materials and Methods). In other words, under similar (warm) spring temperature, warmer winters significantly reduced the EPS. In detail, the correlations were significant for C. avellana (r = −0.60, P = 0.005), L. decidua (r = −0.59, P = 0.006), and F. sylvatica (r = −0.50, P = 0.02), and not significant for P. abies (r = −0.37, P = 0.11).
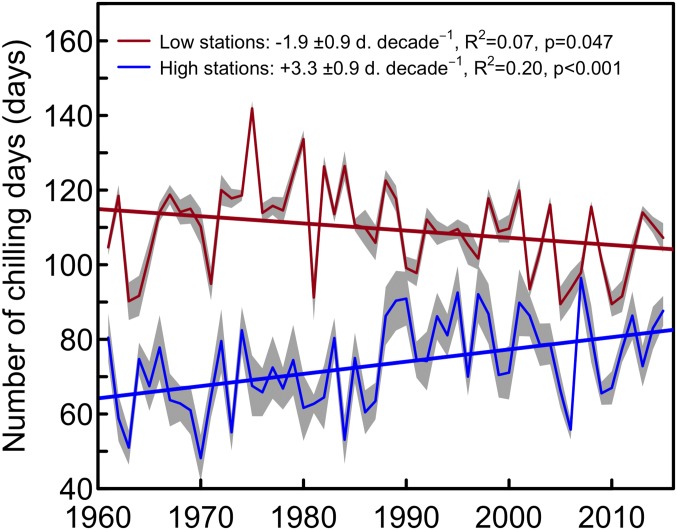
Two subhypotheses may in turn explain these correlations. First, a lack of chilling during exceptionally warm winters may delay phenology at lower elevations. Second, on the contrary, warmer winters may result in longer periods of effective chilling at higher elevations, as daily mean temperatures above 1,000 m are almost always below freezing from November to February in typical winters (see example of one of the low- and high-elevation stations in SI Appendix, Fig. S9), while chilling is usually assumed to range between 0 and 8–10 °C. We therefore calculated the chilling accumulation (Materials and Methods) and indeed found that the number of chilling days has significantly increased at high-elevation sites by 3.3 ± 0.9 d⋅decade−1 (P < 0.001), but has only slightly reduced at low-elevation sites (−1.9 ± 0.9 d⋅decade−1, P = 0.047, Fig. 3).
Fig. 3.
Number of chilling days at low- and high-elevation sites during the study period 1960–2016. Long-term series of homogenized temperatures from stations above 808 m (n = 11, mean elevation 1,360 m) were used to count chilling days for high elevations (mean ± SE), whereas weather stations below 522 m (n = 9, mean elevation 414 m) were used for low elevations. These two thresholds correspond to the 33th and 66th percentile of the elevational range of the phenological stations across species. A chilling day corresponds to a day when daily mean air temperature is between 0 °C and 8 °C from November to the mean date of leaf-out across years and species.
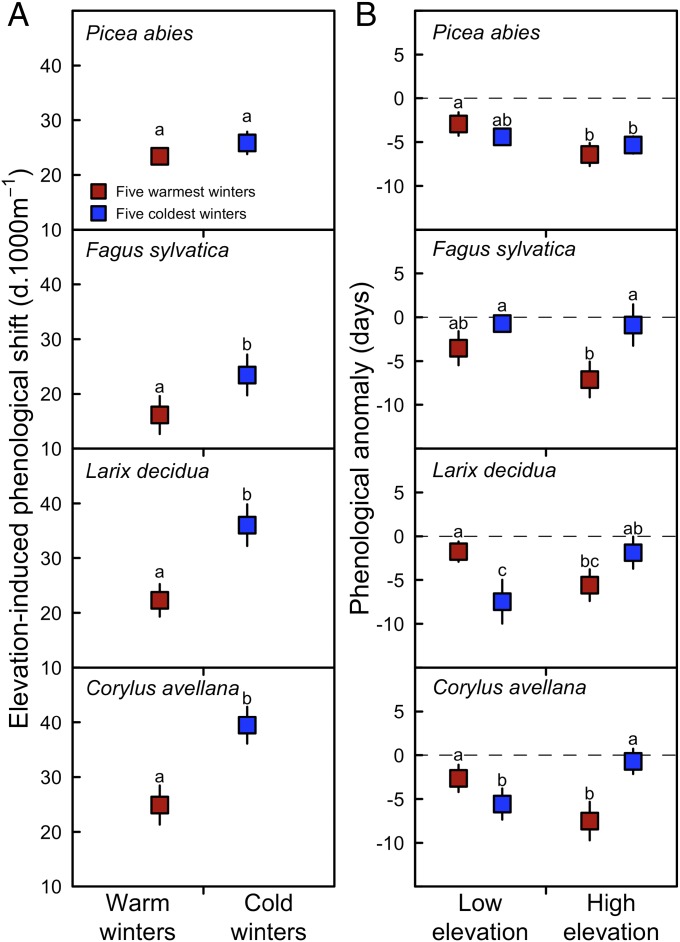
Furthermore, we checked the EPS difference with similar (warm) spring temperature, but under the five warmest winters versus the five coldest winters (Materials and Methods), and found that, on average, the EPS was about 21 d⋅1,000 m−1 under the warmest winters, but 32 d⋅1,000 m−1 under the coldest winters, and this difference in the EPS was significant for all species except P. abies (Fig. 4A). Thus, for all species but spruce, these results strongly support the third hypothesis that the winter warming resulted in larger advancement of leaf-out at higher elevations, but unchanged or even delayed spring phenology at lower elevations (Fig. 4B), finally resulting in a smaller EPS.
Fig. 4.
Elevation-induced phenological shift (EPS) (A) and phenological anomalies (B) during the five warmest and five coldest winters for the warmest springs for each species at low- and high-elevation sites. Low- and high-elevation stations were determined by using the 33th and 66th percentile of the species-specific elevational range. A total of 33% of the warmest springs (based on species-specific preseason temperature) were selected and among them we extracted the five warmest and five coldest winters for both low- and high-elevation sites. The difference in mean temperature between the five warmest and five coldest winters was about 0.6 °C in spring and 3.4 °C in winter across species, respectively. Different letters mean different values at P < 0.10 using nonparametric Kruskal–Wallis tests followed by Conover–Iman post hoc tests.
Discussion
In mountain ecosystems, numerous animals rely on snowmelt and spring vegetation onset and migrate progressively upward with the progress of spring in relation to food availability and quality (21). Our study provides striking evidence that the phenological discrepancy between lower and higher elevations has largely reduced over the last decades and is likely to decline further under future warmer conditions, as, paradoxically, chilling will likely continue to increase at higher elevations but decrease at lower elevations. It is now assumed that insufficient chilling may slow down further phenological advance in response to warming at lower elevations and/or lower latitudes (13, 22). Our study also shows that warmer winters paradoxically provide increasing chilling exposure to trees growing at higher elevations in the Alps as it was also recently found (18), leading to earlier dormancy break, and further reducing the EPS. For spruce, which exhibits the smallest reduction of the EPS (but perhaps also for the other species), nonexplored hypotheses may have additionally prevented or slackened further phenological advance at lower elevations, such as progressively shorter photoperiod at the time when buds become sensitive to warmer temperatures. Shorter photoperiod can slow down cell growth during budburst (23) so that the advance of phenology in response to spring warming may also be slowing down due to shorter daylength during the ecodormancy phase (22). We cannot test and therefore cannot exclude or support this hypothesis in this analysis. However, it is likely that at the warmest sites at lower elevations, photoperiod, in addition to the progressive lack of chilling, will slacken the advance of leaf-out dates in the future warmer climate, but this hypothesis remains largely debated (24). The impact of such reduction in the EPS for mountain ecosystems is, however, largely unknown but may disrupt numerous plant–animal interactions. For instance, observations in the same area show an increasing risk of frost exposure over the last decades only for trees growing at elevation above 800 m due to the larger phenological advance (25), which may considerably affect plant vitality and mountain food web ecosystems. Although the declining EPS could disrupt numerous plant–animal interactions synchronized on food availability and snowmelt timing, it may also help high-elevation plant populations to adapt to warmer climate by gathering genes from warmer locations due to higher overlap in their flowering time. We encourage researchers to explore further the potential implications of such reduction of the EPS for plants, animals, and their interactions.
Conclusions
Our results provide empirical evidence for a declining EPS over the last six decades and also suggest that future climate warming is likely to reduce further the EPS, mainly through changes in the time when chilling requirement is fulfilled to break winter dormancy. Insufficient chilling and shorter photoperiod may slow down further phenological advance in response to warming at lower elevations and/or lower latitudes, whereas increasing chilling may accelerate plant phenology at higher elevation. In other words, spring vegetation onset in the temperate mountain ecosystems may continue to get more uniform across elevations and the Hopkins’ bioclimatic law requires revision to accurately estimate tree leaf-out shifts of these regions. Given the importance of spring phenology for the structure and functioning of plants, mountain ecosystems, and Earth’s climate, this major change in the spring phenological pattern across elevations may have knockon effects in ecosystems and should be explored further both in other mountainous regions or along different bioclimatic gradients, such as across latitudes, but also through experiments to tease apart the importance of the different hypotheses explored here.
Materials and Methods
Phenological Data.
Citizen science-based phenological observations are provided by MeteoSwiss (SPN). The country-based phenology network provides a unique advantage to test the bioclimatic law due to the homogeneous protocols used for the phenological observations, and the very large elevational gradients covered by the observations. We used leaf-out dates of F. sylvatica, C. avellana, P abies, and L. decidua, which have been collected since 1951; the other species monitored in this program have many less data (their monitoring started mostly in the 1990s). Phenological observations were conducted weekly on single or several individuals by one to eight observers per site, applying the same protocol for phenology monitoring. Leaf-out is defined as the date when 50% of the leaves or needles are unfolded and out. We excluded phenological observations before 1960 because the number of stations was insufficient to calculate accurately the elevation-induced phenological shift during these years, and we excluded stations having fewer than 30 y of observations during the period 1960–2016.
Quality controls of phenological data were conducted, and we removed observations corresponding to more than 2.5 times the median absolute deviation (moderately conservative threshold) within a station across the study period (26). In addition, we excluded stations for which the SDs of all phenological observations across years were higher than 15 for C. avellana, L. decidua, and P. abies, and higher than 10 for F. sylvatica because its spring phenology is known to fluctuate much less than other species (27). This was done to remove unreliable stations or stations having large variations, possibly due to observer changes. These data cleaning resulted in 128 phenological stations ranging from 200 to 1,800 m a.s.l. (SI Appendix, Fig. S1). For analyses, we included 98 stations for F. sylvatica (4,132 observations), 56 stations for C. avellana (2,328 observations), 115 stations for L. decidua (5,110 observations), and 96 stations for P. abies (4,080 observations).
Temperature Data.
We used 23 long-term series of daily air temperature that were measured at 2 m height over the whole study period, i.e., from 1960 to 2016. The elevations of the climate stations range from 273 m to 1,804 m, matching well the elevation range of the phenological stations (SI Appendix, Fig. S1). The climate data were provided by MeteoSwiss and have been homogenized by using monthly homogeneity adjustments, and daily data were then derived by applying a spline function (28). Homogenization is important to adjust historic measured values to current measuring condition. Climate and phenology data are available from the Swiss Federal Office of Meteorology and Climatology upon request (https://gate.meteoswiss.ch/idaweb).
Data Analysis.
The EPS was calculated for each year and species as the slope of the linear regression between leaf-out dates and elevations of the phenology stations during the study period 1960–2016. For all species, slopes were significantly different from zero in all tested years (SI Appendix, Tables S2–S5). The amplitude of elevations between the lowest and highest phenology stations for calculating these slopes was always higher than 895 m, 1,035 m, 1,255 m, and 1,470 m for F. sylvatica, C. avellana, P. abies, and L. decidua, respectively, with a minimum of stations for a given year between 15 and 42, and a maximum between 51 and 110, depending on species (SI Appendix, Tables S2–S5).
Throughout the paper, we referred to low- and high-elevation sites as being the 33% lowest and highest phenological stations for a given species. On average across species, low- and high-elevation sites correspond to locations below 522 m and above 808 m, respectively. We calculated the optimal preseason length for each species across years at both low- and high-elevation sites, as the period before the mean leaf unfolding across the low- and high-elevation phenology sites, for which the correlation coefficient between leaf-out and air temperature was highest (13) (with 5-d steps; SI Appendix, Fig. S7). Although climate warming may have shortened this period of bud sensitivity to temperature (18), we calculated the preseason length over the entire study period, as it was not used to calculate temperature sensitivity of leaf-out dates but only to approximate this period to compare warming within this period between low and high elevations.
To test the hypothesis that change in the EPS is associated with winter warming, we first selected the top 33% of years with the warmest springs (based on the species-specific mean preseason length and the mean leaf-out dates calculated across all sites) to avoid inhibition of bud development by spring cold temperatures, which would substantially delay leaf-out timing. This resulted in 20 selected years. Then, using these 20 y, we correlated the EPS with winter temperatures (November to January). Finally, within these 20 y with warmest springs, we also selected the five warmest winters (November to January) and five coldest winters. On average across species mean winter temperatures differed by 3.4 °C between the five warmest and five coldest winters, whereas spring temperatures differed only by 0.6 °C, allowing us to compare the effect of winter temperature at rather similar spring temperatures.
Chilling temperatures that are responsible for breaking dormancy are commonly assumed to be most effective at a temperature range between 0 and 5 °C (29) or more generally between 0 and 8–10 °C (20). As a proxy for the amount of chilling experienced by buds during winter, we calculated for each year the number of days with daily mean temperature falling within 0 to +8 °C from November 1 to the mean leaf-out date across years and species (DOY 121). We did the same calculations restricted to a temperature range between 0 and 5 °C and it yielded similar results. All data analyses were performed using Rstudio version 0.99.489 (30).
Supplementary Material
Acknowledgments
We thank all volunteers who carried out phenological observations and Lindsey Norgrove for improving the English. The data have been provided by MeteoSwiss, the Swiss Federal Office of Meteorology and Climatology. Y.H.F. received support from the General Program of the National Nature Science Foundation of China (Grant 31770516), the National Key Research and Development Program of China (Grant 2017YFA06036001), and the Thousand Talents Program for Young Professionals. This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The climate and phenology data are available from the Swiss federal office of meteorology and climatology upon request (https://gate.meteoswiss.ch/idaweb).
See Commentary on page 833.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1717342115/-/DCSupplemental.
References
- 1.Richardson AD, et al. Climate change, phenology, and phenological control of vegetation feedbacks to the climate system. Agric For Meteorol. 2013;169:156–173. [Google Scholar]
- 2.Chuine I, Beaubien EG. Phenology is a major determinant of tree species range. Ecol Lett. 2001;4:500–510. [Google Scholar]
- 3.Hopkins AD. Periodical events and natural law as guides to agricultural research and practice. Mon Weather Rev. 1918;9:1–42. [Google Scholar]
- 4.Hopkins AD. The bioclimatic law. Mon Weather Rev. 1920;48:355. [Google Scholar]
- 5.Hopkins AD. Bioclimatics: A Science of Life and Climate Relations. US Department of Agriculture; Washington, DC: 1938. p. 188. [Google Scholar]
- 6.Vitasse Y, et al. Leaf phenology sensitivity to temperature in European trees: Do within-species populations exhibit similar responses? Agric For Meteorol. 2009;149:735–744. [Google Scholar]
- 7.Liang L. Beyond the bioclimatic Law. Prog Phys Geogr. 2016;40:811–834. [Google Scholar]
- 8.Zhang X, Tan B, Yu Y. Interannual variations and trends in global land surface phenology derived from enhanced vegetation index during 1982-2010. Int J Biometeorol. 2014;58:547–564. doi: 10.1007/s00484-014-0802-z. [DOI] [PubMed] [Google Scholar]
- 9.Wang H, Ge Q, Dai J, Tao Z. Geographical pattern in first bloom variability and its relation to temperature sensitivity in the USA and China. Int J Biometeorol. 2015;59:961–969. doi: 10.1007/s00484-014-0909-2. [DOI] [PubMed] [Google Scholar]
- 10.Menzel A, Sparks T, Estrella N, Roy D. Altered geographic and temporal variability in phenology in response to climate change. Glob Ecol Biogeogr. 2006;15:498–504. [Google Scholar]
- 11.Memmott J, Craze PG, Waser NM, Price MV. Global warming and the disruption of plant-pollinator interactions. Ecol Lett. 2007;10:710–717. doi: 10.1111/j.1461-0248.2007.01061.x. [DOI] [PubMed] [Google Scholar]
- 12.Peñuelas J, Filella I. Phenology. Responses to a warming world. Science. 2001;294:793–795. doi: 10.1126/science.1066860. [DOI] [PubMed] [Google Scholar]
- 13.Fu YH, et al. Declining global warming effects on the phenology of spring leaf unfolding. Nature. 2015;526:104–107. doi: 10.1038/nature15402. [DOI] [PubMed] [Google Scholar]
- 14.Menzel A, Fabian P. Growing season extended in Europe. Nature. 1999;397:659. [Google Scholar]
- 15.Menzel A, et al. European phenological response to climate change matches the warming pattern. Glob Change Biol. 2006;12:1969–1976. [Google Scholar]
- 16.Fu YH, et al. Recent spring phenology shifts in Western Central Europe based on multiscale observations. Glob Ecol Biogeogr. 2014;23:1255–1263. [Google Scholar]
- 17.Mountain Research Initiative EDW Working Group Elevation-dependent warming in mountain regions of the world. Nat Clim Chang. 2015;5:424–430. [Google Scholar]
- 18.Güsewell S, Furrer R, Gehrig R, Pietragalla B. Changes in temperature sensitivity of spring phenology with recent climate warming in Switzerland are related to shifts of the preseason. Glob Change Biol. 2017;23:5189–5202. doi: 10.1111/gcb.13781. [DOI] [PubMed] [Google Scholar]
- 19.Murray MB, Cannell MGR, Smith RI. Date of budburst of fifteen tree species in Britain following climatic warming. J Appl Ecol. 1989;26:693–700. [Google Scholar]
- 20.Hänninen H. Boreal and Temperate Trees in a Changing Climate: Modelling the Ecophysiology of Seasonality. Springer; Dordrecht, The Netherlands: 2016. The annual phenological cycle; pp. 35–138. [Google Scholar]
- 21.Gaudry W, et al. Partial migration or just habitat selection? Seasonal movements of roe deer in an Alpine population. J Mammal. 2015;96:502–510. [Google Scholar]
- 22.Körner C, Basler D. Plant science. Phenology under global warming. Science. 2010;327:1461–1462. doi: 10.1126/science.1186473. [DOI] [PubMed] [Google Scholar]
- 23.Basler D, Körner C. Photoperiod and temperature responses of bud swelling and bud burst in four temperate forest tree species. Tree Physiol. 2014;34:377–388. doi: 10.1093/treephys/tpu021. [DOI] [PubMed] [Google Scholar]
- 24.Zohner CM, Benito BM, Svenning J-C, Renner SS. Day length unlikely to constrain climate-driven shifts in leaf-out times of northern woody plants. Nat Clim Chang. 2016;6:1120–1123. [Google Scholar]
- 25.Vitasse Y, Schneider L, Rixen C, Christen D, Rebetez M. Increase in the risk of exposure of forest and fruit trees to spring frosts at higher elevations in Switzerland over the last four decades. Agric For Meteorol. 2018;248:60–69. [Google Scholar]
- 26.Leys C, Ley C, Klein O, Bernard P, Licata L. Detecting outliers: Do not use standard deviation around the mean, use absolute deviation around the median. J Exp Soc Psychol. 2013;49:764–766. [Google Scholar]
- 27.Vitasse Y, Basler D. What role for photoperiod in the bud burst phenology of European beech. Eur J For Res. 2013;132:1–8. [Google Scholar]
- 28.Begert M, Schlegel T, Kirchhofer W. Homogeneous temperature and precipitation series of Switzerland from 1864 to 2000. Int J Climatol. 2005;25:65–80. [Google Scholar]
- 29.Coville FV. The influence of cold in stimulating the growth of plants. Proc Natl Acad Sci USA. 1920;6:434–435. doi: 10.1073/pnas.6.7.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.R Development Core Team 2015. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.