Significance
Enzymes that activate dioxygen typically rely on flavins or transition metals as redox cofactors. We describe the discovery of the enzyme catalyst Cap15 that converts 5′-C-glycyluridine (GlyU), an unusual β–hydroxy-α-amino acid, to the modified nucleoside uridine-5′-carboxamide. In contrast to expectations, pyridoxal-5'-phosphate (PLP) is the sole cofactor that serves as the initiating reducing agent to activate dioxygen for incorporation into GlyU prior to decarboxylation. Thus, Cap15 is now classified as an O2- and PLP-dependent monooxygenase-decarboxylase. Also in contrast to expectations, phosphate and the (5′S,6′R) isomer of GlyU—and not (5′S,6′S)-GlyU, the established pathway intermediate—are essential for Cap15 activity. In total, Cap15 utilizes an enzymatic strategy for oxygen incorporation during conversion of an α-amino acid to a carboxamide.
Keywords: natural products, biosynthesis, antibiotic, enzyme function
Abstract
Capuramycins are antimycobacterial antibiotics that consist of a modified nucleoside named uridine-5′-carboxamide (CarU). Previous biochemical studies have revealed that CarU is derived from UMP, which is first converted to uridine-5′-aldehyde in a reaction catalyzed by the dioxygenase CapA and subsequently to 5′-C-glycyluridine (GlyU), an unusual β–hydroxy-α-amino acid, in a reaction catalyzed by the pyridoxal-5′-phosphate (PLP)-dependent transaldolase CapH. The remaining steps that are necessary to furnish CarU include decarboxylation, O atom insertion, and oxidation. We demonstrate that Cap15, which has sequence similarity to proteins annotated as bacterial, PLP-dependent l-seryl-tRNA(Sec) selenium transferases, is the sole catalyst responsible for complete conversion of GlyU to CarU. Using a complementary panel of in vitro assays, Cap15 is shown to be dependent upon substrates O2 and (5′S,6′R)-GlyU, the latter of which was unexpected given that (5′S,6′S)-GlyU is the isomeric product of the transaldolase CapH. The two products of Cap15 are identified as the carboxamide-containing CarU and CO2. While known enzymes that catalyze this type of chemistry, namely α-amino acid 2-monooxygenase, utilize flavin adenine dinucleotide as the redox cofactor, Cap15 remarkably requires only PLP. Furthermore, Cap15 does not produce hydrogen peroxide and is shown to directly incorporate a single O atom from O2 into the product CarU and thus is an authentic PLP-dependent monooxygenase. In addition to these unusual discoveries, Cap15 activity is revealed to be dependent upon the inclusion of phosphate. The biochemical characteristics along with initiatory mechanistic studies of Cap15 are reported, which has allowed us to assign Cap15 as a PLP-dependent (5′S,6′R)-GlyU:O2 monooxygenase-decarboxylase.
Pyridoxal-5′-phosphate (PLP)-dependent enzymes account for more than 230 distinct catalytic functions, most of which involve transamination, decarboxylation, deamination, or racemization of α-amino acids (1). Although ostensibly less common, other types of PLP-dependent enzymes are known, including those catalyzing β- or γ-elimination or -substitution, aldol- and Claisen-type reactions, and even reactions involving O2-dependent oxidation (2–4). Collectively, PLP-dependent enzymes currently occupy five of the six classes used by the Enzyme Commission, with synthetases the lone category yet to be reported. The power and versatility inherent in PLP is chiefly due to the ability to form an imine—first with the side-chain amine of a conserved Lys of the enzyme to form an internal aldimine and then with a substrate to form an external aldimine—and subsequently serve as an electron sink during the chemical transformation of the substrate. Despite steady advances in our understanding of the factors dictating the chemistry downstream of aldimine formation (2), the prediction of a PLP-dependent enzyme activity solely based on sequence remains—for the most part—an inexact science.
The A-500359s from Streptomyces griseus SANK 60196 (5), the A-503083s from Streptomyces sp. SANK 62799 (6), and the A-102395 from Amycolotopsis sp. SANK 60206 (7) are capuramycin-type secondary metabolites (8) that are potent inhibitors of bacterial translocase I and have promising antimycobacterial activity. This group of nucleoside antibiotics is characterized by two rarely observed structural components: a uridine-5′-carboxamide (CarU) nucleoside core and an unsaturated hexuronic acid appended to the core through a 5′-O-glycosidic bond (Fig. 1). The hexuronic acid is subsequently modified with a third component, an l-aminocaprolactam in the case of A-500359s/A-503083s and an unusual arylamine-containing polyamide in the case of A-102395, both of which are attached to the hexuronic acid via an amide bond (Fig. 1). The biosynthetic gene clusters for all three capuramycin-type metabolites have been cloned and characterized (9–11). Comparative bioinformatic analysis revealed 18 homologous open reading frames (orfs) between the capuramycin clusters, consistent with an important role in resistance, regulation, and the biosynthesis of the shared CarU-hexuronic acid disaccharide core (11).
Fig. 1.
Structure and biosynthesis of capuramycin-type metabolites. Proposed biosynthesis of the shared CarU core of capuramycin-type metabolites A-500359s and A-503083s starting from UMP. CapA and CapH, along with the homologs LipL and LipK from the biosynthetic pathway for A-90289 that contains an intact (5′S,6′S)-GlyU in the final metabolite, have been previously assigned in vitro. *LipK was previously shown to stereospecifically generate (5′S,6′S)-GlyU. αKG, α-ketoglutarate.
Of the 18 shared orfs in the gene clusters for the capuramycin-type metabolites, two encode sequence-unrelated proteins that are each predicted to be PLP-dependent enzymes: CapH, with primary sequence similarity to proteins annotated as serine hydroxymethyltransferases, and ORF15, with sequence similarity to bacterial proteins annotated as l-seryl-tRNA(Sec) selenium transferases, SelA. CapH—the discovery of which added to the catalog of reported PLP-dependent catalytic activities—was assigned as a transaldolase that generates acetaldehyde and 5′-C-glycyluridine (GlyU) from l-Thr and uridine-5′-aldehyde (U5′A), the last of which is generated from UMP in a reaction catalyzed by a nonheme Fe(II)-dependent α-ketoglutarate:UMP dioxygenase CapA (Fig. 1) (11, 12). The uncovering of the tandem reactions catalyzed by CapA and CapH revealed GlyU to be the likely biosynthetic precursor of CarU, which was further supported by isotopic enrichment studies that were consistent with l-Thr as the source of the carbon and nitrogen atoms of the carboxamide of CarU and, significantly, that the C-N bond of GlyU remains intact during the transformation to CarU (11). The totality of the results suggested that the remaining steps to furnish CarU minimally involve (i) decarboxylation of GlyU, (ii) incorporation of an oxygen atom via a hydratase or oxygenase activity, and (iii) oxidation to the carboxamide. In lieu of biochemical precedence of PLP-dependent enzymes, it seemed reasonable that ORF15 might serve as the catalyst for the decarboxylation step, despite inference from sequence analysis.
In our attempt to delineate the remaining steps that “trim” GlyU to CarU, we now report the function of ORF15, herein named Cap15. As anticipated, Cap15 catalyzes decarboxylation of GlyU, but also has an unexpected oxygenase activity and is identified as the sole catalyst responsible for the complete conversion of GlyU to the signature CarU nucleoside of the capuramycin-type metabolites. Specifically, the data are consistent with the functional assignment of Cap15 as a PLP-dependent (5′S,6′R)-GlyU monooxygenase-decarboxylase. Additional biochemical studies have enabled us to propose a mechanistic framework for this PLP-dependent enzyme-catalyzed transformation, adding to the already remarkable catalytic diversity of PLP-dependent enzymes found in living systems.
Results
Bioinformatic Analysis of Cap15.
The gene product of cap15 has moderate sequence identity (45–50%) to proteins annotated as putative l-seryl-tRNA(Sec) selenium transferase, SelA. Secondary structure-based predictions mirrored this analysis with the highest similarity to Aquifex aeolicus SelA (13), although sequence identity by pairwise alignment was significantly lower (24%, SI Appendix, Fig. S1A). Bacterial SelA catalyzes the PLP-dependent production of selenocysteinyl-tRNA(Sec) and phosphate from substrates seryl-tRNA(Sec) and selenophosphate (SI Appendix, Fig. S2). Studies with AaSelA have revealed that this enzyme functions as a pentamer of dimers, with residues from each dimer subunit (termed A and B subunits) contributing to catalysis (13), and the reaction is initiated by formation of an internal aldimine between Lys285A and PLP. Based on sequence comparison, Lys230 is predicted to be the respective residue in Cap15 (SI Appendix, Fig. S1A). Only two additional Lys residues are conserved (Lys318 and Lys362 of AaSelA corresponding to Lys265 and Lys303 of Cap15, respectively). As part of the B subunit, the former Lys is located within ∼12 Å of the PLP and selenophosphate binding site of the A subunit; in contrast, the latter Lys is located distant from the active site. The importance of either residue has not been established. The B subunit of the AaSelA dimer also has two essential Arg residues that are proposed to establish an important electrostatic interaction for binding of PLP and the substrate selenophosphate (SI Appendix, Fig. S1B). While one of the Arg residues is conserved in Cap15 (Arg259), the second is substituted with a Lys (Lys262) (SI Appendix, Fig. S1A).
Functional Assignment of Cap15.
Recombinant His6-Cap15 was soluble upon production in Escherichia coli and purified to near homogeneity using immobilized metal affinity chromatography (SI Appendix, Fig. S3A). Cap15(K230A) was also prepared as a potential control (SI Appendix, Fig. S3A). Both purified protein solutions were colorless and lacked a UV/Vis spectrum indicative of a PLP cofactor. The hypothetical substrate (5′S,6′S)-GlyU, which was identified as the stereoisomer produced by the CapH-homolog LipK (47% sequence identity via pairwise alignment) that is involved in the biosynthesis of A-90289 (Fig. 1) (14), was synthesized (SI Appendix, SI Materials and Methods and Fig. S4) and tested with Cap15 in Hepes buffer with PLP. Following overnight reactions, HPLC analysis did not reveal any new peaks compared with the controls. In contrast to LipK (14), the stereochemistry of the CapH product was not previously assigned (11). Therefore, two additional stereoisomers of GlyU—(5′R,6′S) and (5′S,6′R)—that could hypothetically arise from the transaldolase reaction (14) were tested for activity with Cap15. We have detailed the stereocontrolled synthesis and analytic characterization of these two diastereomers elsewhere (15–17). Once again, however, no new peaks were observed with Cap15.
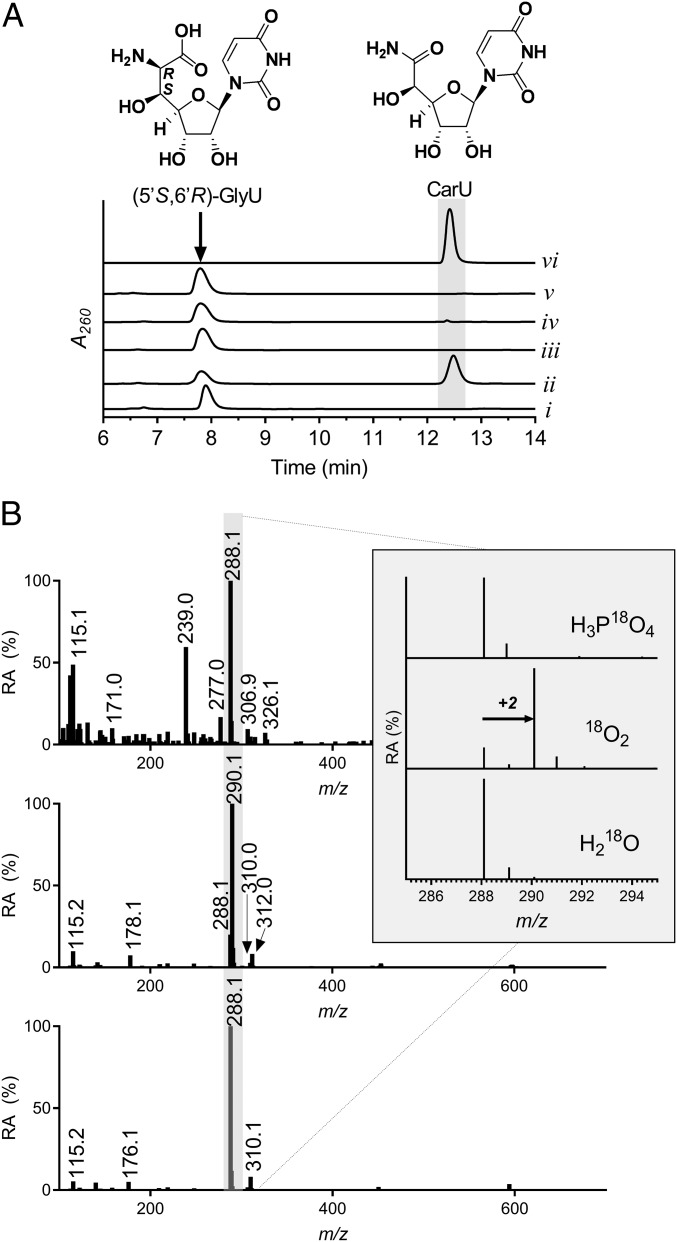
We next explored a variety of reaction conditions including different pH and buffer systems. When reactions were performed in phosphate buffer with the supposedly unnatural (5′S,6′R)-GlyU isomer, a new peak was observed by HPLC, and formation of the new peak was dependent upon the inclusion of Cap15 and PLP (Fig. 2A). A trace amount of the new peak appeared following reactions with Cap15(K230A) (Fig. 2A). No new peaks were detected using the (5′S,6′S) or (5′R,6′S) isomers of GlyU as potential substrates under the phosphate-buffered conditions (SI Appendix, Fig. S5). The new peak was analyzed by LC-MS yielding an (M + H)+ ion at m/z = 288.0, unexpectedly consistent with the molecular formula for CarU [expected (M + H)+ ion at m/z = 288.1 and (M + Na)+ ion at m/z = 310.0]. To simplify product identification, CarU was synthesized and purified, and authentic CarU coeluted with the Cap15 product and had identical mass and NMR spectroscopic properties (Fig. 2A and SI Appendix, Figs. S6–S11). HPLC analysis of the Cap15 reaction revealed the enzyme was inactive under anaerobic conditions (Fig. 2A) and inclusion of EDTA had no effect (SI Appendix, Fig. S12). Thus, Cap15 catalyzes a PLP-, phosphate-, and O2-dependent conversion of (5′S,6′R)-GlyU to CarU.
Fig. 2.
Biochemical characterization of Cap15. (A) HPLC traces of the reaction catalyzed by Cap15 using substrate (5′S,6′R)-GlyU with (i) exclusion of enzyme; (ii) complete reaction mixture containing phosphate, (5′S,6′R)-GlyU, O2, PLP, and Cap15; (iii) exclusion of PLP; (iv) substitution of Cap15(K230A) for the wild-type enzyme; (v) exclusion of O2; and (vi) synthetic CarU. A260, absorbance at 260 nm. (B) LC-MS analysis of the CarU product following Cap15-catalyzed reactions in the presence of the indicated isotopically labeled molecule. Data shown are representative of duplicates. RA, relative abundance.
The unanticipated requirement of O2 and phosphate for Cap15 activity led us to more closely examine their role in the reaction. Using a sealed vessel and monitoring the reaction with an O2 electrode, dissolved O2 was steadily consumed over time (SI Appendix, Fig. S3B), consistent with Cap15 functioning as an oxygenase. We subsequently probed the origin of the oxygen atom of the carboxamide of CarU, which hypothetically could be derived from phosphate, O2, or H2O. Three independent reactions were performed with isotopically enriched H3P18O4, H218O, or 18O2, and CarU production was monitored by LC-MS (Fig. 2B). An (M + H)+ ion at m/z = 290.1 was detected only in the presence of 18O2, which corresponds to a 2-amu increase compared with unlabeled CarU. An estimated 93% enrichment was calculated based on peak intensities under these conditions. Finally, hydrogen peroxide was not detected under optimized reaction conditions. Thus, the data are consistent with the classification of Cap15 as a monooxygenase and also suggest that phosphate does not participate directly in the chemistry of the Cap15 reaction.
The identification of CarU as the product suggested that CO2 was concomitantly formed during the reaction. To confirm this, we used a commercial enzyme-linked bicarbonate detection system for in situ detection of CO2. Using reactions without PLP or phosphate as controls, CO2 was confirmed as the second product of the Cap15-catalyzed reaction (SI Appendix, Fig. S3C). Contrastingly, reactions under anaerobic conditions did not produce detectable CO2. Furthermore, reactions with Cap15(K230A) resulted in only trace amounts of CO2. In total, the data are consistent with the functional assignment of Cap15 as a PLP-dependent (5′S,6′R)-GlyU:O2 monooxygenase-decarboxylase that generates carboxamide-containing CarU and CO2.
Biochemical Properties.
Cap15 was active only in phosphate buffer, wherein the pH profile resembled a bell-shaped curve with optimal activity at pH 7.5 (Fig. 3A). The specific activity of Cap15 was (5.2 ± 0.2) × 10−3 μmol/min/mg under the optimized conditions. The specific activity with Cap15(K230A) was (2.4 ± 0.1) × 10−4 μmol/min/mg, an approximately 20-fold decrease compared with the wild-type enzyme. Using HPLC to detect CarU formation, single-substrate kinetic analysis with variable (5′S,6′R)-GlyU revealed Michaelis–Menten kinetics yielding a Km = (56 ± 7) × 101 μM and kcat = (9.3 ± 0.5) × 10−1 min−1 (Fig. 3B). Increasing the concentration of phosphate from 50 mM to 1 M had little impact with respect to the overall catalytic efficiency [Km = (42 ± 4) × 101 μM and kcat = (6.2 ± 0.2) × 10−1 min−1] (SI Appendix, Fig. S13). Furthermore, nearly identical kinetic parameters were calculated when activity was measured by CO2 detection instead of CarU detection by HPLC (Fig. 3B).
Fig. 3.
Activity analysis of Cap15 and CapH. (A) Dependence of Cap15 activity on buffer and pH. (B) Single-substrate kinetic analysis with variable (5′S,6′R)-GlyU by detection of CarU product by HPLC (○) or by detection of coproduct CO2 (■). (C) HPLC traces of (i) phosgene-modified, synthetic (5′S,6′R)-GlyU (●); (ii) phosgene-modified, synthetic (5′S,6′S)-GlyU (♦); (iii) phosgene-modified product of CapH-catalyzed reaction; and (iv) negative control without CapH.
As previously noted, the activity of Cap15 was stereoselective for the (5′S,6′R)-GlyU diastereomer. Thus, the reaction catalyzed by CapH was re-examined to identify the stereochemistry of the GlyU product. Initially, a one-pot reaction with CapH and Cap15 starting from U5′A and l-Thr was analyzed by HPLC, revealing the formation of a peak coeluting with GlyU (SI Appendix, Fig. S14). However, no CarU was observed, suggesting that the CapH product—presumably (5′S,6′S)-GlyU—is not a substrate for Cap15. Subsequently, the stereochemistry of the CapH product was directly assessed. As described elsewhere (14), phosgene modification of the β–hydroxy-amino acid of GlyU enabled the analytical separation of the three synthetic diastereomers in hand. Following the CapH reaction using synthetic U5′A and l-Thr as substrates, the phosgene-modified GlyU coeluted with authentic, phosgene-modified (5′S,6′S)-GlyU (Fig. 3C). Modification of the LipK product as a control confirmed this result (14). Thus, CapH stereospecifically generates (5′S,6′S)-GlyU, which must undergo epimerization before the Cap15-catalyzed reaction.
Initiatory Mechanistic Analysis.
The K230A mutation of Cap15 did not abolish activity as initially predicted, and thus site-directed mutants of the two remaining conserved Lys residues (Lys265 and Lys303) were prepared, and the mutant proteins analyzed for activity. Cap15-Lys262 was also targeted for mutagenesis due to the importance of the corresponding Arg residue in AaSelA (SI Appendix, Fig. S1). The three additional Lys → Ala mutant proteins were soluble when expressed in E. coli (SI Appendix, Fig. S3A), and activity analysis by HPLC revealed that Cap15(K303A) retained activity comparable to the wild-type enzyme (SI Appendix, Fig. S15). Contrastingly, the K262A and K265A mutant proteins did not produce detectable levels of CarU.
The UV/Vis absorption spectra for the wild-type and mutant proteins were examined with the goal of establishing a mechanistic framework for Cap15 (2, 18, 19). Wild-type Cap15 and the mutant variants did not have an obvious absorbance at wavelengths greater than 300 nm, consistent with the production of the recombinant enzyme in an apo-form (Fig. 4A, trace I, and SI Appendix, Fig. S16A). The lone exception was Cap15(K265A), which displayed a λmax = 411 nm that is consistent with some protein copurifying with PLP as the internal aldimine (SI Appendix, Fig. S16A). Identical results were obtained when including excess PLP before protein purification by affinity chromatography. Following the addition of substoichiometric PLP, which has a λmax at 388 nm representing the aldehyde form (Fig. 4A, trace II), a subtle red shift to λmax at 391 nm was detected for wild-type Cap15 (Fig. 4A, trace III), Cap15(K262A), and Cap15(K303A) (SI Appendix, Fig. S16B); the spectrum for Cap15(K230A) was essentially identical to free PLP. The λmax = 411 nm for Cap15(K265A) did not shift but increased in intensity following the addition of PLP (SI Appendix, Fig. S16B). Upon addition of excess (5′S,6′R)-GlyU to the wild-type Cap15-PLP solution, a red shift was observed to λmax = 415 nm (Fig. 4A, trace IV). Cap15(K230A) and Cap15(K303A), both active as catalysts, had similar spectral shifts (SI Appendix, Fig. S16C). In contrast, the UV/Vis spectra for Cap15(K262A) and Cap15(K265A) did not change (SI Appendix, Fig. S16C). As expected, nonsubstrates (5′S,6′S)-GlyU and (5′R,6′S)-GlyU did not significantly alter the spectral profile when added to the wild-type Cap15-PLP mixture (SI Appendix, Fig. S17). Closer examination of the Cap15 UV/Vis spectrum revealed a second λmax at 503 nm, which appeared immediately following the addition of (5′S,6′R)-GlyU and remained nearly constant over the course of 24 min (Fig. 4B, trace V). In contrast to the other mutant proteins, only Cap15(K303A) displayed the identical λmax at 503 nm (SI Appendix, Fig. S16D).
Fig. 4.
Mechanism of Cap15. (A) UV/Vis spectra of PLP in phosphate pH 7.5 (dotted line); Cap15 before (dashed line) and after (black solid line) the addition of PLP; and the ternary complex after addition of (5′S,6′R)-GlyU (rainbow lines). (B) UV/Vis spectra for Cap15-PLP (black solid line) and ternary complex (rainbow lines) at longer wavelength revealing the λmax at 503 nm. Spectral datasets were zeroed at 600 nm. (C) Proposed mechanism for Cap15. The roman numerals under the chemical species are proposed in part based on interpretation of the spectra highlighted in A and B.
Discussion
The functional assignment of Cap15 has revealed unprecedented enzyme chemistry that involves PLP as an oxygenase cofactor, an unusual role for an already well-known and highly versatile cofactor. Several enzymes involved in primary metabolism that generate carbanionic intermediates have previously been reported to catalyze off-pathway side reactions—so-called paracatalytic reactions—that involve oxidation of the carbanionic intermediate with O2 (20, 21). Given that most members of the PLP-dependent transferase superfamily are characterized by their ability to stabilize a carbanion through electron delocalization within PLP, it is not surprising that five distinct PLP-dependent enzymes are included in the paracatalytic reaction list (20). These five PLP-dependent enzymes use an amino acid substrate to catalyze oxidative deamination in addition to decarboxylation, the former of which was not detected with Cap15. For three of these PLP-dependent enzyme “paracatalysts,” H2O2 is stoichiometrically produced, and thus they are classified as oxidases (20, 22–25). The fate of the oxygen atoms for the other two paracatalysts, however, remains unknown.
In contrast to PLP-dependent enzymes that catalyze paracatalytic reactions, a few enzymes have recently been reported to catalyze PLP-dependent oxidations as their primary function (3, 4, 26, 27). Ind4, involved in indolymycin biosynthesis, was shown to catalyze tandem 2,3- and 4,5-dehydrogenation of l-Arg, concomitantly reducing O2 to H2O2 (3). CcdF, involved in celesticin biosynthesis, was shown to catalyze an oxidative decarboxylation and deamination of an α–amino-acid component of a celesticin precursor, also concomitantly reducing O2 to H2O2 (26). Thus, both Ind4 and CcdF are bona fide PLP-dependent oxidases. Contrastingly, MppP involved in l-enduracidine biosynthesis was shown to catalyze an O2 and PLP-dependent α-deamination and 4-hydroxylation of l-Arg, the latter suggesting that this enzyme is an oxygenase (4). However, neither the fate of the oxygen atoms of O2 nor the origin of the oxygen atom in the hydroxylated product was determined, and thus the enzyme classification of MppP is not clear. Our results definitively establish the use of PLP as a redox cofactor that enables Cap15 to function as a true monooxygenase, incorporating one O atom of O2 directly into the carboxamide-containing product CarU. Interestingly, a similar PLP-dependent, α–amino-acid-to-carboxamide transformation has been proposed during pyoverdine biosynthesis, although the activity of the probable catalyst (PvdN) remains to be demonstrated in vitro (27).
Bioinformatically, Cap15 has primary sequence homology to bacterial proteins annotated as SelA, which is a fold-type I PLP-dependent enzyme that catalyzes the transformation of seryl-tRNA(Sec) to selenocysteinyl-tRNA(Sec) using selenophosphate as the selenium donor (13). The discovery that the Cap15 reaction not only requires O2 but also depends on phosphate, in this case the standard oxygenated variant, gave rise to the possibility that the O atom incorporated into CarU could originate directly from phosphate in a reaction analogous to that catalyzed by SelA. However, the isotopic enrichment studies with 18O-labeled phosphate, compared with 18O-labeled O2, clearly revealed that this was not the case, thus leaving the role of phosphate in the Cap15-catalyzed reaction unknown. Interestingly, the dioxygenase CapA, which initiates the CarU biosynthetic pathway, produces phosphate via an unusual oxidative dephosphorylation mechanism that could potentially participate as a feed-forward allosteric modulator (11). Preliminary analysis suggests that Cap15 does in fact differentially oligomerize in the presence of phosphate (SI Appendix, Fig. S18), although ongoing kinetic and structural studies will be essential to clarify the quaternary structure and role of phosphate.
Wild-type Cap15 (I) does not copurify with a detectable amount of PLP (Fig. 4B) and is inactive without an exogenous supply of PLP. Furthermore, the addition of PLP to Cap15 (III) resulted in a UV/Vis spectrum that is nearly indistinguishable from the aldehyde form of PLP (II), which is in contrast to most other PLP-dependent enzymes that display a significant red shift (λmax of 410–440 nm) that is indicative of an internal aldimine (19). As a result, we initially considered a mechanism that bypasses the internal aldimine. However, studies with PLP-dependent aspartate aminotransferase (28) and 1-aminocyclopropane-1-carboxylate synthase (29), among others (2, 19), have revealed an internal aldimine that—depending upon the orientation and protonation state of PLP in the active site—can display a UV/Vis spectrum with a λmax ranging from 390 nm (corresponding to an unprotonated imine) to 420 nm (corresponding to a protonated imine). Additionally, mutational analysis of Cap15 revealed that the inactive mutant Cap15(K265A) displays a spectrum with a λmax of 411 nm following purification and addition of exogenous PLP, which is consistent with formation of a protonated internal aldimine (30, 31). In lieu of the literature precedent along with these data, we propose a mechanism wherein Cap15 does indeed form a protonated internal aldimine (III) with an unidentified Lys that is characterized by a λmax of 391 nm (Fig. 4B). Mutation of Lys265 is proposed to promote orientation of the internal aldimine to favor protonation, hence displaying the more traditional spectrum associated with PLP-dependent enzymes. Regardless, the red shift to λmax at 416 nm following the addition of (5′S,6′R)-GlyU to the Cap15-PLP complex strongly suggests the formation of an external aldimine (IV) that undergoes Cα-deprotonation to form a resonance-stabilized quininoid (V), which is characterized by the appearance of a second λmax at 503 nm in the spectrum for wild-type Cap15 and Cap15(K303A). Similar to the results with MppP and Ind4 (3, 4), the quininoid appears to reach a steady state during the reaction that would suggest that downstream chemistry is rate-limiting (2).
The most intriguing aspect of the Cap15 reaction is the oxidative steps that follow aldimine formation. Other enzymes that catalyze oxidative decarboxylation of α-amino acids to form carboxamides—namely Trp (32), Lys (33), Arg (34), and Phe (35) 2-monooxygenases–require flavin adenine dinucleotide (FAD) to initiate electron transfer to dioxygen (36). For Trp-2-monooxygenase, the best characterized of the carboxamide-forming flavoproteins, the reaction begins by hydride transfer from Trp to FAD to generate an imine and reduced FAD, respectively (32). The reduced FAD subsequently donates an electron to O2 to yield a caged radical pair that, following spin inversion, recombines to form a C(4a)-hydroperoxide intermediate (34). Contrastingly, the oxidative steps of the Cap15-catalyzed reaction do not require a flavin cofactor, nor does it appear that a redox active metal cofactor is involved. Consequently, we propose a hydroperoxide mechanism for Cap15 (Fig. 4B), wherein the carbanion resonance form of the quininoid (V) serves a role that is comparable to the reduced FAD for Trp-2-monooxygenase. Thus, an electron is first transferred from the carbanion to O2 to form a resonance-stabilized GlyU-PLP radical and superoxide, the latter of which can undergo protonation and rebound with the carbon-centered radical to form the hydroperoxide species. Subsequently, decarboxylation occurs with elimination of the distal hydroperoxyl O atom in the form of water to generate a CarU-PLP aldimine that is ultimately hydrolyzed to release CarU. Although it remains possible that the initial carbanion is generated by decarboxylation instead of deprotonation of the aldimine, we were unable to detect CO2 under anaerobic conditions, thus suggesting that O2 reduction precedes decarboxylation.
In addition to the unusual role of PLP as a redox cofactor, the finding that Cap15 is stereospecific for the (5′S,6′R)-diastereomer of GlyU was unexpected. This led us to reexamine the stereochemical outcome of the transaldolase CapH. Although it is mechanistically feasible for CapH to produce (5′S,6′R)-GlyU, the product was clearly identified as (5′S,6′R)-GlyU upon comparisons with synthetic standards and the product of the well-characterized transaldolase LipK (14). Thus, the discovery of Cap15 activity—despite filling a major gap regarding the biosynthetic mechanism of CarU—introduces another biochemical step in the pathway and suggests the involvement of an additional, unidentified epimerase. Intriguingly, a putative NAD-dependent NDP-hexose epimerase is among the few remaining unassigned proteins within the gene cluster; however, other possibilities cannot be excluded at this time.
In summary, we have provided evidence to support the functional assignment of Cap15 as a PLP-dependent (5′S,6′R)-GlyU:O2 monooxygenase-decarboxylase that generates the carboxamide functionality of CarU, the signature nucleoside core of the capuramycins. Generally, enzymes known to activate O2 do so by employing a flavin or transition metal cofactor or, in some instances, are substrate-assisted (also known as cofactor independent). Indeed, enzymes that catalyze a comparable α-amino acid→carboxamide conversion require a reduced FAD to achieve this chemical outcome. Characterization of Cap15 now provides a definitive example of an enzyme whose native function requires PLP as an oxygenase cofactor. With the mechanistic framework provided here, it is now possible to compare the role of the flavin and PLP cofactors as it relates to the oxidative decarboxylation of amino acids to produce the respective carboxamide-containing products. Furthermore, the abundance of predicted PLP-dependent enzymes per genome with as-of-yet-unknown function hints at the possibility that other PLP-dependent oxygenases not unlike Cap15 remain to be discovered.
Materials and Methods
Cloning, mutatgenesis, heterologous production, and activity analysis for Cap15 were performed using standard procedures and are described in the SI Appendix, SI Materials and Methods. Reactions were monitored by C18 reverse phase HPLC, LC-MS, a Carbon Dioxide Enzymatic Assay kit (Diazyme Laboratories), and a Profiling Oxygen Microsensor PM-PSt7 equipped with Microx 4 oxygen meter (PreSens Precision Sensing), and UV-visible spectroscopy was performed with a Shimadzu UV/Vis-1800 spectrophotometer. The synthesis of (5′R,6′S)-GlyU, (5′S,6′R)-GlyU, and U5′A was previously described (14–17). The syntheses and analytical analyses of (5′S,6′S)-GlyU and CarU are described in the SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Koichi Nonaka (Daiichi-Sankyo Co.) for intellectual contributions and for providing strains and cosmids. This work was supported in part by National Institutes of Health Grants AI128862 and AI087849 (to S.G.V.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1718667115/-/DCSupplemental.
References
- 1.Percudani R, Peracchi A. The B6 database: A tool for the description and classification of vitamin B6-dependent enzymatic activities and of the corresponding protein families. BMC Bioinformatics. 2009;10:273. doi: 10.1186/1471-2105-10-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toney MD. Controlling reaction specificity in pyridoxal phosphate enzymes. Biochim Biophys Acta. 2011;1814:1407–1418. doi: 10.1016/j.bbapap.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Du Y-L, et al. A pyridoxal phosphate-dependent enzyme that oxidizes an unactivated carbon-carbon bond. Nat Chem Biol. 2016;12:194–199. doi: 10.1038/nchembio.2009. [DOI] [PubMed] [Google Scholar]
- 4.Han L, Schwabacher AW, Moran GR, Silvaggi NR. Streptomyces wadayamensis MppP is a pyridoxal 5′-phosphate-dependent l-arginine α-deaminase, γ-hydroxylase in the enduracidine biosynthetic pathway. Biochemistry. 2015;54:7029–7040. doi: 10.1021/acs.biochem.5b01016. [DOI] [PubMed] [Google Scholar]
- 5.Muramatsu Y, et al. Studies on novel bacterial translocase I inhibitors, A-500359s. I. Taxonomy, fermentation, isolation, physico-chemical properties and structure elucidation of A-500359 A, C, D and G. J Antibiot (Tokyo) 2003;56:243–252. doi: 10.7164/antibiotics.56.243. [DOI] [PubMed] [Google Scholar]
- 6.Muramatsu Y, et al. A-503083 A, B, E and F, novel inhibitors of bacterial translocase I, produced by Streptomyces sp. SANK 62799. J Antibiot (Tokyo) 2004;57:639–646. doi: 10.7164/antibiotics.57.639. [DOI] [PubMed] [Google Scholar]
- 7.Murakami R, et al. A-102395, a new inhibitor of bacterial translocase I, produced by Amycolatopsis sp. SANK 60206. J Antibiot (Tokyo) 2007;60:690–695. doi: 10.1038/ja.2007.88. [DOI] [PubMed] [Google Scholar]
- 8.Yamaguchi H, et al. Capuramycin, a new nucleoside antibiotic. Taxonomy, fermentation, isolation and characterization. J Antibiot (Tokyo) 1986;39:1047–1053. doi: 10.7164/antibiotics.39.1047. [DOI] [PubMed] [Google Scholar]
- 9.Funabashi M, et al. Identification of the biosynthetic gene cluster of A-500359s in Streptomyces griseus SANK60196. J Antibiot (Tokyo) 2009;62:325–332. doi: 10.1038/ja.2009.38. [DOI] [PubMed] [Google Scholar]
- 10.Funabashi M, et al. An ATP-independent strategy for amide bond formation in antibiotic biosynthesis. Nat Chem Biol. 2010;6:581–586. doi: 10.1038/nchembio.393. [DOI] [PubMed] [Google Scholar]
- 11.Cai W, et al. The biosynthesis of capuramycin-type antibiotics: Identification of the A-102395 biosynthetic gene cluster, mechanism of self-resistance, and formation of uridine-5′-carboxamide. J Biol Chem. 2015;290:13710–13724. doi: 10.1074/jbc.M115.646414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goswami A, et al. Evidence that oxidative dephosphorylation by the nonheme Fe(II), α-ketoglutarate:UMP oxygenase occurs by stereospecific hydroxylation. FEBS Lett. 2017;591:468–478. doi: 10.1002/1873-3468.12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Itoh Y, et al. Decameric SelA•tRNA(Sec) ring structure reveals mechanism of bacterial selenocysteine formation. Science. 2013;340:75–78. doi: 10.1126/science.1229521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnard-Britson S, et al. Amalgamation of nucleosides and amino acids in antibiotic biosynthesis: Discovery of an L-threonine:uridine-5′-aldehyde transaldolase. J Am Chem Soc. 2012;134:18514–18517. doi: 10.1021/ja308185q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spork AP, Ducho C. Stereocontrolled synthesis of 5′- and 6′-epimeric analogues of muraymycin nucleoside antibiotics. Synlett. 2013;24:343–346. [Google Scholar]
- 16.Spork AP, Koppermann S, Dittrich B, Herbst-Irmer R, Ducho C. Efficient synthesis of the core structure of muraymycin and caprazamycin nucleoside antibiotics based on a stereochemically revised sulfur ylide reaction. Tetrahedron Asymmetry. 2010;21:763–766. [Google Scholar]
- 17.Spork AP, et al. Lead structures for new antibacterials: Stereocontrolled synthesis of a bioactive muraymycin analogue. Chemistry. 2014;20:15292–15297. doi: 10.1002/chem.201404775. [DOI] [PubMed] [Google Scholar]
- 18.Johnson RJ, Metzler DE. Analyzing spectra of vitamin B6 derivatives. Methods Enzymol. 1970;18:433–471. [Google Scholar]
- 19.Kallen RG, et al. Chemical and spectroscopic properties of pyridoxal and pyridoxamine phosphates. In: Christen P, Metzler DE, editors. Transaminases. Wiley; New York: 1985. pp. 37–108. [Google Scholar]
- 20.Bunik VI, Schloss JV, Pinto JT, Dudareva N, Cooper AJL. A survey of oxidative paracatalytic reactions catalyzed by enzymes that generate carbanionic intermediates: Implications for ROS production, cancer etiology, and neurodegenerative diseases. Adv Enzymol Relat Areas Mol Biol. 2011;77:307–360. doi: 10.1002/9780470920541.ch7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abell LM, Schloss JV. Oxygenase side reactions of acetolactate synthase and other carbanion-forming enzymes. Biochemistry. 1991;30:7883–7887. doi: 10.1021/bi00246a002. [DOI] [PubMed] [Google Scholar]
- 22.Sakai K, et al. L-ornithine decarboxylase from Hafnia alvei has a novel L-ornithine oxidase activity. J Biochem. 1997;122:961–968. doi: 10.1093/oxfordjournals.jbchem.a021858. [DOI] [PubMed] [Google Scholar]
- 23.Kaminaga Y, et al. Plant phenylacetaldehyde synthase is a bifunctional homotetrameric enzyme that catalyzes phenylalanine decarboxylation and oxidation. J Biol Chem. 2006;281:23357–23366. doi: 10.1074/jbc.M602708200. [DOI] [PubMed] [Google Scholar]
- 24.Bertoldi M, Cellini B, Montioli R, Borri Voltattorni C. Insights into the mechanism of oxidative deamination catalyzed by DOPA decarboxylase. Biochemistry. 2008;47:7187–7195. doi: 10.1021/bi800478s. [DOI] [PubMed] [Google Scholar]
- 25.Bertoldi M, Borri Voltattorni C. Reaction of dopa decarboxylase with L-aromatic amino acids under aerobic and anaerobic conditions. Biochem J. 2000;352:533–538. [PMC free article] [PubMed] [Google Scholar]
- 26.Wang M, Zhao Q, Zhang Q, Liu W. Differences in PLP-dependent cysteinyl processing lead to diverse S-functionalization of lincosamide antibiotics. J Am Chem Soc. 2016;138:6348–6351. doi: 10.1021/jacs.6b01751. [DOI] [PubMed] [Google Scholar]
- 27.Ringel MT, Dräger G, Brüser T. PvdN enzyme catalyzes a periplasmic pyoverdine modification. J Biol Chem. 2016;291:23929–23938. doi: 10.1074/jbc.M116.755611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldberg JM, et al. Structure of the complex between pyridoxal 5′-phosphate and the tyrosine 225 to phenylalanine mutant of Escherichia coli aspartate aminotransferase determined by isotope-edited classical Raman difference spectroscopy. Biochemistry. 1993;32:8092–8097. doi: 10.1021/bi00083a006. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Feng L, Kirsch JF. Kinetic and spectroscopic investigations of wild-type and mutant forms of apple 1-aminocyclopropane-1-carboxylate synthase. Biochemistry. 1997;36:15477–15488. doi: 10.1021/bi971625l. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J, Cheltsov AV, Ferreira GC. Conversion of 5-aminolevulinate synthase into a more active enzyme by linking the two subunits: Spectroscopic and kinetic properties. Protein Sci. 2005;14:1190–1200. doi: 10.1110/ps.041258305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vacca RA, Giannattasio S, Capitani G, Marra E, Christen P. Molecular evolution of B6 enzymes: Binding of pyridoxal-5′-phosphate and Lys41Arg substitution turn ribonuclease A into a model B6 protoenzyme. BMC Biochem. 2008;9:17. doi: 10.1186/1471-2091-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emanuele JJ, Fitzpatrick PF. Mechanistic studies of the flavoprotein tryptophan 2-monooxygenase. 1. Kinetic mechanism. Biochemistry. 1995;34:3710–3715. doi: 10.1021/bi00011a028. [DOI] [PubMed] [Google Scholar]
- 33.Matsui D, et al. Mutational and crystallographic analysis of l-amino acid oxidase/monooxygenase from Pseudomonas sp. AIU 813: Interconversion between oxidase and monooxygenase activities. FEBS Open Bio. 2014;4:220–228. doi: 10.1016/j.fob.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hong H, Fill T, Leadlay PF. A common origin for guanidinobutanoate starter units in antifungal natural products. Angew Chem Int Ed Engl. 2013;52:13096–13099. doi: 10.1002/anie.201308136. [DOI] [PubMed] [Google Scholar]
- 35.Ida K, et al. Structural basis of proteolytic activation of L-phenylalanine oxidase from Pseudomonas sp. P-501. J Biol Chem. 2008;283:16584–16590. doi: 10.1074/jbc.M800366200. [DOI] [PubMed] [Google Scholar]
- 36.Massey V. Activation of molecular oxygen by flavins and flavoproteins. J Biol Chem. 1994;269:22459–22462. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.