Significance
Although mutations in the neuroligin-3 and neuroligin-4 genes are implicated in autism syndromes, very little is known about the contribution of neuroligin-2 to neuropsychiatric disease states. We report a decrease in neuroligin-2 gene expression in the postmortem nucleus accumbens (NAc) of depressed patients. Reverse translation of this finding in chronic social defeat stress, an animal model of depression that enables investigation of both susceptibility and resiliency mechanisms, uncovers an important functional role for NAc neuroligin-2 in stress susceptibility. We detail a cell-type-specific role for NAc neuroligin-2 in modulating social avoidance behavior and dominance behaviors important for resiliency. Together, these findings describe a role for NAc neuroligin-2 in depression and chronic stress behaviors.
Keywords: neuroligin-2, depression, social defeat stress, dominance, medium spiny neuron
Abstract
Behavioral coping strategies are critical for active resilience to stress and depression; here we describe a role for neuroligin-2 (NLGN-2) in the nucleus accumbens (NAc). Neuroligins (NLGN) are a family of neuronal postsynaptic cell adhesion proteins that are constituents of the excitatory and inhibitory synapse. Importantly, NLGN-3 and NLGN-4 mutations are strongly implicated as candidates underlying the development of neuropsychiatric disorders with social disturbances such as autism, but the role of NLGN-2 in neuropsychiatric disease states is unclear. Here we show a reduction in NLGN-2 gene expression in the NAc of patients with major depressive disorder. Chronic social defeat stress in mice also decreases NLGN-2 selectively in dopamine D1-positive cells, but not dopamine D2-positive cells, within the NAc of stress-susceptible mice. Functional NLGN-2 knockdown produces bidirectional, cell-type-specific effects: knockdown in dopamine D1-positive cells promotes subordination and stress susceptibility, whereas knockdown in dopamine D2-positive cells mediates active defensive behavior. These findings establish a behavioral role for NAc NLGN-2 in stress and depression; provide a basis for targeted, cell-type specific therapy; and highlight the role of active behavioral coping mechanisms in stress susceptibility.
Behavioral coping strategies used during stressful events play an important role in determining the subsequent development of neuropsychiatric disease (1, 2). This same phenomenon is observed in rodent stress models, where susceptibility or resilience to social stress is strongly influenced by the subordinate’s selection of either submissive or dominant coping styles (3–5). However, the underlying synaptic mechanisms promoting the selection of such coping behaviors are unclear (6) and present a therapeutic approach to the treatment of stress-related disorders.
Neuroligin-2 (NLGN-2) is a structural constituent of the inhibitory synapse (7, 8), and although it is also associated with maladaptive social behavior (9–13), its direct role in neuropsychiatric disease is unclear (14, 15). NLGN-2 is a key postsynaptic cell adhesion protein that supports the functional integrity of the inhibitory synapse (7, 14, 16). Studies of developmental NLGN-2 knockout mice have established a distinct role for NLGN-2 in maintaining inhibitory synapse function and modulating anxiety behaviors (8, 17–19). Outside of this developmental window, very little is known about the role of NLGN-2 in regulating adult social behavior. Recent NLGN-2 manipulations in the adult mouse prefrontal cortex and hippocampus describe varying effects on anxiety, aggression, and fear memory (9, 20–22).
The NAc, an integral hub in the mesolimbic dopamine circuit, is well established as a critical brain region regulating social stress behaviors (23). Synaptic plasticity within the dopaminergic mesolimbic circuit is implicated in both major depressive disorder (MDD) and increased susceptibility to stress (24–30). The NAc contains primarily GABAergic medium spiny neurons (MSNs) divided into two subpopulations that often have distinct functional effects on stress- and reward-mediated behaviors: dopamine receptor 1-positive medium spiny neurons (D1-MSNs), expressing primarily dopamine D1 receptors, and D2-MSNs, expressing primarily dopamine D2 receptors (27, 28, 31, 32). Two recent studies have examined NLGN-2 expression in striatal MSNs (33, 34), confirming its role as a constituent of the MSN inhibitory synapse and quantifying NLGN-2 expression within MSN populations. Here, we provide evidence that NLGN-2 is reduced in the NAc of patients with MDD and describe a cell-type-specific role for NLGN-2 in NAc MSNs in modulating active behavioral coping mechanisms in stress susceptibility and resilience in a mouse model of depression.
Results
NLGN-2 Decreases in NAc of Depressed Patients.
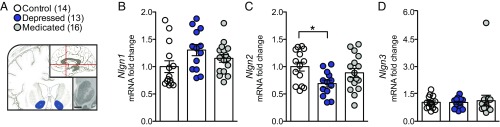
Transcriptional profiling of postmortem human NAc was conducted to identify changes in neuroligin gene expression in MDD, revealing a selective down-regulation of NLGN-2 in subjects with MDD that is not significantly reversed by antidepressant medication being present at the time of death (Fig. 1 and SI Appendix, Table S1 for demographics). The change in NAc NLGN-2 does not generalize to other neuropsychiatric disorders such as chronic cocaine abuse (SI Appendix, Fig. S1). This is evidence for neuroligin gene regulation in the NAc in MDD and presents a unique role for NLGN-2 in neuropsychiatric disease and antidepressant strategies.
Fig. 1.
NLGN-2 in the human postmortem nucleus accumbens changes in MDD. (A) Schematic indicating blue region of dissection of postmortem human NAc. Transcriptional profiling of postmortem human NAc (B) NLGN-1, (C) NLGN-2, and (D) NLGN-3 gene expression revealed a selective decrease in NLGN-2 in depressed subjects (one-way ANOVA: F2,42 = 3.876; *P < 0.05). All data are represented as mean ± SEM. See SI Appendix, Table S1 for detailed demographic data.
NLGN-2 Decreases in NAc of Stress-Susceptible Mice.
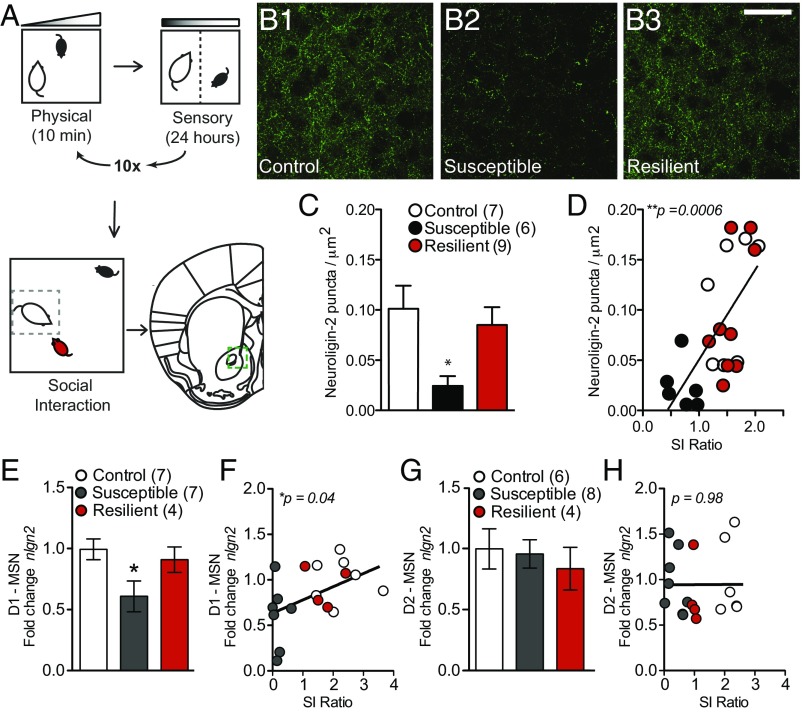
Reverse translation of these findings to chronic social defeat stress, a mouse model of depression-like behavior, recapitulates a decrease in NAc NLGN-2 only in mice that are susceptible to stress (Fig. 2). After stress, mice are classified as susceptible or resilient based on social interaction (35–37) (SI Appendix, Fig. S2). We report a decrease in NAc NLGN-2 protein that significantly correlates with lower social interaction ratio, or increased susceptibility to stress (Fig. 2 B–D). To characterize the cell-type specificity of social-defeat-induced changes, NLGN-2 mRNA was assayed in the principal NAc neuronal populations, D1-MSNs and D2-MSNs, using RiboTag methodology (32). NLGN-2 mRNA levels decreased specifically in D1-MSNs of stress-susceptible mice, and levels correlated with reduced social interaction (Fig. 2 E and F). In D2-MSNs, there were no significant stress-induced changes in NLGN-2 transcription (Fig. 2 G and H).
Fig. 2.
NLGN-2 in the mouse nucleus accumbens changes in chronic stress. (A) The chronic social defeat stress paradigm models depression-like symptoms in mice and results in resilient or susceptible subpopulations. (B) Representative images of NLGN-2 immunohistochemistry in the NAc after social defeat stress in control (B1), susceptible (B2), and resilient (B3) mice. (Scale bar, 20 µm.) (C) NLGN-2 puncta are reduced in susceptible mice (one-way ANOVA: F2,19 = 4.922; *P < 0.05; n = 7, 6, 9), and (D) lower NLGN-2 puncta correlates with decreased social interaction ratio (r2 = 0.4497; ***P = 0.0006). (E) Transcriptional profiling of NLGN-2 in D1-MSNs vs. D2-MSNs after social defeat stress showed a reduction in NLGN-2 mRNA selectively in D1-MSNs of stress-susceptible mice (one-way ANOVA: F2,17 = 3.758; *P < 0.05; n = 7, 7, 4) and (F) the NLGN2 levels in D1-MSNs correlated with reduced interaction time with a novel animal (r2 = 0.238; *P = 0.040). (G) NLGN-2 transcription did not significantly change in D2-MSNs after stress (one-way ANOVA, P > 0.05; n = 6, 8, 4) and (H) did not correlate with changes in social interaction time (r2 = 0.00002; P = 0.98). All data are represented as mean ± SEM.
Cell-Type-Specific NLGN-2 Knockdown Affects Stress-Related Behaviors.
To further explore the behavioral role of NLGN-2 in the NAc, a synthetic microRNA (miRNA) against NLGN-2 was designed, expressed, and validated in a Cre-conditional adeno-associated virus (AAV) vector both in vitro and in vivo (SI Appendix, Fig. S3). Whole-cell recordings of mini inhibitory postsynaptic currents from virus-infected cells demonstrated a significant and bidirectional effect of NLGN-2 knockdown on mini inhibitory postsynaptic currents recorded from D1- and D2-positive cells (SI Appendix, Fig. S4). Stereotactic injection of the miRNA virus or a control virus expressing a nontargeting sequence was performed in the NAc of D1-Cre and D2-Cre transgenic mice, enabling knockdown in NAc MSN subpopulations. This approach allows for temporal and spatially targeted cell-type-specific knockdown in the adult animal. Animals subsequently underwent a subthreshold social defeat stress, which is used to reveal prosusceptibility factors (24–26, 35, 36, 38), followed by a social interaction test (SI Appendix, Fig. S5 for experimental design). Mice were compared with Cre littermates in all behavioral studies.
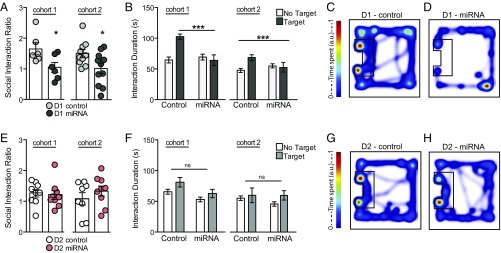
After subthreshold social defeat, D1-Cre mice expressing the NLGN-2 miRNA virus (D1-miRNA) display stress-susceptible behavior, quantified as a lower social interaction ratio and less time spent in the interaction zone with a novel animal present compared with animals expressing the nontargeting miRNA control virus (D1-control; Fig. 3 A–D and SI Appendix, Fig. S5). Conversely, D2-Cre mice expressing the miRNA virus in the NAc (D2-miRNA) exhibit social interaction behaviors similar to D2-Cre mice expressing the nontargeting miRNA control virus (D2-control; Fig. 3 E–H and SI Appendix, Fig. S5). These data are replicated in two independent cohorts shown side by side (Fig. 3). Animals that received AAV-miRNA in D1 and D2 neurons, but did not undergo social defeat stress, showed no deficits in social interaction behavior (SI Appendix, Fig. S6). Last, D2 miRNA mice also display reduced anxiety after social defeat stress, as measured by the open field test, a standard test of exploratory-based anxiety behavior (SI Appendix, Fig. S7). Thus, NLGN-2 plays a distinct, cell-type-specific role in NAc D1-MSNs to modulate stress susceptibility.
Fig. 3.
Cell-type-specific knockdown of NLGN-2 in the NAc alters stress behaviors. (A) D1-Cre mice expressing the miRNA virus (D1-miRNA) have reduced social interaction ratios [Student’s t test cohort 1, *P < 0.05 (n = 6 per group); cohort 2, *P < 0.05 (n = 10, 11 per group)] and (B) spend less time in the interaction zone with a target animal present [two-way ANOVA, cohort 1, F1,20 = 13.22 (***P < 0.001); cohort 2, F1,38 = 51.79 (***P < 0.0001)]. (C) Representative heat maps of D1-Cre control virus-expressing mouse (D1-control) and (D) D1-miRNA mouse in the target present trial. No significant difference in (E) social interaction ratio [Student’s t test, cohort 1, P > 0.05 (n = 9 per group); cohort 2, P > 0.05 (n = 8–9 per group)] or (F) interaction duration was observed between D2-Cre mice expressing the control virus (D2-control) or miRNA virus (D2-miRNA; two-way ANOVA, cohort 1, P > 0.05; cohort 2: P > 0.05). (G) Representative heat maps of D2-control and (H) D2-miRNA animals in the target present trial. All data are represented as mean ± SEM.
NAc NLGN-2 Knockdown Modulates Active Coping Behavior.
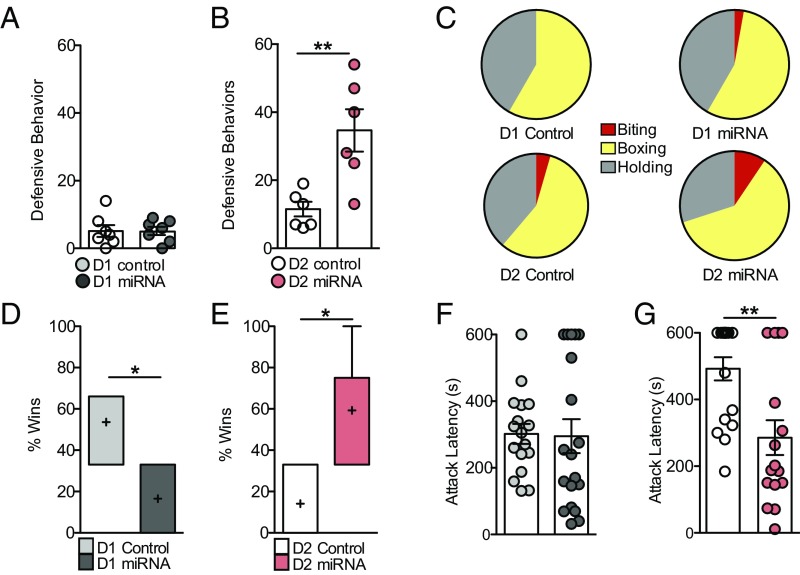
Interestingly, NLGN-2 knockdown in D1 versus D2-MSNs promotes differential social behaviors during social defeat sessions with CD-1 aggressor mice. D2-miRNA mice display significantly more active defensive behaviors against CD-1 aggressors, whereas D1-miRNA mice exhibit minimal active defensive behaviors (Fig. 4 A–C) and are more vulnerable to stress. These behaviors include biting, boxing, and holding down the CD-1 aggressor mouse, which in other studies has been interpreted as active coping and associated with better behavioral outcomes (39, 40) (Fig. 2C). Detailed analysis of CD-1 aggressors and test mice during social defeat stress elucidates no significant differences in CD-1 attack behavior or baseline social behavior between CD-1 and Cre mice across groups, although D1-miRNA animals also show increased baseline social approach behavior toward CD-1 mice (SI Appendix, Tables S2 and S3). These data indicate there are no overall baseline social behavior deficits between CD-1 mice and D1-Cre or D2-Cre mice after NLGN-2 knockdown (SI Appendix, Table S4); however, D1-miRNA mice fail to employ an active behavioral coping strategy.
Fig. 4.
Cell-type-specific knockdown of NLGN-2 in the NAc alters dominance behaviors. (A) There was no difference in defensive behaviors between D1-miRNA and D1-control mice against CD-1 aggressor mice (P > 0.05). (B) D2-miRNA mice engaged in a significantly increased number of defensive behaviors (Student’s t test, **P > 0.01). (C) Distribution of defensive behaviors observed against CD-1 aggressor mice. Please see SI Appendix, Tables S8 and S9 for detailed automated behavioral analyses. (D) D1-miRNA mice win a fewer percentage of tube test trials against control animals (Student’s t test, *P < 0.05). (E) D2-miRNA mice win a greater percentage of tube test trials against control animals (Student’s t test, *P < 0.05). (F) No significant difference in attack latency was observed between D1-control and D1-miRNA mice in the home cage aggression test (P > 0.05). (G) D2-miRNA mice displayed decreased latency to attack an intruder mouse (Student’s t test, **P < 0.01). All data are represented as mean ± SEM.
NAc NLGN-2 Knockdown Affects Dominance and Aggression Behaviors.
To further investigate the role of D1-MSN and D2-MSN NLGN-2 in dominant versus subordinate behaviors related to stress, experimental mice were examined in the tube test, an established model for probing dominance hierarchies among inbred mice (41, 42). Mice are placed at opposite ends of a narrow tube that permits only unidirectional movement. The dominant mouse is defined as the mouse that succeeds in forcing its opponent to retreat from the end of the tube. D1-miRNA mice display decreased dominance behavior, winning ∼30% of tube test trials, whereas D2-miRNA mice display significantly greater dominance behavior in the tube test, winning ∼80% of tube test trials (Fig. 4 D and E). In a test of home cage aggression, experimental D1- or D2-Cre mice injected with NLGN-2 miRNA or control virus were singly housed and exposed to a novel, younger conspecific intruder for 10 min. D2-miRNA mice show decreased latency to attack an intruder mouse compared with D2-control animals, and no significant effects on home cage aggression behavior are seen in D1-miRNA mice (Fig. 4 F and G).
Overall, NLGN-2 knockdown in NAc D1-MSNs supports a behavioral profile marked by decreased dominance and reduced defensive coping behaviors against aggressor mice, together increasing susceptibility to stress. In contrast, NLGN-2 knockdown in NAc D2-MSNs promotes dominance, active defensive aggression, and reduced anxiety-like behavior, which may help to prevent stress susceptibility.
Discussion
Although it is well established in the clinical literature that active coping styles play a critical role in patient outcome (43, 44), there have been few reports examining the molecular mechanisms supporting this observation. Our data describe a mechanism whereby chronic stress or depression regulates the transcriptional profile of NLGN-2 in the NAc of humans and rodents, and that the functional manipulation of NLGN-2 promotes an active behavioral coping mechanism. The NLGN family has been heavily implicated in the etiology of social neuropsychiatric disorders such as autism and schizophrenia (14, 15), and more recently, a case report identified a nonsense mutation of NLGN-2 as possibly underlying anxiety behavior (11). A greater understanding of the role of NGLN-2 within brain reward circuitry in mediating stress-induced behaviors will aid in interpreting its significance for neuropsychiatric disease states.
We provide neuropathological evidence for reduced levels of NLGN-2 in human NAc from depressed subjects. In mice, reduced NLGN-2 in D1-MSNs underlies and promotes a stress-susceptible phenotype characterized by decreased dominance behavior. Conversely, mice with reduced NLGN-2 in D2-MSNs do not develop significant social avoidance, a finding that may be in part a result of their display of active defensive behaviors and greater dominance. Whole-cell recordings of mini inhibitory postsynaptic current from D1- and D2-positive virus-infected cells showed that knockdown of NLGN-2 produces increased mini inhibitory postsynaptic current frequency on D1-positive cells and decreased mini inhibitory postsynaptic current frequency on D2-positive cells. Prior studies of cultured hippocampal cells with total knockout of NLGN-2 demonstrated that NLGN-2 knockout promotes overall decreased inhibitory synaptic responses (8). Only one prior study has examined the role of neuroligins in the NAc (33), and all prior studies used total knockout models, not a knockdown of the gene as produced by our viral manipulation. The bidirectional modulation of inhibitory tone demonstrated by viral knockdown, producing increased inhibition of D1-positive cells, is consistent with previous behavioral studies demonstrating that pharmacogenetic inhibition of NAc D1-positive cells promotes increased social avoidance behavior after social defeat stress (27). We propose that achieving a cell-type-specific balance in NAc NLGN-2 may be necessary to drive adaptive behavioral responses to stress. More work is needed to understand the cell-type-specific functional balance in NAc circuitry mediating stress-susceptible or stress-resilient behaviors.
One limitation of cell-type-specific studies is the restriction to male bacterial artificial chromosome (BAC) transgenic inbred mice, which are known to display variable home cage aggression behaviors compared with validated outbred mouse strains such as the CD-1 retired male breeder (45–47). Inbred strain-specific differences in aggressive behavior have been the focus of research for many years and have been used to identify genetic contributions to aggressive behavior (48–50). Our laboratory and others have noted baseline behavioral differences in BAC transgenic mice that may be a result of off-target effects of the BAC or founder effects from which the lines were derived (51). Interpretation of the data in Figs. 3 and 4 across groups is specifically limited by the baseline differences in behavior between D1-Cre and D2-Cre cohorts. Because viral-mediated strategies to target D1- or D2-positive cells with AAV-mediated gene knockdown have been largely unsuccessful, we are limited to the available transgenic lines for cell-type-specific studies. Future research will be aimed at extending these studies to other mouse models to better characterize the behavioral phenotypes produced by NAc NLGN-2 manipulation.
Interestingly, although not statistically significant, antidepressant treatment increased NLGN-2 transcription in the postmortem human NAc of some depressed subjects. This trend possibly reflects individual variability in behavioral responses to antidepressant treatment, with a subpopulation of responders showing up-regulation of NLGN-2 levels. Approximately one-third of depressed patients are resistant to the current standard antidepressant therapies (52). Thus, modulation of NLGN-2 may be an additional substrate reflecting improved antidepressant efficacy. Future studies should also be aimed at collecting data on additional behavioral endpoints, including aggressive behaviors, in depressed human subjects to better analyze the intersection of postmortem molecular changes with stress coping behaviors.
In summary, we provide evidence that chronic stress in both humans and rodents induces a robust down-regulation of NLGN-2 expression in the NAc. Functional studies in mice uncover a cell-type specific role of NAc NLGN-2 that informs differential behavioral coping strategies to stress. An improved cell-type-specific understanding of depression pathophysiology may aid in the future development of targeted treatments to promote active resilience mechanisms for therapeutic relief.
Materials and Methods
See SI Appendix, SI Materials and Methods, for additional detailed information about procedures used in these studies.
Experimental Animals.
D1-Cre and D2-Cre BAC transgenic heterozygous male mice (courtesy of Eric Nestler, Icahn School of Medicine at Mount Sinai) were bred to c57bl6/j wild-type females from Jackson Laboratories, and the resulting 7–10-wk-old heterozygous male offspring were used for behavioral studies. Eight-week-old c57bl6/j male mice from Jackson were used for immunohistochemistry and mRNA profiling of NLGN-2 after chronic social defeat stress. Singly-housed male CD-1 mice (4-mo-old sexually experienced retired breeders) from Charles River Laboratories were used as aggressors in social defeat experiments, as previously described (35). All mice were group housed in a controlled environment (12 h light/dark cycle) with food and water available ad libitum. Behavioral assessments and tissue collections were performed during the animals’ light phase. All experiments were performed in accordance with the Icahn School of Medicine and the University of Maryland Institutional Animal Care and Use Committees.
Social Defeat Stress and Social Interaction Test.
Chronic social defeat stress, subthreshold social defeat stress, and social interaction test was performed as previously described (24, 25, 29, 35–38).
microRNA Vector.
Commercially available mouse short hairpin RNA against the NLGN-2 gene was purchased (TG516880; Origene). Short hairpin RNA sequences were then used to create microRNA oligos using the BLOCK-iTTM Pol II miR RNAi Expression Vector Kit (K4936-00; Invitrogen). miR constructs were next subcloned into a bicistronic AAV-IRES-GFP vector (VPK-418; Cell Biolabs, Inc.). To construct a Cre-dependent version, we modified an AAV-FLEX-rev-ChR2-Tdtomato vector (#18917; Addgene), using the Gibson assembly method (Cat# 2611; New England Biolabs). AAV constructs were validated in Neuro2A cells (ATCC), using common procedures before being packaged into high-titer viral particles by the University of North Carolina at Chapel Hill Gene Therapy Center (UNC Vector Core). For a detailed description, see SI Appendix.
Defensive Behavior.
Experimental mice were exposed for 10 min to a novel, CD-1 aggressor, as previously described (35). Defensive behaviors by the experimental mice against the CD-1 aggressor were scored during post hoc video analysis by a blinded experimenter. Aggressive behavior by CD-1 aggressors and social behaviors by CD-1, D1-Cre, and D2-Cre were scored in an automated manner using AggressionScan (CleverSys Inc) software.
Tube Test.
The tube test protocol was performed as previously described in reports using a nonautomated test (6, 42, 53).
Home Cage Aggression.
Experimental mice were singly housed and exposed for 10 min to a novel, D1-Cre or D2-Cre conspecific intruder of ∼5 wk age and 15 g weight. Attack latency was manually scored by a blinded experimenter.
Open Field Test.
The open field test was performed as previously described (38). Experimental mice were placed inside a novel box, and movements inside the box were tracked over the course of 10 min, using Ethovision (Noldus) software.
Immunohistochemistry.
After behavioral studies, mice underwent transcardial perfusion, and brains were dissected and postfixed as previously described (24, 38). Next, 1:500 NLGN-2 (rabbit, ab36602; Abcam) primary antibody was used in blocking solution (3% normal donkey serum and 0.3% Triton X in PBS) with free-floating incubation overnight. Images were acquired on a Zeiss LSM 780 confocal microscope using a 100× oil immersion objective. Images were deconvoluted with AutoQuant, and protein puncta were quantified using Image J 3D Object Counter.
RiboTag.
The RiboTag procedure to immunoprecipitate ribosomes from NAc of D1-Cre-RiboTag (D1-Cre-RT) and D2-Cre-RiboTag (D2-Cre-RT) mice was performed as previously described (32). NAc from three mice was pooled to generate each sample. Each mouse met control, susceptible, or resilient criteria, as indicated by social interaction after social defeat stress, as described previously (35).
qPCR.
qPCR was performed as previously described (24). Analysis was performed using the ∆∆C(t) method, with sample normalization to GAPDH. IDT PrimeTime primers were purchased from Integrated DNA Technologies. Please see SI Appendix, Table S4 for a list of primer pairs.
Western Blotting.
Neuro2A cells were collected and protein was isolated in RIPA buffer, using common procedures. Membranes were blocked in 5% milk and incubated overnight in milk with goat polyclonal antibody against NLGN-2 (1:1,000, ab77595; Abcam).
Stereotaxic Surgery.
Surgical procedures were performed according to previously published methods (24, 38, 54) in accordance with guidelines from the Icahn School of Medicine Institutional Animal Care and Use Committee. Nucleus accumbens bregma coordinates: anteroposterior, +1.5 mm; mediolateral, +1.6 mm; dorsoventral, −4.4 mm, 10° angle.
Acquisition of Postmortem Human Tissue.
Nucleus accumbens whole-tissue resections were collected at the local medical examiners offices, after obtaining next-of-kin permission, by the Quebec Suicide Brain Bank at the Douglas Hospital Research Center under an approval of the Douglas Hospital Research Center’s Research Ethics Committee.
Electrophysiology.
Five-week-old D1-Cre and A2A-Cre BAC transgenic mice were injected with AAV-control or AAV-miRNA viruses, and whole-cell recordings were obtained 10–14 d postinfection from NAc medium spiny neurons expressing GFP. MSNs were voltage clamped at +0 mV. Miniature inhibitory postsynaptic currents were recorded in the presence of tetrodotoxin (500 nM), d-APV (100 µM), and NBQX (100 µM), using 3–5 MΩ patch pipettes filled with cesium methanesulfonate-based internal solution. For a detailed description, see SI Appendix.
Statistical Analysis.
All data are expressed as the mean ± SEM. Mean differences between groups were determined using two-tailed Student’s t test, one-way analysis of variance (ANOVA), or two-way ANOVA, followed by Bonferroni posttests if the main effect was significant at P < 0.05. Statistical analyses were performed using Prism 5.0 (GraphPad Software).
Supplementary Material
Acknowledgments
We thank Carmen Ferrer, Yu Feng, Naemal Bhatti, and Kathryn McCabe for technical assistance. This work was funded by National Institutes of Health Grants R01 MH090264, R01 MH114882, P50 MH096890, P50 AT008661 (to S.J.R.), F30 MH100835 (to M.H.), T32 MH087004 (to M.H., M.L.P., and M.E.F.), F31 MH105217 (to M.L.P.), T32 MH096678 (to M.L.P.), and 1F12GM117583-01 (to S.A.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1719014115/-/DCSupplemental.
References
- 1.Russo SJ, Murrough JW, Han MH, Charney DS, Nestler EJ. Neurobiology of resilience. Nat Neurosci. 2012;15:1475–1484. doi: 10.1038/nn.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nat Rev Neurosci. 2009;10:446–457. doi: 10.1038/nrn2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coppens CM, de Boer SF, Koolhaas JM. Coping styles and behavioural flexibility: Towards underlying mechanisms. Philos Trans R Soc Lond B Biol Sci. 2010;365:4021–4028. doi: 10.1098/rstb.2010.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laredo SA, et al. Effects of defeat stress on behavioral flexibility in males and females: Modulation by the mu-opioid receptor. Eur J Neurosci. 2015;41:434–441. doi: 10.1111/ejn.12824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wommack JC, Delville Y. Repeated social stress and the development of agonistic behavior: Individual differences in coping responses in male golden hamsters. Physiol Behav. 2003;80:303–308. doi: 10.1016/j.physbeh.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Briand LA, et al. Mouse model of OPRM1 (A118G) polymorphism increases sociability and dominance and confers resilience to social defeat. J Neurosci. 2015;35:3582–3590. doi: 10.1523/JNEUROSCI.4685-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varoqueaux F, Jamain S, Brose N. Neuroligin 2 is exclusively localized to inhibitory synapses. Eur J Cell Biol. 2004;83:449–456. doi: 10.1078/0171-9335-00410. [DOI] [PubMed] [Google Scholar]
- 8.Chubykin AA, et al. Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron. 2007;54:919–931. doi: 10.1016/j.neuron.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Kooij MA, et al. Impaired hippocampal neuroligin-2 function by chronic stress or synthetic peptide treatment is linked to social deficits and increased aggression. Neuropsychopharmacology. 2014;39:1148–1158. doi: 10.1038/npp.2013.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blundell J, et al. Increased anxiety-like behavior in mice lacking the inhibitory synapse cell adhesion molecule neuroligin 2. Genes Brain Behav. 2009;8:114–126. doi: 10.1111/j.1601-183X.2008.00455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parente DJ, et al. Neuroligin 2 nonsense variant associated with anxiety, autism, intellectual disability, hyperphagia, and obesity. Am J Med Genet A. 2017;173:213–216. doi: 10.1002/ajmg.a.37977. [DOI] [PubMed] [Google Scholar]
- 12.Pettem KL, Yokomaku D, Takahashi H, Ge Y, Craig AM. Interaction between autism-linked MDGAs and neuroligins suppresses inhibitory synapse development. J Cell Biol. 2013;200:321–336. doi: 10.1083/jcb.201206028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun C, et al. Identification and functional characterization of rare mutations of the neuroligin-2 gene (NLGN2) associated with schizophrenia. Hum Mol Genet. 2011;20:3042–3051. doi: 10.1093/hmg/ddr208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Südhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455:903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jamain S, et al. Paris Autism Research International Sibpair Study Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34:27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101:657–669. doi: 10.1016/s0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- 17.Varoqueaux F, et al. Neuroligins determine synapse maturation and function. Neuron. 2006;51:741–754. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Gibson JR, Huber KM, Südhof TC. Neuroligin-2 deletion selectively decreases inhibitory synaptic transmission originating from fast-spiking but not from somatostatin-positive interneurons. J Neurosci. 2009;29:13883–13897. doi: 10.1523/JNEUROSCI.2457-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Babaev O, et al. Neuroligin 2 deletion alters inhibitory synapse function and anxiety-associated neuronal activation in the amygdala. Neuropharmacology. 2016;100:56–65. doi: 10.1016/j.neuropharm.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 20.Liang J, et al. Conditional neuroligin-2 knockout in adult medial prefrontal cortex links chronic changes in synaptic inhibition to cognitive impairments. Mol Psychiatry. 2015;20:850–859. doi: 10.1038/mp.2015.31. [DOI] [PubMed] [Google Scholar]
- 21.Kohl C, et al. Hippocampal neuroligin-2 links early-life stress with impaired social recognition and increased aggression in adult mice. Psychoneuroendocrinology. 2015;55:128–143. doi: 10.1016/j.psyneuen.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 22.Kohl C, et al. Hippocampal neuroligin-2 overexpression leads to reduced aggression and inhibited novelty reactivity in rats. PLoS One. 2013;8:e56871. doi: 10.1371/journal.pone.0056871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14:609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golden SA, et al. Epigenetic regulation of RAC1 induces synaptic remodeling in stress disorders and depression. Nat Med. 2013;19:337–344. doi: 10.1038/nm.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dias C, et al. β-catenin mediates stress resilience through Dicer1/microRNA regulation. Nature. 2014;516:51–55. doi: 10.1038/nature13976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christoffel DJ, et al. Excitatory transmission at thalamo-striatal synapses mediates susceptibility to social stress. Nat Neurosci. 2015;18:962–964. doi: 10.1038/nn.4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Francis TC, et al. Nucleus accumbens medium spiny neuron subtypes mediate depression-related outcomes to social defeat stress. Biol Psychiatry. 2015;77:212–222. doi: 10.1016/j.biopsych.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khibnik LA, et al. Stress and cocaine trigger divergent and cell type-specific regulation of synaptic transmission at single spines in nucleus accumbens. Biol Psychiatry. 2016;79:898–905. doi: 10.1016/j.biopsych.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christoffel DJ, et al. IκB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J Neurosci. 2011;31:314–321. doi: 10.1523/JNEUROSCI.4763-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McEwen BS, Nasca C, Gray JD. Stress effects on neuronal structure: Hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology. 2016;41:3–23. doi: 10.1038/npp.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lobo MK, Nestler EJ. The striatal balancing act in drug addiction: Distinct roles of direct and indirect pathway medium spiny neurons. Front Neuroanat. 2011;5:41. doi: 10.3389/fnana.2011.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chandra R, et al. Opposing role for Egr3 in nucleus accumbens cell subtypes in cocaine action. J Neurosci. 2015;35:7927–7937. doi: 10.1523/JNEUROSCI.0548-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rothwell PE, et al. Autism-associated neuroligin-3 mutations commonly impair striatal circuits to boost repetitive behaviors. Cell. 2014;158:198–212. doi: 10.1016/j.cell.2014.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uchigashima M, Ohtsuka T, Kobayashi K, Watanabe M. Dopamine synapse is a neuroligin-2-mediated contact between dopaminergic presynaptic and GABAergic postsynaptic structures. Proc Natl Acad Sci USA. 2016;113:4206–4211. doi: 10.1073/pnas.1514074113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Golden SA, Covington HE, 3rd, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6:1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krishnan V, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 37.Berton O, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 38.Christoffel DJ, et al. Effects of inhibitor of kappaB kinase activity in the nucleus accumbens on emotional behavior. Neuropsychopharmacology. 2012;37:2615–2623. doi: 10.1038/npp.2012.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veenema AH, Neumann ID. Neurobiological mechanisms of aggression and stress coping: A comparative study in mouse and rat selection lines. Brain Behav Evol. 2007;70:274–285. doi: 10.1159/000105491. [DOI] [PubMed] [Google Scholar]
- 40.Blanchard RJ, Odonnell V, Blanchard DC. Attack and defensive behaviors in the albino mouse. Aggress Behav. 1979;5:341–352. [Google Scholar]
- 41.Lindzey G, Winston H, Manosevitz M. Social dominance in inbred mouse strains. Nature. 1961;191:474–476. doi: 10.1038/191474a0. [DOI] [PubMed] [Google Scholar]
- 42.Wang F, et al. Bidirectional control of social hierarchy by synaptic efficacy in medial prefrontal cortex. Science. 2011;334:693–697. doi: 10.1126/science.1209951. [DOI] [PubMed] [Google Scholar]
- 43.Southwick SM, Vythilingam M, Charney DS. The psychobiology of depression and resilience to stress: Implications for prevention and treatment. Annu Rev Clin Psychol. 2005;1:255–291. doi: 10.1146/annurev.clinpsy.1.102803.143948. [DOI] [PubMed] [Google Scholar]
- 44.Iacoviello BM, Charney DS. Psychosocial facets of resilience: Implications for preventing posttrauma psychopathology, treating trauma survivors, and enhancing community resilience. Eur J Psychotraumatol. 2014;5:23970. doi: 10.3402/ejpt.v5.23970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Golden SA, et al. Basal forebrain projections to the lateral habenula modulate aggression reward. Nature. 2016;534:688–692. doi: 10.1038/nature18601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Golden SA, et al. Persistent conditioned place preference to aggression experience in adult male sexually-experienced CD-1 mice. Genes Brain Behav. 2017;16:44–55. doi: 10.1111/gbb.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miczek KA, Maxson SC, Fish EW, Faccidomo S. Aggressive behavioral phenotypes in mice. Behav Brain Res. 2001;125:167–181. doi: 10.1016/s0166-4328(01)00298-4. [DOI] [PubMed] [Google Scholar]
- 48.Guillot PV, Chapouthier G. Intermale aggression and dark/light preference in ten inbred mouse strains. Behav Brain Res. 1996;77:211–213. doi: 10.1016/0166-4328(95)00163-8. [DOI] [PubMed] [Google Scholar]
- 49.Dow HC, et al. Genetic dissection of intermale aggressive behavior in BALB/cJ and A/J mice. Genes Brain Behav. 2011;10:57–68. doi: 10.1111/j.1601-183X.2010.00640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nehrenberg DL, et al. Genomic mapping of social behavior traits in a F2 cross derived from mice selectively bred for high aggression. BMC Genet. 2010;11:113. doi: 10.1186/1471-2156-11-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kramer PF, et al. Dopamine D2 receptor overexpression alters behavior and physiology in Drd2-EGFP mice. J Neurosci. 2011;31:126–132. doi: 10.1523/JNEUROSCI.4287-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rush AJ, Trivedi M, Fava M. Depression, IV: STAR*D treatment trial for depression. Am J Psychiatry. 2003;160:237. doi: 10.1176/appi.ajp.160.2.237. [DOI] [PubMed] [Google Scholar]
- 53.Jiang-Xie LF, et al. Autism-associated gene Dlgap2 mutant mice demonstrate exacerbated aggressive behaviors and orbitofrontal cortex deficits. Mol Autism. 2014;5:32. doi: 10.1186/2040-2392-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Russo SJ, et al. Nuclear factor kappa B signaling regulates neuronal morphology and cocaine reward. J Neurosci. 2009;29:3529–3537. doi: 10.1523/JNEUROSCI.6173-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.