Significance
Lymphangioleiomyomatosis (LAM) is a destructive lung disease driven by neoplastic LAM cells with a mutated tumor suppressor gene TSC1 or TSC2, leading to increased activity of the mechanistic target of rapamycin (mTOR), which is inhibited by sirolimus (rapamycin). Beta-agonists may treat asthma-like symptoms due to LAM. We observed stabilization of forced expiratory volume in 1 s in patients receiving sirolimus and long-acting beta-agonists with short-acting rescue inhalers compared with patients receiving only sirolimus. Human TSC2+/− skin fibroblasts and LAM cells from explanted lungs treated with sirolimus and the short-term, but not long-term, beta-agonist isoproterenol showed increased phospho-S6 levels and cell growth due to activation of a cAMP/PKA-dependent pathway. Long-acting beta-agonists affect phospho-S6 content, leading to stabilization of lung function in LAM patients.
Keywords: cyclic AMP, sirolimus, lymphangioleiomyomatosis, tuberous sclerosis complex, bronchodilators
Abstract
Lymphangioleiomyomatosis (LAM), a rare disease of women, is associated with cystic lung destruction resulting from the proliferation of abnormal smooth muscle-like LAM cells with mutations in the tuberous sclerosis complex (TSC) genes TSC1 and/or TSC2. The mutant genes and encoded proteins are responsible for activation of the mechanistic target of rapamycin (mTOR), which is inhibited by sirolimus (rapamycin), a drug used to treat LAM. Patients who have LAM may also be treated with bronchodilators for asthma-like symptoms due to LAM. We observed stabilization of forced expiratory volume in 1 s over time in patients receiving sirolimus and long-acting beta-agonists with short-acting rescue inhalers compared with patients receiving only sirolimus. Because beta-agonists increase cAMP and PKA activity, we investigated effects of PKA activation on the mTOR pathway. Human skin TSC2+/− fibroblasts or LAM lung cells incubated short-term with isoproterenol (beta-agonist) showed a sirolimus-independent increase in phosphorylation of S6, a downstream effector of the mTOR pathway, and increased cell growth. Cells incubated long-term with isoproterenol, which may lead to beta-adrenergic receptor desensitization, did not show increased S6 phosphorylation. Inhibition of PKA blocked the isoproterenol effect on S6 phosphorylation. Thus, activation of PKA by beta-agonists increased phospho-S6 independent of mTOR, an effect abrogated by beta-agonist–driven receptor desensitization. In agreement, retrospective clinical data from patients with LAM suggested that a combination of bronchodilators in conjunction with sirolimus may be preferable to sirolimus alone for stabilization of pulmonary function.
Lymphangioleiomyomatosis (LAM), a rare multisystem disease affecting primarily women, is characterized by cystic lung destruction, which can lead to respiratory failure, abdominal tumors [e.g., renal angiomyolipomas (AMLs)], and lymphatic involvement (e.g., lymphangioleiomyomas, adenopathy) (1–4). Depending on organ involvement, patients may exhibit progressive dyspnea on exertion, pneumothoraces, chylous pleural effusions, ascites, and abdominal hemorrhage (5). LAM is often mistakenly diagnosed as another respiratory disease, such as asthma, emphysema, chronic bronchitis, or chronic obstructive pulmonary disease (6–8). Many patients are treated with bronchodilators to alleviate asthma-like symptoms due to LAM disease. In fact, in a study of 235 patients who had LAM, about 49% used bronchodilators regularly (9).
LAM is characterized by proliferation of abnormal smooth muscle-like LAM cells in the lung, resulting in parenchymal cystic destruction. LAM cells are believed to proliferate in axial lymphatics and lung interstitium, leading to airway and lymphatic obstruction (2, 10). While LAM cells may be largely parenchymal, Hayashi et al. (11) showed bronchial involvement by LAM cells in explanted lungs of all 30 patients examined. A significant portion of these patients also had markers of chronic inflammation (e.g., mononuclear cell infiltration, goblet cell hyperplasia, squamous cell metaplasia, thickening of basal lamina) (11). Although LAM lesions were originally considered to represent a benign neoplasm, LAM is now accepted as a cancer with metastatic dissemination of cancer-like LAM cells (12).
LAM cells are characterized by mutations in the tuberous sclerosis complex (TSC) TSC1 or TSC2 gene that encodes, respectively, hamartin and tuberin (13–15). TSC is a rare genetic disease that affects multiple organ systems and results from mutations in one of the same two TSC genes (16). Hamartin and tuberin form a cytosolic complex with Tre2-Bub2-Cdc16 domain family member 7 (TBC1D7) (17). This complex inhibits the mechanistic target of rapamycin (mTOR) pathway, a promoter of cell growth through the GTPase-activating protein (GAP) activity of tuberin toward Ras homolog enriched in brain (Rheb) (17). Rheb in its GTP-bound form is a critical activator of mTOR; tuberin converts active Rheb-GTP to inactive Rheb-GDP (18).
mTOR, a serine/threonine kinase, is found in mTORC1 and mTORC2 complexes (19). mTORC1 comprises regulatory-associated protein of mTOR (Raptor), mammalian lethal with Sec13 protein 8 (mLST8; also known as GβL), proline-rich AKT substrate of 40 kDa (PRAS40), and DEP domain-containing mTOR-interacting protein (Deptor) (20). mTORC2 is also a multimer, sharing proteins, such as mLST8 and Deptor, with mTORC1, whereas the defining component of mTORC2 is Raptor-independent companion of mTOR (Rictor) (21, 22). The mTORC1 substrates [e.g., P70 S6 kinase (P70), 4E-binding protein 1 (4EBP1), unc-51–like autophagy-activating kinase 1 (ULK1)] regulate cell size, proliferation, and autophagy in a phosphorylation-dependent fashion (23). Activation of S6 kinases by mTOR promotes phosphorylation of several substrates, including ribosomal protein S6, eukaryotic initiation factor 4B (eIF4B), programmed cell death 4 (PDCD4), eukaryotic elongation factor 2 kinase (eEF-2K), and S6K1 Aly/REF-like target (SKAR) (23). Phosphorylation of S6, a component of the 40S ribosomal subunit, is associated with increased protein synthesis and cell proliferation (24). mTORC2 regulates metabolism and cytoskeletal organization by phosphorylating AGC kinases, such as Akt and PKC (22, 25, 26).
The absence of functional tuberin leads to persistence of Rheb in its GTP-bound state with mTORC1 activation, as was observed in LAM lung lesions and AMLs. Sirolimus (rapamycin), bound to FK506-binding protein 12 (FKBP12), interacts directly with mTORC1, inhibiting its kinase activity (27, 28), and is now frequently used to treat patients with moderate to severe pulmonary LAM (29). In patients with LAM, sirolimus stabilized forced expiratory volume in 1 s (FEV1) (29); decreased levels of the serum biomarker VEGF-D (30); and reduced the sizes of AMLs (31), chylous effusions, and lymphangioleiomyomas (32). In May 2015, sirolimus was approved by the US Food and Drug Administration for use in LAM, based on the results of the Multicenter International LAM Efficacy and Safety of Sirolimus Trial (29).
While sirolimus is the treatment of choice for patients with LAM who have rapidly progressive disease, some patients respond better than others (32). Since disease progression on sirolimus can be variable, we examined other pathways that might be involved in LAM disease progression. The cAMP/PKA pathway, as activated by chronic stress through beta2-adrenergic receptors, may be involved in tumor progression and metastasis (33, 34). A significant fraction of patients with LAM have reversible airflow obstruction that is treated with short- or long-acting bronchodilators (9). A response to bronchodilators was more common in patients with lung LAM nodules that line the lung cysts (2, 7, 9, 35), and was associated with an accelerated decline in FEV1 (9, 35). Here, we found that brief incubation of human TSC2+/− skin fibroblasts and LAM lung cells with the beta-agonist isoproterenol increased phosphorylation of S6 via the cAMP/PKA pathway, independent of sirolimus. Short-term incubation with a beta-agonist increased cell proliferation and phospho-S6 content more than prolonged incubation. These data were consistent with our retrospective review of a longitudinal study of patients with LAM that showed the rate of FEV1 change may be dependent on the type of beta-agonist used by the patient.
Results
Retrospective Study of the Effects of Bronchodilators on Pulmonary Function.
We have previously reported that a significant number of patients with LAM have partially reversible airflow obstruction, as measured by a positive bronchodilator response to nebulized albuterol, which was associated with a more rapid decline in pulmonary function (9). We retrospectively analyzed the effect of bronchodilator use on pulmonary function in 426 patients with sporadic LAM or LAM/TSC over 3,102 visits (Tables S1 and S2). Clinical records were examined for reported bronchodilator use by the patient. Bronchodilator groups included beta-agonists, steroids, anticholinergics, and other [e.g., leukotriene receptor antagonists, cyclic nucleotide phosphodiesterase inhibitors (PDEs), mast cell stabilizers] (Table S3). Use of bronchodilators was not significantly associated with percent predicted FEV1 (P = 0.128; adjusted for initial FEV1, sirolimus treatment, time of visit, and age) and was somewhat associated with diffusing capacity of the lung for carbon monoxide (DLCO; P = 0.0417; adjusted for initial DLCO, sirolimus treatment, and time of visit). A statistical interaction was seen between use of bronchodilators and sirolimus treatment, such that the effect of bronchodilator use on pulmonary function was different in patients not receiving sirolimus compared with those being treated with the drug. In patients not receiving sirolimus, those taking bronchodilators tended to have lower percent predicted FEV1 compared with those not using bronchodilators, whereas subjects on sirolimus had the opposite pattern (P < 0.001). Subjects not on sirolimus and on bronchodilators had lower percent predicted DLCO compared with those not using bronchodilators, whereas subjects on sirolimus had similar DLCO regardless of bronchodilator use (P = 0.002). Overall, the rate of change of FEV1 was −1.187 ± 0.077 (mean ± SE) percent predicted FEV1 per year in those without bronchodilator use and −1.358 ± 0.104 with bronchodilator use, and the rate of change of DLCO was −1.618 ± 0.065 percent predicted DLCO per year without bronchodilator use and −1.563 ± 0.093 with bronchodilator use. These values are not significantly different.
Since the interaction of sirolimus treatment and bronchodilator use was significant, we analyzed the use of bronchodilators by patients not receiving sirolimus separately from those receiving sirolimus. A total of 405 patients were not receiving sirolimus for 2,642 visits (Table S1). Patients averaged 6.5 ± 0.2 visits (range: 1–28) with a follow-up time of 4.6 ± 0.2 y (range: 0.0–17.8). Patients reported no use of bronchodilators at 1,525 (57.7%) visits to the NIH and the use of bronchodilators at 1,117 (42.3%) visits. Beta-agonist use comprised 407 (36.4%) visits with reported bronchodilator use, followed by use of beta-agonists plus steroids (376 or 33.7% of visits), beta-agonists plus steroids plus anticholinergics (137 or 12.3% of visits), beta-agonists plus anticholinergics (121 or 10.8% of visits), and fewer than 30 visits of each of the other combinations of bronchodilators. Use of bronchodilators was significantly associated with FEV1 and DLCO (both P < 0.001; adjusted for initial FEV1 or DLCO and time of visit). Patients with no bronchodilator use averaged 70.1 ± 0.5 and 64.7 ± 0.4 percent predicted FEV1 and DLCO, respectively, while those using bronchodilators averaged 66.9 ± 0.5 and 62.1 ± 0.4 percent predicted FEV1 and DLCO, respectively. Subjects not receiving sirolimus but using bronchodilators showed a faster decline in FEV1 than those not using bronchodilators (P < 0.001; no bronchodilator use: −1.397 ± 0.070 vs. −1.9534 ± 0.111 percent predicted FEV1 per year with bronchodilator use). The yearly rate of decline of DLCO was also faster in patients using bronchodilators (−2.009 ± 0.111 percent predicted DLCO per year) versus those not using bronchodilators (−1.817 ± 0.070 percent predicted DLCO per year); however, this difference was not significant (P = 0.143).
The analysis of the effect of bronchodilators on pulmonary function in patients receiving sirolimus included 108 patients with 460 visits (Table S2). Patients averaged 3.0 ± 0.2 y of sirolimus use (range: 0.08–10.45 y). Patients reported no use of bronchodilators at 163 (35.4%) of visits to the NIH and use of bronchodilators at 297 (64.6%) visits. Use of beta-agonists plus steroids comprised 99 (21.5%) of visits with reported bronchodilator use, followed by beta-agonists (82 or 17.8% of visits) and beta-agonists plus steroids plus anticholinergics (75 or 16.3% of visits). Use of bronchodilators in this group did not significantly affect the rate of change of FEV1 or DLCO.
Retrospective Study of the Effects of Beta-Agonists on Pulmonary Function.
We next looked specifically at the effects of beta-agonists on pulmonary function by retrospectively analyzing beta-agonist use of 426 patients with sporadic LAM or LAM/TSC over 3,080 visits (Tables S4 and S5). Use of beta-agonists was significantly associated with percent predicted FEV1 (P = 0.043; adjusted for initial FEV1, sirolimus treatment, time of visit, and age) and with DLCO (P = 0.006; adjusted for initial DLCO, sirolimus treatment, and time of visit). As with bronchodilator use, a statistical interaction was seen between use of beta-agonists and sirolimus treatment, such that the effect of beta-agonist use on pulmonary function was different in patients not receiving sirolimus compared with those receiving sirolimus. Subjects not receiving sirolimus but being treated with beta-agonists tended to have lower FEV1 compared with those not using beta-agonists, whereas subjects receiving sirolimus had the opposite pattern (P < 0.001). Subjects not receiving sirolimus but using beta-agonists had lower DLCO compared with those not using beta-agonists, whereas subjects receiving sirolimus had similar DLCO regardless of beta-agonist use (P = 0.004). Overall, the rate of change of FEV1 was −1.198 ± 0.077 percent predicted FEV1 per year without beta-agonist use and −1.322 ± 0.108 with beta-agonist use, and the rate of change of DLCO was −1.618 ± 0.065 percent predicted DLCO per year without beta-agonist use and −1.478 ± 0.098 with beta-agonist use. These values are not significantly different.
Since the interaction of sirolimus treatment and beta-agonist use was significant, we analyzed the visits of patients not receiving sirolimus separately from the visits of those on sirolimus. A total of 404 patients were not on sirolimus for 2,620 visits (Table S4). Beta-agonist groups included short-acting, long-acting, and both. Patients reported no use of beta-agonists at 1,594 (60.8%) of visits to the NIH and the use of beta-agonists at 1,026 (39.2%) visits. Short-acting beta-agonist use comprised 408 (39.8%) of visits with reported beta-agonist use, followed by both (386 or 37.6% of visits) and long-acting beta-agonists (232 or 22.6% of visits). Use of beta-agonists was significantly associated with FEV1 and DLCO (both P < 0.001; adjusted for initial FEV1 or DLCO and time of visit). Patients with no beta-agonist use averaged 70.3 ± 0.4 and 65.0 ± 0.4 percent predicted FEV1 and DLCO, respectively, while those using beta-agonists averaged 66.9 ± 0.5 and 62.2 ± 0.4 FEV1 and DLCO, respectively. Subjects not receiving sirolimus but using beta-agonists showed a faster decline in FEV1 than those not using beta-agonists (P < 0.001; no beta-agonist use: −1.470 ± 0.070 vs. −1.939 ± 0.118 percent predicted FEV1 per year with beta-agonist use). The yearly rate of change of DLCO was not different in patients using beta-agonists (−1.791 ± 0.125) versus those not using beta-agonists (−1.732 ± 0.081) (P = 0.689).
The analysis of the effect of beta-agonists on pulmonary function in patients receiving sirolimus included 107 patients with 460 visits (Table S5). Patients reported no use of beta-agonists at 178 (38.7%) visits to the NIH and use of beta-agonists at 282 (61.3%) visits. Short- and long-acting beta-agonist use comprised 154 (54.6%) of visits with reported beta-agonist use, followed by short-acting beta-agonists (71 or 25.2% of visits) and long-acting beta-agonists (57 or 20.2%). Use of beta-agonists in this group did not significantly affect the rate of change of FEV1 or DLCO (P = 0.415 and P = 0.053, respectively).
Analysis of the Effect of Beta-Agonist Subtypes on FEV1 in Patients Not Receiving Sirolimus.
A multivariate model predicting FEV1 and adjusting for initial FEV1, time of visit, age, and type of beta-agonist (none and long-acting, short-acting, or both) examining all of the visits by patients not receiving sirolimus resulted in a significant (P < 0.01) contribution of all variables considered. The rate of change of FEV1 for those not using beta-agonists was −1.304 ± 0.082 percent predicted per year, while that for short-acting beta-agonists was −1.677 ± 0.238, that for both was −0.783 ± 0.255, and that for long-acting beta-agonists was −2.443 ± 0.391. The rate of change of FEV1 of patients on long-acting beta-agonists was significantly different from that of patients on both long-acting and short-acting beta-agonists (P < 0.001) and from that of patients not on beta-agonists (P = 0.004) (Table 1 and Table S6).
Table 1.
Rate of change of FEV1 categorized by beta-agonist subtype in patients not receiving sirolimus
| Beta-agonist type | FEV1 rate of change, mean percent predicted per year ± SE |
| None | −1.470 ± 0.070 (1,594 visits) |
| Beta-agonist | −1.939 ± 0.118 (962 visits) |
| P < 0.001 | |
| None | −1.304 ± 0.082 (1,594 visits) |
| Short-acting | −1.677 ± 0.238 (391 visits) |
| Both short-acting and long-acting | −0.783 ± 0.255 (349 visits) |
| Long-acting | −2.443 ± 0.391 (222 visits) |
| N vs. S | P = 0.1291 |
| N vs. SL | P = 0.0457 |
| N vs. L | P = 0.0040 |
| S vs. SL | P = 0.0095 |
| S vs. L | P = 0.0908 |
| SL vs. L | P = 0.0003 |
With statistical correction, significant P values are those less than 0.0083. L, long-acting beta-agonist; N, no beta agonist; S, short-acting beta-agonist; SL, short- and long-acting beta-agonists.
Analysis of the Effect of Beta-Agonist Subtypes on FEV1 in Patients Receiving Sirolimus.
Since 51 of the 107 patients on sirolimus only had one or two visits receiving sirolimus, we refined the dataset to exclude visits before 2010 and then those patients without at least three visits approximately 6 mo apart. A total of 53 patients with 349 visits were included in this study; 22 of them had information from visits while not receiving sirolimus, for a total of 40 visits (Tables S7 and S8). The use of beta-agonists was not significantly associated with percent predicted FEV1 (P = 0.174) or DLCO (P = 0.186) after adjusting for initial FEV1 or DLCO and sirolimus treatment. In this study population, the interaction of sirolimus treatment and beta-agonist use was not significant, indicating that the effect of beta-agonists on FEV1 or DLCO was not different in patients receiving or not receiving sirolimus treatment. The use of beta-agonists had no significant effect on the rate of change of DLCO (−0.761 ± 0.285 percent predicted per year for no beta-agonist use versus −0.458 ± 0.222 percent predicted per year for those using beta-agonists; P = 0.391). Beta-agonist use did significantly affect the yearly rate of change of FEV1, however, with a rate of −1.297 ± 0.371 percent predicted FEV1 per year for those not using beta-agonists (134 visits) versus 0.125 ± 0.288 for those using beta-agonists (215 visits) (P = 0.002). Use of beta-agonists may have stabilized the FEV1 compared with that in patients not using beta-agonists, who continue to decline.
The type of beta-agonist was significantly associated with percent predicted FEV1 (P < 0.001) after adjusting for initial FEV1, sirolimus treatment, and time of visit. Here again, the interaction of sirolimus treatment and type of beta-agonist used was significant (P < 0.001), indicating that the effect of the different types of beta-agonists was different in patients receiving or not receiving sirolimus. The type of beta-agonist was not a predictor of percent predicted FEV1 for patients not receiving sirolimus after adjusting for initial FEV1 and time of visit. In this study group, this analysis contained only 22 patients with 40 visits (Table S7). The type of beta-agonist was a predictor of percent predicted FEV1 (P = 0.003) in patients receiving sirolimus treatment, after adjusting for initial FEV1 and time of visit. This subpopulation included 53 patients with 309 visits receiving sirolimus (Table S8): patients did not use beta-agonists for 122 (39.5%) of these visits and used beta-agonists for 187 (60.5%) of these visits. The patients used short-acting beta-agonists for 25.7% of the beta-agonist visits, long-acting beta-agonists for 18.7%, and both for 55.6%. Interestingly, for the patients receiving sirolimus treatment, those with no beta-agonist use showed the fastest rate of decline of FEV1 (−1.311 ± 0.346 percent predicted FEV1 per year), compared with those using short-acting beta-agonists alone (−0.393 ± 0.665 percent predicted FEV1 per year), those using long-acting beta-agonists alone (0.880 ± 0.782 percent predicted FEV1 per year), and those using both (0.493 ± 0.365 percent predicted FEV1 per year). When comparing the rates of change of FEV1 pairwise, only the comparison of no beta-agonist use versus use of both was significant (P = 0.0004; the corrected level of significance is 0.0083) (Table 2). These data suggest that the use of both short- and long-acting beta-agonists stabilizes the rate of decline of FEV1 for patients undergoing sirolimus treatment.
Table 2.
Rate of change of FEV1 categorized by beta-agonist subtype in patients receiving sirolimus
| Beta-agonist type | FEV1 rate of change, mean percent predicted per year ± SE |
| None | −1.297 ± 0.371 (122 visits) |
| Beta-agonist | 0.125 ± 0.288 (187 visits) |
| P = 0.002 | |
| None | −1.311 ± 0.346 (122 visits) |
| Short-acting | −0.393 ± 0.665 (48 visits) |
| Both short-acting and long-acting | 0.493 ± 0.365 (104 visits) |
| Long-acting | 0.880 ± 0.782 (35 visits) |
| N vs. S | P = 0.2253 |
| N vs. SL | P = 0.0004 |
| N vs. L | P = 0.0113 |
| S vs. SL | P = 0.2399 |
| S vs. L | P = 0.2103 |
| SL vs. L | P = 0.6525 |
With statistical correction, significant P values are those less than 0.0083. L, long-acting beta-agonist; N, no beta agonist; S, short-acting beta-agonist; SL, short- and long-acting beta-agonists.
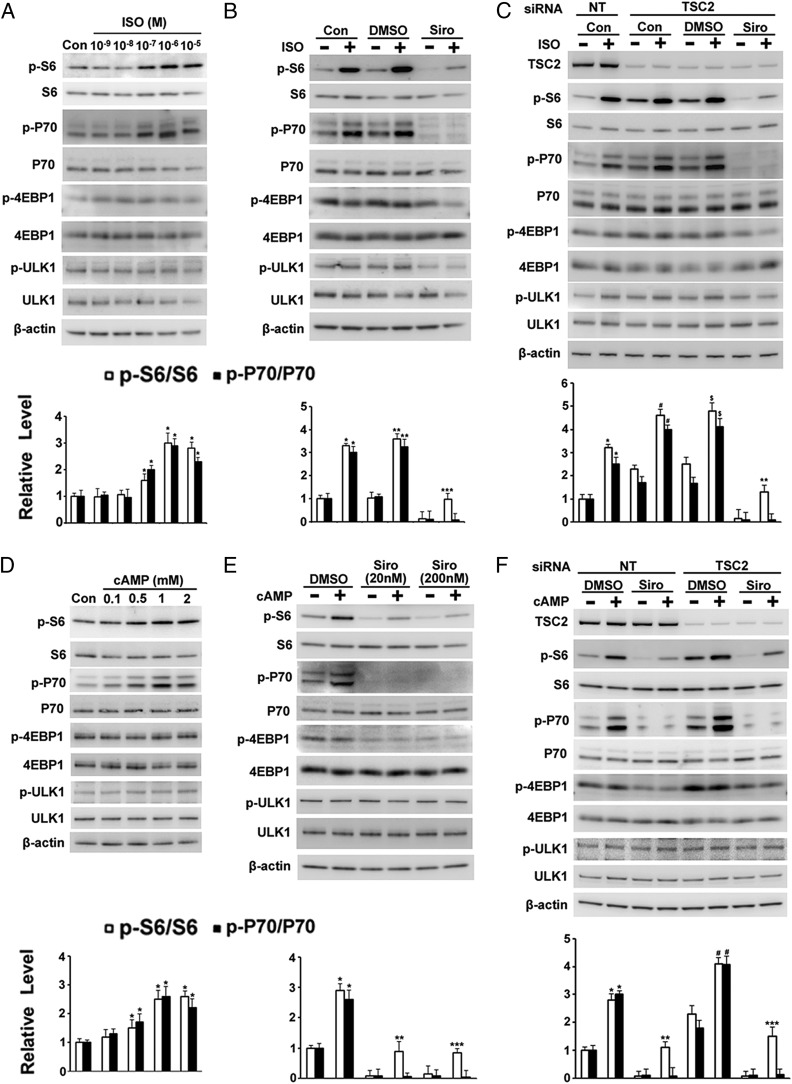
Phosphorylation of S6 and P70 in TSC2+/− Cells Was Increased by Isoproterenol or cAMP.
Since pulmonary function was affected by bronchodilators, and especially by beta-agonists, we decided to examine the effect of beta-agonists (represented by isoproterenol) on the mTOR pathway. To establish experimental conditions, we first studied skin TSC2+/− cells (germline mutation TSC2 c.4830G > A, p.W1610*), which are more homogeneous than LAM lung cultures. Incubation of human skin TSC2+/− fibroblasts with isoproterenol for 1 h increased phosphorylation of S6 and P70 in a concentration-dependent manner (Fig. 1A), with no effect on phospho-4EBP1 or phospho-ULK1 levels. Sirolimus completely prevented the effects of isoproterenol on phospho-P70, but only partially inhibited S6 phosphorylation (Fig. 1B), suggesting that isoproterenol-stimulated phosphorylation of S6 was due to a pathway in addition to mTORC1. As expected, since the TSC2 gene product tuberin acted as a negative regulator of the mTORC1 pathway, levels of phospho-S6 and phospho-P70 were higher in cells transfected with TSC2 siRNA than with nonspecific siRNA (36) (Fig. 1C). Interestingly, S6 phosphorylation was still increased in cells depleted of TSC2 in the presence of sirolimus after incubation with isoproterenol (Fig. 1C). Beta-agonists activate adenylyl cyclase, increasing formation of the second messenger cAMP (37). Incubation of cells with the cAMP analog 8-Br-cAMP also increased phosphorylation of S6 in the presence of sirolimus or in cells depleted of TSC2 (Fig. 1 D–F), consistent with the role of cAMP in the effects of isoproterenol. As seen with isoproterenol, the cAMP-dependent increase in phospho-p70 was blocked by sirolimus. All data supported the view that isoproterenol and cAMP altered S6 phosphorylation via both mTOR-dependent and -independent pathways.
Fig. 1.
Effects of isoproterenol (ISO) and 8-Br-cAMP on phosphorylation of S6 and P70 in human skin TSC2+/− fibroblasts incubated without and with sirolimus (Siro). (A and D) Human skin TSC2+/− cells were incubated for 1 h with control (Con) or the indicated concentration of ISO or 8-Br-cAMP (cAMP) before analysis by Western blotting with indicated bodies and densitometric quantification. *P < 0.01 vs. Con. (B and E) Cells were incubated for 1 h with DMSO or 20 or 200 nM Siro, followed by 1 h with additional 1 μM ISO or 1 mM cAMP before analysis of indicated proteins by Western blotting and densitometric quantification. (B) *P < 0.01 vs. Con; **P < 0.05 vs. DMSO; ***P < 0.01 vs. Siro. (E) *P < 0.05 vs. DMSO; **P < 0.05 vs. 20 nM Siro; ***P < 0.05 vs. 200 nM Siro. (C and F) After a 72-h incubation with nontargeted (NT) or TSC2 siRNA, TSC2+/− cells were incubated for an additional hour with DMSO or 200 nM Siro, followed by 1 h with additional 1 μM ISO or 1 mM cAMP before analysis of indicated proteins by Western blotting and densitometric quantification. (C) *P < 0.01 vs. Con + NT siRNA; #P < 0.005 vs. Con + TSC2 siRNA; $P < 0.01 vs. DMSO + TSC2 siRNA; **P < 0.05 vs. Siro + TSC2 siRNA. (F) *P < 0.05 vs. DMSO + NT siRNA; **P < 0.01 vs. Siro + NT siRNA; #P < 0.01 vs. DMSO + TSC2 siRNA; ***P < 0.05 vs. Siro + TSC2 siRNA.
We also explored effects of bronchodilators ipratropium, an anticholinergic drug, and Montelukast, a leukotriene receptor antagonist. Incubation with ipratropium for 1 h increased S6 and P70 phosphorylation in a concentration-dependent manner. The effects on phospho-S6, however, were completely blocked by sirolimus, suggesting that effects on S6 phosphorylation were via an mTOR-dependent pathway (Fig. S1 A and B). Montelukast caused no obvious change in phospho-S6 or phospho-P70 content (Fig. S1C).
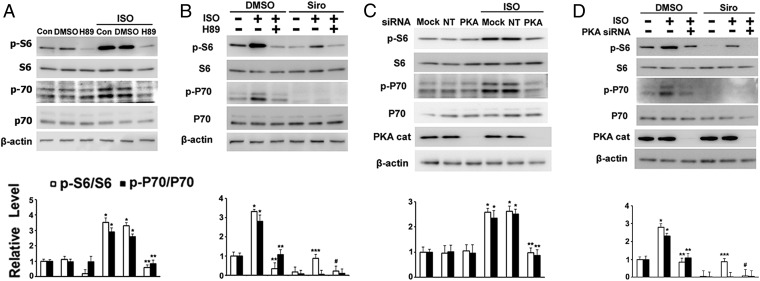
Effects of Isoproterenol or cAMP on S6 or P70 Phosphorylation in TSC2+/− Cells in the Presence of H89 or Following PKA Catalytic Subunit α Depletion.
We hypothesized that effects of isoproterenol and cAMP were mediated through PKA, which comprises two regulatory and two catalytic subunits (38). To explore the role of PKA in regulation of S6 phosphorylation, we used H89, a PKA inhibitor, and PKA catalytic subunit α (Cα) siRNA. Incubation of cells with H89 blocked the effects of isoproterenol or cAMP on phospho-S6 and phospho-p70, with or without sirolimus (Fig. 2 A and B and Fig. S2A). H89 also blocked basal phosphorylation of S6, 4EBP1, and ULK1, although we questioned whether this was due to inhibition of PKA (Fig. 2 A and B and Figs. S2A and S3), since H89 inhibits several kinases (39). To assess more specifically the role of PKA, we knocked down a PKA catalytic subunit. In contrast to H89, incubation with PKA Cα siRNA blocked only the phosphorylation of S6 and p70 induced by isoproterenol or cAMP, and had no effect on phosphorylation of 4EBP1 and ULK1 (Fig. 2 C and D and Figs. S2B and S3). All data are consistent with a model in which isoproterenol and cAMP regulate S6 phosphorylation via mTOR-dependent (mTORC1/P70/S6 pathway that is inhibited by sirolimus) and -independent (cAMP/PKA pathway) pathways.
Fig. 2.
Effects of H89 or PKA Cα knockdown on phosphorylation of S6 and P70 in TSC2+/− cells. (A) TSC2+/− cells were incubated for 1 h with DMSO or 10 μM H89, followed by 1 h with addition of 1 μM isoproterenol (ISO) before analysis of indicated proteins by Western blotting and densitometric quantification. *P < 0.01 vs. DMSO; **P < 0.01 vs. DMSO + ISO. (B) After a 1-h incubation with DMSO, 10 μM H89, or 200 nM sirolimus (Siro), cells were incubated for 1 h with addition of 1 μM ISO before analysis of indicated proteins by Western blotting and densitometric quantification. *P < 0.05 vs. DMSO; **P < 0.005 vs. DMSO + ISO; ***P < 0.01 vs. Siro; #P < 0.01 vs. Siro + ISO. (C) After a 72-h incubation with vehicle alone (Mock) or with nontargeted (NT) or PKA Cα siRNA (PKA), cells were incubated for 1 h with 1 μM ISO before analysis by Western blotting with indicated antibodies and densitometric quantification. *P < 0.05 vs. NT siRNA; **P < 0.01 vs. NT siRNA + ISO. (D) After a 72-h depletion of PKA Cα, cells were incubated for 1 h with DMSO or 200 nM Siro, followed by a 1-h incubation with additional 1 μM ISO before analysis by Western blotting with indicated antibodies and densitometric quantification. *P < 0.01 vs. DMSO; **P < 0.05 vs. DMSO + ISO; ***P < 0.01 vs. Siro; #P < 0.05 vs. Siro + ISO. Fig. S3 shows the effects on 4EBP1 and ULK1.
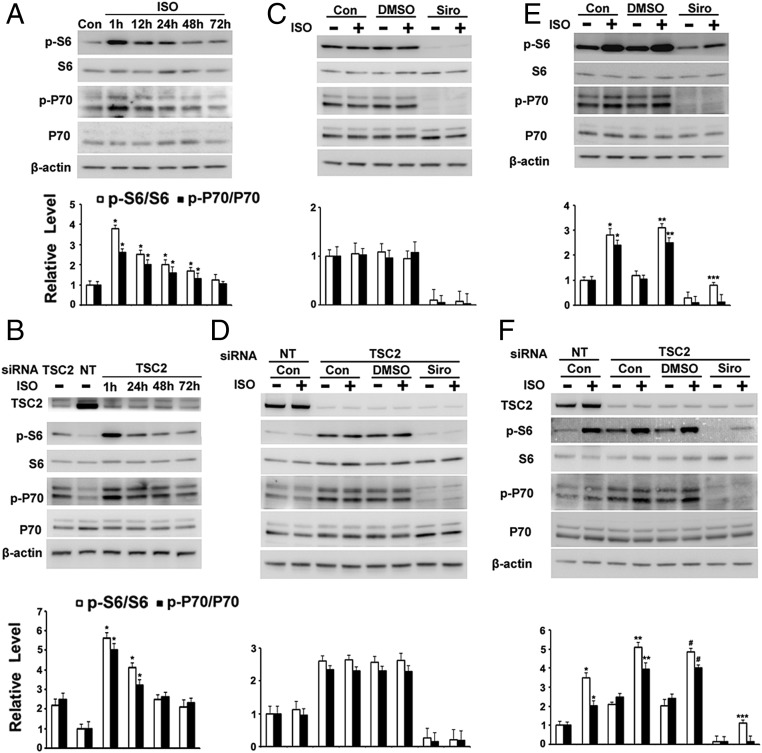
Long-Term Incubation with Isoproterenol Did Not Increase Phospho-S6 or Phospho-P70 in Human TSC2+/− Cells or TSC2-Depleted Cells.
To mimic the effects of long-acting beta-agonists, human TSC2+/− cells were incubated with isoproterenol or cAMP for up to 72 h. Both agents failed to increase S6 or P70 phosphorylation after a 48-h incubation despite producing an early response (Fig. 3A and Figs. S4 and S5). Results were similar in TSC2-depleted cells (Fig. 3B and Fig. S4). As shown in Fig. 3 C and D, after continuous incubation of cells with isoproterenol for 3 d, no effect on S6 or p70 phosphorylation was observed with or without sirolimus, even in TSC2-depleted cells. However, when cells were treated with isoproterenol for 1 h each day for 3 d (mimicking short-acting beta-agonists), S6 or p70 phosphorylation was increased, even in the presence of sirolimus (Fig. 3 E and F), suggesting the presence of a sirolimus-resistant or partially mTOR-dependent pathway.
Fig. 3.
Effects of long-term incubation of TSC2+/− cells with isoproterenol (ISO) on phosphorylation of S6 and P70. (A) TSC2+/− cells were incubated with 1 μM ISO for the indicated time before analysis by Western blotting with indicated antibodies and densitometric quantification. *P < 0.01 vs. control (Con). (B) After a 72-h knockdown of TSC2, cells were incubated with 1 μM ISO for the indicated time before analysis of indicated proteins by Western blotting and densitometric quantification. *P < 0.005 vs. TSC2 siRNA. Cells were incubated with DMSO or sirolimus (Siro) for 3 d, with or without ISO (C) or with ISO for 1 h each day for 3 d (E) before analysis by Western blotting with indicated antibodies and densitometric quantification. *P < 0.01 vs. Con; **P < 0.005 vs. DMSO; ***P < 0.01 vs. Siro. After a 72-h knockdown of TSC2, cells were incubated with DMSO or 200 nM Siro for 3 d, with or without 1 μM ISO (D) or with 1 μM ISO for 1 h each day for 3 d (F) before analysis of indicated proteins by Western blotting and densitometric quantification. *P < 0.01 vs. Con + nontargeted (NT) siRNA; **P < 0.01 vs. Con + TSC2 siRNA; #P < 0.01 vs. DMSO + TSC2 siRNA; ***P < 0.01 vs. Siro + TSC2 siRNA. Fig. S4 shows the effects on 4EBP1 and ULK1.
To exclude the possibility that isoproterenol or cAMP loses its activity after 24 h in medium at 37 °C, human TSC2+/− cells were serum-starved for 72 h and then incubated with media that contained isoproterenol or cAMP that had been incubated with similar cells for 24 h. The results illustrated in Fig. S6A indicate that isoproterenol or cAMP was still active after exposure to cells in medium for 24 h at 37 °C. Cells lost responsiveness, however, to isoproterenol or cAMP after exposure to the agents for 72 h at 37 °C (Fig. S6B).
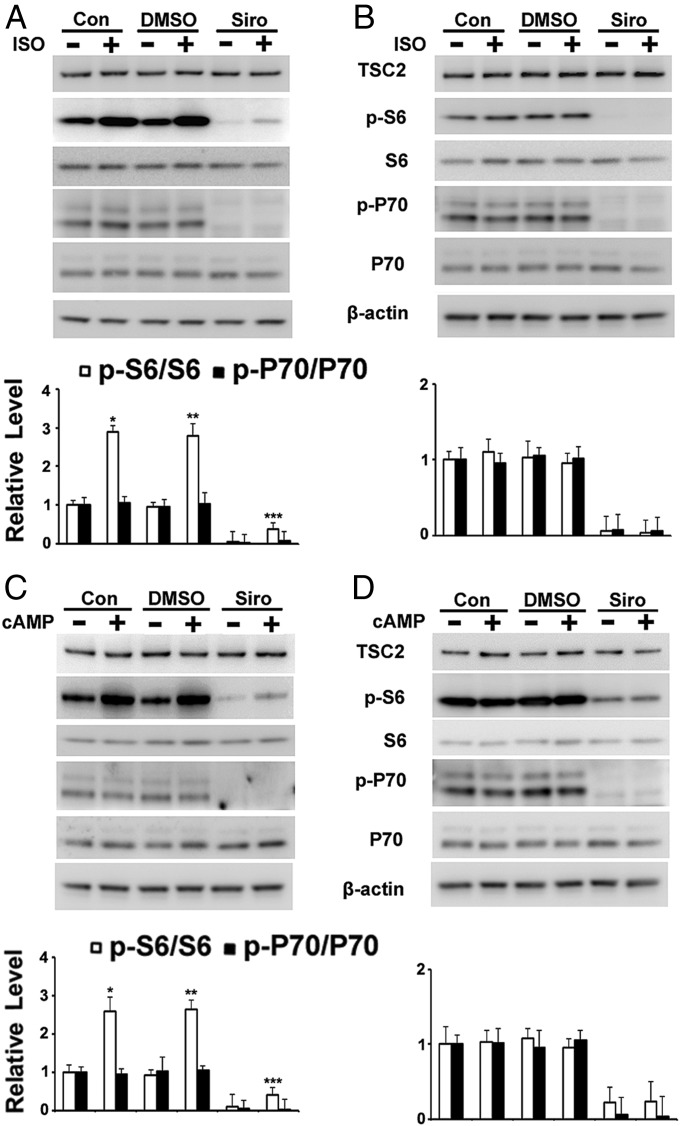
Isoproterenol or cAMP Increased Phospho-S6 in Cells Cultured from Lungs of Patients with LAM.
LAM lung cell cultures are mixtures of cells, which include both TSC2 WT and TSC2-negative cells. Incubation of LAM cell cultures with isoproterenol or cAMP for 1 h with or without sirolimus increased phospho-S6 content (Fig. 4 A and C and Fig. S7). Incubation with isoproterenol, however, did not increase phospho-P70 content (Fig. 4A). In addition, incubation with isoproterenol or cAMP continuously for 3 d did not increase the resulting phospho-S6 content, as was observed with the TSC2+/− cells (Fig. 4 B and D). Furthermore, H89 blocked the effects of isoproterenol on S6 phosphorylation, suggesting that cAMP-PKA, but not the mTOR pathway, was involved in the regulation of phospho-S6 by isoproterenol in cultured lung LAM cells, a result that differed from the effects seen with skin TSC2+/− cells (Fig. S8).
Fig. 4.
Effects of isoproterenol (ISO) or 8-Br-cAMP on phosphorylation of S6 in cells cultured from explanted lungs of patients with LAM who underwent transplantation. (A and C) Cells grown from LAM lung explants were incubated for 1 h with DMSO or 200 nM sirolimus (Siro), followed by 1 h with 1 μM ISO or 1 mM cAMP before analysis of indicated proteins by Western blotting and densitometric quantification. *P < 0.05 vs. control (Con); **P < 0.01 vs. DMSO; ***P < 0.01 vs. Siro. (B and D) Cells were incubated with DMSO or 200 nM Siro for 3 d, with or without 1 μM ISO or 1 mM cAMP, before analysis by Western blotting with indicated antibodies. Fig. S7 shows the effects on 4EBP1 and ULK1.
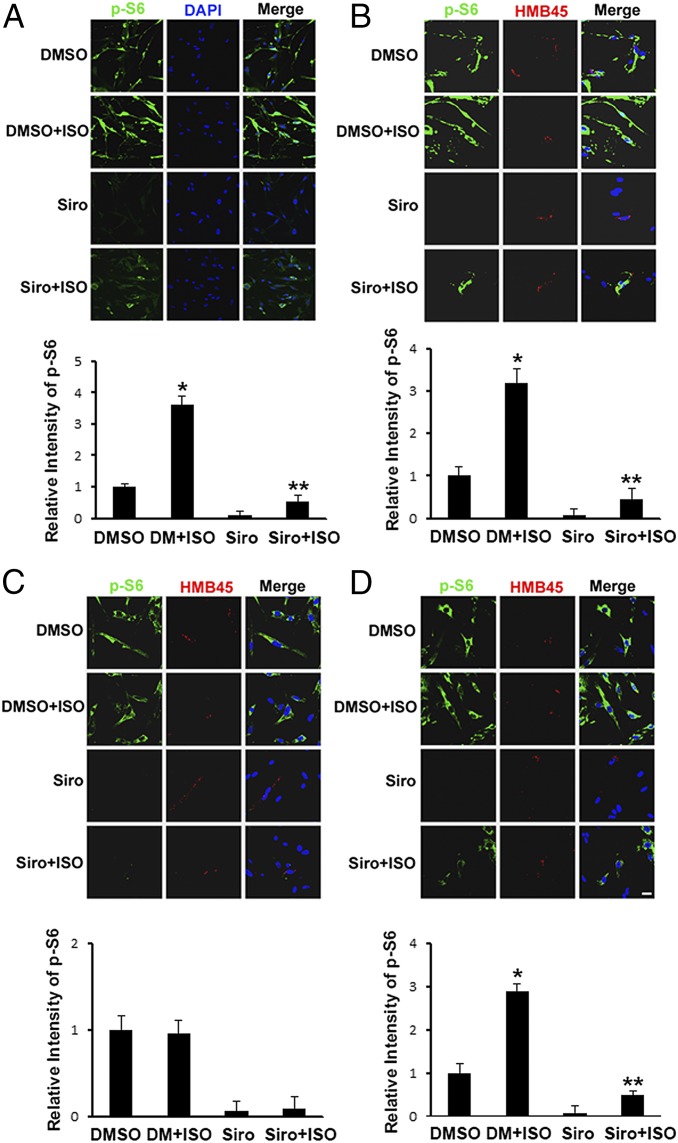
Isoproterenol or cAMP Increased Phospho-S6 in HMB45-Positive Human Lung LAM Cells.
Incubation with isoproterenol or cAMP for 1 h increased the number of human lung LAM cells with high cytosolic reactivity to anti–phospho-S6 antibodies (Fig. 5A and Fig. S9A). Sirolimus almost completely abolished detection of phospho-S6. Sirolimus effects were reversed by a 1-h incubation with isoproterenol or cAMP (Fig. 5A and Fig. S9A). To determine whether LAM cells among the cultured LAM lung cell mixture were affected by isoproterenol or cAMP, we used antibodies that discriminate between LAM and non-LAM cells. The monoclonal antibody HMB45 reacted with LAM cells, recognizing Pmel17, a 100-kDa glycoprotein originally identified in human melanoma cells (40). In the LAM lung cell mixture, HMB45 reacted with cytoplasmic granules resembling immature melanosomes (40). Few positive cells were found, indicating a low percentage of LAM cells in the mixture (Fig. 5B). Incubation with isoproterenol or cAMP for 1 h increased phospho-S6 (green fluorescence) in sirolimus-treated, HMB45-reactive cells (Fig. 5B and Fig. S9B). LAM lung-cultured cells were then treated with isoproterenol or cAMP for 3 d either continuously or for 1 h each day. Continued incubation of the mixed culture with isoproterenol or cAMP for 3 d did not result in increased phospho-S6 content, whereas incubation with isoproterenol for 1 h each day increased phospho-S6 in HMB45-reactive cells (Fig. 5 C and D and Fig. S9 C and D), consistent with effects observed in human skin TSC2+/− cells from patients with TSC.
Fig. 5.
Effects of isoproterenol (ISO) on phosphorylation of S6 in HMB45-positive lung LAM cells. (A and B) Lung LAM cells after a 1-h incubation with DMSO or 200 nM sirolimus (Siro), followed by a 1-h incubation with 1 μM ISO, were fixed and stained with indicated antibodies. After incubation with DMSO or 200 nM Siro for 3 d, with or without 1 μM ISO (C) or with ISO for 1 h each day for 3 d (D), cells were fixed, reacted with rabbit anti–phospho-S6 polyclonal antibodies and mouse HMB45 monoclonal antibodies, and then prepared for confocal immunofluorescence microscopy. (Scale bar: 10 μm.) Mean fluorescence intensity of phospho-S6 in outlined area of each cell (>50 in each population) was measured in pixels by ImageJ software (NIH). *P < 0.01 vs. DMSO; **P < 0.05 vs. Siro. Similar results were obtained from three independent experiments.
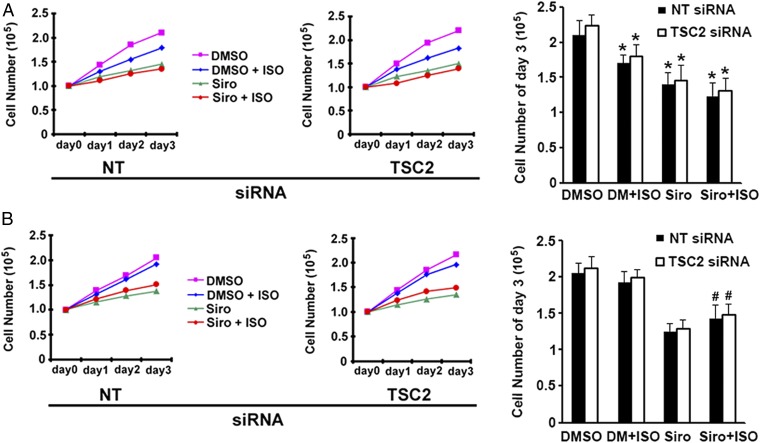
Isoproterenol Affected Proliferation of TSC2+/− Cells.
S6, which is phosphorylated by P70, is a component of the 40S ribosomal subunit and thought to be involved in the regulation of cell size and proliferation (41). Isoproterenol was reported to inhibit cancer cell proliferation and tumor growth, which are mediated by inhibition of ERK1/2 phosphorylation through the cAMP/PKA pathway (42). In Fig. 6A, after transfection with nontarget or TSC2 siRNA, TSC2+/− cells were incubated continuously for 3 d as indicated with DMSO, sirolimus, and/or isoproterenol. Consistent with the earlier report (42), isoproterenol inhibited proliferation of TSC2+/− cells or TSC2+/− cells depleted of TSC2 with siRNA after 3 d of incubation (Fig. 6A). In cells incubated with isoproterenol for 1 h each day for 3 d, the inhibitory effects of isoproterenol on cell proliferation were much less than those after 3 d of continuous isoproterenol exposure (Fig. 6B). Inhibition of cell proliferation by sirolimus was reduced when coupled with isoproterenol (Fig. 6B). We postulate that these effects may have resulted from the increase in phospho-S6 by short-term exposure to isoproterenol, which led to increased cell proliferation.
Fig. 6.
Effects of isoproterenol (ISO), sirolimus (Siro), and TSC2 depletion on cell proliferation. (A) After transfection with nontargeted (NT) or TSC2 siRNA for 72 h, followed by incubation with DMSO, 200 nM Siro, and/or 1 μM ISO for the indicated days, human TSC2+/− cells were trypsinized and counted by microscopy. (B) After transfection with NT or TSC2 siRNA for 72 h, TSC2+/− cells were incubated with DMSO or 200 nM Siro for the indicated days, along with 1 μM ISO for 1 h each day. Cells were counted as in A. *P < 0.05 vs. DMSO; #P < 0.05 vs. Siro. Similar results were obtained from three independent experiments.
Discussion
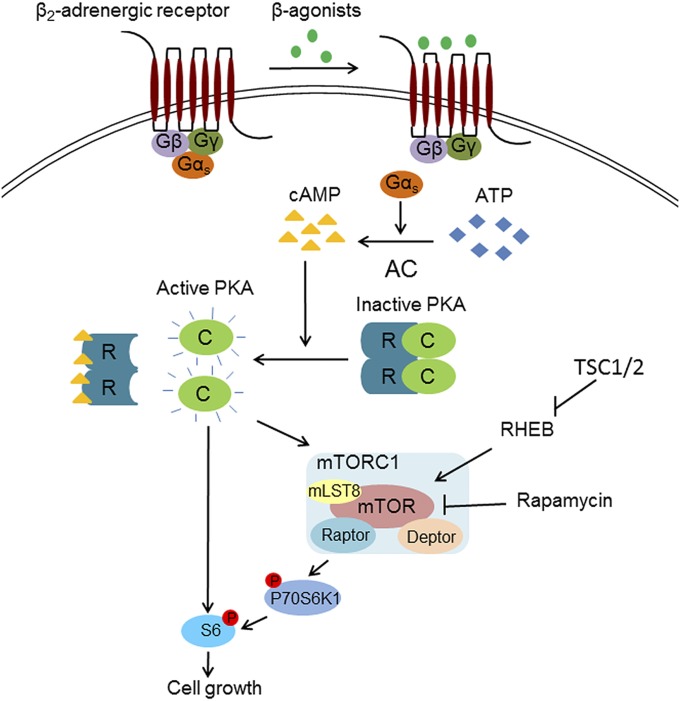
The past two decades have seen significant advances in LAM research and patient care, including both diagnosis and treatment (4). In 2000, TSC2 gene mutations in LAM cells were reported (14), and increased mTOR activity was attributed to the dysregulated growth of these smooth muscle-like cells (36). Thus, activation of mTORC1 was postulated to be crucial for LAM cell growth. Consistent with these data, lung function of patients was stabilized by sirolimus treatment (29). In some cases, however, lung function continued to decline even in the presence of sirolimus (32), suggesting that other pathways, in addition to mTOR, can regulate LAM cell growth. Many patients who have LAM use bronchodilators, such as beta-adrenergic agents affecting the cAMP/PKA pathway, to treat airway obstruction. We questioned whether disease progression both with and without sirolimus treatment was affected by use of bronchodilators. We found that patients using the combination of short- and long-acting beta-agonists without sirolimus had the slowest rate of decline in FEV1 (Table 1), while those taking both sirolimus and short- and long-acting beta-agonists enjoyed a stabilization of FEV1 compared with patients on sirolimus alone (Table 2). To determine why patients respond to these agents, we tested isoproterenol, a nonselective beta-agonist, on TSC2+/− and TSC2-deficient cells. We showed that the inhibitory effects of sirolimus on human skin TSC2+/− cell proliferation and S6 phosphorylation could be bypassed by short-term incubation of cells with isoproterenol (Figs. 1 and 6). We also observed that the short-term effects of isoproterenol or cAMP on HMB45-reactive cells among those grown from lungs of LAM patients bypassed mTORC1 inhibition by sirolimus (Fig. 5). These data showed that the cAMP/PKA pathway may play a critical role in LAM cell growth and suggest that bronchodilators working through cAMP may affect LAM cell phospho-S6 content and proliferation, and thereby disease progression (Fig. 7).
Fig. 7.
Proposed model for regulation of S6 phosphorylation (P) by the cAMP/PKA pathway. PKA, after activation by cAMP, regulates P of S6 via two different routes. One, involving the mTORC1/P70/S6 pathway, is inhibited by sirolimus, and the other, bypassing mTOR, involves P of S6 by PKA. R, regulatory subunit.
Human TSC2+/− cells incubated with isoproterenol for 1 h had markedly increased phosphorylation of P70 S6K and S6 (Fig. 1), consistent with prior reports that cAMP/PKA can activate the mTOR pathway (43–45). cAMP/PKA directly phosphorylates mTOR and RAPTOR, which leads to the activation of P70 S6K (46). In our study, only the increase of phospho-P70, not phospho-S6, was completely blocked by sirolimus, which indicated that isoproterenol increased phospho-S6 via other pathways, in addition to that through mTOR-P70 S6K.
We then used H89 to show that the cAMP/PKA pathway was involved in phospho-S6 formation. However, phospho-4EBP1 and phospho-ULK1 levels were also reduced by H89, which might reflect a nonspecific inhibitory effect. H89 blocks PKA actions through competitive inhibition of the ATP site on the PKA catalytic subunit (47–49). H89 inhibited at least eight other kinases (e.g., MAPKAP-K1b, MSK1, KBα, SGK, S6K1, ROCK II, AMPK, CHK1) (39), thus potentially exhibiting a relatively large number of PKA-independent effects. The fact that H89 inhibited S6K1 and PKA (48) is consistent with the data in Fig. 2A, in which H89 decreased the basal phosphorylation of S6.
Because H89 is a somewhat nonspecific kinase inhibitor, we used siRNA-induced knockdown of a PKA catalytic subunit to confirm a role of PKA. PKA is a heterotetramer comprising two regulatory subunits and two catalytic subunits. Three catalytic subunits have been identified, designated Cα, Cβ, and Cγ (49–52). Cα and Cβ are expressed in most tissues, whereas Cγ is expressed only in testis (52, 53). Cα is thought to be the predominant isoform, and its deletion resulted in phenotypic changes in mice. Targeted deletion of PRKACA caused growth retardation and sperm dysfunction. In contrast, deletion of Cβ resulted in phenotypically normal mice (54). PRKACA mutation was also found in cortisol-producing adrenocortical adenomas (55). Our experiments with PKA Cα siRNA showed that the cAMP/PKA pathway was involved in the regulation of S6 phosphorylation (Fig. 2), consistent with a previous report that PKA is an S6 kinase (56).
In contrast to data from a 1-h short-term incubation, no effects of a beta-agonist on phospho-S6, phospho-P70, phospho-4EBP1, or phospho-ULK1 were seen when TSC2+/− cells or LAM lung cells were incubated with isoproterenol continuously for 3 d. We hypothesize that this effect is due to desensitization of the receptor and perhaps other components in the signal transduction pathway (e.g., cyclic nucleotide PDEs as noted below). Among pathways leading to desensitization, PKA and G protein-coupled receptor kinase phosphorylate, and thus induce, the internalization of the beta-adrenergic receptor (57, 58). After more prolonged agonist exposure, a net loss of cellular receptors occurs through mechanisms independent of receptor phosphorylation, such as ubiquitination, which results in receptor degradation (59). This model (Fig. 7) could explain our data showing that 1-h short-term incubation each day with isoproterenol increased S6 phosphorylation, whereas the modification was not seen after a 3-d continuous, long-term incubation (Figs. 3 and 5).
Increased phospho-S6 and -P70 was not observed following long-term incubation with 8-Br-cAMP, which bypassed the beta-adrenergic receptor. An increase in cAMP produced activation of several PDEs, such as PDE3A, PDE3B, and PDE4s, which catalyze cAMP hydrolysis (60–62). With increased time of exposure to cAMP, PKA-mediated PDE phosphorylation and activation result in negative feedback regulation of cAMP signaling, by decreasing cAMP levels (63, 64). This offers another possible explanation of why prolonged incubation with isoproterenol led to loss of its effect on S6 or P70 phosphorylation.
Our data suggest that short-term, but not long-term, incubation with beta-agonists increased phospho-S6 levels and cell growth. These results may provide an explanation of why patients taking both short- and long-acting beta-agonists had stabilization of FEV1 compared with patients not taking beta-agonists. Inhaled bronchodilators have multiple potential targets in the lungs, including LAM cells, airway smooth muscle cells, mast cells, endothelial cells, eosinophils, neutrophils, macrophages, T lymphocytes, and type I and II pneumocytes (65). Each cell type may have a different response to a bronchodilator (e.g., differences in receptor desensitization, effects on mTOR as shown here in LAM lung cells versus skin cells) (65). In addition, many patients may take more than one type of bronchodilator. Patients with asthma on long-acting beta-agonists receive steroids as well to control inflammation (66). Interestingly, patients not receiving sirolimus but taking long-acting beta-agonists showed a faster rate of decline in FEV1 than patients using both short- and long-acting beta-agonists. A total of 73.9% of the visits with both short- and long-acting beta-agonist use also included steroid use, while only 55.4% of the long-acting beta-agonist visits also included steroid use. Thus, this result may be due to the addition of steroids to the bronchodilator therapy. While this pattern is even greater in the study of beta-agonist subtype in patients receiving sirolimus (87.5% of use of short- and long-acting beta-agonists is in conjunction with steroids versus 42.9% for long-acting beta-agonists plus steroids), the total number of long-acting beta-agonist visits (35 visits) is far fewer than that of both short- and long-acting beta-agonists (104 visits) and may not accurately reflect the effect on pulmonary function of long-acting beta-agonists alone. Late-stage LAM lungs have been shown to have chronic inflammation (11), and so a combination of beta-agonists to reduce airway obstruction plus a corticosteroid may be appropriate in some cases of LAM. Chronic inflammation in chronic obstructive pulmonary disease may be resistant to corticosteroids (67); peripheral blood mononuclear cells from these patients showed increased mTOR activity (68). Treatment of these cells with sirolimus restored sensitivity to corticosteroids (68), showing that the choice of bronchodilator to use with sirolimus may be important.
We have shown here that beta-agonists affect phosphorylation of S6 and proliferation of LAM cells in both the presence and absence of sirolimus. We have shown in a retrospective study that the type of beta-agonist used may affect the stability of pulmonary function over time. Since use of a bronchodilator is important for the quality of life of a patient who has LAM, selection of a bronchodilator, such as a short-acting beta-agonist versus long-acting beta-agonist, or use of alternative bronchodilator therapy, including steroids, should be evaluated in a controlled clinical trial.
Materials and Methods
Sources of antibodies and reagents are given in SI Materials and Methods, along with details of the study population, pulmonary function testing, and statistical analysis. The protocol was approved by the National Heart, Lung, and Blood Institute Institutional Review Board (NHLBI protocols 95-H-0186 and 96-H-0100), and written informed consent was obtained from all participants. Culture of human TSC2+/− skin fibroblasts and LAM cells from explanted lungs, as well as confocal immunofluorescence microscopy and Western blotting, was performed as described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Drs. Daniela Malide and Christian Combs (Light Microscopy Core Facility, National Heart, Lung, and Blood Institute) for their assistance with confocal microscopy. This research was supported by the Intramural Research Program, NIH, NHLBI.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1719960115/-/DCSupplemental.
References
- 1.Matsui K, et al. Extrapulmonary lymphangioleiomyomatosis (LAM): Clinicopathologic features in 22 cases. Hum Pathol. 2000;31:1242–1248. doi: 10.1053/hupa.2000.18500. [DOI] [PubMed] [Google Scholar]
- 2.Ferrans VJ, et al. Lymphangioleiomyomatosis (LAM): A review of clinical and morphological features. J Nippon Med Sch. 2000;67:311–329. doi: 10.1272/jnms.67.311. [DOI] [PubMed] [Google Scholar]
- 3.McCormack FX. Lymphangioleiomyomatosis: A clinical update. Chest. 2008;133:507–516. doi: 10.1378/chest.07-0898. [DOI] [PubMed] [Google Scholar]
- 4.Taveira-DaSilva AM, Moss J. Management of lymphangioleiomyomatosis. F1000Prime Rep. 2014;6:116. doi: 10.12703/P6-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taveira-DaSilva AM, Steagall WK, Moss J. Lymphangioleiomyomatosis. Cancer Contr. 2006;13:276–285. doi: 10.1177/107327480601300405. [DOI] [PubMed] [Google Scholar]
- 6.Taylor JR, Ryu J, Colby TV, Raffin TA. Lymphangioleiomyomatosis. Clinical course in 32 patients. N Engl J Med. 1990;323:1254–1260. doi: 10.1056/NEJM199011013231807. [DOI] [PubMed] [Google Scholar]
- 7.Kitaichi M, Nishimura K, Itoh H, Izumi T. Pulmonary lymphangioleiomyomatosis: A report of 46 patients including a clinicopathologic study of prognostic factors. Am J Respir Crit Care Med. 1995;151:527–533. doi: 10.1164/ajrccm.151.2.7842216. [DOI] [PubMed] [Google Scholar]
- 8.Chu SC, et al. Comprehensive evaluation of 35 patients with lymphangioleiomyomatosis. Chest. 1999;115:1041–1052. doi: 10.1378/chest.115.4.1041. [DOI] [PubMed] [Google Scholar]
- 9.Taveira-DaSilva AM, et al. Reversible airflow obstruction in lymphangioleiomyomatosis. Chest. 2009;136:1596–1603. doi: 10.1378/chest.09-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson SR, et al. Review Panel of the ERS LAM Task Force European Respiratory Society guidelines for the diagnosis and management of lymphangioleiomyomatosis. Eur Respir J. 2010;35:14–26. doi: 10.1183/09031936.00076209. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi T, et al. Bronchial involvement in advanced stage lymphangioleiomyomatosis: Histopathologic and molecular analyses. Hum Pathol. 2016;50:34–42. doi: 10.1016/j.humpath.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 12.McCormack FX, Travis WD, Colby TV, Henske EP, Moss J. Lymphangioleiomyomatosis: Calling it what it is: A low-grade, destructive, metastasizing neoplasm. Am J Respir Crit Care Med. 2012;186:1210–1212. doi: 10.1164/rccm.201205-0848OE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smolarek TA, et al. Evidence that lymphangiomyomatosis is caused by TSC2 mutations: Chromosome 16p13 loss of heterozygosity in angiomyolipomas and lymph nodes from women with lymphangiomyomatosis. Am J Hum Genet. 1998;62:810–815. doi: 10.1086/301804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carsillo T, Astrinidis A, Henske EP. Mutations in the tuberous sclerosis complex gene TSC2 are a cause of sporadic pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci USA. 2000;97:6085–6090. doi: 10.1073/pnas.97.11.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu J, Astrinidis A, Henske EP. Chromosome 16 loss of heterozygosity in tuberous sclerosis and sporadic lymphangiomyomatosis. Am J Respir Crit Care Med. 2001;164:1537–1540. doi: 10.1164/ajrccm.164.8.2104095. [DOI] [PubMed] [Google Scholar]
- 16.Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355:1345–1356. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- 17.Dibble CC, et al. TBC1D7 is a third subunit of the TSC1-TSC2 complex upstream of mTORC1. Mol Cell. 2012;47:535–546. doi: 10.1016/j.molcel.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jewell JL, Russell RC, Guan KL. Amino acid signalling upstream of mTOR. Nat Rev Mol Cell Biol. 2013;14:133–139. doi: 10.1038/nrm3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosner M, Hanneder M, Siegel N, Valli A, Hengstschläger M. The tuberous sclerosis gene products hamartin and tuberin are multifunctional proteins with a wide spectrum of interacting partners. Mutat Res. 2008;658:234–246. doi: 10.1016/j.mrrev.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Peterson TR, et al. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 2009;137:873–886. doi: 10.1016/j.cell.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Zoncu R, Efeyan A, Sabatini DM. mTOR: From growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010;40:310–322. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gressner AM, Wool IG. The phosphorylation of liver ribosomal proteins in vivo. Evidence that only a single small subunit protein (S6) is phosphorylated. J Biol Chem. 1974;249:6917–6925. [PubMed] [Google Scholar]
- 25.Sarbassov DD, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 26.Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253:905–909. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- 28.Huang S, Bjornsti MA, Houghton PJ. Rapamycins: Mechanism of action and cellular resistance. Cancer Biol Ther. 2003;2:222–232. doi: 10.4161/cbt.2.3.360. [DOI] [PubMed] [Google Scholar]
- 29.McCormack FX, et al. National Institutes of Health Rare Lung Diseases Consortium; MILES Trial Group Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med. 2011;364:1595–1606. doi: 10.1056/NEJMoa1100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young L, et al. MILES Trial Group Serum VEGF-D a concentration as a biomarker of lymphangioleiomyomatosis severity and treatment response: A prospective analysis of the Multicenter International Lymphangioleiomyomatosis Efficacy of Sirolimus (MILES) trial. Lancet Respir Med. 2013;1:445–452. doi: 10.1016/S2213-2600(13)70090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bissler JJ, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med. 2008;358:140–151. doi: 10.1056/NEJMoa063564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taveira-DaSilva AM, Hathaway O, Stylianou M, Moss J. Changes in lung function and chylous effusions in patients with lymphangioleiomyomatosis treated with sirolimus. Ann Intern Med. 2011;154:797–805. doi: 10.1059/0003-4819-154-12-201106210-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krizanova O, Babula P, Pacak K. Stress, catecholaminergic system and cancer. Stress. 2016;19:419–428. doi: 10.1080/10253890.2016.1203415. [DOI] [PubMed] [Google Scholar]
- 34.Le CP, et al. Chronic stress in mice remodels lymph vasculature to promote tumour cell dissemination. Nat Commun. 2016;7:10634. doi: 10.1038/ncomms10634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taveira-DaSilva AM, et al. Reversible airflow obstruction, proliferation of abnormal smooth muscle cells, and impairment of gas exchange as predictors of outcome in lymphangioleiomyomatosis. Am J Respir Crit Care Med. 2001;164:1072–1076. doi: 10.1164/ajrccm.164.6.2102125. [DOI] [PubMed] [Google Scholar]
- 36.Goncharova EA, et al. Tuberin regulates p70 S6 kinase activation and ribosomal protein S6 phosphorylation. A role for the TSC2 tumor suppressor gene in pulmonary lymphangioleiomyomatosis (LAM) J Biol Chem. 2002;277:30958–30967. doi: 10.1074/jbc.M202678200. [DOI] [PubMed] [Google Scholar]
- 37.Wallukat G. The beta-adrenergic receptors. Herz. 2002;27:683–690. doi: 10.1007/s00059-002-2434-z. [DOI] [PubMed] [Google Scholar]
- 38.Francis SH, Corbin JD. Cyclic nucleotide-dependent protein kinases: Intracellular receptors for cAMP and cGMP action. Crit Rev Clin Lab Sci. 1999;36:275–328. doi: 10.1080/10408369991239213. [DOI] [PubMed] [Google Scholar]
- 39.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsumoto Y, et al. Markers of cell proliferation and expression of melanosomal antigen in lymphangioleiomyomatosis. Am J Respir Cell Mol Biol. 1999;21:327–336. doi: 10.1165/ajrcmb.21.3.3693. [DOI] [PubMed] [Google Scholar]
- 41.Magnuson B, Ekim B, Fingar DC. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. Biochem J. 2012;441:1–21. doi: 10.1042/BJ20110892. [DOI] [PubMed] [Google Scholar]
- 42.Pérez Piñero C, Bruzzone A, Sarappa MG, Castillo LF, Lüthy IA. Involvement of α2- and β2-adrenoceptors on breast cancer cell proliferation and tumour growth regulation. Br J Pharmacol. 2012;166:721–736. doi: 10.1111/j.1476-5381.2011.01791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kwon G, Marshall CA, Pappan KL, Remedi MS, McDaniel ML. Signaling elements involved in the metabolic regulation of mTOR by nutrients, incretins, and growth factors in islets. Diabetes. 2004;53:S225–S232. doi: 10.2337/diabetes.53.suppl_3.s225. [DOI] [PubMed] [Google Scholar]
- 44.Blancquaert S, et al. cAMP-dependent activation of mammalian target of rapamycin (mTOR) in thyroid cells. Implication in mitogenesis and activation of CDK4. Mol Endocrinol. 2010;24:1453–1468. doi: 10.1210/me.2010-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Joussineau C, et al. mTOR pathway is activated by PKA in adrenocortical cells and participates in vivo to apoptosis resistance in primary pigmented nodular adrenocortical disease (PPNAD) Hum Mol Genet. 2014;23:5418–5428. doi: 10.1093/hmg/ddu265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu D, et al. Activation of mTORC1 is essential for β-adrenergic stimulation of adipose browning. J Clin Invest. 2016;126:1704–1716. doi: 10.1172/JCI83532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Engh RA, Girod A, Kinzel V, Huber R, Bossemeyer D. Crystal structures of catalytic subunit of cAMP-dependent protein kinase in complex with isoquinolinesulfonyl protein kinase inhibitors H7, H8, and H89. Structural implications for selectivity. J Biol Chem. 1996;271:26157–26164. doi: 10.1074/jbc.271.42.26157. [DOI] [PubMed] [Google Scholar]
- 48.Lochner A, Moolman JA. The many faces of H89: A review. Cardiovasc Drug Rev. 2006;24:261–274. doi: 10.1111/j.1527-3466.2006.00261.x. [DOI] [PubMed] [Google Scholar]
- 49.Murray AJ. Pharmacological PKA inhibition: All may not be what it seems. Sci Signal. 2008;1:re4. doi: 10.1126/scisignal.122re4. [DOI] [PubMed] [Google Scholar]
- 50.Uhler MD, et al. Isolation of cDNA clones coding for the catalytic subunit of mouse cAMP-dependent protein kinase. Proc Natl Acad Sci USA. 1986;83:1300–1304. doi: 10.1073/pnas.83.5.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uhler MD, Chrivia JC, McKnight GS. Evidence for a second isoform of the catalytic subunit of cAMP-dependent protein kinase. J Biol Chem. 1986;261:15360–15363. [PubMed] [Google Scholar]
- 52.Turnham RE, Scott JD. Protein kinase A catalytic subunit isoform PRKACA; History, function and physiology. Gene. 2016;577:101–108. doi: 10.1016/j.gene.2015.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beebe SJ, et al. Molecular cloning of a tissue-specific protein kinase (C gamma) from human testis–Representing a third isoform for the catalytic subunit of cAMP-dependent protein kinase. Mol Endocrinol. 1990;4:465–475. doi: 10.1210/mend-4-3-465. [DOI] [PubMed] [Google Scholar]
- 54.Skålhegg BS, et al. Mutation of the Calpha subunit of PKA leads to growth retardation and sperm dysfunction. Mol Endocrinol. 2002;16:630–639. doi: 10.1210/mend.16.3.0793. [DOI] [PubMed] [Google Scholar]
- 55.Cao Y, et al. Activating hotspot L205R mutation in PRKACA and adrenal Cushing’s syndrome. Science. 2014;344:913–917. doi: 10.1126/science.1249480. [DOI] [PubMed] [Google Scholar]
- 56.Moore CE, Xie J, Gomez E, Herbert TP. Identification of cAMP-dependent kinase as a third in vivo ribosomal protein S6 kinase in pancreatic beta-cells. J Mol Biol. 2009;389:480–494. doi: 10.1016/j.jmb.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 57.January B, et al. Beta2-adrenergic receptor desensitization, internalization, and phosphorylation in response to full and partial agonists. J Biol Chem. 1997;272:23871–23879. doi: 10.1074/jbc.272.38.23871. [DOI] [PubMed] [Google Scholar]
- 58.Tran TM, et al. Characterization of agonist stimulation of cAMP-dependent protein kinase and G protein-coupled receptor kinase phosphorylation of the beta2-adrenergic receptor using phosphoserine-specific antibodies. Mol Pharmacol. 2004;65:196–206. doi: 10.1124/mol.65.1.196. [DOI] [PubMed] [Google Scholar]
- 59.Shenoy SK, McDonald PH, Kohout TA, Lefkowitz RJ. Regulation of receptor fate by ubiquitination of activated beta 2-adrenergic receptor and beta-arrestin. Science. 2001;294:1307–1313. doi: 10.1126/science.1063866. [DOI] [PubMed] [Google Scholar]
- 60.Sette C, Conti M. Phosphorylation and activation of a cAMP-specific phosphodiesterase by the cAMP-dependent protein kinase. Involvement of serine 54 in the enzyme activation. J Biol Chem. 1996;271:16526–16534. doi: 10.1074/jbc.271.28.16526. [DOI] [PubMed] [Google Scholar]
- 61.Sette C, Iona S, Conti M. The short-term activation of a rolipram-sensitive, cAMP-specific phosphodiesterase by thyroid-stimulating hormone in thyroid FRTL-5 cells is mediated by a cAMP-dependent phosphorylation. J Biol Chem. 1994;269:9245–9252. [PubMed] [Google Scholar]
- 62.Omori K, Kotera J. Overview of PDEs and their regulation. Circ Res. 2007;100:309–327. doi: 10.1161/01.RES.0000256354.95791.f1. [DOI] [PubMed] [Google Scholar]
- 63.Gettys TW, Blackmore PF, Redmon JB, Beebe SJ, Corbin JD. Short-term feedback regulation of cAMP by accelerated degradation in rat tissues. J Biol Chem. 1987;262:333–339. [PubMed] [Google Scholar]
- 64.Oki N, Takahashi SI, Hidaka H, Conti M. Short term feedback regulation of cAMP in FRTL-5 thyroid cells. Role of PDE4D3 phosphodiesterase activation. J Biol Chem. 2000;275:10831–10837. doi: 10.1074/jbc.275.15.10831. [DOI] [PubMed] [Google Scholar]
- 65.Barnes PJ. Distribution of receptor targets in the lung. Proc Am Thorac Soc. 2004;1:345–351. doi: 10.1513/pats.200409-045MS. [DOI] [PubMed] [Google Scholar]
- 66.Johnston SL, Edwards MR. Mechanisms of adverse effects of beta-agonists in asthma. Thorax. 2009;64:739–741. doi: 10.1136/thx.2009.119230. [DOI] [PubMed] [Google Scholar]
- 67.To Y, et al. Targeting phosphoinositide-3-kinase-delta with theophylline reverses corticosteroid insensitivity in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;182:897–904. doi: 10.1164/rccm.200906-0937OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mitani A, Ito K, Vuppusetty C, Barnes PJ, Mercado N. Restoration of corticosteroid sensitivity in chronic obstructive pulmonary disease by inhibition of mammalian target of rapamycin. Am J Respir Crit Care Med. 2016;193:143–153. doi: 10.1164/rccm.201503-0593OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.