Mosquitoes are often referred to as the deadliest animals on Earth because of the devastating pathogens they are able to transmit when females bite and then feed on blood from human hosts (male mosquitoes don’t bite). In 2015 alone there were an estimated 212 million cases of malaria, resulting in 429,000 deaths (1). Approximately one-third of Earth’s population is considered at risk for infection by the dengue virus (2). Furthermore, the rapid emergence and global spread of mosquito-borne viruses, such as West Nile, Zika, and chikungunya, are of increasing public health concern (3, 4). Because effective vaccines and drug therapies are not available for the majority of these mosquito-borne pathogens, efforts to reduce disease transmission have traditionally focused on suppressing or eliminating the mosquito vector, usually by reducing larval habitats (source reduction) or applying insecticides. However, the effectiveness of these traditional approaches is limited by the proliferation of man-made habitats (e.g., discarded tires and cisterns), the rapid geographic spread of vector species associated with human commerce and travel, and the evolution of insecticide resistance. Novel approaches to control are desperately needed. Recently, a variety of exciting strategies to disrupt disease transmission have emerged based on genetic modification of vectors or infection of vectors with bacterial symbionts (5, 6). These strategies seek to either suppress vector populations to sufficiently low numbers that pathogen transmission cannot be sustained (population suppression), or to introduce and spread genetic modifications or bacterial symbiont infections through natural populations so the mosquitoes are incapable of transmitting pathogens (population replacement). Current population replacement strategies focus on preventing the mosquito from transmitting a pathogen once it has already taken a bite and ingested blood. In PNAS, Bradshaw et al. (7) establish the foundation of an intriguing alternative approach based on the potent logic that mosquitoes that don’t bite cannot transmit disease.
Nonbiting Mosquitoes Have Evolved from Biting Ancestors Multiple Times in Nature
The benefit of blood feeding (biting) by female mosquitoes is obvious; blood provides a rich and abundant nutritional resource that can be allocated to egg production. The costs of blood feeding are less well appreciated, but likely substantial. A blood-feeding (biting) mosquito must allocate sensory and energetic resources to locate a host and then survive while feeding. Additionally, ingesting warm blood elicits a protective heat-shock response in several species of mosquitoes (8), and blood digestion produces toxic by-products that must be sequestered or metabolically processed (9). In fact, three genera of mosquitoes are entirely nonblood feeding (Malaya, Topomyia, and Toxorhynchites), and nonblood-feeding species occur in at least eight additional mosquito genera containing mostly blood-feeding species (10, 11). Thus, nonblood-feeding (nonbiting) mosquitoes have evolved from biting ancestors multiple times independently in nature. In most cases this evolutionary transformation must require, at least in part, that resources for female reproduction are acquired at the larval rather than adult stage. However, the physiological and molecular mechanisms underlying the evolutionary transition from a biting to nonbiting lifestyle in mosquitoes remain largely unresolved (but see ref. 12).
The Pitcher Plant Mosquito, Wyeomyia smithii
W. smithii presents a particularly powerful experimental system to determine the molecular mechanisms underlying the evolution of a nonbiting lifestyle in mosquitoes. W. smithii is not a vector, but it is the only known mosquito species in which some populations opportunistically blood-feed (bite) while other geographically disparate and genetically distinct populations are obligate nonbiters. This difference is apparent under common garden conditions and thus is genetically based. The fact that this profound evolutionary transformation can be studied within a single species is important because it means that classical genetics approaches can be applied to determine the cause of differences in biting behavior. Furthermore, the comparison between populations of the same species reduces the confounding effects of divergence over longer time scales between different species. Diverse evidence indicates that W. smithii has evolved from south to north throughout its range in eastern North America (13). In southern (ancestral) populations, females emerge as adults with undeveloped ovaries, are able to produce one egg batch without blood feeding but require a blood meal for all subsequent egg batches, and exhibit variation in propensity to bite. In contrast, females from northern (derived) populations emerge with partially developed ovaries, possess biting mouthparts but will not bite when offered a blood-meal host, and can produce multiple egg batches without a blood meal (14). Nevertheless, all populations are fully interfertile (15). Thus, the evolutionary divergence in biting behavior between southern and northern populations of W. smithii represents a natural experiment that has occurred over millennia. However, southern and northern populations that exhibit genetically based differences in biting behavior also differ genetically for additional reasons, including natural selection on other phenotypes, the evolution of genetically correlated traits, and random differences caused by genetic drift. Therefore, the central challenge addressed by Bradshaw et al.’s (7) work is to identify genetic differences between southern and northern populations that are directly related to differences in biting behavior.
The Transcriptional Basis of Evolutionary Divergence in Nature
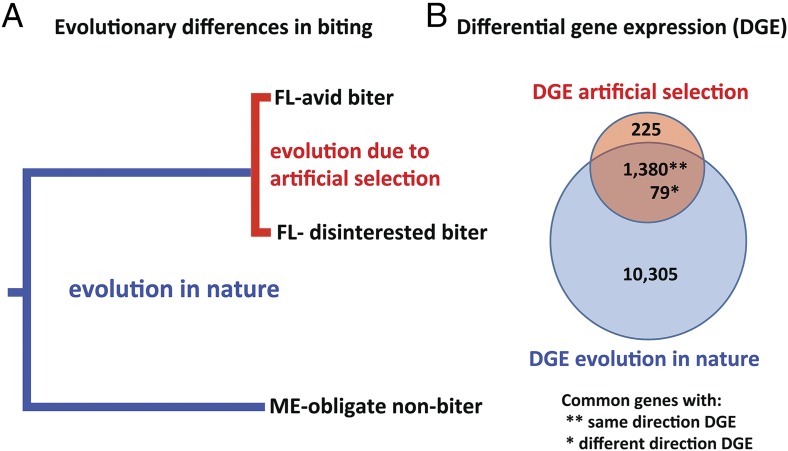
The experimental approach of Bradshaw et al.’s (7) study is illustrated in Fig. 1. The strategy was to perform artificial selection on a southern population that exhibited variation in biting behavior to recapitulate differences that have evolved in nature between a biting population from Florida and an obligately nonbiting population from Maine (ME-obligate nonbiter) (Fig. 1A). To do so, the authors selected from the behaviorally polymorphic Florida population a line of avid biters. Bradshaw et al. then quantified differential gene expression (DGE) in the presence of a blood-meal host. They measured DGE between mosquitoes from the artificially selected biting line that initiated biting (FLavid) and mosquitoes from the unselected Florida laboratory colony that exhibited no biting behavior (Florida-disinterested, or “FLdis”). The authors also compared DGE under the same conditions between FLavid and ME-obligate nonbiters (Fig. 1B). Because DGE was measured in presence of the blood-feeding host but before blood was actually ingested, the gene-expression differences are expected to reflect anticipatory elements of the blood-feeding (biting) response.
Fig. 1.
Experimental approach using artificial selection to determine the gene-expression differences contributing to naturally evolved differences in biting behavior of the pitcher plant mosquito, W. smithii. (A) Cladogram representing evolutionary relationships among populations. (B) Venn diagram representing DGE between FL avid biters vs. FL disinterested biters (DGE artificial selection) and between FL avid biter vs. ME-obligate nonbiter (DGE evolution in nature). See text for details.
As expected based on relative divergence times, more genes were significantly differentially expressed between the naturally evolved FLavid and ME-obligate nonbiting mosquitoes (11,764) than between the artificially selected FLavid and FLdis mosquitoes (1,684). Surprisingly, 87% of the genes that were differentially expressed between the artificially selected mosquitoes (FLavid vs. FLdis) were also differentially expressed between the naturally diverged mosquitoes (FLavid vs. ME-obligate nonbiting). An even more stunning result is that 95% (1,380) of these overlapping differentially expressed genes show DGE in the same direction with strong positive association in the magnitude of DGE (Fig. 1B). These results provide powerful evidence for genetic parallelism, indicating a common molecular basis underlying the differences in biting behavior caused by artificial selection in the laboratory and evolution in the wild. In addition to identifying candidate genes affecting biting behavior, this genetic parallelism is compelling evidence that the evolution of obligate nonbiting in the Maine population was the result of natural selection.
Anticipatory Costs in Biters and Metabolic Flexibility in Nonbiters
Pathway enrichment analysis indicates that relative to nonbiting females, biting females transcriptionally up-regulate several physiological processes with clear functional significance to blood feeding, even before blood is actually ingested. These processes include protein degradation, mRNA processing, mRNA translation, cell proliferation, and ovarian development. These results emphasize that metabolic costs of blood-feeding (biting) are incurred before the point at which blood is actually ingested, and are provocative because they stimulate questions about additional physiological costs of blood-feeding that may occur during other stages of the life cycle that have not previously been considered. In contrast to biting females, the transcriptional response of nonbiting females appears to involve a shift toward intermediary metabolites associated with energy utilization and storage, but no clear commitment to any single pathway. For example, the pyruvate metabolism pathway was enriched for genes up-regulated in nonbiters, including genes related to the conversion of pyruvate to Acetyl-CoA. However, genes directly linking Acetyl-CoA to the citric acid cycle or fatty acid metabolism were not differentially expressed. Bradshaw et al. (7) interpret these results to represent “flexible metabolic opportunism in nonbiting females,” which could be another factor favoring the evolution of a nonblood-feeding (nonbiting) lifestyle.
Implications and Future Directions
Reducing the devastating impact of vector-borne diseases remains an urgent challenge. The innovative study by Bradshaw et al. (7) establishes the foundation for a novel approach to addressing this challenge based on the simple but powerful rationale that nonbiting mosquitoes cannot transmit pathogens. Their study establishes a powerful experimental paradigm for combining artificial selection and genomics to identify the genetic basis of evolutionary divergence among natural populations for biting, a behavior that is essential for disease transmission in mosquitoes. Furthermore, Bradshaw et al.’s results provide detailed insight into the molecular pathways acted on by natural selection in wild populations that contribute to the evolutionary transformation from a blood-feeding to obligately nonbiting lifestyle. Nevertheless, significant challenges remain. One is to determine whether homologous genes exist and have similar effects in important vector species. With rapidly advancing genomic technologies, including the increasing availability of well-assembled and annotated mosquito genomes and exciting progress utilizing CRISPR/Cas9 in mosquitoes, this challenge should be readily surmountable. Another important goal is to identify upstream regulatory genes that affect host localization and the initiation of biting behavior. Interrogating physiological processes that might be important at other life stages, such as larval resource acquisition and allocation, will also be important. Bradshaw et al.’s intriguing study is sure to inspire further research that will drive advances in our understanding of fundamental physiological and behavioral processes that can be used to develop novel approaches for combating vector-borne diseases. Their study raises the tantalizing prospect that one of these novel approaches might be to manipulate natural vector populations to favor the evolution of a nonbiting lifestyle, a transition that has already occurred multiple times in nature.
Footnotes
The author declares no conflict of interest.
See companion article on page 1009.
References
- 1.World Health Organization . World Malaria Report 2016. World Health Organization; Geneva: 2016. [Google Scholar]
- 2.Bhatt S, et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kilpatrick AM, Randolph SE. Drivers, dynamics, and control of emerging vector-borne zoonotic diseases. Lancet. 2012;380:1946–1955. doi: 10.1016/S0140-6736(12)61151-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fauci AS, Morens DM. Zika virus in the Americas—Yet another arbovirus threat. N Engl J Med. 2016;374:601–604. doi: 10.1056/NEJMp1600297. [DOI] [PubMed] [Google Scholar]
- 5.McGraw EA, O’Neill SL. Beyond insecticides: New thinking on an ancient problem. Nat Rev Microbiol. 2013;11:181–193. doi: 10.1038/nrmicro2968. [DOI] [PubMed] [Google Scholar]
- 6.Wang S, et al. Driving mosquito refractoriness to Plasmodium falciparum with engineered symbiotic bacteria. Science. 2017;357:1399–1402. doi: 10.1126/science.aan5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradshaw WE, et al. Evolutionary transition from blood feeding to obligate nonbiting in a mosquito. Proc Natl Acad Sci USA. 2018;115:1009–1014. doi: 10.1073/pnas.1717502115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benoit JB, et al. Drinking a hot blood meal elicits a protective heat shock response in mosquitoes. Proc Natl Acad Sci USA. 2011;108:8026–8029. doi: 10.1073/pnas.1105195108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graça-Souza AV, et al. Adaptations against heme toxicity in blood-feeding arthropods. Insect Biochem Mol Biol. 2006;36:322–335. doi: 10.1016/j.ibmb.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Clements AN. The Physiology of Mosquitoes. Pergamon; Oxford, UK: 1963. [Google Scholar]
- 11.Clements AN. The Biology of Mosquitoes. Vol. 1. Development, Nutrition, and Reproduction. Chapman and Hall; London: 1992. [Google Scholar]
- 12.Gulia-Nuss M, Elliot A, Brown MR, Strand MR. Multiple factors contribute to anautogenous reproduction by the mosquito Aedes aegypti. J Insect Physiol. 2015;82:8–16. doi: 10.1016/j.jinsphys.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merz C, et al. Replicate phylogenies and post-glacial range expansion of the pitcher-plant mosquito, Wyeomyia smithii, in North America. PLoS One. 2013;8:e72262. doi: 10.1371/journal.pone.0072262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Meara GF. Interactions in mosquitoes: Kicking the blood-feeding habit. Fla Entomol. 1985;68:122–133. [Google Scholar]
- 15.Armbruster P, Bradshaw WE, Holzapfel CM. Evolution of the genetic architecture underlying fitness in the pitcher- plant mosquito, Wyeomyia smithii. Evolution. 1997;51:451–458. doi: 10.1111/j.1558-5646.1997.tb02432.x. [DOI] [PubMed] [Google Scholar]