ABSTRACT
The protein composition of an Outer Membrane Vesicle (OMV) preparation that constitutes the active pharmaceutical ingredient of VA-MENGOC-BC®, an effective vaccine against Neisseria meningitidis serogroups B, and C is presented. This preparation has a high lipid content and five abundant membrane proteins (FetA, PorA, PorB, RmpM, and Opc), constituting approximately 70% of the total protein mass. The protein composition was determined by combining the use of the Hexapeptide Ligand Library and an orthogonal tandem fractionation of tryptic peptides by reverse-phase chromatography at alkaline and acid pH. This approach equalizes the concentration of tryptic peptides derived from low- and high-abundance proteins as well as considerably simplifying the number of peptides analyzed by LC-MS/MS, enhancing the possibility of identifying low-abundance species. Fifty-one percent of the proteins originally annotated as membrane proteins in the genome of the MC58 strain were identified. One hundred and sixty-eight low-abundance cytosolic proteins presumably occluded within OMV were also identified. Four (NadA, NUbp, GNA2091, and fHbp), out of the five antigens constituting the Bexsero® vaccine, were detected in this OMV preparation. In particular, fHbp is also the active principle of the Trumenba® vaccine developed by Pfizer. The HpuA and HpuB gene products (not annotated in the MC58 genome) were identified in the CU385 strain, a clinical isolate that is used to produce this OMV. Considering the proteins identified here and previous work done by our group, the protein catalogue of this OMV preparation was extended to 266 different protein species.
KEYWORDS: OMV, vaccine, Neisseria meningitidis, peptide library, equalization, proteomics, dynamic concentration range, VAMENGO BC
Introduction
Meningococcal infectious disease is still a cause of morbidity and mortality worldwide. Six serogroups (A, B, C, W, X, and Y) defined by the capsular polysaccharide, are mainly responsible for the cases reported globally.1 Two age-related peaks of the disease have been documented, with the first peak in children under 1 year of age and a second peak in adolescents and young adults (15 to 25 years old).2 Although the incidence of meningococcal disease changes according to the geographical area, the overall incidence was of 1.2 million cases per year, with about 135,000 deaths worldwide.3
While successful vaccines based on this immunogen have been developed against four meningococcus serogroups (A, C, Y, W135), serogroup B polysaccharide resembles certain native human molecules and that makes it poorly immunogenic, therefore limiting its possible use for vaccine production.4
The vaccines against Neisseria meningitidis in humans include those of Outer Membrane Vesicles (OMV), which are of particular importance against meningococcal serogroup B. Although the OMV vaccines were studied for the first time in humans at the end of the 1970´s, during the 1990´s they were tested in several efficacy clinical trials in Cuba, Norway, Brazil and Chile.5 The first-generation vaccines based on OMV were able to contain strain-specific epidemics.6 Following the same approach, in the first five years of this century a strain-specific vaccine was developed and used to control an epidemic of group B meningococcal disease in New Zealand.7 Because immune responses were induced against hypervariable membrane proteins, these vaccines showed limited results in settings where heterologous strains were also circulating.8 This limitation stressed the need to work on vaccines prepared from immunogenic and conserved membrane proteins to achieve more extensive protection. Although several candidates have been studied, OMV is still an important constituent for vaccine preparations. For example, the 5-component meningococcal serogroup B Bexsero® vaccine that includes OMV from the NZ98/254 strain was authorized by regulatory agencies in several regions.6 Results obtained with this vaccine are promising, but not enough to guarantee a complete coverage of the circulating strains.9
VAMENGOC-BC® was the first highly effective OMV-based vaccine10 against N. meningitidis serogroup B, and since it was licensed in Cuba in 1989 it has been applied in more than 17 countries, mainly in Latin America and the Caribbean.11 Here, the OMV obtained by a deoxycholate extraction process are combined with serogroup C polysaccharide and absorbed onto aluminum hydroxide gel with phosphates, sodium chloride and 0.01% thimerosal as the preservative.12
This OMV preparation was produced at the Finlay Institute in Havana using the CU385 strain that was originally obtained from a clinical isolate in the Cuban epidemic outbreak in 1987.10,12 The active pharmaceutical ingredient is a preparation enriched in membrane proteins (MPs) and lipids.10,11
Because it is a detergent extracted OMV-based vaccine, the protective activity induced by this preparation is mainly targeted against homologous strains.10,13–15 Nevertheless, some cross-reactive immune responses can be observed in clinical trials with this vaccine candidate, which are presumably induced by minor proteins. This hypothesis is supported by the finding of Williams et al (2014),16 who observed this type of cross-response against proteins other than the major antigens of meningococcus.
Therefore, our group has been systematically applying different proteomic tools to identify low-abundance proteins present in the OMV-based vaccine VAMENGOC-BC®. Additionally, the identification of low-abundance proteins of immunological relevance could be used to establish quality control indicators for the vaccine, and to develop vaccines based on specific compositions after considering their sequence conservation in clinical isolates.
In spite of the well-known limitations of two-dimensional gel electrophoresis (2DE-PAGE) for identifying MPs17,18, our group initially applied this technique combined with ESI-MS/MS to identify the proteins present in the VA-MENGOC-BC® as well as to demonstrate the reproducibility of the batch-to-batch production process.19 Seventy-eight spots were processed, but only 31 non-redundant proteins were identified.
Additionally, a method developed in our laboratory named DF-PAGE20 was applied to identify the proteins found in this OMV-based vaccine. This method, based on dual fractionation (DF) of proteins and peptides by polyacrylamide gel electrophoresis (PAGE), enabled the identification of 97 different proteins.20
A gel-free method called SCAPE (Selective CApture of PEptides)21 developed for 2DE-free proteome studies, was also applied to the characterization of this OMV preparation22 and 106 non-redundant proteins were identified, including thirty-one, which were annotated or predicted to be membrane proteins.22
Overlapping the information obtained in terms of protein identification, after applying the 2DE, DF-PAGE and SCAPE methods to the characterization of the OMV preparation, a total of 160 proteins were identified in this preparation.
None of the above mentioned methods can decrease the wide dynamic protein concentration range of this OMV preparation and its derived peptides. In fact, this is a serious limitation in identifying low-abundance proteins.
Hexapeptide ligand libraries (HPLL)23 were applied in proteomics with excellent results in reducing the wide dynamic concentration range of soluble proteins24 found in fluids such as serum,25 bile,26 urine27 and other complex biological samples.28–31 The application of HPLL to study a mixture of membrane proteins has not been reported, despite the importance of this sub-proteome for discovering new receptors and vaccine candidates. This application may not have been explored deeply because the buffers required for solubilizing MPs contain detergents and chaotropic agents that could interfere with the protein-HPLL interactions required for an effective equalization. The equalization of proteolytic peptides derived from a mixture with high dynamic protein concentration range in peptide-based proteomics has not yet been published.
Fractionation of proteolytic peptides with orthogonal chromatographic techniques has been used before mass spectrometric (MS) analysis to increase protein identification.32–34 RP-HPLC eliminates the salts that interfere with the efficiency of electrospray ionization and it also has excellent resolving power for separating complex peptide mixtures. The combination of two consecutive steps of RP fractionation at alkaline and acid pH (RP OH−/H+) has been underexploited in spite of the wide difference between the selectivity for separating peptides in each chromatographic step.35,36
For all these reasons, we decided to combine the use of HPLL with tandem orthogonal fractionation by reverse-phase chromatography at alkaline/acid pH to extend the catalogue of proteins found in an OMV preparation that constitutes the active pharmaceutical ingredient of VA-MENGOC-BC®.
Results
General strategy for extending the protein catalogue of an OMV preparation obtained from the clinical isolate CU385 of N. meningitidis serogroup B
The SDS-PAGE analysis (Fig. 1, OMV lane) of this OMV revealed the presence of five major membrane proteins identified as: FetA, PorA, PorB, RmpM, and OpC.19 They represent nearly 70% of the total protein mass estimated by gel densitometry scanning. Also, Fig. 1 shows a considerable number of other faint non-assigned bands distributed across the entire separation range. LC-MS/MS analysis of the tryptic peptides derived from this OMV preparation after reduction and S-alkylation identified the above-mentioned proteins with very high sequence coverage (60-80%, data not shown). This conventional approach did not yield new protein identification, considering the previous results of our group.19–22
Figure 1.
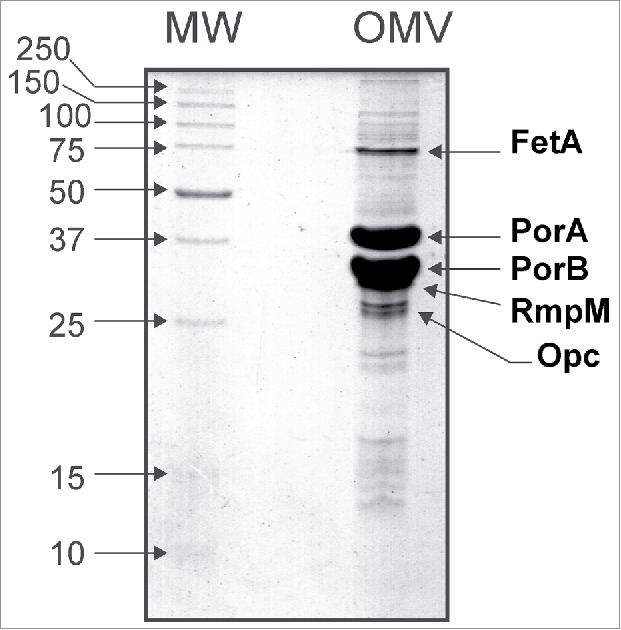
SDS-PAGE analysis of the Outer Membrane Vesicles (OMV) of Neisseria meningitidis serogroup B. The most abundant membrane proteins (FetA, PorA, PorB, RmpM and Opc) representing approximately 70% of the total protein mass in the sample analyzed are indicated.
To extend the protein catalogue of this OMV preparation we used the general strategy (HPLL&RP(OH−/H+)) shown in Fig. 2. The proteolytic peptides derived from this OMV were desalted, divided into four identical parts, and lyophilized. Two of them were dissolved in sodium acetate buffer and the remaining in Tris/HCl buffer. Two of these parts containing the peptide mixture dissolved in two different buffers were directly separated by RP at basic pH (RP/OH−), collected in 20 fractions that were acidified and analyzed by LC-MS/MS. The two other parts were treated separately with two different “copies” of the HPLL synthesized in our laboratory, which were previously equilibrated in the same two buffers. The non-retained peptides were discarded and not analyzed while the peptides retained by the HPLL were eluted, fractionated by RP/OH− and collected in 20 fractions and analyzed by LC-MS/MS at acid pH. The subcellular location of the identified proteins in the strategy shown in Fig. 2 was predicted through the PSortb software.37
Figure 2.
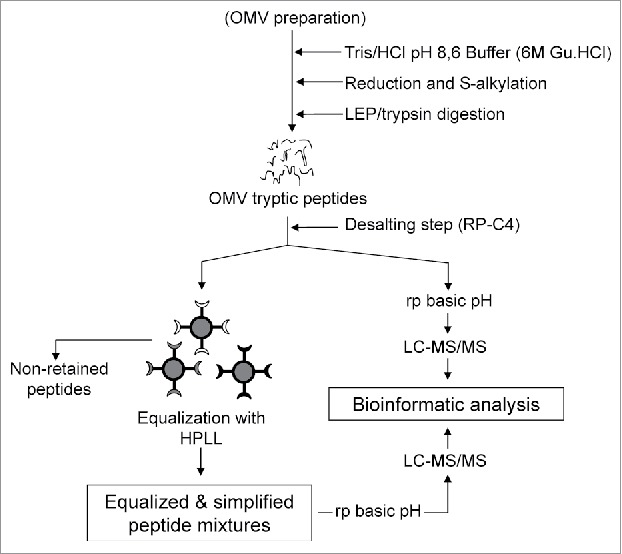
The general strategy proposed for a detailed characterization of protein composition of the OMV preparation from N. meningitidis by combining equalization and simplification at the level of tryptic peptides using a hexapeptide ligand library (HPLL) and a further fractionation of equalized peptides at alkaline and acid pH (HPLL+RP OH−/H+). Two different portions of the hexapeptide ligand library are used to equalize the concentration of the tryptic peptides derived from the OMV preparation.
At our laboratory we have seen that HPLL can be also used for equalizing the concentration of tryptic peptides derived from an artificial mixture of three proteins prepared within a range of two orders of magnitude in terms of concentration (see Supplementary Material 1). Additionally, we have also found that the HPLL treatment simplifies the complexity of a mixture of tryptic peptides once the peptides retained by the beads are analyzed by LC-MS/MS (see Supplementary Material 2).
In the strategy shown in Fig. 2, we included a direct fractionation of the tryptic peptides using reverse-phase chromatography at basic pH,34,35 without the HPLL treatment before the LC-MS/MS experiment. In principle this may avoid the exclusion from the identification process of peptides that are not retained by the HPLL. This would be particularly crucial for the low-abundance proteins that are identified in proteomic experiments by only a few peptides and in many cases by just one.
Additionally, we have noticed (Supplementary Material 2) that the set of peptides retained by HPLL depend on the equilibrium buffer used during the equalization step. Therefore, during the application of the strategy described in Fig. 2 we explored equalization using Tris/HCl and acetate buffers.
Proteins identified in the OMV analyzed on combining equalization and dual fractionation of peptides
The number of peptides and proteins identified using the incubation buffers (Acetate and Tris/HCl) were compared. Data of Fig. 3 combine the results obtained after treating tryptic peptides with HPLL (+HPLL in Fig. 3), their fractionation by reverse-phase chromatography at basic pH and their analysis by LC-MS/MS. Also, Fig. 3 shows the results of carrying out fractionation of the tryptic peptides by reverse-phase chromatography at basic pH and their direct analysis by LC-MS/MS without the HPLL treatment (−HPLL in Fig. 3).
Figure 3.
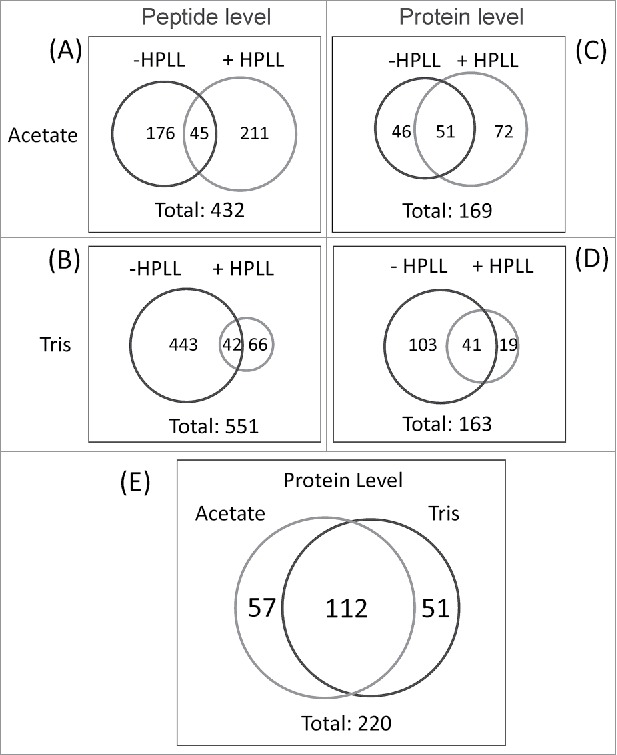
(A) Venn diagram representing the number of peptides identified after the equalization process with two buffers (buffer A: 25 mM sodium acetate, pH 5.0 and buffer B: 25 mM Tris/HCl, pH 9.0). (B) Venn diagram representing the amount of proteins identified after the equalization process with the same two buffers.
A total of 432 peptides were identified in acetate buffer; 256 and 221 under +HPLL and −HPLL conditions, respectively (Fig. 3A and Supplementary Material 3). Forty-five peptides were identified by both experiments (Fig. 3A).
In Tris/HCl buffer, 551 peptides were identified, 443 were exclusively identified when the mixture of tryptic peptides were directly fractionated by RP/OH− and analyzed by LC-MS/MS (-HPLL, Fig. 3B, Supplementary Material 3). Only 66 peptides were identified when the tryptic peptides were incubated with HPLL and those retained were fractionated using reverse-phase chromatography at alkaline pH and analyzed by LC-MS/MS (+HPLL, Fig. 3B and Supplementary Material 3). In that experiment, 42 peptides were identified under both conditions: +HPLL and −HPLL (Fig. 3B).
In the equalization experiments, 169 proteins were identified in acetate, and the Tris/HCl incubation buffers identified 163. (Fig. 3C and D; and Supplementary Material 3). In acetate buffer, the same 51 proteins were identified under both conditions: − HPLL and +HPLL (Fig. 3C). Forty-six and seventy-two proteins were exclusively identified in -HPLL and +HPLL experiments, respectively (Fig. 3C).
Only 19 proteins were exclusively identified in the Tris/HCl buffer when the proteolytic peptides were treated with HPLL (Fig. 3D, Supplementary Material 3). On considering that this result was obtained after the LC-MS/MS analysis of twenty fractions collected at RP (basic pH) and run in triplicate, the need to equalize the concentration of tryptic peptides in this buffer seems to be questionable.
In contrast, 144 proteins were identified by the direct fractionation of the tryptic peptides when combining the analysis by RP/OH− with LC-MS/MS (Fig. 3D, Supplementary Material 3). Only 41 proteins were identified equally in both experiments (Fig. 3D, Supplementary Material 3).
Overlapping all results on protein identification from the experiments described above, it was found that 220 proteins were identified, with a false discovery rate of less than 1%. This represents more than twice the individual contributions of any of the methods previously reported by our group.19–22 One hundred and twelve proteins were identified in both incubation buffers (Fig. 3E, and Supplementary Material 4), which is a cross confirmation of the presence of these proteins in the OMV preparation.
Fifty-seven and fifty-one proteins were exclusively identified in the experiments conducted in acetate and Tris/HCl buffers, respectively (Fig. 3E, and Supplementary Material 4). More than half of the proteins (112 proteins) that were identified (Fig. 3E and Supplementary Material 4) were detected using both incubation buffers, Tris/HCl and Acetate.
Integrating results of all proteins identified in this OMV preparation using different methods
A total of 260 different proteins were identified on jointly considering the work done by our group using three different methods,19–22 for a more in-depth characterization of this OMV-based vaccine preparation, and the results obtained here (Supplementary Material 3). Ten, forty, and seventy-nine different proteins were identified by the combination of four, three, and two different methodologies, respectively (Fig. 4A). Nearly 50% of all proteins were identified by applying at least two different methods.19–22 One hundred and thirty-one proteins (Fig. 4A) were identified by applying only one methodology.
Figure 4.
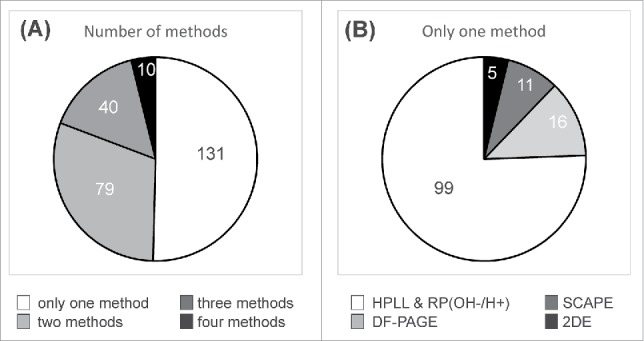
(A) Identification of the proteins by applying different methods to the characterization of the OMV isolated from N. meningitidis. The color legend indicates the number of proteins that were identified by only one, or by two, three and four methods (SCAPE, DF-PAGE, 2DE and HPLL+RP OH−/H+). (B) The graph shows the number of proteins that were identified by applying only one method.
We further analyzed the individual contribution of the methodologies (2DE, SCAPE, DF-PAGE and HPLL&RP(OH−/H+)) to extend the protein catalogue of this OMV preparation by studying the proteins that were identified by a single method (Fig. 4B, Supplementary Material 5). Only five proteins were identified exclusively by 2DE combined with ESI-MS/MS19 (Fig. 4B, Supplementary Material 5). Sixteen and eleven proteins were solely identified by using either DF-PAGE20 or SCAPE21,22 methods, respectively (Fig. 4B, Supplementary Material 5). Ninety-nine proteins were exclusively identified by applying the HPLL and RP OH−/H+ method (Fig. 4B, Supplementary Material 5) described in this paper (Fig. 2).
Amino acid changes and proteins not observed in the MC58 strain
Several high quality MS/MS spectra did not identify any protein when only the proteins derived from the MC58 genome were considered.38 Nonetheless, when a second search was carried out with proteins reported for other non-MC58 Neisseria meningitidis strains, the proteins having several amino acid changes were identified, and the results are summarized in the Supplementary Material 6A.
The proteins showing sequence variations include well-known, highly variable antigens such as major outer membrane proteins: PorA, PorB, and FrpB. We also identified bactericidal outer membrane exposed MafA protein39 and the hypothetical protein NMB1465 that belongs to the Opa adhesin family.40 The NMB1971 protein, with no function assigned, was identified as the product of an essential gene in bacteraemic disease or colonization, included in the adhesin family.41 Additionally, following the same approach we identified six new proteins that were not annotated in the MC58 genome38 (Supplementary Material 6B) together with several peptides having a MASCOT score above the identity threshold, considering an FDR lower than 1%.
In conclusion, by overlapping the identification results provided by the application of the different methods to the characterization of this OMV preparation, constituting the active pharmaceutical ingredient of VAMENGO-BC®, we have extended the protein catalogue to a total of two hundred and sixty-six proteins.
Subcellular location predicted by PSortb software for the identified proteins
The subcellular location of all proteins identified here were predicted by the PSortb program37 and results are shown in Fig. 5 (Supplementary Material 5). Fourteen (5.2%) and nine proteins (3.4%) are located in the cytoplasmic membrane and in the periplasmic compartment, respectively. Thirty-three proteins, representing 12.4% still have unknown subcellular location. Forty-one are classified as outer membrane proteins and represent 15.4%. Four out of six proteins not found in the MC58 genome were predicted by Psortb as membrane proteins (Supplementary Material 6B). For example, HpuB is annotated as an outer membrane protein and interacts with the extracellular lipoprotein HpuA. Both proteins are included in one of the meningococcal mechanisms considered, using hemoglobin as a source of iron for growth, which is important for the survival of the pathogen during its invasion into the bloodstream.42 The absence of HpuA and HpuB in the N. meningitidis MC58 genome38 is seemingly due to a recombination event mediated by an Nf1-encoded integrase; and its presence has been identified in the genome of the serogroup A Z2491 strain and the serogroup B α710 strain as part of the so-called pathogenic islands. The protein codified by gene gi|316985066 is identified as a filamentous hemagglutinin family N-terminal domain protein in the serogroup B H44/76 genome.43 The conserved domain analysis reveals that this protein belongs to that of COG3210, where FhaB proteins are grouped.44 The members of this domain are mediators of pathogen adhesion and also carry out many other functions related to virulence. For example, the corresponding homologue protein in Bordetella plays a crucial role in bacterial persistence in the lower respiratory tract, presumably participating in a mechanism of resistance to early immune-mediated clearance.45 The gi|308389763 gene coding for a protein annotated as a putative integral membrane in the Neisserial strain alpha710, is identified as a tRNA modification GTPase mnmE protein in the N. meningitidis alpha522 strain. The E. coli and Salmonella MnmE homologue has been involved in the pathogenesis caused by these bacteria and its deletion leads to a significantly attenuated mutant in animal models for infection.46
Figure 5.
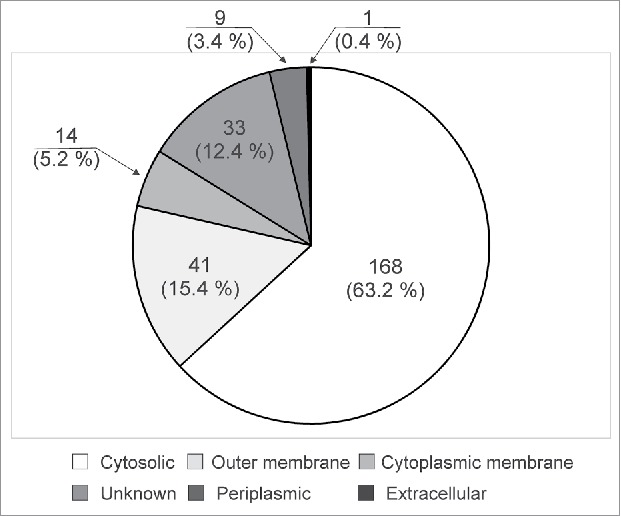
Subcellular distribution of identified proteins, according to the PSORTb v.3.0 software.37
Taking into account that a large part of the proteins identified in this OMV preparation have been previously described (160/260 proteins),19–22 we limited our search in the literature to information regarding proteins that were exclusively identified by the method implemented here, and which had been previously tested as vaccine candidates or classified as outer membrane proteins (Supplementary Material 7). In addition to the previously mentioned five major porins, other proteins studied as vaccine candidates that induced bactericidal activity in preclinical stages, were also identified by the method developed here (Supplementary Material 7, highlighted in bold and shadowed in gray). Bactericidal activity is an accepted correlate of protection for meningococcal infection.47 These antigens include lactoferrin binding protein A (lbpA),48 Omp85,49,50 FrpB,51 secretin PilQ,52 App,53 TonB-dependent receptor,54 MspA,55 IgA-specific serine endopeptidase,56 GNA33,57 NspA,58 and others. Combining the results of the methodologies applied, most meningococcal antigens forming part of the two recombinant vaccines approved in the USA and Europe (Trumenba® and Bexsero®, Supplementary Material 8), are also found in this OMV preparation. The factor H binding protein (fHbp, also referred to as NMB1870), is contained in the Bexsero® vaccine (Novartis) as well as in Trumenba® (Pfizer59). Two other components of Bexsero®, such as Neisseria adhesin A (NadA, also referred as NMB1994 and gi|7227256), and GNA2091 (referred as NMB2091 and gi|7227353) were identified by at least two different methods. The GNA1030 antigen (referred to as NMB1030, NUbp and gi|7226269), also present in Bexsero®, was identified only by the method described in this paper. The lipoprotein coded by the NMB2132 gene (gi|7227388), renamed as Neisserial heparin-binding antigen (NHbp),60 which is the fifth component of the Novartis vaccine, was not identified in this OMV preparation. In short, we detected in this preparation four out of the five vaccine antigens included in the Bexsero® vaccine developed by Novartis.61 Other vaccine candidates evaluated in clinical trials are shown in Supplementary Material 8. Although most of the proteins identified here are predicted to be cytosolic proteins (168 proteins, 63.2%) it is important to point out that they are low-abundance proteins and represent only 14.6% of the 1148 proteins annotated as being cytosolic in the entire genome of the MC58 strain.38
Discussion
VAMENGOC-BC® has been a widely used OMV anti-meningococcal vaccine in Latin America.10–12 Its role in controlling serogroup B epidemics has been extensively documented, so the characterization of its composition is of great interest in terms of immunological responses, regulatory aspects, and basic knowledge.
It has been well described that the major component of immunological responses against OMV based vaccines is targeted against the most abundant surface protein antigens that because of selective pressure generally show hypervariable sequences. Consequently, specific responses are mainly induced against the strain that was used to prepare the OMV (i.e., homologous strain). Nevertheless, a certain amount of cross-responses against heterologous strains have been observed with VAMENGOC-BC®, presumably caused by minor antigens, thus making the identification of these antigens relevant.16,62 This hypothesis led us to implement a series of proteomic and bioinformatic tools to identify these least represented proteins.19–22,63–65
To extend the protein catalogue of this OMV preparation, the identification of low-abundance proteins was mandatory. As expected, the wide dynamic concentration range of proteins found in this OMV preparation is also an inherent attribute of their derived proteolytic peptides and it introduces a bias in the LC-MS/MS analysis for the identification of the five most abundant proteins (FetA, PorA, PorB, RmpM, and Opc).
The alternative approach of increasing the amount of tryptic peptides analyzed by LC-MS/MS for the identification of low-abundance proteins was excluded because the resolution of the chromatographic separation would be seriously compromised, and the carry-over would damage the column and pre-column.
Therefore, after reaching this point we believed that in order to increase the number of proteins or peptides identified, we would need to apply a strategy based on the LC-MS/MS analysis of peptides that combines: (1) the reduction of the dynamic concentration range of tryptic peptides; (2) the simplification of the complexity of this mixture in terms of the number of peptides to be analyzed and (3) a peptide fractionation technique orthogonal to the LC-MS/MS analysis performed at an acid pH. All these features can be attributed to the methodology applied here.
These aspects give equal opportunity to the peptides retained by HPLL to be analyzed during the LC-MS/MS experiments regardless of whether they come from either high-abundance or low-abundance proteins. Additionally, a peptide-based strategy after tryptic digestion may yield non-hydrophobic peptides that can even be derived from hydrophobic proteins, particularly the peptides contained in regions exposed to cytosol or located at extracellular domains which are easier to handle and show higher recoveries.32,66,67
The results obtained here represent an increase of approximately 100 new proteins identified. Although this would suggest the application of this methodology for the full characterization of several batches of this OMV, it was not conceived for quality control experiments for several reasons. On one hand, the considerable number of LC-MS/MS runs required to do this hinders its routine implementation. On the other hand, this methodology involves the treatment of tryptic peptides with the HPLL and the reproducibility of the HPLL is difficult to demonstrate. Particularly, in our case to ensure the wide application of HPLL in proteomic experiments we synthesized ninety copies of the library in the same test tube (500 mL). Although the weight of the HPLL was constant for all equalization experiments, it is impossible to guarantee the same ligand composition for any new portion taken for that purpose even after homogenizing. As expected, the composition of the resulting peptides from the equalization step is sensitive not only to the equalization buffer but also to the composition of the library. Therefore, the unsuccessful identification of a given protein in individual experiments cannot be taken as a demonstration of their absence in the sample. This would be even more crucial for minor proteins, which are frequently identified by a single peptide. Finally, it is not recommended to reuse the same portion of the library to characterize several batches of this OMV preparation, in order to avoid cross contamination, mainly when the presence of sticky peptides during equalization has been demonstrated in this paper.
Our group has developed several methodologies that are based on different principles,19–22 and have also been applied to the characterization of this OMV, through which we have identified 160 unique proteins. Combining all these results, the protein catalogue of this OMV has been extended up to 260 proteins. Half of these proteins were consistently identified with a considerable overlapping by the application of at least two different methodologies.19–22 We believe that this very valuable protein list could be used as a starting point to select the immunologically relevant antigens that must be assessed for the quality control of this vaccine. A methodology based on Selective Reaction Monitoring (SRM), and not on the methodology described in this paper, could be the tool of choice to assess the quality control of this OMV preparation as will be discussed later on in this section.
The amino acid changes detected here for ten proteins seem to be reasonable, considering the known variability between the reference MC58 strain and clinical isolates such as the case of the CU385 strain. This may be linked to mutational events needed as escape mechanisms in response to selective immunological pressure.
The proteins not identified in the MC58 genome38 is not an infrequent event considering that the genome of N. meningitidis is characterized by its high plasticity due to frequent exchanges of genetic material. Entire genome sections containing numerous virulence genes are frequently imported through genetic exchange.68,69 Interestingly, four out of these six proteins (gi|308390070 HpuA, gi|308390069 HpuB, gi|308389763, and gi|316985066) have primary annotations that classify them as possibly having virulence attributes, and their incorporation into the CU385 genome could represent an advantage for the pathogenic processes.
The active pharmaceutical ingredient of VA-MENGOC-BC® 10 is a preparation rich in membrane proteins because approximately 54% of the membrane proteins predicted by the PSortB software37 in the MC58 genome38 were detected.
The identification of several membrane proteins in the CU385 OMV-based vaccine that are also present in the Bexsero® and Trumenba® vaccines is of relevance because they are the unique proteins of Neisseria meninigitidis serogroup B with significant bactericidal and protective properties, and were approved by regulatory agencies for human vaccination.
The selection of these antigens as components of the above mentioned vaccines was based on their conservation in the meningococcal population for at least fifty years, thus expecting the induction of a broad protection.70 Sequence analyses of FHbp and NadA show that the corresponding proteins of the CU385 strain are similar to those included in the Bexsero vaccine.71 This supports the possibility of that antigens may contribute to the cross-reactive response induced by VAMENGOC BC.
An understandable doubt is whether these antigens remain in the OMV in sufficient amounts to induce a protective response, considering that Koeberling et al. have shown that these proteins are largely removed by the detergent extraction process. That same article, however, showed that a certain level of response by serum antibodies induced by a detergent extracted OMV from the H44/76 strain against FHbp, was detected.72 Because the wild type H44/76 and CU385 have similar FHbp expression levels,73 we would expect that the experimental behavior in this animal model for the Cuban strain could be similar. Koeberling et al. reported undetectable levels of antibodies against NadA when mice were immunized with a detergent extracted OMV. Similar results were observed for a mutant OMV although NadA-specific bactericidal responses were reported for the animal group receiving this immunogen.72
The presence of non-outer membrane proteins, mainly classified as cytosolic, have been systematically identified in this OMV preparation19–22 and in other proteomic studies.74,75 For example, Knaust et al.76 reported that several plasminogen receptors were detected in crude outer membrane preparations of N. meningitidis, by using specific anti-sera, although most of these proteins were found in total cellular lysates, as would be expected from proteins primarily present in the cytosol. Also, Tunio et al.77 found that meningococcal fructose-1,6-bisphosphate aldolases are located both in the cytoplasm and in the outer membrane. Others examples were documented by Ferrari et al.,41 using fluorescence-activated cell sorting (FACs) analysis of whole meningococcal cells where they demonstrated the presence of cytosolic proteins in the outer membrane of the meningococci.
Here we found that certain proteins that were predicted as being cytoplasmic by PsortB, had been documented as surface exposed proteins. For instance, the products of genes of NMB0017, NMB0707, NMB0554, NMB1285, NMB1347 and NMB1372 that are mainly related to enzyme and regulatory cytoplasmic functions have also been identified in previous papers as outer membrane components, exposed on the cell surface or secreted homologues.69,78–81
These results reinforce our previous discussion and the need to encourage a more critical evaluation of the use of subcellular localization predictor software, taking into account that proteins of apparently cytoplasmic origin should not be eliminated from studies without careful consideration and validation.
A group of cytoplasmic and inner membrane proteins NMB0143,82,83 NMB146784 and NMB020785 previously identified by experiments with sera from patients recovering from meningococcal disease, or that were vaccinated or in persons infected by N. meningitides, also point toward this conclusion.
OMV based vaccines are thought to provide strain specific protection as a result of the high-abundance hypervariable surface antigens such as PorA and the other four major proteins previously mentioned. Thus, current quality control methods are based on the scanning intensity of major Coomassie Blue-stained protein bands analyzed by SDS-PAGE, enabling the comparison of protein patterns to standards, as well as the quantification of major proteins.69,86,87 Nevertheless, published results showed that OMV vaccines are also able to induce a certain level of cross-reactivity against heterologous strains. From this perspective, the detection and quantification of minor proteins in a batch-to-batch approach could be advantageous for ensuring not only consistency in protein content but also a high quality immune response. At this point, monitoring proteins of known protective activity such as the components of the Trumenba® and Bexsero® vaccines and other conserved antigens may be relevant.
Although analytical methods based on immune detection can be made for this purpose, selective reaction monitoring (SRM)88 may hypothetically allow the specific quantification of individual proteins with key contributions in protective immunological response, even in the complex context of OMV. This is affordable through the use of state-of-the-art mass spectrometers combined with the stable isotope dilution concept.89,90 SRM technology solves the bias in the identification of the most abundant proteins, while being very sensitive, because it does not require a scanning technique.88 A hypothesis-driven approach may also be useful to demonstrate the presence of low-abundance proteins that are immunologically relevant, and that cannot be identified by mass spectrometry in this study due to the stochastic selection of precursor ions in LC-MS/MS experiments. The list of target proteins used to demonstrate batch-to-batch reproducibility may also include all membrane proteins predicted by the PSort program as well as all conserved antigens included in other vaccines. Additionally, on considering that the most abundant proteins are determinants for the protein content of a vaccine and that they play a key role in the protection against specific strains, their inclusion in the target list may also be mandatory.
Although this study is valid in a more in-depth evaluation of the protein composition of this OMV preparation, it was not designed to demonstrate batch-to-batch reproducibility. The method developed here has this limitation and it cannot therefore be used as a routine tool for quality control. Our method generates several fractions, and it also includes equalization steps which are time consuming due to the multiple LC-MS/MS runs required to complete the analysis.
Previous studies by our group demonstrated the batch-to batch reproducibility of this OMV preparation using SDS-PAGE as well as two-dimensional gel electrophoresis.19 However, the sample processing for 2DE analysis requires an extraction step using organic solvent that may contribute to the removal of hydrophobic proteins of immunological relevance present in this OMV. SDS-PAGE analysis, although assesses the contribution of the major surface proteins to the total protein content, is very simple method to characterize such complex preparation. These reasons excluded the application of these methodologies for a detailed analysis of this OMV preparation.
The method presented here should be seen as a first necessary step for making the list of proteins present in this OMV preparation, so as to determine in a second step, their quantitation in several batches to assess the reproducibility of the production process using the SRM. The key proteins in charge of inducing a protective response after vaccination must be known to implement this approach.
The fact that some MPs were not identified in this study could be due to several reasons: (a) they are not expressed under the culture conditions of the CU385 strain used to manufacture the VAMENGOC-BC® vaccine, (b) they were removed during the production process of the OMV22 that uses detergent (deoxycholate), (c) all the tryptic peptides of non-identified MP were irreversibly attached to the RP column during the desalting step required prior to equalization or (d) simply that the HPLL was not able to enrich the tryptic peptides derived from the MP at levels above the detection limits of the analytical tools used in this study.
Although the HPLL used in the experiments described here contains 64 million sequences, this diversity is not enough to retain all tryptic peptides generated by the proteolysis of the OMV proteins and a considerable part of them are discarded. This may be crucial for the identification of low-abundance proteins that are often identified with very few peptides, and sometimes with just a single peptide. Therefore, although the simplification of mixture complexity is useful in identifying more proteins when discarding the peptides derived from abundant proteins, it is also valid for low-abundance proteins and this potential limitation should be taken into account. The interaction between tryptic peptides and HPLL has few structural constraints imposed by the generally random structure of interacting partners.
Additionally, we have noticed that some peptides stick strongly to the HPLL, suggesting that a general phenomenon may be driving their interaction, instead of a specific affinity with a particular partner bead, as hypothesized earlier for the case of proteins.26–31 Peptides with this particular characteristic are not equalized by a single bead as ideally expected and they will be favored in their identification during the LC-MS/MS experiments.
The use of a state-of-the art mass spectrometer such as an Orbitrap, which can handle samples within a broader dynamic concentration range, may provide superior results in terms of the number of protein identified.
In spite of the above mentioned limitations, we believe that HPLL combined with dual fractionation by reverse-phase chromatography performed at alkaline and acid pH is another choice in the arsenal of analytical tools used for the challenging characterization of MPs in proteomics.
Conclusions
The inclusion of HPLL in peptide-based proteomics has a dual effect: (1) equalizing the concentration of a complex mix of proteolytic peptides derived from a protein mixture with a wide dynamic concentration range, and also (2) simplifying the complexity of the analyzed peptide mixture because a considerable group of peptides are not retained during the equalization process. These two properties of HPLL combined with dual fractionation by reverse-phase chromatography at basic and acid pH during the LC-MS/MS experiments, increased the number of protein identified in the OMV preparation characterized, which constitutes the active pharmaceutical ingredient of an effective vaccine against N.meningitidis, VAMENGOC-BC®.
Equalization using HPLL at the level of proteolytic peptides enables the study of MPs, which constitute a non-deeply mined subproteome due to its scarce solubility, although this type of protein is important in discovering receptors, biomarkers, and vaccine candidates. Two hundred and sixty-six proteins were reported in this OMV preparation. Fifty percent of the proteins reported in the MC58 strain as membrane proteins were found in this preparation including four out of five conserved antigen components of the Bexsero® vaccine developed by Novartis61 and the active pharmaceutical ingredient of the Trumenba® vaccine developed by Pfizer.59 These two vaccines have been licensed by regulatory agencies in Europe and the US.
The antigens identified by the present methodology also grouped virulence-related attributes that could participate in pathogenic processes or could confer advantages in colonization or invasion steps. Other polypeptides have been directly involved in immune responses, which have been experimentally recognized through the sera of patients or human carriage. Additionally, several outer membrane proteins and previously studied vaccine candidates are reported. In several cases we also identified homologies with proteins that share some of the previously mentioned characteristics, indicating the potential participation in immune responses of proteins that were not previously studied for this purpose in meningococci. All this information could be used by others groups in the search for novel vaccine candidates, since up to now, no universal vaccine against serogroup B has been found.
Materials and methods
Sequencing grade proteases, trypsin and lysyl-endopeptidase (LEP) were provided by Promega (USA) and Wako (Japan), respectively. The OMV preparation from Neisseria meningitidis serogroup B from strain CU385 was produced at the Finlay Institute in Havana, Cuba.10 Other solvents and reagents were of analytical grade and were provided by well-known suppliers.
Synthesis of the hexapeptide ligand library (HPLL)
Ninety copies of the HPLL were synthesized on 10 μm TentaGel resin (Rapp Polymere) by a split-mix synthesis approach.23 The hexapeptide ligand library (HPLL) was synthesized by using the standard solid phase peptide synthesis method with 9-fluorenylmethyoxycarbonyl chemistry.91 All the natural L-amino acids were included in the synthesis, except Trp, Met and Cys, because they tend to oxidize and cross-link during a prolonged storage of the library. Once the ¨split-mix¨ cycles were completed, the side-chain protecting groups were removed by adding a mixture containing trifluoroacetic acid/triisopropylsilane/water (95:2.5:2.5, v/v/v). The peptide beads were subsequently washed with ethanol and water, and stored dry at 4°C until use.
Conditioning HPLL for the equalizing mixture of tryptic peptides
Before the equalization of peptide mixtures, the HPLL were conditioned by successive 10 minute washing steps with methanol, TFA 10% in methanol, 6 mol/L guanidinium chloride, milliQ water. These washing steps are essential to obtain a good signal-to-noise ratio at m/z below 1000 in the ESI-MS spectra. The portions of HPLL were independently equilibrated with two incubation buffers (10 mL) previously reported in the literature92 (buffer 1: 25 mM sodium acetate buffer pH 5.0, buffer 2: 25 mM Tris/HCl, pH 9.0) but sodium chloride was excluded in their composition.
SDS-PAGE analysis
The electrophoretic separation of proteins was done on 12% SDS-PAGE. Five and ten μg of molecular weight markers and the OMV preparation of N. meninigitidis serogroup B (the active pharmaceutical ingredient of VA-MENGOC-BC®), respectively, were dissolved in 20 μL Laemmli buffer containing bromophenol blue.93 Samples were heated at 100°C for 5 minutes and separation was performed in a Mini–Protean II 2-D Cell (Biorad), using 25 mA, for 45 minutes. The protein bands were visualized by Coomassie blue staining.
Processing the OMV preparation from N. meningitidis serogroup B
The equivalent to six milligrams of proteins contained in the OMV preparation were completely dissolved in 100 μL of 250 mM pH 8.6 Tris/HCl buffer containing 6 mo/L guanidinium chloride and 10 mM of DTT. The solution was incubated at 40°C, in a nitrogen atmosphere. After three hours the solution was cooled at room temperature and acrylamide was added to reach a final concentration of 20 mM. The reaction proceeded for one hour and the mixture was finally diluted three times with milliQ water and digested with LEP (Wako, Japan) at an enzyme to substrate ratio of 1:100 for 8 hours at 37°C. The LEP peptides were further diluted with an equal volume of water and digested with sequencing grade trypsin (Promega, USA) for an additional 16 hours at 37°C, using a 1:100 enzyme-to-substrate ratio. The peptide pool was divided into four equal parts that were desalted in a RP-C4 column (20 × 2 mm id), collected into a single fraction and lyophilized. Two fractions were dissolved in 25 mM, sodium acetate pH 5.0, and the remaining two fractions in 25 mM Tris/HCl pH 9.0 buffers. Both peptide mixtures were individually equalized with two ¨copies¨ of the combinatorial hexapeptide library previously equilibrated in the same buffers. The beads were centrifuged at 10 000 rpm for five minutes, and the supernatant mainly containing the LEP/tryptic peptides, derived from the most abundant proteins, was discarded. The peptides retained on the beads were washed with the same incubation buffer, centrifuged at 10 000 rpm for five minutes, and the supernatant was discarded. The retained tryptic peptides were eluted in the same incubation buffers containing 6 mol/L guanidium hydrochloride. The resultant equalized and simplified mixture of LEP/tryptic peptides was directly fractionated on a POROS-R2 column (200 × 2 mm id) from Applied Biosystems, previously equilibrated in solution A (0.1% NH4OH pH 10.9), at 0.2 mL/min. The elution gradient of solution B (ACN/water 1/1, containing 0.1% NH4OH pH 10.9), from 1% to 60% at an increasing rate of 0.5% of B/min, was used to collect the peptides in twenty fractions that were neutralized with 1% formic acid, then dried and frozen at −20°C until LC-MS/MS analysis. The two other fractions of tandem digestion (LEP+trypsin) were separately dissolved in the two incubation buffers previously described and were directly fractionated by reverse-phase chromatography at alkaline pH on a POROS R-2 column, as described above, and analyzed by LC-MS/MS.
NanoLC separation of peptides
The nanoLC-MS analyses of the individual samples were performed on a nanoLC Agilent 1100 (Agilent, USA) equipped with a microwell plate autosampler. The loaded peptides (40 μL) were trapped and desalted on a PS-DVB monolithic trap column (5 mm × 200 μm id) from Dionex (USA) for 10 minutes with 0.2% formic acid delivered by an auxiliary pump operated at 20 μL/min. By valve switching the retained peptides were back-flushed and loaded onto the capillary PS-DVB monolithic column (100 μm x 50 mm) from Dionex (USA). The separation column was previously equilibrated in solution A (formic acid 0.2% in water) and peptides were eluted with a linear gradient of solution B (acetonitrile/water/formic acid 80/20/0.2 v/v/v) at 0.75% of B/min. The eluate at a flow-rate of 300 nL/min was transferred to the QTOF-2™ mass spectrometer (Micromass, UK).
Mass Spectrometry
The ESI-MS spectra were acquired using a hybrid quadrupole orthogonal acceleration tandem mass spectrometer QTOF-2™ from Micromass (UK) fitted with a Z-spray nanoflow electrospray ion source. Samples were dissolved in 2–3 microliters and directly loaded onto the metal coated borosilicate nanocapillary (Proxeon, Netherlands). To obtain the low-energy ESI-MS/MS spectra, the precursor ions were selected with a mass window (4-6 Da) that was wide enough to transmit all the components of the isotopic distribution of peptides to the collision chamber. Argon was used as the collision gas and collision energies were ramped (15-45 eV) according to the m/z and z of the precursor ions selected for MS/MS analysis to obtain an efficient fragmentation upon CID and a reliable protein identification of peptides in sequence databases. In LC-MS/MS experiments only doubly and triply-charged ions, with intensities higher than 15 counts/sec, were selected for the MS/MS analysis. Data acquisition and processing were performed using MassLynx (v 4.0) software (Micromass, UK). All samples were measured by triplicate.
Protein identification and data analysis
The raw files containing the LC-MS/MS spectra were smoothed, centered and exported as pkl files using the Micromass ProteinLynx Global Server (v 2.0). The pkl files were analyzed with the MASCOT (v 2.2) software.94 Propionamide cysteine was set as a fixed modification while methionine sulfoxide and deamidation were considered variable modifications. The mass tolerances were 0.5 and 0.08 Da for the precursor and fragment ions, respectively. Two missed cleavage sites were allowed. Protein identification was performed using two different databases, one containing 2001 predicted proteins from the N. meningitidis serogroup B genome (strain MC58, Proteome ID UP00000042538) downloaded from the Uniprot site (http://www.uniprot.org)95; while the other is a compendium of 6856 proteins derived from non-MC58 strains (downloaded from http://www.ncbi.nlm.nih.gov/protein/). Target-decoy strategy with a False Discovery Rate lower than 1% was used for data quality assessment. The subcellular location of identified proteins was predicted by PSORTb v3.0.2 software (http://www.psort.org/psortb/).37
Supplementary Material
Abbreviations
- 2-DE
two-dimensional gel electrophoresis
- DF-PAGE
dual fractionation by polyacrylamide gel electrophoresis
- HPLL
Hexapeptide ligand library
- LC-MS/MS
liquid chromatography coupled to tandem mass spectrometry
- LEP
lysyl-endopeptidase
- MPs
membrane proteins
- m/z
mass-to-charge ratio
- nHnR
peptides devoid of histidine and arginine
- OMV
outer membrane vesicles
- SCAPE
selective capture of peptides
- SCX
strong cation exchange
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors are deeply grateful to Ms. Miriam Ribas for the corrections of the style and grammar of this manuscript.
Funding
The authors acknowledge CIGB for giving the financial support.
References
- [1].Harrison OB, Claus H, Jiang Y, Bennett JS, Bratcher HB, Jolley KA, Corton C, Care R, Poolman JT, Zollinger WD, et al.. Description and nomenclature of Neisseria meningitidis capsule locus. Emerg Infect Dis. 2013;19:566–73. doi: 10.3201/eid1904.111799. PMID:23628376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gabutti G, Stefanati A, Kuhdari P. Epidemiology of Neisseria meningitidis infections: case distribution by age and relevance of carriage. J Prev Med Hyg. 2015;56:E116–20. PMID:26788731. [PMC free article] [PubMed] [Google Scholar]
- [3].Rouphael NG, Stephens DS. Neisseria meningitidis: Biology, Microbiology, and Epidemiology. Methods Mol Biol. 2012;799:1–20. doi: 10.1007/978-1-61779-346-2_1. PMID:21993636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Finne J, Leinonen M, Makela PH. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet. 1983;2(8346):355–7. doi: 10.1016/S0140-6736(83)90340-9. PMID:6135869. [DOI] [PubMed] [Google Scholar]
- [5].Sadarangani M, Pollard AJ. Serogroup B meningococcal vaccines—an unfinished story. Lancet Infect Dis. 2010;10:112–24. doi: 10.1016/S1473-3099(09)70324-X. PMID:20113980. [DOI] [PubMed] [Google Scholar]
- [6].Gasparini R, Amicizia D, Domnich A, Lai PL, Panatto D. Neisseria meningitidis B vaccines: recent advances and possible immunization Policies. Expert Rev Vaccine. 2014;13:345–64. doi: 10.1586/14760584.2014.880341. [DOI] [PubMed] [Google Scholar]
- [7].Loring BJ, Turner N, Petousis-Harris H. MeNZB™ vaccine and epidemic control: When do you stop vaccinating? Vaccine. 2008;26:5899–904. doi: 10.1016/j.vaccine.2008.08.062. PMID:18804134. [DOI] [PubMed] [Google Scholar]
- [8].Tappero JW, Lagos R, Ballesteros AM, Plikaytis B, Williams D, Dykes J, Gheesling LL, Carlone GM, Høiby EA, Holst J, et al.. Immunogenicity of 2 serogroup B outer-membrane protein meningococcal vaccines: a randomized controlled trial in Chile. JAMA. 1999;281(16):1520–7. doi: 10.1001/jama.281.16.1520. PMID:10227322. [DOI] [PubMed] [Google Scholar]
- [9].Donnelly J, Medini D, Boccadifuoco G, Biolchi A, Ward J, Frasch C, Richard-Moxon E, Stella M, Comanducci M, Bambini S, et al.. Qualitative and quantitative assessment of meningococcal antigens to evaluate the potential strain coverage of protein-based vaccines. PNAS 9. 2010;107:19490–5. doi: 10.1073/pnas.1013758107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sierra GV, Campa HC, Varcacel NM, Garcia IL, Izquierdo PL, Sotolongo PF, Casanueva GV, Rico CO, Rodriguez CR, Terry MH. Vaccine against group B Neisseria meningitidis: protection trial and mass vaccination results in Cuba. NIPH Ann. 1991;14:195–210. PMID:1812432. [PubMed] [Google Scholar]
- [11].Sotolongo F, Campa C, Casanueva V, Fajardo EM, Cuevas IE, González N Cuban Meningococcal BC Vaccine: Experiences & Contributions from 20 Years of Application. MEDICC Rev. 2017:9(1):16–22. PMID:21487356. [DOI] [PubMed] [Google Scholar]
- [12].Sierra G, Campa C, García IL, Sotolongo F, Izquierdo L, Valcárcel M, Casanueva V, Baró M, Leguen F, Rodríguez R, Terry H. Efficacy evaluation of the Cuban vaccine VA-MENGOC-BC against disease caused by serogroup B Neisseria meningitidis. In: Atchman M et al., Neisseria. Berlin: Walter Gruyter; 1990. p. 129–34. [Google Scholar]
- [13].Costa EA. On the Controversy about the Efficacy of the Antimeningococcal B Vaccine: Methodological Pitfalls. Cad Saúde Públ. 1995;11(2):332–5. doi: 10.1590/S0102-311X1995000200018. [DOI] [PubMed] [Google Scholar]
- [14].Costa E, Martins HJ, Klein CH. Avaliação da proteção conferida pela vacina antimeningocócica BC no Estado de Santa Catarina, Brazil, 1990/92. Rev Saúde Pública. 1996;30(5):460–70. doi: 10.1590/S0034-89101996000500009. [DOI] [PubMed] [Google Scholar]
- [15].Galeano LA, Echeverry ML. Efectividad de una vacuna antimeningocócica en una cohorte de Itaguí Colombia, 1995. Boletín Epidemiológico de Antioquia. 1996;20(2):110–8. [Google Scholar]
- [16].Williams JN, Weynants V, Poolman JT, Heckels JE, Christodoulides M. Immuno-proteomic analysis of human immune responses to experimental Neisseria meningitides outer membrane vesicle vaccines identifies potential cross-reactive antigens. Vaccine. 32;2014:1280–86. doi: 10.1016/j.vaccine.2013.12.070. PMID:24486354. [DOI] [PubMed] [Google Scholar]
- [17].Santoni V, Molloy M, Rabilloud T. Membrane proteins and proteomics: Un amour impossible? Electrophoresis. 2000;21:1054–70. doi: 10.1002/(SICI)1522-2683(20000401)21:6%3c1054::AID-ELPS1054%3e3.0.CO;2-8 ;2-. PMID:10786880. [DOI] [PubMed] [Google Scholar]
- [18].Wu CC, Yates JR III. The application of mass spectrometry to membrane proteomics. Nat Biotechnol. 2003;21:262–7. doi: 10.1038/nbt0303-262. PMID:12610573. [DOI] [PubMed] [Google Scholar]
- [19].Uli L, Castellanos-Serra L, Betancourt L, Domínguez F, Barberá R, Sotolongo F, Guillén G, Pajón Feyt R. Outer membrane vesicles of the VA-MENGOC-BC vaccine against serogroup B of Neisseria meningitidis: Analysis of protein components by two-dimensional gel electrophoresis and mass spectrometry. Proteomics. 2006;6:3389–99. doi: 10.1002/pmic.200500502. PMID:16673438. [DOI] [PubMed] [Google Scholar]
- [20].Ramos Y, Gutierrez E, Machado Y, Sanchez A, Castellanos-Serra L, González LJ, Fernandez-de-Cossio J, Perez Y, Betancourt L, Gil J, et al.. Proteomics based on peptide fractionation by SDS-free PAGE. J Proteome Res. 2008;7:2427–34. doi: 10.1021/pr700840y. PMID:18422305. [DOI] [PubMed] [Google Scholar]
- [21].Betancourt L, Gil J, Besada V, González LJ, Fernández-de-Cossio J, García L, Pajón R, Sanchez A, Alvarez F, Padrón G. SCAPE: a new tool for the Selective CApture of PEptides in protein identification. J Proteome Res. 2005;4:491–6. doi: 10.1021/pr049794x. PMID:15822926. [DOI] [PubMed] [Google Scholar]
- [22].Gil J, Betancourt L, Sardiñas G, Yero D, Niebla O, Delgado M, García D, Pajón R, Sanchez A, González LJ, et al.. Proteomic study via a non-gel based approach of meningococcal outer membrane vesicle vaccine obtained from strain CU385: a road map for discovery new antigens. Hum Vaccin. 2009;5:347–56. doi: 10.4161/hv.5.5.7367. PMID:19377283. [DOI] [PubMed] [Google Scholar]
- [23].Furka A, Sebestyén F, Asgedom M, Dibó G. General method for rapid synthesis of multicomponent peptide mixtures. Int J Pept Protein Res. 1991;37:487–93. doi: 10.1111/j.1399-3011.1991.tb00765.x. PMID:1917305. [DOI] [PubMed] [Google Scholar]
- [24].Thulasiraman V, Lin S, Gheorghiu L, Lathrop J, Lomas L, Hammond D, Boschetti E. Reduction of the concentration difference of proteins in biological liquids using a library of combinatorial ligands. Electrophoresis. 2005;26:3561–71. doi: 10.1002/elps.200500147. PMID:16167368. [DOI] [PubMed] [Google Scholar]
- [25].Sennels L, Salek M, Lomas L, Boschetti E, Righetti PG, Rappsilber J. Proteomic analysis of human blood serum using peptide library beads. J Proteome Res. 2007;6:4055–62. doi: 10.1021/pr070339l. PMID:17877382. [DOI] [PubMed] [Google Scholar]
- [26].Guerrier L, Claverol S, Finzi L, Paye F, Fortis F, Boschetti E, Housset C. Contribution of solid-phase hexapeptide ligand libraries to the repertoire of human bile proteins. J Chromatogr A. 2007;1176:192–205. doi: 10.1016/j.chroma.2007.11.007. PMID:18036598. [DOI] [PubMed] [Google Scholar]
- [27].Castagna A, Cecconi D, Sennels L, Rappsilber J, Guerrier L, Fortis F, Boschetti E, Lomas L, Righetti PG. Exploring the hidden human urinary proteome via ligand library beads. J Proteome Res. 2005;4:1917–30. doi: 10.1021/pr050153r. PMID:16335936. [DOI] [PubMed] [Google Scholar]
- [28].Guerrier L, Claverol S, Fortis F, Rinalducci S, Timperio AM, Antonioli P, Jandrot-Perrus M, Boschetti E, Righetti PG. Exploring the platelet proteome via combinatorial, hexapeptide ligand libraries. J Proteome Res. 2007;6:4290–303. doi: 10.1021/pr0703371. PMID:17918985. [DOI] [PubMed] [Google Scholar]
- [29].Roux-Dalvai F, Gonzalez de Peredo A, Simó C, Guerrier L, Bouyssié D, Zanella A, Citterio A, Burlet-Schiltz O, Boschetti E, Righetti PG, et al.. Extensive analysis of the cytoplasmic proteome of human erythrocytes using the peptide ligand library technology and advanced mass spectrometry. Mol Cell Proteomics. 2008;7:2254–69. doi: 10.1074/mcp.M800037-MCP200. PMID:18614565. [DOI] [PubMed] [Google Scholar]
- [30].Boschetti E, Righetti PG. The art of observing rare protein species in proteomes with peptide ligand libraries. Proteomics. 2009;9:1492–510. doi: 10.1002/pmic.200800389. PMID:19235170. [DOI] [PubMed] [Google Scholar]
- [31].D'Ambrosio C, Arena S, Scaloni A, Guerrier L, Boschetti E, Mendieta ME, Citterio A, Righetti PG. Exploring the chicken egg white proteome with combinatorial peptide ligand libraries. J Proteome Res. 2008;7:3461–74. doi: 10.1021/pr800193y. PMID:18570458. [DOI] [PubMed] [Google Scholar]
- [32].Washburn MP, Wolters D, Yates JR III. Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nat Biotechnol. 2001;19:242–47. doi: 10.1038/85686. PMID:11231557. [DOI] [PubMed] [Google Scholar]
- [33].Hörth P, Miller CA, Preckel T, Wenz C. Efficient fractionation and improved protein identification by peptide OFFGEL electrophoresis. Mol Cell Proteomics. 2006;5:1968–74. doi: 10.1074/mcp.T600037-MCP200. PMID:16849286. [DOI] [PubMed] [Google Scholar]
- [34].Geiger T, Velic A, Macek B, Lundberg E, Kampf C, Nagaraj N, Uhlen M, Cox J, Mann M. Initial quantitative proteomic map of 28 mouse tissues using the SILAC mouse. Mol Cell Proteomics. 2013;12:1709–22. doi: 10.1074/mcp.M112.024919. PMID:23436904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gilar M, Olivova P, Daly AE, Gebler JC. Two-dimensional separation of peptides using RP- RP-HPLC system with different pH in first and second separation dimension. J Sep Sci. 2005;28:1694–703. doi: 10.1002/jssc.200500116. PMID:16224963. [DOI] [PubMed] [Google Scholar]
- [36].Mihailova A, Lundanes E, Greibrok T. Determination and removal of impurities in 2-DLC-MS of peptides. J Sep Sci. 2006;29:576–81. doi: 10.1002/jssc.200500496. PMID:16583696. [DOI] [PubMed] [Google Scholar]
- [37].Yu NY, Wagner JR, Laird MR, Melli G, Rey S, Lo R, Dao P, Sahinalp SC, Ester M, Foster LJ, et al.. PSORTb 3.0: Improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics. 2010;26:1608–15. doi: 10.1093/bioinformatics/btq249. PMID:20472543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Tettelin H, Saunders NJ, Heidelberg JF, Jeffries AC, Nelson KE, Eisen JA, Ketchum KA, Hood DW, Peden JF, Dodson RJ, et al.. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science. 2000;287:1809–15. doi: 10.1126/science.287.5459.1809. PMID:10710307. [DOI] [PubMed] [Google Scholar]
- [39].Grifantini R, Bartolini E, Muzzi A, Draghi M, Frigimelica E, Berger J, Ratti G, Petracca R, Galli G, Agnusdei M, et al.. Previously unrecognized vaccine candidates against group B meningococcus identified by DNA microarrays. Nat Biotechnol. 2002;20:914–21. doi: 10.1038/nbt728. PMID:12172557. [DOI] [PubMed] [Google Scholar]
- [40].Callaghan MJ, Jolley KA, Maiden MC. Opacity-associated adhesin repertoire in hyperinvasive Neisseria meningitidis. Infect Immun. 2006;74:5085–94. doi: 10.1128/IAI.00293-06. PMID:16926400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ferrari G, Garaguso I, Adu-Bobie J, Doro F, Taddei AR, Biolchi A, Brunelli B, Giuliani MM, Pizza M, Norais N, et al.. Outer membrane vesicles from group B Neisseria meningitidis delta gna33 mutant: proteomic and immunological comparison with detergent-derived outer membrane vesicles. Proteomics. 2006;6:1856–66. doi: 10.1002/pmic.200500164. PMID:16456881. [DOI] [PubMed] [Google Scholar]
- [42].Rohde KH, Gillaspy AF, Hatfield MD, Lewis LA, Dyer DW. Interactions of hemoglobin with the Neisseria meningitidis receptor HpuAB: the role of TonB and an intact proton motive force. Mol Microbiol. 2002;43:335–54. doi: 10.1046/j.1365-2958.2002.02745.x. PMID:11985713. [DOI] [PubMed] [Google Scholar]
- [43].Piet JR, Huis int Veld RAG, van Schaik BDC, van Kampen AHC, Baas F, van de Beek D, Pannekoek Y, van der Ende A. Genome Sequence of Neisseria meningitidis Serogroup B Strain H44/76 J. Bacteriol. 2011;193:2371–72. doi: 10.1128/JB.01331-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Marchler-Bauer A, Derbyshire MK, Gonzales NR, Lu S, Chitsaz F, Geer LY, Geer RC, He J, Gwadz M, Hurwitz DI, et al.. CDD: NCBI's conserved domain database. Nucleic Acids Res. 2015;43:222–6. doi: 10.1093/nar/gku1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Melvin JA, Scheller EV, Noël CR, Cotter PA. New insight into filamentous hemagglutinin secretion reveals a role for full-length FhaB in Bordetella virulence. mBio. 2015;6:e01189–15. doi: 10.1128/mBio.01189-15. PMID:26286694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Shippy DC, Fadl AA. tRNA modification enzymes GidA and MnmE: potential role in virulence of bacterial pathogens. Int J Mol Sci. 2014;15:18267–80. doi: 10.3390/ijms151018267. PMID:25310651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Frasch CE, Borrow R, Donelly J. Bactericidal antibody is the immunologic surrogate of protection against meningococcal disease. Vaccine. 2009;27:B112–6. doi: 10.1016/j.vaccine.2009.04.065. PMID:19464093. [DOI] [PubMed] [Google Scholar]
- [48].Pettersson A, Kortekaas J, Weynants VE, Pierre Poolman VJT, Bos MP, Tommassen J. Vaccine potential of the Neisseria meningitidis lactoferrin-binding proteins LbpA and LbpB. Vaccine. 2006;17:243545–57. [DOI] [PubMed] [Google Scholar]
- [49].Norheim G, Aase A, Caugant DA, Høiby A, Fritzsønn E, Tangen T, Kristiansen P, Heggelund U, Rosenqvist E. Development and characterisation of outer membrane vesicle vaccines against serogroup A Neisseria meningitidis. Vaccine. 2005;23:3762–74. doi: 10.1016/j.vaccine.2005.02.021. PMID:15893613. [DOI] [PubMed] [Google Scholar]
- [50].Weynants VE, Feron CM, Goraj KK, Bos MP, Denoe PA, Verlant VG, Tommassen J, Peak IRA, Judd RC, et al.. Additive and synergistic bactericidal activity of antibodies directed against minor outer membrane proteins of neisseria meningitidis. Infect Immun. 2007;75:5434–42. doi: 10.1128/IAI.00411-07. PMID:17664268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Pettersson A, Kuipers B, Pelzer M, Verhagen E, Tiesjema RH, Tommassen J, Poolman JT. Monoclonal antibodies against the 70-kilodalton iron-regulated protein of Neisseria meningitidis are bactericidal and strain specific. Infect Immun. 1990;58:3036–41. PMID:1696939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Haghi F, Peerayeh SN, Siadat SD, Zeighami H. Recombinant outer membrane secretin PilQ(406-770) as a vaccine candidate for serogroup B Neisseria meningitidis. Vaccine. 2012;30:1710–4. doi: 10.1016/j.vaccine.2011.12.076. PMID:22234267. [DOI] [PubMed] [Google Scholar]
- [53].Serruto D, Adu-Bobie J, Scarselli M, Veggi D, Pizza M, Rappuoli R, Arico B. Neisseria meningitidis App, a new adhesin with autocatalytic serine protease activity. Mol Microbiol. 2003;48:323–34. doi: 10.1046/j.1365-2958.2003.03420.x. PMID:12675794. [DOI] [PubMed] [Google Scholar]
- [54].Turner PC, Thomas CE, Stojiljkovic I, Elkins C, Kizel G, Ala'Aldeen DAA, Sparling PF. Neisserial TonB-dependent outer-membrane proteins: detection, regulation and distribution of three putative candidates identified from the genome sequences. Microbiology. 2001;147:1277–90. doi: 10.1099/00221287-147-5-1277. PMID:11320131. [DOI] [PubMed] [Google Scholar]
- [55].Turner DPJ, Marietou AG, Johnston L, Ho KKL, Rogers AJ, Wooldridge KG, Ala'Aldeen DAA. Characterization of MspA, an immunogenic autotransporter protein that mediates adhesion to epithelial and endothelial cells in Neisseria meningitidis. Infect Immun. 2006;74:2957–64. doi: 10.1128/IAI.74.5.2957-2964.2006. PMID:16622234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Gupta SK, Smita S, Sarangi AN, Srivastava M, Akhoon BA, Rahman Q, Gupta SK. In silico CD4+ T-cell epitope prediction and HLA distribution analysis for the potential proteins of Neisseria meningitidis Serogroup B—A clue for vaccine development. Vaccine. 2010;28:7092–97. doi: 10.1016/j.vaccine.2010.08.005. PMID:20716448. [DOI] [PubMed] [Google Scholar]
- [57].Adu-Bobie J, Lupetti P, Brunelli B, Granoff D, Norais N, Ferrari G, Grandi G, Rappuoli R, Pizza M. GNA33 of Neisseria meningitidis is a lipoprotein required for cell separation, membrane architecture, and virulence. Infect Immun. 2004;72:1914–9. doi: 10.1128/IAI.72.4.1914-1919.2004. PMID:15039310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Martin D, Brodeur BR, Hamel J, Couture F, Alwis U, Lian Z, Martin S, Andrews D, Ellis RW. Candidate Neisseria meningitidis NspA vaccine. J Biotechnol. 2000;83:27–31. doi: 10.1016/S0168-1656(00)00294-7. PMID:11000456. [DOI] [PubMed] [Google Scholar]
- [59].Shirley M, Dhillon S. Bivalent rLP2086 vaccine (Trumenba®): A review in active immunization against invasive meningococcal group B disease in individuals aged 10–25 years. BioDrugs. 2015;29(5):353–61. doi: 10.1007/s40259-015-0139-0. PMID:26394633. [DOI] [PubMed] [Google Scholar]
- [60].Serruto D, Spadafina T, Ciucchi L, Lewis LA, Ram S, Tontini M, Santini L, Biolchi A, Seib KL, Giuliani MM, et al.. Neisseria meningitidis GNA2132, a heparin-binding protein that induces protective immunity in humans. Proc Natl Acad Sci U S A. 2010;107:3770–75. doi: 10.1073/pnas.0915162107. PMID:20133713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Esposito S, Principi N. Vaccine profile of 4CMenB: a four-component Neisseria meningitidis serogroup B vaccine. Expert Rev Vaccines. 2014;13:193–202. doi: 10.1586/14760584.2014.874949. PMID:24393061. [DOI] [PubMed] [Google Scholar]
- [62].Ochoa-Azze R, García-Imia L. Efectividad de la vacuna VA-MENGOC-BC® contra cepas heterólogas de meningococo B. VacciMonitor. 2016;25(2):43–48. [Google Scholar]
- [63].Pajon R, Yero D, Niebla O, Climent Y, Sardiñas G, García D, Perera Y, Llanes A, Delgado M, Cobas K, et al.. Identification of new meningococcal serogroup B surface antigens through a systematic analysis of neisserial genomes. Vaccine. 28;2009:532–41. doi: 10.1016/j.vaccine.2009.09.128. PMID:19837092. [DOI] [PubMed] [Google Scholar]
- [64].Sardiñas G, Yero D, Climent Y, Caballero E, Cobas K, Niebla O. Neisseria meningitidis antigen NMB0088: sequence variability, protein topology and vaccine potential. J Med Microbiol. 2009;58:196–208. doi: 10.1099/jmm.0.004820-0. PMID:19141737. [DOI] [PubMed] [Google Scholar]
- [65].Delgado M, Yero D, Niebla O, González S, Climent Y, Pérez Y, Cobas K, Caballero E, García D, Pajón R. Lipoprotein NMB0928 from Neisseria meningitidis serogroup B as a novel vaccine candidate. Vaccine. 2007;25:8420–31. doi: 10.1016/j.vaccine.2007.09.053. PMID:17996338. [DOI] [PubMed] [Google Scholar]
- [66].Wolters DA, Washburn MP, Yates JR III. An automated multidimensional protein identification technology for shotgun proteomics. Anal Chem. 2001;73:5683–5690. doi: 10.1021/ac010617e. PMID:11774908. [DOI] [PubMed] [Google Scholar]
- [67].Millar AH, Heazlewood JL. Genomic and proteomic analysis of mitochondrial carrier proteins in Arabidopsis. Plant Physiol. 2003;131:443–53. doi: 10.1104/pp.103.028399 10.1104/pp.009985. PMID:12586869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Joseph B, Schneiker-Bekel S, Schramm-Gluck A, Blom J, Claus H, Linke B, Schwarz RF, Becker A, Goesmann A, Frosch M, et al.. Comparative genome biology of a serogroup B carriage and disease strain supports a polygenic nature of meningococcal virulence. J Bacteriol. 2010;192:5363–77. doi: 10.1128/JB.00883-10. PMID:20709895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Parkhill J, Achtman M, James KD, Bentley SD, Churcher C, Klee SR, Morelli G, Basham D, Brown D, Chillingworth T, et al.. Complete DNA sequence of a serogroup A strain of Neisseria meningitidis Z2491. Nature. 2000;404:502–06. doi: 10.1038/35006655. PMID:10761919. [DOI] [PubMed] [Google Scholar]
- [70].Bambini S, Piet J, Muzzi A, Keijzers W, Comandi S, De Tora L, Pizza M, Rappuoli R, van de Beek D, van der Ende A, et al.. An analysis of the sequence variability of meningococcal fHbp, NadA and NHBA over a 50-year period in the Netherlands. PLoS ONE. 2013;8:e65043. doi: 10.1371/journal.pone.0065043. PMID:23717687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Muzzi A, Mora M, Pizza M, Rappuoli R, Donati C. Conservation of meningococcal antigens in the genus Neisseria. mBio. 2013;4:e00163–13. doi: 10.1128/mBio.00163-13. PMID:23760461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Koeberling O, Seubert A, Granoff DM. Bactericidal antibody responses elicited by a meningococcal outer membrane vesicle vaccine with overexpressed factor H–binding protein and genetically attenuated endotoxin. J Infect Dis. 2008;198:262–70. doi: 10.1086/589308. PMID:18505380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Masignani V, Comanducci M, Giuliani MM, Bambini S, Adu-Bobie J, Aricò B, Brunelli B, Pieri A, Santini L, Savino S, et al.. Vaccination against neisseria meningitides using three variants of the lipoprotein GNA1870. J Exp Med. 2003;197:789–99. doi: 10.1084/jem.20021911. PMID:12642606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Vipond C, Wheeler JX, Jones C, Feavers IM, Suker J. Characterization of the protein content of a meningococcal outer membrane vesicle vaccine by polyacrylamide gel electrophoresis and mass spectrometry. Hum Vaccine. 2005;1:80–84. doi: 10.4161/hv.1.2.1651. [DOI] [PubMed] [Google Scholar]
- [75].Williams JN, Skipp PJ, Humphries HE, Christodoulides M, O'Connor CD, Heckels JE. Proteomic analysis of outer membranes and vesicles from wild-type serogroup B Neisseria meningitidis and a lipopolysaccharide-deficient mutant. Infect Immun. 2007;75:1364–72. doi: 10.1128/IAI.01424-06. PMID:17158897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Weber MVR, Hammerschmidt S, Bergmann S, Frosch M, Kurzai O. Cytosolic proteins contribute to surface plasminogen recruitment of neisseria meningitidis. J Bacteriol. 2007;189:3246–55. doi: 10.1128/JB.01966-06. PMID:17307854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Tunio SA, Oldfield NJ, Berry A, Ala'Aldeen DAA, Wooldridge KG, Turner DPJ. The moonlighting protein fructose-1, 6-bisphosphate aldolase of Neisseria meningitidis: surface localization and role in host cell adhesion. Mol Microbiol. 2010;76:605–15. doi: 10.1111/j.1365-2958.2010.07098.x. PMID:20199602. [DOI] [PubMed] [Google Scholar]
- [78].Snyder LAS, Saunders NJ. The majority of genes in the pathogenic Neisseria species are present in non-pathogenic Neisseria lactamica, including those designated as virulence genes. BMC Genomics. 2006;7:128. doi: 10.1186/1471-2164-7-128. PMID:16734888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Bos MP, Tommassen J. The LptD chaperone LptE is not directly involved in lipopolysaccharide transport in Neisseria meningitidis. J Biol Chem. 2011;286:28688–96. doi: 10.1074/jbc.M111.239673. PMID:21705335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Dutta A, Bhattacharyya S, Kundu A, Dutta D, Das AK. Macroscopic amyloid fiber formation by staphylococcal biofilm associated SuhB protein. Biophys Chem. 2016;217:32–41. doi: 10.1016/j.bpc.2016.07.006. PMID:27497060. [DOI] [PubMed] [Google Scholar]
- [81].Zhong W, Xu W, Wang H, Huang Y, Cao J, Gong Y, Xu X, Min X, Zhang Y, Dong J, et al.. Mucosal immunization with caseinolytic protease X elicited cross-protective immunity against pneumococcal infection in mice. Exp Biol Med (Maywood). 2012;237:694–702. doi: 10.1258/ebm.2012.011383. PMID:22728705. [DOI] [PubMed] [Google Scholar]
- [82].Mendum TA, Newcombe J, McNeilly CL, McFadden J. Towards the immunoproteome of Neisseria meningitidis. PLOS ONE. 2009;4:e5940. doi: 10.1371/journal.pone.0005940. PMID:19529772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Williams JN, Skipp PJ, O'Connor CD, Christodoulides M, Heckels JE. Immunoproteomic analysis of the development of natural immunity in subjects colonized by neisseria meningitidis reveals potential vaccine candidates. Infect Immun. 2009;77:5080–9. doi: 10.1128/IAI.00701-09. PMID:19737898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Williams JN, Weynants V, Poolman JT, Heckels JE, Christodoulides M. Immuno-proteomic analysis of human immune responses to experimental Neisseria meningitidis outer membrane vesicle vaccines identifies potential cross-reactive antigens. Vaccine. 2014;32:1280–6. doi: 10.1016/j.vaccine.2013.12.070. PMID:24486354. [DOI] [PubMed] [Google Scholar]
- [85].Grifantini R, Bartolini E, Muzzi A, Draghi M, Frigimelica E, Berger J, Ratti G, Petracca R, Galli G, Agnusdei M, et al.. Previously unrecognized vaccine candidates against group B meningococcus identified by DNA microarrays. Nat Biotechnol. 2002;20:914–21. doi: 10.1038/nbt728. PMID:12172557. [DOI] [PubMed] [Google Scholar]
- [86].Finney M, Vaughan T, Taylor S, Hudson MJ, Pratt C, Wheeler JX, Vipond C, Feavers I, Jones C, Findlow J, et al.. Characterization of the key antigenic components and pre-clinical immune responses to a meningococcal disease vaccine based on Neisseria lactamica outer membrane vesicles. Hum Vaccines. 2008;4:23–30. doi: 10.4161/hv.4.1.4806. [DOI] [PubMed] [Google Scholar]
- [87].Tsolakos N, Lie K, Bolstad K, Maslen S, Kristiansen PA, Hoiby EA, Wallington A, Vipond C, Skehel M, Tang CM, et al.. Characterization of meningococcal serogroup B outer membrane vesicle vaccines from strain 44/76 after growth in different media. Vaccine. 2010;28,3211–18. doi: 10.1016/j.vaccine.2010.02.023. PMID:20188677. [DOI] [PubMed] [Google Scholar]
- [88].Galien S, Duriez E, Domon B. Selective reaction monitoring applied to proteomics. J Mass Spectrom. 2011;46:298–312. doi: 10.1002/jms.1895. PMID:21394846. [DOI] [PubMed] [Google Scholar]
- [89].Desiderio DE, Kai M. Preparation of stable isotope-incorporated peptide internal standards for field desorption mass spectrometry quantification of peptides in biologic tissue. Biomed Mass Spectrom. 1983;10, 471–9. doi: 10.1002/bms.1200100806. PMID:6616023. [DOI] [PubMed] [Google Scholar]
- [90].Barr JR, Magio VL, Patterson DG, Cooper GR Jr, Henderson LO, Turner WE, Smith SJ, Hannon WH, Needham LL, Sampson EJ. Isotope dilution-mass spectrometric quantification of specific proteins: model application with apolipoprotein A-I. Clin Chem. 1996;42:1676–82. PMID:8855153. [PubMed] [Google Scholar]
- [91].Atherton E, Sheppard RC. Solid phase peptide synthesis: a practical approach. In: Coligan JE, Dunn BM, Ploegh HL, Current protocols in protein science. Oxford: IRL Press; 1989; pp. 1–216. [Google Scholar]
- [92].Guerrier L, Thulasiraman V, Castagna A, Fortis F, Lin S, Lomas L, Righetti PG, Boschetti E. Reducing protein concentration range of biological samples using solid-phase ligand libraries. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;833:33–40. doi: 10.1016/j.jchromb.2005.12.048. PMID:16455314. [DOI] [PubMed] [Google Scholar]
- [93].Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. PMID:5432063. [DOI] [PubMed] [Google Scholar]
- [94].Perkins DN, Pappin DJ, Creasy DM, Cottrell JS. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis. 1999;20:3551–67. doi: 10.1002/(SICI)1522-2683(19991201)20:18%3c3551::AID-ELPS3551%3e3.0.CO;2-2. PMID:10612281. [DOI] [PubMed] [Google Scholar]
- [95].The UniProt Consortium UniProt: a hub for protein information. Nucleic Acids Res. 2015;43:D204–12. doi: 10.1093/nar/gku989. PMID:25348405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


