Abstract
Accurate assessment of the quantity and chemical type of phosphorus (P) content in processed meat products may have major clinical implications for management of kidney disease patients.
We examined 40 lots of cooked ham including 20 without and 20 with P-containing preservatives. Novel spectro-photometrical methods were employed to measure total P and 3 different P subtypes, i.e., water-soluble (inorganic) P including added preservatives and natural P derived from phospholipids and phosphoproteins separately. Total Nitrogen and fat contents were assayed, as well.
There was 66% more inorganic P in preserved vs. non-enhanced ham, i.e., 169±36 vs. 102±16 mg/100g (p<0.001), respectively; there were no significant differences in P contents derived from proteins or lipids. The P-to-protein ratio in preserved and non-enhanced ham was 16.1±4.0 and 9.8±0.8 mg/g, respectively (p<0.001). The sum of measured inorganic P and P from phospholipids and phosphoproteins was 91%±4 % of measured total P (207.1±50.7 vs 227.2±54.4 mg/100g, p>0.05), indicating a small portion of unspecified P and/or undermeasurement
Novel differential dietary P measurement detects added P-containing preservatives. Processed cooked ham has 66% more measurable inorganic P and 64% higher P-to-protein ratio than non-enhanced product. The contribution of processed food to global dietary phosphorus burden can negatively influence CKD outcome and counteract the efficacy of P-binder medications.
Keywords: Preservatives, enhanced food, phosphate, nutrition, diet, ESRD
Introduction
Phosphorus (P), an abundant element in nature, is an important component of food proteins and other nutrients. In form of phosphate or other chemical compounds, P plays an instrumental role in the structure and function of enzymes and vital metabolic pathways of living organisms. Adequate dietary P is required to survive and to remain healthy. However, emerging research suggests that the contemporary diet in form of processed or enhanced food contains too much unnatural or added P including as “preservatives”. The inorganic P in most food preservatives has higher digestibility index and is, hence, by far more readily (almost 100%) absorbable through the gastrointestinal tract as compared to natural (mostly organic) P which is usually only 40% to 60% absorbable. [1,2] Higher dietary P burden in processed foods can lead to deleterious health consequences, since it may engender or aggravate diseases of the endocrine system, bones and kidneys and be a risk factor for cardiovascular disease and malignancy.[1,2] In particular uncontrolled dietary P burden may be associated with poor outcomes across all stages of chronic kidney disease (CKD) [3,4]. Patients who suffer from this condition have tendency to exhibit positive P balance, which is a main physiopathological factor leading to secondary hyperparathyroidism, vascular calcification and increased cardiovascular morbidity and mortality [5,6].
Dietary P is introduced via inorganic salt or as bound to organic substances [7]. Organic P is present in foods as phosphor-proteins, phospholipids and other sources of phosphorus like phytate or starch phosphate mono-ester in vegetable foods. Inorganic phosphorus is naturally present as phosphate anions in interstitial and intracellular fluids, bone tissue and teeth. In addition to the previously listed substances, which are naturally present in foods in rather small amounts compared to organic P, extra amount of inorganic P can be added as a functional food preservatives in relatively large proportions to the natural P[8,9].
Previously, we and others used spectrophotometric determination of P in mineralized water extracts from common foods as source of protein, without and with P-containing preservatives.[10] Whereas this method yields a relatively accurate estimate of the total P, the differential measurements of diverse types of P in food and in particular quantification of added inorganic P is a difficult task.[11] Poly-phosphates hydrolysis occurs during the shelf life of meat products and they may ultimately be converted to orthophosphate. It has long been believed that dietary P is strictly correlated with the dietary protein content [9, 12], and protein-rich food, such as meat-products, contain more organic P molecules (nucleotides, phospholipids, etc.), along with orthophosphate salts. Accordingly, until very recently the amount of added P was determined by mathematical calculations of the difference between the measured total P and P estimated on the basis of protein content.[13] A more reliable method to measure added P is urgently needed for public health nutrition research but practical and reproducible methods to measure dietary P differentially and to identify the different P-containing molecules in food have been lacking.
The aim of our current study is to quantify different type of P present in meat-products and to compare them between processed and non-enhanced foods using a novel biochemical procedure derived from independent spectro-photometry technology. Throughout this manuscript, “enhanced” or ”processed” food indicated a food product that contains added “preservatives” according to its visible label.
Results
Table 1 shows the content of dry matter, nitrogen and total P as well as the differential P measurements in processed and non-enhanced cooked ham samples, i.e., with or without listed P containing preservatives, respectively. Dry matter, total nitrogen, protein and fat contents were 14%, 8%, 8% and 43% lower, respectively, in each 100 grams of preserved compared to non-enhanced cooked ham products. Whereas non-enhanced ham contained on average 185 mg of total P per 100 g of serving, in the processed ham 85 mg or 46% more total P was found per same unit. However, the larger standard deviation in preserved ham (+/−46 mg/100g) compared to non-enhanced product (+/−17 mg/100g) demonstrated a wider variability in P content in the former. Given the higher total P and lower protein content of the processed ham, the P-to-protein ratio was 64% higher in the preserved ham compared to non-enhanced product
Table 1.
Dry Matter, Total Nitrogen (N), Total Phosphorus (P) and Protein content in 100 grams of edible cooked ham in 20 non-enhanced (without preservatives) and 20 processed (with added preservatives) ham products. Measurements of Inorganic P, of P in protein and of P in lipids are also reported.
| WITH preservatives |
WITHOUT preservatives |
p-value | |
|---|---|---|---|
| Dry matter, g/100g | 25.6 ± 1.6 | 29.6 ± 2.6 | < 0.001 |
| Total N, g/100g | 2.82 ± 0.37 | 3.07 ± 0.43 | < 0.001 |
| Protein, g/100g | 17.6 ± 2.3 | 19.2 ± 2.7 | < 0.05 |
| Fat, g/100g | 4.3 ± 1.8 | 7.5 ± 3.3 | < 0.001 |
| Total P, mg/100g | 270.2 ± 46.0 | 185.4 ± 17.3 | < 0.001 |
| P / Protein, mg/g | 16.1 ± 4.0 | 9.8 ± 0.8 | < 0.001 |
| Inorganic P, mg/100g | 169.3 ± 36.6 | 101.7 ± 16.0 | < 0.001 |
| P in protein, mg/100g | 40.2 ± 13.1 | 37.5 ± 11.9 | n.s. |
| P in Lipids, mg/100g | 36.1 ± 16.9 | 30.5 ± 12.6 | n.s. |
| P unspecified*, mg/100g | 16.0 ± 8.0 | 24.0 ± 11.0 | <0.01 |
As “unspecified P” we mean the difference between total P and the sum of 3 measured types of P.
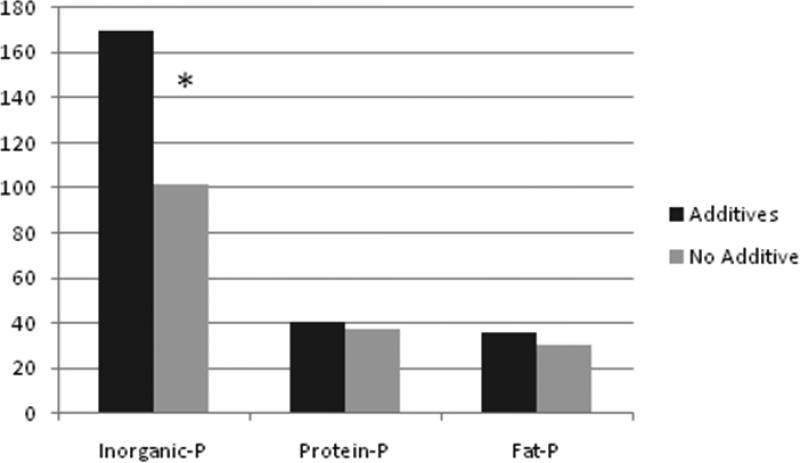
To examine the contributions of different types of P to the total dietary P burden of the ham products, we used novel modified spectrophotometric methods as described above. Table 1 and Figure 1 show the differential P measurements indicating that inorganic phosphorus was 66% higher in the processed ham (169+/−37 mg/100g) compared to non-enhanced ham (102+/−16 mg/100g). However, in the separately measured organic P from phosphoproteins there was only 7% difference between the two ham products (p-value>0.05). The extracted organic P from fat in form of phospholipids was slightly higher in the enhanced food but the difference was not statistically significant either.
Figure 1.
Milligram of Inorganic Phosphorus (P) versus phosphorus bound to Protein and Lipids in 100 g of edible cooked ham. * p < 0.001
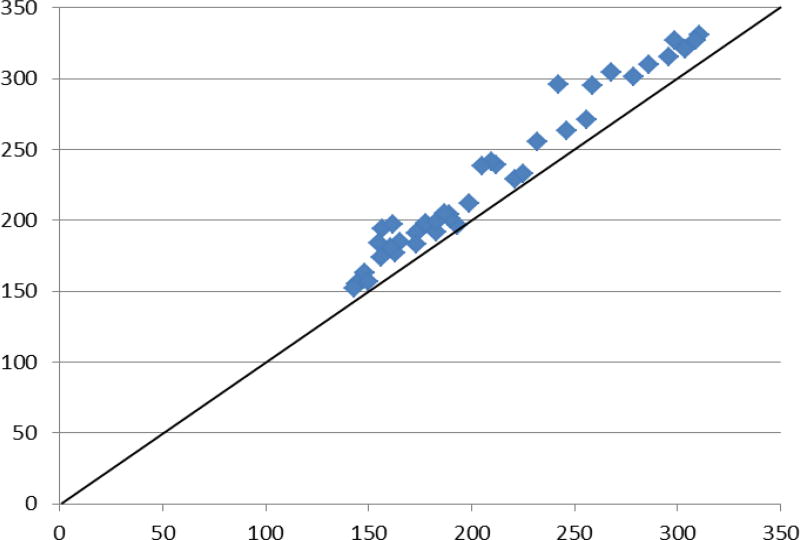
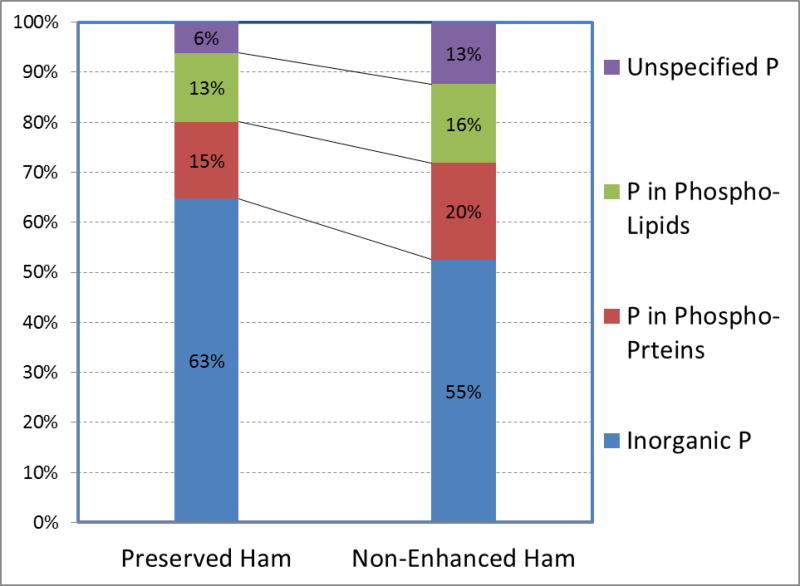
Figures 2 shows the scatter plot of the correlation between the measured total P (Y axis) and the sum of the subtypes of P in form of inorganic P plus P extracted from proteins and lipids (X axis). In our series of experiments, the sum of measured inorganic P and P from phospholipids and phosphoproteins provided aggregate values which were slightly lower than the measured total P (207.1±50.7 vs 227.2±54.4 mg/100g, p>0.05), indicating that we likely managed to measure 91% ± 4 % the constituents of the total P accurately. This gap, herewith referred to as unspecified P, may be due to the technical difficulties of completely extracting the inorganic P from the food matrix, as reported by Jastrzębska et al [14] or due to other unknown or unmeasured types of P. After subtracting the sum of separately measured P types from total P, the resultant unspecified (non-classified) P was lower in the processed food (see Figure 3).
Figure 2.
the scatter plot of the correlation between the measured total P (Y axis) and the sum of the subtypes of P in form of inorganic P plus P extracted from proteins and lipids (X axis).
Figure 3.
Comparison P proportions in preserved vs. non-enhanced ham products\
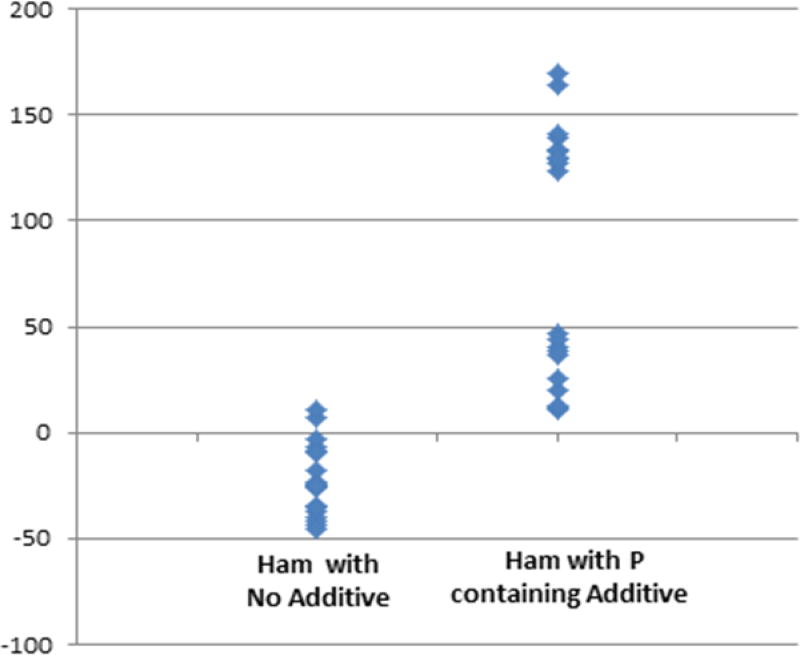
We also estimated total P based on the assumption of 10.92 mg P (or mg P2O5) per gram of protein in ham[13,15]. Figure 4 shows the difference between the measured and expected total P content in each 100 g of cooked ham in both samples without and with listed P-containing preservatives. In respect to the amount of P expected on the basis of protein content, the extra-P content from preservatives ranged from 11 to 164 mg P per 100 g of cooked ham, which was consistent with a larger standard deviation of these products as shown in Table 1.
Figure 4.
Difference of total phosphorus content (mg) in respect to the amount of expected phosphorus content of 100 g of cooked ham (estimated as 25 mg P2O5 per g of protein, i.e. 10,92 mg P per g of protein) in samples without or with listed P-containing additives
Discussion
Examining the total and differential P contents via novel spectro-photometric methods in 20 processed and 20 non-enhanced ham products purchased randomly in a grocery store in Italy, we found that compared to non-enhanced hams, in hams with P containing preservatives the dry matter and protein and fat contents were significantly lower per 100 grams of serving. There was 66% more inorganic P in the processed vs. non-processed ham. Whereas we found no significant difference in the P content derived from proteins or lipids, the P to protein ratio in the preserved ham was 64% higher than non-enhanced ham. The sum of measured inorganic P and P from phospholipids and phosphoproteins was only slightly lower than the measured total P indicating that we managed to measure 91% of the subtypes of the total P accurately in these meat products. These findings may have major technical, clinical, public health implications in the management of global dietary P burden and disease management in both the general population and groups with certain disease states as CKD.
In the present study, not only the total P content was measured but also its principal alternative forms, namely inorganic phosphorus and P from phospholipids and from phosphoproteins, in that each one was determined by separately by independent spectro-photometry analyses. The method of inorganic P used here is based upon extraction with water solution 1 mM NaOH: Hence, the measured inorganic P (Pi) includes any types of P that is soluble in water, which is mainly PO4-3 (phosphate) anions, but also all other inorganic P such as polyphosphates. On the other hand, it does not include phospholipids and phosphoproteins which are not soluble in water (or in only negligible amount <1%).
It is important to note that for other complex P-containing molecules such as ATP or ADP, the P solubility is not well defined. In addition, it should be noted that the process of food cooking and preparation can modify these molecules. Meat products per se undergo additional chemical modification after death of the animal. For example, an almost complete conversion of ATP or ADP occurs in most cells that die due to lack of oxygen supply upon death. Therefore it is likely, although not clearly proven, that the P that was originally in ATP be recovered as inorganic P in our measurements, hence, explaining why in our study the non-enhanced ham without preservatives still contained 55% inorganic P. In addition, all ham samples we studied were cooked, and the cooking process may have "modified" other molecules in which the P was bound, releasing more P ions that we measured together as inorganic ions.
We chose the cooked ham for this investigation because it is an example of a meat product which is widely consumed, the product of a well-defined anatomical part of an animal (i.e. pork thigh), and it is considered to have moderately low P-to-protein ratio as opposed to other animal based protein such as beef, salmon or egg yolk.[2, 9] As expected, inorganic P found in the preserved samples was almost twice that found in the sample group which did not. Meanwhile, the amounts of P bound to proteins (as phosphoproteins) or to lipids (as phosphor-lipids) were not found to be different between the 2 groups of cooked ham samples i.e. with or without listed P containing preservatives. (Figure 1). Dry matter was found to be significantly lower in the ham samples with P-containing additives : this may be the natural consequence of higher water content in the preservatives containing samples, which may be consistent with some of the objectives of food processing.
In the current nutritional and dietary practice, the amount of added P is usually determined by the difference between the measured total P and the P estimated on the basis of protein content. The estimated amount of P in each food item can be calculated from the estimated protein concentration (measured as total nitrogen by the Kjeldahl method) using the known P to protein ratio of a given food item. The total P–to-Protein ratio (mg/g) for different raw meats are shown by Dušek et al [16]: for example, pork thigh is assumed to have 25 mg P2O5 per g of protein [13]. The actual P to protein ratio is a function of not only different animal species but it is also influenced also by any other substance which may be added during food processing; in our series, on average, P2O5 resulted 22.4 mg per gram of protein.
P containing preservatives, also known as additives in lay language, contribute to an important extra-load of dietary P that may be particularly risky because this type of P is almost completely (>90%) absorbed by the gut. Therefore, it contributes significantly to the dietary P burden and may engender or aggravates such complications as vascular calcification and hyperparathyroidism especially in CKD patients. On average, foods with listed preservatives that contain P have an extra-P content of about 60–70 mg per 100 g of products which is more readily absorbable than the natural P. However, in our study there was a relatively wide range, i.e., from 11 to 164 mg P per 100 g of cooked ham, which exhibits wide ranges of hidden or unknown P intake upon ingesting such meat products. Egg, milk or plant protein extracts or isolates can be added to modify either total P or net protein content. Of consequence, the P to protein ratio can decrease or increase, leading to errors in the estimation of added P [10, 13, 16]. As a whole, this is one more important reason to encourage state governments to obligate food manufacturers to report the total P as well as the amount of added P on food labels.[17]. Such measures can help consumers make more appropriate choices upon purchasing meat products. We believe that implementation of nutritional education and counselling about phosphorus dietary sources is needed [9,18,19]. Indeed, the restriction of known and hidden phosphorous intake to gain better control of serum phosphorus is of crucial importance to chronic kidney disease patients, but is also beneficial for cardiac patients and even for the general population [20–22].
Our study should be qualified for its relatively small sample size (20+20 samples) and its restriction to Italian ham products. Moreover, we did not compare our measurements to another gold standard. However, there is currently no gold standard of differential P measurement, so that the verification of the sum of 3 P compartments comprising 91% of the total P should be considered as a quality assurance and reproducibility approach. To our knowledge, this is the first study that uses 3 novel differential P measurement methods combined with other nutrient extractions.
Conclusion
Measurement of different subtypes of dietary P using our novel procedures is feasible and can accurately detect the added P-containing preservatives in meat products. Both processed and non-enhanced cooked ham products contain measurable inorganic P, although the former has 66% more measurable inorganic P than the latter. The P to protein ratio in the preserved ham is 64% higher than non-enhanced product. Our pioneering findings show that it is possible to differentiate among the various types of P -containing molecules in cooked ham and likely other food products. In our opinion, these procedures can be extended to all common foods of both animal and plant origin and can also include determination of P from phytin in certain plant products including legumes. Since the main differentiating component is inorganic P, the amount of which increases dramatically when P containing preservatives are added during food processing. and since P bound to natural proteins and to lipids (i.e. included in phosphoproteins or phospholipids) represents a lower but relatively fixed part of dietary P.
Indeed, the restriction of known and hidden phosphorous intake to gain better control of serum phosphorus is of crucial importance to chronic kidney disease patients, but is also beneficial for cardiac patients and even for the general population. The contribution of processed food to global dietary P and disease burden warrants additional investigations.
Methods
Samples
Forty lots of cooked ham (20 brands without and 20 with listed phosphates preservatives in the food label), were randomly purchased in a typical grocery market in Pisa, Italy.
Foods with phosphate preservatives were recognized by the phrase “containing polyphosphates” or by the initials “ E338–E341, E450–452 “ on the food label according to European Union Food Labeling Legislation (Directive 2000/13/EC). The purchased food samples were minced, homogenized with a plate of 3 mm diameter holes, packed into polyethylene bags, frozen and stored at −20°C until assayed.
Analytical Procedures
Dry matter and total nitrogen (TN) were determined as previously described [23]. In brief, dry matter was determined accordingly to the International Standards Organization 1442:1973. Total nitrogen was determined by the Kjeldhal method using a mixture of K2SO4 and CuSO4 as catalyst. Samples (0,3 g) were digested with a mixture of sulphuric acid and Hydrogen peroxide and the content of nitrogen was determined by titolation with 0,05 N NaOH versus blank. Total Protein was calculated using the formula total nitrogen × 6,25.
Total Lipids (TL)
Extraction of lipids was performed as follows: samples (2 ± 0.0001 g fresh mass) were mixed with 20,0 ml of chloroform:methanol (2:1, v/v) and immediately homogenized for 2 min at medium speed in a Ultra-turrax homogenizer. Apparatus was rinsed twice with a portion of 5,0 ml chloroform:methanol (2:1, v/v) and all the extracts were then combined. The pooled extract was centrifuged (10 min, 2000g) and filtered through Whatman n°1 filter paper. Residue in filter paper was washed with one portion of 5,0 mL chloroform:methanol (2:1, v/v) and all the filtrates were then combined. Subsequently, 4,0 ml of distilled water was added and the new mixture was shaken vigorously. The final biphasic system was allowed to separate by centrifugation (10 min, 2000g). The upper aqueous phase was eliminated, 1,0 g of anhydrous sodium sulphate was added and the mixture was shaken vigorously. The chloroform phase was filtered through Whatman n°1 filter paper and collected. Residue in filter paper was washed with 5,0 mL of chloroform and the filtrate was combined. The solvent was further evaporated with a rotary evaporator under vacuum and finally, the glass vessels with the residue, which contained the lipids, were dried in a conventional oven (100 °C for 30 minutes.). Cool lipid extract content was then gravimetrically determined against blank.
Total and different subtypes of Phosphorus
Total and inorganic P, as well as P from phospholipids and phosphoproteins were determined by the following procedures of sample processing and final P measurement by molybdenum blue method on wet ashing samples.
Total phosphorous (TP)
Total phosphorus was determined as previously described [23]. In brief sample (0,4 g) was wet mineralized with sulphuric acid and hydrogen peroxide. Cool digest was diluted to 100 mL with bi-distilled water and underwent spectrophotometric Molybdenum blue procedure with ascorbic acid for phosphorus concentration measurement.
Inorganic phosphorous (IP)
Soluble phosphate ions, which usually include either natural or, to a greater extent, added inorganic phosphorus, were extracted according to Jastrzębska’s method [14], which was modified by us [10]: the samples (5 ± 0.0001 g fresh mass) were extracted with 10.0 mL of 1 mM NaOH using an orbital shaker for 60 min. The extracts were separated using centrifuge at 4800g for 30 min. Supernatant was filtered with a Whatman n°1 filter paper, and then the filtrate were transferred into a 50 mL volumetric flasks, made up to the mark with bi-distilled water. Since the filtration step was rather difficult (polyphosphates bind the water), this step was accomplished by using a vacuum system. Five millilitres sample solution was then pipetted in a 125 mL digestion flask and digested at a final temperature of 420 °C with 5 mL of sulphuric acid 96% and 5 mL of H2O2 35%, until mixture was clear. Cool digest was diluted to 100 mL with bi-distilled water and underwent spectrophotometric Molybdenum blue procedure with ascorbic acid. Cool digest was diluted to 100 mL with bi-distilled water and underwent spectrophotometric Molybdenum blue procedure with ascorbic acid for phosphorus concentration measurement.
Phosphorous from phospholipids (PL)
Based on the results reported by Pérez-Palacios et al. [15], lipid extraction was performed according to the method of Folch (Folch et al. 1957). Samples (2 ± 0.0001 g fresh mass) were subjected to the lipid extraction procedure as described above and ccool lipid extract content was then gravimetrically determined. About half of it (from 0,04–0,08 g) was then placed into a 125 mL digestion flask and digested at a final temperature of 420 °C with 5 mL of sulphuric acid 96% and 5 mL of H2O2 35%, until mixture was clear. Cool digest was diluted to 100 mL with bi-distilled water and underwent spectrophotometric Molybdenum blue procedure with ascorbic acid for phosphorus concentration measurement.
Phosphorous from Phosphoproteins (PP)
Phosphorous from phosphoproteins were determined according to Dušek et al [16] with slight modifications: samples (1,5000 ± 0.0001 g fresh mass) was homogenized in 36,5 ml bi-distilled water for 2 min at medium speed by Ultraturrax homogenizer. Apparatus was rinsed with 1 mL of bidistilled water and the solutions were then combined. The mixture thus obtained was boiled for 30 min on a hot plate. After cooling, 10 ml of 10% trichloracetic acid was added. The precipitate was filtered through Whatman n° 1 filter paper and then was dried in a conventional oven (100 °C for 30 minutes.). After cooling, the filter was gravimetrically determined and about 40% of it (equivalent to 0,6 g fresh mass) was placed in a 125 mL digestion flask and digested at a final temperature of 420 °C with 5,0 mL of sulphuric acid 96% and 5,0 mL of H2O2 35%, until mixture was clear. Cool digest was diluted to 100 mL with bi-distilled water and underwent spectrophotometric Molybdenum blue procedure with ascorbic acid for phosphorus concentration measurement.
Final Measurement of Phosphorus
Orthophosphate ions content into mineralized sample solutions from TP, IP, PP and PL was determined by Molybdenum Blue method according to AOAC and Italian Dairy Product Official Methods of Analysis. In brief (0,800 mL) were transferred to 10 mL volumetric flask and the following were added: 0,400 mL of ammonium molibdate solution 1.80 %, 0,400 mL of ascorbic acid solution 2,5%, 7,00 mL of bi-distilled water and 0,390 mL of sulphuric acid solution 8N. Each flask was gently swirled and finally placed in boiling water for 30 minutes to form characteristic molybdenum blue species. The solution was then cooled to room temperature, made up to the mark with bi-distilled water and absorbance was measured by spectrophotometry at 650 nm against blank
Measurement Settings
All the laboratory technicians were blinded to food labelled nutritional content. In every TN, TL, TP, IP, PP and PL determination batch there was a blank (N=2). All analyses were performed in duplicate.
Expected Phosphorus content
The expected amount of phosphorus is calculated by the estimated protein concentration (measured as total nitrogen by the Kjeldahl method) multiplied by the known phosphate to protein ratio of a food. Total P2O5 /Protein (mg/g) ratio for different raw meats are shown by Dušek et al [16]. Pork thigh is assumed to have 25 mg P2O5 per g of protein [13], by the Italian National Institute of Health.
Statistical analysis
All the results are given as Mean ± Standard Deviation. Statistical analysis was performed using Student “t” test for unpaired data. Differences were considered as statistically significant when p <0.05.
Acknowledgments
Funding: KKZ is supported by NIH grants K24-DK091419, R01-DK078106 and R21-DK078012
Footnotes
Disclosure:
No Conflict of interest is declared by the authors
References
- 1.Noori N, Kalantar-Zadeh K, Kovesdy CP, Bross R, Benner D, Kopple JD. Association of dietary phosphorus intake and phosphorus to protein ratio with mortality in hemodialysis patients. Clin J Am Soc Nephrol. 2010;5:683–92. doi: 10.2215/CJN.08601209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cupisti A, Morelli E, D’Alessandro C, Lupetti S, Barsotti G. Phosphate control in chronic uremia: don’t forget diet. J Nephrol. 2003;16:29–33. [PubMed] [Google Scholar]
- 3.Zoccali C, Ruggenenti P, Perna A, Leonardis D, Tripepi R, Tripepi G, Mallamaci F, Remuzzi G, for the REIN Study Group Phosphate, CKD progression and ACE-inhibition. A post-hoc analysis of the REIN trial. JASN. in press. [Google Scholar]
- 4.Spasovski G, Massy Z, Vanholder R. Phosphate Metabolism in Chronic Kidney Disease: From Pathophysiology to Clinical Management. Seminars in Dialysis. 2009;22:357–62. doi: 10.1111/j.1525-139X.2009.00580.x. [DOI] [PubMed] [Google Scholar]
- 5.Mathew S, Tustison KS, Sugatani T, Chaudhary LR, Rifas L, Hruska KA. The mechanism of phosphorus as a cardiovascular risk factor in CKD. J Am Soc Nephrol. 2008;19:1092–105. doi: 10.1681/ASN.2007070760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burke SK. Phosphate Is a Uremic Toxin. J Ren Nutr. 2008;18:27–32. doi: 10.1053/j.jrn.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Weiner ML, Salminenb WF, Larsonc PR, Barterd RA, Kranetz JL, Simon GS. Toxicological review of inorganic phosphates. Food and Chemical Toxicology. 2001;39:759–86. doi: 10.1016/s0278-6915(01)00028-x. [DOI] [PubMed] [Google Scholar]
- 8.Sherman RA, Mehta O. Dietary phosphorus in dialysis patients: potential impact of processed meat, poultry, and fish products as protein sources. Am J Kidney Dis. 2009;54:18–23. doi: 10.1053/j.ajkd.2009.01.269. [DOI] [PubMed] [Google Scholar]
- 9.Kalantar-Zadeh K, Gutekunst L, Mehrotra R, et al. Understanding Sources of Dietary Phosphorus in the Treatment of Patients with Chronic Kidney Disease. Clin J Am Soc Nephrol. 2010;5:519–30. doi: 10.2215/CJN.06080809. [DOI] [PubMed] [Google Scholar]
- 10.Benini O, D'Alessandro C, Gianfaldoni D, Cupisti A. Extra-phosphate load from food additives in commonly eaten foods: a real and insidious danger for renal patients. J Ren Nutr. 2011;21:303–8. doi: 10.1053/j.jrn.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 11.Jastrzębska A. Determination of sodium tripolyphosphate in meat samples by capillary zone electrophoresis with on-line isotachophoretic sample pre-treatment. Talanta. 2006;69:1018–24. doi: 10.1016/j.talanta.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Boaz M, Smetana S. Regression equation predicts dietary phosphorus intake from estimate of dietary protein intake. J Am Diet Assoc. 1996;96:1268–70. doi: 10.1016/S0002-8223(96)00331-8. [DOI] [PubMed] [Google Scholar]
- 13.Baldini M, Fabietti F, Giammarioli S, Onori R, Orefice L, Stacchini A. Metodi di analisi utilizzati per il controllo chimico degli alimenti. Rapporti ISTISAN. 1996;34 [Google Scholar]
- 14.Jastrzębska A, Hol A, Szłyka E. Simultaneous and rapid determination of added phosphorus(V) compounds in meat samples by capillary isotachophoresis. LWT - Food Science and Technology. 2008;41:2097–103. [Google Scholar]
- 15.Pérez-Palacios T, Ruiz J, Martín D, Muriel E, Antequera T. Comparison of different methods for total lipid quantification in meat and meat products. Food Chemistry. 2008;110:1025–29. doi: 10.1016/j.foodchem.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 16.Dušek M, Kvasnika F, Lukášková L, Krátká J. Isotachophoretic determination of added phosphate in meat products. Meat Science. 2003;65:765–9. doi: 10.1016/S0309-1740(02)00279-6. [DOI] [PubMed] [Google Scholar]
- 17.Karalis M. Food and Drug Administration Petition on Food Labeling: An Update From the American Dietetic Association and National Kidney Foundation. J Ren Nutr. 2007;17:423–4. doi: 10.1053/j.jrn.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Cupisti A, D'Alessandro C, Baldi R, Barsotti G. Dietary habits and counseling focused on phosphate intake in hemodialysis patients with hyperphosphatemia. J Ren Nutr. 2004;14:220–5. [PubMed] [Google Scholar]
- 19.Caldeira D, Amaral T, David C, Sampaio C. Educational Strategies to Reduce Serum Phosphorus in Hyperphosphatemic Patients With Chronic Kidney Disease: Systematic Review With Meta-analysis. J Ren Nutr. 2011;21:285–94. doi: 10.1053/j.jrn.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Young B, Sherrard DJ, Andress DL. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol. 2005;16:520–28. doi: 10.1681/ASN.2004070602. [DOI] [PubMed] [Google Scholar]
- 21.Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation. 2005;112:2627–33. doi: 10.1161/CIRCULATIONAHA.105.553198. [DOI] [PubMed] [Google Scholar]
- 22.Dhingra R, Sullivan LM, Fox CS, Wang TJ, D’Agostino RB, Graziano JM, Vasan RS. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med. 2007;167:879–85. doi: 10.1001/archinte.167.9.879. [DOI] [PubMed] [Google Scholar]
- 23.Cupisti A, Comar F, Benini O, Lupetti S, D'Alessandro C, Barsotti G, Gianfaldoni D. Effect of Boiling on Dietary Phosphate and Nitrogen Intake. J Ren Nutr. 2006;16:36–40. doi: 10.1053/j.jrn.2005.10.005. [DOI] [PubMed] [Google Scholar]