Abstract
Objectives
Objective measures of clinical improvement in patients with acute heart failure (AHF) are lacking. The aim of this study was to determine whether repeated lung ultrasound could semi-quantitatively capture changes in pulmonary edema (B-lines) in patients with hypertensive AHF early in the course of treatment.
Methods
We conducted a feasibility study in a cohort of adults with acute onset of dyspnea, severe hypertension in the field or at triage (systolic blood pressure ≥ 180 mm Hg), and a presumptive diagnosis of AHF. Subjects underwent repeated dyspnea and lung ultrasound assessments using a 10-cm visual analog scale (VAS) and an 8-zone scanning protocol. Lung ultrasound assessments were performed at the time of triage, initial VAS improvement, and disposition from the emergency department. Sonographic pulmonary edema (SPE) was independently scored offline in randomized and blinded fashion using a scoring method that accounted for both the sum of discrete B-lines and degree of B-line fusion.
Results
SPE scores from significantly decreased from initial to final ultrasound assessments (p<0.001). The median percentage decrease among the 20 included patient encounters was 81% [IQR 55%, 99%]). While SPE scores correlated with VAS scores (ρ=0.64, p<0.001), the magnitude of change in these scores did not correlate with each other (ρ=−0.04, p=0.89).
Conclusions
Changes in SPE can be semi-quantitatively measured by serial 8-zone lung ultrasound using a scoring method that accounts for B-line fusion. SPE improves in patients with hypertensive AHF during the initial hours of treatment.
Keywords: pulmonary edema, ultrasound, congestive heart failure, hypertension, dyspnea, hypoxia
A primary diagnosis of acute heart failure (AHF) accounts for approximately one million annual emergency department (ED) visits in the United States1. Considering the high rates of 30-day readmission2 and 30-day mortality3 following AHF hospitalization, accurate assessment of treatment efficacy is critical. However, objective non-invasive measures of clinical improvement in AHF patients are lacking. Changes in B-type natriuretic peptide (BNP) levels are unlikely to rapidly decrease4, and incorporating serial BNP levels into inpatient management has not been shown to improve hospital length of stay, 30-day mortality, or readmission rates5. The most common symptom prompting AHF patients to seek care is dyspnea6,7, and its improvement is an important measure for ascertaining therapeutic efficacy in clinical practice and clinical trials8–10. Dyspnea, however, is subjective, multifactorial, and language used to describe dyspnea is subject to cultural and racial differences11.
A more objective measure of pulmonary edema, the primary pathophysiologic derangement underlying dyspnea in AHF patients, might help clinicians better gauge therapeutic success. The chest radiograph has been the conventional imaging tool used to identify and follow changes in pulmonary edema. Radiographic signs of pulmonary edema, however, are insensitive12, subject to variability in interpretation13, and may not change contemporaneously with rapid fluid changes occurring in the pulmonary interstitium. Lung ultrasound, in contradistinction, identifies pulmonary edema with high sensitivity12 and has demonstrated utility in capturing changes in pulmonary edema in real time14,15. The dynamics of pulmonary decongestion using lung ultrasound in AHF patients are not well defined and have not been studied during the earliest phases of treatment.
Semi-quantitative measures of sonographic pulmonary edema (SPE) have been based on the grand sum of discrete vertical artifacts called B-lines in an intercostal space from each of 28 thoracic zones14–20. When pulmonary edema is severe, B-line quantification becomes less straightforward because B-lines fuse together (Figure 1). An alternative method for quantifying B-lines based on estimates of B-line confluence has been proposed21 and found to have good inter-rater reliability22, but it has not been applied to serial sonographic measures of pulmonary edema. The objective of this study was to demonstrate the feasibility of measuring sonographic pulmonary decongestion in AHF patients during the course of their treatment in the ED with a simplified 8-zone scanning protocol and a measure of B-lines that accounts for B-line fusion. We chose to study patients with hypertensive AHF who presented with acute onset of dyspnea in order to ensure that lung ultrasound could capture the most rapid changes in pulmonary edema. In this phenotype of AHF, dyspnea is often severe and improves dramatically in response to treatment targeting cardiac loading conditions23,24.
Figure 1.
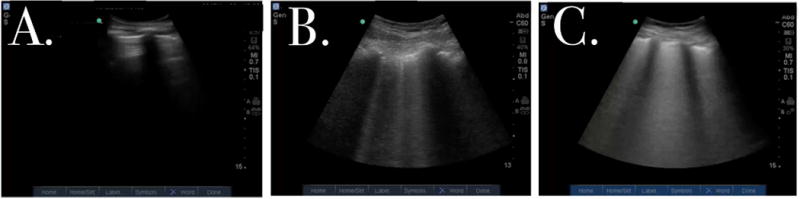
Still images obtained from lung ultrasound video clips.
A. Absence of B-lines
B. Discrete B-lines.
C. Complete B-line fusion
Materials and Methods
Study population
This is a prospective, observational cohort study designed to evaluate the extent to which sonographic B-lines disappear with treatment in a convenience sample of subjects presenting to the ED with presumed hypertensive AHF and acute dyspnea. Enrollment took place between October 2015 and September 2016 in two academic EDs with a combined annual census of 195,000 patients. This study was conducted in accordance with the amended Declaration of Helsinki and was approved by the Institutional Review Boards at both hospitals. Patients with an early working diagnosis of hypertensive AHF were selected based on an initial systolic blood pressure ≥ 180 mm Hg obtained either in the prehospital setting or at ED triage, acute onset of dyspnea, and the presence of pathologic B-lines (≥ 3 lines per intercostal space) in both lungs on a rapid anterior two-region lung ultrasound21,25. Exclusion criteria were fever, ST-elevation myocardial infarction, primary lung malignancy, pulmonary fibrosis, sarcoidosis, a working diagnosis of acute lung disease (COPD, asthma, pneumonia) as the primary cause of dyspnea, pneumothorax, acute respiratory distress syndrome (ARDS), acute pulmonary embolism, and end-stage renal disease (ESRD). The study sample was limited to those subjects who could be scanned within 45 minutes of ED triage. Subjects were not excluded if they received prehospital vasodilator or diuretic therapy or if non-invasive ventilation (NIV) was initiated by emergency medical services (EMS) prior to ED arrival.
Study flow
Immediately after eligible subjects consented to participate in the study, the investigator performed an initial 8-zone lung ultrasound and dyspnea assessment, and measured concurrent vital signs. Thereafter, dyspnea severity was repeatedly assessed at fixed time intervals during the ED course until the subject showed a 2-cm improvement on a visual analog scale. A second lung ultrasound and set of vital signs were obtained at the time of this initial substantial improvement. A third and final lung ultrasound, dyspnea assessment, and set of vital signs were obtained at the time the treating clinician decided on the subject’s disposition (regardless of the subject’s score on the visual analog scale). The treating clinicians were blinded to SPE scores and not present in the room for the repeated 8-zone lung ultrasounds. The treating team was able to perform their own lung ultrasound exams if they wished to. In a few cases in which the treating clinician hadn’t yet performed their own diagnostic lung ultrasound, the clinician was in the room for the rapid anterior two-region lung ultrasound used to determine study eligibility performed by the investigator. The treating clinicians were not asked to follow any specific treatment protocol.
Dyspnea Assessment
Dyspnea severity was evaluated using an uncalibrated, horizontal 10-cm visual analog scale (VAS) that has been previously used in heart failure research26. Subjects were asked to mark the line based on their self-perceived proximity to the two statements flanking the line. If subjects reported feeling “less short of breath” (they were asked at 20-minute intervals from the time of initial sonogram), they were given the same VAS that they initially marked and asked to make a second mark. A second ultrasound was performed if and when a 2-cm improvement was measured on the VAS. This has previously been defined as a clinically meaningful difference27. Dyspnea severity was also serially administered with a 5-point Likert scale at the of each lung ultrasound. Measures on this ordinal scale have previously been shown to correlate with B-line severity28.
Lung ultrasound
Investigators performing lung ultrasound were board-certified emergency physicians with variable levels of formal training in point-of-care ultrasound. All investigators underwent a 30-minute training session on the lung ultrasound protocol before enrollment. As described by Volpicelli et al29, lung ultrasound was performed by scanning 8 thoracic zones (2 anterior and 2 lateral per hemithorax) with the head of the stretcher at a minimum of 45°. Scans were performed using a 3.5-MHz curvilinear transducer (Sonosite Inc, Bothell, WA) on abdominal settings set to a depth of 11–15 cm. A 6-second video clip of an intercostal space from each of the 8 thoracic zones was recorded for future off-line interpretation.
Video segments were recorded as QuickTime mp4 files, which were then numbered and arranged by a random sequence generator before compilation into a single randomized file for review after subject enrollment was completed. Two investigators fellowship-trained in sonography (JFK, MS) independently analyzed the de-identified video clips and recorded their assessments in separate spreadsheet files (Excel 2014, version 15.18 Microsoft, Redmond, WA). Each investigator analyzed every video clip, and comparisons of the scores for all clips were used to measure inter-rater reliability. A subset of 40 video clips from the data set was randomly selected using a random number generator, then duplicated and mixed into the data set to measure intra-rater reliability. The investigators performing clip analysis were blinded to subject identity, clinical information, and timing of video clip acquisition.
B-lines were defined as vertical hyperechoic lines arising from the pleural line, extending down to the bottom of the screen, and moving synchronously with respirations. A sonographic pulmonary edema (SPE) score was based on the greatest number of B-lines (or degree of B-line fusion) noted in a single intercostal space at any instant during each video clip. Confluent B-lines were scored by dividing the percentage of pleural line occupied by confluence by the number 1021. This number was added to any other discrete B-lines that might be simultaneously noted in the intercostal space. A score of 10 would be applicable if the entire pleural line were occupied by B-line confluence22. The overall SPE score for each 8-zone scan was defined as the sum of individual SPE scores. Final SPE scores were based on the average sum between the two investigators’ independent scores. Median imputation was performed for video-clips that were missing or classified as uninterpretable.
Statistical analysis
A sample size of 20 subjects was calculated using a two-tailed alpha of 0.05, a beta error of 0.1, and estimations of effect size and the standard deviation of effect size. Based on prior studies that measured change in B-line sums across 28 scanned thoracic regions in response to inpatient treatment, we estimated that the average initial B-line score in our study would be smaller in value (30) and decrease by 50% from the time of initial scan to self-reported improvement in dyspnea17,30. An estimated standard deviation of the change in effect size of 20 was based on standard deviations reported for raw B-line scores in these studies. Continuous variables are expressed as median (interquartile range), as appropriate. Categorical variables are presented as counts (percentages). Comparisons among the three SPE scores and among the three dyspnea VAS scores were performed using the Friedman test. Spearman analysis was used to correlate SPE scores with VAS scores and change in SPE scores with change in VAS scores. Inter-rater and intra-rater reliability were determined by intraclass correlation coefficients (ICC) with absolute agreement, two-way random effects. Clips in which one or both raters deemed to be uninterpretable were excluded from ICC estimates. Statistical analysis was performed using statistical software (IBM® SPSS®, Version 20, IBM Corp., Armonk, NY).
Results
A total of 19 subjects were enrolled and 2 subjects were excluded: one subject was scanned with a different ultrasound machine than the one selected for the study protocol; another subject was determined not to have initial bilateral pathologic B-lines. Two subjects returned to the ED during the enrollment period, contributing more than once to the 20 total ED presentations that were analyzed (Figure 2). IRB approval was obtained at both enrollment sites, and all subjects provided informed consent.
Figure 2.
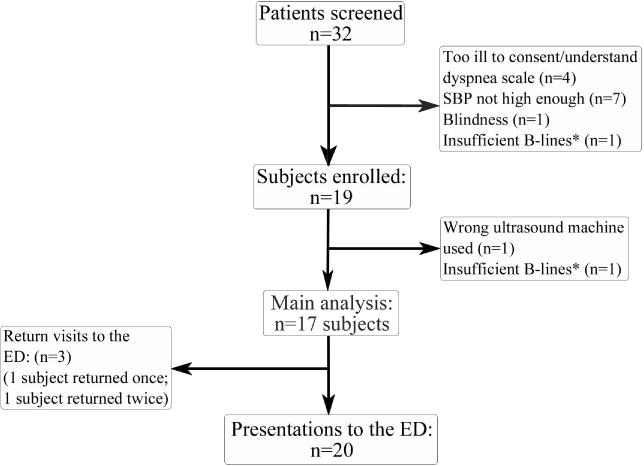
Study flow chart.
*Insufficient B-lines: <3 B-lines/intercostal space, bilaterally
Clinical characteristics of the subjects included in the final analysis are shown in Table 1. All subjects were African American, and 6 of the 17 patients were male. Mean arterial pressure at the time of ED triage was high (139 mm Hg [IQR 123,152]) in spite of the fact that prehospital treatment was administered prior to half of the ED encounters (Table 2). Non-invasive ventilation was administered during every patient encounter. Based on data from formal inpatient echocardiograms following enrollment (n=16), 8 subjects had reduced ejection fraction (<40%), 4 had mid-range ejection fraction (40–49%), and 4 had preserved ejection fraction (≥50%) (Table 1). Four subjects had echocardiograms performed within the 6 months preceding study enrollment. Ejection fraction was preserved in 3 of these subjects and reduced in one. Evidence of diastolic dysfunction was present on echocardiogram for each subject with mid-range or preserved ejection fraction.
Table 1.
Clinical characteristics of study population
| Demographics | |
| Age | 67.5 (57.8,77.3) |
| Male (n,%) | 6 (35) |
| Race, Black (n,%) | 17 (100) |
| BMI (kg/m2) | 29 (24.6,32.0) |
| Past Medical History, n (%) | |
| Hypertension | 16 (94) |
| Diabetes mellitus | 10 (59) |
| Congestive Heart Failure | 13 (76) |
| Myocardial Infarction | 4 (24) |
| CABG | 2 (12) |
| PCI | 3 (18) |
| Pacemaker | 1 (6) |
| AICD | 4 (24) |
| Renal insufficiency | 3 (18) |
| Stroke | 3 (18) |
| Atrial fibrillation | 1 (6) |
| COPD | 4 (24) |
| Asthma | 1 (6) |
| EMS Data | |
| Systolic blood pressure (n=12) | 202 (190,213) |
| Diastolic blood pressure (n=12) | 133 (109,141) |
| Room Air Oxygen Saturation (n=9) | 89 (88,90) |
| Triage Vital Signs | |
| Heart rate | 101 (90,113) |
| Systolic Blood Pressure | 188 (159,199) |
| Diastolic Blood Pressure | 113 (97,134) |
| MAP | 139 (123,152) |
| Respiratory rate | 30 (23,32) |
| Laboratory data | |
| BNP | 894 (483,1414) |
| Sodium | 139 (136,141) |
| BUN | 24 (17.3,31.8) |
| Creatinine | 1.4 (1.0,2.3) |
| Hemoglobin | 12.1 (11.0,13.7) |
| pH | 7.34 (7.25,7.37) |
| Lactate | 1.6 (1.3,3.2) |
| Echocardiographic Data (n=16) n, (%) | |
| Reduced ejection fraction (< 40%) | 8 (50) |
| Mid-range ejection fraction (40–49%) | 4 (25) |
| Preserved ejection fraction (≥ 50%) | 4 (25) |
Data are presented as median (IQR) unless otherwise indicated. BMI, body mass index; CABG, coronary artery bypass graft; PCI, percutaneous coronary intervention; AICD, automatic implantable cardioverter defibrillator; COPD, chronic obstructive pulmonary disease; EMS, emergency medical services; MAP, mean arterial pressure
Table 2.
Treatment and disposition
| EMS Treatment, n (%) | |
| Administration of nitroglycerin (tab or spray) | 9 (45) |
| Initiation of NIV | 4 (20) |
| Administration of furosemide | 2 (10) |
| ED Treatment, n (%) | |
| Nitroglycerin (tab or spray) | 11 (55) |
| Nitroglycerin continuous infusion | 12 (60) |
| Non-invasive ventilation | 20 (100) |
| Enalapril (intravenous) | 13 (65) |
| Hydralazine | 3 (15) |
| Furosemide (intravenous) | 7 (35) |
| Disposition, n (%) | |
| CCU | 7 (35) |
| Step-down Unit | 1 (5) |
| Telemetry | 6 (30) |
| Floor | 6 (30) |
| Hospitalization | |
| Median length of stay (days) (IQR) | 5 (3.0,7.5) |
EMS, emergency medical services; NIV, non-invasive ventilation; CCU, coronary care unit
Lung ultrasound was performed by five of the study investigators and was feasible in every subject. Two video clips (zones 4 and 6 on the initial ultrasound study of a subject) were inadvertently not recorded. Of the 924 video clips reviewed by the two investigators (each reviewed the same data set of 462 recorded clips), 824 (97%)could be analyzed and scored. Inter-rater reliability (0.83 [95% CI 0.75–0.88], N=439 clips) and intra-rater reliability (ICC 0.97 [95%CI 0.96–0.98]) for B-line scoring were excellent. Median time from ED triage to the first lung ultrasound was 12.5 minutes (IQR 3.25,24.5).
SPE scores decreased from initial assessment to the time of disposition decision in every subject (Figure 3). The presence of B-line fusion was observed in 40% of video clips. Median SPE scores for initial, second, and third ultrasound studies were 47 (IQR 40,51), 25 (IQR 11,35), and 8 (IQR 4,19). The decrease in the number of B-lines among the three lung ultrasound studies was statistically significant, χ2 = 31.6, (2, n=18), p<0.001. SPE scores decreased by a median of 81% (IQR 44%, 99%) from the initial to the final lung ultrasound.
Figure 3.
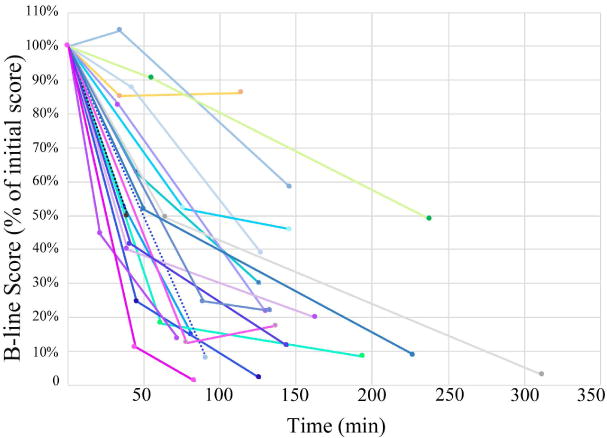
Percentage of initial B-line score over time.
Dotted lines represent the two subject encounters that were scanned only twice (failed to improve by >2 cm on the dyspnea scale before treatment disposition)
Vital signs improved during the course of ED treatment in this cohort of subjects (Table 3). All subjects demonstrated a minimum improvement of 2 cm on the 10-cm VAS between triage and disposition decision. Improvement in VAS scores was statistically significant (χ2 = 36, (2, n=18), p<0.001). Two subjects failed to demonstrate at least 2 cm of improvement at a time earlier than disposition decision. Lung ultrasound was performed only at triage and disposition in these two subjects. In one of the two subjects, SPE score improved by 91% in spite of only a 2.8 cm improvement in dyspnea at the time of disposition. The other subject had a low initial VAS score (2.3 cm) in spite of a high initial SPE score (50). A disposition decision was made early into the ED course of this subject (39 minutes after the initial ultrasound study) at which time the SPE score improved by 50%. While there was a correlation between B-line score and dyspnea as measured by the VAS (ρ=0.64, p<0.001), there was no correlation between the magnitude of change in SPE and VAS scores (final score − initial score) (ρ=−0.04, p=0.89).
Table 3.
Clinical and sonographic profiles of patients at ED triage, initial self-reported improvement of dyspnea, and disposition.
| Phase 1 (ED Triage) |
Phase 2 (Improved dyspnea) |
Phase 3 (Disposition) |
|
|---|---|---|---|
| N | 20 | 18 | 20 |
| Time from triage (min) | 12.5 (3.3,24.5) |
56.5 (42,67.8) |
138 (103.5,170.5) |
| Vital Signs | |||
| Systolic blood pressure | 192 (157,208) |
153 (114,185) |
150 (122,180) |
| Diastolic blood pressure | 106 (95,126) |
86 (74,100) |
83 (72,100) |
| Heart rate | 101 (85,115) |
92 (79,104) |
83 (77,98) |
| Respiratory rate | 32 (27,34) |
25 (21,28) |
26 (22,28) |
| Lung ultrasound | |||
| Raw B-line score | 47 (40,51) |
25 (11,35) |
8 (4,19) |
| Percentage of initial B-line score | 1 | 50% (25%,84%) |
19% (9%,45%) |
| Dyspnea Scores | |||
| Visual analog scale | 7.5 (5.5,9.0) |
3.0 (1.8,4.5) |
0.7 (0,1.8) |
| 5-Point Likert scale | 4 (3,4) |
2 (2,3) |
2 (1,2) |
Data are presented as median (IQR) unless otherwise indicated.
Discussion
Our study demonstrates that serial lung ultrasound can capture rapid changes in pulmonary edema in a cohort of hypertensive patients presenting to the ED with acute pulmonary edema. Reliable estimates of SPE were obtained using an 8-zone scanning approach and a scoring system that incorporated estimates of B-line fusion. This study adds to previous work investigating changes in SPE in response to longer durations of AHF treatment. In a cohort of 25 patients with systolic heart failure presenting to an ED with clinical signs of pulmonary congestion, Facchini et al30 performed 28-zone lung ultrasound assessments before and after a 24-hour infusion of diuretic therapy. B-line scores decreased by 41% (53.4 ± 17.2 to 31.7 ± 13.5). Gargani et al20 also demonstrated partial pulmonary decongestion in response to inpatient treatment of AHF using the same scanning protocol. B-line scores in this cohort of 100 patients decreased by 58% (48 ± 48 to 20 ± 23) from hospital admission to discharge. Using a simplified lung ultrasound score based on the number of positive thoracic areas (a positive area defined as ≥ 3 B-lines), Volpicelli et al31 demonstrated a decrease in the median number of positive lung zones from 8 to 1 (out of the 11 thoracic zones interrogated) from hospital admission to discharge (after 4.2 ±1.7 days) among 70 AHF patients. Dichotomizing individual SPE scores for each of the 8 lung zones interrogated in our study (positive defined as SPE score ≥ 3) produces similar results: a decrease from a median of 8 to 1 positive lung zones (out of the 8 interrogated) from triage to ED disposition (after a median of 138 min [IQR 103.5,170.5]). While this scoring method might be most easily incorporated into clinical practice, it might lack the sensitivity to detect more gradual and less dramatic improvement in pulmonary decongestion during the initial phase of AHF treatment.
In a heterogeneous cohort of acute heart failure patients, Cortellaro et al32 evaluated the dynamics of pulmonary decongestion over the initial 24 hours of treatment using a simplified scoring system that incorporated B-line coalescence (0= no B-lines, 1= multiple B-lines, 2=confluent B-lines) across 11 lung zones. At 3 hours from admission, hemodynamic and respiratory vital signs improved significantly, and the B-line score improved by 54%. The most commonly identified trigger for the patients in this cohort was hypertension (defined in this study as diastolic pressure > 120 mmHg). This study further supports the feasibility of performing serial lung ultrasound throughout the acute phase of treatment of AHF and in quantifying rapid changes in pulmonary edema using a simplified scale.
The rate of improvement in SPE in our study was more rapid than that observed by Cortellaro et al32, likely because of differences in our studied populations. While our study was not designed to evaluate the rate of SPE improvement, it is notable that the median time for an 80% or greater reduction in SPE (in the 10 subjects that attained this degree of improvement in the ED), was 86 minutes (IQR 69,149). Acute pulmonary edema in the hypertensive phenotype of AHF that we studied is thought to result from sympathetically mediated vascular redistribution of fluid from vasoconstricted splanchnic and peripheral vascular beds into the pulmonary circulation rather than from volume overload24,33. Based on this conceptual model of disease, therapies aimed at reducing cardiac filling pressures are likely to redistribute blood volume and decrease pulmonary edema. In addition to its pulmonary benefits, NIV may be particularly useful in hypertensive AHF through its hemodynamic effects on reducing cardiac loading conditions34,35. NIV alone has been shown to resolve pulmonary edema in a patient with both congestive heart failure and end stage renal disease over several hours36. Every subject received NIV during the ED treatment course. Vasodilator therapy in the form of nitrates can rapidly improve cardiac filling pressures and redistribute blood volume. Nitrate therapy was administered by EMS or in the ED to all but one subject enrolled in this study. This subject demonstrated the least amount of SPE improvement (14%). Subjects seemed to benefit from vasodilator therapy and non-invasive ventilation regardless of their ejection fraction. An 86% reduction in SPE was observed in the 11 subjects in this study who did not receive loop diuretic therapy.
Measures of pulmonary edema after therapeutic intervention in the ED have the potential to help risk-stratify patients and assist in disposition decisions. Patients with hypertensive AHF who present with the most severe dyspnea and degree of pulmonary edema may have complete resolution of pulmonary edema in a short period of time. Persistent pulmonary edema after treatment, however, may indicate a need for further therapeutic intervention. Pulmonary decongestion is incomplete in some AHF patients even after inpatient treatment, and residual pulmonary edema at hospital discharge has been show to predict rehospitalization19,20.
Symptomatic improvement is an important clinical goal in treating patients with acute heart failure and improved dyspnea, as measured by reduced VAS scores, has been used as an outcome measure in major clinical trials8,26. Despite a correlation between raw VAS scores and SPE scores in our study, we did not find a correlation between the magnitudes of change in these measures. The severity of dyspnea that subjects presented with (7.5 cm [95% CI 5.5–9.0 cm]) likely made this self-assessment more challenging than it would have been in subjects who were in less respiratory distress. Self-perceived relief of dyspnea may also partially reflect decreased underlying anxiety and adrenergic tone, and thus might be exaggerated in subjects reassured by treating clinicians of their anticipated clinical improvement and averted intubation. One subject reported an 8-cm improvement on the VAS despite minimal improvement in SPE (14%). In the majority of subjects, the % magnitude change in VAS exceeded that observed in SPE. Changes in VAS might be larger than those observed on lung ultrasound, if as suggested by previous studies19,37, subclinical SPE is significant. Persistent SPE was observed in the 6 subjects in our study who reported complete resolution of dyspnea on the VAS.
Our study has several important limitations. The small sample size of this feasibility study precludes subgroup analysis. Analyses of SPE resolution stratified by different treatment approaches (administration of diuretic, dosage of nitroglycerin) and clinical variables (ejection fraction) in future studies would be informative. Because our study objective was to capture rapid and dramatic changes in SPE, we chose to limit our studied population to hypertensive AHF patients with acute dyspnea. Our results are therefore not generalizable to AHF patients with lower presenting blood pressures or gradual dyspnea. The presence of bilateral pulmonary edema was an inclusion criterion in this study, and selection bias may have played a role in selecting patients with a more severe degree of pulmonary edema on initial exam and subsequently a greater improvement in SPE. The dyspnea scale used in this study, while used in other heart failure research, is not a validated instrument, so correlations between pulmonary decongestion and dyspnea improvement may be affected by limitations of the scale itself. Another methodological limitation in this study was allowing for a variable image depth as part of our scanning protocol. The vast majority (87%) of our video clips were set to a depth of 15 cm, and the authors view it unlikely that image depths several cm short of this would lead to an overestimation of B-line number. This study does not reflect true before-and-after changes in SPE following therapeutic intervention, as half of enrolled subjects received prehospital treatment by EMS. A strength of this study, however, was the short time between ED triage and initial lung ultrasound. The same sonographer performed each of the serial scans for a single patient during their treatment course, and this may have introduced some degree of observer bias. To limit the effects of observer bias, SPE scoring was performed in blinded fashion, and video clips were randomized by patient and scan sequence. Our results would be more applicable to clinical practice if scoring were concurrent with scanning and both scoring and scanning were performed by clinicians lacking advanced training in ultrasound.
In conclusion, this study demonstrates the dynamics of pulmonary edema in dyspneic patients with hypertensive AHF early in their treatment course. Real-time feedback from serial sonographic assessments of pulmonary edema may be informative to the clinician titrating AHF treatments and making disposition decisions. Validation of this simplified scanning protocol and semi-quantitative approach to measuring changes in sonographic pulmonary edema in a larger cohort of patients with different phenotypes of AHF is needed. The reliability of measuring pulmonary edema while scanning in comparison to offline analysis also merits study.
Acknowledgments
Sources of support: Supported in part by the NYU CTSA grant UL1 TR001445 from the National Center for Advancing Translational Sciences, National Institutes of Health.
Funding Sources:
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Storrow AB, Jenkins CA, Self WH, et al. The burden of acute heart failure on U.S. emergency departments. JACC Heart failure. 2014;2(3):269–277. doi: 10.1016/j.jchf.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. The New England journal of medicine. 2009;360(14):1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 3.Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nature reviews Cardiology. 2011;8(1):30–41. doi: 10.1038/nrcardio.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu AH, Smith A, Apple FS. Optimum blood collection intervals for B-type natriuretic peptide testing in patients with heart failure. The American journal of cardiology. 2004;93(12):1562–1563. doi: 10.1016/j.amjcard.2004.02.072. [DOI] [PubMed] [Google Scholar]
- 5.Singer AJ, Birkhahn RH, Guss D, et al. Rapid Emergency Department Heart Failure Outpatients Trial (REDHOT II): a randomized controlled trial of the effect of serial B-type natriuretic peptide testing on patient management. Circulation Heart failure. 2009;2(4):287–293. doi: 10.1161/CIRCHEARTFAILURE.108.826685. [DOI] [PubMed] [Google Scholar]
- 6.Fonarow GC, Abraham WT, Albert NM, et al. Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF): rationale and design. American heart journal. 2004;148(1):43–51. doi: 10.1016/j.ahj.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Adams KF, Jr, Fonarow GC, Emerman CL, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) American heart journal. 2005;149(2):209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Teerlink JR, Cotter G, Davison BA, et al. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet. 2013;381(9860):29–39. doi: 10.1016/S0140-6736(12)61855-8. [DOI] [PubMed] [Google Scholar]
- 9.Metra M, O’Connor CM, Davison BA, et al. Early dyspnoea relief in acute heart failure: prevalence, association with mortality, and effect of rolofylline in the PROTECT Study. European heart journal. 2011;32(12):1519–1534. doi: 10.1093/eurheartj/ehr042. [DOI] [PubMed] [Google Scholar]
- 10.O’Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with acute decompensated heart failure. The New England journal of medicine. 2011;365(1):32–43. doi: 10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]
- 11.Hardie G, Liu R, Darden J, Gold WM. Ethnic differences in methacholine responsiveness and word descriptors in African Americans, Hispanic-Mexican Americans, Asian-Pacific Islanders, and Whites with mild asthma. The Journal of asthma: official journal of the Association for the Care of Asthma. 2010;47(4):388–396. doi: 10.3109/02770903.2010.481341. [DOI] [PubMed] [Google Scholar]
- 12.Martindale JL, Wakai A, Collins SP, et al. Diagnosing Acute Heart Failure in the Emergency Department: A Systematic Review and Meta-analysis. Academic emergency medicine: official journal of the Society for Academic Emergency Medicine. 2016;23(3):223–242. doi: 10.1111/acem.12878. [DOI] [PubMed] [Google Scholar]
- 13.Martindale JL, Noble VE, Liteplo A. Diagnosing pulmonary edema: lung ultrasound versus chest radiography. European journal of emergency medicine: official journal of the European Society for Emergency Medicine. 2013;20(5):356–360. doi: 10.1097/MEJ.0b013e32835c2b88. [DOI] [PubMed] [Google Scholar]
- 14.Mallamaci F, Benedetto FA, Tripepi R, et al. Detection of pulmonary congestion by chest ultrasound in dialysis patients. JACC Cardiovascular imaging. 2010;3(6):586–594. doi: 10.1016/j.jcmg.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Noble VE, Murray AF, Capp R, Sylvia-Reardon MH, Steele DJ, Liteplo A. Ultrasound assessment for extravascular lung water in patients undergoing hemodialysis. Time course for resolution. Chest. 2009;135(6):1433–1439. doi: 10.1378/chest.08-1811. [DOI] [PubMed] [Google Scholar]
- 16.Agricola E, Bove T, Oppizzi M, et al. “Ultrasound comet-tail images”: a marker of pulmonary edema: a comparative study with wedge pressure and extravascular lung water. Chest. 2005;127(5):1690–1695. doi: 10.1378/chest.127.5.1690. [DOI] [PubMed] [Google Scholar]
- 17.Frassi F, Gargani L, Gligorova S, Ciampi Q, Mottola G, Picano E. Clinical and echocardiographic determinants of ultrasound lung comets. European journal of echocardiography: the journal of the Working Group on Echocardiography of the European Society of Cardiology. 2007;8(6):474–479. doi: 10.1016/j.euje.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Jambrik Z, Monti S, Coppola V, et al. Usefulness of ultrasound lung comets as a nonradiologic sign of extravascular lung water. The American journal of cardiology. 2004;93(10):1265–1270. doi: 10.1016/j.amjcard.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Coiro S, Rossignol P, Ambrosio G, et al. Prognostic value of residual pulmonary congestion at discharge assessed by lung ultrasound imaging in heart failure. European journal of heart failure. 2015;17(11):1172–1181. doi: 10.1002/ejhf.344. [DOI] [PubMed] [Google Scholar]
- 20.Gargani L, Pang PS, Frassi F, et al. Persistent pulmonary congestion before discharge predicts rehospitalization in heart failure: a lung ultrasound study. Cardiovascular ultrasound. 2015;13(1):40. doi: 10.1186/s12947-015-0033-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Volpicelli G, Elbarbary M, Blaivas M, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive care medicine. 2012;38(4):577–591. doi: 10.1007/s00134-012-2513-4. [DOI] [PubMed] [Google Scholar]
- 22.Anderson KL, Fields JM, Panebianco NL, Jenq KY, Marin J, Dean AJ. Inter-rater reliability of quantifying pleural B-lines using multiple counting methods. Journal of ultrasound in medicine: official journal of the American Institute of Ultrasound in Medicine. 2013;32(1):115–120. doi: 10.7863/jum.2013.32.1.115. [DOI] [PubMed] [Google Scholar]
- 23.Levy P, Compton S, Welch R, et al. Treatment of severe decompensated heart failure with high-dose intravenous nitroglycerin: a feasibility and outcome analysis. Ann Emerg Med. 2007;50(2):144–152. doi: 10.1016/j.annemergmed.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 24.Viau DM, Sala-Mercado JA, Spranger MD, O’Leary DS, Levy PD. The pathophysiology of hypertensive acute heart failure. Heart. 2015 doi: 10.1136/heartjnl-2015-307461. [DOI] [PubMed] [Google Scholar]
- 25.Liteplo AS, Marill KA, Villen T, et al. Emergency thoracic ultrasound in the differentiation of the etiology of shortness of breath (ETUDES): sonographic B-lines and N-terminal pro-brain-type natriuretic peptide in diagnosing congestive heart failure. Academic emergency medicine: official journal of the Society for Academic Emergency Medicine. 2009;16(3):201–210. doi: 10.1111/j.1553-2712.2008.00347.x. [DOI] [PubMed] [Google Scholar]
- 26.McMurray JJ, Teerlink JR, Cotter G, et al. Effects of tezosentan on symptoms and clinical outcomes in patients with acute heart failure: the VERITAS randomized controlled trials. Jama. 2007;298(17):2009–2019. doi: 10.1001/jama.298.17.2009. [DOI] [PubMed] [Google Scholar]
- 27.Ander DS, Aisiku IP, Ratcliff JJ, Todd KH, Gotsch K. Measuring the dyspnea of decompensated heart failure with a visual analog scale: how much improvement is meaningful? Congestive heart failure. 2004;10(4):188–191. doi: 10.1111/j.1527-5299.2004.03475.x. [DOI] [PubMed] [Google Scholar]
- 28.Weber CK, Miglioranza MH, Moraes MA, et al. The five-point Likert scale for dyspnea can properly assess the degree of pulmonary congestion and predict adverse events in heart failure outpatients. Clinics. 2014;69(5):341–346. doi: 10.6061/clinics/2014(05)08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Volpicelli G, Mussa A, Garofalo G, et al. Bedside lung ultrasound in the assessment of alveolar-interstitial syndrome. The American journal of emergency medicine. 2006;24(6):689–696. doi: 10.1016/j.ajem.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 30.Facchini C, Malfatto G, Giglio A, Facchini M, Parati G, Branzi G. Lung ultrasound and transthoracic impedance for noninvasive evaluation of pulmonary congestion in heart failure. J Cardiovasc Med (Hagerstown) 2015 doi: 10.2459/JCM.0000000000000226. [DOI] [PubMed] [Google Scholar]
- 31.Volpicelli G, Caramello V, Cardinale L, Mussa A, Bar F, Frascisco MF. Bedside ultrasound of the lung for the monitoring of acute decompensated heart failure. The American journal of emergency medicine. 2008;26(5):585–591. doi: 10.1016/j.ajem.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 32.Cortellaro F, Ceriani E, Spinelli M, et al. Lung ultrasound for monitoring cardiogenic pulmonary edema. Internal and emergency medicine. 2016 doi: 10.1007/s11739-016-1510-y. [DOI] [PubMed] [Google Scholar]
- 33.Fallick C, Sobotka PA, Dunlap ME. Sympathetically mediated changes in capacitance: redistribution of the venous reservoir as a cause of decompensation. Circulation Heart failure. 2011;4(5):669–675. doi: 10.1161/CIRCHEARTFAILURE.111.961789. [DOI] [PubMed] [Google Scholar]
- 34.Rasanen J, Heikkila J, Downs J, Nikki P, Vaisanen I, Viitanen A. Continuous positive airway pressure by face mask in acute cardiogenic pulmonary edema. The American journal of cardiology. 1985;55(4):296–300. doi: 10.1016/0002-9149(85)90364-9. [DOI] [PubMed] [Google Scholar]
- 35.Moritz F, Benichou J, Vanheste M, et al. Boussignac continuous positive airway pressure device in the emergency care of acute cardiogenic pulmonary oedema: a randomized pilot study. European journal of emergency medicine: official journal of the European Society for Emergency Medicine. 2003;10(3):204–208. doi: 10.1097/00063110-200309000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Liteplo AS, Murray AF, Kimberly HH, Noble VE. Real-time resolution of sonographic B-lines in a patient with pulmonary edema on continuous positive airway pressure. The American journal of emergency medicine. 2010;28(4):541 e545–548. doi: 10.1016/j.ajem.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 37.Gargani L, Pang PS, Frassi F, et al. Persistent pulmonary congestion before discharge predicts rehospitalization in heart failure: a lung ultrasound study. Cardiovascular ultrasound. 2015;13:40. doi: 10.1186/s12947-015-0033-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


