Abstract
Clear cell sarcoma (CCS) of tendons and aponeuroses / malignant melanoma of soft parts is a rare tumor and in the majority of cases presents a characteristic reciprocal translocation t(12;22)(q13;q12) that results in fusion of the EWS and ATF1 genes. Although the melanocytic differentiation of CCS is indisputable, its precise lineage remains unclear. Typically, the slowly growing tumor affects the extremities of adolescents or young adults, especially around the ankle and foot. CCS is classically regarded as a deep soft tissue tumor associated with tendons or aponeuroses. This traditional view is put into perspective by the description of primary CCS of the gastrointestinal tract, which may have a variant fusion gene EWSR1-CREB1. We describe 12 cases of cutaneous CCS and discuss the differential diagnoses. These 12 cases share an identical immunohistochemical profile with malignant melanoma (MM) and thus can easily be confused with a dermal variant of spindle cell MM or metastasis of MM. The patients' ages ranged from 6 to 74 years (median: 25 y), and there was a female predominance (10 females, 2 males). Most tumors (n = 9) were located on the extremities, 2 tumors arose on the back, and 1 on the abdomen. The mean tumor size was 0,97 cm (range, 0,4 to 1,7 cm). Six cases showed invasion of the subcutis, the other 6 cases were entirely dermal. Tumor necrosis was evident in 2 cases, melanin pigment in 2 cases, and ulceration in 1 tumor. All cases showed uniform nests and fascicles of pale spindled or slightly epitheloid cells with finely granular eosinophilic or clear cytoplasm. There was fair pleomorphism with plump spindled nuclei and significantly prominent nucleoli. Multinucleated wreath-like tumor giant cells were observed in two-thirds of cases, but were usually present only focally. The dense cellular aggregates were encased by delicate fibrous septa. The stroma showed a sclerotic reticulated pattern. Partly, the nests of spindle cells bordered the epidermis, prima vista mimicking junctional nests of melanocytes. The specific translocation pattern was confirmed in all cases by fluorescence in situ hybridization. Local recurrences and metastases developed in 2 and 3 patients, respectively, and 1 patient was dead of the disease.
Keywords: Clear cell sarcoma, Melanoma of soft parts, Melanoma
Introduction
Clear cell sarcoma (CCS) of tendons and aponeuroses / malignant melanoma of soft parts is a unique sarcoma initially described by Franz Enzinger in 1965.10 The tumor has a proclivity to involve the tendons and aponeuroses of distal extremities of adolescents or young adults, with a peak incidence in the third and fourth decade and a slight female predominance. The tumor is usually deep seated and characterized by multiple local recurrences with late metastases and a high rate of tumor deaths. Pathologic findings include fascicles and nests of pale fusiform and epitheloid tumor cells with clear or finely granular, eosinophilic cytoplasm and elongated oval nuclei with prominent nucleoli and scattered mitoses. The cellular aggregates are encased by delicate fibrous septa and in two-thirds of cases multinucleated giant cells are observed. Clear cell sarcoma shows consistent evidence of melanocytic differentiation. By electron microscopy melanosomes in varying stages of development can be identified in the majority of the cases.3,13,15,19 Immunohistochemically, distinction from malignant melanoma is not possible since both tumors share positivity for the melanocytic markers S-100, HMB-45, MelanA, and microphthalmia transcription factor (MITF). Because the histologic and immunohistochemical features of clear cell sarcoma overlap with those of cutaneous spindle cell melanoma and occult metastatic melanoma, the differential diagnosis between these entities is still problematic with profound clinical consequences.8,26 Characteristically, cytogenetic analysis of clear cell sarcoma indicates in most cases the presence of a reciprocal translocation, t(12;22)(q13;q12) that has not yet been identified in malignant melanoma.23 The gene fusion product involves the EWS (22q12) and ATF1 (12q13) genes. This translocation has been detected cytogenetically in about 75% of reported cases of CCS. In addition, another fusion of EWS to CREB1, a gene at 2q13, has been found in a subset of CCS that arouse preferentially in the gastrointestinal tract.2 Besides, both molecular genetic changes have been reported in cases of angiomatoid fibrous histiocytoma.1,12 Adverse prognostic factors identified to date include larger tumor size as a continuous variable and any microscopic tumor necrosis.9,22 No correlation was found between clinical course, histologic and immunohistochemical features, including proliferative activity.19,21 CCS frequently develops recurrences and metastases five years and more subsequent to initial therapy, indicating the need for long-term follow-up and treatment.6,10 Surgery is the mainstay of treatment, with chemotherapy having little effect.17 Early diagnosis and initial wide excision are essential for a favorable outcome of CCS.14 Because of the rarity of CCS further characterization of the tumor is necessary to strengthen the understanding of this particular sarcoma and to facilitate making accurate diagnosis. In this study we included only cutaneous cases of CCS with typical morphologic findings and proven EWSR1 re-arrangement by fluorescence in situ hybridization technique.
Materials and Methods
For this study, all cases were collected from the routine diagnostic files and consultation cases of the authors. The patients, 10 female and 2 male, were 6 to 74 years old (median: 25 years). Detailed clinical features are summarized in Table 1. Tumors were assessed as entirely dermal when there was neither infiltration of the subcutis in step sections nor evidence of any tumor rest by reexcision. Each patient was staged meticulously by its clinicians to rule out the facts that primary tumors were not metastases to the skin by prior deep soft tissue CCS as well as metastases from the detected primary cutaneous CCS.
Table 1. Patients with cutaneous clear cell sarcoma.
| Case | Age (y)/Sex | Site | Size (cm) | EWSR1 | Follow-up (mo) |
|---|---|---|---|---|---|
| 1 | 31/F | Back | 1,0 | 16/65 | ANED 52 |
| 2 | 7/M | Foot | 0,9 | 18/76 | REC 12, ANED 67 |
| 3 | 13/F | Lower Arm | 0,7 | 27/98 | ANED 54 |
| 4 | 12/F | Sole | 1,2 | 21/83 | REC 12; MET; DOD 38 |
| 5 | 19/F | Foot | 1,7 | 10/43 | MET 2, AWD 37 |
| 6 | 50/F | Abdomen | 0,4 | 16/65 | ANED 40 |
| 7 | 15/M | Sole | 0,5 | 18/69 | ANED 17 |
| 8 | 61/F | Upper Leg | 1,6 | 52/73 | NA 17 |
| 9 | 74/F | Upper Arm | 1,3 | 26/61 | ANED 14 |
| 10 | 65/F | Palm | 0,6 | 29/54 | MET 1; AWD 14 |
| 11 | 25/F | Upper Leg | 0,7 | 09/51 | ANED 20 |
| 12 | 50/F | Back | 1,2 | 11/36 | ANED 12 |
EWSR1 indicates number of positive cells by fluorescence in situ hybridization in relation to the number of all counted nuclei; ANED indicates alive with no evidence of disease; AWD, alive with disease; DOD, dead of disease; MET, metastasis; REC, local recurrence; NA, not available
All specimens were fixed in 4% buffered formalin, routinely processed and embedded in paraffin; 4 μm thick sections were stained routinely with hematoxylin and eosin. In addition, sections in all cases were stained immunohistochemically by the labeled Streptavidin Biotin (LSAB) technique using Anti-Human Melanosome (Clone HMB-45, 1:300, DAKO, Glostrup, Denmark, no pretreatment), anti-S-100 protein (polyclonal, 1:2000, DAKO, Glostrup, Denmark, no pretreatment), anti-Human Melan-A (Clone A103, 1:50, DAKO, Glostrup, Denmark, pretreatment with standard antigen retrieval), and anti-Melanoma associated antigen (Clone NKI/C3 (1:200, BioGenex, SanRamon, USA, no pretreatment). Local ethical guidelines were followed for this study.
FISH analysis
In order to detect the translocation of the EWSR1 gene on 22q11 a directly FITC/Rhodamine-labeled break apart probe was used (Abbott, Bergisch Gladbach, Germany). Fluorescence in situ hybridization was performed on 3 μM sections of formalin-fixed, paraffin-embedded tissue after heating at 65°C for 16 hours, deparaffinization with xylene and dehydration with ethanol. All tissue sections were pretreated with a 30% solution of Oncor pretreatment powder in 2×SSC and digested for 10 minutes with Proteinase K following the instructions of the suppliers (Q-Biogene, Heidelberg, Germany). After a second dehydration step, the probes were applied to the sections and the covered slides were sealed with rubber cement, heat-denatured and hybridized at 37°C for 16 hours. All sections were counterstained with DAPI II in mounting medium (125 ng/ml, Vysis Bergisch Gladbach, Germany) and visualized under a Zeiss Axioplan microscope using a HBO100 lamp and the appropriate filters for the three fluorescent dyes. All EWS FISH analyses were performed in the same lab. At least 50 nuclei were counted with the exception of two cases (see table 1). Identification and enumeration of nuclei was performed by a dermatopathologists and a molecular biologist simultaneously. The number of counted nuclei varies because only intact nuclei of tumor cells showing the four signals (two orange and two green signals) were counted. In addition, only nuclei were evaluated in which the whole membrane was visible in order to avoid incorrect results derived from nuclei lying closely together which cause difficulties in distinguishing the nuclei and identifying the appropriate signals. All EWS FISH analyses were performed with one positive and two negative controls. For the positive control Ewing's sarcomas with a proven EWS translocation were used, the negative controls included melanomas and synovial sarcomas.
Results
The clinical features of patients with cutaneous CCS are summarized in Table 1. There was a female predominance with 10 females and 2 males with ages ranging from 6 to 74 years (median: 25 years). Most tumors (9) were located on the extremities, and 2 tumors arose on the back, 1 on the abdomen. The mean tumor size was 0,97 cm (range, 0,4 -1,7 cm) measured under the microscope. A sentinel lymph node operation was performed in two cases (Cases 1 and 10). There was a metastasis to a sentinel lymph node in Case 10 in the left axilla (3-4 cm of tumor), but the subsequent completion lymphadenectomy showed no additional involvement (n=34 lymph nodes). The tumors locally recurred in 2 cases (Cases 2 and 4). Metastases developed postoperatively in 2 cases (Cases 4 and 5), and the patient in Case 4 died of CCS as a result of extensive metastatic disease.
Histologically, six neoplasms were confined to the dermis. An infiltration of superficial parts of the subcutis was noted in the remaining six cases. The salient histologic features of all tumors were dense nests and fascicles divided by fine fibrous tissue septa and a pronounced hyalinized stroma. In six cases the nests bordered the epidermis mimicking junctional nests of a melanocytic tumor. None of these cases showed increase of single melanocytes in the basal epidermal layer or transepidermal spread of atypical melanocytes. One case presented ulceration, necrosis was observed in two cases, and melanin pigment in two other tumors. The pale fusiform and epitheloid cells showed clear or finely granular, eosinophilic cytoplasm. There was fair pleomorphism with plump spindled nuclei and prominent nucleoli. The mitotic activity ranged from 2 to 20 mitotic figures (MF)/10 high-power fields (HPF) (mean: 8MF/10 HPF). In the current immunohistochemical assessment, the tumors expressed the individually performed melanocytic markers, except negativity for MelanA in Cases 2 and 9. EWSR1 re-arrangement was proven in all cases by fluorescence in situ hybridization technique. Case 10, recently illustrated in a different study,31 demonstrated by RT-PCR the fusion gene EWSR1-CREB1. Detailed histologic and immunohistochemical findings are summarized in Table 2.
Table 2. Histologic and immunohistochemical features of cutaneous clear cell sarcoma.
| Case | Dermal only | JPN | Ulceration | Necrosis | Pigment | MGC | MF/HPF | S100 | MelanA | HMB-45 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | + | + | - | - | - | - | 12 | *** | *** | ** |
| 2 | - | + | - | - | - | + | 3 | *** | - | * |
| 3 | - | + | + | - | - | - | 9 | *** | NA | *** |
| 4 | - | + | - | - | - | + | 5 | NA | *** | * |
| 5 | + | + | - | - | - | + | 14 | *** | * | ** |
| 6 | + | - | - | - | - | ++ | 4 | ** | ** | NA |
| 7 | + | - | - | - | - | + | 2 | *** | NA | * |
| 8 | - | + | - | + | + | + | 10 | *** | *** | *** |
| 9 | - | - | - | + | - | - | 20 | * | - | NA |
| 10 | + | - | - | - | - | ++ | 3 | *** | NA | NA |
| 11 | - | - | - | - | + | - | 3 | * | NA | NA |
| 12 | + | + | - | - | - | + | 14 | *** | *** | *** |
JPN indicates junctional pseudo nests simulating junctional nests of a melanocytic tumor; MGC, multinucleated giant cells: +, few; ++, many; MF, mitotic figures; HPF, 10 high-power fields; NA, not available; ***, more than 80% cells positive; **, 40% - 80% cells positive; *, 5% - 40% cells positive;
Discussion
The cytogenetic hallmark of CCS is t(12;22)(q13;q12), resulting in a chimeric EWSR1/ATF1 gene in which the 3′-terminal part of EWS at 22q is replaced by the 3′-terminal part of ATF1 at 12q.4,8,16,27,29,30 CCS can also display additional chromosomal aberrations including extra copies of chromosomes 2, 7, and 8 and additional abnormalities of chromosome 22.4,5 The EWSR1/ATF1 fusion transcript can be identified by reverse transcription-polymerase chain reaction and fluorescence in situ hybridization techniques.15 Four types of EWSR1/ATF1 chimeric transcripts have been identified in CCS, designated type 1 to 4.24 No significant association between the transcript type and patient's outcome was found.7 A subset of CCS that occurs preferentially in the gastrointestinal tract may have a variant fusion gene EWSR1-CREB1 and can show little or no melanocytic differentiation.2 This is in contradiction with an earlier study of partly the same authors in which gene expression profiles were supposed to support the classification of CCS as a distinct genomic subtype of melanoma characterized by its deep soft tissue primary location, lack of cutaneous invasion, and is distinguished from a primary cutaneous melanoma primarily by its anatomic location and clinical features.28 Case 10 in our study of cutaneous CCS also demonstrated by RT-PCR the fusion gene EWSR1-CREB1. In contrast to melanoma, mutations of exon 11 and 15 of the BRAF gene are consistently negative in clear cell sarcoma, suggesting different genetic pathways for these tumors.25 Microsatellite instability, a variation in the length of short repeat DNA segments in the genome, has been implicated in melanoma tumorigenesis, but is rare or absent in CCS.11
Because the histologic and immunohistochemical features overlap, cutaneous CCS can be confused with cutaneous spindle cell melanoma or metastasis of melanoma. Immunohistochemically, distinction from malignant melanoma is not possible since both tumors share positivity for the common melanocytic markers like S-100, HMB-45, MelanA and NKI/C3. Histologically, some tumors even present melanin pigment such as two cases in our series and the dense fascicles of spindle cells closely bordering the epidermis and invading the papillary dermis mimic junctional nests of melanocytes. Still, there are reliable criteria for the accurate histological diagnosis of cutaneous CCS before involving molecular techniques for evidence. Cutaneous CCS shows a uniform pattern of fascicles of spindle cells throughout the entire tumor. The fascicles are encased by delicate fibrous septa and mostly show a characteristic hyalinized sclerotic and reticulated stroma, a pattern which is unlikely to be found in melanoma and should lead to the working diagnosis of cutaneous CCS. Melanoma and its metastases often show more than one population of cells within one lesion. None of the tumors in our study, even those mimicking junctional melanocytic nests, show a pagetoid spread with atypical melanocytes scattered in neither the epidermis nor any significant increase of melanocytes within the basal epidermis layer. Multinucleated giant cells are usually not a feature of melanoma, but can occur sporadically. We found multinucleated giant cells in two-thirds of our cases, albeit sparse in most tumors. Giant cells in cutaneous CCS posses a wreath of multiple peripherally placed nuclei with relatively monomorphic appearance. Multinucleated cells in melanoma show a rather unarranged distribution of usually more pleomorphic nuclei. Transversely cut fascicles with densely arranged nuclei of cells between sclerotic septa should not be confused with multinucleated giant cells. Melanoma with features of blue nevus often has a junctional component; the nuclei are hyperchromatic and have coarse chromatin. In atypical cellular blue nevus cytologically atypical melanocytes are scattered among inconspicuous dendritic melanocytes with thin elongated nuclei, a characteristic biphasic growth pattern not to be found in cutaneous CCS. Cellular blue nevus can be a differential diagnosis, particularly in small biopsies, but cells are bland and homogenous displaying a tiny inconspicuous nucleolus and no mitotic figures.
The original description of clear cell sarcoma of tendons and aponeuroses is without doubt an immense merit of Dr. Enzinger.21 Clear cell sarcoma of tendons and aponeuroses / malignant melanoma of soft parts is not only a rather long and therefore inconvenient term but also not the ideally suited as a name for this tumor. Additionally, it might become a misnomer with primary gastrointestinal 2,8,18 and primary dermal cases of CCS. The tumor is unrelated to pediatric lesions currently known as clear cell sarcoma of the kidney, clear cell myelomelanocytic tumor, and clear cell carcinomas. In contrast to the original name of clear cell sarcoma, neither all of the tumors nor all of the tumor cells have clear cytoplasm. Often spindle cells with a pale eosinophilic cytoplasm dominate.
In his original work Enzinger concluded that the cell of origin of CCS was unclear stating that “…the term clear cell sarcoma of tendons and aponeuroses …is purely descriptive and reflects the uncertainty of histogenesis. He further stated that it was not related to fibrosarcoma or synovial sarcoma, and that it was not a spindle cell melanoma. In a subsequent paper co-authored with Chung in 1983 6 he reassessed his point of view and they stated that “… the presence of melanin was overlooked …”, and thus concluded that a more appropriate term would be “… malignant melanoma of soft parts, rather than the purely descriptive term of clear cell sarcoma.” Later, other authors stated that clear cell sarcoma was a preferable term, since clear cell sarcoma was clinically very unlikely to be cutaneous melanoma.17 This original dilemma has continued to date and CCS is still viewed as a tumor of uncertain differentiation despite the detection of intracellular melanin and melanosomes. Further characterization of this neoplasm is required to strengthen the understanding of this particular sarcoma. Long-term follow-up studies are necessary to analyze if there are statistical differences in the prognosis of cutaneous CCS and comparable melanoma. This present series expands the spectrum of CCS to include lesions that are based in the dermis and should be distinguished from cutaneous melanoma.
In summary, the histologic criteria stated above can lead to the rare diagnosis of cutaneous CCS. It has to be distinguished from melanoma. In questionable cases, definitive diagnosis is possible with molecular confirmation of the sarcoma defining translocation.
Figure 1.
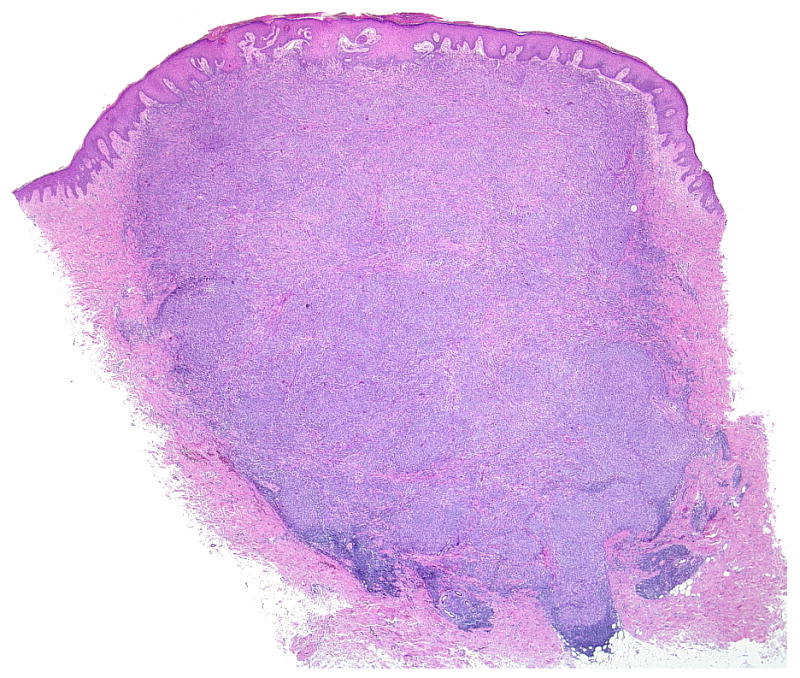
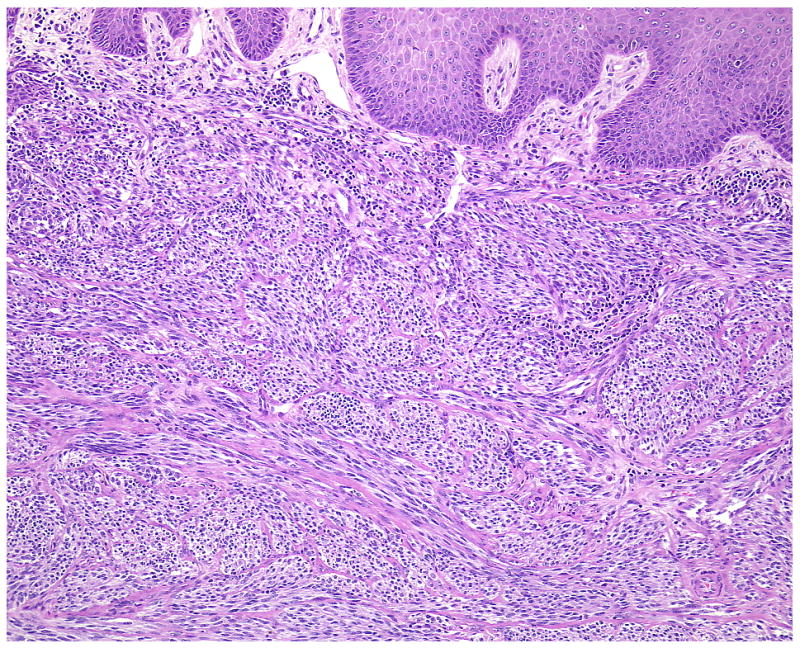
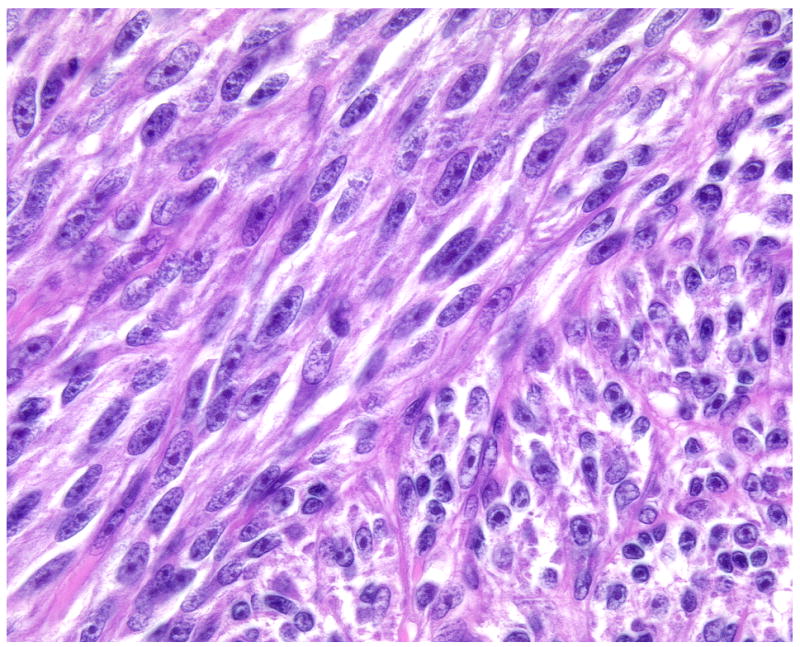
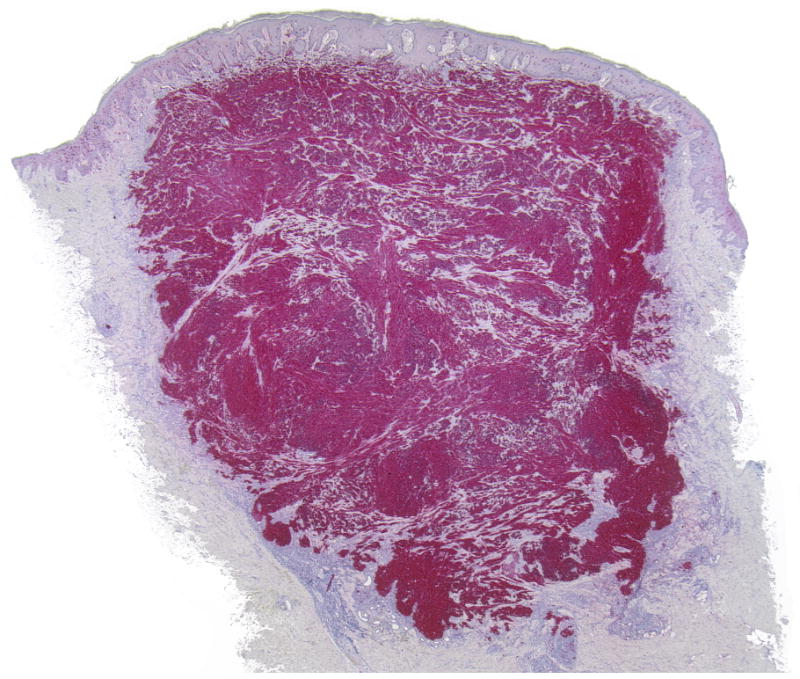
(A-D) A primary dermal CCS (Case 1) with fascicles of spindle cells bordering the epidermis. The spindle cells show pale eosinophilic or clear cytoplasm and elongated moderately pleomorphic oval nuclei with prominent nucleoli. In transversely cut fascicles and cells nuclei appear round. The cells show a homogenous expression of S100.
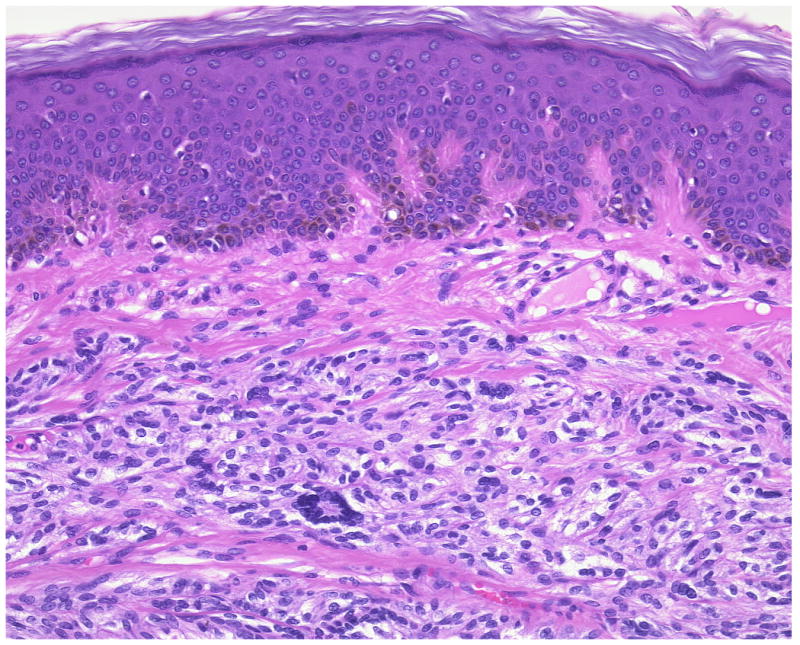
Figure 2.
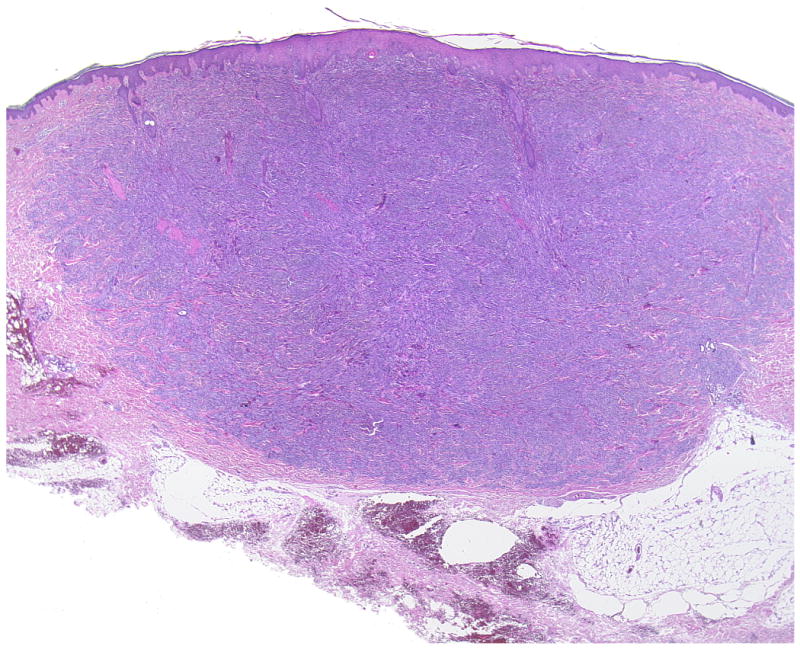
(A, B) A primary dermal CCS (Case 12). Fascicles of S100-positive spindle cells border closely the epidermis mimicking prima vista junctional nests. Melanocytes are neither increased nor scattered in the epidermis.
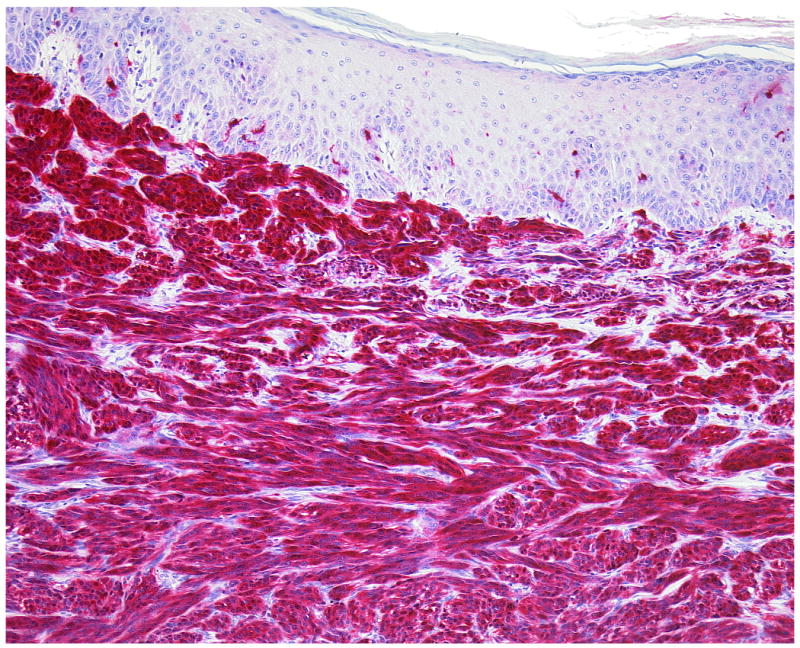
Figure 3.
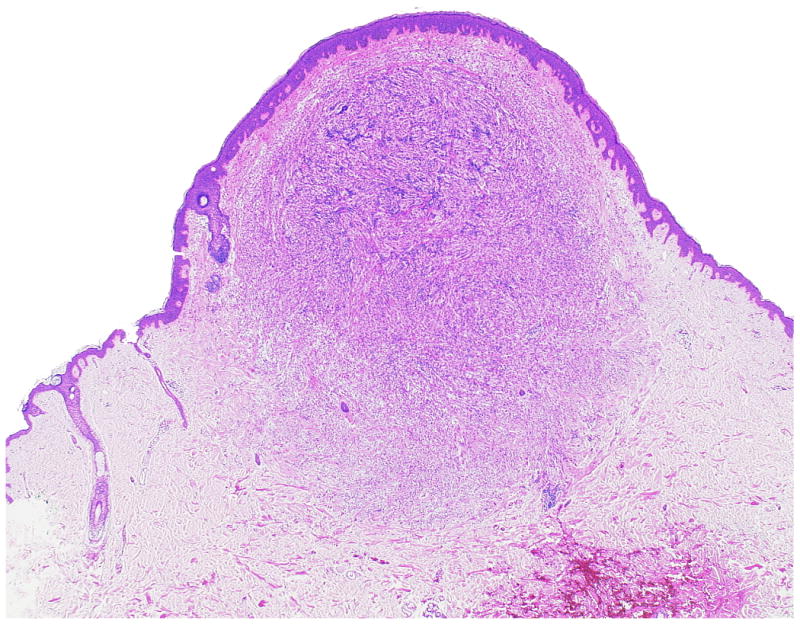
(A, B) The smallest primary dermal CCS in our series (Case 6) presents few multinucleated giant cells among the fascicles of spindle cells.
Figure 4.
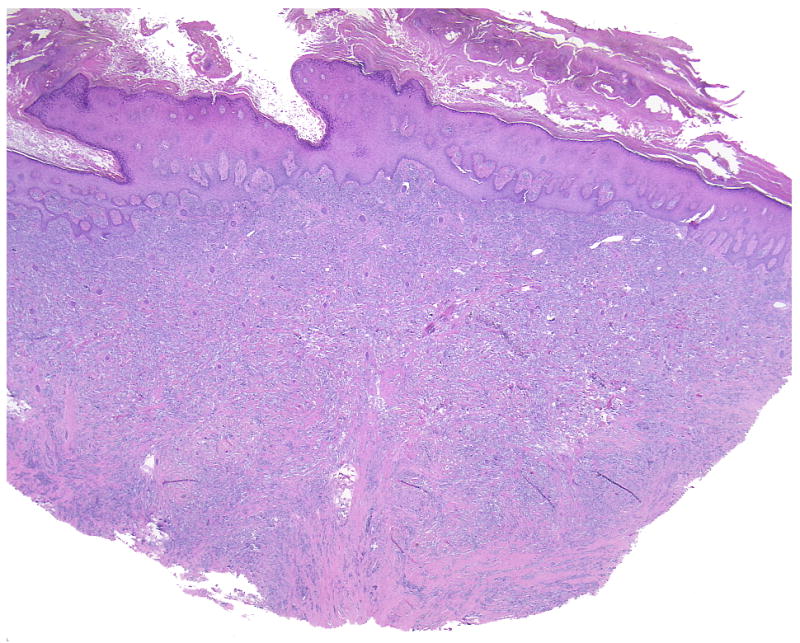
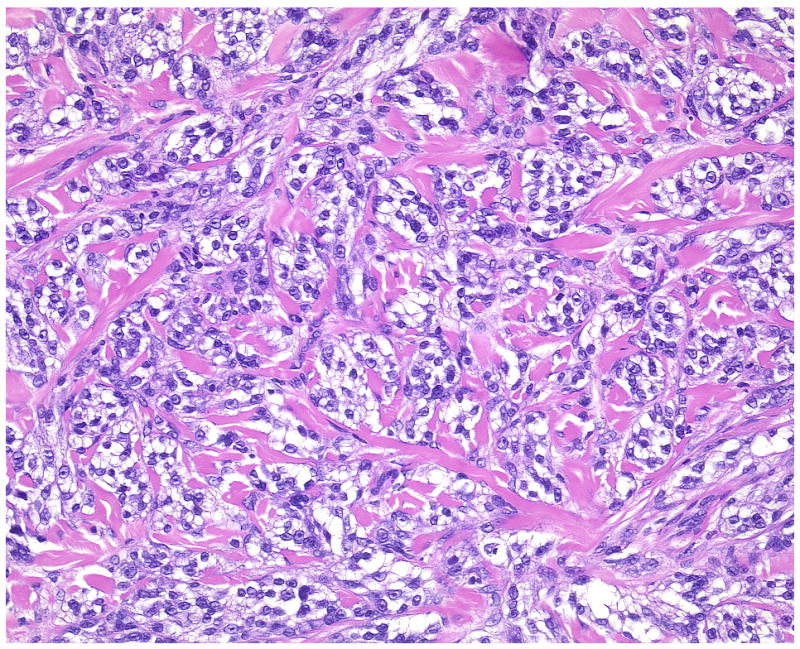
A characteristic feature of cutaneous CCS is fascicles of spindle cells encased by a reticulated pronounced hyalinized and sclerotic stroma (Case 4, overview and detail).
Figure 5.
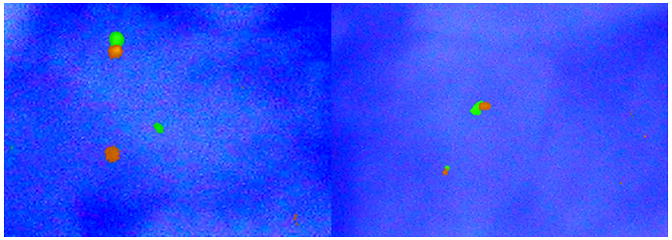
FISH analysis for the detection of EWSR1 translocation by EWSR1-break apart probe. Left half of the picture shows a positive result in Case 1 where signals are in different areas of the nucleus. In the right half (negative control) signals are very close together.
Acknowledgments
The authors are grateful to the following pathologists, dermatopathologists and clinicians who kindly provided case material and clinical follow-up information: Dr. Donner (Aachen, Germany); Dr. Golz (Wuppertal, Germany), Professor Dr. Handt (Würselen-Bardenberg, Germany); Dr. Henschel (Freiberg, Germany); Dr. Hofman (Memmingen, Germany); Professor Dr. Werner Kempf (Zürich, Switzerland); Professor Dr. Dieter Metze (Münster, Germany), Dr. Pommerenke (Güstrow, Germany); Professor Dr. Störkel (Wuppertal, Germany).
Funding: This work partially supported by NIH 1P50CA09345-01A1 (Lazar)
References
- 1.Antonescu CR, Dal Cin P, Nafa K, et al. EWS-CREB1 is the predominant gene fusion in angiomatoid fibrous histiocytoma. Genes Chromosomes Cancer. 2007;46:1051–1060. doi: 10.1002/gcc.20491. [DOI] [PubMed] [Google Scholar]
- 2.Antonescu CR, Nafa K, Segal NH, et al. EWS-CREB1: a recurrent variant fusion in clear cell sarcoma-association with gastrointestinal location and absence of melanocytic differentiation. Clin Cancer Res. 2006;(12):5356–5362. doi: 10.1158/1078-0432.CCR-05-2811. [DOI] [PubMed] [Google Scholar]
- 3.Bearman RM, Noe J, Kempson RL. Clear-cell sarcoma with melanin pigment. Cancer. 1975;3:977–984. doi: 10.1002/1097-0142(197509)36:3<977::aid-cncr2820360321>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 4.Bridge JA, Borek DA, Neff JR, et al. Chromosomal abnormalities in clear cell sarcoma. Implications for histogenesis. Am J Clin Pathol. 1990;93:26–31. doi: 10.1093/ajcp/93.1.26. [DOI] [PubMed] [Google Scholar]
- 5.Bridge JA, Sreekantaiah C, Neff JR, et al. Cytogenetic findings in clear cell sarcoma. Malignant melanoma of soft parts. Cancer Genet Cytogenet. 1991;52:101–106. doi: 10.1016/0165-4608(91)90059-4. [DOI] [PubMed] [Google Scholar]
- 6.Chung EB, Enzinger FM. Malignant melanoma of soft parts. A reassessment of clear cell sarcoma. Am J Surg Pathol. 1983;7:405–413. doi: 10.1097/00000478-198307000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Coindre JM, Hostein II, Terier P, et al. Diagnosis of clear cell sarcoma by real-time reverse transcriptase-polymerase chain reaction analysis of paraffin embedded tissues. Clinicopathologic and molecular analysis of 44 patients from the French sarcoma group. Cancer. 2006;107:1055–1064. doi: 10.1002/cncr.22099. [DOI] [PubMed] [Google Scholar]
- 8.Covinsky M, Gong S, Rajram V, et al. EWS-ATF1 fusion transcripts in gastrointestinal tumors previously diagnosed as malignant melanoma. Hum Pathol. 2005;36:74–81. doi: 10.1016/j.humpath.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 9.Deenik W, Mooi WJ, Rutgers EJ, et al. Clear cell sarcoma (malignant melanoma) of soft parts: a clinicopathologic study of 30 cases. Cancer. 1999;86:969–975. [PubMed] [Google Scholar]
- 10.Enzinger FM. Clear-cell sarcoma of tendons and aponeuroses. An analysis of 21 cases. Cancer. 1965;18:1163–1174. doi: 10.1002/1097-0142(196509)18:9<1163::aid-cncr2820180916>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 11.Garcia JJ, Kramer MJ, O'Donnell RJ, et al. Mismatch repair protein expression and microsatellite instability: a comparison of clear cell sarcoma of soft parts and metastatic melanoma. Mod Pathol. 2006;19:950–957. doi: 10.1038/modpathol.3800611. [DOI] [PubMed] [Google Scholar]
- 12.Haller KH, Mertens F, Jin Y, et al. Fusion of the EWSR1 and ATF1 genes without expression of the MITF-M transcript in angiomatoid histiocytoma. Genes Chromosomes Cancer. 2005;44:97–193. doi: 10.1002/gcc.20201. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman GJ, Carter D. Clear-cell sarcoma oftendons and aponeuroses with melanin. Arch Pathol. 1973;95:22–25. [PubMed] [Google Scholar]
- 14.Kawai A, Hosono A, Nakayama R, et al. Clear cell sarcoma of tendons and aponeuroses: a study of 75 patients. Cancer. 2007;109:109–116. doi: 10.1002/cncr.22380. [DOI] [PubMed] [Google Scholar]
- 15.Kindblom LG, Lodding P, Angervall L. Clear-cell sarcoma of tendons and aponeuroses. An immunohistochemical and electron microscopic analysis indicating neural crest origin. Virchows Arch [Pathol Anat] 1983;401:109–128. doi: 10.1007/BF00644794. [DOI] [PubMed] [Google Scholar]
- 16.Langezaal SM, Graadt van Roggen JF, Cleton-Jansen AM, et al. Malignant melanoma is genetically distinct from clear cell sarcoma of tendons and aponeuroses (malignant melanoma of soft parts) Br J Cancer. 2001;84:535–538. doi: 10.1054/bjoc.2000.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lucas DR, Nascimento AG, Sim FH. Clear cell sarcoma of soft tissues. Mayo Clinic experience with 35 cases. Am J Surg Pathol. 1992;16:1197–1204. doi: 10.1097/00000478-199212000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Lyle PL, Amato CM, Fitzpatrick JE, et al. Gastrointestinal melanoma or clear cell sarcoma? Molecular evaluation of 7 cases previously diagnosed as malignant melanoma. Am J Surg Pathol. 2008;32:858–866. doi: 10.1097/PAS.0b013e31815b8288. [DOI] [PubMed] [Google Scholar]
- 19.MacKenzie DH. Clear-cell sarcoma of tendons and aponeuroses with melanin production. J Pathol. 1974;114:231–232. doi: 10.1002/path.1711140407. [DOI] [PubMed] [Google Scholar]
- 20.Marques B, Terrier P, Voigt JJ, et al. Clear cell soft tissue sarcoma. Clinical, histopathological and prognostic study of 36 cases. Ann Pathol. 2000;20:298–303. [PubMed] [Google Scholar]
- 21.Meis-Kindblom JM. Clear cell sarcoma of tendons and aponeuroses: A historical perspective and tribute to the man behind the entity. Adv Anat Pathol. 2006;13:286–292. doi: 10.1097/01.pap.0000213052.92435.1f. [DOI] [PubMed] [Google Scholar]
- 22.Montgomery EA, Meis JM, Ramos AG, et al. Clear cell sarcoma of tendons and aponeuroses. A clinicopathologic study of 58 cases with analysis of prognostic factors. Int J Surg Pathol. 1993;1:89–100. [Google Scholar]
- 23.Mrozek K, Karakousis CP, Perez-Mesa C, et al. Translocation t(12;22)(q13;q12.2-q12.3) in a clear cell sarcoma of tendons and aponeuroses. Genes Chromosomes Cancer. 1993;6:249–252. doi: 10.1002/gcc.2870060412. [DOI] [PubMed] [Google Scholar]
- 24.Panagopoulos I, Mertens F, Debiec-Rychter M, et al. Molecular genetic characterization of the EWS/ATF1 fusion gene in clear cell sarcoma of tendons and aponeuroses. Int J Cancer. 2002;99:560–567. doi: 10.1002/ijc.10404. [DOI] [PubMed] [Google Scholar]
- 25.Panagopoulos I, Mertens F, Isaksson M, et al. Absence of mutations of the BRAF gene in malignant melanoma of soft parts [clear cell sarcoma of tendons and aponeuroses) Cancer Genet Cytogenet. 2005;156:74–76. doi: 10.1016/j.cancergencyto.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 26.Patel RM, Down-Kelly E, Weiss SW, et al. Dual-color, break-apart fluorescence in situ hybridization for EWS gene rearrangement distinguishes clear cell sarcoma of soft tissue from malignant melanoma. Mod Pathol. 2005;18:1585–1590. doi: 10.1038/modpathol.3800503. [DOI] [PubMed] [Google Scholar]
- 27.Reeves BR, Fletcher CDM, Gusterson BA. The translocation t(12;22)(q13;q13) is a nonrandom rearrangement in clear cell sarcoma. Cancer Genet Cytogenet. 1992;64:101–103. doi: 10.1016/0165-4608(92)90336-7. [DOI] [PubMed] [Google Scholar]
- 28.Segal NH, Pavlidis P, Noble WS, et al. Classification of clear-cell sarcoma as a subtype of melanoma by genomic profiling. J Clin Oncol. 2003;21:1775–1781. doi: 10.1200/JCO.2003.10.108. [DOI] [PubMed] [Google Scholar]
- 29.Stenman G, Kindblom L, Angervall L. Reciprocal translocation t(12;22)(q13;q13) in clear cell sarcoma of tendons and aponeuroses. Genes Chromosomes Cancer. 1992;4:122–127. doi: 10.1002/gcc.2870040204. [DOI] [PubMed] [Google Scholar]
- 30.Travis JA, Bridge JA. Significance of both numerical and structural chromosomal abnormalities in clear cell sarcoma. Cancer Genet Cytogenet. 1992;64:104–106. doi: 10.1016/0165-4608(92)90337-8. [DOI] [PubMed] [Google Scholar]
- 31.Wei-Lien W, Empar M, Wenyong Z, et al. Detection and characterization of EWSR1/ATF1 and EWSR1/CREB1 chimeric transcripts in clear cell sarcoma (melanoma of soft parts) Mod Pathol. 2009;22:1201–1209. doi: 10.1038/modpathol.2009.85. [DOI] [PubMed] [Google Scholar]


