Key Points
Question
Can oral insulin delay or prevent clinically diagnosed type 1 diabetes?
Findings
In this randomized clinical trial that included 389 participants in the primary analysis who were first- and second-degree relatives of patients with type 1 diabetes, oral insulin compared with placebo did not significantly reduce the risk of diabetes onset over a median of 2.7 years (insulin group, 28.5% and placebo group, 33%; hazard ratio, 0.87).
Meaning
Oral insulin as used in this study was not effective in prevention of type 1 diabetes.
Abstract
Importance
Type 1 diabetes requires major lifestyle changes and carries increased morbidity and mortality. Prevention or delay of diabetes would have major clinical effect.
Objective
To determine whether oral insulin delays onset of type 1 diabetes in autoantibody-positive relatives of patients with type 1 diabetes.
Design, Setting, and Participants
Between March 2, 2007, and December 21, 2015, relatives with at least 2 autoantibodies, including insulin autoantibodies and normal glucose tolerance, were enrolled in Canada, the United States, Australia, New Zealand, the United Kingdom, Italy, Sweden, Finland, and Germany. The main study group (n = 389) had first-phase insulin release on an intravenous glucose tolerance test that was higher than the threshold. The 55 patients in the secondary stratum 1 had an identical antibody profile as the main study group except they had first-phase insulin release that was lower than the threshold. Secondary strata 2 (n = 114) and strata 3 (n = 3) had different autoantibody profiles and first-phase insulin release threshold combinations. Follow-up continued through December 31, 2016.
Interventions
Randomization to receive 7.5 mg/d of oral insulin (n = 283) or placebo (n = 277), including participants in the main study group who received oral insulin (n = 203) or placebo (n = 186).
Main Outcome and Measures
The primary outcome was time to diabetes in the main study group. Significance was based on a 1-sided threshold of .05, and 1-sided 95% CIs are reported.
Results
Of a total of 560 randomized participants (median enrollment age, 8.2 years; interquartile range [IQR], 5.7-12.1 years; 170 boys [60%]; 90.7% white non-Hispanic; 57.6% with a sibling with type 1 diabetes), 550 completed the trial including 389 participants (median age, 8.4 years; 245 boys [63%]), 382 (96%) in the main study group. During a median follow-up of 2.7 years (IQR, 1.5-4.6 years) in the main study group, diabetes was diagnosed in 58 participants (28.5%) in the oral insulin group and 62 (33%) in the placebo group. Time to diabetes was not significantly different between the 2 groups (hazard ratio [HR], 0.87; 95% CI, 0-1.2; P = .21). In secondary stratum 1 (n = 55), diabetes was diagnosed in 13 participants (48.1%) in the oral insulin group and in 19 participants (70.3%) in the placebo group. The time to diabetes was significantly longer with oral insulin (HR, 0.45; 95% CI, 0-0.82; P = .006). The HR for time to diabetes for the between-group comparisons for the 116 participants in the other secondary stratum was 1.03 (95% CI, 0-2.11; P = .53) and for the entire cohort of 560 participants was 0.83 (95% CI, 0-1.07; P = .11), which were not significantly different. The most common adverse event was infection (n = 254), with 134 events in the oral insulin group and 120 events in the placebo group, but no significant study-related adverse events occurred.
Conclusions and Relevance
Among autoantibody-positive relatives of patients with type 1 diabetes, oral insulin at a dose of 7.5 mg/d, compared with placebo, did not delay or prevent the development of type 1 diabetes over 2.7 years. These findings do not support oral insulin as used in this study for diabetes prevention.
Trial Registration
clinicaltrials.gov Identifier: NCT00419562
This randomized trial tests the effects of oral insulin on time to new-onset type 1 diabetes in relatives of patients with type 1 diabetes with autoantibodies or impaired glucose tolerance..
Introduction
In the Diabetes Prevention Trial–Type 1 (DPT-1), oral insulin compared with placebo did not show a reduction in the development of diabetes, but a post hoc analysis identified an at-risk subgroup with higher insulin autoantibody titers that suggested benefit. Consequently, the Type 1 Diabetes TrialNet clinical trials network, which succeeded the DPT-1 trial group, sought to further explore the role of oral insulin in delaying diabetes among relatives who were not significantly different from those in the subgroup who had experienced apparent benefit from oral insulin in DPT-1.
The DPT-1 oral insulin trial enrolled relatives who were positive for islet cell antibodies by immunofluorescence and positive for insulin autoantibodies by radioimmunoassay, had first-phase insulin release on intravenous glucose tolerance test higher than threshold (defined below), and had a normal oral glucose tolerance test result. Since the DPT-1 oral study was conducted, the microinsulin autoantibody assay, which requires much less blood volume, was developed.
Thus, TrialNet screened participants for eligibility for this protocol using a strategy of initially testing samples for the presence of microinsulin autoantibodies and antibodies to glutamic acid decarboxylase and insulinoma-associated antigen-2, with subsequent testing for islet cell autoantibodies among patients who had tested positive for the antibody. This trial, therefore, differed from the DPT-1 oral study by using the microinsulin autoantibody assay instead of the radioimmunoassay to detect insulin autoantibodies and by initially testing for microinsulin autoantibodies, glutamic acid decarboxylase autoantibodies, and insulinoma-associated antigen-2 autoantibodies compared with initial testing for islet cell autoantibodies and subsequent testing for insulin autoantibodies using the radioimmunoassay in islet cell autoantibody-positive participants. Additionally, to test whether the outcome would extend to microinsulin autoantibody–positive individuals not eligible for enrollment in the previous DPT-1 trial, this study included 4 separate strata according to additional antibodies and first-phase insulin release status.
Methods
Protocols for this trial were approved by institutional review boards or ethics committees at all 87 participating locations in the United States, Canada, Sweden, Finland, Italy, United Kingdom, Australia, Germany, and New Zealand. Study coordination, laboratory tests, and data management were conducted centrally. Participants, their parents, or both provided written informed consent.
Screening and Eligibility
Potential participants were identified through participation in the TrialNet Natural History Study (subsequently renamed the TrialNet Pathway to Prevention Study). Nondiabetic relatives of probands with type 1 diabetes were screened at institutional review board–approved sites after informed consent was obtained. These included first-degree relatives (sibling, parent, or child) who were aged 3 through 45 years, as well as second- or third-degree relatives (niece, nephew, aunt, uncle, cousin) who were aged 3 through 20 years. Initial screening was for diabetes autoantibodies—antibodies to microinsulin, glutamic acid decarboxylase, and insulinoma-associated antigen-2. Islet cell autoantibodies were measured if at least 1 other antibody tested positive. Race/ethnicity was included as part of federal reporting requirements, based on participant self-report of fixed categories determined by the National Institutes of Health.
Eligible participants were nondiabetic relatives of patients with type 1 diabetes, who had normal glucose tolerance on an oral glucose tolerance test, who were confirmed to have tested positive for the microinsulin autoantibody on 2 sample collections, who did not have the diabetes-protective human leukocyte antigen haplotypes DQA1*0102 and DQB1*0602, and who met criteria for the following primary and secondary study strata based on other autoantibodies and metabolic characteristics.
Primary Analysis Stratum
The primary objective of the study was to assess the effects of treatment within this stratum.
Eligible participants had to have either islet cell autoantibodies (≥10 juvenile diabetes foundation units) confirmed positive on 2 sample collections, or if not confirmed for islet cell autoantibodies, both glutamic acid decarboxylase and insulinoma-associated antigen-2 autoantibody tested positive on the same sample with confirmation of at least 1 of these autoantibodies required on a separate sample. Participants had to have first-phase insulin release higher than the threshold determined from the sum of the 1- and 3-minute insulin values from an intravenous glucose tolerance test. For participants aged 3 through 7 years or parents of probands with type 1 diabetes, the threshold was 60 μU/mL or higher. For siblings or offspring aged 8 through 45 years or other relatives aged 8 through 20 years, the threshold was 100 μU/mL or higher. These were the same thresholds used in the DPT-1 study.
The secondary objectives were to assess the effects of treatment in each stratum and in the entire group enrolled (the 4 strata combined).
Secondary Stratum 1: Identical to the primary stratum except participants had first-phase insulin release lower than the thresholds defined above.
Secondary Stratum 2: Islet cell autoantibodies not confirmed, or glutamic acid decarboxylase or insulinoma-associated antigen-2 autoantibody positive and confirmed on a separate sample (those confirmed for islet cell autoantibodies are in the primary stratum). In this stratum, participants also had first-phase insulin release higher than the thresholds defined in the primary stratum.
Secondary Stratum 3: Identical to secondary stratum 2 except participants had first-phase insulin release lower than the thresholds defined in the primary stratum above. Other entry criteria included normal glucose tolerance by oral glucose tolerance test, or if they had a previous abnormal glucose tolerance, 2 consecutive oral glucose tolerance tests with normal glucose tolerance. Abnormal glucose tolerance was defined as it was in DPT-1 using the standard criteria in effect at the initiation of DPT-1: fasting plasma glucose of more than 110 mg/dL; and/or 2-hour plasma glucose of more than 140 mg/dL; and/or 30-, 60-, or 90-minute plasma glucose of more than 200 mg/dL (to convert glucose from mg/dL to mmol/L, multiply by 0.0555).
Full exclusion criteria are available in the protocol (Supplement 1).
Randomization and Masking
After participants signed the consent form, completed screening visits, met all of the inclusion criteria and none of the exclusion criteria, and completed the baseline procedures, they were randomized in equal allocations to each treatment group via a computerized random-number generator. Randomization was stratified by study site, and block size was a variation of size 2 and 4. Randomization was not stratified by stratum. Treatment assignment was double masked. Outcome assessments were conducted without knowledge of treatment assignment.
Intervention
Participants were assigned to receive capsules of either oral insulin, 7.5 mg of recombinant human insulin crystals (Eli Lilly), or matched placebo. This was the same dose used in the DPT-1 study. Capsules were prepared with methylcellulose filler at a compounding pharmacy (Eminent Services Corp) and masked bottles were shipped to the clinical sites. All participants were requested to take 1 capsule of study medication daily for the duration of the study. Study medication was dispensed at each 6-month visit. Participants consumed the capsule as a single daily dose, either by taking the capsule or, if the participant could not swallow capsules, sprinkling its contents in juice or on food.
Assessments
Participants were seen every 6 months. At those visits, an oral glucose tolerance test was performed to assess whether diabetes had developed. Criteria for diabetes onset were, as defined by the American Diabetes Association (ADA), based on glucose testing or the presence of unequivocal hyperglycemia with acute metabolic decompensation. Specific criteria for diabetes onset is defined as the presence of symptoms of diabetes plus casual (random) plasma glucose of 200 mg/dL or higher or fasting plasma glucose of 126 mg/dL or higher, or 2-hour plasma glucose of 200 mg/dL or higher. The criteria must have been met on 2 occasions as soon as possible but no less than 1 day apart for diabetes to be diagnosed. It was preferred that at least 1 of the 2 testing occasions involved an oral glucose tolerance test. Tolerance tests were performed after an overnight fast. Blood samples were drawn through a temporary indwelling intravenous catheter. For the oral glucose tolerance test, the oral glucose dose was 1.75 g/kg (maximum, 75 g).
Outcomes
The primary outcome was the elapsed time from random treatment assignment to the development of diabetes among those enrolled in the primary analysis stratum, consisting of participants with insulin autoimmunity and first-phase insulin release that was higher than the threshold. Secondary outcomes included the effects of oral insulin treatment vs placebo in each stratum and in all strata combined, the consistency of oral insulin vs placebo treatment effect among strata, various subgroup analyses, and longitudinal analyses to assess the effects of oral insulin vs placebo over time. Other secondary outcomes such as the association of demographic, genetic, immunologic, metabolic, and lifestyle factors are not reported herein.
Additional exploratory analyses were planned and developed by the TrialNet protocol committee and finalized before the study results were unblinded and included analyses that incorporates factors such as duration of oral insulin use, age of enrollment, specific autoantibody pairs, year of enrollment, site of enrollment, sex, time to dysglycemia as a time-dependent covariate, baseline insulin autoantibody titer, time to dysglycemia, changes in autoantibody levels or positivity, hemoglobin A1c as an outcome, C-peptide levels at diagnosis, the presence of symptoms at the time of diagnosis, consistency of hazard rates, and association between adherence to oral insulin and results. Of these additional analyses, the relationship between adherence to oral insulin and results is reported herein and in eFigure 2 in Supplement 2. Additionally, a post hoc hazard rate comparison with the DPT-1 study was also completed and is reported herein.
Statistical Methods
Diabetes Risk
The cumulative incidence of diabetes onset over time since randomization within each group was estimated from a modified Kaplan-Meier estimate of the “diabetes-free” survival function. The difference between groups in the cumulative incidence functions and the associated hazard functions was tested using the Mantel log-rank test on discrete time to type 1 diabetes (6-month intervals). The relative risk of diabetes onset between groups was estimated from the discrete Cox proportional hazards model. The critical value for the test statistic, P = .047, and CIs in the primary analysis were adjusted for a single interim analysis based on the Lan-DeMets spending function.
The effect of treatment with oral insulin vs placebo was tested using the intention-to-treat principle in the primary and secondary analysis strata, each consisting of participants defined using different combinations of autoantibodies and metabolic status using the same analyses as above for the primary analysis. An additional analysis assessed the effect of treatment within all strata combined using a Cox proportional hazards model stratified by stratum. Significance was based on a 1-sided threshold of .05, and 1-sided 95% CIs are reported.
The study was designed as a maximum information trial, which did not include a fixed sample size. Instead, participants were recruited and followed up until the required amount of statistical information was achieved. At any point during the study, the information in the data for a log-rank test is provided by I = (DOI×DC)/(DOI+DC), for which DOI and DC refer to the number of participants who developed diabetes in the oral insulin and control groups, respectively. The information required to provide 85% power to detect a 40% risk reduction (identical to the DPT-1 Oral Insulin Trial) with a 1-sided log-rank test at the .05 significance level is I = 27.551. All but 7 participants contributed to the analysis in the primary stratum. No attempt was made to impute missing data and no adjustment has been made for multiple comparisons, except the interim monitoring and multivariate analyses. Consequently, all but the primary analysis should be considered exploratory. Except for the post hoc hazard rate comparison with the DPT-1 study, the primary and secondary analyses were prespecified, and the exploratory analyses were preplanned.
Statistical analyses were performed using TIBCO Spotfire S+ 8.2 (PerkinElmer). Data on adverse events and efficacy were evaluated twice yearly by an independent data and safety monitoring board with predefined stopping rules.
Results
Participants in this study were relatives of patients with type 1 diabetes who tested positive for multiple autoantibodies and had normal glucose tolerance at the time of randomization. Screening of 138 385 individuals for potential enrollment began in March 2004. The first participant was randomized March 2, 2007, and the last participant, December 21, 2015. For the entire cohort, the randomization rate was a mean of 5 participants per month, and for the primary stratum, 3.5 participants per month. The database for this report was locked January 20, 2017.
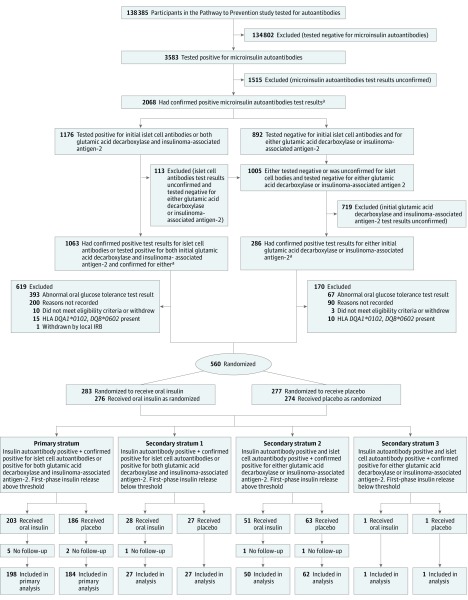
Of those screened, 3583 (2.56%) tested positive for microinsulin autoantibodies so were potentially eligible for this study. Of these, 2068 tested positive for microinsulin autoantibodies (Figure 1). A total of 560 participants were randomized, including 389 in the primary stratum. In the secondary strata, 55 participants were randomized in secondary stratum 1, 114 participants in secondary stratum 2, and 2 participants in secondary stratum 3. In the entire cohort, the median age at enrollment was 8.2 years (IQR, 5.7-12.1 years), 60% were boys, more than 90.7% were non-Hispanic white, and 57.6% had a sibling diagnosed with type 1 diabetes. The Table shows the baseline participant characteristics by treatment group for the entire cohort, for the primary stratum, and for the secondary strata. There were no substantial imbalances between treatment groups.
Figure 1. Participant Flow Through Type 1 Diabetes TrialNet Study.
Table. Distribution of Participant Characteristics by Treatment Group and Strata.
| Participant Characteristicsa | Entire Cohort | Primary Stratum | Secondary Stratum 1 | Secondary Strata 2 and 3 | ||||
|---|---|---|---|---|---|---|---|---|
| Oral Insulin (n = 283) |
Placebo (n = 277) |
Oral Insulin (n = 203) |
Placebo (n = 186) |
Oral Insulin (n = 28) |
Placebo (n = 27) |
Oral Insulin (n = 52) |
Placebo (n = 64) |
|
| Age, median (IQR), y | 8.2 (5.9-12.5) |
8.2 (5.4-11.5) |
8.6 (6.1 -12.8) |
8.2 (5.5-11.8) |
9.1 (5.9-13.7) |
8.5 (6.5-10.8) |
7.3 (5.1 -10.3) |
8.3 (5.1-11.5) |
| Boys, No. (%) | 170 (60.1) | 170 (61.4) | 128 (63.1) | 117 (62.9) | 19 (67.9) | 19 (70.4) | 23 (44.2) | 34 (53.1) |
| Race/ethnicity, No. (%)b | ||||||||
| White | 252 (95.5) | 249 (94.3) | 181 (95.3) | 172 (94.5) | 25 (96.2) | 25 (100.0) | 46 (95.8) | 52 (91.2) |
| Black | 8 (3.0) | 9 (3.4) | 6 (3.2) | 4 (2.2) | 0 | 0 | 2 (4.2) | 5 (8.8) |
| Asian/Pacific Islander | 4 (1.5) | 6 (2.3) | 3 (1.6) | 6 (3.3) | 1 (3.8) | 0 | 0 | 0 |
| Non-Hispanic, No. (%) | 256 (90.5) | 252 (91.0) | 182 (89.7) | 171 (91.9) | 26 (92.9) | 26 (96.3) | 48 (92.3) | 55 (85.9) |
| BMI, median (IQR)c | 17.1 (15.3-19.5) |
16.9 (15.5-19.2) |
17.4 (15.5-20.0) |
17.1 (15.6-19.6) |
16.2 (15-18.1) |
16.9 (15.5-17.8) |
16.4 (15.0-18.4) |
16.8 (15.3-19.2) |
| Family members with type 1 diabetes, No. (%) | ||||||||
| Sibling | 153 (54.1) | 162 (58.5) | 110 (54.2) | 111 (59.7) | 15 (53.6) | 19 (70.4) | 28 (53.8) | 32 (50.0) |
| Identical twin | 6 (2.1) | 3 (1.1) | 3 (1.5) | 2 (1.1) | 2 (7.1) | 1 (3.7) | 1 (1.9) | 0 |
| Offspring | 3 (1.1) | 7 (2.5) | 2 (1.0) | 5 (2.7) | 0 | 0 | 1 (1.9) | 2 (3.1) |
| Parent | 71 (25.1) | 57 (20.6) | 45 (22.2) | 40 (21.5) | 7 (25.0) | 3 (11.1) | 19 (36.5) | 14 (21.9) |
| Parent and sibling | 10 (3.5) | 13 (4.7) | 9 (4.4) | 6 (3.2) | 1 (3.6) | 3 (11.1) | 0 | 4 (6.2) |
| Offspring and another first-degree relative | 2 (0.7) | 0 | 2 (1.0) | 0 | 0 | 0 | 0 | 0 |
| Second-degree relative | 33 (11.7) | 30 (10.8) | 27 (13.3) | 18 (9.7) | 3 (10.7) | 1 (3.7) | 3 (5.8) | 11 (17.2) |
| Third-degree or further removed relative | 5 (1.8) | 5 (1.8) | 5 (2.5) | 4 (2.2) | 0 | 0 | 0 | 1 (1.6) |
| Autoantibodies positive, No. (%) | ||||||||
| Glutamic acid decarboxylase | 235 (83.0) | 236 (85.2) | 171 (84.2) | 156 (83.9) | 21 (75.0) | 23 (85.2) | 43 (82.7) | 57 (89.1) |
| Insulinoma-associated antigen-2 | 157 (55.5) | 146 (52.7) | 131 (64.5) | 126 (67.7) | 20 (71.4) | 18 (66.7) | 6 (11.5) | 2 (3.1) |
| Micro insulin autoantibodies | 253 (89.4) | 241 (87.0) | 186 (91.6) | 163 (87.6) | 24 (85.7) | 24 (88.9) | 43 (82.7) | 54 (84.4) |
| Islet cell autoantibodies | 198 (70.0) | 178 (64.3) | 171 (84.2) | 152 (81.7) | 25 (89.3) | 22 (81.5) | 2 (3.8) | 4 (6.2) |
| Hemoglobin A1c, median (IQR), %d | 5.0 (4.8-5.2) |
5.1 (4.8-5.2) |
5.0 (4.8-5.2) |
5.1 (4.8-5.2) |
5.1 (4.9-5.3) |
5.2 (5.0-5.5) |
5.0 (4.9-5.2) |
5.0 (4.9-5.1) |
| First-phase insulin release, median (IQR), μU/mL | 145 (102-221) |
150 (98.4-232) |
153 (112-230) |
152 (106-236) |
50.8 (45.4-67.1) |
57.8 (44.8-71.9) |
163 (119-243) |
174 (132-285) |
| C-peptide, median (IQR), nmol/Le | 1.35 (1.00-1.81) |
1.34 (1.03-1.82) |
1.42 (1.04-1.91) |
1.34 (1.04-1.96) |
1.02 (0.93 -1.3) |
1.1 (0.76-1.52) |
1.36 (1.06-1.75) |
1.43 (1.11-1.8) |
| Human leukocyte antigen alleles, No.(%)f | ||||||||
| DR3 | 119 (42.2) | 102 (37.0) | 88 (43.3) | 74 (39.8) | 9 (32.1) | 9 (33.3) | 22 (43.1) | 19 (30.2) |
| DR4 | 199 (70.6) | 182 (65.9) | 144 (70.9) | 135 (72.6) | 22 (78.6) | 18 (66.7) | 33 (64.7) | 29 (46.0) |
Abbreviations: BMI, body mass index, calculated as weight in kilograms divided by height in meters squared; IQR, interquartile range.
The primary stratum included participants with normal glucose tolerance, first-phase insulin release higher than threshold, the presence of microinsulin autoantibodies, and islet cell autoantibodies on 2 separate samples or both glutamic acid decarboxylase and insulinoma-associated antigen-2 autoantibodies on the same sample. Secondary stratum 1 included participants with the same profile as those in the primary stratum except they had first-phase insulin release that was lower than threshold. Secondary stratum 2 participants had normal glucose tolerance, first-phase insulin release higher than threshold, the presence of microinsulin autoantibodies, and islet cell autoantibodies on 1 sample or glutamic acid decarboxylase or insulinoma-associated antigen-2 autoantibodies on 2 separate samples. Secondary stratum 3 included participants whose profiles were identical to those in secondary stratum 2 except for their first-phase insulin release was lower than threshold, defined in the Methods section.
Self-reported race was not provided by 32 participants.
Not reported for 3 participants.
Values were missing for 1 participant.
C-peptide from oral glucose tolerance test not available for 1 participant. Area under the curve (AUC) mean, oral glucose tolerance test.
Not available for 2 participants.
Follow-up
Participants were followed up for a median of 2.7 years (IQR, 1.5-4.7 years) for the entire cohort; a median of 2.7 years (IQR, 1.5-4.6 years) for the primary stratum. A total of 173 participants (31%) were diagnosed with type 1 diabetes in the entire cohort and 120 (31%) in the primary stratum, were diagnosed with type 1 diabetes. Based on the number of participants diagnosed with type 1 diabetes, study design parameters were met (I = 30.0).
Lost or Withdrawn From Study
The lost-to-follow-up rate was 4.0% per year. Ten randomized participants were never evaluated for type 1 diabetes and never underwent an oral glucose tolerance test after randomization (7 in the oral insulin; 3 in the placebo group; 5 and 2, respectively, for just the primary stratum). If these participants are removed, the rate of lost to follow-up was 3.3% per year.
Outcome Data
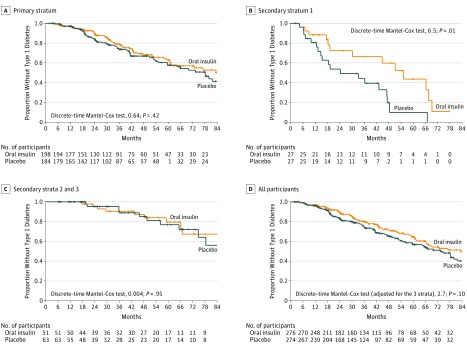
In the primary analysis stratum, diabetes was diagnosed in 120 participants—58 in the oral insulin group and 62 in the placebo group (Figure 2A). The annualized rate of diabetes was not significantly different between the 2 groups: 8.8% (95% CI, 6.7%-11.2%) with oral insulin and 10.2% (95% CI, 7.8%-12.9%) with placebo (hazard ratio [HR], 0.87; 95% CI, 0-1.2; P = .21). Treatment site was not a significant factor when tested either as a fixed effect in a mixed-effects model or in the Cox proportional hazards model.
Figure 2. The Proportion of Study Individuals Who Had Not Developed Type 1 Diabetes as a Function of Follow-up.
The plus marks indicate that the participants were censored.
A, The quartiles of time to diabetes were 18.4, 31.9, and 55.1 months for oral insulin and 18.9, 32.7, and 54.4 months for placebo. Of those in the oral insulin group, 140 participants did not develop type 1 diabetes and 58 did. Of those in the placebo group, 122 participants did not develop type 1 diabetes and 62 did. The median months of follow-up was 32.0 (1.87-114).
B, The quartiles of time to diabetes were 12.9, 19.2, and 52.8 months for oral insulin and 10.5, 18.5, and 39.8 months for placebo. Of those in the oral insulin group, 14 did not develop type 1 diabetes and 13 did. Of those in the placebo group, 8 did not develop type 1 diabetes and 19 did. The median months of follow-up was 18.9 (2.33-77.5).
C, The quartiles of time to diabetes were 25.3, 49.2, and 65.8 months for oral insulin and 18.1, 30.2, and 63.3 months for placebo. Of those in the oral insulin group, 40 participants did not develop type 1 diabetes and 11 did. Of those in the placebo group, 53 participants did not develop type 1 diabetes and 10 did. The median months of follow-up was 39.4 (6.21-115).
D, The quartiles of time to diabetes were 18.4, 34.9, and 59.7 months for oral insulin and 17.8, 31.2, and 54.0 months for placebo. Of those in the oral insulin group, 194 participants did not develop type 1 diabetes and 82 did. Of those in the placebo group, 183 participants did not develop type 1 diabetes and 91 did. The median months of follow-up was 32.4 (1.87-115).
Of the 55 patients in secondary stratum 1 (ie, with first-phase insulin release lower than threshold), diabetes was diagnosed in 32 participants—13 in the oral insulin group and 19 in the placebo group (Figure 2B). The annualized rate of diabetes was 18.1% (95% CI, 9.6%-29.1%) for the oral insulin group and 34.1% (95% CI, 20.6%-51.1%) for the placebo group (HR, 0.45; 95% CI, 0-0.82; P = .006). Thus, the median time to diabetes for the oral insulin group was 55.3 months (IQR, 19.2-67.5 months) and was 24.3 months (IQR, 13.3-47.3 months) for the placebo group, a difference of 31.0 months.
In secondary strata 2 and 3 (combined), diabetes was diagnosed in 21 participants—11 in the oral insulin group and 10 in the placebo group. The annualized rate of diabetes for this combined stratum was 5.1% (95% CI, 2.6%-8.6%) in the oral insulin group and 4.7% (95% CI, 2.2%-8.0%) for the placebo group (HR, 1.03; 95% CI, 0-2.11; P = .53; Figure 2C).
In the entire cohort, diabetes was diagnosed in 173 participants—82 in the oral insulin group and 91 in the placebo group. The annualized rate of diabetes was 8.7% (95% CI, 6.9%-10.7%) and 10.4% (95% CI, 8.3%-12.6%) in the oral and placebo treatment groups, respectively (HR, 0.83; 95% CI, 0-1.07; P = .11; Figure 2D).
Because the primary stratum in this study was designed to be consistent with the DPT-1 Study subgroup with baseline radioimmunoassay insulin autoantibodies of more than 80, a post hoc analysis of the HR of the primary stratum in the current study to the DPT-1 subgroup was conducted (DPT-1 to oral insulin, 1.04; 95% CI, 0.71-1.52; eFigure 2 in Supplement 2), which was not statistically different at P = .84, 2-sided. Thus, the entry criteria succeeded in replicating the risk seen in the DPT-1 cohort.
Analysis of Additional Preplanned Exploratory Outcomes
In an exploratory analysis of the primary stratum that included participants with 85% or more adherence with treatment (n = 215), the annualized rate of diabetes was 6.9% with oral insulin and 9.7% with placebo (HR, 0.69; 95% CI, 0-1.04; P = .06). In examining Kaplan-Meier curves for the primary stratum that included participants with varying degrees of adherence among more adherent participants, there was separation between the oral insulin and placebo groups during the first 24 months after randomization. Therefore, in an analysis of the first 24 months’ follow-up of adherent participants in the primary stratum, a protective association was observed among those taking oral insulin with fewer participants progressing to diabetes than among those taking placebo—the annualized rate of diabetes was 2.4% with oral insulin and 6.9% with placebo (HR, 0.348; 95% CI, 0-0.855; P = .02). This exploratory analysis is included in the eAppendix in Supplement 2.
Adverse Events
There were no serious adverse events and no reported episodes of severe hypoglycemia. The most common adverse event was categorized as infection, with 134 and 120 events reported in this category in the oral insulin and placebo groups, respectively, over the duration of the study (eTable 1 and 2 in Supplement 2).
Discussion
In this randomized clinical trial, oral insulin at a dose of 7.5 mg/d did not prevent the development of clinical type 1 diabetes in antibody-positive relatives of patients with type 1 diabetes in the primary stratum or in the entire randomized group.
Oral antigen administration has had small and inconsistent benefits in clinical trials involving patients with multiple sclerosis and rheumatoid arthritis, despite success in animal models of those autoimmune diseases. Among patients who have been newly diagnosed with type 1 diabetes, oral insulin, at the same or lower doses than the dose levels studied in this trial, failed to preserve pancreatic islet β-cell function. In the DPT-1 study, 7.5 mg/d of oral insulin failed to delay or prevent type 1 diabetes in the study as a whole. However, a post hoc analysis of data from DPT-1 revealed that autoantibody-positive relatives with higher confirmed radioimmunoassay insulin autoantibody titers progress to diabetes at a faster rate than participants with lower or unconfirmed insulin autoantibodies, and this subgroup may have benefitted from oral insulin. Participants with insulin autoantibodies that were 80 nU/mL or higher and who were treated with 7.5/d of oral insulin had an apparent 4- to 5-year delay in the onset of disease.
Because this study was undertaken to replicate the DPT-1 subgroup finding, the same 7.5-mg/d of oral insulin was used. However, immunomodulatory effects were reported with higher doses of oral insulin in preclinical studies and in a small-dose escalation study of children early in the disease process. The Pre-Point study found potentially protective immunomodulatory effects only in the 6 children who received the highest dose of oral insulin studied (67.5 mg/d). Therefore, as a companion study to the clinical trial reported herein, TrialNet has recently completed enrollment of more than 90 antibody-positive relatives in a study aimed at determining whether higher doses of oral insulin administered daily or every other week have measurable immunomodulatory effects (NCT02580877).
In a prespecified, secondary analysis in the current trial, a significant protective effect was found in the stratum consisting of 55 individuals in whom the first-phase insulin response was lower than the threshold needed for entry into the primary stratum. The placebo group in this stratum had a higher rate of progression to type 1 diabetes than participants taking placebo in the primary stratum (34.1%-10.2%), attesting to the added diabetes risk among those with diminished first-phase insulin release. However, because there was no adjustment for multiple comparisons, this analysis must be considered exploratory and hypothesis generating.
Although requiring a large, multicenter network, this trial emphasizes the feasibility of identifying and treating individuals early in the type 1 diabetes disease process. Individuals with 2 or more antibodies are destined (>80% risk at 10 years) to develop clinical type 1 diabetes, albeit at various rates of progression. Individuals with 2 or more antibodies and normal glucose tolerance are now considered to have stage 1 type 1 diabetes. Without intervention, most of these individuals will develop stage 2 type 1 diabetes, manifested by abnormal glucose tolerance and eventually clinically apparent, or stage 3 type 1 diabetes. As noted in this study, the overall rate of progression from stage 1 to stage 3 was approximately 9.5% per year; not significantly different from the rate of progression in genetically at-risk infants who were followed up from birth.
Thus, trials of disease-modifying therapies among individuals with multiple antibodies could be considered treatment trials, in which the therapy aims to treat early type 1 diabetes or islet autoimmunity as distinct from prevention trials, which implies testing of therapies in healthy individuals to prevent disease. Most randomized clinical trials investigating type 1 diabetes–modifying therapy have been conducted among individuals with clinically overt disease. Disease-modifying therapies including the anti-CD3 monoclonal antibody, rituximab, abatacept, and alefacept significantly preserved insulin production (as measured by C-peptide levels) as well having immunological effects in the first 1 to 2 years after diagnosis of clinical disease. As demonstrated in the Diabetes Control and Complications Trial (DCCT), improved short-term diabetes control associated with sustained C-peptide production decreased the risk of complications. However, an effect at earlier stages of the disease in delaying onset of clinical diagnosis of diabetes would have a much more profound effect in relieving the burden of disease. Despite marked advances in insulin delivery and glucose monitoring, there are still significant unmet needs for disease modifying therapy in type 1 diabetes.
Limitations
This study has several limitations. First, there was a change in insulin autoantibody assay methods from the DPT-1 Oral Insulin study. The change in assay may have contributed to an inability to replicate previous results from the study using a 7.5-mg fixed dose of oral insulin because the selected study population for this trial may differ slightly from the population in the previous study that showed apparent benefit. Second, as in other type 1 diabetes prevention trials, there is the limited knowledge about and the ability to incorporate heterogeneity in the pathogenesis of disease. This trial enrolled participants based on evidence of autoimmunity but did not take into account genetic background, age at onset, and type of first-appearing diabetes-related autoantibody. The emerging literature now suggests that future trials need to consider these factors, and this trial adds to that, suggesting that first-phase insulin release status and treatment adherence should also be incorporated into study designs. Third, due to lack of adjustment for multiple comparisons, all secondary analyses need to be interpreted as exploratory.
Conclusions
Among autoantibody-positive relatives of patients with type 1 diabetes, oral insulin at a dose of 7.5 mg/d, compared with placebo, did not delay or prevent the development of type 1 diabetes over 2.7 years. These findings do not support oral insulin as used in this study for diabetes prevention.
Trial protocol
eFigure 1. Proportion of Study Individuals With 85% Adherence to Medication Remaining Type 1 Diabetes Free
eFigure 2. Proportion of Participants in TrialNet vs DPT-1 Remaining Type 1 Diabetes Free
eAppendix. Adherence Methods
eTable 1. Adverse Events by Severity Grade
eTable 2. Adverse Events by Category
References
- 1.Diabetes Prevention Trial—Type 1 Diabetes Study Group Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med. 2002;346(22):1685-1691. [DOI] [PubMed] [Google Scholar]
- 2.Skyler JS, Krischer JP, Wolfsdorf J, et al. Effects of oral insulin in relatives of patients with type 1 diabetes: the Diabetes Prevention Trial—Type 1. Diabetes Care. 2005;28(5):1068-1076. [DOI] [PubMed] [Google Scholar]
- 3.Williams AJK, Bingley PJ, Bonifacio E, Palmer JP, Gale EAM. A novel micro-assay for insulin autoantibodies. J Autoimmun. 1997;10(5):473-478. [DOI] [PubMed] [Google Scholar]
- 4.Bingley PJ. Clinical applications of diabetes antibody testing. J Clin Endocrinol Metab. 2010;95(1):25-33. [DOI] [PubMed] [Google Scholar]
- 5.Mahon JL, Sosenko JM, Rafkin-Mervis L, et al. ; TrialNet Natural History Committee; Type 1 Diabetes TrialNet Study Group . The TrialNet Natural History Study of the Development of Type 1 Diabetes: objectives, design, and initial results. Pediatr Diabetes. 2009;10(2):97-104. [DOI] [PubMed] [Google Scholar]
- 6.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(suppl 1):S62-S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Therneau TMGP. Modeling Survival Data: Extending the Cox Model. New York, NY: Springer; 2000. [Google Scholar]
- 8.Gordon Lan KK, Demets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70(3):659-663. [Google Scholar]
- 9.Lachin JM. Maximum information designs. Clin Trials. 2005;2(5):453-464. [DOI] [PubMed] [Google Scholar]
- 10.Chaillous L, Lefèvre H, Thivolet C, et al. ; Diabète Insuline Orale group . Oral insulin administration and residual β-cell function in recent-onset type 1 diabetes: a multicentre randomised controlled trial. Lancet. 2000;356(9229):545-549. [DOI] [PubMed] [Google Scholar]
- 11.Bonifacio E, Ziegler AG, Klingensmith G, et al. ; Pre-POINT Study Group . Effects of high-dose oral insulin on immune responses in children at high risk for type 1 diabetes: the Pre-POINT randomized clinical trial. JAMA. 2015;313(15):1541-1549. [DOI] [PubMed] [Google Scholar]
- 12.Insel RA, Dunne JL, Atkinson MA, et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care. 2015;38(10):1964-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herold KC, Burton JB, Francois F, Poumian-Ruiz E, Glandt M, Bluestone JA. Activation of human T cells by FcR nonbinding anti-CD3 mAb, hOKT3γ1(Ala-Ala). J Clin Invest. 2003;111(3):409-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herold KC, Gitelman SE, Masharani U, et al. A single course of anti-CD3 monoclonal antibody hOKT3γ1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes. 2005;54(6):1763-1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herold KC, Gitelman SE, Ehlers MR, et al. ; AbATE Study Team . Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes. 2013;62(11):3766-3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herold KC, Hagopian W, Auger JA, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346(22):1692-1698. [DOI] [PubMed] [Google Scholar]
- 17.Sherry N, Hagopian W, Ludvigsson J, et al. ; Protégé Trial Investigators . Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo-controlled trial. Lancet. 2011;378(9790):487-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hagopian W, Ferry RJ Jr, Sherry N, et al. ; Protégé Trial Investigators . Teplizumab preserves C-peptide in recent-onset type 1 diabetes: two-year results from the randomized, placebo-controlled Protégé trial. Diabetes. 2013;62(11):3901-3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keymeulen B, Vandemeulebroucke E, Ziegler AG, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005;352(25):2598-2608. [DOI] [PubMed] [Google Scholar]
- 20.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, et al. ; Type 1 Diabetes TrialNet Anti-CD20 Study Group . Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med. 2009;361(22):2143-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pescovitz MD, Greenbaum CJ, Bundy B, et al. ; Type 1 Diabetes TrialNet Anti-CD20 Study Group . B-lymphocyte depletion with rituximab and β-cell function: two-year results. Diabetes Care. 2014;37(2):453-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orban T, Bundy B, Becker DJ, et al. ; Type 1 Diabetes TrialNet Abatacept Study Group . Costimulation modulation with abatacept in patients with recent-onset type 1 diabetes: follow-up 1 year after cessation of treatment. Diabetes Care. 2014;37(4):1069-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rigby MR, Harris KM, Pinckney A, et al. Alefacept provides sustained clinical and immunological effects in new-onset type 1 diabetes patients. J Clin Invest. 2015;125(8):3285-3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nathan DM, Genuth S, Lachin J, et al. ; Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977-986. [DOI] [PubMed] [Google Scholar]
- 25.Nathan DM; DCCT/EDIC Research Group . The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. 2014;37(1):9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eFigure 1. Proportion of Study Individuals With 85% Adherence to Medication Remaining Type 1 Diabetes Free
eFigure 2. Proportion of Participants in TrialNet vs DPT-1 Remaining Type 1 Diabetes Free
eAppendix. Adherence Methods
eTable 1. Adverse Events by Severity Grade
eTable 2. Adverse Events by Category