Abstract
Bradykinin (BK), a component of the kallikrein-kininogen-kinin system exerts multiple effects via B1 and B2 receptor activation. In the cardiovascular system, bradykinin has cardioprotective and vasodilator properties. We investigated the effect of BK on cardiac-projecting neurons of nucleus ambiguus, a key site for the parasympathetic cardiac regulation. BK produced a dose-dependent increase in cytosolic Ca2+ concentration. Pretreatment with HOE140, a B2 receptor antagonist, but not with R715, a B1 receptor antagonist, abolished the response to BK. A selective B2 receptor agonist, but not a B1 receptor agonist, elicited an increase in cytosolic Ca2+ similarly to BK. Inhibition of N-type voltage-gated Ca2+ channels with ω-conotoxin GVIA had no effect on the Ca2+ signal produced by BK, while pretreatment with ω-conotoxin MVIIC, a blocker of P/Q-type of Ca2+ channels, significantly diminished the effect of BK. Pretreatment with xestospongin C and 2-aminoethoxydiphenyl borate, antagonists of inositol 1,4,5-trisphosphate receptors, abolished the response to BK. Inhibition of ryanodine receptors reduced the BK-induced Ca2+ increase, while disruption of lysosomal Ca2+ stores with bafilomycin A1 did not affect the response. BK produced a dose-dependent depolarization of nucleus ambiguus neurons, which was prevented by the B2 receptor antagonist. In vivo studies indicate that microinjection of BK into nucleus ambiguus elicited bradycardia in conscious rats via B2 receptors. In summary, in cardiac vagal neurons of nucleus ambiguus, BK activates B2 receptors promoting Ca2+ influx and Ca2+ release from endoplasmic reticulum, and membrane depolarization; these effects are translated in vivo by bradycardia.
Keywords: calcium signaling, cardiovascular regulation, microinjection, vagal tone
Introduction
Bradykinin (BK) is an endogenous nonapeptide released from proteolytic cleavage of kininogens by tissue and plasma kallicreins (Sainz et al., 2007). BK is rapidly degraded by the endopeptidases, mainly angiotensin converting enzyme (ACE, kininase II), into inactive fragments (Erdos, 1990, 2006). BK exerts its effects by interacting with two GPCRs namely B1 and B2 receptors (Alexander et al., 2015). Both types of receptors are Gq/11-coupled leading to phosphoinositide hydrolysis and intracellular Ca2+ mobilization, and with Gi proteins to inhibit adenylate cyclase (Leeb-Lundberg et al., 2005). B1 and B2 receptors have a wide tissue distribution in the vascular, cardiac and nervous system (Leeb-Lundberg et al., 2005). B1 receptor is expressed at a very low level in healthy tissues and induced in pathophysiological conditions such as inflammation and pain, while B2 receptor is ubiquitous and constitutively expressed (Calixto et al., 2004, Leeb-Lundberg et al., 2005).
BK, similar to other kinins, has been involved in cardiovascular homeostasis, smooth muscle contractility, nociception and inflammation. BK has been shown to have cardioprotective effects (Erdos, 1990), especially in ischemic conditions (Yang et al., 1997, Ito et al., 2003). In the peripheral circulation, BK has a vasodilator effect, opposite to the vasoconstrictor effect of angiotensin II (Regoli et al., 2012). Cardiovascular effects produced by central microinjection of BK include both pressor and depressor responses indicating multiple sites at which BK modulates the cardiovascular activity (Diz and Jacobowitz, 1984, Buccafusco and Serra, 1985). Intracerebroventricular (i.c.v.) injection of BK, produced a dose-dependent increase in arterial pressure and heart rate in conscious rats, by interaction with the central cholinergic system (Buccafusco and Serra, 1985). Depletion of brain acetylcholine by treatment with hemicholinium 3 abolished the BK-induced pressor response and converted the tachycardia to bradycardia (Buccafusco and Serra, 1985). On the other hand, in anesthetized rats, microinjection of BK in the paraventricular hypothalamic nucleus produced bradycardia, while microinjection of BK into preoptic suprachiasmatic nucleus or anterior hypothalamus produced tachycardia (Diz and Jacobowitz, 1984), indicating that BK modulates both the sympathetic and parasympathetic cardiac tone.
Autoradiography studies identified B2 receptor binding sites in human and rat nucleus ambiguus (de Sousa Buck et al., 2002), a key area in parasympathetic cardiac regulation (Dyavanapalli et al., 2016), however the effect of BK at this level has not been characterized. Our study investigates the effect of BK on nucleus ambiguus at the cellular and whole organism level.
Experimental procedures
Ethical approval
Animal protocols were approved by the Institutional Animal Care and Use Committee from Temple University and Thomas Jefferson University.
Chemicals
Chemicals were from Sigma-Aldrich (St. Louis, MO) unless otherwise mentioned. The selective B1 (9-BK, Lys-[des-Arg9]-bradykinin) and B2 (8,9-BK, [Phe8ψ(CH-NH)-Arg9]-bradykinin) receptor agonists, as well as the selective B1 antagonist R715, and selective B2 antagonist HOE140 (Hock et al., 1991) were from Tocris Bioscience (Bio-Techne, Minneapolis, MN). In the in vitro studies, the antagonist was administered for 20 min before and for the entire duration of agonist administration: for the in vivo studies, the antagonist was loaded in the cannula immediately before the agonist.
Animals
Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA), neonates and adults, were used in this study. Neonatal rats were used for retrograde labeling of nucleus ambiguus and neuronal culture for in vitro studies, and adult male rats were used for in vivo studies.
Retrograde labeling and neuronal culture
Cardiac vagal neurons of nucleus ambiguus were retrogradely labeled by intrapericardial injection of rhodamine [X-rhodamine-5-(and-6)-isothiocyanate; 5(6)-XRITC], 40 µl, 0.01%, (Invitrogen, ThermoFisher Scientific, Grand Island, NY), as previously reported (Brailoiu et al., 2014a, Brailoiu et al., 2014b). 24 h after rhodamine injection, rats were euthanized by decapitation, and the brains quickly removed and immersed in ice-cold Hanks’ balanced salt solution (HBSS; Mediatech, Manassas, VA). Nucleus ambiguus neurons were dissociated by enzymatic and mechanical dissociation and cultured on poly-lysine-coated glass coverslips in Neurobasal-A medium containing GlutaMax (1%), antibiotic-antimycotic (2%), and fetal bovine serum (10%). Cultures were maintained at 37 °C, in a humidified atmosphere with 5% CO2. Glial cell proliferation was inhibited by adding cytosine β-arabino furanoside (1 µM).
Calcium imaging
The intracellular Ca2+ concentration, [Ca2+]i, was assessed, as previously described, in neurons loaded with Fura-2 AM (Brailoiu et al., 2014a, Brailoiu et al., 2014b). Neurons were incubated with Fura-2 AM (5 µM in HBSS, 45 min, at room temperature). After wash in dye-free HBSS, coverslips were mounted in an open bath chamber on the stage of an inverted microscope Nikon Eclipse TiE (Nikon Inc., Melville, NY). The microscope was equipped with a Perfect Focus System and a Photometrics CoolSnap HQ2 CCD camera (Photometrics, Tucson, AZ). Fura-2 AM fluorescence (excitation - 340 and 380 nm, emission 510 nm) was acquired and analyzed using NIS-Elements AR software (Nikon), and the fluorescence ratio (340/380 nm) was converted to Ca2+ concentrations.
Measurement of membrane potential
The changes of membrane potential were assessed in neurons loaded with bis-(1,3-dibutylbarbituric acid)-trimethine-oxonol, DiBAC4(3), a voltage-sensitive dye, as reported (Brailoiu et al., 2014a, Brailoiu et al., 2014b). Neurons were incubated with DiBAC4(3) (0.5 mM in HBSS, 30 min) and the fluorescence (excitation/emission 480nm/540nm) was monitored. Calibration of DiBAC4(3) fluorescence was performed using gramicidin in Na+-free physiological solution, and various concentrations of K+ and N-methylglucamine.
Surgical procedures
Adult male rats (250–300 g) were anesthetized with ketamine hydrochloride (100–150 mg/kg) and acepromazine maleate (0.2 mg/kg), as previously reported (Brailoiu et al., 2014a, Brailoiu et al., 2014b). Nucleus ambiguus was identified based on stereotaxic coordinates (12.24 mm posterior to bregma, 2.1 mm from midline and 8.2 mm ventral to the dura mater); a guide C315G cannula was bilaterally inserted into the nucleus ambiguus. A calibrated transmitter (E-mitters, series 4000; Mini Mitter, Sunriver, OR) was inserted in the intraperitoneal space, as reported (Brailoiu et al., 2014a, Brailoiu et al., 2014b).
Telemetric heart rate monitoring
Series 4000 receivers (Mini Mitter, Sunriver, OR), and VitalView™ software (Mini Mitter, Sunriver, OR) were used to collect the signal from transmitters, as previously reported (Brailoiu et al., 2014a, Brailoiu et al., 2014b). Each data point represents the heart rate average per 30 s.
Microinjection into nucleus ambiguus
Bilateral microinjections into the nucleus ambiguus were performed one week after surgery, using the C315I internal cannula (PlasticsOne) and a Neuros Hamilton syringe, without animal handling. At least two hours were allowed between two injections. The functional identification of nucleus ambiguus was based on the bradycardia induced by microinjection of L-glutamate (L-Glu, 5 mM, 50 nL) at this site, as reported (Brailoiu et al., 2014a, Brailoiu et al., 2014b).
Statistical analysis
Data were expressed as mean ± standard error of mean. Data sets were compared for statistically significant differences using one-way ANOVA followed by post hoc Bonferroni test in Origin 7 (Origin Lab Corporation, Northampton, MA); P < 0.05 was considered statistically significant.
RESULTS
BK elevates cytosolic Ca2+ concentration by activating B2 receptors in cardiac preganglionic nAmb neurons
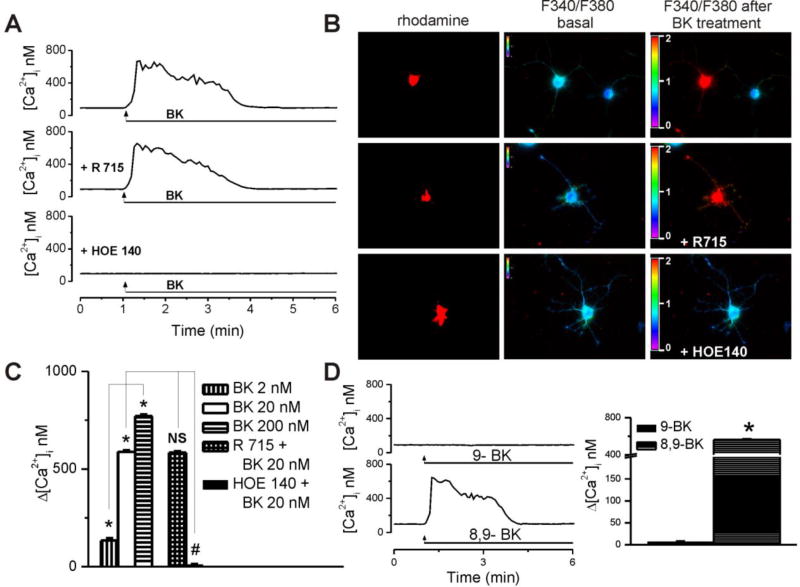
In rhodamine-labeled neurons, BK (20 nM) produced a fast and sustained increase in intracellular Ca2+ concentration, [Ca2+]i (Fig 1A, B). In neurons pretreated for 20 min with the B1 receptor antagonist R715 (2 µM), BK produced a similar response, while the response was absent in neurons pretreated with the B2 receptor blocker HOE140 (200 nM, 20 min, Fig 1A, B). Increasing concentrations of BK (2 nM, 20 nM, and 200 nM) produced concentration-dependent effects, measuring 134 ± 11 nM, 588 ± 7.4 nM and 771 ± 9.1, respectively, at the peak of the response (Fig. 1C). Six cells were examined in each treatment group. Blocking B1 receptors with R715 did not affect the response induced by BK (Δ[Ca2+]i = 584 ± 6.7 nM), while pretreatment with B2 receptor antagonist HOE140 reduced it to insignificant levels of 11 ± 2.8 nM (Fig. 1 B,C). To further validate the involvement of B2 receptors in BK-induced Ca2+ increase, we tested whether selective B1 and B2 agonists could produce responses comparable to that of BK. Indeed, a selective B2 receptor agonist (8,9-BK, [Phe8ψ(CH-NH)-Arg9]-bradykinin) produced a similar Ca2+ response as BK (Δ[Ca2+]i = 573 ± 7.9 nM, n = 6 neurons), as opposed to a B1 receptor agonist (9-BK, Lys-[des-Arg9]-bradykinin), which was virtually inactive (Δ[Ca2+]i = 7 ± 1.3 nM, n = 6 neurons, Fig. 1D).
Figure 1. BK produced concentration-dependent, B2 receptor-mediated [Ca2+]i elevations in cardiac vagal of nucleus ambiguus.
A, Typical Ca2+ responses induced by administration of BK alone (top) or in presence of either B1 receptor antagonist R715 (2 µM, middle), or B2 receptor blocker HOE140 (200 nM, bottom). B, Representative examples of changes in Fura-2 fluorescence ratio (340 nm/380 nm) of rhodamine-labeled neurons upon administration of 20 nM bradykinin (BK) alone (top) and in presence of R715 (middle) or HOE140 (bottom). C, Concentration-dependent effect of BK (2, 20, 200 nM) on [Ca2+]i of cardiac vagal of nucleus ambiguus and differential effect of R715 (2 µM, 20 min) and HOE140 (200 nM, 20 min) on the effect of BK (20 nM); *P < 0.05 when compared with basal Ca2+ levels and within the group; NS (not significant) and #P < 0.05 when compared with the effect of 20 nM BK. D, Characteristic tracings (left panel) and means comparison (right panel) of the Ca2+ responses induced by a selective B1 receptor agonist (9-BK, Lys-[des-Arg9]-bradykinin, 20 nM) and a selective B2 receptor agonist (8,9-BK, [Phe8ψ(CH-NH)-Arg9]-bradykinin, 20 nM); n = 6 neurons for each condition. *P < 0.05 as compared with basal Ca2+ levels.
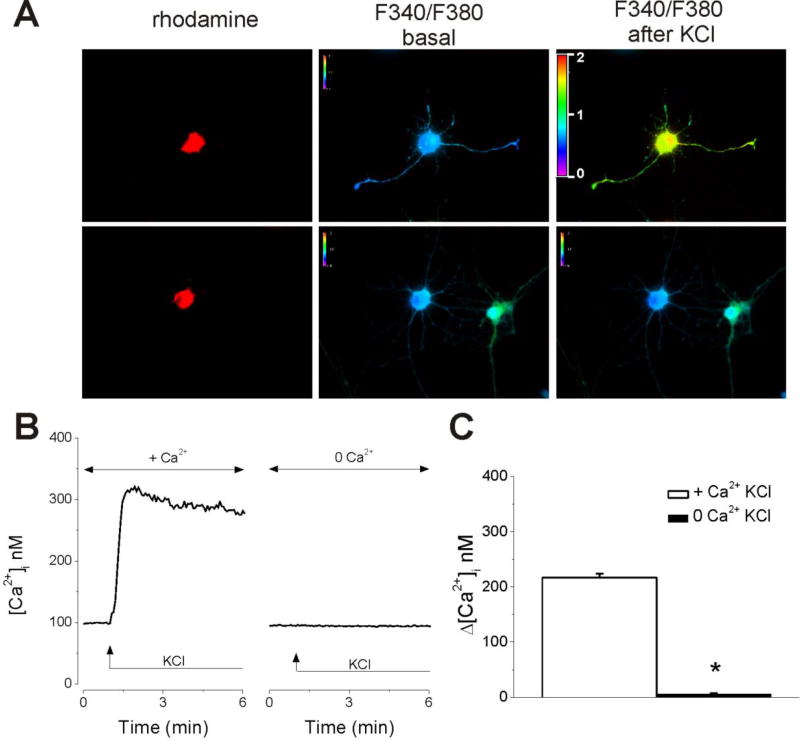
We tested the sensitivity of rhodamine-labeled neurons of nucleus ambiguus to KCl (6 mM) (Fig. 2). In Ca2+-containing saline, KCl elicited an increase in [Ca2+]i by 217 ± 6.8 nM (n = 6), while in Ca2+-free saline the response to KCl was abolished (Δ[Ca2+]i = 6 ± 1.4 nM, n = 6 neurons, Fig. 2), indicating that Ca2+ influx was elicited in response to high K+ concentration.
Figure 2. Retrogradely labeled nucleus ambiguus neurons are sensitive to high K+ concentration.
A, Representative examples of changes in Fura-2 fluorescence ratio (340 nm/380 nm) of rhodamine-labeled neurons upon application of KCl (6 mM) in Ca2+-containing and Ca2+-free HBSS. B. Illustration of [Ca2+]i increased produced by KCl in Ca2+-containing HBSS, and lack of response in Ca2+-free HBSS. C. Comparison of the amplitude of the increase in [Ca2+]i produced by KCl in nucleus ambiguus neurons; n = 6 neurons for each condition. *P < 0.05 as compared with the response in Ca2+-containing HBSS.
BK-induced Ca2+ elevation is subject to tachyphylaxis
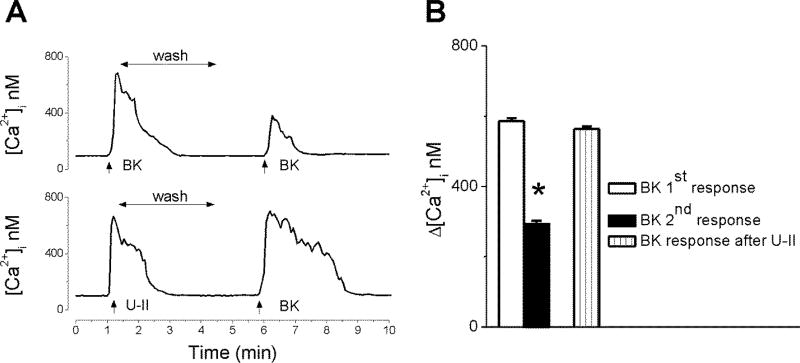
To investigate the effect of repeated treatment with BK, we monitored the Ca2+ response during two consecutive administrations of BK (20 nM). The second application of BK, after ~5 min wash, produced an increase in [Ca2+]i with a lower amplitude than the first application (Fig. 3A). The initial response induced by BK measured 586 ± 8.7 nM (n = 6), while the Ca2+ response promoted by a subsequent administration was significantly reduced to 294 ± 8.9 nM (n = 6), indicating receptor desensitization (Fig. 3B). On the other hand, when neurons were treated with BK (20 nM) after urotensin II (100 nM), the response to BK was not significantly affected (Δ [Ca2+]i by 564 ± 7.3 nM, n = 6) (Fig. 3)
Figure 3. Repetitive BK administration produces receptor desensitization.
Representative tracings (A) and mean amplitude comparison (B) of the Ca2+ responses induced by two consecutive applications of BK (20 nM), or by BK after urotensin II (U-II, 100 nM); n = 6 neurons for each condition. *P < 0.05 compared with the first BK-induced response.
BK triggers Ca2+ entry via P/Q voltage-gated Ca2+ channels (VGCC)
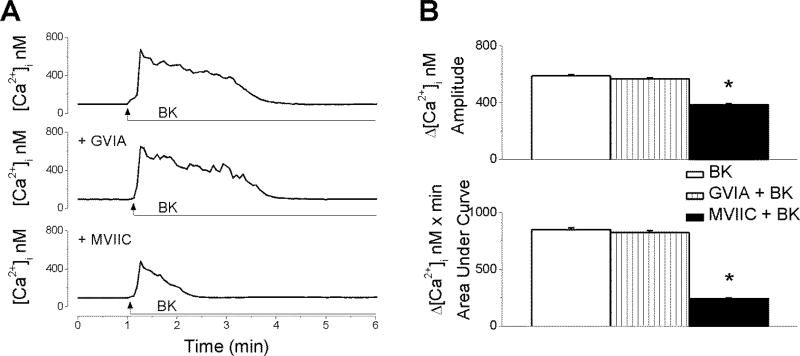
Next, we sought to identify the Ca2+ pools mobilized by BK in cardiac preganglionic neurons of nAmb. Inhibition of N-type VGCC with ω-conotoxin GVIA (100 nM, 20 min) had no effect on the Ca2+ signal produced by BK in rhodamine-labeled preganglionic neurons (Fig. 4A). The amplitude of the increase in [Ca2+]i produced by BK measured 588 ± 7.4 nM (n = 6) in the absence versus 567 ± 8.1 nM (n = 6) in the presence of N-type Ca2+ channel blocker (Fig. 4B). The area under curve (AUC) of BK-induced Ca2+ response was 849.4 ± 14 nM × min in the absence versus 823 ± 17 nM × min (n = 6) in the presence of ω-conotoxin GVIA. Pretreatment of neurons with ω-conotoxin MVIIC (100 nM, 20 min), a blocker of P/Q-type of Ca2+ channels, significantly diminished the effect of BK (Δ[Ca2+]i was 385 ± 7.4 nM, and ΔAUC = 241 ± 8 nM × min; n = 6 (Fig. 4A, B). Notably, when P/Q channels were blocked, the kinetics of the BK-induced Ca2+ release was also drastically altered, in that the effect was no longer sustained, but rather returned rapidly to basal levels (Fig. 4A).
Figure 4. BK promotes P/Q-type Ca2+ channel-mediated Ca2+ influx.
A, Representative examples of Ca2+ responses triggered by BK (20 nM) in the absence and presence of blockers of voltage-activated Ca2+ channels (N-type, ω-conotoxin GVIA; P/Q-type, ω-conotoxin MVIIC). B, Quantification of the increase in [Ca2+]i amplitude and Area Under Curve (AUC) produced by BK in each of the conditions mentioned in A; n = 6 neurons for each condition. *P < 0.05 when compared with the effect of BK administration alone.
BK releases Ca2+ via inositol 1,4,5-trisphosphate (IP3) and ryanodine receptors
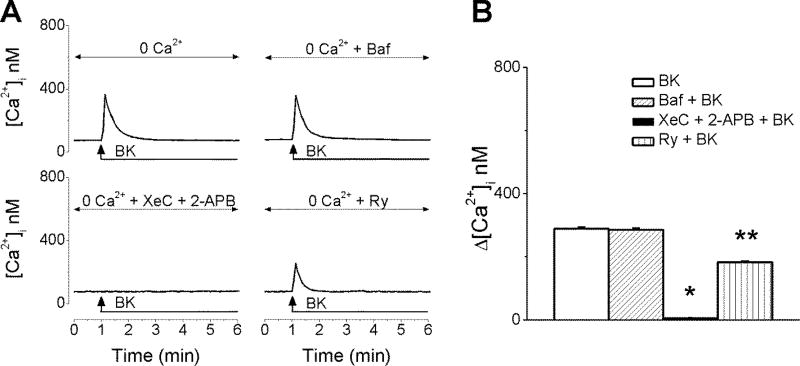
The fact that Ca2+ influx only partially accounted for BK-promoted Ca2+ elevation in cardiac vagal neurons prompted us to test the involvement of intracellular Ca2+ stores. In neurons incubated with Ca2+-free saline, BK (20 nM) induced a fast and transient [Ca2+]i elevation measuring 289 ± 4.1 nM (n = 6) at the peak of the response (Fig. 5A, B). The sustained phase that characterized BK-triggered effect in presence of extracellular Ca2+ (Fig.1A and 4A) was absent in Ca2+-free saline (Fig. 5A), further supporting Ca2+ influx as the underlying mechanism of the prolonged kinetics. Disruption of lysosomal Ca2+ stores with bafilomycin A1 (1 µM, 1h pre-incubation), a V-type ATPase that inhibits lysosomal acidification did not significantly interfere with BK-promoted Ca2+ increase (Δ[Ca2+]i was 286 ± 4.6 nM (n = 6). In contrast, pretreatment with xestospongin C (10 µM, 15 min) and 2-aminoethoxydiphenyl borate (2-APB, 100 µM, 15 min), which block IP3 receptors (IP3Rs), completely abolished the response of rhodamine-labeled cardiac vagal neurons to BK (Δ[Ca2+]i =7.8 ± 1.3 nM, n = 6 cells, Fig. 5A, B). Inhibition of ryanodine receptors with ryanodine (10 µM, 1h) significantly attenuated the effect of BK to 183 ± 3.9 nM (n = 6, Fig. 5A, B), indicating that ryanodine-sensitive Ca2+ stores are recruited, most likely, via a Ca2+-induced Ca2+-release mechanism.
Figure 5. BK releases Ca2+ from endoplasmic reticulum Ca2+ stores.
A, Characteristic increases in [Ca2+]i produced by BK (20 nM) in Ca2+-free saline, in the absence and presence of lysosomal disruptor bafilomycin A1 (Baf); IP3R inhibitors xestospongin C (XeC) and 2-APB; or ryanodine receptor blocker, ryanodine (Avemary and Diener). B, Comparison of the effects of BK on [Ca2+]i induced by the treatments indicated in A, in cardiac vagal neurons; n = 6 neurons for each condition. P < 0.05 compared with the effect of BK alone or in the presence of Baf or Ry (*), and compared with the effect of BK alone and in the presence of other antagonists (Baf, XeC + 2-APB) (**).
BK elicits depolarization of cultured cardiac vagal neurons
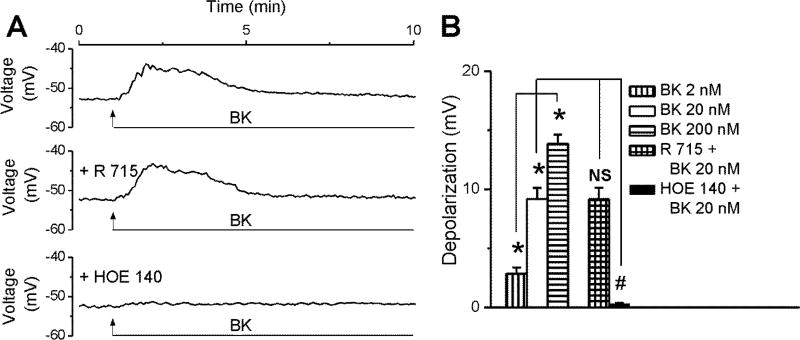
BK (20 nM) depolarized rhodamine-labeled nucleus ambiguus neurons with rather slow kinetics: the effect peaked within 30–40 s, maintained as a plateau for another minute and then receded gradually (Fig. 6A). BK-induced depolarization was not affected by pretreatment with the B1 antagonist R715 (2 µM, 20 min), but largely abolished upon blocking B2 receptors with HOE140 (200 nM, 20 min, Fig. 6A, B). Increasing concentrations of BK (2 nM, 20 nM, 200 nM) depolarized neurons by 2.85 ± 0.52 mV, 9.18 ± 0.94 mV, 13.88 ± 0.76 mV, respectively (Fig. 6B, n = 6 neurons per each condition). In the presence of R715 (2 µM), the amplitude of the depolarization induced by BK (20 nM) was 9.16 ± 0.97 mV (n = 6), similar to the response to BK in absence of any pretreatment, while BK effect was basically absent upon pretreatment with B2 receptor antagonist HOE140 (ΔVm = 0.31 ± 0.07 mV, n = 6, Fig. 6B).
Figure 6. BK depolarizes cardiac-projecting nucleus ambiguus neurons.
A, Characteristic changes in membrane potential elicited by BK (20 nM) alone or after B1 (R715) or B2 (HOE140) receptor antagonist pretreatment in rhodamine-labeled cardiac vagal nAmb neurons. B, Response quantifications reveal concentration-dependent depolarizing effect of BK (2–200 nM) on the resting membrane potential of cardiac preganglionic neurons;; n = 6 neurons for each condition. *P < 0.05 when compared with resting membrane potential and within the group; NS, not significant and #P < 0.05 when compared with the depolarizing effect of BK (20 nM).
Microinjection of BK into the nucleus ambiguus produces bradycardia in conscious rats
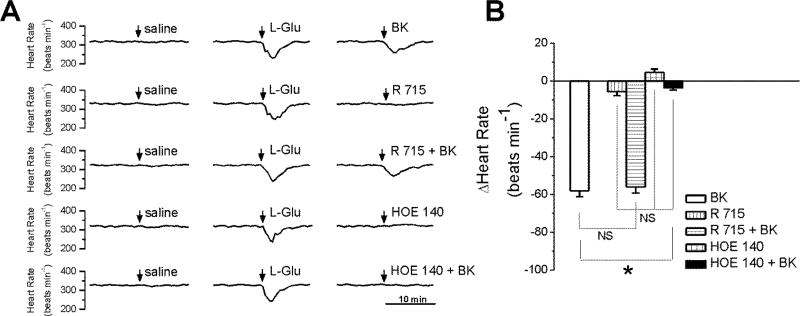
In conscious, freely moving rats, with cannula implanted into the nucleus ambiguus, microinjection of control saline (50 nL) produced negligible effects on heart rate. Microinjection of L-glutamate (5 mM, 50 nL) elicited bradycardia (Fig. 7A), indicating the correct placement of the cannula into the nucleus ambiguus (Brailoiu et al., 2013a, Brailoiu et al., 2014a). Two hours after L-glutamate administration, BK microinjection (20 nM, 50 nL) transiently and markedly reduced animal heart rate by 58 ± 3.11 beats per minute (bpm) (Fig. 7A,B). Microinjection of the B1 receptor antagonist R715 (2 µM, 50 nL) alone or of the B2 receptor antagonist HOE140 (200 nM, 50 nL) did not elicit a significant change to heart rate (Fig. 7A,B). Co-administration of BK and B1 receptor antagonist R715 (2 µM) induced a similar decrease in heart rate as BK alone (56 ± 3.26 bpm), while B2 receptor antagonist HOE140 (200 nM) abolished the BK-induced bradycardia (Fig. 7B). Six adult rats were used per each treatment group.
Figure 7. Bradycardic effects of BK administration in the nucleus ambiguus of conscious rats.
A, Typical heart rate recordings after microinjection of saline, L-glutamate (L-Glu, 5 mM, 50 nL), BK (20 nM, 50 nL), B1 receptor blocker, R715 (2 µM, 50 nL) alone, or in combination with BK (20 nM, 50 nL), B2 antagonist HOE140 (200 nM, 50 nL) alone or in combination with BK (20 nM, 50 nL). B, Quantifications of the heart rate changes induced by the treatments mentioned in A; n = 6 rats for each condition. *P < 0.05 when compared with the effect of BK; NS, not significant.
Discussion
Bradykinin, an endogenous nonapeptide, has vasodilator and cardioprotective effects by acting on vascular smooth muscle cells and cardiac myocytes (Leeb-Lundberg et al., 2005). However, its effects on central cardiovascular regulation have been less studied. We examined the effects of bradykinin on retrogradely-labeled neurons of nucleus ambiguus, that modulate the vagal cardiac tone (Dyavanapalli et al., 2016).
We first identified that BK increased cytosolic Ca2+ concentration, [Ca2+]i in these neurons. Likewise, previous studies indicate that BK increased [Ca2+]i in rat submucosal plexus (Avemary and Diener, 2010), myenteric neurons (Wurner et al., 2014), sensory neurons of trigeminal ganglia (Szteyn et al., 2015), or liver cells (Garcia-Sainz and Avendano-Vazquez, 1999). In cardiac vagal neurons, the effect of BK was mediated by B2 receptors, similarly to the response in rat submucosal plexus (Avemary and Diener, 2010) or liver cells (Garcia-Sainz and Avendano-Vazquez, 1999), while in myenteric neurons both B1 and B2 receptors were involved in the response (Wurner et al., 2014).
When BK was applied twice to cultured nucleus ambiguus neurons, the second Ca2+ response to BK had a lower amplitude than the first one, as previously reported (Garcia-Sainz and Avendano-Vazquez, 1999), indicating that BK, like other GPCR ligands, promoted tachyphylaxis. The most common mechanism of tachyphylaxis is desensitization of the receptor, due to receptor phosphorylation and internalization, as reported for B2 receptor in other cellular models (Blaukat et al., 1996, Haasemann et al., 1998). Previous administration of urotensin II, another peptide that activates nucleus ambiguus neurons (Brailoiu et al., 2014b) did not affect the response to bradykinin.
We next investigated the source of Ca2+ increase produced by BK. In cardiac vagal neurons of nucleus ambiguus, similarly to sensory neurons (Szteyn et al., 2015) or subcutaneous fibroblasts (Pinheiro et al., 2013), both Ca2+ influx and Ca2+ release from internal stores contributed to the increase in [Ca2+]i produced by BK. Using a pharmacological approach, we identified that in nucleus ambiguus neurons BK triggers Ca2+ entry via P/Q-type voltage-gated Ca2+ channels. Neurons of nucleus ambiguus are endowed with P/Q Ca2+ channels (Mendelowitz, 2004) and we found that several other peptides, such as urocortin 3 (Brailoiu et al., 2012), nesfatin-1 (Brailoiu et al., 2013b), urotensin II (Brailoiu et al., 2014b) promoted Ca2+ influx through a similar mechanism. In other neuronal populations, BK elicited Ca2+ influx via different mechanisms. For example, BK activated L-, N- and Q-types of Ca2+ channels in neurons from rat submucosal plexus (Avemary and Diener, 2010) or produced store-operated calcium entry (SOCE) via Orai1 activation and TRPC3 channels in rat trigeminal neurons (Szteyn et al., 2015).
In nucleus ambiguus neurons, BK releases Ca2+ from endoplasmic reticulum mainly via inositol 1,4,5-trisphosphate (IP3)- receptors, supporting the Gq-coupling of B2 receptors as reported before in other cellular models (de Weerd and Leeb-Lundberg, 1997, Leeb-Lundberg et al., 2005). In addition, in cardiac vagal neurons of ambiguus, BK elicited Ca2+ release via ryanodine receptors indicating that ryanodine-sensitive Ca2+ stores are recruited via a Ca2+-induced Ca2+-release mechanism. In rat hepatocytes, BK also produced Ca2+ release from endoplasmic reticulum and Ca2+ influx (Garcia-Sainz and Avendano-Vazquez, 1999).
BK produced a dose-dependent depolarization of cultured nucleus ambiguus neurons, which was prevented by the B2 receptor antagonist. Previous studies indicate a B2 receptor-mediated depolarization produced by bradykinin in rat submucosal plexus (Avemary and Diener, 2010) or in respiratory parasympathetic ganglion (Mochidome et al., 2001). A depolarization of cardiac vagal neurons of nucleus ambiguus leads to acetylcholine release in cardiac ganglia, and consequent bradycardia (Mendelowitz, 1999, 2004).
We next investigated the in vivo significance of our findings, by examining the changes in heart rate elicited by microinjection of BK into the rat nucleus ambiguus. To avoid the eventual effects of anesthetics on autonomic cardiovascular responses (Shimokawa et al., 1998, Akine et al., 2001, Lee et al., 2002), we performed the in vivo experiments in conscious rats, with a cannula implanted into nucleus ambiguus one week prior. Microinjection of BK into nucleus ambiguus produced bradycardia, that was abolished by the microinjection of B2 but not of B1 receptor antagonist. Notably, the B2 receptor antagonist, when microinjected into nucleus ambiguus, did not elicit any notable effect on the heart rate by itself. These findings indicate that there is not a release of BK in rat nucleus ambiguus in basal conditions. This in agreement with previous studies reporting that neurons of nucleus ambiguus are inherently silent (Mendelowitz, 1996). To our knowledge, this is the first study characterizing the effect of BK on neurons of nucleus ambiguus.
Previous studies reported that microinjection of BK in the hypothalamic and brainstem autonomic nuclei produced bradycardia or tachycardia depending on the site of injection, indicating that BK modulates both the sympathetic and parasympathetic tone (Diz and Jacobowitz, 1984, Buccafusco and Serra, 1985). For example, microinjection of BK in the dorsomedial and posterior hypothalamic nuclei, increased both heart rate and blood pressure (Diz and Jacobowitz, 1984), while BK microinjection into the nucleus tractus solitarius, an important afferent relay, elicited bradycardia (Caligiorne et al., 1996)
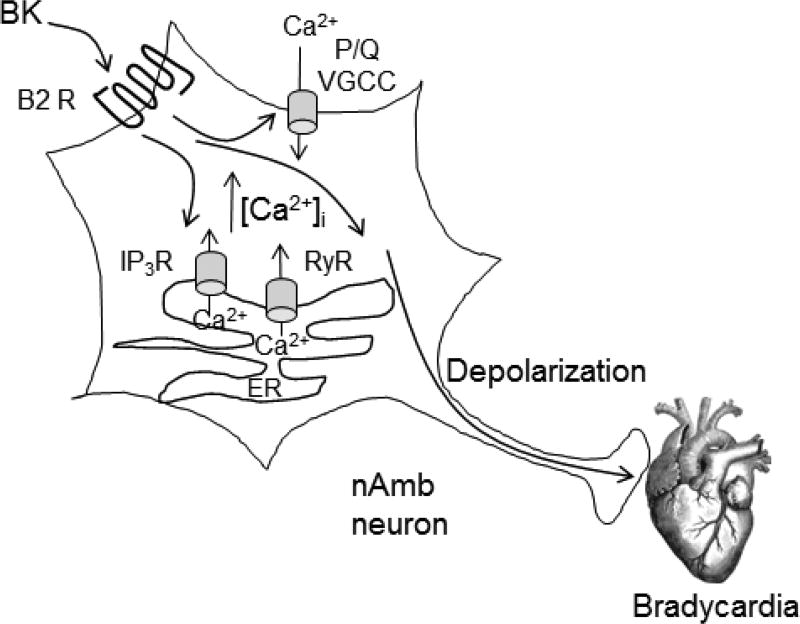
Our results support a role for bradykinin in the modulation of parasympathetic cardiac tone by acting on B2 receptors in nucleus ambiguus; the proposed mechanism is summarized in Figure 8. We characterize the cellular effects of BK on nucleus ambiguus, and unravel a new site of action for BK relevant for cardiovascular regulation. While the physiologic and pathophysiologic significance of our findings remain to be determined, they suggest a role in central cardiovascular regulation. Interestingly, previous studies reported changes in BK receptors levels in brain cardiovascular nuclei in various pathological conditions such as hypertension and diabetes (de Sousa Buck et al., 2002, Cloutier et al., 2004). For example, the B2 receptor binding density was about 10 times greater in nucleus ambiguus of spontaneously hypertensive rats as compared to age-matched Wistar Kyoto rats (Cloutier et al., 2004), suggesting a possible role for bradykinin via B2 receptor activation in ambiguus as a compensatory mechanism in hypertension.
Figure 8. Diagram summarizing the mechanism proposed for BK on nucleus ambiguus neurons.
BK, acting on B2 receptors (B2R) of nAmb increases [Ca2+]i by promoting Ca2+ influx via P/Q voltage-gated Ca2+ channels (VGCC) at the plasma membrane, and Ca2+ release from endoplasmic reticulum, via IP3 receptor (IP3R) and ryanodine receptor (RyR). BK also depolarizes Amb neurons, leading to acetylcholine release to the cardiac ganglia and subsequent bradycardia.
Acknowledgments
This study was supported by startup funds from the Jefferson College of Pharmacy, and by the National Institutes of Health (grants R01 DA035926 and P30 DA 013429).
Abbreviations
- AUC
area under curve
- BK
bradykinin
- B1 receptor
bradykinin receptor 1
- B2 receptor
bradykinin receptor 2
- [Ca2+]i
cytosolic Ca2+ concentration
- DiBAC4(3)
bis-(1,3-dibutylbarbituric acid)-trimethine-oxonol
- HBSS
Hank’s balanced salt solution
- IP3
1,4,5-trisphosphate
- nAmb
nucleus ambiguus
References
- 1.Akine A, Suzuka H, Hayashida Y, Kato Y. Effects of ketamine and propofol on autonomic cardiovascular function in chronically instrumented rats. Autonomic neuroscience : basic & clinical. 2001;87:201–208. doi: 10.1016/S1566-0702(00)00271-X. [DOI] [PubMed] [Google Scholar]
- 2.Alexander SP, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Southan C, Davies JA, Collaborators C. The Concise Guide to PHARMACOLOGY 2015/16: G protein-coupled receptors. British journal of pharmacology. 2015;172:5744–5869. doi: 10.1111/bph.13348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avemary J, Diener M. Bradykinin-induced depolarisation and Ca(2+) influx through voltage-gated Ca(2+) channels in rat submucosal neurons. European journal of pharmacology. 2010;635:87–95. doi: 10.1016/j.ejphar.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Blaukat A, Alla SA, Lohse MJ, Muller-Esterl W. Ligand-induced phosphorylation/dephosphorylation of the endogenous bradykinin B2 receptor from human fibroblasts. The Journal of biological chemistry. 1996;271:32366–32374. doi: 10.1074/jbc.271.50.32366. [DOI] [PubMed] [Google Scholar]
- 5.Brailoiu E, Deliu E, Sporici RA, Benamar K, Brailoiu GC. HIV-1-Tat excites cardiac parasympathetic neurons of nucleus ambiguus and triggers prolonged bradycardia in conscious rats. American journal of physiology Regulatory, integrative and comparative physiology. 2014a;306:R814–822. doi: 10.1152/ajpregu.00529.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brailoiu GC, Benamar K, Arterburn JB, Gao E, Rabinowitz JE, Koch WJ, Brailoiu E. Aldosterone increases cardiac vagal tone via G protein-coupled oestrogen receptor activation. The Journal of physiology. 2013a;591:4223–4235. doi: 10.1113/jphysiol.2013.257204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brailoiu GC, Deliu E, Rabinowitz JE, Tilley DG, Koch WJ, Brailoiu E. Urotensin II promotes vagal-mediated bradycardia by activating cardiac-projecting parasympathetic neurons of nucleus ambiguus. Journal of neurochemistry. 2014b;129:628–636. doi: 10.1111/jnc.12679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brailoiu GC, Deliu E, Tica AA, Chitravanshi VC, Brailoiu E. Urocortin 3 elevates cytosolic calcium in nucleus ambiguus neurons. Journal of neurochemistry. 2012;122:1129–1136. doi: 10.1111/j.1471-4159.2012.07869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brailoiu GC, Deliu E, Tica AA, Rabinowitz JE, Tilley DG, Benamar K, Koch WJ, Brailoiu E. Nesfatin-1 activates cardiac vagal neurons of nucleus ambiguus and elicits bradycardia in conscious rats. Journal of neurochemistry. 2013b;126:739–748. doi: 10.1111/jnc.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buccafusco JJ, Serra M. Role of cholinergic neurons in the cardiovascular responses evoked by central injection of bradykinin or angiotensin II in conscious rats. European journal of pharmacology. 1985;113:43–51. doi: 10.1016/0014-2999(85)90341-3. [DOI] [PubMed] [Google Scholar]
- 11.Caligiorne SM, Santos RA, Campagnole-Santos MJ. Cardiovascular effects produced by bradykinin microinjection into the nucleus tractus solitarii of anesthetized rats. Brain research. 1996;720:183–190. doi: 10.1016/0006-8993(95)01498-5. [DOI] [PubMed] [Google Scholar]
- 12.Calixto JB, Medeiros R, Fernandes ES, Ferreira J, Cabrini DA, Campos MM. Kinin B1 receptors: key G-protein-coupled receptors and their role in inflammatory and painful processes. British journal of pharmacology. 2004;143:803–818. doi: 10.1038/sj.bjp.0706012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cloutier F, Ongali B, Campos MM, Thibault G, Neugebauer W, Couture R. Correlation between brain bradykinin receptor binding sites and cardiovascular function in young and adult spontaneously hypertensive rats. British journal of pharmacology. 2004;142:285–296. doi: 10.1038/sj.bjp.0705759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Sousa Buck H, Ongali B, Thibault G, Lindsey CJ, Couture R. Autoradiographic detection of kinin receptors in the human medulla of control, hypertensive, and diabetic donors. Canadian journal of physiology and pharmacology. 2002;80:249–257. doi: 10.1139/y02-050. [DOI] [PubMed] [Google Scholar]
- 15.de Weerd WF, Leeb-Lundberg LM. Bradykinin sequesters B2 bradykinin receptors and the receptor-coupled Galpha subunits Galphaq and Galphai in caveolae in DDT1 MF-2 smooth muscle cells. The Journal of biological chemistry. 1997;272:17858–17866. doi: 10.1074/jbc.272.28.17858. [DOI] [PubMed] [Google Scholar]
- 16.Diz DI, Jacobowitz DM. Cardiovascular effects of discrete intrahypothalamic and preoptic injections of bradykinin. Brain research bulletin. 1984;12:409–417. doi: 10.1016/0361-9230(84)90113-8. [DOI] [PubMed] [Google Scholar]
- 17.Dyavanapalli J, Dergacheva O, Wang X, Mendelowitz D. Parasympathetic Vagal Control of Cardiac Function. Current hypertension reports. 2016;18:22. doi: 10.1007/s11906-016-0630-0. [DOI] [PubMed] [Google Scholar]
- 18.Erdos EG. Angiotensin I converting enzyme and the changes in our concepts through the years. Lewis K. Dahl memorial lecture. Hypertension. 1990;16:363–370. doi: 10.1161/01.hyp.16.4.363. [DOI] [PubMed] [Google Scholar]
- 19.Erdos EG. The ACE and I: how ACE inhibitors came to be. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2006;20:1034–1038. doi: 10.1096/fj.06-0602ufm. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Sainz JA, Avendano-Vazquez SE. Activation of bradykinin B2 receptors increases calcium entry and intracellular mobilization in C9 liver cells. Biochemistry and molecular biology international. 1999;47:927–933. doi: 10.1080/15216549900202043. [DOI] [PubMed] [Google Scholar]
- 21.Haasemann M, Cartaud J, Muller-Esterl W, Dunia I. Agonist-induced redistribution of bradykinin B2 receptor in caveolae. Journal of cell science. 1998;111(Pt 7):917–928. doi: 10.1242/jcs.111.7.917. [DOI] [PubMed] [Google Scholar]
- 22.Hock FJ, Wirth K, Albus U, Linz W, Gerhards HJ, Wiemer G, Henke S, Breipohl G, Konig W, Knolle J, et al. Hoe 140 a new potent and long acting bradykinin-antagonist: in vitro studies. Br J Pharmacol. 1991;102:769–773. doi: 10.1111/j.1476-5381.1991.tb12248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ito H, Hayashi I, Izumi T, Majima M. Bradykinin inhibits development of myocardial infarction through B2 receptor signalling by increment of regional blood flow around the ischaemic lesions in rats. British journal of pharmacology. 2003;138:225–233. doi: 10.1038/sj.bjp.0705013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JS, Morrow D, Andresen MC, Chang KS. Isoflurane depresses baroreflex control of heart rate in decerebrate rats. Anesthesiology. 2002;96:1214–1222. doi: 10.1097/00000542-200205000-00026. [DOI] [PubMed] [Google Scholar]
- 25.Leeb-Lundberg LM, Marceau F, Muller-Esterl W, Pettibone DJ, Zuraw BL. International union of pharmacology. XLV. Classification of the kinin receptor family: from molecular mechanisms to pathophysiological consequences. Pharmacological reviews. 2005;57:27–77. doi: 10.1124/pr.57.1.2. [DOI] [PubMed] [Google Scholar]
- 26.Mendelowitz D. Firing properties of identified parasympathetic cardiac neurons in nucleus ambiguus. The American journal of physiology. 1996;271:H2609–2614. doi: 10.1152/ajpheart.1996.271.6.H2609. [DOI] [PubMed] [Google Scholar]
- 27.Mendelowitz D. Advances in Parasympathetic Control of Heart Rate and Cardiac Function. News Physiol Sci. 1999;14:155–161. doi: 10.1152/physiologyonline.1999.14.4.155. [DOI] [PubMed] [Google Scholar]
- 28.Mendelowitz D. Brainstem premotor cardiac vagal neurons. Boston/Dordrecht/London: Kluwer Academic Publishers; 2004. [Google Scholar]
- 29.Mochidome T, Ishibashi H, Takahama K. Bradykinin activates airway parasympathetic ganglion neurons by inhibiting M-currents. Neuroscience. 2001;105:785–791. doi: 10.1016/s0306-4522(01)00211-1. [DOI] [PubMed] [Google Scholar]
- 30.Pinheiro AR, Paramos-de-Carvalho D, Certal M, Costa C, Magalhaes-Cardoso MT, Ferreirinha F, Costa MA, Correia-de-Sa P. Bradykinin-induced Ca2+ signaling in human subcutaneous fibroblasts involves ATP release via hemichannels leading to P2Y12 receptors activation. Cell communication and signaling : CCS. 2013;11:70. doi: 10.1186/1478-811X-11-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Regoli D, Plante GE, Gobeil F., Jr Impact of kinins in the treatment of cardiovascular diseases. Pharmacol Ther. 2012;135:94–111. doi: 10.1016/j.pharmthera.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Sainz IM, Pixley RA, Colman RW. Fifty years of research on the plasma kallikrein-kinin system: from protein structure and function to cell biology and in-vivo pathophysiology. Thrombosis and haemostasis. 2007;98:77–83. [PubMed] [Google Scholar]
- 33.Shimokawa A, Kunitake T, Takasaki M, Kannan H. Differential effects of anesthetics on sympathetic nerve activity and arterial baroreceptor reflex in chronically instrumented rats. J Auton Nerv Syst. 1998;72:46–54. doi: 10.1016/s0165-1838(98)00084-8. [DOI] [PubMed] [Google Scholar]
- 34.Szteyn K, Gomez R, Berg KA, Jeske NA. Divergence in endothelin-1- and bradykinin-activated store-operated calcium entry in afferent sensory neurons. ASN neuro. 2015;7 doi: 10.1177/1759091415578714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wurner L, Pouokam E, Diener M. The effect of bradykinin on the electrical activity of rat myenteric neurons. European journal of pharmacology. 2014;738:158–169. doi: 10.1016/j.ejphar.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 36.Yang XP, Liu YH, Scicli GM, Webb CR, Carretero OA. Role of kinins in the cardioprotective effect of preconditioning: study of myocardial ischemia/reperfusion injury in B2 kinin receptor knockout mice and kininogen-deficient rats. Hypertension. 1997;30:735–740. doi: 10.1161/01.hyp.30.3.735. [DOI] [PubMed] [Google Scholar]