SUMMARY
Intestinal stem cells (ISCs) maintain and repair the intestinal epithelium. While regeneration after ISC-targeted damage is increasingly understood, injury-repair mechanisms that direct regeneration following injuries to differentiated cells remain uncharacterized. The enteric pathogen, rotavirus, infects and damages differentiated cells while sparing all ISC populations, thus allowing the unique examination of the response of intact ISC compartments during injury-repair. Upon rotavirus infection in mice, ISC compartments robustly expand and proliferating cells rapidly migrate. Infection results specifically in stimulation of the active crypt-based columnar ISCs, but not alternative reserve ISC populations, as is observed after ISC-targeted damage. Conditional ablation of epithelial WNT secretion diminishes crypt expansion and ISC activation, demonstrating a previously unknown function of epithelial-secreted WNT during injury-repair. These findings indicate a hierarchical preference of crypt-based columnar cells (CBCs) over other potential ISC populations during epithelial restitution and the importance of epithelial-derived signals in regulating ISC behavior.
In Brief
Using rotavirus infection as an intestinal epithelial injury model, Zou et al. demonstrate that crypt-based columnar cells are the predominant cell type responding to epithelial villus injury, and WNT ligands secreted from the intestinal epithelium are essential for this regenerative process.
INTRODUCTION
The small intestinal epithelium is one of the fastest renewing tissues in the human body, regenerating every 4–5 days (van der Flier and Clevers, 2009; Mezoff and Shroyer, 2015). This regenerative capacity is critical for maintaining the epithelium, protecting against constant insults from the luminal environment. Intestinal stem cells (ISCs) in the crypts maintain and repair the epithelial surface by giving rise to differentiated cells on the villi. Differentiation of ISCs in the crypts produces daughter cells that migrate up in a “conveyer belt” fashion to the villi, where they mature into both absorptive and secretory cells that play a major role in nutrient absorption and other intestinal functions (Potten, 1997; van der Flier and Clevers, 2009; Mezoff and Shroyer, 2015; Henning and von Furstenberg, 2016; Beumer and Clevers, 2016). One exception to this migratory pathway is the mature Paneth cells, which remain in the crypts instead of migrating upward, interact closely with the ISCs, and secrete stem cell maintenance factors, including WNT (Henning and von Furstenberg, 2016; Mezoff and Shroyer, 2015; Beumer and Clevers, 2016).
The crypts are thought to contain two types of ISCs (Henning and von Furstenberg, 2016; Mezoff and Shroyer, 2015; Beumer and Clevers, 2016). The best studied is the crypt-based columnar cells (CBCs) located at the base of the crypt. CBCs express the cell-surface marker leucine-rich repeat-containing G-protein-coupled receptor 5 (LGR5), among others, and continually proliferate under homeostasis (Cheng and Leblond, 1974a, 1974b; Barker et al., 2007). There is also evidence for an alternative reserve ISC population, which can be referred to as +4 cells or quiescent ISCs, as well as early absorptive and secretory progenitors that reside above the base of the crypt (Potten and Hendry, 1975; Potten, 1977; van der Flier and Clevers, 2009; Mezoff and Shroyer, 2015). Traditionally, tissue renewal after injury in the intestine has been studied using γ-irradiation, chemotherapy treatments, or genetic ablation, in which proliferating crypt-based columnar ISCs are ablated (Beumer and Clevers, 2016). CBC loss can activate reserve ISCs or the dedifferentiation of committed progenitors to repopulate the CBC pool and provide epithelial restoration (May et al., 2008; Potten et al., 2009; Takeda et al., 2011; Tian et al., 2011; Hua et al., 2012; Powell et al., 2012; van Es et al., 2012; Van Landeghem et al., 2012; Yan et al., 2012; Yu, 2013; Metcalfe et al., 2014; Poulin et al., 2014; Roche et al., 2015; Tetteh et al., 2016; Buczacki et al., 2013). These studies highlight the dynamic nature of the ISC niche that can readily regenerate following functional stem cell loss. However, while ISCs are well characterized under homeostatic conditions or situations where they are damaged directly, very little is known about the differential activation of these populations under intestinal epithelial dysbiosis in which ISCs remain undamaged.
The maintenance and regeneration of the intestinal epithelium is regulated, at least in part, by canonical WNT/β-catenin signaling (Clevers et al., 2014; Clevers and Nusse, 2012; Kühl and Kühl, 2013; Nusse and Varmus, 2012; Shroyer et al., 2015; Yan et al., 2017b). Extracellular WNT ligands bind to membrane Frizzled (FZD) receptors to trigger intracellular translocation of the transcriptional co-activator β-catenin, which can then drive the expression of well-established WNT pathway target genes (Nusse and Varmus, 2012). Studies in mice have indicated that cells in the intestinal epithelium and mesenchyme are two independent sources of WNT secretion (Farin et al., 2012; Gregorieff et al., 2005; Valenta et al., 2016). Paneth cells in the intestinal epithelium secrete WNT3, WNT6, and WNT9B; and myofibroblasts in the mesenchyme express WNT2, WNT4, and WNT5A (Gregorieff et al., 2005; Farin et al., 2012; Aoki et al., 2016; Stzepourginski et al., 2017; Valenta et al., 2016). Importantly, the epithelium and the mesenchyme are thought to be redundant sources of WNT secretion, with epithelial secreted WNT ligands being nonessential (Farin et al., 2012; Kabiri et al., 2014; San Roman et al., 2014; Valenta et al., 2016). When WNT3 or genes essential for WNT secretion (e.g., Porcupine and Wntless) were ablated in the intestinal epithelium, no apparent phenotype was observed (Farin et al., 2012). Knockouts (KOs) of genes specific to Paneth cells (e.g., Atoh1 and Gfi1), the primary cellular source of epithelial WNT, also do not affect WNT pathway activation (Durand et al., 2012; Kim et al., 2012; Shroyer et al., 2005, 2007). These studies led to the prevailing notion that the epithelium is a redundant source of WNT and is not essential for CBC maintenance. By contrast, in vitro, mouse intestinal enteroids that contain only epithelial cells can be propagated without supplementing WNT ligands (Sato et al., 2009, 2011), suggesting that epithelial-secreted WNT is sufficient for stem cell proliferation. These discordant results warrant additional analyses on the role of epithelial-secreted WNT under nonhomeostatic situations.
We use rotavirus (RV) infection as an infection/injury model to test ISC responses to villus damage and whether mesenchymal or epithelial WNT plays a role in ISC activation. RV, a well-characterized, noninflammatory small intestinal viral pathogen, infects terminally differentiated mature cells, but not ISCs, in the small intestinal epithelium, resulting in watery diarrhea with vomiting, fever, and abdominal pain (Greenberg and Estes, 2009). RV infection alters the cytoskeleton and impairs host secretory pathways, leading to mislocalization of brush border enzymes and malabsorption, disruption of cell-cell junctions, increased permeability, cytotoxic effects, and activation of cell death (Ramig, 2004; Beau et al., 2007; Jourdan et al., 1998; Burns et al., 1995). Infection in adult mice is accompanied by a notable lack of villus blunting or epithelial ulceration, suggesting the induction of epithelial repair mechanisms that compensate and presumably replace the infected, lost cells (Ward et al., 1990; Burns et al., 1995; O’Neal et al., 1997; Blutt et al., 2012). Importantly, both the active and reserve ISC populations remain uninfected and undamaged during RV infection, providing an elegant model system to examine the ISC response within an intact stem cell compartment after injury. Our studies show that epithelial-derived signals regulate the hierarchical stem cell response when the niche remains intact and provide evidence for a functional role for epithelial WNT signaling.
RESULTS
RV Infection Does Not Infect the Crypt Compartment and Induces Crypt Expansion and Crypt-Villus Migration
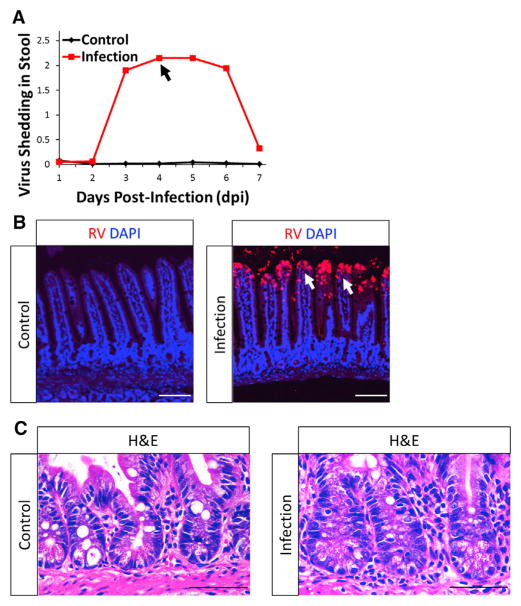
Oral inoculation of adult mice with murine RV results in viral infection that is detected by viral shedding in stool (Figure 1A) (Ward et al., 1990; Burns et al., 1995; O’Neal et al., 1997; Blutt et al., 2012). Infected cells detected by anti-RV antibody were limited to the tips of the villi from 2 to 4 days post-infection (dpi) (Figures 1B and S1A). Maximum levels of viral shedding were observed 4 dpi (Figure 1A); therefore, all subsequent studies were performed at this time point. Importantly, the stem cell compartment is never infected (Figure 1B and S1A), and all cell types, including CBCs, reserve ISCs, and Paneth cells within the crypts, remained intact in RV-infected animals (Figures 1C, 3D, 3H, and S2).
Figure 1. RV Infection Is Limited to the Tip of the Villi, Preserving an Intact Crypt Compartment.
(A) Representative stool ELISA monitoring RV infections. Points represent means of respective groups (n = 2). Peak viral shedding was observed at 4 days post-infection (dpi; black arrow).
(B) Representative confocal images of control- and RV-infected mouse epithelium. RV-infected differentiated cells were detected using a laboratory-generated, polyclonal anti-RV antibody. Infected villi are noted by white arrows.
(C) Representative H&E images showing intact crypts in both control- and RV-infected mouse epithelium.
See also Figures S1 and S2.
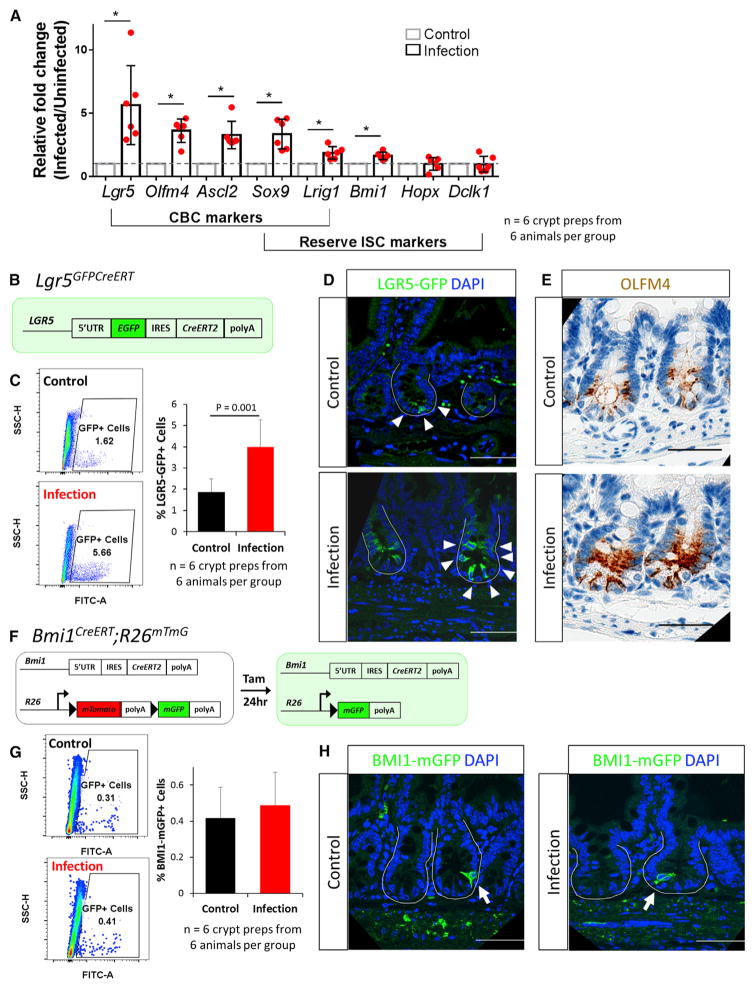
Figure 3. CBCs Are Induced following RV Infection.
(A) qRT-PCR results showed upregulation of all CBC markers and some reserve ISC markers.
(B) Schematic of Lgr5GFPCreERT mice. Cells expressing LGR5 are green.
(C) Representative flow cytometry analysis on control- and RV-infected Lgr5GFPCreERTmice. Quantification of GFP+ cells using flow cytometry analysis on crypt-enriched epithelial preparations.
(D) Representative confocal images of Lgr5GFPCreERT mice following control- and RV-infection. White arrowheads denote GFP+ cells.
(E) Representative light microscopy images of OLFM4 immunohistochemistry staining in control- and RV-infected animals.
(F) Schematic of Bmi1CreERT;R26mTmG mice.
(G) Representative flow cytometry analysis on control- and RV-infected Bmi1CreERT;R26mTmG mice. Quantification of BMI1-mGFP+ cells using flow cytometry analysis on crypt-enriched epithelial preparations.
(H) Representative confocal images of BMI1-mGFP+ mice following control- and RV-infection. White arrows denote BMI1-mGFP+ cells.
Scale bars, 50 μm.
To determine whether infected cells at the tip of the villus are damaged by infection, we examined cell morphology via H&E staining (Figure S1B), which showed increased cell shedding, nuclear mislocalization, and distorted and enlarged nuclei with hypodense hematoxylin staining. Immunohistochemical staining of junctional protein E-cadherin showed its mislocalization from the cell membrane into the cytoplasm in addition to necrotic shedding cells (based on fragmented nuclei) (Figure S1C). To characterize the integrity of the brush border after infection, we evaluated villin, sodium-hydrogen antiporter 3 (NHE3), and ezrin, which are all normally located on the villus brush border (Figures S1D–S1F). On the infected villi, irregular and intensified villin staining was present on the apical cell surface with protein mis-localization into the cytoplasm (Figure S1D). NHE3 and ezrin protein staining was lost in parts of RV-infected villi (Figures S1E and S1F). To decipher the mechanism of cell death following infection, we probed for the anoikis marker phosphorylated myosin light chain (P-MLC) (Bullen et al., 2006) and the apoptosis marker cleaved caspase-3 (Figures S1G and S1H). We found that the number of P-MLC-labeled cells increased following infection but found few cleaved-caspase-3-labeled cells, suggesting that the majority of RV- infected cells undergo anoikis related cell death (Figures S1G and S1H).
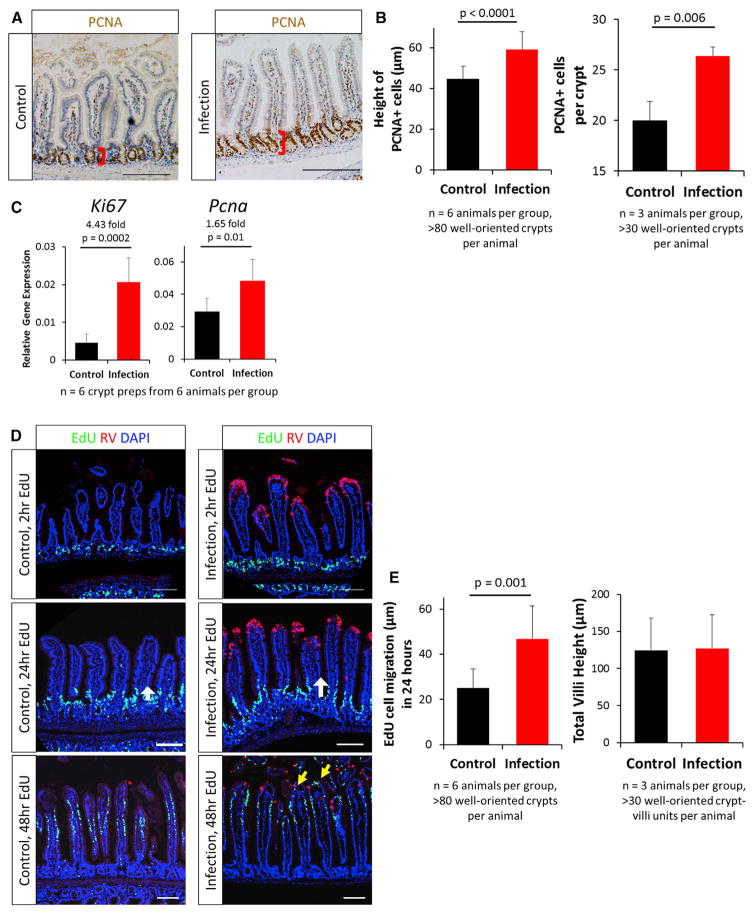
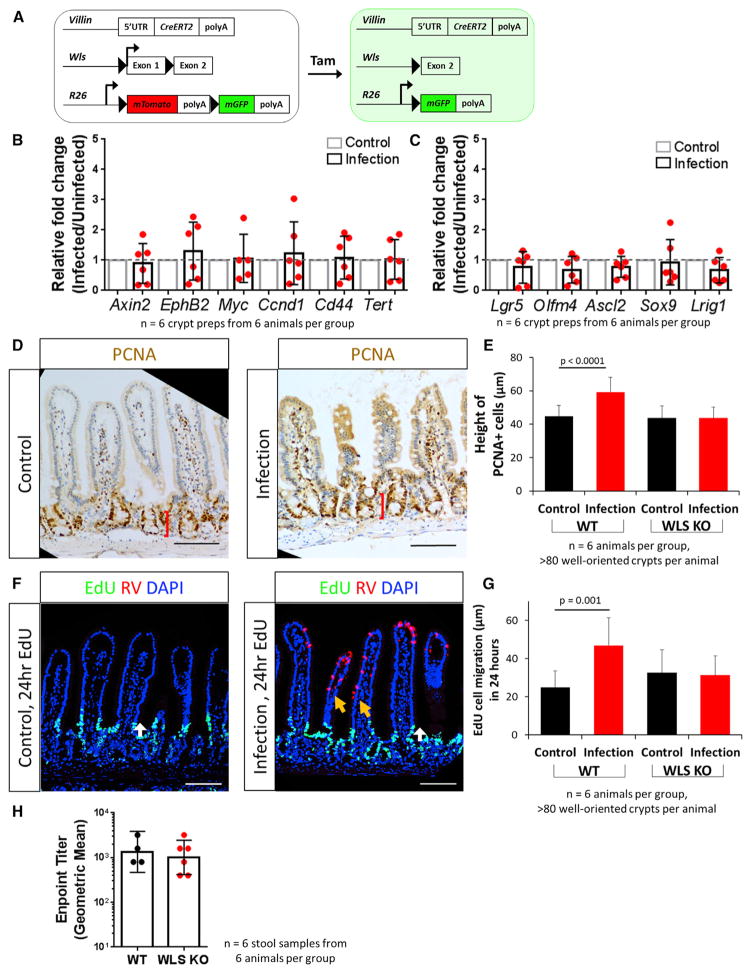
To determine whether viral infection at the tip of the villi produced any response within the crypt region, we first examined proliferation of the crypts following RV infection. Expression of the proliferative marker PCNA indicated that crypts in RV-infected animals remained intact and that the PCNA+ compartment was expanded (Figure 2A). Quantification of this expansion showed a 15-μm (or nearly one-third) increase in the height of PCNA+ cells, with increased PCNA+ cells per crypt following RV-infection (Figure 2B). The proliferative markers Ki67 and Pcna were also transcriptionally upregulated in isolated crypts following infection (Figure 2C). Together, these experiments demonstrate an expanded and more proliferative PCNA+ zone following RV infection.
Figure 2. RV Infection Led to Increased Epithelial Proliferation and Increased Migration.
(A) Representative immunohistochemistry images of PCNA+ proliferating compartments. Red brackets represent the height of the PCNA+ cells measured in (B).
(B) Quantification of height of PCNA+ cells (left) and PCNA+ cells per crypt (right).
(C) qRT-PCR results showing upregulation of the proliferative markers Ki67 and Pcna in isolated epithelial crypts.
(D) Representative confocal images of cell migration in control- and RV-infected animals with 2-, 24-, and 48-hr EdU labeling. EdU+ cells were restricted to the crypts after 2-hr EdU labeling. The length of 24-hr EdU migration was measured from the crypt-villi junction to the farthest EdU-labeled cells (white arrows). After 48 hr of labeling, EdU+ cells were observed near RV-infected cells (yellow arrows).
(E) Quantification of cell migration 24 hr after EdU labeling (left) and total villi height (right). EdU cell migration distance is an absolute measure of distance from the crypt-villus junction (just outside of the transient amplifying [TA] zone) to the cell that had migrated the furthest.
Bars represent means ± SD. All statistical analyses were performed using Student’s t test. Scale bars, 100 μm. See also Figures S3–S6.
Renewal of the intestinal epithelium depends on an upward migration of proliferating and differentiating cells from the crypt to the villus compartment. Previous studies have indicated that RV infection can affect cell migration in neonatal mice (Boshuizen et al., 2003; Preidis et al., 2012). Having documented that RV infection results in increased proliferation within the crypt region, we examined whether this expansion might impact upward migration of the differentiated progeny (Figure 2D). 2 hr after injecting the thymidine analog EdU, EdU+ cells were restricted to the proliferating crypt zone in both control- and RV-infected animals (Figure 2D). 24 hr after EdU labeling, EdU+ cells were observed to migrate out of the crypts and onto the villi (Figure 2D). Cell migration was assessed by measuring the absolute distance from the crypt-villus junction (just outside of the trans-amplifying zone) to the EdU+ cell that had migrated the furthest. We determined the migration distance to be 22 μm, or 87%, faster in the RV-infected group than in the control group in 24 hr (Figures 2D and 2E, left). 48 hr after EdU injection, positive cells were already present at the tip of the villi in RV-infected mice, replacing RV-infected, damaged cells that had been shed lumenally, whereas EdU+ cells were still migrating up the villi in control mice. (Figure 2D). Despite this more rapid cell migration up the villi, the total villus height was not different between RV-and control-infected animals, indicating that faster-migrating cells in RV-infected mice were replacing shed cells rather than extending the villus length (Figure 2E, right). These experiments demonstrate that RV infection stimulates a faster migration of proliferating cells from the crypt to the villi.
Active CBCs, but Not Alternative Reserve ISCs, Respond to RV Infection
In addition to the active CBC population, reserve ISCs have been postulated to exist within the small intestinal crypt (Potten, 1977; Potten and Hendry, 1975; Cheng and Leblond, 1974a; Henning and von Furstenberg, 2016; Mezoff and Shroyer, 2015; Beumer and Clevers, 2016). To determine whether a specific cell type responds to the injury from RV-induced damage, we evaluated the expression of putative ISC markers. Markers associated with CBCs alone (Lgr5, Olfm4, and Ascl2) (Barker et al., 2007; van der Flier et al., 2009a, 2009b) and with both CBCs and reserve ISCs (Sox9 and Lrig1) (Van Landeghem et al., 2012; Powell et al., 2012) were significantly upregulated in crypts isolated from RV-infected mice, whereas markers associated with reserve ISC populations (Bmi1, Hopx, and Dclk1) (Yan et al., 2012; Takeda et al., 2011; Giannakis et al., 2006) showed minimal upregulation following infection (Figure 3A). These data suggest that the predominant response following RV infection occurs in the CBC population.
Given that some CBC markers would also be direct targets of the WNT and other signaling pathways, we tested whether transcriptional upregulation of CBC markers during RV infection indicates an increase in the CBC population. We utilized Lgr5GFPCreERT mice (Barker et al., 2007) in which LGR5-expressing cells are directly tagged with GFP (Figure 3B). A significant increase in the percentage of crypt cells expressing LGR5GFP and LGR5GFP-high was seen following infection by flow cytometry (Figures 3C and S3). Immunofluorescence staining indicated that LGR5-labeled CBCs were increased in number in crypts from RV-infected animals compared to control animals (Figure 3D). While the number of crypts per micrometer of intestine is unchanged (Figure S4C), an increase in LGR5-labeled crypts per micrometer of intestine was also observed when the entire small intestine was examined from infected mice (Figures S4A and S4B). It is possible that the increase in LGR5+ crypts might be an artifact of the mosaic nature of the Lgr5GFPCreERT mice; however, assessment of OLFM4 (van der Flier et al., 2009a), another marker of CBCs, showed increased expression in crypts when comparing RV-infected and control animals (Figure 3E). Together, these data indicate an expansion of crypt-based columnar ISCs in response to RV infection.
To assess whether expansion of reserve ISCs also occurred in small intestinal crypts in response to RV infection, we first examined the reserve ISC marker BMI1. In Bmi1CreERT;R26mTmG mice (Yan et al., 2012), BMI1-labeled and reserve ISCs can be tagged with membrane-GFP following a dose of tamoxifen injection 1 day before harvest (Figure 3F). The BMI1+ cell population remained very stable and comparable in control and RV-infected animals (Figures 3G and 3H). While Bmi1 transcription was significantly upregulated with a small change (1.6-fold), a similar percentage of BMI1 cells in control- and RV-infected animals was observed by flow cytometry and immunofluorescence (Figures 3G and 3H). Lineage tracing also showed no change in BMI1 lineage following RV infection (Figure S5). Together, our data suggest that alternative reserve ISC populations do not respond to RV infection. These results demonstrate that, when intact, the CBC population remains the primary source of epithelial restitution and does not rely on alternative reserve ISC populations.
Epithelial WNT Secretion Is Essential for ISC Induction following RV Infection
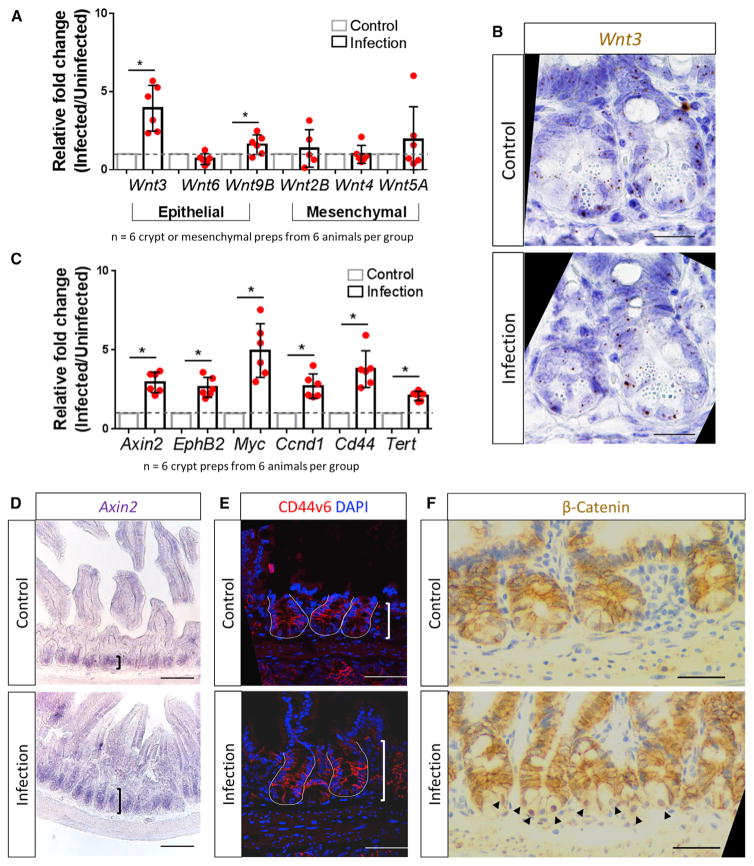
The WNT signaling pathway is known to play a major role in stem cell proliferation and expansion (Fevr et al., 2007; Kuhnert et al., 2004; Pinto et al., 2003; Yan et al., 2017b). To determine whether RV infection induces WNT signaling, we examined the transcriptional profile of WNT ligands in isolated epithelial crypt and mesenchymal compartments of the small intestine (Gregorieff et al., 2005; Farin et al., 2012). Epithelial-expressed Wnt3 and Wnt9B in crypt preparations were significantly upregulated in RV-infected mice compared with control mice (Figure 4A); however, the expression pattern of Wnt3 in Paneth cells and other crypt cell types did not change when comparing control and RV-infected mice (Figure 4B). Wnt2B, Wnt4, and Wnt5A expression in mesenchymal preparations of infected mice were not affected (Figure 4A). Additionally, when we examined the WNT signaling amplifier R-spondin (R-spo) family members, we saw that R-spo1 is upregulated in the mesenchymal preparations of infected mice (Figure S6). The expression of several well-established downstream WNT target genes (Axin2, EphB2, Myc, Ccnd1, Cd44, and Tert) was also upregulated in the crypts isolated from RV-infected animals (Figure 4C). RNA in situ hybridization confirmed WNT signaling upregulation by the expansion of Axin2 expression in the crypts of the infected animals (Figure 4D). Immunofluorescence showed that expression of CD44v6, a pan crypt cell-surface marker that is regulated through the WNT pathway, was expressed on more crypt cells following infection (Figure 4E). Additionally, β-catenin, the intra-cellular transducer of the WNT signaling pathway, translocated from the cytoplasm to the nucleus in the crypts of RV-infected animals (Figure 4F). Collectively, these experiments show that RV-induced epithelial damage results in the increased transcriptional expression of epithelial WNT molecules, and they provide evidence of WNT signaling within the crypts.
Figure 4. WNT Signaling Pathway Is Stimulated in Crypts following RV Infection.
(A) qRT-PCR results showing upregulation of the epithelial-expressed Wnt3 and Wnt9B in isolated epithelial crypt preparations. Wnt2B, Wnt4, and Wnt5A remained stable in mesenchymal preparations.
(B) RNAScope analysis showing Wnt3 expression in Paneth cells and other crypt cell types in both control and RV infection.
(C) qRT-PCR results showing upregulation of well-established WNT signaling pathway target genes in isolated epithelial crypt preparations after RV infection.
(D) Representative RNA in situ hybridization images showed expansion of Axin2 expression in RV infected animals.
(E) Representative immunofluorescence images showing that the WNT-target gene CD44v6 is expressed on more cells in the RV-infected animal.
(F) Representative immunohistochemistry staining of β-catenin, the intracellular transducer of the WNT signaling pathway. RV infection induces cytoplasm to nuclear translocation of β-catenin in the crypts (black arrowheads).
Scale bars represent 50 μm in (B), (E), and (F) and 100 μm in (D). See also Figure S6.
Traditionally, the epithelium and the mesenchyme are thought to be redundant sources of WNT secretion during homeostasis, with the important WNT pathway signals derived from mesenchymal cells (Kabiri et al., 2014; Farin et al., 2012; Valenta et al., 2016; San Roman et al., 2014; de Groot et al., 2013). Whether there are unique functions of epithelial-secreted WNT ligands is currently unknown. The transcriptional upregulation of the epithelial-secreted WNT ligands Wnt3 and Wnt9B (Figure 4A) suggested that epithelial WNTs might play an important role in the activation of LGR5+ stem cells within the crypt following villus damage. To assess whether secretion of epithelial WNT was related to crypt expansion following infection, VillinCreERT;WLSf/f;R26mTmG (WLS KO) mice, in which WNT secretion in the epithelium is conditionally impaired, were infected with RV (Figures 5A and S7A) (Bänziger et al., 2006; Carpenter et al., 2010). Although RV infection induced upregulation of Wnt5A in mesenchymal preparations from WLS KO mice (Figure S7B), WNT pathway target genes were no longer upregulated in small intestinal crypts following infection (Figure 5B) compared to the upregulation seen in wild-type (WT) infected mice (Figure 4C). Further, CBC markers, including Lgr5 and Olfm4, were not induced when epithelial WNT secretion was impaired (Figure 5C). The crypts were not expanded (Figures 5D and 5E), nor was there faster migration induced by infection, suggesting that epithelial WNT secretion plays an important role in these processes (Figures 5F and 5G). Additionally, while no overt phenotype was observed in the RV-infected WLS KO mice and RV shedding remained similar in the WT and WLS conditional KO model (Figure 5H), RV-infected cells in the WLS KO model were no longer localized to the tips of the villi (Figures 5F and S7A) compared to infected WT mice (Figures 1B and S1A). These findings indicate that epithelial-secreted WNT ligands are important for the regenerative crypt response following epithelial damage, and they may help restrict RV infection to the tip of the villi.
Figure 5. Epithelial-Secreted WNT Ligands Are Essential for RV-Induced Stem Cell Regeneration.
(A) Schematic of VillinCreERT;WLSf/f;R26mTmG (WLS KO) mouse. Injections of tamoxifen allows for the conditional knockout of the Wntless gene in villin-expressing cells, impairing WNT secretion specifically in the epithelium.
(B) qRT-PCR results showed expression of WNT pathway target genes remain stable in isolated epithelial crypts following RV infection in WLS KO mice.
(C) qRT-PCR results showed expression of putative CBC markers remain stable in isolated epithelial crypts following RV infection in WLS KO mice.
(D) Representative immunohistochemistry staining of PCNA in control- and RV-infected WLS KO animals.
(E) Quantification of height of PCNA+ cell measurement in WT and WLS KO mice. Bars represent means ± SD.
(F) Representative image of EdU-labeled cell migration in control- and RV-infected animals in WLS KO mice. There was no induction of EdU+ cell migration (white arrows). RV-infected cells were no longer restricted to the tip of the villi (orange arrows).
(G) Quantification of cell migration with 24 hr of EdU labeling in WT and WLS KO mice (white arrows in E). Bars represent means ± SD.
(H) Endpoint titer from stool ELISA of WT and WLS KO animals. Bars represent geometric mean ± 95% CI.
Scale bars, 100 μm. See also Figure S7.
DISCUSSION
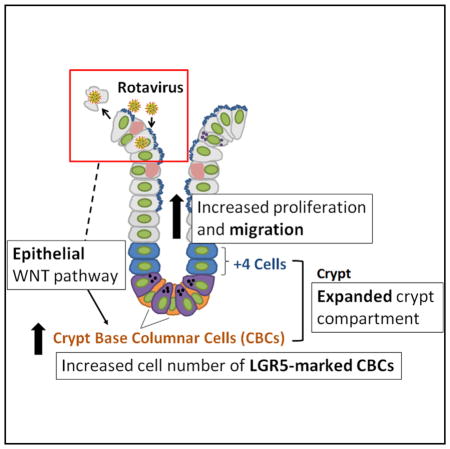
Understanding activation of stem cells within the niche environment is important for dissecting pathways that regulate epithelial repair. Intestinal regeneration after injury has been studied extensively using several models. For example, radiation-induced damage targets and kills LGR5+ cycling CBCs. In this situation, the epithelium regenerates due to the plasticity of the reserve ISCs labeled by BMI1, HOPX, LRIG1, SOX9, and others, which repopulate the LGR5+ cycling population within the base of the crypt (Hua et al., 2012; May et al., 2008; Metcalfe et al., 2014; Potten et al., 2009; Powell et al., 2012; Takeda et al., 2011; Yan et al., 2012; Yu, 2013). Chemotherapy treatments, such as doxorubicin, elicit injury patterns similar to radiation treatment, with loss of LGR5+ populations within the crypt. Activation and increased proliferation of surface glycoprotein CD24-labeled ISCs following injury can reconstitute the damaged epithelium (Dekaney et al., 2009; Seiler et al., 2015). Genetic mouse models resulting in the direct ablation of LGR5-labeled CBCs have also shown the importance of the reserve ISCs as well as the newly characterized secretory and absorptive progenitors after LGR5+ cell loss (Tetteh et al., 2016; van Es et al., 2012; Buczacki et al., 2013). Together, these studies suggest a plastic ISC environment in which many different stem cell types and progenitor cells can fulfill the demand of injury repair during CBC damage and loss. In the present work, we extend these studies by exploring how the crypt stem cell populations respond to villus-specific injury. RV infection has been linked to increased proliferation in the epithelium (Boshuizen et al., 2003; Preidis et al., 2012). Our data demonstrate the LGR5+ CBC population within the crypt environment is the one that expands during RV infection. In contrast to radiation, chemotherapy, and direct ablation, BMI1-marked cells do not respond to villus-specific damage (Figure 3). Because the crypt compartment is highly plastic, it is possible that BMI1-marked cells may have reverted back to the CBC state following RV infection, resulting in LGR5+ cell expansion. However, this is unlikely, as we did not observe BMI1+ cells at the CBC position (Figure 3), nor did we observe increased lineage tracing of BMI1+ daughter cells following RV infection (Figure S5). Recent work suggests that BMI1+ cells bear signatures of enteroendocrine cells, a differentiated, secretory cell type in the small intestine, making BMI1+ cells a possible early progenitor committed to the enteroendocrine lineage (Jadhav et al., 2017; Yan et al., 2017a). Together, these results argue that RV-induced villus damage also does not affect the enteroendocrine progenitor population. These data build a more complex model of regeneration initiated in the stem cell niche in which LGR5+ CBCs are important for homeostatic maintenance of the intestinal epithelium and are preferred to respond to villus epithelial damage by increasing proliferation and migration, while reserve ISCs and other progenitor cell types are only recruited during CBC loss and play an important role in regeneration of the crypt itself. Importantly, these data indicate there is a hierarchical control of the niche and the stem cells that comprise it.
WNT signaling is an important component of the ISC niche. Inhibition of global WNT secretion reduces proliferation and impairs epithelial homeostasis (Fevr et al., 2007; Kuhnert et al., 2004; Pinto et al., 2003; Valenta et al., 2016). While both the epithelium and mesenchyme secrete WNT molecules, epithelial-secreted WNT ligands are thought to be redundant and nonessential (Farin et al., 2012; Kabiri et al., 2014; San Roman et al., 2014; Valenta et al., 2016). When WNT3 and WNT secretion pathway genes (e.g., Porcupine and Wntless) were ablated in the intestinal epithelium, no apparent phenotype was observed (Farin et al., 2012). KOs of Atoh1 and other genes specific to the Paneth cell, a known source of epithelial WNT secretion, also give no aberrant loss in WNT signaling activation (Durand et al., 2012; Kim et al., 2012). These findings have led to the hypothesis that mesenchymal WNT secretion is the primary mechanism for regulating the proliferative response in the stem cell niche. This idea is supported by several recent studies that have identified key mesenchymal cell types, marked by transcription factor Foxl1 and surface antigen CD34, which can potentiate WNT signaling (Aoki et al., 2016; Stzepourginski et al., 2017). By contrast, our results show that epithelial-secreted WNT ligands are essential for the expansion of LGR5+ cells and that proliferation and migration occur following virus-induced villus damage (Figures 3 and 4). Thus, although epithelial WNTs are thought to be nonessential for maintaining homeostasis, they are an important component of epithelial repair. It is important to note that our findings do not exclude the involvement of the mesenchyme. The upregulation of the WNT signaling amplifier R-spo1 in the mesenchyme following infection raises the interesting possibility that epithelial WNT may be one arm of a broad inter-compartmental response to RV infection in which the induction of R-spo family proteins in the mesenchyme requires the priming by epithelial WNT to carry out injury repair after RV infection. This is in line with the recent publication on the nonequivalent yet cooperative role of R-spo and WNT on stem cell self-renewal (Yan et al., 2017b). Further, while RV-infected cells are restricted to the tip of the villi in WT mice, epithelial Wntless deletion resulted in an aberrant infection pattern in which infected cells can be observed midlength on the villi (Figure 5F). Since RV infects the terminally differentiated enterocytes that are present at the tips of the villi (Figures 1 and S1A) (Ward et al., 1990; Burns et al., 1995; O’Neal et al., 1997; Blutt et al., 2012), one possibility may be that the differentiation status of the enterocytes has been altered in the absence of epithelial-secreted WNT. Although previous studies did not find any significant differences in the differentiation pattern of epithelial-WNT impaired mice compared to WT mice (Kabiri et al., 2014; San Roman et al., 2014), it is possible that subtle cellular changes that are relevant to RV susceptibility and pathogenesis have occurred that were not detected in the initial comparison studies. Another explanation may be that epithelial WNT ligands, through their proliferative effects, affect the rate of cell migration and epithelial repair (Figures 2 and 5). Therefore, in the absence of epithelial WNT ligands, mature cells simply do not migrate as fast to the tip of the villi; thus, RV-infected cells are found in nontraditional locations. Future studies are needed to determine why RV-infected Wntless KO mice exhibit an altered infection pattern.
One of the key aspects to determining the mechanisms through which villus damage results in WNT secretion will be identifying which cell type is producing WNT following RV infection. A likely candidate is the Paneth cell, which has already been shown to secrete WNT (Sato et al., 2009, 2011). Our experiments, however, did not show changes in this cell type (Figure S2). Hence, there may exist an additional cell type with WNT-secreting ability that produces WNT only in the context of epithelial damage. Apoptotic cells during hydra head injury have been shown to be an unexpected source of WNT3 and are able to drive regeneration (Chera et al., 2009; Lengfeld et al., 2009). Similar mechanisms may exist in RV infection, where infected, apoptotic cells directly secrete WNT ligands, leading to increased stem cell proliferation within the crypt. Alternatively, other nonapoptotic damage signals produced by infected villus cells may indirectly stimulate WNT secretion from other cell types (e.g., Paneth cells), leading to stem cell activation. Such feedback mechanisms from differentiated cells to stem cells are not well understood in mammalian systems, but several studies in the Drosophila gut have shown the importance of the JAK-STAT pathway (Beebe et al., 2010; Buchon et al., 2009; Jiang et al., 2016; Lin et al., 2010). Stress, injury, and bacterial infection can lead to cytokine production in damaged cells, stimulating the JAK-STAT pathway in WNT-producing cell types, which manifests into ultimate ISC proliferation (Jiang et al., 2016; Xu et al., 2011). Our previous study observed a robust upregulation of the interferon pathways in the intestinal epithelium following RV infection in the human intestinal enteroids (Saxena et al., 2017). Interferon production, with the subsequent activation of the JAK-STAT pathway, may serve as a bridge from infected cells to epithelial WNT production and CBC expansion (Saxena et al., 2017; Nava et al., 2010; Arnold et al., 2013). Future studies will focus on elucidating the cell type and mechanisms that elicit WNT signaling in response to RV infection.
Finally, while our data point to a necessary role in epithelial-secreted WNT ligands, we were not able to study whether epithelial-secreted WNT ligands are sufficient for the proliferative responses seen following RV infection. Future studies using the intestinal enteroid cultures that do not contain the mesenchyme will address the sufficiency of epithelial-signals in stem cell induction following infection. In addition, these cultures may facilitate the identification of additional epithelial sources of WNT ligands that are induced following infection. Alternatively, the mesenchyme may still potentiate regenerative responses in villus damage. Such close communication between epithelial and mesenchymal WNT signaling pathways has been shown in both the Drosophila midgut as well as mammalian small intestine (Kux and Pitsouli, 2014; Le Guen et al., 2015; Roulis and Flavell, 2016; Karlsson et al., 2000). Well-established feedback loops from Hedgehog and WNT signal pathways could serve as a potential link between the mesenchyme and the epithelium after RV infection (Büller et al., 2012; Kosinski et al., 2010). Several cell types recently identified to secrete R-spo and other growth factors in the mesenchyme could also serve as important catalysts for the WNT signaling pathway following epithelial injury (Stzepourginski et al., 2017; Aoki et al., 2016).
In summary, this study used RV infection as a model to study the injury-repair response from an intact ISC niche. We showed that LGR5+ CBCs, when present, remain the primary source of epithelial restitution, and no other reserve cell types were needed for the response. In addition, epithelial-secreted WNT ligands are nonredundant, essential components of injury repair. These discoveries provide a framework for how cell types communicate in the intestinal tract and lay a foundation for developing regenerative treatments for intestinal injury.
EXPERIMENTAL PROCEDURES
Genetic Mouse Lines and Viral Infection
Lgr5GFPCreERT, R26mTmG, Bmi1CreERT, and VillinCreERT mice were obtained from The Jackson Laboratory (Bar Harbor, ME). WLSf/f mice were a generous gift from Dr. Jim Wells (Cincinnati Children’s Hospital) (Carpenter et al., 2010). Mice aged 8–16 weeks (both males and females) were randomly assigned to control- or RV-infected groups with at least three mice per group per experiment. RV strain ECWT (P[17], G3) or PBS-control gut homogenate from previously infected mice was administered by oral gavage (O’Neal et al., 1997). All animals were housed in a physically separated BSL-2 animal facility. A 1 × 105 50% infectious dose (ID50) was used to obtain adequate infection (O’Neal et al., 1997). Fecal samples from each mouse were collected daily. Fecal ELISA was used to monitor RV infection as described previously (O’Neal et al., 1997). Mice were sacrificed 4 days following infection at the peak of viral shedding. To induce lineage tracing through CreERT, tamoxifen in 10% EtOH/corn oil was intraperitoneally injected. To label BMI1+ cells and BMI1 lineage, 1 dose of 1 mg tamoxifen was injected in Bmi1CreERT;R26mTmG mice 1 or 7 days before harvest, respectively. To conditionally knock out Wntless, 3 daily doses of 1 mg tamoxifen were injected in VillinCreERT;WLSf/f;R26mTmG mice 3 days before infection. To determine proliferation in the intestinal epithelium, 1 mg EdU in 10% DSMO/PBS was intraperitoneally injected at 2, 24, or 48 hr before harvest. All protocols were approved by the Baylor College of Medicine Institutional Animal Care and Use Committee (IACUC).
Crypt Purification and Mesenchymal Isolation
Intestinal crypts were prepared using previously described protocols, with modifications (Mahe et al., 2013; Gracz et al., 2012). Briefly, the entire small intestine was dissected out and flushed with ice-cold Ca2+/Mg2+-free Dulbecco’s PBS (DPBS). Intestines were opened lengthwise and cut into 1-cm pieces. Tissues were incubated with chelating buffer (2 mM EDTA in DPBS) on ice for 30 min by gentle shaking. Chelating buffer was then replaced with shaking buffer (1% [43.3 mM] sucrose, 1% [54.9 mM] sorbitol in DPBS) and manually shaken for ~1–2 min to disassociate crypts. Intestinal crypts were filtered through a 70-μm cell strainer (BD Falcon) and then spun down at 150 × g, 4°C for 5 min. Remaining tissue after epithelial purification was harvested as mesenchyme.
Statistical Analysis
Data are presented as means ± SD. The n in all experiments refers to biological replicates (different animals) in each group. n for each experiment is listed in each figure. Statistical comparisons between two groups were analyzed using the Student’s t test. Significance was taken as p < 0.05. All analyses were performed using GraphPad Prism version 5.01 (GraphPad Software, La Jolla, CA).
DATA AND SOFTWARE AVAILABILITY
The accession numbers for the flow cytometry data reported in this paper are Flow Repository: FR-FCM-ZYF8 and FR-FCM-ZYF9.
Supplementary Material
Highlights.
Rotavirus infection is an excellent model for investigating intestinal villus injury
Villus damage stimulates the active crypt-based columnar intestinal stem cells
Reserve intestinal stem cells play a minimal role in repair of villus injury
Regeneration following villus injury depends on epithelial-secreted WNT
Acknowledgments
We thank Susan Henning for insightful consultation throughout the project and the members of the Estes, Donowitz, and Shroyer labs for critical input on experiments and data analysis. We thank Joel Sederstrom, Dr. Fabio Stossi, Dr. Milton Finegold, and Dr. Patricia Castro for their expert assistance. This work was performed as part of the Intestinal Stem Cell Consortium with funding from the NIH through grants U01 DK103168, U01 DK103168-03S1, and U01 DK103168-02S1 to M.K.E. and grant F30 DK107173 to W.Y.Z. This project was also supported by the Cellular and Molecular Morphology Core of the Texas Medical Center Digestive Disease Center (funded by NIH grant P30DK056338) and the Advanced Technology Core Laboratories at Baylor College of Medicine, including the Cytometry and Cell Sorting Core (funded by NIH NIAID grant P30AI036211, NCI grant P30CA125123, and NCRR grant S10RR024574), the Pathology and Histology Core (funded by NCI grant P30CA125123), and the Integrated Microscopy Core (funded by NIH grants DK56338 and CA125123, CPRIT grant RP150578, the Dan L. Duncan Comprehensive Cancer Center [P30CA125123], and the John S. Dunn Gulf Coast Consortium for Chemical Genomics [CPRIT RP150578]).
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, seven figures, and two tables and can be found with this article online at https://doi.org/10.1016/j.celrep.2017.12.093.
AUTHOR CONTRIBUTIONS
S.E.B. and M.K.E. conceptualized the study. M.K.E., S.E.B., and N.F.S. supervised all studies performed. W.Y.Z., M.-S.C., X.-L.Z., and D.C.-A. designed and performed experiments. W.Y.Z., Y.-H.L, S.E.B., N.F.S., and O.D.K. critically analyzed experimental data. W.Y.Z. wrote the manuscript. S.E.B., N.F.S., M.K.E., and M.D. provided critical input in manuscript revision. All authors contributed to the writing or editing of the final manuscript.
DECLARATION OF INTERESTS
The authors declare no competing interests.
References
- Aoki R, Shoshkes-Carmel M, Gao N, Shin S, May CL, Golson ML, Zahm AM, Ray M, Wiser CL, Wright CV, Kaestner KH. Foxl1-expressing mesenchymal cells constitute the intestinal stem cell niche. Cell Mol Gastroenterol Hepatol. 2016;2:175–188. doi: 10.1016/j.jcmgh.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold MM, Sen A, Greenberg HB, Patton JT. The battle between rotavirus and its host for control of the interferon signaling pathway. PLoS Pathog. 2013;9:e1003064. doi: 10.1371/journal.ppat.1003064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bänziger C, Soldini D, Schütt C, Zipperlen P, Hausmann G, Basler K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125:509–522. doi: 10.1016/j.cell.2006.02.049. [DOI] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Beau I, Berger A, Servin AL. Rotavirus impairs the biosynthesis of brush-border-associated dipeptidyl peptidase IV in human enterocyte-like Caco-2/TC7 cells. Cell Microbiol. 2007;9:779–789. doi: 10.1111/j.1462-5822.2006.00827.x. [DOI] [PubMed] [Google Scholar]
- Beebe K, Lee WC, Micchelli CA. JAK/STAT signaling coordinates stem cell proliferation and multilineage differentiation in the Drosophila intestinal stem cell lineage. Dev Biol. 2010;338:28–37. doi: 10.1016/j.ydbio.2009.10.045. [DOI] [PubMed] [Google Scholar]
- Beumer J, Clevers H. Regulation and plasticity of intestinal stem cells during homeostasis and regeneration. Development. 2016;143:3639–3649. doi: 10.1242/dev.133132. [DOI] [PubMed] [Google Scholar]
- Blutt SE, Miller AD, Salmon SL, Metzger DW, Conner ME. IgA is important for clearance and critical for protection from rotavirus infection. Mucosal Immunol. 2012;5:712–719. doi: 10.1038/mi.2012.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boshuizen JA, Reimerink JH, Korteland-van Male AM, van Ham VJ, Koopmans MP, Büller HA, Dekker J, Einerhand AW. Changes in small intestinal homeostasis, morphology, and gene expression during rotavirus infection of infant mice. J Virol. 2003;77:13005–13016. doi: 10.1128/JVI.77.24.13005-13016.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N, Broderick NA, Poidevin M, Pradervand S, Lemaitre B. Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe. 2009;5:200–211. doi: 10.1016/j.chom.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Buczacki SJ, Zecchini HI, Nicholson AM, Russell R, Vermeulen L, Kemp R, Winton DJ. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature. 2013;495:65–69. doi: 10.1038/nature11965. [DOI] [PubMed] [Google Scholar]
- Büllen TF, Forrest S, Campbell F, Dodson AR, Hershman MJ, Pritchard DM, Turner JR, Montrose MH, Watson AJ. Characterization of epithelial cell shedding from human small intestine. Lab Invest. 2006;86:1052–1063. doi: 10.1038/labinvest.3700464. [DOI] [PubMed] [Google Scholar]
- Buller NV, Rosekrans SL, Westerlund J, van den Brink GR. Hedgehog signaling and maintenance of homeostasis in the intestinal epithelium. Physiology (Bethesda) 2012;27:148–155. doi: 10.1152/physiol.00003.2012. [DOI] [PubMed] [Google Scholar]
- Burns JW, Krishnaney AA, Vo PT, Rouse RV, Anderson LJ, Greenberg HB. Analyses of homologous rotavirus infection in the mouse model. Virology. 1995;207:143–153. doi: 10.1006/viro.1995.1060. [DOI] [PubMed] [Google Scholar]
- Carpenter AC, Rao S, Wells JM, Campbell K, Lang RA. Generation of mice with a conditional null allele for Wntless. Genesis. 2010;48:554–558. doi: 10.1002/dvg.20651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. I. Columnar cell. Am J Anat. 1974a;141:461–479. doi: 10.1002/aja.1001410403. [DOI] [PubMed] [Google Scholar]
- Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am J Anat. 1974b;141:537–561. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- Chera S, Ghila L, Dobretz K, Wenger Y, Bauer C, Buzgariu W, Martinou JC, Galliot B. Apoptotic cells provide an unexpected source of Wnt3 signaling to drive hydra head regeneration. Dev Cell. 2009;17:279–289. doi: 10.1016/j.devcel.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Clevers H, Loh KM, Nusse R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014;346:1248012. doi: 10.1126/science.1248012. [DOI] [PubMed] [Google Scholar]
- de Groot RE, Farin HF, Macůurková M, van Es JH, Clevers HC, Korswagen HC. Retromer dependent recycling of the Wnt secretion factor Wls is dispensable for stem cell maintenance in the mammalian intestinal epithelium. PLoS ONE. 2013;8:e76971. doi: 10.1371/journal.pone.0076971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekaney CM, Gulati AS, Garrison AP, Helmrath MA, Henning SJ. Regeneration of intestinal stem/progenitor cells following doxorubicin treatment of mice. Am J Physiol Gastrointest Liver Physiol. 2009;297:G461–G470. doi: 10.1152/ajpgi.90446.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand A, Donahue B, Peignon G, Letourneur F, Cagnard N, Slomianny C, Perret C, Shroyer NF, Romagnolo B. Functional intestinal stem cells after Paneth cell ablation induced by the loss of transcription factor Math1 (Atoh1) Proc Natl Acad Sci USA. 2012;109:8965–8970. doi: 10.1073/pnas.1201652109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farin HF, Van Es JH, Clevers H. Redundant sources of Wnt regulate intestinal stem cells and promote formation of Paneth cells. Gastroenterology. 2012;143:1518–1529. e7. doi: 10.1053/j.gastro.2012.08.031. [DOI] [PubMed] [Google Scholar]
- Fevr T, Robine S, Louvard D, Huelsken J. Wnt/beta-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol Cell Biol. 2007;27:7551–7559. doi: 10.1128/MCB.01034-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakis M, Stappenbeck TS, Mills JC, Leip DG, Lovett M, Clifton SW, Ippolito JE, Glasscock JI, Arumugam M, Brent MR, Gordon JI. Molecular properties of adult mouse gastric and intestinal epithelial progenitors in their niches. J Biol Chem. 2006;281:11292–11300. doi: 10.1074/jbc.M512118200. [DOI] [PubMed] [Google Scholar]
- Gracz A, Puthoff B, Magness ST. Identification, isolation, and culture of intestinal epithelial stem cells from murine intestine. Methods Mol Biol. 2012;879:89–107. doi: 10.1007/978-1-61779-815-3_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg HB, Estes MK. Rotaviruses: from pathogenesis to vaccination. Gastroenterology. 2009;136:1939–1951. doi: 10.1053/j.gastro.2009.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorieff A, Pinto D, Begthel H, Destrée O, Kielman M, Clevers H. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology. 2005;129:626–638. doi: 10.1016/j.gastro.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Henning SJ, von Furstenberg RJ. GI stem cells - new insights into roles in physiology and pathophysiology. J Physiol. 2016;594:4769–4779. doi: 10.1113/JP271663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua G, Thin TH, Feldman R, Haimovitz-Friedman A, Clevers H, Fuks Z, Kolesnick R. Crypt base columnar stem cells in small intestines of mice are radioresistant. Gastroenterology. 2012;143:1266–1276. doi: 10.1053/j.gastro.2012.07.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav U, Saxena M, O’Neill NK, Saadatpour A, Yuan GC, Herbert Z, Murata K, Shivdasani RA. Dynamic reorganization of chromatin accessibility signatures during dedifferentiation of secretory precursors into Lgr5+ intestinal stem cells. Cell Stem Cell. 2017;21:65–77. e5. doi: 10.1016/j.stem.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Tian A, Jiang J. Intestinal stem cell response to injury: lessons from Drosophila. Cell Mol Life Sci. 2016;73:3337–3349. doi: 10.1007/s00018-016-2235-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdan N, Brunet JP, Sapin C, Blais A, Cotte-Laffitte J, Forestier F, Quero AM, Trugnan G, Servin AL. Rotavirus infection reduces sucrase-isomaltase expression in human intestinal epithelial cells by perturbing protein targeting and organization of microvillar cytoskeleton. J Virol. 1998;72:7228–7236. doi: 10.1128/jvi.72.9.7228-7236.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabiri Z, Greicius G, Madan B, Biechele S, Zhong Z, Zaribafzadeh H, Edison Aliyev J, Wu Y, Bunte R, et al. Stroma provides an intestinal stem cell niche in the absence of epithelial Wnts. Development. 2014;141:2206–2215. doi: 10.1242/dev.104976. [DOI] [PubMed] [Google Scholar]
- Karlsson L, Lindahl P, Heath JK, Betsholtz C. Abnormal gastrointestinal development in PDGF-A and PDGFR-(alpha) deficient mice implicates a novel mesenchymal structure with putative instructive properties in villus morphogenesis. Development. 2000;127:3457–3466. doi: 10.1242/dev.127.16.3457. [DOI] [PubMed] [Google Scholar]
- Kim TH, Escudero S, Shivdasani RA. Intact function of Lgr5 receptor-expressing intestinal stem cells in the absence of Paneth cells. Proc Natl Acad Sci USA. 2012;109:3932–3937. doi: 10.1073/pnas.1113890109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosinski C, Stange DE, Xu C, Chan AS, Ho C, Yuen ST, Mifflin RC, Powell DW, Clevers H, Leung SY, Chen X. Indian hedgehog regulates intestinal stem cell fate through epithelial-mesenchymal interactions during development. Gastroenterology. 2010;139:893–903. doi: 10.1053/j.gastro.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühl SJ, Kühl M. On the role of Wnt/β-catenin signaling in stem cells. Biochim Biophys Acta. 2013;1830:2297–2306. doi: 10.1016/j.bbagen.2012.08.010. [DOI] [PubMed] [Google Scholar]
- Kuhnert F, Davis CR, Wang HT, Chu P, Lee M, Yuan J, Nusse R, Kuo CJ. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dick-kopf-1. Proc Natl Acad Sci USA. 2004;101:266–271. doi: 10.1073/pnas.2536800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kux K, Pitsouli C. Tissue communication in regenerative inflammatory signaling: lessons from the fly gut. Front Cell Infect Microbiol. 2014;4:49. doi: 10.3389/fcimb.2014.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Guen L, Marchal S, Faure S, de Santa Barbara P. Mesenchymal-epithelial interactions during digestive tract development and epithelial stem cell regeneration. Cell Mol Life Sci. 2015;72:3883–3896. doi: 10.1007/s00018-015-1975-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengfeld T, Watanabe H, Simakov O, Lindgens D, Gee L, Law L, Schmidt HA, Ozbek S, Bode H, Holstein TW. Multiple Wnts are involved in Hydra organizer formation and regeneration. Dev Biol. 2009;330:186–199. doi: 10.1016/j.ydbio.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Lin G, Xu N, Xi R. Paracrine unpaired signaling through the JAK/STAT pathway controls self-renewal and lineage differentiation of Drosophila intestinal stem cells. J Mol Cell Biol. 2010;2:37–49. doi: 10.1093/jmcb/mjp028. [DOI] [PubMed] [Google Scholar]
- Mahe MM, Aihara E, Schumacher MA, Zavros Y, Montrose MH, Helmrath MA, Sato T, Shroyer NF. Establishment of gastrointestinal epithelial organoids. Curr Protoc Mouse Biol. 2013;3:217–240. doi: 10.1002/9780470942390.mo130179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May R, Riehl TE, Hunt C, Sureban SM, Anant S, Houchen CW. Identification of a novel putative gastrointestinal stem cell and adenoma stem cell marker, doublecortin and CaM kinase-like-1, following radiation injury and in adenomatous polyposis coli/multiple intestinal neoplasia mice. Stem Cells. 2008;26:630–637. doi: 10.1634/stemcells.2007-0621. [DOI] [PubMed] [Google Scholar]
- Metcalfe C, Kljavin NM, Ybarra R, de Sauvage FJ. Lgr5+ stem cells are indispensable for radiation-induced intestinal regeneration. Cell Stem Cell. 2014;14:149–159. doi: 10.1016/j.stem.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Mezoff E, Shroyer N. Anatomy and physiology of the small and large intestines. In: Wyllie R, Hyams JS, Kay M, editors. Pediatric Gastrointestinal and Liver Disease. 5. Elsevier; 2015. pp. 345–359. [Google Scholar]
- Nava P, Koch S, Laukoetter MG, Lee WY, Kolegraff K, Capaldo CT, Beeman N, Addis C, Gerner-Smidt K, Neumaier I, et al. Interferon-γ regulates intestinal epithelial homeostasis through converging β-catenin signaling pathways. Immunity. 2010;32:392–402. doi: 10.1016/j.immuni.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R, Varmus H. Three decades of Wnts: a personal perspective on how a scientific field developed. EMBO J. 2012;31:2670–2684. doi: 10.1038/emboj.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neal CM, Crawford SE, Estes MK, Conner ME. Rotavirus virus-like particles administered mucosally induce protective immunity. J Virol. 1997;71:8707–8717. doi: 10.1128/jvi.71.11.8707-8717.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto D, Gregorieff A, Begthel H, Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17:1709–1713. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten CS. Extreme sensitivity of some intestinal crypt cells to X and gamma irradiation. Nature. 1977;269:518–521. doi: 10.1038/269518a0. [DOI] [PubMed] [Google Scholar]
- Potten CS. Epithelial cell growth and differentiation. II. Intestinal apoptosis. Am J Physiol. 1997;273:G253–G257. doi: 10.1152/ajpgi.1997.273.2.G253. [DOI] [PubMed] [Google Scholar]
- Potten CS, Hendry JH. Differential regeneration of intestinal proliferative cells and cryptogenic cells after irradiation. Int J Radiat Biol Relat Stud Phys Chem Med. 1975;27:413–424. doi: 10.1080/09553007514550411. [DOI] [PubMed] [Google Scholar]
- Potten CS, Gandara R, Mahida YR, Loeffler M, Wright NA. The stem cells of small intestinal crypts: where are they? Cell Prolif. 2009;42:731–750. doi: 10.1111/j.1365-2184.2009.00642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin EJ, Powell AE, Wang Y, Li Y, Franklin JL, Coffey RJ. Using a new Lrig1 reporter mouse to assess differences between two Lrig1 antibodies in the intestine. Stem Cell Res (Amst) 2014;13(3 Pt A):422–430. doi: 10.1016/j.scr.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell AE, Wang Y, Li Y, Poulin EJ, Means AL, Washington MK, Higginbotham JN, Juchheim A, Prasad N, Levy SE, et al. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell. 2012;149:146–158. doi: 10.1016/j.cell.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preidis GA, Saulnier DM, Blutt SE, Mistretta TA, Riehle KP, Major AM, Venable SF, Barrish JP, Finegold MJ, Petrosino JF, et al. Host response to probiotics determined by nutritional status of rota-virus-infected neonatal mice. J Pediatr Gastroenterol Nutr. 2012;55:299–307. doi: 10.1097/MPG.0b013e31824d2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramig RF. Pathogenesis of intestinal and systemic rotavirus infection. J Virol. 2004;78:10213–10220. doi: 10.1128/JVI.78.19.10213-10220.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche KC, Gracz AD, Liu XF, Newton V, Akiyama H, Magness ST. SOX9 maintains reserve stem cells and preserves radioresistance in mouse small intestine. Gastroenterology. 2015;149:1553–1563. e10. doi: 10.1053/j.gastro.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roulis M, Flavell RA. Fibroblasts and myofibroblasts of the intestinal lamina propria in physiology and disease. Differentiation. 2016;92:116–131. doi: 10.1016/j.diff.2016.05.002. [DOI] [PubMed] [Google Scholar]
- San Roman AK, Jayewickreme CD, Murtaugh LC, Shivdasani RA. Wnt secretion from epithelial cells and subepithelial myofibroblasts is not required in the mouse intestinal stem cell niche in vivo. Stem Cell Reports. 2014;2:127–134. doi: 10.1016/j.stemcr.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena K, Simon LM, Zeng XL, Blutt SE, Crawford SE, Sastri NP, Karandikar UC, Ajami NJ, Zachos NC, Kovbasnjuk O, et al. A paradox of transcriptional and functional innate interferon responses of human intestinal enteroids to enteric virus infection. Proc Natl Acad Sci USA. 2017;114:E570–E579. doi: 10.1073/pnas.1615422114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler KM, Schenhals EL, von Furstenberg RJ, Allena BK, Smith BJ, Scaria D, Bresler MN, Dekaney CM, Henning SJ. Tissue underlying the intestinal epithelium elicits proliferation of intestinal stem cells following cytotoxic damage. Cell Tissue Res. 2015;361:427–438. doi: 10.1007/s00441-015-2111-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shroyer NF, Wallis D, Venken KJ, Bellen HJ, Zoghbi HY. Gfi1 functions downstream of Math1 to control intestinal secretory cell subtype allocation and differentiation. Genes Dev. 2005;19:2412–2417. doi: 10.1101/gad.1353905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shroyer NF, Helmrath MA, Wang VYC, Antalffy B, Henning SJ, Zoghbi HY. Intestine-specific ablation of mouse atonal homolog 1 (Math1) reveals a role in cellular homeostasis. Gastroenterology. 2007;132:2478–2488. doi: 10.1053/j.gastro.2007.03.047. [DOI] [PubMed] [Google Scholar]
- Shroyer NF, Bell K, Lo Y-H. Biology of intestinal epithelial stem cells. In: Yang VW, Bialkowska AB, editors. Intestinal Tumorigenesis: Mechanisms of Development & Progression. Springer International Publishing; 2015. pp. 55–99. [Google Scholar]
- Stzepourginski I, Nigro G, Jacob JM, Dulauroy S, Sansonetti PJ, Eberl G, Peduto L. CD34+ mesenchymal cells are a major component of the intestinal stem cells niche at homeostasis and after injury. Proc Natl Acad Sci USA. 2017;114:E506–E513. doi: 10.1073/pnas.1620059114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda N, Jain R, LeBoeuf MR, Wang Q, Lu MM, Epstein JA. Interconversion between intestinal stem cell populations in distinct niches. Science. 2011;334:1420–1424. doi: 10.1126/science.1213214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetteh PW, Basak O, Farin HF, Wiebrands K, Kretzschmar K, Begthel H, van den Born M, Korving J, de Sauvage F, van Es JH, et al. Replacement of lost Lgr5-positive stem cells through plasticity of their enterocyte-lineage daughters. Cell Stem Cell. 2016;18:203–213. doi: 10.1016/j.stem.2016.01.001. [DOI] [PubMed] [Google Scholar]
- Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255–259. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenta T, Degirmenci B, Moor AE, Herr P, Zimmerli D, Moor MB, Hausmann G, Cantù C, Aguet M, Basler K. Wnt ligands secreted by subepithelial mesenchymal cells are essential for the survival of intestinal stem cells and gut homeostasis. Cell Rep. 2016;15:911–918. doi: 10.1016/j.celrep.2016.03.088. [DOI] [PubMed] [Google Scholar]
- van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- van der Flier LG, Haegebarth A, Stange DE, van de Wetering M, Clevers H. OLFM4 is a robust marker for stem cells in human intestine and marks a subset of colorectal cancer cells. Gastroenterology. 2009a;137:15–17. doi: 10.1053/j.gastro.2009.05.035. [DOI] [PubMed] [Google Scholar]
- van der Flier LG, van Gijn ME, Hatzis P, Kujala P, Haegebarth A, Stange DE, Begthel H, van den Born M, Guryev V, Oving I, et al. Transcription factor achaete scute-like 2 controls intestinal stem cell fate. Cell. 2009b;136:903–912. doi: 10.1016/j.cell.2009.01.031. [DOI] [PubMed] [Google Scholar]
- van Es JH, Sato T, van de Wetering M, Lyubimova A, Yee Nee AN, Gregorieff A, Sasaki N, Zeinstra L, van den Born M, Korving J, et al. Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nat Cell Biol. 2012;14:1099–1104. doi: 10.1038/ncb2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Landeghem L, Santoro MA, Krebs AE, Mah AT, Dehmer JJ, Gracz AD, Scull BP, McNaughton K, Magness ST, Lund PK. Activation of two distinct Sox9-EGFP-expressing intestinal stem cell populations during crypt regeneration after irradiation. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1111–G1132. doi: 10.1152/ajpgi.00519.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RL, McNeal MM, Sheridan JF. Development of an adult mouse model for studies on protection against rotavirus. J Virol. 1990;64:5070–5075. doi: 10.1128/jvi.64.10.5070-5075.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu N, Wang SQ, Tan D, Gao Y, Lin G, Xi R. EGFR, Wingless and JAK/STAT signaling cooperatively maintain Drosophila intestinal stem cells. Dev Biol. 2011;354:31–43. doi: 10.1016/j.ydbio.2011.03.018. [DOI] [PubMed] [Google Scholar]
- Yan KS, Chia LA, Li X, Ootani A, Su J, Lee JY, Su N, Luo Y, Heilshorn SC, Amieva MR, et al. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci USA. 2012;109:466–471. doi: 10.1073/pnas.1118857109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan KS, Gevaert O, Zheng GXY, Anchang B, Probert CS, Larkin KA, Davies PS, Cheng ZF, Kaddis JS, Han A, et al. Intestinal enteroendocrine lineage cells possess homeostatic and injury-inducible stem cell activity. Cell Stem Cell. 2017a;21:78–90. e6. doi: 10.1016/j.stem.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan KS, Janda CY, Chang J, Zheng GXY, Larkin KA, Luca VC, Chia LA, Mah AT, Han A, Terry JM, et al. Non-equivalence of Wnt and R-spondin ligands during Lgr5+ intestinal stem-cell self-renewal. Nature. 2017b;545:238–242. doi: 10.1038/nature22313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J. Intestinal stem cell injury and protection during cancer therapy. Transl Cancer Res. 2013;2:384–396. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.