Abstract
Purpose
Treatment of neuropathic corneal pain (NCP) is intricate, and involves a long-term combined multistep approach. The self-retained cryopreserved amniotic membrane (PROKERA®, Bio-Tissue, Miami, FL) has been utilized for multiple ocular surface disorders. We evaluate the efficacy, safety, and tolerability of ProKera® Slim [PKS] and ProKera® Clear [PKC] in the treatment of NCP.
Methods
Retrospective case series of 9 patients who received PKS/PKC for the acute treatment of NCP. Patient demographics, prior therapies, clinical examination, duration of PKS/PKC retention, changes in pain severity, corneal subbasal nerve density and morphology by in vivo confocal microscopy (IVCM; HRT3/RCM, Heidelberg Engineering, Heidelberg, Germany), and adverse events were recorded.
Results
PKS/PKC were placed in 10 eyes of 9 patients. Pain severity improved by 72.5±8.4% (from 6.3±0.8 to 1.9±0.6, scale 1–10, p=0.0003) after retention for 6.4±1.1 days. Despite shorter retention for 4.0±0.7 days in patients with ring dysesthesia (4 eyes) or premature implant disengagement (2 eyes), pain severity still improved by 63.1±12.5% (from 6.8±1.0 to 2.4±0.9, p=0.009). During a follow-up of 9.3±0.8 months, two patients reported recurrence of pain after 2.3 and 9.6 months respectively, which was treated effectively with additional PKS/PKC placement. IVCM showed a 36.6±17.6% increase in total nerve density, from 17,700.9±1,315.7 to 21,891.3±2,040.5 μm/mm2 (p=0.047), while the fellow PKS/PKC-untreated eyes did not show a significant interval change. Main nerve trunk and branch nerve densities were not statistically different. Dendritiform cell density decreased from 46.0±8.2 to 32.0±6.0 cells/mm2 (p=0.01).
Conclusions
PKS/PKC provide a safe and effective treatment approach to achieve sustained pain control in patients with NCP.
Keywords: Amniotic membrane, Cornea, Neuralgia, Neuropathic pain, Pain, Prokera
1. INTRODUCTION
Neuropathic corneal pain (also termed corneal neuralgia, keratoneuralgia, or corneal neuropathy) results from damaged trigeminal nerve terminals, eliciting disproportionate sensation of pain in response to both innocuous stimuli (hyperalgesia) and non-noxious triggers (allodynia).1–5 Patients with neuropathic corneal pain usually experience non-specific perception of pain, such as photoallodynia (painful sensitivity to light), severe eye-ache, burning, irritation, and sensation of eye pressure, with none to minimal signs observed on slit-lamp examination.6,7 This has posed a great diagnostic and therapeutic challenge to the ophthalmology community, particularly considering the detrimental effects of severe pain, which can result in significant decrease in quality of life and autonomy, impaired physical and social function, and even existential agony.8–11 Fortunately, as a result of recent scientific advances in the understanding of the pathophysiology of neuropathies and development of pain, both awareness and knowledge about neuropathic corneal pain have increased among ophthalmologists and other vision care providers. Moreover, neurosensory abnormalities have recently been introduced as part of the definition and pathophysiology of dry eye disease.12
Given that the diagnosis of neuropathic pain requires the demonstration of nerve damage, typically attained by skin biopsies in cases of non-ocular neuropathic disease, slit-lamp examination alone does not suffice. As such, in vivo confocal microscopy (IVCM), a noninvasive real-time imaging device that provides high-resolution images of the corneal nerve plexus at a cellular level, has enabled us to document the presence of corneal nerve alterations through optical biopsies.13–15 IVCM in patients with neuropathic corneal pain has demonstrated decreased nerve density, presence of microneuromas (engorged abrupt endings of the subbasal nerves), increased nerve tortuosity and beading (small beads along the nerve fibers), as well as various degrees of inflammation, confirming the underlying diagnosis and allowing for objective assessment of responses to therapeutic interventions.4,16,17
Treatment is often complex, refractory, and involves a multistep and potentially multi-disciplinary approach, involving the exclusion of other causes of pain and management of co-morbidities (e.g., ocular allergies, conjunctivochalasis, exposure keratopathy), employing neuro-regenerative and anti-inflammatory therapies, systemic pharmacotherapy, and even alternative medicine.2,18 Neuro-regenerative therapies, such as autologous serum tears, can lead to the restoration of nerve density and morphology, as well as slow symptomatic improvement of neuropathic pain.4 Interestingly, amniotic membranes have been found to have regenerative effects, since amniotic epithelial cells synthesize multiple anti-inflammatory, antiscarring, and mitogenic factors, as well as neurotransmitters and neurotrophins, which play significant roles in neuronal development and survival.19–24 The commercially available self-retaining cryopreserved amniotic membranes (ProKera® Slim [PKS] and ProKera® Clear [PKC], Bio-Tissue, Miami, FL) have largely been utilized with positive clinical results for the treatment of multiple ocular surface disorders, including limbal stem cell deficiency,25 chemical burns,26 bacterial keratitis,27 refractory dry eye disease,28 and neurotrophic ulcers, among others.29,30 Its safety, availability in the clinics, and promising regenerative effects prompted us to study the efficacy, tolerability, and safety of PKS/PKC in the treatment of patients with acute neuropathic corneal pain.
2. METHODS
The Institutional Review Board of Tufts Medical Center/Tufts University Health Sciences approved the retrospective single-center medical record review of patients who received PKS/PKC for the treatment of neuropathic corneal pain between July 2015 and May 2016. Patients were seen at the Cornea Service of the New England Eye Center, Tufts Medical Center, Boston, MA. The protocol conformed to the Declaration of Helsinki and adhered to the Health Insurance Portability and Accountability Act (HIPAA).
Diagnosis of neuropathic corneal pain was made in the presence of confirmed corneal nerve damage by IVCM with an unremarkable clinical slit-lamp examination, along with the absence of other active ocular surface disease or any other concomitant causes of pain or co-morbidities. Central cause of pain (central sensitization) was ruled out by the proparacaine challenge test as previously described,18 since PKS/PKC placement would theoretically help with peripheral but not central pain. Patients initially received PKS. When PKC, which provides a central aperture that maintains clarity of the visual axis, became available, we switched to the use of PKC. Patient demographics, ocular and systemic medical history, underlying etiology of neuropathic pain, prior treatment regimens, clinical examination, duration of PKS/PKC retention, adverse events, and corneal IVCM findings were reviewed. Charts were also reviewed for pain severity, which was based on patient self-assessment and measured on the visual analogue scale from 1 to 10, ten being the greatest. Pain level had been registered before and after placement of PKS/PKC. Esthesiometry was not routinely checked in patients with neuropathic corneal pain, as the hyperalgesia and allodynia may yield inaccurate measurements.
In vivo confocal microscopy (Heidelberg Retina Tomograph 3 with the Rostock Cornea Module, Heidelberg Engineering GmbH, Heidelberg, Germany) was performed routinely on all patients with pain and discomfort for confirmation of corneal subbasal nerve alterations, as previously described.4 IVCM provides 400 × 400 μm full-thickness coronal corneal scans, which allow visualization of the central cornea, and assessment of corneal nerve alterations. In order to evaluate changes in subbasal corneal nerves with placement of PKS/PKC, quantitative analysis of subbasal nerve density and morphology was performed in a masked fashion on 4 representative images of 4 patients, in which images were available both before and after PKC/PKS placement as imaging was not routinely performed after PKS/PKC placement. Images were selected by a masked observer as previously described.4,31 These were traced with Image J using the previously described semiautomatic plug-in Neuron J32 (available online at http://www.imagescience.org/meijering/software/neuronj/) to assess total nerve density, main nerve trunk density, and branch nerve density. Dendritiform cell (DC) density was assessed manually using Image J. These measurements were then compared to reference controls.4
Statistical analysis was performed in STATA TM (StataCorp, College Station, TX- version 13) with paired Student’s t-test to compare the pre- and post-treatment findings, and with unpaired t-test to analyze differences in baseline conditions between groups. Statistically significant difference was defined as P values of less than 0.05.
3. RESULTS
3.1. Patient Demographics
Ten eyes of 9 patients with diagnosis of neuropathic corneal pain were included in the study. Eight of them were females and one was a male, with a mean age of 58.8 ± 4.3 years (range 24 to 72). Details are summarized in Table 1.
Table 1.
Demographics
| Patient no. | Age/Gender | Eye | Causes | Prior treatments |
|---|---|---|---|---|
| 1 | 51/F | OS | DED | ML, WC/LM, FSO, loteprednol |
| 2 | 72/F | OD | DED, conjunctivochalasis | ML, WC/LM, PP, loteprednol, AST, nortriptyline |
| 3 | 68/F | OS | DED, MGD/blepharitis | ML, WC/LM, loteprednol, AST |
| 4 | 59/F | OS | DED, TED | ML, WC/LM, FSO, doxycycline, loteprednol, AST, TD |
| 5 | 66/F | OD | DED, s/p strabismus surgery | ML, WC/LM, PP, loteprednol, TD |
| 6 | 63/F | OD | MGD | ML, WC/LM, FSO, MG, MGP, loteprednol, AST, nortriptyline |
| OS | ||||
| 7 | 64/F | OD | MGD | ML, WC/LM, FSO, azithromycin, doxycycline, MGP, loteprednol |
| 8 | 24/M | OD | s/p PRK | ML, WC/LM, doxycycline, loteprednol, nortriptyline |
| 9 | 55/F | OS | DED, conjunctivochalasis, blepharospasm | ML, WC/LM, PP, loteprednol, AST, trihexyphenidyl, BTx |
AST= autologous serum tears; BTx= botulinum toxin injection; DED= dry eye disease; F= female; FSO= flaxseed oil; M= male; MG= moisture goggles; MGD= meibomian gland dysfunction; MGP= meibomian gland probing; ML= maximal lubrication; OD= right eye; OS= left eye; PP= punctal plug/s; PRK= photorefractive keratectomy; TD= testosterone drops; TED= thyroid eye disease; WC/LM= warm compresses/lid massage
3.2. Clinical Features
The underlying etiology of neuropathic corneal pain included prior history of dry eye disease (six eyes), prior history of obstructive meibomian gland dysfunction (three eyes), conjunctivochalasis (two eyes), post-surgical (two eyes, status post strabismus surgery and photorefractive keratectomy, respectively), thyroid eye disease (one eye), and blepharospasm secondary to Meige syndrome (one eye). All patients had been treated for these conditions, which had resolved and were deemed not to be causative of pain at the time of PKS/PKC placement. Prior therapeutic regimens included maximal lubrication with preservative-free artificial tears, emulsion-based drops or ointment in all cases, as well as hot compresses with lid massage, and medical therapy and/or intraductal meibomian gland probing for patients with obstructive meibomian gland dysfunction. All patients had also received trials of topical steroid treatment with an initial regimen of loteprednol 0.5% QID, followed by a bi-weekly taper until a maintenance dose of loteprednol once or twice weekly. Five patients were under treatment with 20% autologous serum tears eight times daily: two had been on these drops for 6 months, two for 1.5 years, and one for 4 years. They had all improved with the autologous serum tears, but complained of recurrence of pain while on autologous serum tears on the day PKS/PKC placement was chosen. Three of these patients had shown decreased corneal nerve density prior to PKS/PKC insertion. Two patients had poor compliance with the treatment regimen (and one of these had decreased nerve density on IVCM). Two patients were receiving topical compounded off-label 0.03% testosterone drops in both eyes three times daily. Furthermore, three patients had been treated with a trial of oral nortriptyline. Oral trihexyphenidyl therapy and botulinum toxin injection had been implemented for the treatment of one case with concomitant Meige syndrome and blepharospasm. None of the treatments had more recently resulted in complete control of symptoms, with an average pain severity of 6.3 ± 0.8 on a scale from 0 to 10 prior to PKS/PKC placement. Best-corrected visual acuity in logMAR (logarithmic minimum angle of resolution) was 0.176 (20/30) in 3 eyes, 0.097 (20/25 Snellen equivalent) in 7 eyes, and 0.0 (20/20) in 3 eyes. IVCM had been performed on all patients for the diagnosis of neuropathic corneal pain. Baseline characteristics included decreased nerve density and presence of microneuromas in all the eyes.
3.3. Efficacy of treatment
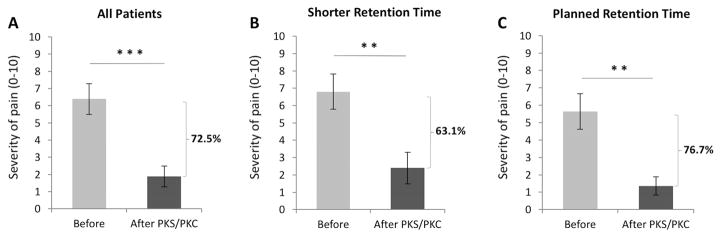
Eight PKS and 2 PKC were initially placed in 10 eyes of 9 patients (Table 2). Mean severity of pain prior to amniotic membrane placement was 6.3 ± 0.8. PKS/PKC were removed after a mean of 6.4 ± 1.1 days (range 2–14 days). The average pain severity was found to improve by 72.5 ± 8.4%, reducing the mean pain level to 1.9 ± 0.6 (p=0.0003; Fig. 1A). Interestingly, one patient (patient #4) reported simultaneous resolution of pain in the fellow PKC-untreated eye as well.
Table 2.
Tolerance, Retention Times, and Changes in Pain Severity
| Intolerance to ring | Patient no. | Eye | ProKera | Days of retention | Pain | Follow-up (months) | |
|---|---|---|---|---|---|---|---|
| Before | After | ||||||
| − | 1 | OS | PKS | 7 | 4 | 2 | 9.0 |
| − | 2 | OD | PKS | 5 | 9 | 0 | 9.0 |
| − | 4 | OS | PKC | 14 | 1.5 | 0 | 7.6 |
| − | 5 | OD | PKC | 6 | 5 | 0 | 8.0 |
| − | 6 | OS | PKS | 7 | 9 | 3 | 10.1 |
| − | 9 | OS | PKS | 6 | 5 | 1.5 | 13.8 |
| Mean ± SEM | 7.5 ± 1.34 | 5.58 ± 1.20 | 1.08 ± 0.52 | ||||
| + | 3 | OS | PKS | 6 | 8 | 1.5 | 8.1 |
| + | 6 | OD | PKS | 5 | 9 | 4 | 10.0 |
| + | 8 | OD | PKS | 2 | 7 | 5 | 8.4 |
| Mean ± SEM | 4.33 ± 1.20 | 8 ± 0.58 | 3.5 ± 1.04 | ||||
| +++ | 7 | OD | PKS | 0 | 5 | same-day removal | - |
OD= right eye; OS= left eye; PKC= ProKera Clear; PKS= ProKera Slim; SEM= standard error of the mean
Figure 1.
Average severity of pain before and after first PKS/PKC placement in all patients (A), in whom pain severity improved by 72.5 ± 8.4 % after treatment. Average severity of pain before and after PKS/PKC placement improved by 63.1 ± 12.5 % in patients where the amniotic membrane was removed before complete dissolution of the membrane (B), and 76.7 ± 8.7 % in patients who retained the membrane for the planned period of time (C). **p<.01, ***p<.001, compared to the pre-treatment group by paired t-test.
3.4. Tolerability and Retention
One patient required PKS removal minutes after insertion due to intolerance to the ring (Table 2). In addition, late onset discomfort resulted in the removal of PKS/PKC prior to complete dissolution of the membrane in 3 eyes, while the amniotic membrane fell out spontaneously in 2 eyes. Nevertheless, despite a slightly shorter retention time of 4.0 ± 0.7 days (range 2–6 days) in these 5 patients, the pain severity still improved by 63.1 ± 12.5% (from 6.8 ± 1.0 to 2.4 ± 0.9; p= 0.009; Fig. 1B). In contrast, patients with the planned ring retention period experienced a 76.7 ± 8.7 % improvement in pain, from 5.6 ± 1.0 to 1.4 ± 0.5 (p= 0.002; Fig. 1C). The difference in pain level prior to PKS/PKC placement was not statistically significant between patients who had removal of PKS/PKC upon dissolution of the membrane (5.6 ± 1.2) and patients who requested earlier removal due to discomfort (7.3 ± 0.9, p=0.17), although increased pain levels were observed in this last group.
3.5. Recurrence of Pain and Re-treatment
Over the average follow-up period of 9.3 ± 0.8 months (range 7.6–13.8 months), only two patients reported recurrence of pain after a mean of 6.0 ± 2.1 months. PKS placement in the first patient resulted in improvement in pain levels from 9/10 to 0/10. Pain recurred to a level of 3/10 after 2.3 months. Placement of a second PKC resulted in improvement to 1.5/10. Pain recurred to 7/10 after 6.1 months, at which point a third PKC was placed, resulting in improvement of pain to 0/10.
PKS placement in both eyes in the second patient resulted in improvement of pain from 9/10 to 4/10 in the right eye and 3/10 in the left eye. Pain recurred in the left eye after 9.6 months, at a level of 6/10. A second PKS placement resulted in improvement of pain to 3/10.
No other eye experienced recurrence of pain during the follow-up period.
3.6. Safety
Best-corrected distance visual acuity pre- and post-PKS/PKC remained statistically unchanged in all eyes immediately after removal and at last follow-up visit (0.099 ± 0.021 logMAR at baseline vs. 0.099 ± 0.023 after removal; p= 0.49, and 0.086 ± 0.021 at last follow-up; p= 0.31). No significant adverse events were experienced by the treated patients.
3.7. IVCM Findings
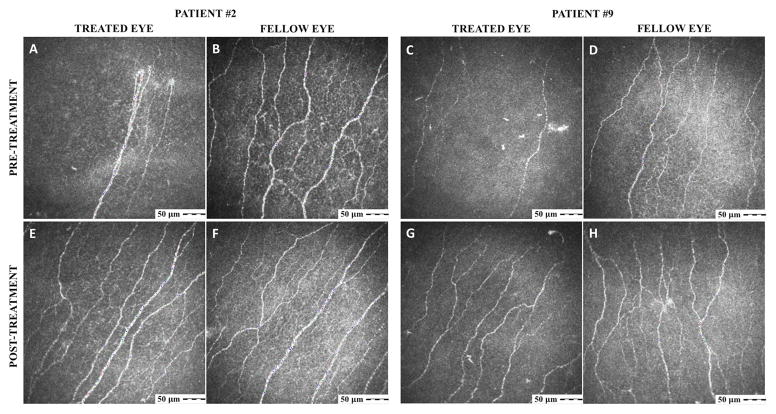
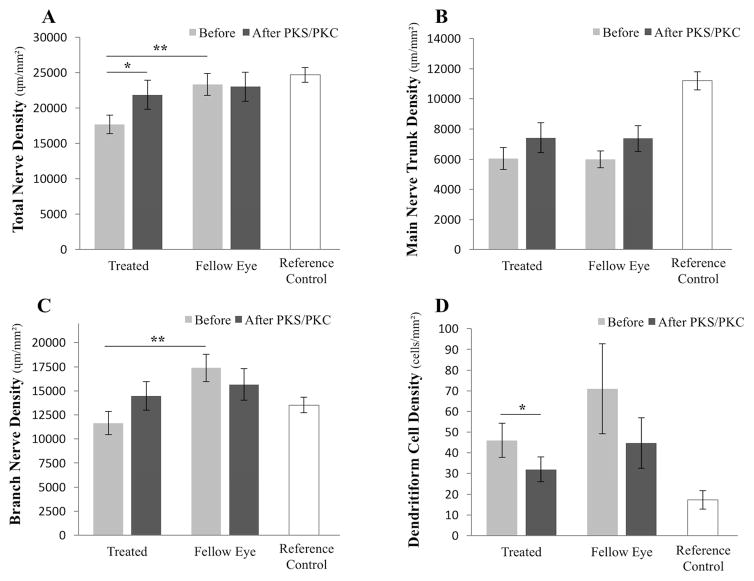
IVCM was performed before and after treatment on 4 patients, on average 48.8 ± 16.5 days (range 34–63 days) after placement of the PKS/PKC. Analysis of those patients showed a 36.6 ± 17.6% increase in total subbasal nerve density, rising from a mean density of 17,700.9 ± 1,315.7 to 21,891.3 ± 2,040.5 μm/mm2 (p=0.047; Figs. 2 and 3A). The fellow untreated eyes of these same patients did not show a significant simultaneous interval change (23,358.0 ± 1,546.4 vs. 23,038.2 ± 2,054.6 μm/mm2, p=0.45). In comparison, our reference controls have a subbasal nerve density of 24,714.0 ± 1,056.0 μm/mm2.4 Main nerve trunk density measurements were statistically unchanged after treatment (6,050.2 ± 727.9 μm/mm2 before PKS/PKC vs. 7,425.8 ± 989.7 after PKS/PKC; p= 0.104; Fig. 3B). Branch nerve density appeared to increase from 11,650.7 ± 1,190.8 to 14,465.5 ± 1,486.5 μm/mm2, but similarly, the difference was not statistically significant (p= 0.09; Fig. 3C). Lastly, DC density decreased in the PKS/PKC-treated eyes from 46.0 ± 8.2 to 32.0 ± 6.0 cells/mm2 (p= 0.01), while the density was found not to change in the untreated eyes (71.0 ± 21.8 vs. 44.8 ± 12.2 cells/mm2; p= 0.16; Fig. 3D). In comparison, DC density is 17.3 ± 4.4 cells/mm2 in reference controls.4
Figure 2.
Representative IVCM pictures of patients #2 and 9, who were imaged before (A–D) and then again 36 (E, F) and 63 (G, H) days after PKS/PKC placement, respectively. For each patient, treated eyes are presented on the left column and untreated eyes, on the right.
Figure 3.
Comparison of total nerve density in the central corneal subbasal plexus pre- and post-self-retained cryopreserved amniotic membrane in treated and untreated eyes imaged with IVCM, as well as reference controls, showing a 36.6 ± 17.6% increment after PKS/PKC treatment (A). Main nerve trunk (B) and branch nerve (C) densities, as well as presence of microneuromas (D) and dendritiform cell density (E) were also compared before and after PKS/PKC placement in treated and untreated eyes, and to reference controls. *p<.05, **p<.01, compared to fellow eyes by unpaired t-test, and to the pre-treatment group by paired t-test. n= 4 patients.
4. DISCUSSION
Previous studies have validated the efficacy of self-retained cryopreserved amniotic membrane placement in the treatment of multiple ocular surface disorders, including persistent neurotrophic epithelial defects and ulcers, attributing its healing properties primarily to the promotion of epithelialization and inhibition of fibrosis.21,33–38 Likewise, persistent epithelial defects have been effectively treated with tissue-cultured human amniotic epithelial cells seeded on collagen shields.39 To our knowledge, the use of amniotic membrane transplantation in the treatment of neuropathic corneal pain has previously not been explored. Therefore, the current study is the first report demonstrating positive clinical and IVCM-based results of self-retained cryopreserved amniotic membrane placement in the acute treatment of neuropathic corneal pain.
The amniotic membrane closely covers the embryo as the innermost layer delineating the amniotic cavity, and is formed by three main layers: the epithelial layer, a basement membrane, and the avascular mesenchymal stromal matrix.40,41 The amnion exchanges many nutritional, gaseous, immunologic, and hormonal elements with the mother through the surrounding chorion, providing a protective developmental environment for the fetus. However, the exact biochemical mechanisms behind the amniotic membrane’s ocular healing properties have not been completely elucidated. To date, it is known that amniotic membrane epithelial and mesenchymal cells express numerous anti-inflammatory, anti-fibrotic, and mitogenic mediators, such as interleukin (IL)-1 receptor antagonist (IL-1 RA), IL-10, tissue inhibitors of metalloproteinases (TIMPs), transforming growth factor (TGF)-β, and epidermal growth factor (EGF).42–45 Of note, uncontrolled inflammation can contribute to nerve degeneration, a key component of corneal neuropathy, required for the development of neuropathic pain.31,46 Interestingly, amniotic cells have been found to produce neurotrophic factors, such as nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and ephrin-A2, all of which are fundamental for neuronal development and cell survival.23,24 These factors are also of great importance for corneal nerve regeneration and maintenance.47–49 This has been demonstrated clinically through topical application of autologous serum tears, which owing to the supply of multiple neurotrophic and immunomodulatory factors,50 have been shown to improve photoallodynia in patients with corneal neuropathy even in the presence of intact epithelium, and to result in concomitant regeneration of damaged subbasal corneal nerves.4
We postulate that the neurotrophic and anti-inflammatory microenvironment induced by the placement of amniotic membranes is responsible for the regeneration of subbasal corneal nerves as seen in a subset of our patients. Thus, it is plausible that the subjacent mechanism of pain alleviation results from induction of neurotropism and restoration of nerve function, which could be effective in both patients with neurotrophic keratopathy and neuropathic corneal pain, as these entities share in common the underlying dysfunction and damage to the trigeminal nerve endings. Ultimately, further morphological, functional, and biochemical alterations may lead to a higher threshold for pain. Sustained pain control in the majority of patients for more than 9 months suggests that the amniotic membrane treatment restored a certain degree of neuronal integrity and functionality, then conceivably maintained by the baseline topical and systemic therapies for neuropathic pain, which patients continued using in the interim.
IVCM findings have previously not been studied in patients with neuropathic corneal pain, treated with amniotic membrane placement, and further reinforce our clinical findings, potentially by induction of neurotropism and a higher threshold for pain. However, the fact that IVCM was repeated in average almost 2 months after PKS/PKC placement potentially allows for concurrent regenerative therapies, such as autologous serum tears, to influence our findings on nerve density. Nevertheless, when compared to the fellow eyes not treated with amniotic membrane placement, which simultaneously also received autologous serum tears, nerve density was significantly higher only in the eyes with PKS/PKC placement. The non-treated fellow eyes did not show a statistically significant increase in nerve density. Of note, patients on autologous serum tears had been on this treatment for at least 6 months. Given the retrospective nature of this study, only a subset of patients had IVCM images before and after amniotic membrane placement. However, the results warrant larger randomized controlled trials to confirm the results of our pilot study in order to further characterize morphological and functional changes following amniotic membrane treatment.
Self-retained cryopreserved amniotic membrane placement has not been associated with any significant adverse reactions or unexpected long-term effects.51 However, PKS/PKC have been found to cause discomfort in the form of foreign body sensation given the addition of the surrounding polycarbonate ring.52 This can be particularly pronounced in neuropathic corneal pain patients with hyperalgesia. Nevertheless, even patients with much shorter retention of PKS/PKC demonstrated significant improvement in pain intensity. Assessment of baseline pain did not seem to be a predictor of discomfort caused by the ring, suggesting that hyperalgesia does not directly correlate to pain severity. Moreover, one of the 4 patients who required earlier removal of the ring in one eye, was able to tolerate 2 placements in the fellow eye without complains of discomfort, suggesting that discomfort due to hyperalgesia may be asymmetric.
A limitation of amniotic membrane implantation is the transient decrease in vision by an average of 1.26 logMAR compared to baseline, which recovers immediately after removal of the membrane.52 In the present study, our patients did not complain of that transient decreased vision, presumably from the unaffected visual acuity in the fellow eyes due to the nature of their disease. Also, patients who underwent PKC placement had an additional advantage: the central aperture in the amniotic membrane is intended to maintain visual acuity during treatment.
The limitations of the current study include its retrospective nature, small sample size, and limited number of patients who underwent IVCM imaging both before and after PKS/PKC placement. However, given the rare population of these patients, the severity of pain and impact on quality of life, and the constrained availability of effective treatment options, the current study is of significant importance in the management of these patients.
5. CONCLUSIONS
The use of self-retained cryopreserved amniotic membranes represents a novel and promising therapeutic option for patients with neuropathic corneal pain based on rapid patient-reported improvement of pain severity and increase in corneal nerve density as shown by IVCM. Patients seemed to tolerate treatment well, except for ring intolerance in a subset of patients and earlier removal in 40% of the eyes, with nevertheless significant reduction of pain after shorter retention. The present study further demonstrates sustained pain control in 80% of the treated eyes for more than 9 months after a single placement of PKS/PKC. Therefore, we conclude that PKS/PKC placement is a safe and effective therapeutic approach for acute management of neuropathic corneal pain, a challenging and undertreated condition.
Acknowledgments
Financial Support: NIH R01-EY022695 (PH), NIH R21-EY025393 (PH), Tissue-Tech (PH). The funding organizations had no role in the design or conduct of this research.
Footnotes
Disclosures: Pedram Hamrah holds a consulting and non-remunerative relationship with Tissue-Tech. This work has been presented, in part, at the 19th International Ocular Surface Society Meeting, May 2016, Seattle, WA.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marfurt CF, Cox J, Deek S, Dvorscak L. Anatomy of the human corneal innervation. Exp Eye Res. 2010;90(4):478–92. doi: 10.1016/j.exer.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Cohen SP, Mao J. Neuropathic pain: mechanisms and their clinical implications. BMJ. 2014;348:7656. doi: 10.1136/bmj.f7656. [DOI] [PubMed] [Google Scholar]
- 3.Belmonte C, Aracil A, Acosta C, Luna C, Gallar J. Nerves and sensations from the eye surface. Ocul Surf. 2004;2(4):248–53. doi: 10.1016/s1542-0124(12)70112-x. [DOI] [PubMed] [Google Scholar]
- 4.Aggarwal S, Kheirkhah A, Cavalcanti BM, Cruzat A, Colon C, Brown E, et al. Autologous serum tears for treatment of photoallodynia in patients with corneal neuropathy: efficacy and evaluation with in vivo confocal microscopy. Ocul Surf. 2015;13(3):250–62. doi: 10.1016/j.jtos.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kovács I, Luna C, Quirce S, Mizerska K, Callejo G, Riestra A, et al. Abnormal activity of corneal cold thermoreceptors underlies the unpleasant sensations in dry eye disease. Pain. 2016;157(2):399–417. doi: 10.1097/j.pain.0000000000000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenthal P, Baran I, Jacobs DS. Corneal pain without stain: is it real? Ocul Surf. 2009;7(1):28–40. doi: 10.1016/s1542-0124(12)70290-2. [DOI] [PubMed] [Google Scholar]
- 7.Rosenthal P, Borsook D. The corneal pain system. Part I: the missing piece of the dry eye puzzle. Ocul Surf. 2012;10(1):2–14. doi: 10.1016/j.jtos.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Miljanović B, Dana R, Sullivan DA, Schaumberg DA. Impact of dry eye syndrome on vision-related quality of life. Am J Ophthalmol. 2007;143(3):409–15. doi: 10.1016/j.ajo.2006.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman NJ. Impact of dry eye disease and treatment on quality of life. Curr Opin Ophthalmol. 2010;21(4):310–6. doi: 10.1097/ICU.0b013e32833a8c15. [DOI] [PubMed] [Google Scholar]
- 10.Na KS, Han K, Park YG, Na C, Joo CK. depression, stress, quality of life, and dry eye disease in Korean women: a population-based study. Cornea. 2015;34(7):733–8. doi: 10.1097/ICO.0000000000000464. [DOI] [PubMed] [Google Scholar]
- 11.Qazi Y, Hurwitz S, Khan S, Jurkunas UV, Dana R, Hamrah P. Validity and reliability of a novel ocular pain assessment survey (OPAS) in quantifying and monitoring corneal and ocular surface pain. Ophthalmology. 2016;123(7):1458–68. doi: 10.1016/j.ophtha.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belmonte C, Nichols JJ, Cox SM, Brock JA, Begley CG, Bereiter DA, et al. TFOS DEWS II pain and sensation report. Ocul Surf. 2017 Jul;15(3):404–437. doi: 10.1016/j.jtos.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petroll WM, Robertson DM. In vivo confocal microscopy of the cornea: new developments in image acquisition, reconstruction, and analysis using the HRT-Rostock Corneal Module. Ocul Surf. 2015;13(3):187–203. doi: 10.1016/j.jtos.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villani E, Baudouin C, Efron N, Hamrah P, Kojima T, Patel SV, et al. In vivo confocal microscopy of the ocular surface: from bench to bedside. Curr Eye Res. 2014;39(3):213–31. doi: 10.3109/02713683.2013.842592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel DV, McGhee CN. Quantitative analysis of in vivo confocal microscopy images: a review. Curr Eye Res. 2014;39(3):213–31. doi: 10.3109/02713683.2013.842592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cruzat A, Qazi Y, Hamrah P. In vivo confocal microscopy of corneal nerves in health and disease. Ocul Surf. 2017;15(1):15–47. doi: 10.1016/j.jtos.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamrah P, Qazi Y, Shahatit B, Dastjerdi MH, Pavan-Langston D, Jacobs DS, et al. corneal nerve and epithelial cell alterations in corneal allodynia: an in vivo confocal microscopy case series. Ocul Surf. 2017;15(1):139–151. doi: 10.1016/j.jtos.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Goyal S, Hamrah P. Understanding neuropathic corneal pain--gaps and current therapeutic approaches. Semin Ophthalmol. 2016;31(1–2):59–70. doi: 10.3109/08820538.2015.1114853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouchard CS, John T. Amniotic membrane transplantation in the management of severe ocular surface disease: indications and outcomes. Ocul Surf. 2004;2(3):201–11. doi: 10.1016/s1542-0124(12)70062-9. [DOI] [PubMed] [Google Scholar]
- 20.Dua HS, Gomes JA, King AJ, Maharajan VS. The amniotic membrane in ophthalmology. Surv Ophthalmol. 2004;49:51–77. doi: 10.1016/j.survophthal.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Park JH, Jeoung JW, Wee WR, Lee JH, Kim MK, Lee JL. Clinical efficacy of amniotic membrane transplantation in the treatment of various ocular surface diseases. Cont Lens Anterior Eye. 2008;31(2):73–80. doi: 10.1016/j.clae.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Tseng SC, Espana EM, Kawakita T, Di Pascuale MA, Li W, He H, et al. How does amniotic membrane work? Ocul Surf. 2004;2(3):177–87. doi: 10.1016/s1542-0124(12)70059-9. [DOI] [PubMed] [Google Scholar]
- 23.Uchida S, Inanaga Y, Kobayashi M, Hurukawa S, Araie M, Sakuragawa N. Neurotrophic function of conditioned medium from human amniotic epithelial cells. J Neurosci Res. 2000;62(4):585–90. doi: 10.1002/1097-4547(20001115)62:4<585::AID-JNR13>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 24.Marvin KW, Keelan JA, Eykholt RL, Sato TA, Mitchell MD. Expression of angiogenic and neurotrophic factors in the human amnion and choriodecidua. Am J Obstet Gynecol. 2002;187(3):728–34. [PubMed] [Google Scholar]
- 25.Kheirkhah A, Casas V, Raju VK, Tseng SC. Sutureless amniotic membrane transplantation for partial limbal stem cell deficiency. Am J Ophthalmol. 2008;145(5):787–94. doi: 10.1016/j.ajo.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kheirkhah A, Johnson DA, Paranjpe DR, Raju VK, Casas V, Tseng SC. Temporary sutureless amniotic membrane patch for acute alkaline burns. Arch Ophthalmol. 2008;126(8):1059–66. doi: 10.1001/archopht.126.8.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheha H, Liang L, Li J, Tseng SC. Sutureless amniotic membrane transplantation for severe bacterial keratitis. Cornea. 2009;28(10):1118–23. doi: 10.1097/ICO.0b013e3181a2abad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng AM, Zhao D, Chen R, Yin HY, Tighe S, Sheha H, et al. Accelerated restoration of ocular surface health in dry eye disease by self-retained cryopreserved amniotic membrane. Ocul Surf. 2016;14(1):56–63. doi: 10.1016/j.jtos.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pachigolla G, Prasher P, Di Pascuale MA, McCulley JP, McHenry JG, Mootha VV. Evaluation of the role of ProKera in the management of ocular surface and orbital disorders. Eye Contact Lens. 2009;35(4):172–5. doi: 10.1097/ICL.0b013e3181a66a12. [DOI] [PubMed] [Google Scholar]
- 30.Suri K, Kosker M, Raber IM, Hammersmith KM, Nagra PK, Ayres BD, et al. Sutureless amniotic membrane ProKera for ocular surface disorders: short-term results. Eye Contact Lens. 2013;39(5):341–7. doi: 10.1097/ICL.0b013e3182a2f8fa. [DOI] [PubMed] [Google Scholar]
- 31.Cruzat A, Witkin D, Baniasadi N, Zheng L, Ciolino JB, Jurkunas UV, et al. Inflammation and the nervous system: the connection in the cornea in patients with infectious keratitis. Invest Ophthalmol Vis Sci. 2011;52(8):5136–43. doi: 10.1167/iovs.10-7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meijering E, Jacob M, Sarria JC, Steiner P, Hirling H, Unser M. Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytometry A. 2004;58:167–76. doi: 10.1002/cyto.a.20022. [DOI] [PubMed] [Google Scholar]
- 33.Lee SH, Tseng SC. Amniotic membrane transplantation for persistent epithelial defects with ulceration. Am J Ophthalmol. 1997;123(3):303–12. doi: 10.1016/s0002-9394(14)70125-4. [DOI] [PubMed] [Google Scholar]
- 34.Chen HJ, Pires RT, Tseng SC. Amniotic membrane transplantation for severe neurotrophic corneal ulcers. Br J Ophthalmol. 2000;84(8):826–33. doi: 10.1136/bjo.84.8.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanada K, Shimazaki J, Shimmura S, Tsubota K. Multilayered amniotic membrane transplantation for severe ulceration of the cornea and sclera. Am J Ophthalmol. 2001;131(3):324–31. doi: 10.1016/s0002-9394(00)00825-4. [DOI] [PubMed] [Google Scholar]
- 36.Khokhar S, Natung T, Sony P, Sharma N, Agarwal N, Vajpayee RB. Amniotic membrane transplantation in refractory neurotrophic corneal ulcers: a randomized, controlled clinical trial. Cornea. 2005;24:654–660. doi: 10.1097/01.ico.0000153102.19776.80. [DOI] [PubMed] [Google Scholar]
- 37.Iakimento SA, Buznyk OI, Rymgayllo-Jankowska B. Amniotic membrane transplantation in treatment of persistent corneal ulceration after severe chemical and thermal eye injuries. Eur J Ophthalmol. 2013;23:496–503. doi: 10.5301/ejo.5000243. [DOI] [PubMed] [Google Scholar]
- 38.Uhlig CE, Frings C, Rohloff N, Harmsen-Aasman C, Schmitz R, Kiesel L, et al. Long-term efficacy of glycerine-processed amniotic membrane transplantation in patients with corneal ulcer. Acta Ophthalmol. 2015;93(6):e481–7. doi: 10.1111/aos.12671. [DOI] [PubMed] [Google Scholar]
- 39.Parmar DN, Alizadeh H, Awwad ST, Li H, Neelam S, Bowman RW, et al. Ocular surface restoration using non-surgical transplantation of tissue-cultured human amniotic epithelial cells. Am J Ophthalmol. 2006;141(2):299–307. doi: 10.1016/j.ajo.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 40.Toda A, Okabe M, Yoshida T, Nikaido T. The potential of amniotic membrane/amnion-derived cells for regeneration of various tissues. J Pharmacol Sci. 2007;105(3):215–28. doi: 10.1254/jphs.cr0070034. [DOI] [PubMed] [Google Scholar]
- 41.Mamede AC, Carvalho MJ, Abrantes AM, Laranjo M, Maia CJ, Botelho MF. Amniotic membrane: from structure and functions to clinical applications. Cell Tissue Res. 2012;349(2):447–58. doi: 10.1007/s00441-012-1424-6. [DOI] [PubMed] [Google Scholar]
- 42.Paolin A, Trojan D, Leonardi A, Mellone S, Volpe A, Orlandi A, et al. Cytokine expression and ultrastructural alterations in fresh-frozen, freeze-dried and γ-irradiated human amniotic membranes. Cell Tissue Bank. 2016;17(3):399–406. doi: 10.1007/s10561-016-9553-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li H, Niederkorn JY, Neelam S, Mayhew E, Word RA, McCulley JP, et al. Immunosuppressive factors secreted by human amniotic epithelial cells. Invest Ophthalmol Vis Sci. 2005;46(3):900–7. doi: 10.1167/iovs.04-0495. [DOI] [PubMed] [Google Scholar]
- 44.Solomon A, Rosenblatt M, Monroy D, Ji Z, Pflugfelder SC, Tseng SC. Suppression of interleukin 1alpha and interleukin 1beta in human limbal epithelial cells cultured on the amniotic membrane stromal matrix. Br J Ophthalmol. 2001;85(4):444–9. doi: 10.1136/bjo.85.4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hao Y, Ma DH, Hwang DG, Kim WS, Zhang F. Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane. Cornea. 2000;19(3):348–52. doi: 10.1097/00003226-200005000-00018. [DOI] [PubMed] [Google Scholar]
- 46.Benowitz LI, Popovich PG. Inflammation and axon regeneration. Curr Opin Neurol. 2011;24(6):577–83. doi: 10.1097/WCO.0b013e32834c208d. [DOI] [PubMed] [Google Scholar]
- 47.Mohammad J, Shenaq J, Rabinovsky E, Shenaq S. Modulation of peripheral nerve regeneration: a tissue-engineering approach. The role of amnion tube nerve conduit across a 1-centimeter nerve gap. Plast Reconstr Surg. 2000;105(2):660–6. doi: 10.1097/00006534-200002000-00027. [DOI] [PubMed] [Google Scholar]
- 48.Esquenazi S, Bazan HE, Bui V, He J, Kim DB, Bazan NG. Topical combination of NGF and DHA increases rabbit corneal nerve regeneration after photorefractive keratectomy. Invest Ophthalmol Vis Sci. 2005;46(9):3121–7. doi: 10.1167/iovs.05-0241. [DOI] [PubMed] [Google Scholar]
- 49.Aloe L, Tirassa P, Lambiase A. The topical application of nerve growth factor as a pharmacological tool for human corneal and skin ulcers. Pharmacol Res. 2008;57(4):253–8. doi: 10.1016/j.phrs.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 50.Matsumoto Y, Dogru M, Goto E, Ohashi Y, Kojima T, Ishida R, et al. Autologous serum application in the treatment of neurotrophic keratopathy. Ophthalmology. 2004;111(6):1115–20. doi: 10.1016/j.ophtha.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 51.Paolin A, Cogliati E, Trojan D, Griffoni C, Grassetto A, Elbadawy HM, et al. Amniotic membranes in ophthalmology: long term data on transplantation outcomes. Cell Tissue Bank. 2016;17(1):51–8. doi: 10.1007/s10561-015-9520-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ijiri S, Kobayashi A, Sugiyama K, Tseng SCG. Evaluation of visual acuity and color vision in normal human eyes with a sutureless temporary amniotic membrane patch. Am J Ophthalmol. 2007;144:938–942. doi: 10.1016/j.ajo.2007.08.003. [DOI] [PubMed] [Google Scholar]