Abstract
Abnormal behavior occurs in a number of captive nonhuman primate species and is often used as an indicator of welfare. However, reported levels of abnormal behavior often vary across species, making general welfare judgments difficult. The purpose of this study was to assess differences in levels of abnormal behavior and associated risk factors across three species of Old World monkeys in order to identify similarities and differences across species. The subjects were 415 (109 females) cynomolgus macaques (Macaca fascicularis), 365 (181 females) rhesus macaques (Macaca mulatta), and 331 (187 females) baboons (Papio hamadryas) that had been singly-housed for 30–120 days. A 5-min observation using one-zero sampling recorded the presence or absence of abnormal behavior for each animal. Macaques exhibited higher levels of abnormal behavior than baboons (29% vs. 14%; χ2(1) = 24.849, p < 0.001), but there was no difference between macaque species (30% vs. 28%; χ2(1) = 0.263, p = 0.608). Risk factors also varied. Overall, males exhibited greater levels of motor stereotypies (b = 0.425, p < 0.05), females greater levels of abnormal appetitive behavior (b = 1.703, p < 0.05), and older animals greater levels of self-directed behavior (b = 0.065, p < 0.05). However, macaques exhibited greater levels of motor stereotypy (b = 2.527, p < 0.001) and self-directed behavior (b = 2.968, p < 0.005) than did baboons. There was also a genus × sex interaction for abnormal appetitive behavior (b = −2.379, p < 0.01) and a genus × age interaction for motor stereotypy (b = −0.167, p < 0.05). These results demonstrate that differences in abnormal behavior exist across closely-related primate species. Therefore, a single species cannot be used generally as a model for abnormal behavior or animal welfare.
Keywords: Species differences, Behavioral assessment, Welfare
1. Introduction
Abnormal behavior has been noted in a number of captive nonhuman primate species including prosimians (Tarou et al., 2005; Watson et al., 2009), New World monkeys (Berkson et al., 1966; McGrogan and King, 1982), Old World monkeys (Bayne et al., 1992; Camus et al., 2013; Lutz et al., 2003, 2014), as well as lesser and greater apes (Birkett and Newton-Fisher, 2011; Nash et al., 1999; Trollope, 1977; Walsh et al., 1982) housed at both laboratories and zoos. Behavior can be considered to be abnormal if it differs in kind (i.e., qualitatively different) or by degree (i.e., quantitatively different) from those behaviors typically observed in the wild (Erwin and Deni, 1979). However, abnormal behavior is not a strict dichotomy, and the dividing line between normal and abnormal can be difficult to establish (Bayne, 1996; Mason, 1991). Although there are similarities to normal behavior, abnormal behavior is of concern because it can be an indicator of past or present compromised welfare (Mason, 1991) or altered physiological states (Tiefenbacher et al., 2004). Tiefenbacher et al. (2005) presented a “developmental-neurochemical hypothesis” theorizing that adverse early conditions along with later stressful events result in alterations in neuropeptide and neuroendocrine systems that can lead to abnormal behavior such as self-biting. However, with the exception of pathological behaviors that may result in tissue damage (Novak et al., 2012), the extent to which levels of abnormal behaviors are an indicator of wellbeing is unclear (Mason, 1991).
Although abnormal behaviors can be uniquely individualistic, for the purpose of this paper they will be classified into four categories: motor stereotypy, which includes behaviors such as pacing, rocking, flipping, swinging, and head tossing (Camus et al., 2013; Fritz et al., 1992; Gottlieb et al., 2015; Hook et al., 2002; Lutz et al., 2003; Nash et al., 1999; Vandeleest et al., 2011); self-directed behaviors which include hair-pulling, “saluting,” eye-covering, or digit sucking (Fritz et al., 1992; Hook et al., 2002; Jacobson et al., 2016; Lutz et al., 2003; Thierry, 1984); abnormal appetitive behavior which includes regurgitation, hair eating, and coprophagy (Akers and Schildkraut, 1985; Birkett and Newton-Fisher, 2011; Fritz et al., 1992; Gould and Bres, 1986; Hook et al., 2002; Jacobson et al., 2016; Nash et al., 1999; Nevill and Lutz, 2015); and self-injurious behavior which includes behaviors that result in injury or have the potential for injury such as head-banging, self-biting, and self-wounding (Birkett and Newton-Fisher, 2011; Gottlieb et al., 2013; Hosey and Skyner, 2007; Lutz et al., 2003; Rommeck et al., 2009). Various factors play a role in the display of abnormal behavior. These risk factors include a number of environmental conditions such as nursery rearing, single housing, and clinical procedures (Bayne et al., 1992; Bellanca and Crockett, 2002; Crast et al., 2014; Gottlieb et al., 2013, 2015; Lutz et al., 2003; Nash et al., 1999; Rommeck et al., 2009; Vandeleest et al., 2011) as well as variables intrinsic to the animal such as the animal’s species, sex, and age (Crast et al., 2014; Gottlieb et al., 2013, 2015; Lutz et al., 2003; Tarou et al., 2005; Trollope, 1977). This study focuses on intrinsic risk factors.
Surveys of zoo populations have demonstrated that levels of abnormal behavior can vary greatly across species, genera, and/or families of nonhuman primates. For example, in a survey of 108 zoos and 68 species of nonhuman primates, abnormal behavior was reported in 14% of the animals. Apes exhibited the highest percentage (40%), followed by Old World monkeys (14%), prosimians (7%), and New World monkeys (6%) (Bollen and Novak, 2000). In a smaller survey of 35 zoos, self-injurious behavior was reported in 16 species of apes, Old World monkeys, and New World monkeys, but not in prosimians (Hosey and Skyner, 2007). Similarly, an observational study conducted at twenty zoos reported that those in the families Cercopithecidae, Cebidae, Pongidae, and Hylobatidae displayed comparable levels of abnormal behavior (5.7–7.1%), while Callitrichidae, Lemuridae, and Lorisidae displayed no behavioral abnormalities (Trollope, 1977). However, a survey of 48 zoos reported that 13% of prosimians exhibited some form of abnormal behavior (Tarou et al., 2005). Differences in abnormal behavior can also be found across laboratory species. For example, studies of nonhuman primates housed singly in laboratories have reported higher levels of abnormal behavior in macaque monkeys (89–100%; Bayne et al., 1992; Camus et al., 2013; Lutz et al., 2003) and sooty mangabeys (Cercocebus atys) (83%; Crast et al., 2014), than in baboons (14–26%; Kessel and Brent, 2001; Lutz et al., 2014). It should be noted, however, that direct comparisons of abnormal behavior across species housed in different facilities can be confounded by variables such as differences in husbandry practices, housing conditions, as well as differences in observation or data collection methods.
In addition to overall levels, abnormal behavior can also vary in form and frequency across species; certain types of abnormal behavior are more likely to occur in some species than in others. For example, captive gorillas (Gorilla gorilla) are often reported to exhibit coprophagy and regurgitation/reingestion (Akers and Schildkraut, 1985; Gould and Bres, 1986), while coprophagy tends to be the most common abnormal behavior in chimpanzees (Pan troglodytes) (Birkett and Newton-Fisher, 2011; Jacobson et al., 2016; Nash et al., 1999; Walsh et al., 1982). Similarly, hair-pulling and hair-eating is common in baboons (Brent and Hughes, 1997; Mejido et al., 2009; Nevill and Lutz, 2015) and “wiggle digits,” a behavior that is often associated with regurgitation, appears to be limited to the baboon population (Lutz et al., 2014). In contrast, pacing, a motor stereotypy, is a behavior that is commonly performed by many species of captive nonhuman primates (Bellanca and Crockett, 2002; Crast et al., 2014; Lutz et al., 2003, 2014; McGrogan and King, 1982; Pomerantz et al., 2012, 2013; Tarou et al., 2005; Vandeleest et al., 2011). Therefore, a single species or genus may not be an accurate model of abnormal behavior for the primate order as a whole.
The role that sex plays in abnormal behavior can vary both within and between species. For example, two surveys of nonhuman primates housed at zoos assessed the impact of sex on abnormal behavior. In one survey of 630 zoo primates, significantly more males (10.2%) than females (2.7%) exhibited abnormal behavior (Trollope, 1977), while another survey of eight zoo primate species including prosimians, monkeys, and apes reported no sex difference in abnormal stereotyped behavior (Marriner and Drickamer, 1994). In macaque monkeys (e.g., Macaca mulatta, M. cynomolgus, M. nemestrina), when there was a sex difference, males were reported to exhibit more abnormal behavior than were females. This was noted in all categories of abnormal behavior including motor stereotypy, self-directed, and self-injurious behavior (Bayne et al., 1995; Cross and Harlow, 1965; Gottlieb et al., 2013, 2015; Lutz et al., 2003; Novak et al., 2002; Rommeck et al., 2009; Suomi et al., 1971; Thierry, 1984; Vandeleest et al., 2011). However, studies of rhesus macaques (Macaca mulatta) have also reported no sex difference in some (Lutz et al., 2003, 2007) or all (Hook et al., 2002) observed abnormal behaviors. Although less is known about abnormal behavior in baboons, baboon males were also reported to be more likely to exhibit abnormal behaviors such as appetitive behaviors, self-directed behaviors and body movements (Brent and Hughes, 1997; Lutz et al., 2014). However, as with macaques, a sex difference was not reported in all behaviors (Lutz et al., 2014).
Age can also play a role in the levels of abnormal behavior displayed by nonhuman primates. Age differences in behavior may be due to factors such as the animal’s physical abilities and behavioral repertoire (Mason, 1993). For example, a survey of zoos reported that younger animals (i.e., infants and juveniles) had fewer behavioral abnormalities than did older animals (Trollope, 1977). When younger animals did exhibit abnormal behavior, however, the behaviors tended to be more physically active motor stereotypies such as pacing, body flipping, and swinging (Lutz et al., 2003), and the display of these behaviors typically decreased with age (Gottlieb et al., 2013). For example, in rhesus monkeys, the display of motor stereotypic behavior increased until 6 years of age and then declined with increasing age (Gottlieb et al., 2015). Older monkeys were instead more likely to exhibit sedentary abnormal behaviors that do not require a lot of movement such as self-directed behaviors (e.g., eye poke, eye cover, hair-pull), and self-injurious behavior (Lutz et al., 2003; Thierry, 1984). However, not all studies reported an effect of age on abnormal behavior (Birkett and Newton-Fisher, 2011; Hook et al., 2002; Tarou et al., 2005) and age was reported to be negatively correlated with abnormal behavior in group-housed baboons (Brent and Hughes, 1997).
The repertoire of abnormal behavior is often highly individualistic (Paulk et al., 1977; Thierry, 1984). Because of this, displays of abnormal behavior within an individual have been described as a “behavioral fingerprint” for that individual (Bayne and McCully, 1989). However, abnormal behavior also varies across species and has the potential of being utilized as a species-specific “behavioral fingerprint.” Previous studies of species differences in abnormal behavior have often compared animals that were housed at different facilities, under varying conditions, and possibly with different observation methods. In contrast, few studies assessed abnormal behavior across species that were housed in the same facility, under similar conditions, and with the same procedures. The purpose of this study was to utilize a standardized assessment method to survey the presence of abnormal behavior in large populations of captive baboons (Papio hamadryas) and macaques (Macaca mulatta, M. fascicularis) and to identify species differences in abnormal behavior. In addition, the impact of intrinsic risk factors such as sex and age was assessed for comparison. Knowing these species-specific risk factors will help us to better predict vulnerable individuals for directing animal care.
2. Methods
2.1. Subjects
The subjects were 415 (109 females, 306 males) cynomolgus macaques (Macaca fascicularis), between the ages of 1–19 years; 365 (181 females, 184 males) rhesus macaques (Macaca mulatta), between the ages of 1–25 years; and 331 (187 females, 144 males) baboons (Papio hamadryas) consisting mainly of the subspecies olive baboon (Papio hamadryas anubis) and olive/yellow baboon (Papio hamadryas cynocephalus) crosses, along with various other crosses, between the ages of 1–22 years. All of the macaques and 290 of the baboons were mother-reared. Thirty six of the baboons (19 female, 17 male) were nursery-reared and five (4 female, 1 male) had an unknown rearing history. Because nursery rearing can have a differential impact on abnormal behavior (Brent and Hughes, 1997; Conti et al., 2012; Gottlieb et al., 2013; Lutz et al., 2007), the 36 baboons that were nursery-reared and the five with an unknown rearing history were excluded from the data analyses. The baboons, rhesus macaques, and 46 (23 males, 23 females) of the cynomolgus macaques were socially housed in indoor/outdoor enclosures at Southwest National Primate Research Center (SNPRC) prior to their transfer to single housing. The remaining 369 cynomolgus macaques were obtained from an outside supplier. Although their rearing history is known, their housing history after weaning and prior to arrival at SNPRC cannot be confirmed.
At the time of observation, the subjects were singly-housed indoors for either research or clinical reasons at the Southwest National Primate Research Center (SNPRC), San Antonio Texas, USA. They were fed a nutritionally complete diet supplemented with fruits, vegetables, and grains, and were provided with toys, foraging devices, and novel food treats on a rotating schedule. The facility is accredited by AAALAC International and the animals were maintained in accordance with the Guide for the Care and Use of Laboratory Animals (Institute for Laboratory Animal Research, 2011). The research was approved by the Institutional Animal Care and Use Committee and complied with the laws and regulations of the United States Animal Welfare Act.
2.2. Procedures
Behavioral assessments were conducted quarterly from November 2006 through August 2016 on all animals that had been singly-housed for at least 30 days. The animals were alert and mobile at the time of observation. If more than one assessment was conducted on an animal, only the first assessment was used for data analysis. At the time of assessment, the subjects had been singly-housed at SNPRC a minimum of 30 and a maximum of 120 days.
Behavioral assessments consisted of a 5-minute observation using one-zero sampling. The observer stood approximately 1–2 m from the front of the cage, depending on the room configuration, and recorded the presence or absence of abnormal behaviors. Four experienced observers collected the data, and they received annual training on the identification of abnormal behavior. Due to the low number of abnormal behaviors exhibited during observation, the behaviors were grouped into three categories for further analysis: motor stereotypy, self-directed behavior (including self-injurious behavior), and abnormal appetitive behaviors (Table 1).
Table 1.
Ethogram.
| Behavior | Definition |
|---|---|
| Motor Stereotypy | |
| Bizarre posture | Holding a seemingly uncomfortable or contorted position |
| Bounce | Repetitivelya using one’s hind legs or all four limbs to push oneself off the cage surface |
| Flip | Repeateda forward or backwards somersaults, may utilize the cage sides or ceiling |
| Floating limb | An arm or leg rises into the air and may or may not contact the body (e.g., gently stroking the body). The action appears to be non-volitional; the animal may interact with the limb as if it is not part of the body. This behavior may be associated with SIB such as self-biting or self-hitting |
| Head toss | Repetitivelya moving head up and down, side to side, or in a circular manner |
| Pace | Repeated walking in the same pattern (e.g., back and forth) for at least 3 revolutions |
| Rock | Repeateda back and forth or side to side movement of the body |
| Spin | Repetitivea circling of body around a pivot point |
| Stereotypic locomotion | Idiosyncratic repetitivea whole body movements, particular to an individual; does not meet criteria for other behaviors defined above |
| Swing | Repetitivea back-and-forth movement when hanging from the cage side or ceiling |
| Self-directed Behavior | |
| Eye poke | Placement of fingers or toes into, or right next to, the eye for an extended period of time; often appears as if the animal is “saluting” |
| Hair pull | Pulling own hair from body |
| Nipple manipulation | Repeated handling of nipple with hands or mouth |
| Self-biteb | Mouth-to-self contact where teeth contact the skin |
| Self-clasp | Clutching one’s own body with hands or feet |
| Self-oral | Sucking a part of one’s own body |
| Abnormal Appetitive Behavior | |
| Abnormal mouth movements | Repeated movement of mouth, lips, or tongue, not associated with eating or manipulating an object in the mouth. |
| Coprophagy | Ingesting or manipulating feces in the mouth |
| Drink urine | Licking or ingesting urine |
| Feces paint | Smearing and/or rubbing feces on a surface |
| Food smear | Spreading of chewed food on a surface with the mouth; food is often licked off |
| Hair-eat | Chewing or ingestion of hair |
| Regurgitate | The backward flow of already swallowed food |
| Wiggle digits | Repeated movement of fingers or toes usually at, in, or around the mouth, often associated with regurgitation |
Repetitive = a minimum of 3 times.
Self-injurious behavior (i.e., self-bite) was placed in the category of self-directed behavior due to its low occurrence.
2.3. Data analysis
Statistical analyses were conducted using Systat® 13 (Chicago, IL). The level of significance is P < 0.05.
2.3.1. Demographic variables
One-way ANOVAs were conducted to examine species differences in age, and chi-square analyses were conducted to examine species differences in sex ratio. T-tests were then conducted to examine sex differences in age for each species.
2.3.2. Abnormal behavior
Chi-square analyses were conducted to examine species differences in the overall number of individuals exhibiting abnormal behavior. Sign tests were then conducted to compare frequency of behavioral categories within each species.
2.3.3. Contribution of demographic factors to abnormal behavior
For each behavioral category, a logistic regression was conducted to examine the potential effects of the demographic variables on abnormal behavior. For each regression, the variables sex, age, genus (macaque vs. baboon), macaque species (rhesus vs. cynomolgus), and their interactions were first entered into the model and a backwards elimination procedure was used to determine the ‘best fit’ model. Terms with the highest p-values were eliminated first. In case of a significant interaction, the interaction and both main effects were retained in the model.
3. Results
3.1. Demographic variables
Results are presented as mean ± SE. There was a significant species difference in animal age (F(2, 1067) = 138.420, P < 0.001). Post-hoc Tukey tests showed that baboons were significantly older (M = 9.1 ± 0.2 years) than rhesus and cynomolgus macaques (P < 0.001), and that rhesus (M = 6.5 ± 0.3 years) were significantly older than cynomolgus (M = 4.3 ± 0.1 years) macaques (P < 0.001). Chi-square tests showed that there was also a significant species difference in sex ratios (χ2(2) = 75.728, P < 0.001). Baboons had a greater proportion of females (57%) than did macaques (χ2(1) = 32.479, P < 0.001), and rhesus had a greater proportion of females (50%) than did cynomolgus (26%) macaques (χ2(1) = 45.232, P < 0.001). Females were significantly older than males in rhesus macaques (t(363) = 5.347, P < 0.001) and baboons (t(288) = 4.288, P < 0.001), but there was no such age difference in cynomolgus macaques (t(413) = −0.14, P = 0.888).
3.2. Abnormal behavior
There was a significant overall difference by species in the number of animals exhibiting abnormal behavior (χ2(2) = 25.138, P < 0.001). Significantly more macaques exhibited at least one type of abnormal behavior than did baboons (29% vs. 14%; χ2(1) = 24.849, P < 0.001), while there was no difference between rhesus and cynomolgus macaques (30% vs. 28%; χ2(1) = 0.263, P = 0.608).
In baboons, self-directed behavior was exhibited significantly less than motor stereotypy (Z = 4.05, P < 0.001) and abnormal appetitive behavior (Z = 4.59, P < 0.001), but motor stereotypy and appetitive behavior were not significantly different from one another (Z = 0.33, P = 0.74; Fig. 1). In both species of macaques, motor stereotypy was exhibited significantly more than abnormal appetitive behavior (cynomolgus: Z = 9.03, P < 0.001; rhesus: Z = 7.80, P < 0.001) and self-directed behavior (cynomolgus: Z = 8.14, P < 0.001; rhesus: Z = 6.84, p < 0.001), but self-directed and abnormal appetitive behavior were not significantly different from one another (cynomolgus: Z = 1.92, P = 0.052; rhesus: Z = 1.54, P = 0.124; Fig. 1).
Fig. 1.
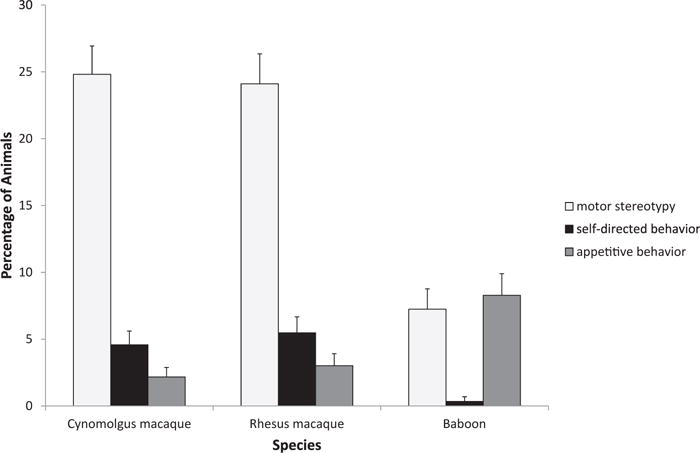
Species differences in the display of abnormal behavior.
3.3. Contribution of demographic factors to abnormal behavior
Age, sex, genus, and macaque species all contributed to the expression of abnormal behavior (Table 2). Those that exhibited motor stereotypies were more likely to be male and macaque. There were also age × genus and age × macaque species interactions. Macaques exhibiting motor stereotypies were younger, and baboons older, than those that did not exhibit these behaviors (Fig. 2). Although there was no age effect in rhesus macaques, cynomolgus macaques that exhibited motor stereotypies were younger than those that did not (Fig. 3). Those that exhibited self-directed behavior were more likely to be macaque and older than those that did not. Those that exhibited appetitive behavior were more likely to be female, but there was also a sex × genus interaction. In macaques, males exhibited more appetitive behavior than did females, but in baboons, females exhibited more appetitive behavior than did males (Fig. 4).
Table 2.
Beta values and associated significance levels for terms included in best fit models.a
| Variables | Behavior Category
|
||
|---|---|---|---|
| Motor Stereotypy | Self-Directed | Appetitive | |
| Sex |
b = 0.425, p < 0.05 |
b = 1.703, p < 0.05 |
|
| Age |
b = −0.077, p = 0.062 |
b = 0.065, p < 0.05 |
|
| Genus |
b = 2.527, p < 0.001 |
b = 2.968, p < 0.005 |
b = −0.433, p = 0.343 |
| Macaque Species |
b = 0.313, p = 0.108 |
||
| Age × Genus |
b = −0.167, p < 0.05 |
||
| Age × Macaque Species |
b = −0.082, p < 0.05 |
||
| Sex × Genus |
b = −2.379, p < 0.01 |
||
Blank = not in best-fit model.
Fig. 2.
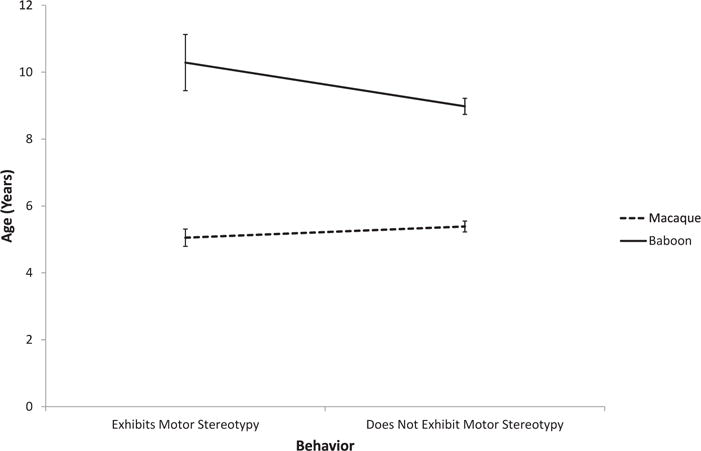
Age × genus interaction in the display of motor stereotypies.
Fig. 3.
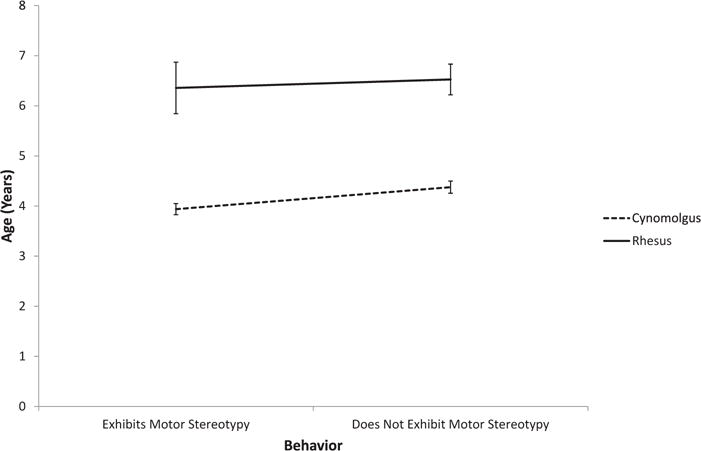
Age × macaque species interaction in the display of motor stereotypies.
Fig. 4.
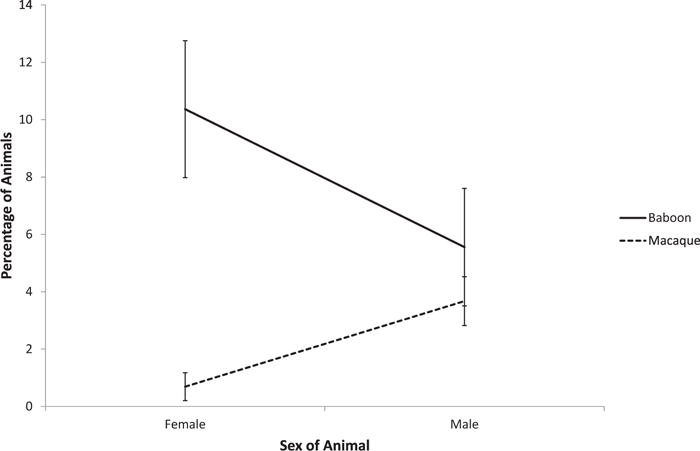
Sex × genus interaction in the display of abnormal appetitive behavior.
4. Discussion
The aim of this study was to further explore species differences in abnormal behavior. The overall objectives were to identify whether there are differences in amount and pattern of abnormal behavior displayed across species and whether these behaviors are differentially impacted by sex and age. Because abnormal behavior may be an indicator of the welfare of nonhuman primates, it is important to understand differences in these behaviors across species. In the present study, macaques exhibited a higher overall level of abnormal behavior in comparison to baboons, but there was no difference when the two macaque species were compared to one another. This same pattern of similarity within a genus but differences across genera also occurred when individual behavioral categories were assessed separately. For example, macaques exhibited higher levels of motor stereotypy and self-directed behaviors than did baboons. In addition, motor stereotypies were the most common behaviors in both species of macaques, but motor stereotypy and abnormal appetitive behavior were equally common in baboons. These results support the theory that, as with species-specific normal behavior, there are also species- or genus- specific patterns of abnormal behavior. Although the term “behavioral fingerprint” (Bayne and McCully, 1989) was initially coined to describe the behavioral patterns of individual animals, patterns of abnormal behavior can also be described as a “behavioral fingerprint” for a species or genus. Previous studies of zoo populations have typically reported species differences in overall levels of abnormal behavior, but not differences in behavioral patterns. In addition, some data were collected via surveys, with no means to determine whether the observations are comparable, and the animals were likely housed in varying conditions across facilities. In the present study, the same data collection method was used for each species, and the animals were housed in similar conditions at the time of observation, allowing for direct comparisons to be made.
In addition to species differences, there were also differences in the influence of sex on abnormal behavior. For example, males exhibited higher levels of motor stereotypies than did females. This male-biased sex difference in motor stereotypy is consistent with previous findings that males typically exhibit more abnormal behavior than do females (Bayne et al., 1995; Brent and Hughes, 1997; Cross and Harlow, 1965; Gottlieb et al., 2013, 2015; Lutz et al., 2003, 2014; Novak et al., 2002; Rommeck et al., 2009; Suomi et al., 1971; Thierry, 1984; Vandeleest et al., 2011). However, for appetitive behavior, more females exhibited this behavior and there was also a sex × genus interaction. As with previous studies, macaque males exhibited higher levels than did macaque females. However, baboon females were more likely to exhibit abnormal appetitive behavior than were baboon males. This result further demonstrates differences between macaques and baboons, but it also contradicts a previous study showing that male baboons exhibited more abnormal appetitive behavior than did females (Lutz et al., 2014). Although the results for baboons in the present study differ from those of the previous study, they add to our overall understanding of abnormal behavior in the baboon population. However, the reason for any sex difference in abnormal behavior remains unclear.
As with sex differences, there were also differences in the impact of age on abnormal behavior, but the direction of the effect depended on the behavior. For example, the levels of self-directed behavior increased with age. This result reflects previous findings that suggest there is a tendency for increased levels of more sedentary and/or self-directed abnormal behaviors (such as eye-poking, hair-pulling, and self-injury) with age (Lutz et al., 2003; Thierry, 1984). Although there was not an overall effect of age on motor stereotypy, there was a genus by age interaction. In macaques, levels of motor stereotypy decreased with age. This result is similar to studies reporting reduced levels of active abnormal behaviors (such as pacing, body flipping, and swinging) in older animals (Gottlieb et al., 2013, 2015; Lutz et al., 2003). In contrast, in the baboon population, there was an increase in motor stereotypy with age, further demonstrating differences in abnormal behavior across genera.
Abnormal behavior is a persistent occurrence in captive animals, whether they are housed in laboratories or zoos. Because abnormal behavior may be associated with an animal’s welfare, it needs to be addressed. In the present study, the observations were relatively brief and the animals were singly-housed, which can limit comparisons to other studies which may have socially-housed populations or more comprehensive methods for estimating the prevalence of abnormal behavior. However, the housing conditions and data collection methods were consistent within the study, allowing for direct comparisons across species. This study demonstrates that abnormal behavior may be impacted by intrinsic variables such as the animal’s species, sex, and age. Intrinsic variables therefore need to be taken into account when addressing behavioral needs of the animal. These results also demonstrate that although abnormal behavior can be individualistic, there are patterns of abnormal behavior that can be associated with a species or genus. However, not all types of abnormal behavior are necessarily equal in terms of welfare concerns, and behavior that is more concerning in one species may be less concerning in another. Therefore, a single species cannot be used as a model for all, and further associations between behavior and welfare need to be assessed. A better understanding of species-specific differences in abnormal behavior is necessary to better tailor animal care to fit the animal’s needs.
5. Conclusions
Differences in abnormal behavior can occur across species of captive nonhuman primates. For example, singly-housed macaque monkeys exhibited higher levels of abnormal behavior than did similarly-housed baboons. In addition, rhesus and cynomolgus macaques exhibited similar types of abnormal behavior, which differed significantly from those exhibited by baboons. These results suggest that there may be species-specific patterns of abnormal behavior. Therefore, differences across species or genera need to be taken into account when utilizing abnormal behavior as a measure of wellbeing in captive nonhuman primates.
Acknowledgments
I would like to thank Heath Nevill, Kim Linsenbardt, and Brittany Peterson for their assistance with data collection. I would also like to thank Kris Coleman and Rose Kroeker for their helpful comments and suggestions.
Funding
This study was supported by National Institutes of Health (grant number 2P51OD011133) to Texas Biomedical Research Institute.
References
- Akers JS, Schildkraut DS. Regurgitation/reingestion and coprophagy in captive gorillas. Zoo Biol. 1985;4:99–109. [Google Scholar]
- Bayne KAL, McCully C. The effect of cage size on the behavior of individually housed rhesus monkeys. Lab Anim. 1989;18:25–28. [Google Scholar]
- Bayne K, Dexter S, Suomi S. A preliminary survey of the incidence of abnormal behavior in rhesus monkeys (Macaca mulatta) relative to housing condition. Lab Anim. 1992;21:38–46. [Google Scholar]
- Bayne K, Haines M, Dexter S, Woodman D, Evans C. Nonhuman primate wounding prevalence: a retrospective analysis. Lab Anim. 1995;24:40–44. [Google Scholar]
- Bayne K. Normal and abnormal behaviors of laboratory animals: what do they mean? Lab Anim. 1996;25:21–26. [Google Scholar]
- Bellanca RU, Crockett CM. Factors predicting increased incidence of abnormal behavior in male pigtailed macaques. Am J Primatol. 2002;58:57–69. doi: 10.1002/ajp.10052. [DOI] [PubMed] [Google Scholar]
- Berkson G, Goodrich J, Kraft I. Abnormal stereotyped movements of marmosets. Percept Mot Skills. 1966;23:491–498. [Google Scholar]
- Birkett LP, Newton-Fisher NE. How abnormal is the behavior of captive, zoo-living chimpanzees? PLoS One. 2011;6:e20101. doi: 10.1371/journal.pone.0020101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollen KS, Novak MA. A survey of abnormal behavior in captive zoo primates. Am J Primatol. 2000;51(S1):47. [Google Scholar]
- Brent L, Hughes A. The occurrence of abnormal behavior in group-housed baboons. Am J Primatol. 1997;42:96–97. [Google Scholar]
- Camus SMJ, Blois-Heulin C, Li Q, Hausberger M, Bezard E. Behavioural profiles in captive-bred cynomolgus macaques: towards monkey models of mental disorders? PLoS One. 2013;4:e62141. doi: 10.1371/journal.pone.0062141. http://dx.doi.org/10.1371/journal.pone.0062141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti G, Hansman C, Heckman JJ, Novak MFX, Ruggiero A, Suomi SJ. Primate evidence on the late health effects of early-life adversity. PNAS. 2012;109:8866–8871. doi: 10.1073/pnas.1205340109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crast J, Bloomsmith MA, Perlman JE, Meeker TL, Remillard CM. Abnormal behaviour in captive sooty mangabeys. Anim Welf. 2014;23:167–177. [Google Scholar]
- Cross HA, Harlow HF. Prolonged and progressive effects of partial isolation on the behavior of macaque monkeys. J Exp Res Pers. 1965;1:39–49. [Google Scholar]
- Erwin J, Deni R. Strangers in a strange land: abnormal behaviors or abnormal environments? In: Erwin J, Maple TL, Mitchell G, editors. Captivity and Behavior, Primates in Breeding Colonies, Laboratories, and Zoos. Van Nostrand Reinhold; New York, NY: 1979. pp. 1–28. [Google Scholar]
- Fritz J, Nash LT, Alford PL, Bowen JA. Abnormal behaviors, with a special focus on rocking, and reproductive competence in a large sample of captive chimpanzees (Pan troglodytes) Am J Primatol. 1992;27:161–176. doi: 10.1002/ajp.1350270302. [DOI] [PubMed] [Google Scholar]
- Gottlieb DH, Capitanio JP, McCowan B. Risk factors for stereotypic behavior and self-biting in rhesus macaques (Macaca mulatta): animal’s history current environment, and personality. Am J Primatol. 2013;75:995–1008. doi: 10.1002/ajp.22161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb DH, Maier A, Coleman K. Evaluation of environmental and intrinsic factors that contribute to stereotypic behavior in captive rhesus macaques (Macaca mulatta) Appl Anim Behav Sci. 2015;171:184–191. doi: 10.1016/j.applanim.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Bres M. Regurgitation and reingestion in captive gorillas: description and intervention. Zoo Biol. 1986;5:241–250. [Google Scholar]
- Hook MA, Lambeth SP, Perlman JE, Stavisky R, Bloomsmith MA, Schapiro SJ. Inter-group variation in abnormal behavior in chimpanzees (Pan troglodytes) and rhesus macaques (Macaca mulatta) Appl Anim Behav Sci. 2002;76:165–176. [Google Scholar]
- Hosey GR, Skyner LJ. Self-injurious behavior in zoo primates. Int J Primatol. 2007;28:1431–1437. [Google Scholar]
- Institute for Laboratory Animal Research. Guide for the Care and Use of Laboratory Animals. 8th. National Academies Press; Washington (DC): 2011. [Google Scholar]
- Jacobson SL, Ross SR, Bloomsmith MA. Characterizing abnormal behavior in a large population of zoo-housed chimpanzees: prevalence and potential influencing factors. PeerJ. 2016;4:e2225. doi: 10.7717/peerj.2225. http://dx.doi.org/10.7717/peerj.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessel A, Brent L. The rehabilitation of captive baboons. J Med Primatol. 2001;30:71–80. doi: 10.1034/j.1600-0684.2001.300201.x. [DOI] [PubMed] [Google Scholar]
- Lutz C, Well A, Novak M. Stereotypic and self-injurious behavior in rhesus macaques: a survey and retrospective analysis of environment and early experiences. Am J Primatol. 2003;60:1–15. doi: 10.1002/ajp.10075. [DOI] [PubMed] [Google Scholar]
- Lutz CK, Davis EB, Ruggiero AM, Suomi SJ. Early predictors of self-biting in socially-housed rhesus macaques (Macaca mulatta) Am J Primatol. 2007;69:584–590. doi: 10.1002/ajp.20370. [DOI] [PubMed] [Google Scholar]
- Lutz CK, Williams PC, Sharp RM. Abnormal behavior and associated risk factors in captive baboons (Papio hamadryas Spp) Am J Primatol. 2014;76:355–361. doi: 10.1002/ajp.22239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriner LM, Drickamer LC. Factors influencing stereotyped behavior of primates in a zoo. Zoo Biol. 1994;13:267–275. [Google Scholar]
- Mason GJ. Stereotypies: a critical review. Anim Behav. 1991;41:1015–1037. [Google Scholar]
- Mason GJ. Forms of stereotypic behaviour. In: Lawrence AB, Rushen J, editors. Stereotypic Animal Behaviour: Fundamentals and Applications to Welfare. Cab International; Wallingford, UK: 1993. pp. 7–40. [Google Scholar]
- McGrogan HJ, King JE. Repeated separations of 2-year-old squirrel monkeys from familiar mother surrogates. Am J Primatol. 1982;3:285–290. doi: 10.1002/ajp.1350030126. [DOI] [PubMed] [Google Scholar]
- Mejido DCP, Dick EJ, Jr, Williams PC, Sharp RM, Andrade MCR, DiCarlo CD, Hubbard GB. Trichobezoars in baboons. J Med Primatol. 2009;38:302–309. doi: 10.1111/j.0047-2565.2009.00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash LT, Fritz J, Alford PA, Brent L. Variables influencing the origins of diverse abnormal behaviors in a large sample of captive chimpanzees (Pan troglodytes) Am J Primatol. 1999;48:15–29. doi: 10.1002/(SICI)1098-2345(1999)48:1<15::AID-AJP2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Nevill CH, Lutz CK. The effect of a feeding schedule change and the provision of forage material on hair eating in a group of captive baboons (Papio hamadryas sp) J Appl Anim Welf Sci. 2015;18:319–331. doi: 10.1080/10888705.2014.980888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak MA, Crockett CM, Sackett GP. Self-injurious behavior in captive macaque monkeys. In: Schroeder SR, Oster-Granite ML, Thompson T, editors. Self-Injurious Behavior Gene-Brain-Behavior Relationships. American Psychological Association; Washington, DC: 2002. pp. 151–161. [Google Scholar]
- Novak MA, Kelly BJ, Bayne K, Meyer JS. Behavioral disorders of nonhuman primates. In: Abee C, Mansfield K, Tardif S, Morris T, editors. Nonhuman Primates in Biomedical Research, Biology and Management. Vol. 1. Elsevier; Waltham, MA: 2012. pp. 177–196. [Google Scholar]
- Paulk HH, Dienske H, Ribbens LG. Abnormal behavior in relation to cage size in rhesus monkeys. J Abnorm Psychol. 1977;86:87–92. doi: 10.1037//0021-843x.86.1.87. [DOI] [PubMed] [Google Scholar]
- Pomerantz O, Paukner A, Terkel J. Some stereotypic behaviors in rhesus macaques (Macaca mulatta) are correlated with both perseveration and the ability to cope with acute stressors. Behav Brain Res. 2012;230:274–280. doi: 10.1016/j.bbr.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantz O, Meiri S, Terkel J. Socio-ecological factors correlate with levels of stereotypic behavior in zoo-housed primates. Behav Processes. 2013;98:85–91. doi: 10.1016/j.beproc.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Rommeck I, Anderson K, Heagerty A, Cameron A, McCowan B. Risk factors and remediation of self-injurious and self-abuse behavior in rhesus macaques. J Appl Anim Welf Sci. 2009;12:61–72. doi: 10.1080/10888700802536798. [DOI] [PubMed] [Google Scholar]
- Suomi SJ, Harlow HF, Kimball SD. Behavioral effects of prolonged partial social isolation in the rhesus monkey. Psychol Rep. 1971;29:1171–1177. doi: 10.2466/pr0.1971.29.3f.1171. [DOI] [PubMed] [Google Scholar]
- Tarou LR, Bloomsmith MA, Maple TL. Survey of stereotypic behavior in prosimians. Am J Primatol. 2005;65:181–196. doi: 10.1002/ajp.20107. [DOI] [PubMed] [Google Scholar]
- Thierry B. Descriptive and contextual analysis of eye covering behavior in captive rhesus macaques (Macaca mulatta) Primates. 1984;25:62–77. [Google Scholar]
- Tiefenbacher S, Novak MA, Marinus LM, Chase WK, Miller JA, Meyer JS. Altered hypothalamic-pituitary-adrenocortical function in rhesus monkeys (Macaca mulatta) with self-injurious behavior. Psychoneuroendocrinology. 2004;29:501–515. doi: 10.1016/s0306-4530(03)00068-4. [DOI] [PubMed] [Google Scholar]
- Tiefenbacher S, Novak MA, Lutz CK, Meyer JS. The physiology and neurochemistry of self-injurious behavior: a nonhuman primate model. Front Biosci. 2005;10:1–11. doi: 10.2741/1500. [DOI] [PubMed] [Google Scholar]
- Trollope J. A preliminary survey of behavioural stereotypes in captive primates. Lab Anim. 1977;11:195–196. doi: 10.1258/002367777780936666. [DOI] [PubMed] [Google Scholar]
- Vandeleest JJ, McCowan B, Capitanio JP. Early rearing interacts with temperament and housing to influence the risk for motor stereotypy in rhesus monkeys (Macaca mulatta) Appl Anim Behav Sci. 2011;132:81–89. doi: 10.1016/j.applanim.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh S, Bramblett CA, Alford PL. A vocabulary of abnormal behaviors in restrictively reared chimpanzees. Am J Primatol. 1982;3:315–319. doi: 10.1002/ajp.1350030131. [DOI] [PubMed] [Google Scholar]
- Watson SL, McCoy JG, Fontenot MB, Hanbury DB, Ward CP. L-tryptophan and correlates of self-injurious behavior in small-eared bushbabies (Otolemur garnettii) J Am Assoc Lab Anim Sci. 2009;48:185–191. [PMC free article] [PubMed] [Google Scholar]


