Abstract
Acne is a multifactorial and inflammatory disease of pilosebaceous follicles, which affects most adolescents. Recent epidemiological data revealed a difference in adults affected by this disease. Women have a high prevalence and incidence when compared with men, especially after 25 years of age. In contrast to what was initially thought, most of these patients do not present endocrinopathy capable of leading to the development of the lesions. When present, polycystic ovarian syndrome is the main cause. However, in these cases, acne is rarely the only dermatological manifestation; hirsutism and acanthosis nigricans are often present. The majority of the normoandrogenic acne patients present a history since adolescence, but in many cases the lesion distribution and intensity change with time. There is often a typical localization of the lesions in the lower third of the face and lateral region of the neck. Another interesting feature is related to the impact on quality of life (QoL), which is always intense. Often there are signs of depression, even when the lesions are mild. As most adult patients are women, in addition to the conventional options, there is also hormone treatment. Combined oral contraceptives and spironolactone are good options. Knowing more about the particularities in etiopathogenesis, impact on QoL, and specific treatment options is important to all dermatologists who face the challenge of treating acne in adults.
Keywords: adult, acne, hormonal, female
Introduction
Acne is one of the most prevalent inflammatory skin diseases. Epidemiological data have disclosed that, on a global scale, it is the eighth most frequent disease, with 9.4% predominance, including both adults and adolescents of several ethnic groups.1 Acne vulgaris, or acne of the adolescent, has a peak incidence in 14- and 17-year-old girls and in 16- and 19-year-old boys.2
Recent research has shown an increase in total adult female acne (AFA) cases. Although with no absolute consensus, the surveys analyzed 26- to 44-year-old women.3–5 Perkins et al published North American data showing that 12%–22% women suffer from acne in their adult life.6 Another study performed by French researchers concluded that acne persists in 41% of adult women; half of them reported dyschromias and presence of scars, 78% described worsening of lesions during their premenstrual period, and above 90% reported manipulating the lesions. The authors pointed out that only 22% of those patients sought medical assistance.7 Upon analyzing statistical data on dermatological consultations to treat acne, Yentzer et al observed that adult patients, mainly women, corresponded to 61.9% of the total attendances.8 A community-based survey of >700 people, older than 25 years, also reported clinical facial acne, in 12% of women and 3% of men.9 In the study published by Goulden et al, besides the increased predominance in adult women, the presence of a clinical picture with more severe lesions was verified compared to that affecting men in the same age group.4
It is not yet clear if there is a real increase in incidence in that age group or if access to information on the disease and/or treatment causes that group to look for specialized medical care.10 In the majority of cases, acne is persistent, meaning that lesions start in adolescence and persist in adult life. Goulden et al, upon the assessment of 200 adult women with acne, reported that only 18.4% presented a clinical picture of late onset, that is, at over 25 years of age. In the same study, a familial history of AFA was detected in 50% of patients.9 Dumont-Wallon and Dréno found similar data in relation to the family history; that is, 53% patients had first-degree relatives with AFA history. They also reported that 20%–40% of cases had late onset.11
For some researchers, there was still a third subtype related to the moment of the onset of the lesions. In such cases, the patient presented acne in adolescence, followed by an interval without the disease, and then a return in adult life of lesions with some different morphological features, mainly related to the distribution along the face.12
In a study by Golden et al to determine the presence of hormonal alterations in acne patients, evidence of biochemical hyperandrogenism was found in only 37%.9 Ozdemir et al’s analysis of the presence of endocrinological diseases in patients with isolated acne showed that over 50% did not present clinical or biochemical evidence of hyperandrogenism.13 Since there is no consensus about the inclusion criteria in studies on AFA, mainly with respect to whether the presence of acne is isolated or associated with other androgenic alterations, such as hirsutism, it remains controversial which hormonal alterations are the most frequent and which could be used as markers for the disease.14 However, there seems to be some agreement in that, for the group of patients presenting with late-onset acne (at over 25 years of age), it would be more important to investigate the presence of endocrinological diseases. Thus, such a division between persistent and late-onset acne would act as a factor for the dermatologist to ascertain whether it is necessary to request complementary examinations.
Etiopathogenesis
The etiopathogenesis of acne is multifactorial, immune-mediated, and androgen-triggered. These hormones increase the production of sebum and stimulate follicular hyperkeratinization resulting in microcomedo.15 The inner glandular layer starts presenting an increase in colonization by Propionibacterium acnes, a Gram-positive bacteria responsible for the onset of the inflammatory process.16–18
New data have disclosed that the development of lesions is related to the activation of the innate immunity, and that this process can occur even before follicular hyperkeratinization.18–26 Histopathological and immunohistochemical investigations confirm that a lymphocytic infiltrate composed of memory and effector T-cells is the first finding at the beginning of the disease.27,28 Jeremy et al showed the existence of a center-follicular inflammatory process before the development of the microcomedo.20
P. acnes is connected to toll-like receptors (TLRs) present in the keratinocytes, sebocytes, and dendritic cells, triggering signalizing cascades that activate transcriptional factors such as nuclear factor kappa B and phosphokinases such as mitogen activators.29,30 Such activation in the monocytes results in the release of two interleukins (ILs), IL-12 and IL-8.19 IL-12 is the major inflammatory cytokine responsible for the immune response against the invasion of Gram-positive bacteria.31
Another function of the TLRs in acne vulgaris could be the promotion of antimicrobial peptide release. There is a higher production of the antimicrobial peptide β-defensin 2 by epithelial cells in response to the stimulation with TLR2 and TLR4 antagonists. Such peptides can also activate the cells. Once there is an increased regulation or upregulation, of the β-defensins 1 and 2 in acne vulgaris lesions compared to the controls, their involvement in the pathogenesis of the disease was suggested.21 Rocha et al’s study of AFA patients showed that the TLR2 expression in sebocytes was increased both in the perilesional area and in the lesions, with superiority of the latter. In the same study, the author compared the expression of that receptor in skin biopsies from women without acne, revealing a low expression when compared with the perilesional area of acne patients.32
IL-8, present in the follicular and surrounding regions of the acne lesions, is essential for the recruitment of neutrophils. These defense cells release lysosomal enzymes that result in the rupture of the follicular epithelium, worsening in the inflammatory process.31
P. acnes is also able to release several exogenous lipases and proteases that connect to the protease-activated receptor 2 (PAR-2) present in the keratinocytes. The activation of the receptor increases the transition of several inflammatory cytokines, including IL-1α, IL-8, and tumor necrosis factor-α. Matrix metalloproteinases and LL-37 are also produced, a cathelicidin that is the only antimicrobial peptide member known in humans. Therefore, at least through the TLR and PAR-2, P. acnes is able to trigger the inflammatory process.19,33
Studying cells obtained from inflammatory lesions in eight patients with acne, Tenaud et al verified an increase in the TLR2 expression when compared with the controls. By performing only an in vitro analysis, the researchers also proved that adapalene in two different concentrations, 10−7 and 10−6 M, succeeded in reducing the TLR2 expression in cultivated keratinocytes. As this topical retinoid modulated the innate immune system in the studied cells, the authors concluded that this is one of the possible mechanisms that would explain its anti-inflammatory action.34 By treating two groups of patients with AFA for 6 months (one group with a combined oral contraceptive [COC] and the other group with 15% azelaic acid gel), Rocha et al demonstrated through immunohistochemistry, a reduction in the TLR2 receptor expression in sebocytes parallel to the reduction of clinical lesions.32
Although several studies have confirmed that the skin of patients with acne has a higher P. acnes density, no relationship to the inflammation severity has been verified.35,36
From a hormonal point of view, androgens are necessary for sebaceous production, and they play a key role in acne development.37 Women with severe acne present higher levels of dehydroepiandrosterone sulfate (DHEAS) than those with mild to moderate acne. Such alteration is specific; it is not found with regard to other androgens, such as testosterone (T).38,39 Earlier studies with a reduced number of participants found increased levels of free testosterone (FT) and dihydrotestosterone (DHT) in adult women with acne.40,41
It has been proved that there is an alteration of the sebum components in acne patients when compared with healthy individuals. Of all components changed, linoleic acid (LA), which is an essential fatty acid, is the most important because it protects the follicular epithelial wall. With the reduction of LA, the epithelium is harmed by the free fatty acids resulting from the hydrolysis of triglycerides through lipases released by P. acnes. This mechanism results in infundibular hyperkeratinization and dermal inflammation.26,42,43
There is no acne development without the sebocytes’ proliferation and differentiation. Such processes are likely related to the correlation between androgens and peroxisome proliferator-activated receptors (PPARs), molecules that regulate lipogenesis along adipocyte differentiation. PPAR activation and hyperexpression can be required for lipogenesis that characterizes the final stage of sebocyte differentiation.44–46
Studies analyzing sebocyte cultures have shown that such cells are able to trigger an inflammatory process through an inherent mechanism by increasing the IL-1α expression.47 IL-1α induces follicular hyperkeratinization, and it is present in high concentrations in the comedo.48
Hence, both IL-1α production by sebocytes and the production of free fatty acids with the ability to irritate the follicular epithelium can contribute as inherent factors to the evolution of acne inflammatory lesions.16
Recent advances in the knowledge of mechanisms involved in triggering the acne inflammatory cascade have also proved the presence of a higher IL-17 expression in mononuclear cells present in the surrounding follicular infiltrate of the comedo, which seems to be induced by the presence of P. acnes. This fact leads to the conclusion that cellular response is not mediated only by T helper 1 lymphocytes but also by T helper 17 lymphocytes.49
In adult women, a positive correlation was observed between DHT, DHEAS levels, and the inflammatory lesions severity, with blood levels of the insulin-like growth factor 1 (IGF-1).40,50 Hyperinsulinemia influences both the IGF-1 concentration in the blood and the insulin-like growth factor-binding protein 3 (IGFBP-3) that acts directly in the keratinocytes’ proliferation and apoptosis. In a hyperinsulinemic state, the IGF-1 level rises and the IGFBP-3 decreases, thus causing a disbalance in keratinocyte hyperproliferation.51 IGF-1 seems to mediate comedogenic factors such as androgens, growth hormone, and glucocorticoids. A study in humans showed that the endogenous androgen increases IGF-1 serum levels and that IGF-1 increases androgen levels. Thus, a vicious circle is set, resulting in continuous sebum production.52,53
External hormonal factors are mainly connected to the use of pro-androgen progestins present in specific oral, injectable, or intrauterine device contraceptives, which may trigger or aggravate acne.4
The use of cosmetics remains controversial. Several studies mention them as worsening factors,7,11,54 while others showed that the withdrawal of cosmetics did not contribute to the improvement of acne.10 Such differences can be explained by the existing diversity of cosmetics, mainly in relation to the vehicles and number of products. In a literature review about cosmetics and acne, Dall’oglio et al conclude that whenever well-indicated and of good quality, cosmetics help to improve the outcome of acne treatment; the researchers recommend the use of makeup to conceal the lesions, which results in higher adherence to the treatment.55
For Goulden et al, the stress reported by 71% patients is a worsening factor for acne.9 Poli et al report that ~50% of women mentioned it as one of the worsening factors.7 The connection between stress and the worsening of acne is now understood through the higher production of neuromodulators, such as the substance P, which connects to the receptors also present in the sebaceous gland stimulating sebum production.10
In some recent studies, smoking habit was considered a factor associated with AFA persistence, which means that women who smoke present a higher frequency of the disease, resistance to treatment, and a higher amount of comedo.10,56,57
Hormones and peripheral metabolism
From the endocrinological point of view, a small percentage of women with AFA have hyperandrogenism. In those cases, the presence of other clinical alterations associated with acne is common, such as hirsutism, alopecia, change of voice timbre, irregularity of the menstrual cycle, and infertility. For those patients, the majority of authors suggest FT and TT, DHEAS, luteinizing hormone, follicular-stimulating hormone serum dosages, and transvaginal ultrasound of the ovaries. Such dosages must always be administered during the follicular phase, preferably between the first and fifth day of the menstrual cycle, and collection must be performed in the morning, between 8:00 and 10:00 am. This way, the hormonal variations of the menstrual cycle interfere less with the analysis of the hormones.58,59 After such investigations, polycystic ovary syndrome (PCOS) is the more frequently associated cause diagnosed.60,61 Other rare endocrinological causes are virilizing tumors and congenital adrenal hyperplasia.
Whenever there is a suspicion of PCOS, the following criteria must be checked:62 biochemical and clinical hyperandrogenism abnormalities, the presence of ultrasound alterations, and amenorrhea or oligomenorrhea. According to the consensus revised in 2004, the presence of two of the three criteria confirm the diagnosis.62 It is important to point out that the most frequently reported dermatological manifestation in PCOS is hirsutism, present in 65%–73% of patients.63
The majority of patients with isolated AFA show no other signs of clinical hyperandrogenism and are therefore considered normoandrogenic.38,64 In contrast, some researchers found only a few high DHEAS levels, even in patients with acne nonrelated to hirsutism.39,65 Thiboutot et al analyzing a small sample (eight adult patients) detected an increase in the levels of DHEAS, androstenedione (A), FT, and DHT when compared with the control group. In this study, there was no description of the presence or absence of other clinical manifestations of hyperandrogenism.41
Although DHT can be considered a primary marker for the peripheral production of androgens, it is metabolized rapidly and has a high affinity for the sex hormone binding globulin.66 Conversely, when quantifying its distal metabolite, 3α, 17β-androstanediol glucuronide (3α-diol), a better correlation is attained with the androgens’ peripheral activity, mainly when related to the idiopathic cases of hirsutism.67 Cappel et al analyzing the dosage of hormones related to the severity of acne verified an increase in the amount of DHT in women with persistent acne and a higher number of lesions.50
It remains unclear in the literature which are the possible abnormalities of the sebaceous gland’s functioning that would justify both the onset and the chronicity of the acne lesions. It is speculated that the androgens circulating at normal levels would act in an exaggerated way through a higher peripheral conversion and/or higher affinity for the receptors. One of the possibilities related to the peripheral conversion would be related to a higher activity of the type 1 steroid 5α-reductase responsible for the conversion of T into DHT.41
Androgenic production originates from the gonads, adrenal glands, and peripheral tissues, including the skin. The main blood sources of androgens in women are A, DHEAS, and T. DHEAS is produced by the adrenal glands, A by adrenals and ovaries, and T by the ovaries and peripheral tissues such as fat, muscle, and skin.59
As to the skin, especially the pilosebaceous unit can synthesize androgens directly from cholesterol68,69 or by the conversion of weak blood androgens into powerful androgens (Figure 1). Similar to other steroid hormone producing organs, there are six enzymes responsible for this metabolism: steroid sulfatase, 3β-hydroxysteroid dehydrogenase (3β-HSD), 17β-HSD, 5α-reductase, 3α-HSD, and aromatase. Type 1 5α-reductase is mainly found in the sebaceous glands, while type 2 isoenzyme is located in the hair follicle.70 The conversion of weak androgens into powerful androgens begins with DHEA generating T and DHT. In the sebocytes particularly, steroid sulfatase, 3β-HSD1, 17β-HSD3, and type 1 5α-reductase are the main enzymes responsible for the production of the potent androgens, while 17β-HSD2, 3α-HSD, and aromatase seem to locally deactivate excessive androgen production, thus assuring homeostasis of that peripheral activity.71 Previous studies have proved that the levels of type 1 5α-reductase increased in the sebaceous glands in some regions prone to acne development.72,73 This way, the androgenic conversion in the skin is activated, and the androgens produced act directly on the intracellular receptors, thus performing an intracrine function.74
Figure 1.
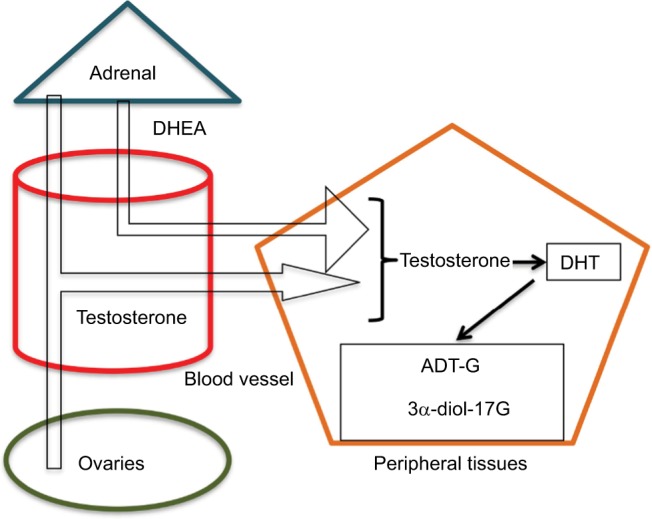
Pilosebaceous unit synthesis of androgens directly from cholesterol or by the conversion of weak blood androgens into powerful androgens.
Abbreviations: DHEA, dehydroepiandrosterone; DHT, dihydrotestosterone.
During the final phase of the metabolization process, the enzyme 3α-hydroxydesidrogenase converts DHT into 3α-androstenediol that suffers glucuronidation. Such modification decreases DHT’s affinity for the receptors, increases its hydrophilic property, and facilitates renal excretion.75 This metabolite can be used as a hyperandrogenism marker, mainly in women with idiopathic hirsutism.70 For cases of isolated acne, this marker did not show any effectiveness. On the other hand, earlier studies showed that ADT-G, another important androgenic metabolite, despite being assessed by techniques presently deemed less accurate, such as radioimmunoassay, presented a significant increase in patients with acne.64,76 ADT-G corresponds to around 93% of the total androgen metabolites, and its serum dosage has already been used to quantify the total androgenic activity.73
Ideally, measurement of the androgenic activity in peripheral tissues should be performed by quantifying the tissue concentration of the active androgens. However, this is usually impossible, except in sampling attained from specific types of cancer after surgical removal, as shown in the studies from the 1980s.77–79 Nevertheless, as it is not possible to access the androgenic activity in specific tissues, the use of more sensitive and specific laboratory techniques, such as liquid chromatography associated with mass spectrometry to dose a glucuronized derivate such as ADT-G, enables the assessment of the total androgenic activity in the body at a high accuracy level. This background is reinforced by the mandatory way of androgenic elimination through its inactivation and later glucuronidation.80–84 Such an option seems to be the best way to assess the total androgenic activity, thus correlating clinical findings to the hormonal quantification.85
Although there is no consensus on the use of those substances as AFA markers, studies carried out by Carmina et al show a significant increase in the ADT-G level in 75% of normoandrogenic women with acne. In the same group, TT, FT, and DHEAS presented normal values. All the remaining patients were treated with a COC (ethinyl estradiol [EE] associated to norgestimate) for 6 months, with a significant reduction in the levels of FT and ADT-G. The authors concluded that even with the androgenic precursors at normal levels, ADT-G acted as an acne marker in women.38 More recent studies performed by Rocha et al in 2017 used liquid chromatography associated to mass spectrometry. The authors were able to prove that women with AFA presented a significant increase in ADT-G level, even when T and DHEA were within reference values. The ADT-G level decreased after treatment with an oral contraceptive containing EE associated to drospirenone.86 Lookingbill et al’s study on 3α-androstenediol showed a significant increase in women with mild to moderate acne.59 This was not confirmed by further studies which have detected, on the contrary, reduced levels of this metabolite in patients presenting the same clinical picture.87,88
Labrie et al analyzing cohort studies performed over 20 years concluded that the correlation between serum T and androgenic clinic conditions remains undetermined. Their observations are related to the serum T dosage’s inability to reflect the androgenic hormonal conversion in the peripheral tissues. Sometimes, these hormones act in the tissues where they are generated without releasing significant amounts into the circulation (intracrine system). Studying the FT and ADT-G dosage in healthy 30- to 35-year-old women, the authors concluded that there is no correlation between the hormone and its metabolite. In their view, T dosage should be replaced by ADT-G through liquid chromatography associated to the mass spectrometry in order to study the androgenic hyperproduction or deficient cases.89
Clinical presentation
With regard to the clinical picture, the majority of studies agree that there are some differences in AFA compared to the typical presentation observed in adolescents. In adult women, the lesions are predominantly inflammatory, with mild to moderate intensity, tending to be located in the lower third of the face (mandibular line, perioral region, and side of the neck). The distribution is not yet completely understood.7,9,56,90–94 The predominance of comedo and macrocomedo in the frontal and side regions of the face usually is observed in patients who are over 40 years and smokers.95
In a recent multicenter prospective observational study, the clinical characteristics of 374 patients with AFA of age 26–66 years were assessed. A facial involvement of multiple regions was observed in 89.8% of cases and a small group representing 11.2% with acne exclusively in the mandibular region. This finding was contrary to the majority of previous studies. Interestingly, in this study, the majority of patients presented comedonal acne.96
Adult women with acne in their adult life have increased sensitivity of the skin with higher frequency of post- inflammatory erythema, hyper and/or hypopigmentation, and scarring, all of which are cosmetically disfiguring changes.10,11 Further, in terms of physical aspects, this is undoubtedly a disease with a strong psychosocial impact, which can lead to the onset of signs and symptoms of depression and anger.97
Quality of life (QoL)
The term QoL can be characterized subjectively as the patient’s perception of the disease and treatment, while the technical concept comprises a series of components related to mental health, physical and functional ability, and the social dimension. Regarding dermatological diseases, studies suggest that psychometric tooling can assist the doctor in finding the best therapeutic proposal and to detect those patients psychologically affected, even when the clinical picture is deemed mild.98 The development of markers, instruments, or questionnaires is based on evidence that there is a disagreement between the assessment by the doctor or the health professional and the patient in relation to the severity of the disease and the success of the treatment. Additionally, there are both different answers regarding the therapy and different satisfaction levels among patients with the same clinical picture.99
Studies related to acne point out a negative influence on QoL, including the presence of signs and symptoms of depression and anxiety, such as anger and low self-esteem.100–102
The use of disease-specific questionnaires, such as the QoL in acne (Acne-QoL) already translated and validated for the Brazilian Portuguese,103 quantifies the impact of acne on QoL.104,105
Literature has demonstrated that the impact on QoL is not always correlated to the severity of acne. In specific cases, individuals present mild disease with a high impact on their QoL.101,106 Furthermore, there are studies proving that the psychological impact caused by the presence of acne seems to affect more female patients than male patients.11
A recent study by Rocha et al confirmed that AFA has a high negative impact on the patients’ QoL, even when they have mild to moderate acne. In the same study, the patients showed an improvement in QoL scores after treatment with a COC or even with topical medication containing azelaic acid. A statistical superiority was observed for the contraceptive group in only two Acne-QoL domains (self-perception and acne symptoms).107
Treatment
Concerning the treatment for AFA, multiple options are available, similar to those used to treat adolescents.108 If the clinical picture is mild, topical retinoid, azelaic acid, and benzoyl peroxide are indicated as monotherapy or associated to topical antibiotics. The latter should not be prescribed in isolation, as they may induce bacterial resistance.94 As in monotherapy, no topical medication is able to treat all the etiopathogenic aspects of acne; frequently, it is required to associate topical medications or topics added to systemic drugs.108
Oral antibiotics, mainly those that derivate from cyclins, help more inflammatory forms, but they have a high relapse rate after they are stopped.109 Oral isotretinoin is also used, but it can fail in keeping adult patients free of lesions after months. There is a higher incidence of irritating symptoms due to an increased mucocutaneous sensibility found in this age group.110,111
A study carried out by Rademaker et al on the use of a low-dose oral isotretinoin (5 mg a day) to treat mild intensity acne in adults for 32 weeks showed therapeutic success, improvement in QoL, and minimal side effects. Regarding relapse rates, the authors followed part of the group and in 2017 published the results, disclosing an absence of relapse of 40%. Among the relapse group (60%), about 50% of patients, after 7 months without treatment, preferred to restart isotretinoin even in doses as low as 5–10 mg, twice a week.112,113
Hormonal treatments, mainly those with an association of estrogens and new antiandrogenic progestins (compounds present in certain contraceptives), can be used by women with no formal contraindications when they also want contraception.114 These drugs promote a reduction in FT levels through an increase of SBHG, a reduction of gonadotropin release, a decrease of ovarian production, and an antiandrogenic effect on sebaceous gland receptors.115–118
COCs are drugs combining an estrogen, usually EE, to synthetic progestins. These medications have undergone several evolutions since their development. One of them is related to the amount of estrogen. Initially, 100 µg per tablet presented a large incidence of severe side effects, such as deep vein thrombosis, cerebrovascular accident, and myocardial infarction.119 Presently, with doses between 20 and 35 µg, they are very safe.120
Another important evolution is related to the progestin contained in the COCs. There are several progestin generations; the most recent are less androgenic, such as norgestimate and desogestrel,121 or they present an antiandrogenic effect, such as drospirenone.122
A recent review by the Cochrane Library analyzed 31 comparative studies among contraceptives used to treat acne. The conclusion was that the COCs were more effective than the placebo, with cyproterone acetate and chlormadinone presenting better results than levonorgestrel, and drospirenone better than norgestimate.123 Favorable estrogen effects can be eliminated by the inclusion of the androgenic progestin, especially the derivate from 19-nortestosterone.124
The more frequent side effects are nausea, headache, breast pain, sporadic bleeding, and decreased libido. The incidence is quite different, depending on the combination of each contraceptive.125
It must be pointed out that the use of COCs offers several benefits to women in addition to the improvement of acne, such as the reduction of dysmenorrhea as well as protection against ovary and endometrium cancer, anemia caused by iron deficiency, and pelvic inflammatory disease.125
In relation to venous thromboembolism, its incidence in young women is low, and there is a small increase in the risk, 0.05%–0.1%, for COC users.126 It must be pointed out that the risk for thromboembolism in the postpartum period is 0.5%; during pregnancy, it is 0.1%.127
The introduction of COC must be performed after careful anamnesis, and it is contraindicated for women with a high risk of deep venous thrombosis and cardiovascular diseases, such as family history and/or smoking habit.125
Spironolactone is an aldosterone antagonist, used since 1957, that has antiandrogenic properties.128 It acts by blocking 5α-reductase activity and the androgen receptor in peripheral tissues.129,130 A study published by Sato et al shows the effectiveness of spironolactone to treat acne in Asian women, with a 47% good response using an initial dose of 200 mg per day, then reducing the dose every 4 weeks. In monotherapy, 80% of patients presented menstrual irregularity.131 Spironolactone’s side effects are dose-dependent, and the most frequent are increase of diuresis, headache, dizziness, menstrual irregularity, breast pain, fatigue, and hyperpotassemia.132
A recent retrospective study found that in young women, who did not have kidney disease and were nonusers of other medications that might increase potassium levels, a periodic monitoring of these levels is not required.133
Although the drug is associated with tumors in animals when the doses were much higher than those prescribed in clinical practice, human studies with a duration of >5 years of use did not verify a carcinogenic effect.134,135
The drug can be used to promote androgenic blockade in patients using an intrauterine device with levonorgestrel or to increase the androgenic blockade in patients using COCs.130 In these cases, 100 mg per day is safe and rarely associated with side effects.136
A systematic review published in 2017 concluded that the evidence for its use to treat acne in adult women is still poor, and a recommendation is still based on the opinion of experts or consensuses. Randomized, double-blind, and controlled studies are required to assess its effectiveness.137
Conclusion
The fact that acne is a self-limiting disease for most patients is not the case for the group of women who present this condition in adult life. Genetic factors that command an excessive activation of the immune system, especially the innate system, seem to justify the persistence of the lesions.
There are clinical characteristics that seem to differentiate the disease in adult life; among them are the predominance of inflammatory lesions, premenstrual worsening, and the lesions distribution on the face. Curiously, although often less intense in adulthood, the negative impact on QoL is greater than in adolescence.
Although there are options for its treatment, in highlight to the hormonal treatment, there are still specific medications for this age group that can be directed to the possible mechanisms implicated in its chronicity.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Tan JK, Bhate K. A global perspective on the epidemiology of acne. Br J Dermatol. 2015;172(Suppl 1):3–12. doi: 10.1111/bjd.13462. [DOI] [PubMed] [Google Scholar]
- 2.Burton JL, Cunliffe WJ, Stafford L. The prevalence of acne vulgaris in adolescence. Br J Dermatol. 1971;85(2):119–126. doi: 10.1111/j.1365-2133.1971.tb07195.x. [DOI] [PubMed] [Google Scholar]
- 3.White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39(2):S34–S37. doi: 10.1016/s0190-9622(98)70442-6. [DOI] [PubMed] [Google Scholar]
- 4.Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. J Am Acad Dermatol. 1999;41(4):577–580. [PubMed] [Google Scholar]
- 5.Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58(1):56–59. doi: 10.1016/j.jaad.2007.06.045. [DOI] [PubMed] [Google Scholar]
- 6.Perkins AC, Maglione J, Hillebrand GG, Miyamoto K, Kimball AB. Acne vulgaris in women: prevalence across the life span. J Womens Health (Larchmt) 2012;21(2):223–230. doi: 10.1089/jwh.2010.2722. [DOI] [PubMed] [Google Scholar]
- 7.Poli F, Dréno B, Verschoore M. An epidemiological study of acne in female adults: results of a survey conducted in France. J Eur Acad Dermatol Venereol. 2001;15(6):541–545. doi: 10.1046/j.1468-3083.2001.00357.x. [DOI] [PubMed] [Google Scholar]
- 8.Yentzer BA, Hick J, Reese EL, Uhas A, Feldman SR, Balkrishnan R. Acne vulgaris in the United States: a descriptive epidemiology. Cutis. 2010;86(2):94–99. [PubMed] [Google Scholar]
- 9.Goulden V, Clark SM, Cunliffe WJ. Post-adolescent acne: a review of clinical features. Br J Dermatol. 1997;136(1):66–70. [PubMed] [Google Scholar]
- 10.Willians C, Layton AM. Persistent acne in women. Am J Clin Dermatol. 2006;7(5):281–290. doi: 10.2165/00128071-200607050-00002. [DOI] [PubMed] [Google Scholar]
- 11.Dumont-Wallon G, Dréno B. Acné de la femme de plus de 25 ans: spécifique par sa clinique et les facteurs favorisants [Specificity of acne in women older than 25 years] Presse Med. 2008;37(4):585–591. doi: 10.1016/j.lpm.2007.07.014. French. [DOI] [PubMed] [Google Scholar]
- 12.Preneau S, Dreno B. Female acne – a different subtype of teenager acne? J Eur Acad Dermatol Venereol. 2012;26(3):277–282. doi: 10.1111/j.1468-3083.2011.04214.x. [DOI] [PubMed] [Google Scholar]
- 13.Ozdemir S, Ozdemir M, Görkemli H, Kiyici A, Bodur S. Specific dermatologic features of the polycystic ovary syndrome and its association with biochemical markers of the metabolic syndrome and hyperandrogenism. Acta Obstet Gynecol Scand. 2010;89(2):199–204. doi: 10.3109/00016340903353284. [DOI] [PubMed] [Google Scholar]
- 14.Choi CW, Lee DH, Kim HS, Kim BY, Park KC, Youn SW. The clinical features of late onset acne compared with early onset acne in women. J Eur Acad Dermatol Venereol. 2011;25(4):454–461. doi: 10.1111/j.1468-3083.2010.03813.x. [DOI] [PubMed] [Google Scholar]
- 15.Cunliffe WJ. The sebaceous gland and acne – 40 years on. Dermatology. 1998;196(1):9–15. doi: 10.1159/000017859. [DOI] [PubMed] [Google Scholar]
- 16.Gollnick HP. Current concepts of pathogenesis of acne – implications for drug treatment. Drugs. 2003;63(15):1579–1596. doi: 10.2165/00003495-200363150-00005. [DOI] [PubMed] [Google Scholar]
- 17.Thiboutot D. Acne: hormonal concepts and therapy. Clin Dermatol. 2004;22(5):419–428. doi: 10.1016/j.clindermatol.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 18.Kurokawa I, Danby WJ, Ju Q, et al. New developments in our understanding of acne pathogenesis and treatment. Exp Dermatol. 2009;18(10):821–832. doi: 10.1111/j.1600-0625.2009.00890.x. [DOI] [PubMed] [Google Scholar]
- 19.Kim J, Ochoa MT, Krutzic SR, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine response. J Immunol. 2002;169(3):1535–1541. doi: 10.4049/jimmunol.169.3.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeremy AH, Holland DB, Roberts SG, Thomson KF, Cunliffe WJ. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol. 2003;12(1):20–27. doi: 10.1046/j.1523-1747.2003.12321.x. [DOI] [PubMed] [Google Scholar]
- 21.McInturff JE, Modlin RL, Kim J. The role of toll-like receptors in the pathogenesis and treatment of dermatological disease. J Invest Dermatol. 2005;125(1):1–8. doi: 10.1111/j.0022-202X.2004.23459.x. [DOI] [PubMed] [Google Scholar]
- 22.Holland DB, Jeremy AH. The role of inflammation in the pathogenesis of acne and acne scarring. Semin Cutan Med Surg. 2005;24(2):79–83. doi: 10.1016/j.sder.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Krishna S, Kim C, Kim J. Innate immunity in the pathogenesis of acne vulgaris. In: Shalita AR, Del Rosso JQ, Webster GF, editors. Acne Vulgaris. 1st ed. London: Informa Healthcare; 2011. pp. 12–27. [Google Scholar]
- 24.Bellew S, Thiboutot D, Del Rosso JQ. Pathogenesis of acne vulgaris: what’s new, what’s interesting and what may be clinically relevant. J Drugs Dermatol. 2011;10(6):582–585. [PubMed] [Google Scholar]
- 25.Heughebaert C, Shalita AR. Comedogenesis. In: Shalita AR, Del Rosso JQ, Webster GF, editors. Acne Vulgaris. 1st ed. London: Informa Healthcare; 2011. pp. 28–42. [Google Scholar]
- 26.Taylor M, Gonzalez M, Porter R. Pathways to inflammation: acne pathophysiology. Eur J Dermatol. 2011;21(3):323–333. doi: 10.1684/ejd.2011.1357. [DOI] [PubMed] [Google Scholar]
- 27.Norris JF, Cunliff WJ. A histological and immunohistochemical study of early acne lesions. Br J Dermatol. 1988;118(5):651–659. doi: 10.1111/j.1365-2133.1988.tb02566.x. [DOI] [PubMed] [Google Scholar]
- 28.Layton AM, Morris C, Cunliff WJ, Ingham E. Immunohistochemical investigation of evolving inflammation in lesions of acne vulgaris. Exp Dermatol. 1998;7(4):191–197. doi: 10.1111/j.1600-0625.1998.tb00323.x. [DOI] [PubMed] [Google Scholar]
- 29.Kadowaki N, Ho S, Antonenko S, et al. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194(6):863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jugeau S, Tenaud I, Knol AC, et al. Induction of toll-like receptor by Propionibacterium acnes. Br J Dermatol. 2005;153(6):1105–1113. doi: 10.1111/j.1365-2133.2005.06933.x. [DOI] [PubMed] [Google Scholar]
- 31.Kim J. Review of the innate response in acne vulgaris: activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. Dermatology. 2005;211(3):193–198. doi: 10.1159/000087011. [DOI] [PubMed] [Google Scholar]
- 32.Marco Rocha AD, Lilia Guadanhim RS, Sanudo A, Bagatin E. Modulation of Toll like receptor-2 on sebaceous gland by the treatment of adult female acne. Dermatoendocrinol. 2017 Oct 4; doi: 10.1080/19381980.2017.1361570. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee SE, Jeong SK, Lee SH. Protease and protease-activated receptor-2 signaling in the pathogenesis of atopic dermatitis. Yonsei Med J. 2010;51(6):808–822. doi: 10.3349/ymj.2010.51.6.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tenaud I, Khammari A, Dréno B. In vitro modulation of TLR-2, CD1d and IL-10 by adapalene on normal human skin and acne inflammatory lesions. Exp Dermatol. 2007;16(6):500–506. doi: 10.1111/j.1600-0625.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- 35.Leyden JJ, McGinley KJ, Mills OH, Kligman AM. Propionibacterium levels in patients with and without acne vulgaris. J Invest Dermatol. 1975;65(4):382–384. doi: 10.1111/1523-1747.ep12607634. [DOI] [PubMed] [Google Scholar]
- 36.Leeming JP, Holland KT, Cunliff WJ. The microbial colonization of inflamed acne vulgaris lesions. Br J Dermatol. 1988;118(2):203–208. doi: 10.1111/j.1365-2133.1988.tb01775.x. [DOI] [PubMed] [Google Scholar]
- 37.Youn SW, Park ES, Lee DH, Huh CH, Park KC. Does facial sebum excretion really affect the development of acne? Br J Dermatol. 2005;153(5):919–924. doi: 10.1111/j.1365-2133.2005.06794.x. [DOI] [PubMed] [Google Scholar]
- 38.Carmina E, Godwin AJ, Stanczyc FZ, Lippman JS, Lobo RA. The association of serum androsterone glucuronide with inflammatory lesions in women with adult acne. J Endocrinol Invest. 2002;25(9):765–768. doi: 10.1007/BF03345509. [DOI] [PubMed] [Google Scholar]
- 39.Seirafi H, Farnaghi F, Vasheghani-Farahani A, et al. Assessment of androgens in women with adult-onset acne. Int J Dermatol. 2007;46(11):1188–1191. doi: 10.1111/j.1365-4632.2007.03411.x. [DOI] [PubMed] [Google Scholar]
- 40.Aizawa H, Niimura M. Elevated serum insulin-like growth factor-1 (IGF-1) levels in women with postadolescent acne. J Dermatol. 1995;22(4):249–252. doi: 10.1111/j.1346-8138.1995.tb03381.x. [DOI] [PubMed] [Google Scholar]
- 41.Thiboutot D, Gilliland K, Light J, Lookingbill D. Androgen metabolism in sebaceous glands from subjects with and without acne. Arch Dermatol. 1999;135(9):1041–1045. doi: 10.1001/archderm.135.9.1041. [DOI] [PubMed] [Google Scholar]
- 42.Summerly R, Yardley HJ, Raymond M, Tabiowo A, Ilderton E. The lipid composition of sebaceous glands as a reflection of gland size. Br J Dermatol. 1976;94(1):45–53. doi: 10.1111/j.1365-2133.1976.tb04340.x. [DOI] [PubMed] [Google Scholar]
- 43.Pye RJ, Meyrick G, Burton JL. Free fatty acids in the early inflammatory papule of acne vulgaris. Clin Exp Dermatol. 1977;2(4):355–359. doi: 10.1111/j.1365-2230.1977.tb01575.x. [DOI] [PubMed] [Google Scholar]
- 44.Rosenfield RL, Deplewski D, Kentsis A, Ciletti N. Mechanisms of androgen induction of sebocyte differentiation. Dermatology. 1998;196(1):43–46. doi: 10.1159/000017864. [DOI] [PubMed] [Google Scholar]
- 45.Rosenfield RL, Kentsis A, Deplewski D, Ciletti N. Rat preputial sebocyte differentiation involves peroxisome proliferator-activated receptors. J Invest Dermatol. 1999;112(2):226–232. doi: 10.1046/j.1523-1747.1999.00487.x. [DOI] [PubMed] [Google Scholar]
- 46.Rosenf ield RL, Deplewski D, Greene ME. Peroxisome proliferator- activated receptors and skin development. Horm Res. 2000;54(5–6):269–274. doi: 10.1159/000053270. [DOI] [PubMed] [Google Scholar]
- 47.Zouboulis CC, Xia L, Akamatsu H, et al. The human sebocyte culture model provides new insights into development and management of seborrhoea and acne. Dermatology. 1998;196(1):21–31. doi: 10.1159/000017861. [DOI] [PubMed] [Google Scholar]
- 48.Ingham E, Eady EA, Goodwin CE, Cove JH, Cunliffe WJ. Pro-inflammatory levels of interleukin-1 alpha-like bioactivity are present in the majority of open comedones in acne vulgaris. J Invest Dermatol. 1992;98(6):895–901. doi: 10.1111/1523-1747.ep12460324. [DOI] [PubMed] [Google Scholar]
- 49.Thiboutot DM, Layton AM, Eady EA. IL-17: a key player in the P. acnes inflammatory cascade? J Invest Dermatol. 2014;134(2):307–310. doi: 10.1038/jid.2013.400. [DOI] [PubMed] [Google Scholar]
- 50.Cappel M, Mauger D, Thiboutot D. Correlation between serum levels of insulin-like growth factor 1, dehydroepiandrosterone sulfate, and dihydrotestosterone and acne lesion counts in adult women. Arch Dermatol. 2005;141(3):333–338. doi: 10.1001/archderm.141.3.333. [DOI] [PubMed] [Google Scholar]
- 51.Ludwig DS. The glycemic index: physiological mechanism relating to obesity, diabetes, and cardiovascular disease. JAMA. 2002;287(18):2414–2423. doi: 10.1001/jama.287.18.2414. [DOI] [PubMed] [Google Scholar]
- 52.Adebamowo CA, Spiegelman D, Danby FW, Frazier AL, Willett WC, Holmes MD. High school dietary dairy intake and teenage acne. J Am Acad Dermatol. 2005;52(2):207–214. doi: 10.1016/j.jaad.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 53.Adebamowo CA, Spiegelman D, Berekey CS, et al. Milk consumption and acne in adolescents girls. Dermatol Online J. 2006;12(4):1. [PubMed] [Google Scholar]
- 54.Kane A, Niang SO, Diagne AC, Ly F, Ndiaye B. Epidemiologic, clinical and therapeutic features of acne in Dakar, Senegal. Int J Dermatol. 2007;46(Suppl 1):36–38. doi: 10.1111/j.1365-4632.2007.03462.x. [DOI] [PubMed] [Google Scholar]
- 55.Dall’oglio F, Tedeschi A, Fabbrocini G, Veraldi S, Picardo M, Micali G. Cosmetics for acne: indications and recommendations for an evidence-based approach. G Ital Dermatol Venereol. 2015;150(1):1–11. [PubMed] [Google Scholar]
- 56.Rivera R, Guerra A. Management of acne in women over 25 years of age. Actas Dermosifiliogr. 2009;100(1):33–37. [PubMed] [Google Scholar]
- 57.Capitanio B, Sinagra JL, Ottaviani M, Bordignon V, Amantea A, Picardo M. Acne and smoking. Dermatoendocrinol. 2009;1(3):129–135. doi: 10.4161/derm.1.3.9638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lucky AW, McGuire J, Rossenfield RL, Lucky PA. Plasma androgens in women with acne vulgaris. J Invest Dermatol. 1983;81(1):70–74. doi: 10.1111/1523-1747.ep12539043. [DOI] [PubMed] [Google Scholar]
- 59.Lookingbill DP, Horton R, Demers RM. Tissue production of androgens in women with acne. J Am Acad Dermatol. 1985;12(3):481–487. doi: 10.1016/s0190-9622(85)70067-9. [DOI] [PubMed] [Google Scholar]
- 60.Derman RJ. Androgen excess in women. Int J Fertil Menopausal Stud. 1996;41(2):172–176. [PubMed] [Google Scholar]
- 61.Yarak S, Bagatin E, Hassun KM, Parada MO, Talarico Filho S. Hiperandrogenismo e pele: síndrome do ovário policístico e resistência periférica à insulina. An Bras Dermatol. 2005;80(4):395–410. Portuguese. [Google Scholar]
- 62.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 63.Archer JS, Chang RJ. Hirsutism and acne in polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 2004;18(5):737–754. doi: 10.1016/j.bpobgyn.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 64.Carmina E, Lobo RA. Evidence for increased androsterone metabolism in some normoandrogenic women with acne. J Clin Endocrinol Metab. 1993;76(5):1111–1114. doi: 10.1210/jcem.76.5.8496299. [DOI] [PubMed] [Google Scholar]
- 65.Slayden SM, Moran C, Sams WM, Jr, Boots LR, Azziz R. Hyperandrogenemia in patients presenting with acne. Fertil Steril. 2001;75(5):889–892. doi: 10.1016/s0015-0282(01)01701-0. [DOI] [PubMed] [Google Scholar]
- 66.Baxendale PM, Jacobs HS, James VH. Plasma and salivary androstenedione and dihydrotestosterone in women with hyperandrogenism. Clin Endocrinol. 1983;18(5):447–457. doi: 10.1111/j.1365-2265.1983.tb02874.x. [DOI] [PubMed] [Google Scholar]
- 67.Wang C, Wakelin K, White J, Wood PJ. Salivary androgens in hirsutism: are they of use in routine evaluation? Ann Clin Biochem. 1986;23(Pt 5):590–595. doi: 10.1177/000456328602300517. [DOI] [PubMed] [Google Scholar]
- 68.Menon GK, Feingold KR, Moser AH, Brown BE, Elias PM. De novo sterologenesis in the skin. II. Regulation by cutaneous barrier requirements. J Lipid Res. 1985;26(4):418–427. [PubMed] [Google Scholar]
- 69.Smythe CD, Greenall M, Kealey T. The activity of HMG-CoA reductase and acetyl-CoA carboxylase in human apocrine sweat glands, sebaceous glands, and hair follicles is regulated by phosphorylation and by exogenous cholesterol. J Invest Dermatol. 1998;111(1):139–148. doi: 10.1046/j.1523-1747.1998.00246.x. [DOI] [PubMed] [Google Scholar]
- 70.Chen W, Thiboutot D, Zouboulis CC. Cutaneous androgen metabolism: basic research and clinical perspectives. J Invest Dermatol. 2002;119(5):992–1007. doi: 10.1046/j.1523-1747.2002.00613.x. [DOI] [PubMed] [Google Scholar]
- 71.Fritsch M, Orfanos CE, Zouboulis CC. Sebocytes are the key regulators of androgen homeostasis in human skin. J Invest Dermatol. 2001;116(5):793–800. doi: 10.1046/j.1523-1747.2001.01312.x. [DOI] [PubMed] [Google Scholar]
- 72.Harris G, Azzolina B, Baginsky W, et al. Identification and selective inhibition of an isozyme of steroid 5 alpha-reductase in human scalp. Proc Natl Acad Sci U S A. 1992;89(22):10787–10791. doi: 10.1073/pnas.89.22.10787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thiboutot D, Harris G, Iles V, Cimis G, Gilliland K, Hagari S. Activity of the type 1 5 alpha-reductase exhibits regional differences in isolated sebaceous glands and whole skin. J Invest Dermatol. 1995;105(2):209–214. doi: 10.1111/1523-1747.ep12317162. [DOI] [PubMed] [Google Scholar]
- 74.Zouboulis CC. Human skin: an independent peripheral endocrine organ. Horm Res. 2000;54(5–6):230–242. doi: 10.1159/000053265. [DOI] [PubMed] [Google Scholar]
- 75.Azzouni F, Godoy A, Li Y, Mohler J. The 5 alpha-reductase isozyme family: a review of basic biology and their role in human diseases. Adv Urol. 2012;2012:530121. doi: 10.1155/2012/530121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Carmina E, Stanczyc FZ, Matteri RK, Lobo RA. Serum androsterone conjugates differentiate between acne and hirsutism in hyperandrogenic women. Fertil Steril. 1991;55(5):872–876. [PubMed] [Google Scholar]
- 77.Poortman J, Thijssen JH, Von Landeghem AA, Wiegerinck MA, Alsbach GP. Subcellular distribution of androgens and oestrogens in target tissue. J Steroid Biochem. 1983;19(1C):939–945. doi: 10.1016/0022-4731(83)90037-7. [DOI] [PubMed] [Google Scholar]
- 78.Labrie F, Dupont A, Belanger A. Complete androgen blockade for the treatment of prostate cancer. Important Adv Oncol. 1985:193–217. [PubMed] [Google Scholar]
- 79.Bélanger B, Bélanger A, Labrie F, Dupont A, Cusan L, Monfette G. Comparison of residual C-19 steroids in plasma and prostatic tissue of human, rat and guinea pig after castration: unique importance of extra-testicular androgens in men. J Steroid Biochem. 1989;32(5):695–698. doi: 10.1016/0022-4731(89)90514-1. [DOI] [PubMed] [Google Scholar]
- 80.Coffman BL, Tephly TR, Irshaid YM, et al. Characterization and primary sequence of a human hepatic microsomal estriol UDP glucuronosyltransferase. Arch Biochem Biophys. 1990;281(1):170–175. doi: 10.1016/0003-9861(90)90428-2. [DOI] [PubMed] [Google Scholar]
- 81.Beaulieu M, Lévesque E, Hum DW, Bélanger A. Isolation and characterization of a novel cDNA encoding a human UDP-glucuronosyltransferase active on C19 steroids. J Biol Chem. 1996;271(37):22855–22862. doi: 10.1074/jbc.271.37.22855. [DOI] [PubMed] [Google Scholar]
- 82.Beaulieu M, Lévesque E, Barbier O, et al. Isolation and characterization of a simian UDP-glucuronosyltransferase UGT2B18 active on 3-hydroxyandrogens. J Mol Biol. 1998;275(5):785–794. doi: 10.1006/jmbi.1997.1486. [DOI] [PubMed] [Google Scholar]
- 83.Carrier JS, Turgeon D, Journault K, Hum DW, Bélanger A. Isolation and characterization of the human UGT2B7 gene. Biochem Biophys Res Commun. 2000;272(2):616–621. doi: 10.1006/bbrc.2000.2795. [DOI] [PubMed] [Google Scholar]
- 84.Turgeon D, Carrier JS, Lévesque E, Beatty BG, Bélanger A, Hum DW. Isolation and characterization of the human UGT2B15 gene, localized within a cluster of UGT2B genes and pseudogenes on chromosome 4. J Mol Biol. 2000;295(3):489–504. doi: 10.1006/jmbi.1999.3374. [DOI] [PubMed] [Google Scholar]
- 85.Labrie F, Luu-The V, Bélanger A, et al. Is dehydroepiandrosterone a hormone? J Endocrinol. 2005;187(2):169–196. doi: 10.1677/joe.1.06264. [DOI] [PubMed] [Google Scholar]
- 86.Rocha M, Karina Cardozo HM, Carvalho VM, Bagatin E. ADT-G as a promising biomarker for peripheral hyperandrogenism in adult female acne. Dermatoendocrinol. 2017 Oct 13; doi: 10.1080/19381980.2017.1361571. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Toscano V, Balducci R, Bianchi P, et al. Two different pathogenetic mechanisms may play a role in acne and in hirsutism. Clin Endocrinol (Oxf) 1993;39(5):551–556. doi: 10.1111/j.1365-2265.1993.tb02408.x. [DOI] [PubMed] [Google Scholar]
- 88.Joura EA, Geusau A, Schneider B, Söregi G, Huber JC. Serum 3 alpha-androstanediol-glucuronide is decreased in nonhirsute women with acne vulgaris. Fertil Steril. 1996;66(6):1033–1035. doi: 10.1016/s0015-0282(16)58705-6. [DOI] [PubMed] [Google Scholar]
- 89.Labrie F, Bélanger A, Bélanger P, et al. Androgen glucuronides, instead of testosterone, as the new markers of androgenic activity in women. J Steroid Biochem Mol Biol. 2006;99(4–5):182–188. doi: 10.1016/j.jsbmb.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 90.Knaggs HE, Wood EJ, Rizer RL, Mills OH. Post-adolescent acne. Int J Cosmet Sci. 2004;26(3):129–138. doi: 10.1111/j.1467-2494.2004.00210.x. [DOI] [PubMed] [Google Scholar]
- 91.Marks R. Acne and its management beyond the age of 35 years. Am J Clin Dermatol. 2004;5(6):459–462. doi: 10.2165/00128071-200405060-00011. [DOI] [PubMed] [Google Scholar]
- 92.Schmitt JV, Masuda PY, Miot HA. Padrões clínicos da acne em mulheres de diferentes faixas etaŕias. An Bras Dermatol. 2009;84(4):349–354. doi: 10.1590/s0365-05962009000400005. Portuguese. [DOI] [PubMed] [Google Scholar]
- 93.Addor FA, Schalka S. Acne da mulher adulta: aspectos epidemiológicos, diagnósticos e terapeûticos. An Bras Dermatol. 2010;85(6):789–795. doi: 10.1590/s0365-05962010000600003. Portuguese. [DOI] [PubMed] [Google Scholar]
- 94.Dréno B, Layton A, Zouboulis CC, et al. Adult female acne: a new paradigm. J Eur Acad Dermatol Venereol. 2013;27(9):1063–1070. doi: 10.1111/jdv.12061. [DOI] [PubMed] [Google Scholar]
- 95.Capitanio B, Sinagra JL, Bordignon V, Cordiali Fei P, Picardo M, Zouboulis CC. Underestimated clinical features of postadolescent acne. J Am Acad Dermatol. 2010;63(5):782–788. doi: 10.1016/j.jaad.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 96.Dréno B, Thiboutot D, Layton AM, Berson D, Perez M, Kang S, Global Alliance to Improve Outcomes in Acne Large-scale international study enhances understanding of an emerging acne population: adult females. J Eur Acad Dermatol Venereol. 2015;29(6):1096–1106. doi: 10.1111/jdv.12757. [DOI] [PubMed] [Google Scholar]
- 97.Yazici K, Baz K, Yazici AE, et al. Disease-specific quality of life is associated with anxiety and depression in patients with acne. J Eur Acad Dermatol Venereol. 2004;18(4):435–439. doi: 10.1111/j.1468-3083.2004.00946.x. [DOI] [PubMed] [Google Scholar]
- 98.Gollnick HP, Cunliffe W, Berson D, et al. Management of acne: a report from a global alliance to improve outcomes in acne. J Am Acad Dermatol. 2003;49(Suppl 1):S1–S38. doi: 10.1067/mjd.2003.618. [DOI] [PubMed] [Google Scholar]
- 99.Berlim MT, Fleck MPA. “Quality of life”: a brand new concept for research and practice in psychiatry. Rev Bras Psiquiatr. 2003;25(4):249–252. doi: 10.1590/s1516-44462003000400013. [DOI] [PubMed] [Google Scholar]
- 100.Kellett SC, Gawkrodger DJ. The psychological and emotional impact of acne and the effect of treatment with isotretinoin. Br J Dermatol. 1999;140(2):273–282. doi: 10.1046/j.1365-2133.1999.02662.x. [DOI] [PubMed] [Google Scholar]
- 101.Rapp DA, Brenes GA, Feldman SR, et al. Anger and acne: implications for quality of life, patient satisfaction and clinical care. Br J Dermatol. 2004;151(1):183–189. doi: 10.1111/j.1365-2133.2004.06078.x. [DOI] [PubMed] [Google Scholar]
- 102.Dalgard F, Gieler U, Holm J, Bjertness E, Hauser S. Self-esteem and body satisfaction among late adolescents with acne: results from a population survey. J Am Acad Dermatol. 2008;59(5):746–751. doi: 10.1016/j.jaad.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 103.Kamamoto CSL, Hassun KM, Bagatin E, Tomimori J. Questionaŕio de qualidade de vida específico para acne (Acne-QoL): tradução, adaptação cultural e validação para lińgua portuguesa usada no Brasil. An Bras Dermatol. 2014;89(1):83–90. doi: 10.1590/abd1806-4841.20142172. Portuguese. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Patrick DL, Deyo RA. Generic and disease-specific measures in assessing health status and quality of life. Med Care. 1989;27(Suppl 3):S217–S232. doi: 10.1097/00005650-198903001-00018. [DOI] [PubMed] [Google Scholar]
- 105.Klassen AF, Newton JN, Mallon E. Measuring quality of life in people referred for specialist care of acne: comparing generic and disease-specific measures. J Am Acad Dermatol. 2000;43(2):229–233. doi: 10.1067/mjd.2000.105507. [DOI] [PubMed] [Google Scholar]
- 106.Martin AR, Lookingbill DP, Batek A, Light J, Thiboutot D, Girman CJ. Health-related quality of life among patients with facial acne – assessment of a new acne-specific questionnaire. Clin Exp Dermatol. 2001;26(5):380–385. doi: 10.1046/j.1365-2230.2001.00839.x. [DOI] [PubMed] [Google Scholar]
- 107.Rocha M, Sanudo A, Bagatin E. The effect on acne quality of life of topical azelaic acid 15% gel versus a combined oral contraceptive in adult female acne: a randomized trial. Dermatoendocrinol. 2017 Oct 13; doi: 10.1080/19381980.2017.1361572. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Savage LJ, Layton AM. Treating acne vulgaris: systemic, local and combination therapy. Expert Rev Clin Pharmacol. 2010;3(4):563–580. doi: 10.1586/ecp.10.27. [DOI] [PubMed] [Google Scholar]
- 109.Shaw JC, White LE. Persistent acne in adult women. Arch Dermatol. 2001;137(9):1252–1253. [PubMed] [Google Scholar]
- 110.Cunliffe WJ, Caputo R. Roaccutane treatment guidelines: results of an international survey. Dermatology. 1997;194(4):351–357. doi: 10.1159/000246134. [DOI] [PubMed] [Google Scholar]
- 111.Seukeran DC, Cunliffe WJ. Acne vulgaris in the elderly: the response to low dose isotretinoin. Br J Dermatol. 1998;139(1):99–101. doi: 10.1046/j.1365-2133.1998.02321.x. [DOI] [PubMed] [Google Scholar]
- 112.Rademaker M, Wishart JM, Birchall NM. Isotretinoin 5 mg daily for low-grade adult acne vulgaris – a placebo-controlled, randomized double-blind study. J Eur Acad Dermatol Venereol. 2014;28(6):747–754. doi: 10.1111/jdv.12170. [DOI] [PubMed] [Google Scholar]
- 113.Rademaker M, Wishart J, Birchall N. Long term remission of persistent adult acne following very low-dose (5 mg/day) isotretinoin. Australas J Dermatol. 2017;58(1):69. doi: 10.1111/ajd.12469. [DOI] [PubMed] [Google Scholar]
- 114.George R, Clarke S, Thiboutot D. Hormonal therapy for acne. Semin Cutan Med Surg. 2008;27(3):992–1007. doi: 10.1016/j.sder.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 115.Redmond GP, Olson WH, Lippman JS, Kafrissen ME, Jones TM, Jorizzo JL. Norgestimate and ethinyl estradiol in the treatment of acne vulgaris: a randomized, placebo-controlled trial. Obstet Gynecol. 1997;89(4):615–622. doi: 10.1016/S0029-7844(97)00059-8. [DOI] [PubMed] [Google Scholar]
- 116.Van Vloten WA, Van Haselen CW, Van Zuuren EJ, Gerlinger C, Heithecker R. The effect of 2 combined oral contraceptives containing either drospirenone or cyproterone acetate on acne and seborrhea. Cutis. 2002;69(4):2–15. [PubMed] [Google Scholar]
- 117.Maloney JM, Dietze P, Jr, Watson D, et al. Treatment of acne using a 3-milligram drospirenone/20-microgram ethinyl estradiol oral contraceptive administered in a 24/4 regimen: a randomized controlled trial. Obstet Gynecol. 2008;112(4):773–781. doi: 10.1097/AOG.0b013e318187e1c5. [DOI] [PubMed] [Google Scholar]
- 118.Zouboulis CC. Sebaceous gland receptors. Dermatoendocrinol. 2009;1(2):77–80. doi: 10.4161/derm.1.2.7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Speroff L, DeCherney A. Evaluation of a new generation of oral contraceptives. The advisory board for new progestins. Obstet Gynecol. 1993;81(6):1034–1047. [PubMed] [Google Scholar]
- 120.Thorneycroft IH. Evolution of progestins. Focus on the novel progestin drospirenone. J Reprod Med. 2002;47(11):975–980. [PubMed] [Google Scholar]
- 121.Greenwood R, Brummitt L, Burke B, Cunliffe WJ. Acne: double blind clinical and laboratory trial on tetracycline, oestrogen-cyproterone acetate, and combined treatment. Br Med J. 1985;291(6504):1231–1235. doi: 10.1136/bmj.291.6504.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lemay A, Dewailly SD, Grenier R, Huard J. Attenuation of mild hyperandrogenic activity in postpubertal acne by a triphasic oral contraceptive containing low doses of ethynyl estradiol and d,l-norgestrel. J Clin Endocrinol Metab. 1990;71(1):8–14. doi: 10.1210/jcem-71-1-8. [DOI] [PubMed] [Google Scholar]
- 123.Arowojolu AO, Gallo MF, Lopez LM, Grimes DA. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012;7:CD004425. doi: 10.1002/14651858.CD004425.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Klipping C, Marr J. Effects of two combined oral contraceptives containing ethinyl estradiol 20 microg combined with either drospirenone or desogestrel on lipids, hemostatic parameters and carbohydrate metabolism. Contraception. 2005;71(6):409–416. doi: 10.1016/j.contraception.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 125.Tyler KH, Zirwas MJ. Contraception and the dermatologist. J Am Acad Dermatol. 2013;68(6):1022–1029. doi: 10.1016/j.jaad.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 126.Raymond EG, Burke AE, Espey E. Combined hormonal contraceptives and venous thromboembolism: putting the risks into perspective. Obstet Gynecol. 2012;119(5):1039–1044. doi: 10.1097/AOG.0b013e31825194ca. [DOI] [PubMed] [Google Scholar]
- 127.Heit JA, Kobbervig CE, James AH, Petterson TM, Bailey KR, Melton LJ., 3rd Trends in the incidence of venous thromboembolism during pregnancy or postpartum: a 30-year population-based study. Ann Intern Med. 2005;143(10):697–706. doi: 10.7326/0003-4819-143-10-200511150-00006. [DOI] [PubMed] [Google Scholar]
- 128.Rathnayake D, Sinclair R. Use of spironolactone in dermatology. Skinmed. 2010;8(6):328–332. [PubMed] [Google Scholar]
- 129.Goodfellow A, Alaghband-Zadeh J, Carter G, et al. Oral spironolactone improves acne vulgaris and reduces sebum excretion. Br J Dermatol. 1984;111(2):209–214. doi: 10.1111/j.1365-2133.1984.tb04045.x. [DOI] [PubMed] [Google Scholar]
- 130.Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5(3):37–50. [PMC free article] [PubMed] [Google Scholar]
- 131.Sato K, Matsumoto D, Iizuka F, et al. Anti-androgenic therapy using oral spironolactone for acne vulgaris in Asians. Aesthetic Plast Surg. 2006;30(6):689–694. doi: 10.1007/s00266-006-0081-0. [DOI] [PubMed] [Google Scholar]
- 132.Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43(3):498–502. doi: 10.1067/mjd.2000.105557. [DOI] [PubMed] [Google Scholar]
- 133.Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151(9):941–944. doi: 10.1001/jamadermatol.2015.34. [DOI] [PubMed] [Google Scholar]
- 134.Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year follow-up study. J Cutan Med Surg. 2002;6(6):541–545. doi: 10.1007/s10227-001-0152-4. [DOI] [PubMed] [Google Scholar]
- 135.Friedman AJ. Spironolactone for adult female acne. Cutis. 2015;96(4):216–217. [PubMed] [Google Scholar]
- 136.Krunic A, Ciurea A, Scheman A. Efficacy and tolerance of acne treatment using both spironolactone and a combined contraceptive containing drospirenone. J Am Acad Dermatol. 2008;58(1):60–62. doi: 10.1016/j.jaad.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 137.Layton AM, Eady EA, Whitehouse H, Del Rosso JQ, Fedorowicz Z, van Zuuren EJ. Oral spironolactone for acne vulgaris in adult females: a hybrid systematic review. Am J Clin Dermatol. 2017;18(2):169–191. doi: 10.1007/s40257-016-0245-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


