Abstract
Background
Two frequent problems associated with titanium (Ti) surfaces of bone/dental implants are their corrosion and lack of native tissue integration.
Methods
Here, we present an anodization-hydrothermal method for coating Ti surfaces with a layer of silicon (Si)- and strontium (Sr)-loaded titania nanotubes (TNs). The Ti surfaces coated with such a layer (Si–Sr-TNs) were characterized with different techniques.
Results
The results indicate that the Si4+ and Sr2+ ions were evenly incorporated into the TNs and that the Si–Sr-TN layer provides good protection against corrosive media like simulated body fluid. The excellent cytocompatibility of the coating was confirmed in vitro by the significant growth and differentiation of MC3T3-E1 osteoblastic cells.
Conclusion
Being easily and economically fabricated, the Si–Sr-TN surfaces may find their niche in clinical applications, thanks to their excellent biological activity and corrosion resistance.
Keywords: TiO2 nanotube, silicon, strontium, cytocompatibility, corrosion resistance
Introduction
The high corrosion resistance of titanium (Ti) results from the growth of a protective titanium dioxide (TiO2) film on its surface (2–6 nm thickness).1 However, this nanothick passive layer is not inert to corrosive attack when subjected to aggressive biological conditions (eg, pH variation, presence of fluorides, and surface wear).2 The intrinsic bioinertness of Ti surfaces and its alloys usually results in poor osseointegration due to insufficient bone formation, which then increases the risk of instability after surgical procedures.3 The osseointegrative potential of commercial pure Ti surfaces has recently been enhanced through tuning their wettability, chemistry, and nanotopography.4 One of such approaches is to cover Ti surfaces with a coating of anodic titania nanotubes (TNs), which effectively promotes bone formation,4,5 a highly ordered TN array with lateral dimensions ⩽100 nm was formed on the surface of a Ti substrate anodized in fluoride-containing electrolytes.6,7
Nanostructured surfaces provide an opportunity to more closely resemble the surface topography of living tissue. It has been shown that TiO2 nanotube (NT) arrays clearly accelerate apatite formation and, moreover, transformation from amorphous into the crystalline TiO2 facilitates this process.8–10 From cell in vitro studies, it has become increasingly evident that surface morphology can influence cell fates. A mechanism is driven by promoting the adsorption of selected proteins (such as fibronectin and vitronectin), which are important for mediating signals between the cell, the material surface, and the cell adhesion.11 Additionally, NTs with open volume nanotubular structures have been shown to be ideal carriers for drug loading and release.12 NTs have great potential as a long-term drug delivery system because they provide a novel and effective strategy by adding bioactive agents to raise implantation success rates. The osteogenic activity of Ti surfaces has been improved through incorporating different elements such as strontium (Sr) and silicon (Si) into the NTs.13 These ions play important roles in the adhesion, growth, and differentiation of osteogenic cells and also in osteoclastogenesis and bone resorption. Si-doped TNs significantly enhance the expression of the genes involved in the osteogenic differentiation;14 the incorporation of Sr into the TNs has improved the initial adhesion, migration, and differentiation of preosteoblast cells;3 therefore, it can be conjectured that the Sr/Si codoping of the TNs may synergistically enhance their osteoblast proliferation and differentiation as compared to the separate loading of Sr or Si.15 However, Sr–Si-codoped TiO2 NTs’ formation on Ti under anodization-hydrothermal condition has not been yet extensively studied. Being easily and economically fabricated, the Si–Sr-TN surfaces may find their niche in clinical applications, thanks to their excellent biological activity and corrosion resistance.
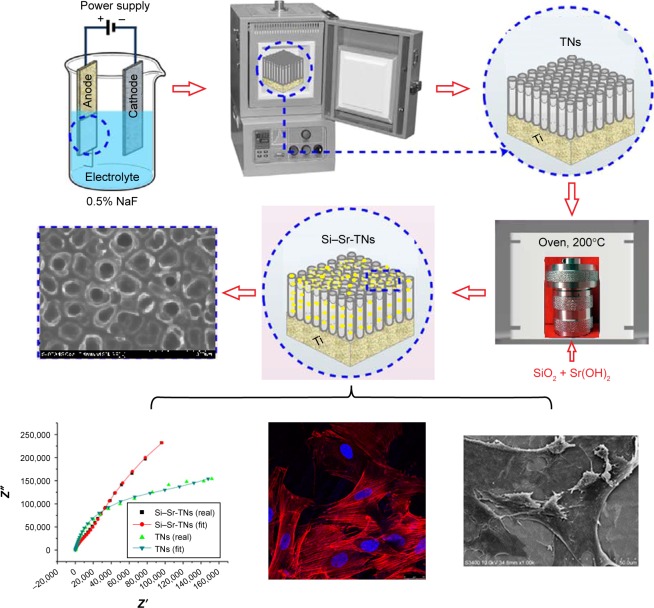
Therefore, the present work is aimed at demonstrating that the simultaneous incorporation of Si and Sr into the TNs can significantly enhance the corrosion resistance of TN-coated Ti substrates and can ultimately stimulate the bone formation around such Sr–Si-codoped TN-coated Ti implants. We here report on the anodization-hydrothermal synthesis of Sr–Si-codoped TN (Si–Sr-TNs) layers and their morphological, physicochemical, and electrochemical characterization. The biological response of the Si–Sr-TN layers has also been understood by exploring the cellular adhesion, cell proliferation, and osteogenic differentiation marker. Figure 1 shows the schematic process for the preparation and characterization of the highly ordered arrays of Si–Sr-TNs on Ti.
Figure 1.
Schematic process for the preparation and characterization of the highly ordered arrays of Si–Sr-TNs on titanium.
Abbreviations: Si, silicon; Sr, strontium; TNs, titania nanotubes.
Experimental details
The TNs were fabricated from commercially pure Ti foils (99.9% purity, 10×10×1 mm; Non-Ferrous Metals, Ltd, Baoji, China) using the anodization process.16,17 The TN arrays were prepared by the standard two-electrode anodization at room temperature in the potentiostatic mode. The cathode was a platinum plate, and the anode was the Ti sample. To create the NT layers, the samples were anodized in an electrolyte containing 0.5 wt% of hydrogen fluoride solution. The anodizing voltage was kept at 20 V during the 50 min process. After the anodization, the samples were sintered at 450°C for 2 h. The purpose of the heat treatment was to enhance the crystallinity of the hydroxyapatite coating and to make the TNs transform from amorphous to anatase phase. Analytical-grade Sr hydroxide and nano-silicon dioxide (SiO2) were adopted as the sources for Sr and Si, respectively. All reagents were purchased from Aladdin Ltd, Shanghai, China. The hydrothermal solution consisted of 1 g/L nano-SiO2 (15±5 nm) and 0.02 M Sr(OH)2 (pH=12.4). The anodized samples were then hydrothermally treated for 4 h at 200°C in a 25 mL Teflon-lined autoclave to fabricate the Si–Sr-TN layers. Then, the samples were rinsed with 0.1 M HCl to eliminate the residual Sr(OH)2.
We characterized the surface topography of the Si-Sr-TN samples using a field-emission scanning electron microscopy (JSM-7500F; JEOL) and analyzed their crystalline structure with X-ray diffraction (XRD) (D/Max-2500; Rigaku); their surface chemical compositions were analyzed with X-ray photoelectron spectroscopy (XPS) (ESCALAB 250XI; Thermo Fisher Scientific, Waltham, MA, USA) and energy-dispersive X-ray spectrometer (Max20 EDAX); the wettability was measured with a contact angle meter (DSA 100; Kruess, Hamburg, Germany).16 The electrochemical measurement process was explained elsewhere.17
The MC3T3-E1 mouse preosteoblasts (Cell Bank of Chinese Academy of Sciences, Shanghai, China) were incubated at 37°C in a 5% CO2 environment in α-minimum essential medium with 10% fetal bovine serum and 1% streptomycin/ penicillin. We conducted a cell-counting kit-8 (CCK-8) assay with MC3T3-E1 cells to identify potential cellular toxicity of the Si–Sr-TN samples after 7 days were passed since the seeding of the cells onto the sample.3 In addition, the preosteoblasts (8×104 cells/well) were seeded onto the surface of the Si–Sr-TN samples in a 24-well plate and were then incubated for 1 day to allow cell adhesion. Thereafter, the unattached cells were removed through gentle washing with phosphate-buffered saline at 37°C; then, the adhering cells were immersed in a 4% paraformaldehyde solution for 10 min and were then stained using phalloidin (5 µg/mL; Sigma-Aldrich Co., St Louis, MO, USA) and 4′,6-diamidino-2-phenylindole. Fluorescence images of the adhering cells were obtained by a stereomicroscope (model SMZ745/745T; Nikon Corporation, Tokyo, Japan) (for the details on the assay processes of cell morphology and alkaline phosphatase [ALP] activity, refer Huang et al16,17).
Results and discussion
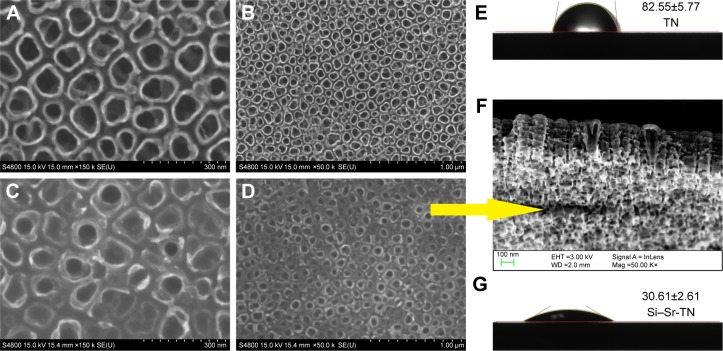
As shown in Figure 2A and C, the Si–Sr-TNs and the TN samples exhibited tubular nanostructures with an average inner diameter of 50 and 100 nm and the average wall thickness of the NTs is 15 and 50 nm, respectively. The total thickness of the NT layer is ~300 nm (Figure 2F). The close-packed arrangement of the NT in the Si–Sr-TN layers (eg, Figure 2D) is denser and smoother than that of the TN samples represented in Figure 2B. As shown in Figure 2E and G, the TN samples exhibit a high contact angle of 82.55°±5.77°; the doped Si and Sr give rise to a considerably lower contact angle of 30.61°±2.61° for the Si–Sr-TN sample; the abundant hydrophilic hydroxyl-terminated groups on the surfaces of the Si–Sr-TN sample give rise to hydrophilicity, making it favorable for cell attachment and proliferation.18
Figure 2.
The water droplet images and the contact angle values of (E) the TNs and (G) the Si–Sr-TNs and the FESEM images of the TNs (A and B) and the Si–Sr-TNs (C and D). Cross-section morphology of the Si–Sr-TNs (F).
Abbreviations: FESEM, field-emission scanning electron microscopy; Si, silicon; Sr, strontium; TN, titania nanotube.
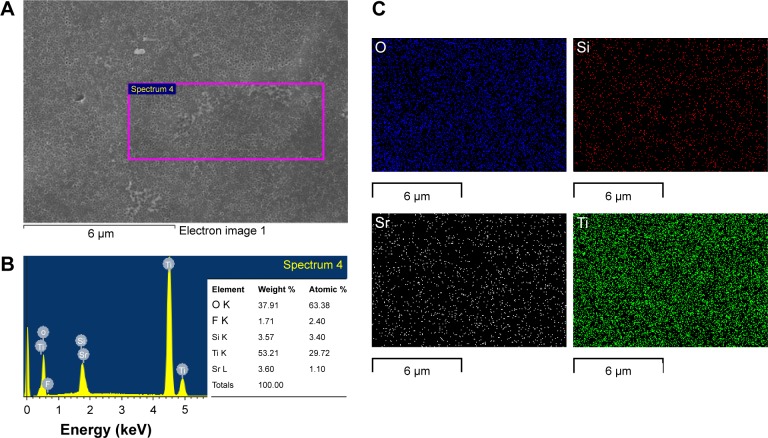
The energy-dispersive spectroscopy (EDS) patterns of the Si–Sr-TNs (Figure 3A) shown in Figure 3B indicate that the Si–Sr-TNs are mainly composed of Sr, Si, O, and Ti; the O and Ti amounts are primarily due to the TNs; the presence of Si and Sr suggests that the Si and Sr had been doped into the TNs; the amounts of the incorporated Si and Sr were found to be ~3.57 and 3.60 wt%, respectively. Figure 3C reflects the uniform distribution of O, Si, Sr, and Ti across the coating surface.
Figure 3.
The FESEM images of the Si–Sr-TNs (A), the EDS elemental spectrum (B), and EDS mapping spectrum (C) of the Si–Sr-TNs.
Abbreviations: EDS, energy-dispersive spectroscopy; FESEM, field-emission scanning electron microscopy; Si, silicon; Sr, strontium; TNs, titania nanotubes.
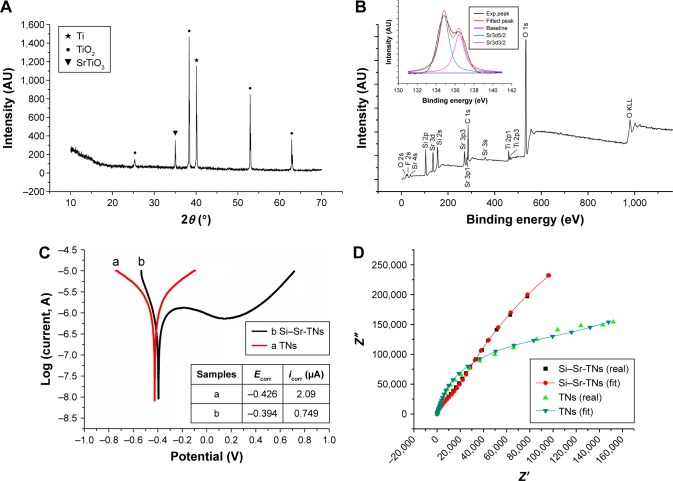
The peak located at 35° of the XRD patterns of the Si–Sr-TNs, given in Figure 4A, can be attributed to cubic SrTiO3, which indicates that the TiO2 in the NTs has partly been converted into SrTiO3 during the hydrothermal process;3 moreover, there are peaks in the spectrum that have originated from anatase TiO2 and the Ti substrate. The full XPS of the Si–Sr-TNs, given in Figure 4B, confirms that the Sr–Si-TN coating layers consisted of Si, Ti, O, and Sr. The fine XPS spectrum of Sr3d in the Si–Sr-TNs includes two peaks at 134.6 and 136.7 eV that are assigned to Sr3d5/2 and Sr3d3/2, respectively; therefore, the Sr in the TNs is in the form of SrTiO3. The fine XPS peaks of Si2p and Si2s, which have been measured at 368.3 and 374.3 eV, respectively, confirm that the deposits within the SrTiO3 NT array consist of SiO2 and Sr2SiO4, which was consistent with the EDS analysis.
Figure 4.
(A) The XRD pattern of the Si–Sr-TNs, (B) the XPS general spectrum of the Si–Sr-TNs, (C) the Tafel polarization curves of the TNs and Si–Sr-TNs, (D) the experimental and fitted Nyquist plots of the TNs and Si–Sr-TNs. The results here belong to only one sample, though we did many measurements on many samples. The parameters Ecorr and icorr stand for the corrosion potential and the corrosion current density, respectively.
Abbreviations: Exp, experimental; Si, silicon; Sr, strontium; TNs, titania nanotubes; XPS, X-ray photoelectron spectroscopy; XRD, X-ray diffraction.
Despite the high corrosion resistance of Ti materials, they are not inert to corrosive attack in the presence of the aggressive biological environment.19 Thus, the study of how Si–Sr-TNs behave when submitted to corrosive conditions is of upmost importance.20 Figure 4C shows that the Si–Sr-TNs exhibit better corrosion resistance than the TN samples. The nanoporous surface of the TNs gives rise to a high corrosion current density (icorr) for the TN sample. The results summarized in the inserted table (Figure 4C) indicate that the incorporation of Si and Sr has improved the corrosion resistance of the TN samples, which is in line with the findings of Ahmadi et al.21 As shown in Figure 4D, the Nyquist plots of the TN and Si–Sr-TN samples indicate that the latter have larger capacitive loops than the former. As the loop radius increases, the corrosion resistance can be enhanced because of higher impedance values.22 The same conclusion can be drawn through a qualitative comparison between the polarization data and the Nyquist plots. It is known that implant surface features significantly influence the electrochemical stability of the metallic implant when in contact with simulated body fluids. Very interestingly, in particular, TiO2 NTs have also shown potential to prevent long-term implant failure due to biocorrosion.23 In general, both the nanotubular surfaces display a fast and effective passivation behavior, which may be related to the properties of the barrier layer formed at TN interfaces during the anodization process.24 The same trend was observed by Grotberg et al23 for NTs grown from Ti6Al4V substrates. Yu et al25 also studied the corrosion behavior of TiO2 NT layers in Hank’s solution, and they concluded that the Ipass density was significantly influenced by Ti oxide NTs grown by anodization. Electrochemical impedance spectroscopy results confirmed the better corrosion resistance of TNs because of a thicker barrier layer present on nanotubular films than on smooth Ti. From electrochemical impedance spectroscopy and potentiodynamic studies, the authors concluded that very low corrosion current densities were recorded on TiO2 NTs due to a strong passive oxide film formation.26 However, after Sr and Si enrichment of NT surfaces by anodization-hydrothermal processes, significantly lower icorr values were found for Si–Sr-TN samples (Figure 4C), suggesting that they display superior corrosion resistance ability. The characterization of the newly formed TN interfaces after hydrothermal method of NTs in Sr/Si electrolyte regarding compact layer morphology, thickness, and elemental composition is of upmost importance. This knowledge will allow to predict the mechanisms behind the barrier layer formation and to understand the properties responsible for the improved corrosion resistance of Sr/Si-doped TiO2 NTs. The enhanced corrosion resistance provides a relatively stable environment for better initial cell attachment and the ensuing cell growth.
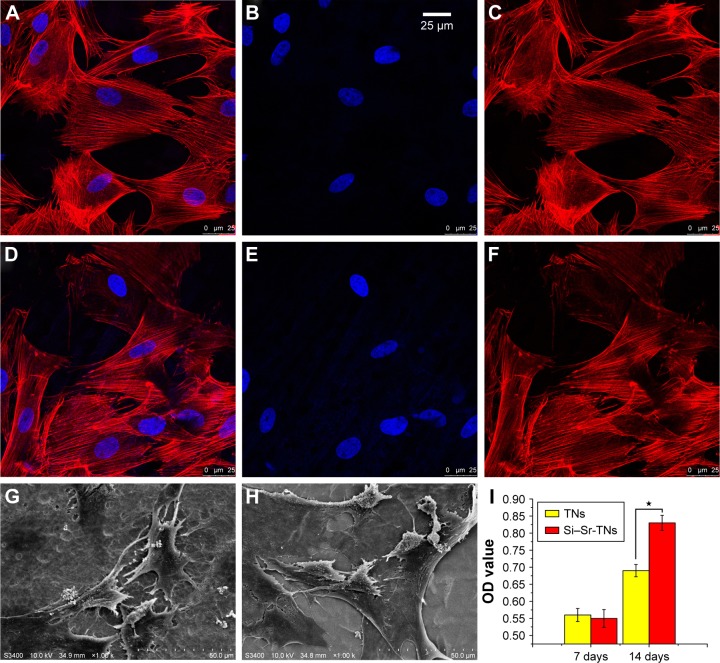
The immunofluorescence images given in Figure 5A–F reveal the cytoskeletal actin of cells, which indicate that the number of adhered cells did not significantly differ after culturing for 1 day; there is even no obvious difference between the cytoskeletons of the TN and Si–Sr-TN samples; that is, all the cells were polygonal and well distributed. Figure 5G and H demonstrates the cellular attachment behavior on the surfaces of the TNs and the Si–Sr-TNs, respectively. The resulted morphologies are not very distinct – the MC3T3-E1 cells are flat and actively spread with a number of filopodia protrusions, which reflect a normal cell attachment and growing process.27
Figure 5.
The confocal microscopic fluorescent images of cells that were immunostained for F-actin (red) and nuclei (blue) after being cultured on the TNs (A–C) and the Si–Sr-TNs (D–F) for 24 h. The scanning electron microscopy images of the morphology of the cells cultured on the TNs (G) and the Si–Sr-TNs (H) for 24 h. (I) The ALP activity of the osteoblasts cultured on the TNs and the Si–Sr-TNs in 7 and 14 days (*P⩽0.05).
Abbreviations: ALP, alkaline phosphatase; OD, optical density; Si, silicon; Sr, strontium; TNs, titania nanotubes.
The proliferation of MC3T3-E1 cells cultured on the TNs and Si–Sr-TNs was measured by CCK-8 assay; the data have not been presented here; no significant difference in both of the sample groups was observed, suggesting that cellular toxicity is not a concern. Figure 5I illustrates the activity of the ALP in the cells on the surface of the TN and the Si–Sr-TN samples; the amount of the ALP increased with increasing the cell incubation time – after 14 days of incubation, the amount of the ALP in the Si–Sr-TNs was much higher than that in the TNs. In conclusion, the MC3T3-E1 cells could keep their typical osteoblastic morphology on the Si–Sr-TNs surface, which is an indication of its minimal cytotoxicity. Furthermore, the released mineral ions such as Sr and Si from the Si–Sr-TNs can promote the osteoblast differentiation. According to Lin et al,15 the codoping of the functional elements like Si/Sr may be of considerable use in designing osteogenic and angiogenic bioactive materials for bone regeneration.
Conclusion
For the first time, through anodization, Ti implant surfaces were coated with TN arrays, which were then loaded with Si and Sr by a hydrothermal treatment. Through in vitro electrochemical measurements, we have learned that the corrosion resistance of the Si–Sr-TN-coated Ti surfaces is superior to that of the TN-coated surfaces of Ti. Both types of coatings have been found to possess similar cytoskeleton and morphology, that is, the trace Si and Sr doping did not change the proliferation of osteoblasts (MC3T3-E1). However, the codoping has improved the osteoblasts’ differentiation, for the ALP secretion has been increased. Being easily and economically fabricated, the Si–Sr-TN surfaces may find their niche in clinical applications, thanks to their excellent biological activity and corrosion resistance.
Acknowledgments
This work was supported by the Second Batch of Young Talents Fund of Hebei Province, China, the Natural Science Foundation of Hebei Province, China (number H2016405008), the Youth Fund of Science and Technology of Hebei Colleges, China (QN2017010), the Population Health Information in Hebei Province Engineering Technology Research Center, the Science and Technology Research Project of Zhangjiakou, China (17120012D), and the Medical Science Research Project Planning Project of Health and Family Planning Commission of Hebei Province (2018).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Yang L, Chinthapenta V, Li Q, et al. Understanding osteoblast responses to stiff nanotopographies through experiments and computational simulations. J Biomed Mater Res A. 2011;97(4):375–382. doi: 10.1002/jbm.a.33094. [DOI] [PubMed] [Google Scholar]
- 2.Mathew MT, Abbey S, Hallab NJ, Hall DJ, Sukotjo C, Wimmer MA. Influence of pH on the tribocorrosion behavior of CpTi in the oral environment: synergistic interactions of wear and corrosion. J Biomed Mater Res B Appl Biomater. 2012;100(6):1662–1671. doi: 10.1002/jbm.b.32735. [DOI] [PubMed] [Google Scholar]
- 3.Cheng H, Xiong W, Fang Z, et al. Strontium (Sr) and silver (Ag) loaded nanotubular structures with combined osteoinductive and antimicrobial activities. Acta Biomater. 2016;31:388–400. doi: 10.1016/j.actbio.2015.11.046. [DOI] [PubMed] [Google Scholar]
- 4.Minagar S, Li Y, Berndt CC, Wen C. The influence of titania-zirconia-zirconium titanate nanotube characteristics on osteoblast cell adhesion. Acta Biomater. 2015;12:281–289. doi: 10.1016/j.actbio.2014.10.037. [DOI] [PubMed] [Google Scholar]
- 5.Lin X, Yang SF, Lai K, et al. Orthopedic implant biomaterials with both osteogenic and anti-infection 3 capacities and associated in vivo evaluation methods. Nanomedicine. 2017;13(1):123–142. doi: 10.1016/j.nano.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Macak JM, Tsuchiya H, Ghicov A, et al. TiO2 nanotubes: self-organized electrochemical formation, properties and applications. Curr Opin Solid State Mater Sci. 2007;11(1–2):3–18. [Google Scholar]
- 7.Lee K, Mazare A, Schmuki P. One-dimensional titanium dioxide nanomaterials: nanotubes. Chem Rev. 2014;114(19):9385–9454. doi: 10.1021/cr500061m. [DOI] [PubMed] [Google Scholar]
- 8.Cipriano AF, Miller C, Liu H. Anodic growth and biomedical applications of TiO2 nanotubes. J Biomed Nanotechnol. 2014;10(10):2977–3003. doi: 10.1166/jbn.2014.1927. [DOI] [PubMed] [Google Scholar]
- 9.Indira K, Kamachi Mudali U, Rajendran N. In-vitro biocompatibility and corrosion resistance of strontium incorporated TiO2 nanotube arrays for orthopaedic applications. J Biomater Appl. 2014;29(1):113–129. doi: 10.1177/0885328213516821. [DOI] [PubMed] [Google Scholar]
- 10.Xin YC, Jiang J, Huo KF, Hu T, Chu PK. Bioactive SrTiO3 nanotube arrays: strontium delivery platform on Ti-based osteoporotic bone implants. ACS Nano. 2009;3(10):3228–3234. doi: 10.1021/nn9007675. [DOI] [PubMed] [Google Scholar]
- 11.Zhong S, Luo RF, Wang X, et al. Effects of polydopamine functionalized titanium dioxide nanotubes on endothelial cell and smooth muscle cell. Colloids Surf B Biointerfaces. 2014;116:553–560. doi: 10.1016/j.colsurfb.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 12.Zhang YX, Dong CF, Yang SF, et al. Alkalescent nanotube films on a titanium-based implant: a novel approach to enhance biocompatibility. Mater Sci Eng C Mater Biol Appl. 2017;72:464–471. doi: 10.1016/j.msec.2016.11.096. [DOI] [PubMed] [Google Scholar]
- 13.Khudhair D, Bhatti A, Li Y, et al. Anodization parameters influencing the morphology and electrical properties of TiO2 nanotubes for living cell interfacing and investigations. Mater Sci Eng C. 2016;59:1125–1142. doi: 10.1016/j.msec.2015.10.042. [DOI] [PubMed] [Google Scholar]
- 14.Zhao XJ, Wang T, Qian S, Liu XY, Sun JY, Li B. Silicon-doped titanium dioxide nanotubes promoted bone formation on titanium implants. Int J Mol Sci. 2016;17(3):292–304. doi: 10.3390/ijms17030292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin KL, Wang XH, Zhang N, Shen YH. Strontium (Sr) strengthens the silicon (Si) upon osteoblast proliferation, osteogenic differentiation and angiogenic factor expression. J Mater Chem B. 2016;4:3632–3638. doi: 10.1039/c6tb00735j. [DOI] [PubMed] [Google Scholar]
- 16.Huang Y, Xu ZW, Zhang XJ, et al. Nanotube-formed Ti substrates coated with silicate/silver co-doped hydroxyapatite as prospective materials for bone implants. J Alloy Compd. 2017;697:182–199. [Google Scholar]
- 17.Huang Y, Zhang XJ, Zhang HL, et al. Fabrication of silver- and strontium-doped hydroxyapatite/TiO2 nanotube bilayer coatings for enhancing bactericidal effect and osteoinductivity. Ceram Int. 2017;43(1 pt B):992–1007. [Google Scholar]
- 18.Bakhsheshi-Rad HR, Hamzah E, Ismail AF, et al. Novel bi-layered nano-structured SiO2/Ag-FHAp coating on biodegradable magnesium alloy for biomedical applications. Ceram Int. 2016;42(10):11941–11950. [Google Scholar]
- 19.Jayaraman M, Meyer U, Bühner M, Joos U, Wiesmann HP. Influence of titanium surfaces on attachment of osteoblast-like cells in vitro. Biomaterials. 2004;25(4):625–631. doi: 10.1016/s0142-9612(03)00571-4. [DOI] [PubMed] [Google Scholar]
- 20.Alves SA, Patel SB, Sukotjo C, et al. Synthesis of calcium-phosphorous doped TiO2 nanotubes by anodization and reverse polarization: a promising strategy for an efficient biofunctional implant surface. Appl Surf Sci. 2017;399:682–701. [Google Scholar]
- 21.Ahmadi S, Mohammadi I, Sadrnezhaad SK. Hydroxyapatite based and anodic titania nanotube biocomposite coatings: fabrication, char-acterization and electrochemical behavior. Surf Coat Technol. 2016;287:67–75. [Google Scholar]
- 22.Roman I, Trusca RD, Soare ML, et al. Titanium dioxide nanotube films: preparation, characterization and electrochemical biosensitivity towards alkaline phosphatase. Mater Sci Eng C. 2014;37:374–382. doi: 10.1016/j.msec.2014.01.036. [DOI] [PubMed] [Google Scholar]
- 23.Grotberg J, Hamlekhan A, Butt A, et al. Thermally oxidized titania nanotubes enhance the corrosion resistance of Ti6Al4V. Mater Sci Eng C. 2016;59:677–689. doi: 10.1016/j.msec.2015.10.056. [DOI] [PubMed] [Google Scholar]
- 24.Monsees TK, Barth K, Tippelt S, et al. Effects of different titanium alloys and nanosize surface patterning on adhesion, differentiation, and orientation of osteoblast-like cells. Cells Tissues Organs. 2005;180(2):81–95. doi: 10.1159/000086749. [DOI] [PubMed] [Google Scholar]
- 25.Yu Wq, Qiu J, Xu L, Zhang Fq. Corrosion behaviors of TiO2 nanotube layers on titanium in Hank’s solution. Biomed Mater. 2009;4(6):065012. doi: 10.1088/1748-6041/4/6/065012. [DOI] [PubMed] [Google Scholar]
- 26.Yu X, Li Y, Wlodarski W, Kandasamy S, Kalantarzadeh K. Fabrication of nanostructured TiO2 by anodization: a comparison between electrolytes and substrates. Sens Actuators B Chem. 2008;130(1):25–31. [Google Scholar]
- 27.Kim SY, Kim YK, Jang YS, et al. Bioactive effect of alkali-heat treated TiO2 nanotubes by water or acid treatment. Surf Coat Technol. 2016;303(Prt A):256–267. [Google Scholar]