Abstract
CD30 is a novel therapeutic target in human mast cell (MC) neoplasms. In this ‘comparative oncology’ study, we examined CD30 expression and regulation in neoplastic canine MC using a panel of immunomodulatory cytokines [interleukin-2 (IL-2), IL-4, IL-5, IL-6, IL-13 and stem cell factor (SCF)] and the canine mastocytoma cell lines NI-1 and C2. Of all cytokines tested IL-4 was found to downregulate expression of CD30 in NI-1 and C2 cells. We also found that the CD30-targeting antibody-conjugate brentuximab vedotin induces growth inhibition and apoptosis in both MC lines. Next, we asked whether IL-4-induced downregulation of CD30 interferes with brentuximab vedotin-effects. Indeed, pre-incubation of NI-1 cells with IL-4 decreased responsiveness towards brentuximab vedotin. To overcome IL-4-mediated resistance, we applied drug combinations and found that brentuximab vedotin synergizes with the Kit-targeting drugs masitinib and PKC412 in inhibiting growth of NI-1 and C2 cells. In summary, CD30 is a new marker and IL-4-regulated target in neoplastic canine MC.
Keywords: brentuximab vedotin, canine mastocytoma, CD30, IL-4, masitinib, PKC412
Introduction
During the past few years, comparative oncology has become a rapidly expanding field with great perspectives and robust clinical models. One of the best studied models may be mast cell (MC) neoplasms. In both the human and the canine system, MC disorders are characterized by an accumulation of neoplastic MC in various organs and by clinical symptoms caused by vasoactive and inflammatory mediators released from MC.1–6 Systemic mastocytosis (SM) is a heterogeneous group of MC neoplasms in humans. Depending on the disease-type and molecular lesions, SM shows an indolent or aggressive clinical course.1–4 Pure cutaneous variants of mastocytosis have also been described in humans, and in a smaller subset of paediatric patients, solitary or multiple skin mastocytomas are found.1,2
In dogs, cutaneous mast cell tumours (MCT) represent the most frequent type of skin neoplasms, accounting for up to 21% of all cutaneous malignancies.5–8 In both species, MC neoplasms exhibit variable biological behaviour and courses, ranging from chronic and indolent with favourable outcomes to highly aggressive, systemic, disease variants with grave prognosis.1–8 In human patients with advanced SM and in dogs with high grade MCT, MC are often immature and show rapid proliferation in various organ systems, with consecutive organ damage.2,9
In humans, a number of different conventional drugs and chemotherapy agents have been tested in advanced SM, including interferon-alpha (IFN-α) and cladribine (2CdA). These drugs have been described to induce responses in a subset of patients with advanced SM. However, in patients with rapidly progressing aggressive SM or mast cell leukemia (MCL), responses are usually short-lived and the prognosis is poor.10–13
In most human patients with SM, transforming Kit mutations are detectable in neoplastic cells. These mutations are considered to be responsible for factor-independent, autonomous growth of MC in these patients. During the past few years, several Kit-targeting drugs have been developed, and several of these agents reportedly suppress the in vitro growth of human neoplastic MC.14–22 Recent data suggest that transforming Kit mutations also develop in canine mastocytomas and MC tumours in other species.5,6,17,23–25 In canine patients, the tyrosine kinase inhibitors (TKIs) masitinib and toceranib received approval for treatment of malignant MCT, and the effects of a number of additional promising targeted drugs have been investigated in canine MCT patients.26–28 However, although clinical responses are seen quite frequently, they are usually short-lived and followed by a relapse, which points to secondary resistance.29–33
The Ki-1 antigen, also known as CD30, has long been recognized as a rather specific marker of neoplastic cells in human Hodgkin’s disease and ALK+ anaplastic large cell lymphomas.34,35 Other haematopoietic neoplasms are usually CD30-negative. However, recent data suggest that neoplastic human MC in advanced SM express substantial amounts of cytoplasmic CD30.36,37 It has also been described that neoplastic human MC express CD30 on their cell surface.38,39
However, so far, the mechanisms underlying aberrant expression of CD30 in neoplastic MC, remain unknown. Moreover, CD30 has not been investigated in the context of canine MC tumours so far. In this study, we examined the expression, function and regulation of CD30 in neoplastic canine MC and asked whether CD30 would serve as a potential therapeutic target in MCT.
Materials and methods
Reagents
Masitinib and midostaurin (PKC412) were purchased from LC Laboratories (Woburn, MA, USA), piceatannol and pimozide from Sigma-Aldrich (St Louis, MO, USA), RDEA119, PD0325901 and NVP-BEZ235 from Selleck (Houston, TX, USA) and RAD001 from ChemieTek (Indianapolis, IN, USA). The antibody-drug conjugate brentuximab vedotin (SGN-35) was kindly provided by Dr P. Veiby and Dr J. V. Garafalo (Millennium Takeda Oncology Company, Cambridge, MA, USA). Stock solutions of drugs were prepared by dissolving in dimethyl sulfoxide (Merck, Darmstadt, Germany). Recombinant human (rh) interleukin (IL)-2 was obtained from ImmunoTools (Friesoythe, Germany), rhIL-4 from Peprotech (Rocky Hill, NJ, USA), rhIL-5 from BD Biosciences (San Jose, CA, USA), rhIL-6 from Novartis (Basel, Switzerland), rhIL-13, rhCD30 ligand, recombinant canine (rc) IL-4, and rc stem cell factor (SCF) from R&D Systems (Minneapolis, MN, USA) and rhSCF from Strathmann Biotech (Hannover, Germany). RPMI 1640 medium and fetal calf serum (FCS) were purchased from PAA Laboratories (Pasching, Austria), 3H-thymidine from Amersham (Buckinghamshire, UK) and the Annexin V-FITC Kit from eBiosciences (San Diego, CA, USA).
Culture of canine cell lines
The canine mastocytoma cell line C2 was kindly provided by Dr W. Gold (Cardiovascular Research Institute, University of California, San Francisco, CA, USA).40 C2 cells were cultured in Iscove’s Modified Dulbecco’s Medium (IMDM) supplemented with 5% FCS, α-thioglycerol (Sigma-Aldrich) and antibiotics at 5% CO2 and 37 °C. The canine mastocytoma cell line NI-1 was established in our lab and was maintained in RPMI 1640 medium supplemented with 10% FCS and antibiotics at 5% CO2 and 37 °C.41
Immunocytochemistry and immunohistochemistry
Immunocytochemistry (ICC) was performed as described using cytospin-slides prepared from NI-1 and C2 cells.42 The mouse anti-human CD30 monoclonal antibody (mAb) Ber-H2 (work dilution, 1:20, at 4 °C for 16 h) and a biotinylated goat anti-mouse IgG were applied in these experiments. Ber-H2 has been validated for veterinary-use to detect CD30-positive activated B- and T-cells as well as CD30-positive canine neoplastic cells by immunohistochemistry (IHC).43–45 In this study, IHC was performed on formalin-fixed and paraffin-embedded tumour sections obtained from 32 patients (Table 1) essentially as described.42,46 Bone marrow sections from human CD30 positive mastocytosis patients were used as positive control, and sections from canine and human patients with normal bone marrow as negative control. In addition, antibody staining was controlled by demonstrating negativity of megakaryocytes and erythroid cells in bone marrow sections (internal negative control) as well as by antibody omission. Finally, we were able to demonstrate expression of CD30 mRNA in neoplastic MC by quantitative polymerase chain reaction (qPCR). The tumour grade in the canine patients examined was evaluated according to the Patnaik scheme.47 In IHC experiments, the indirect immunoperoxidase staining technique and the mouse anti-human CD30 mAb Ber-H2 (work dilution, 1:20; at 4 °C for 16 h) were applied. After immunostaining, slides were counterstained in Mayer’s Hemalaun and mounted in Aquatex. The stained tissue sections were examined by microscopy using the recently published scoring system.33 The numbers of CD30+ MC as well as the intensity of the staining reaction were recorded. The following semi-quantitative score was applied: (−), negative or <10% MC positive; (+), 10–50% MC positive; (++), >50% MC positive. Images were taken using an Olympus DP21 camera connected to an Olympus BX50F4 microscope (Olympus, Hamburg, Germany) (magnification: ×100). Figures were prepared using Adobe Photoshop CS2 software version 10.0 (Adobe Systems, San Jose, CA, USA) to adapt brightness and contrast.
Table 1.
Canine mastocytoma patients’ characteristics and CD30 expression in primary mast cell tumours
| # | Age (years) | Gender (m/f) | Breed | Localization | Stage | Grade | IHC CD30 | mRNA CD30 |
|---|---|---|---|---|---|---|---|---|
| 1 | 13.5 | fc | German Pinscher | Skin; eye lid | I | I | ++ | n.e. |
| 2 | 5 | f | Labrador Retriever | Skin; ear | I | I | ++ | n.e. |
| 3 | 9.5 | f | Golden Retriever | Skin; ear | I | I | + | n.e. |
| 4 | 7 | fc | Beagle | Skin; abdominal region | I | I | ++ | + |
| 5 | 6 | fc | Labrador Retriever | Skin; tail root | I | I | ++ | ++ |
| 6 | 6 | f | Boxer | Skin; thorax region | I | I | n.e. | n.e. |
| 7 | 16 | mc | Maltese | Mucosa; mouth | n.e. | I | ++ | n.e. |
| 8 | 7 | fc | Boxer | Skin; neck | I | II | + | ++ |
| 9 | 10 | m | Golden Retriever | Skin; head | I | II | ++ | n.e. |
| 10 | 8 | f | Maltese | Subcutis; neck | I | II | ++ | n.e. |
| 11 | 11.5 | m | Golden Retriever | Skin; flank | I | II | + | + |
| 12 | 13 | m | Golden Retriever | Skin; neck | I | II | ++ | ++ |
| 13 | 10 | fc | Labrador Retriever | Skin; abdominal region | I | II | ++ | ++ |
| 14 | 6.5 | f | Wire-haired Dachshund | Skin; perianal region | I | II | ++ | ++ |
| 15 | 9 | mc | Mixed breed | Skin; hind leg | II | II | + | + |
| 16 | 9.5 | fc | Miniature Schnauzer | Skin; hind leg | II | II | ++ | + |
| 16a | 9.5 | fc | Miniature Schnauzer | Lymph node metastasis | II | II | ++ | + |
| 17 | 7 | m | Golden Retriever | Multiple skin lesions | III | II | ++ | n.e. |
| 18 | 7.5 | m | Golden Retriever | Multiple skin lesions | III | II | ++ | n.e. |
| 19 | 8 | m | Golden Retriever | Multiple skin lesions | III | II | + | − |
| 20 | 11 | m | Golden Retriever | Multiple skin lesions | III | II | ++ | + |
| 21 | 10 | mc | German Shepherd Dog | Skin; thorax region | I | III | ++ | ++ |
| 22 | 10 | fc | Pit Bull Terrier | Skin; hind leg | III | III | + | n.e. |
| 23 | 13.5 | m | Alpine Dachsbracke | Multiple skin lesions | III | III | + | n.e. |
| 24 | 8.5 | f | Labrador Retriever | Multiple skin lesions | III | III | ++ | n.e. |
| 25 | 11.5 | m | Golden Retriever | Multiple skin lesions | III | III | ++ | n.e. |
| 26 | 12 | fc | Golden Retriever | Multiple skin lesions | III | III | ++ | ++ |
| 27 | 10.5 | fc | Golden Retriever | Subcutaneous lesion | III | III | ++ | ++ |
| 28 | 13 | fc | Mixed breed | Skin | III | III | + | − |
| 29 | 8.5 | m | Mixed breed | Skin; abdominal wall | III | III | + | − |
| 30 | 7 | m | Shar Pei | Multiple skin lesions | IV | III | ++ | ++ |
| 31 | 10.5 | fc | Pit Bull Terrier | Skin | IV | III | ++ | − |
| 32 | 13.5 | f | Golden Retriever | Skin; thorax wall | IV | III | + | ++ |
f, female; fc, female castrated: m, male; mc, male castrated; n.e., not evaluated; Grade according to the Patnaik et al. scheme41; IHC scoring system according to Sotlar et al.33: CD30 IHC staining score: −, negative or <10% MC positive; +, 10–50% MC positive; ++, >50% MC positive. Analysis of the CD30 mRNA expression: −, < 0.005 CD30 mRNA level (% of beta-actin); +, < 0.05 CD30 mRNA level (% of beta-actin); ++, > 0.05 CD30 mRNA level (% of beta-actin).
RNA extraction and quantitative real-time polymerase chain reaction
Total RNA was isolated from primary canine neoplastic MCT (n = 20) using the RNeasy Micro-Kit (Qiagen, Hilden, Germany) or from MC lines using RNeasy MinElute-Cleanup-Kit (Qiagen) according to the manufacturer’s instructions. PCR was performed using primers (Eurofins MWG Operon, Ebersberg, Germany) specific for canine CD30 (forward: 5′-CCAGGGATGGTCACCAAA-3′ and reverse: 5′-GTCTGGGTTGATGCTGCAC-3′); canine IL-4 receptor (IL-4R) (forward: 5′-CAG CACCACGTGGCTTAACT-3′ and reverse: 5′-CA GATGGCCAGGATGACG AG-3′); canine Kit receptor (forward: 5′-GGCTTGAGCAGGTCCATTTA-3′ and reverse: 5′-ACCAGCGTATCATTGCCTTC-3′) and canine beta-actin (forward: 5′-CCA AGGCCAACCGTGAGAAG-3′ and reverse: 5′-AGGGACAGCACAG CCTGGAT-3′). mRNA levels were quantified on a 7900HT Fast Real-Time PCR System (Applied Biosystem, Foster City, CA, USA) using iTAq SYBR Green Supermix with ROX (Bio-Rad, Hercules, CA, USA). While beta-actin is an established dog housekeeping gene expressed at high levels in all cells, Kit is a surface protein (like CD30) expressed in all MC and was not regulated by IL-4 in NI-1 and C2 cells. Expression levels of target genes were normalized to beta-actin or Kit by the 2−ΔΔCT method as described.48 In addition, CD30 mRNA expression was also determined by absolute copy number quantification to adjust for possible differences in PCR efficiency between target gene and control gene that could affect 2−ΔΔCT based relative quantification methods. For absolute quantification of CD30-, beta-actin- and Kit copy numbers, plasmid standards were generated by cloning of PCR products into the pCR2.1-TOPO vector (Life Technologies, Carlsbad, CA, USA). Plasmid DNA was diluted to a 4-point standard curve ranging from 103 to 106 copies per μL for CD30, beta-actin and Kit. Plasmid standards were included in each qPCR run, and standard curves were generated based on linear regression of CT-values and log10 of plasmid DNA copies using MS Excel (Microsoft). Standard curves showed a slope between −3.1 and −3.9 and a correlation coefficient ≥0.98 according to published guidelines.49 Absolute copy number of samples was calculated using slope and intercept of the respective linear regression line. Finally, CD30 copy numbers were normalized to beta-actin or Kit copy numbers and were expressed as percent of canine beta-actin or Kit. In a separate set of experiments, NI-1 cells were treated with various concentrations of rhIL-4 (50–200 ng mL−1) or rcIL-4 (50–200 ng mL−1) at 37 °C for 48 or 96 h before total RNA was isolated.
Flow cytometry analysis
Flow cytometry was performed on NI-1, C2 and MCPV cells using mAb against Kit and CD30. Before being examined, cell lines were incubated with various concentrations (50–200 ng mL−1) of rhSCF, rhIL-2, rhIL-4, rhIL-5, rhIL-6, rhIL-13, rhCD30 ligand, rcSCF or rcIL-4 at 37 °C for 48 or 96 h. Then, surface expression of CD30 and CD117 was determined by flow cytometry on a FACSCalibur (BD Biosciences) as reported.41 MCPV-1.1 cells were used as positive control to verify staining with anti-CD30 antibody. Staining reactions were controlled by isotype-matched control-antibodies. Flow cytometry results were expressed as staining index: ratio of mean fluorescence intensity (MFI) obtained with specific mAb to MFI obtained with control mAb. MFI defines the mean intensity of the fluorescence measured in a specific channel. The staining index is defined by the ratio of the MFI obtained with a specific mAb and of the MFI obtained with the isotype-matched mAb. When analysing cell surface marker expression by flow cytometry, only viable cells defined by forward side scatter characteristics, were included. In a separate set of experiments, NI-1 and C2 cells were incubated with various concentrations of either brentuximab vedotin (10–50 μgmL−1) at 4 or 37 °C (in parallel), or various concentrations of masitinib (0.05–2 μM), or PKC412 (0.05–2 μM) for 3–96 h at 37 °C before CD30 expression was analysed. In another set of experiments, NI-1 and C2 cells were incubated with various concentrations of the MEK inhibitors PD0325901 or RDEA119 (0.001–10 μM), the phosphatidylinositol-3-kinase/mechanistic target of rapamycin (PI3K/mTOR) inhibitor NVP-BEZ235, the mTOR blocker RAD001 (0.001–10 μM) or the STAT5 inhibitors piceatannol and pimozide (0.1–10 μM) at 37 °C for 24h. Subsequently, CD30 cell surface expression was analysed by flow cytometry. We also examined the effect of IL-4 on immunoglobulin E (IgE) receptor expression in NI-1 and C2 cells. In these experiments, cells were incubated with various concentrations of rcIL-4 (50–200 ngmL−1) at 37 °C for 96 h. Thereafter, IgE receptor expression was analysed by staining with fluorescein isothiocyanate (FITC)-labelled anti-IgE antibody A40-125F after preloading cells with 5 μgmL−1 dog IgE at 37 °C for 2 h. Antibody reactivity was determined on a FACSCalibur.
Apoptosis assays
AnnexinV/propidium iodide (PI) staining and active caspase-3 staining were performed by flow cytometry as described.41 Prior to staining, NI-1 and C2 cells were incubated with various concentrations of brentuximab vedotin (2.5–30 μg mL−1) at 37 °C for 96 h. To quantify expression of activated caspase-3 after drug exposure, cells were fixed in formaldehyde (2%) and permeabilized using methanol (100%) at −20 °C for 15 min. After staining with an antibody against active caspase-3 (clone C92-605; BD Biosciences) cells were analysed by flow cytometry on a FACSCalibur.
Measurement of 3H-thymidine uptake
To determine growth-modulating effects of cytokines and drugs, proliferation was analysed by measuring 3H-thymidine uptake as described.14 Briefly, NI-1 and C2 cells were incubated in various concentrations of brentuximab vedotin (0.001–50 μg/mL−1) at 37 °C for 96 h. To examine potential additive or synergistic drug effects, NI-1 and C2 cells were incubated with brentuximab vedotin and masitinib, or with brentuximab vedotin and PKC412 as single agents or in combination at a fixed ratio of drug concentrations at 37 °C for 96 h before 3H-thymidine uptake was measured. In another proliferation experiment, NI-1 cells were pre-incubated with 200 ng mL−1 rcIL-4 or control medium for 48 h. Subsequently, cells were treated with various drug combinations at fixed ratio (brentuximab vedotin + masitinib or brentuximab vedotin + PKC412) plus 200 ng mL−1 rcIL-4 or with drug combinations alone for 96 h. Thereafter, 3H-thymidine uptake was measured. In a further set of experiments, canine cell lines were incubated with various concentrations of rhIL-4 or rcIL-4 (50–200 ng mL−1) for 48 or 96 h, or rhCD30 ligand (50 – 100 ng mL−1) for 48 or 72 h (37 °C). To determine the influence of IL-4 on responsiveness of MC against brentuximab vedotin, NI-1 and C2 cells were pre-incubated with rhIL-4 (200 ng mL−1) or rcIL-4 (200 ng mL−1), or control medium for 48 h. Subsequently, cells were treated with various concentrations of brentuximab vedotin (1 – 50 μg mL−1) plus 200 ng mL−1 rhIL-4 or 200 ng mL−1 rcIL-4, or with brentuximab vedotin alone for 96 h. After incubation, 3H-thymidine uptake was measured. All experiments were performed in triplicates.
Statistical analysis
Data were presented as mean values from at least three independent experiments with standard deviation. Statistical analysis was performed by Student’s t-test. A P value <0.05 was considered to be statistically significant. Drug interactions (additive, synergistic and antagonistic) were determined by calculating combination index (CI) values using CalcuSyn software (Biosoft, Cambridge, UK). A CI value of 1 indicates an additive effect, whereas CI values below 1 indicate synergistic drug effects.
Results
Neoplastic canine MC express CD30
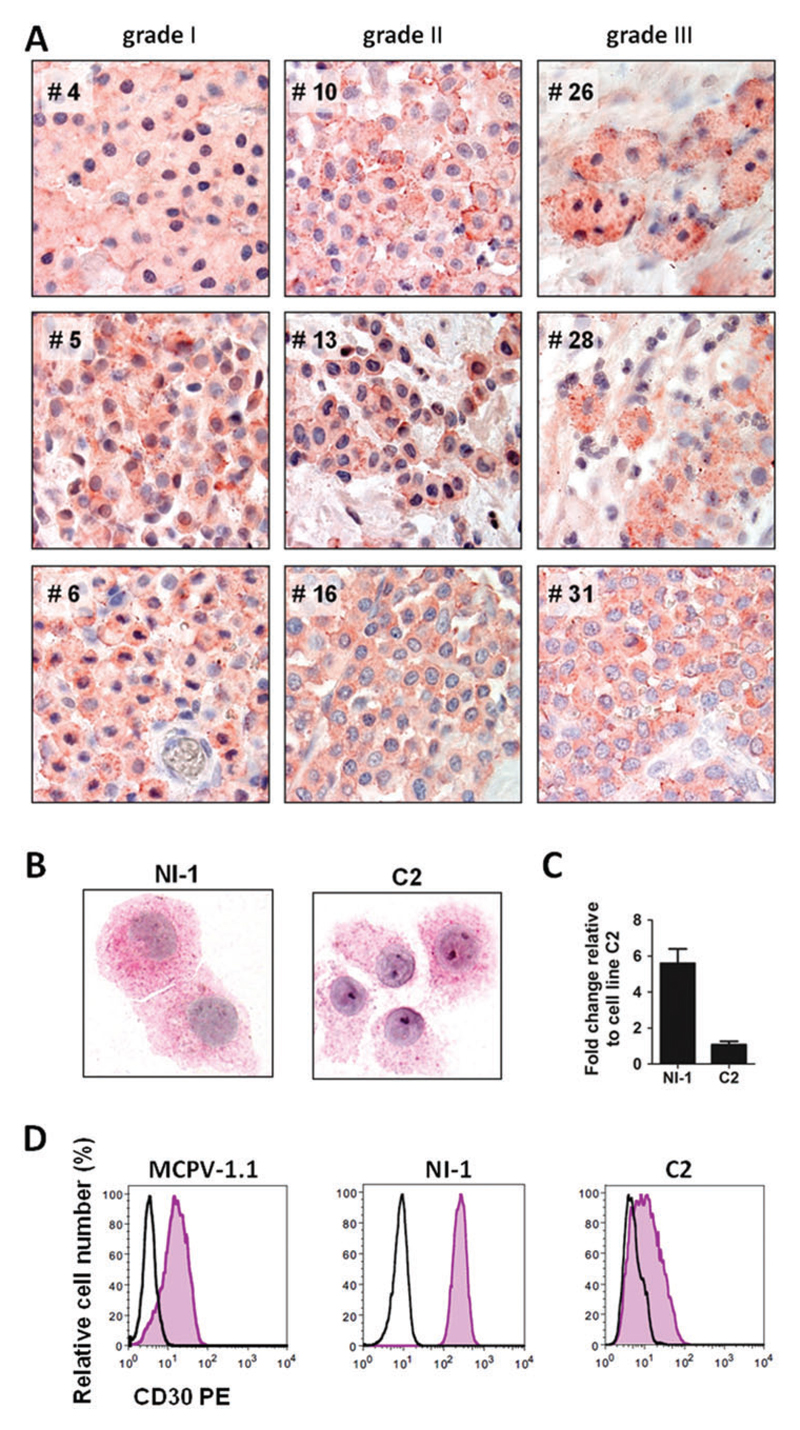
As assessed by IHC, canine neoplastic MC were found to express cytoplasmic and membrane-associated CD30 in all samples tested, independent of the grade of the mastocytoma (Fig. 1A, Table 1). Correspondingly, qPCR analyses revealed that canine neoplastic MCT express CD30 mRNA regardless of the grade of the mastoyctoma (Fig. S1, Supporting information). CD30 expression was also detected in the canine mastocytoma cell lines NI-1 and C2 by ICC (Fig. 1B). qPCR analysis confirmed CD30 mRNA expression in NI-1 and C2 cells (Fig. 1C). Interestingly, however, NI-1 cells expressed higher levels of CD30 mRNA compared with C2 cells (Fig. 1C). We also examined surface expression of CD30 on neoplastic MC by flow cytometry. MCPV-1.1 cells were employed as positive-control and found to express CD30 in these experiments (Fig. 1D). As visible in Fig. 1D, both NI-1 and C2 cells expressed CD30 on their cell surface. Again, NI-1 cells expressed substantially higher levels of CD30 compared with C2 cells (Fig. 1D).
Figure 1.
Expression of CD30 in neoplastic canine mast cells. (A) Immunohistochemical detection of CD30 in primary neoplastic MCs in canine mastocytoma lesions. Tissue sections were prepared from formalin-fixed, paraffin-embedded tumour samples obtained from nine canine patients (left panels: grade I tumours: #4, #5, #6; middle panels: grade II: #10, #13, #16; right panels: grade III: #26, #28, #31). Sections were stained with monoclonal anti-CD30 antibody Ber-H2 as described in the text. Original magnification: ×100. (B) Immunocytochemical detection of CD30 in the canine mastocytoma cell lines NI-1 and C2 using anti-CD30 antibody Ber-H2. NI-1 and C2 cells were spun on cytospin-slides and expression of CD30 was analysed by immunocytochemistry. Images were taken using an Olympus microscope as described in the text. Original magnification: ×100. (C) CD30 mRNA expression in NI-1 and C2 cells. RNA isolation, cDNA synthesis and qPCR analysis were performed as described in the text. Expression levels of CD30 mRNA were calculated by the 2−ΔΔCT method and beta-actin was used as internal control. The figure shows the mean ± SD of three independent experiments. (D) CD30 surface expression on MCPV-1.1, NI-1 and C2 cells was analysed by flow cytometry using phycoerythrin (PE)-labelled monoclonal antibody BerH8 directed against CD30 (pink histograms). The isotype-matched control antibody is also shown (black open histograms). [Colour figure can be viewed at wileyonlinelibrary.com]
Role of Kit and Kit downstream signalling pathways on CD30 expression in the canine MC lines NI-1 and C2
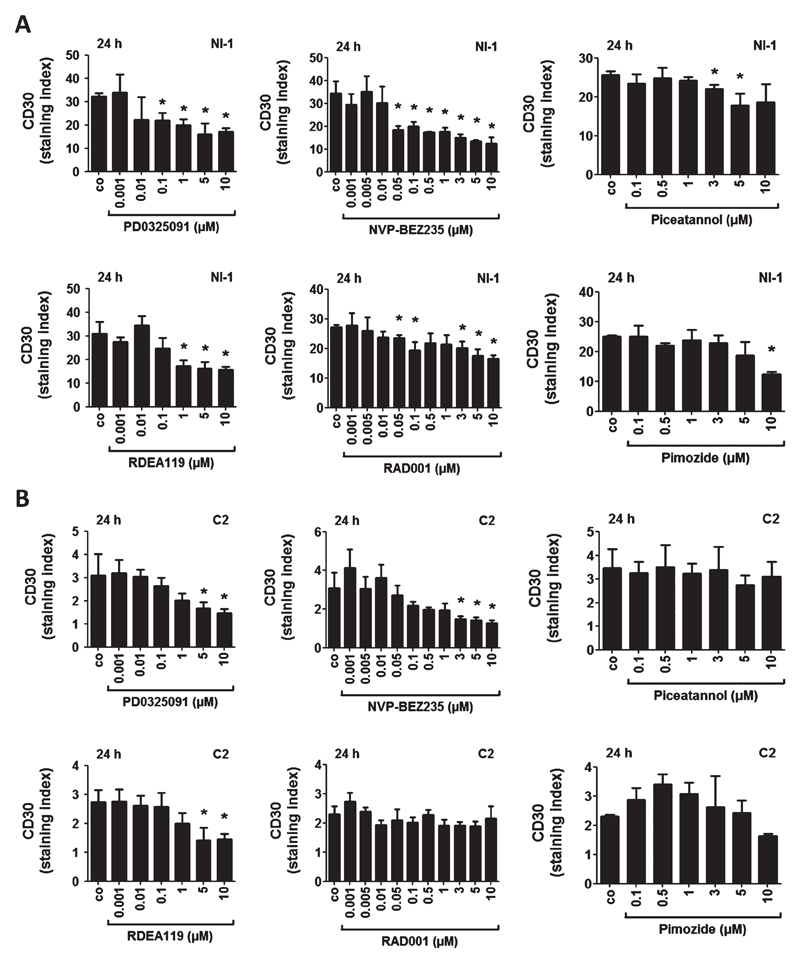
As assessed by flow cytometry, the Kit-targeting drugs masitinib and PKC412 induced a dose-dependent decrease in CD30 surface expression in NI-1 and C2 cells after 16, 24, 48 and 96 h (Fig. S2), whereas no drug effects on CD30 expression were seen after 3 or 6 h (data not shown). Next, we examined the effects of various drugs targeting Kit-downstream signalling molecules in neoplastic canine MC, including the MEK inhibitors PD0325901 and RDEA119, the PI3-kinase/mTOR blocker NVP-BEZ235, the mTOR blocker RAD001 (everolimus) and the STAT5 inhibitors piceatannol and pimozide. Both MEK inhibitors and the mTOR inhibitors decreased CD30 cell surface expression in a significant dose-dependent manner in NI-1 cells after 24 h (Fig. 2A), whereas both STAT5 inhibitors induced only a slight decrease in CD30 expression in NI-1 cells (Fig. 2A). In C2 cells, both MEK inhibitors and the PI3K/mTOR blocker NVP-BEZ235 reduced CD30 cell surface expression in a dose-dependent manner (Fig. 2B). However, RAD001, piceatannol and pimozide did not change CD30 expression after 24 h of treatment in C2 cells (Fig. 2B).
Figure 2.
Effects of various targeted drugs on CD30 expression in NI-1 and C2 cells. NI-1 cells (A) and C2 cells (B) were incubated with various concentrations of the MEK inhibitors PD0325091 and RDEA119, the PI3K/mTOR inhibitor NVP-BEZ235, the mTOR blocker RAD001 (everolimus) and the STAT5 inhibitors piceatannol and pimozide at 37 °C for 24 h. Subsequently, surface expression of CD30 was determined by flow cytometry using anti-CD30 mAb BerH8. Results are expressed as staining index (ratio of mean fluorescence intensity obtained with CD30 mAb and isotype-matched control mAb). Results represent the mean ± SD of three independent experiments. Asterisk (*): P <0.05.
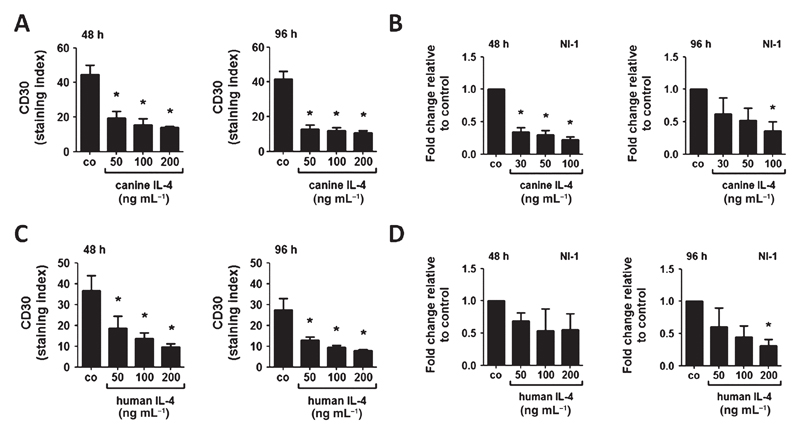
IL-4 downregulates CD30 expression in NI-1 and C2 cells
To further examine mechanisms underlying expression of CD30 on neoplastic MC, we applied various cytokines on NI-1 and C2 cells. Most of the cytokines tested did not affect CD30 expression in NI-1 and C2 cells. However, IL-4 was found to downregulate cell surface CD30 expression and CD30 mRNA expression in NI-1 cells (Fig. 3A – D). The effects of IL-4 were dose-dependent and were detectable after 48 and 96 h. Both rcIL-4 and rhIL-4 suppressed CD30 expression in these cells (Fig. 3A–D). In C2 cells, basal CD30 levels were low, but IL-4 was still able to downregulate CD30 expression (Fig. S3A). The other cytokines tested (rhSCF, rhIL-2, rhIL-5, rhIL-6, rhIL-13 and rcSCF) failed to modulate CD30 expression in NI-1 or C2 cells after 48 or 96 h (data not shown). As assessed by qPCR, NI-1 and C2 cells were found to display IL-4 receptors (data not shown). We also examined the effect of IL-4 on CD30 expression in the CD30+ human MC lines HMC-1.1 and MCPV-1.1. However, neither rhIL-4 nor rcIL-4 suppressed CD30 expression in these cells (Fig. S3B,C).
Figure 3.
Effects of canine and human IL-4 on CD30 expression in NI-1 cells. NI-1 cells were incubated with various concentrations of recombinant canine IL-4 (A, B) or recombinant human IL-4 (C, D) at 37 °C for 48 or 96 h. After incubation, expression of CD30 was determined by flow cytometry using anti-CD30 mAb BerH8; and expression of CD30 mRNA by qPCR analysis as described in the text. Flow cytometry results are expressed as staining index (= ratio of mean fluorescence intensity obtained with anti-CD30 mAb and isotype-matched control mAb); and results represent the mean ± SD of three independent experiments. Expression levels of CD30 mRNA were calculated by the 2−ΔΔCT method and beta-actin was used as internal control. The figures show the mean ± SD of three independent experiments. Asterisk (*): P <0.05.
IL-4 upregulates IgE receptor expression on NI-1 cells
In human MC, expression of several cell surface structures is regulated by IL-4, including the IgE receptor (upregulated) and Kit (CD117) (downregulated).3,4,50,51 In this study, rcIL-4 induced a slight increase in IgE receptor expression in NI-1 cells, whereas no effect of IL-4 on IgE receptor expression in C2 cells was seen (Fig. S4). In advanced mastocytomas, canine patients may also suffer from symptoms caused by MC mediators, including pruritus, flushing, gastrointestinal symptoms (or even bleeding) and other histamine-induced reactions. Therefore, we were interested to examine whether brentuximab vedotin, a CD30 targeting antibody-conjugate, would modulate IgE-dependent histamine release in canine MC. We have recently shown that brentuximab vedotin inhibits IgE-mediated histamine release in human MC.36 However, in this study, brentuximab vedotin failed to suppress IgE-dependent histamine release from NI-1 cells (Fig. S5). As C2 cells do not express a functionally active IgE receptor, we did not examine histamine release in these cells. Treatment with IL-4 (rcIL-4 or rhIL-4) did not change surface expression of Kit in NI-1 or C2 cells (Fig. S6A,B). In control experiments, rhIL-4 reduced Kit (CD117) surface expression in HMC-1 cells (Fig. S6C), confirming previous studies.52 Additionally, we tested the phosphorylation status of Kit by Western blot analysis. However, rcIL4 did not alter the levels of phosphorylated Kit in NI-1 cells (Fig. S7).
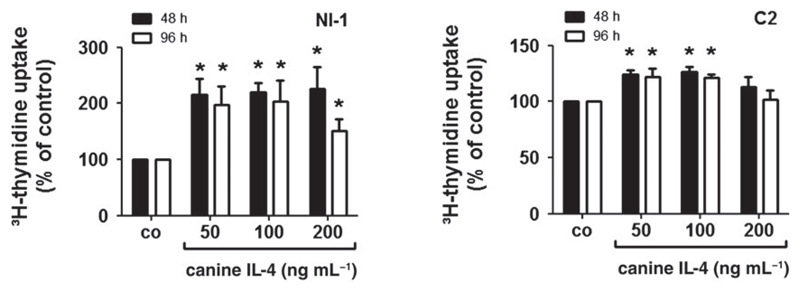
IL-4 augments proliferation of NI-1 and C2 cells
Next, we asked whether IL-4 is able to modulate proliferation in neoplastic canine MC. We found that rcIL-4 slightly promotes growth in NI-1 and C2 cells (Fig. 4). We also asked whether the CD30 ligand (CD30L) modulates CD30 expression or proliferation in canine MC. However, CD30L did not regulate CD30 expression or proliferation in NI-1 or C2 cells (data not shown).
Figure 4.
Effects of IL-4 on proliferation of NI-1 and C2 cells. NI-1 and C2 cells were incubated with various concentrations of recombinant canine IL-4 at 37 °C for 48 or 96 h. Thereafter, 3H-thymidine uptake was measured. Results are expressed as percent of control (co) and represent the mean ± SD of four independent experiments. Asterisk (*): P < 0.05.
Identification of CD30 as a novel target in NI-1 and C2 cells
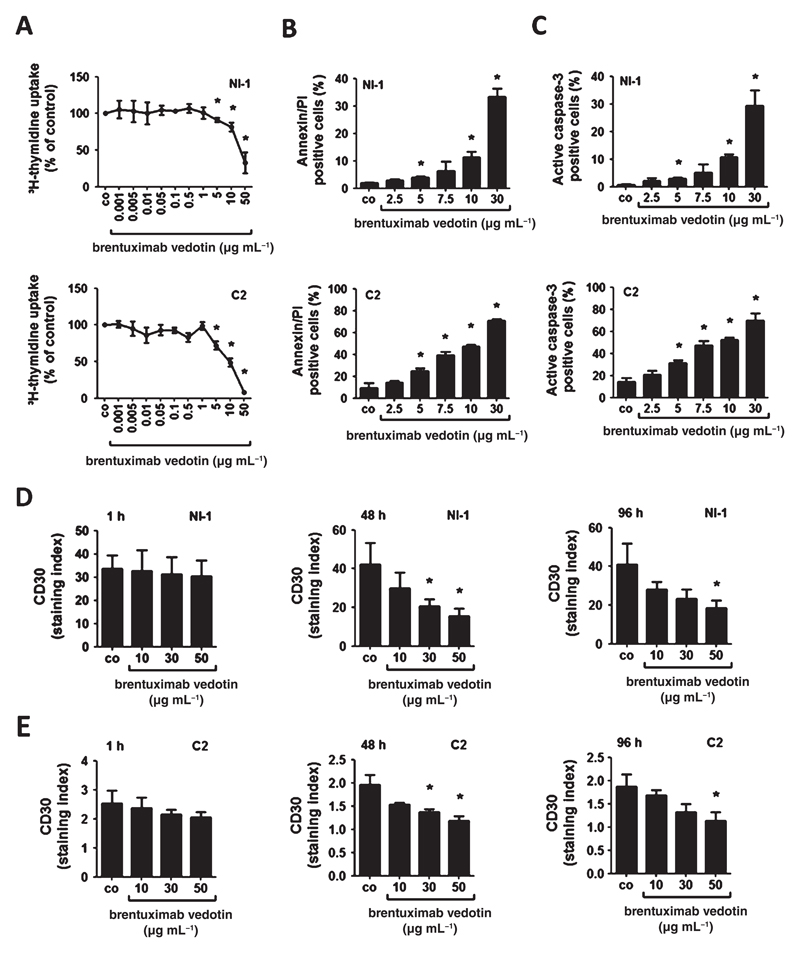
Brentuximab vedotin, a CD30-targeting antibody–drug conjugate, has been described to inhibit the growth of CD30+ lymphoma cells.33,34 We applied brentuximab vedotin in order to test its anti-neoplastic effects on neoplastic canine MC lines. In proliferation experiments, brentuximab vedotin induced dose-dependent growth inhibition in NI-1 and C2 cells, with higher IC50 values obtained in NI-1 cells (30 μg mL−1) compared with C2 cells (10 μg mL−1) (Fig. 5A). We also asked whether the growth-inhibitory effects of brentuximab vedotin are associated with induction of apoptosis. Indeed, brentuximab vedotin induced a dose-dependent increase in apoptosis in NI-1 and C2 cells as determined by AnnexinV/PI staining and active caspase-3 staining (Fig. 5B,C).
Figure 5.
Effects of brentuximab vedotin on proliferation, apoptosis and CD30 expression of NI-1 and C2 cells. (A) NI-1 and C2 cells were incubated with various concentrations of brentuximab vedotin at 37 °C for 96 h. Thereafter, 3H-thymidine uptake was measured. Results are expressed as percent of control (co) and represent the mean ± SD of three independent experiments. Asterisk (*): P < 0.05. (B, C) NI-1 and C2 cells were incubated in various concentrations of brentuximab vedotin at 37 °C for 96 h. Then, cells were examined by flow cytometry to determine the percentage of AnnexinV/PI-positive cells (B) and the percentage of active caspase-3 positive cells (C). Technical details are described in the text. Results represent the mean ± SD of three independent experiments. Asterisk (*): P < 0.05. (D, E) NI-1 and C2 cells were incubated in various concentrations of brentuximab vedotin at 37 °C for 1, 48 or 96 h. Thereafter, surface expression of CD30 was determined by flow cytometry. Results are expressed as staining index as described in the text. Results represent the mean ± SD of three independent experiments. Asterisk (*): P <0.05.
Effects of brentuximab vedotin on CD30 expression of canine MC line NI-1 and C2
Interestingly, incubation with brentuximab vedotin resulted in a dose-dependent decrease in CD30 surface expression in NI-1 and C2 cells at 37 °C after 1, 48 or 96 h (Fig. 5D). However, CD30 cell surface expression in NI-1 and C2 cells was not influenced by exposure to brentuximab vedotin at 4 °C (data not shown).
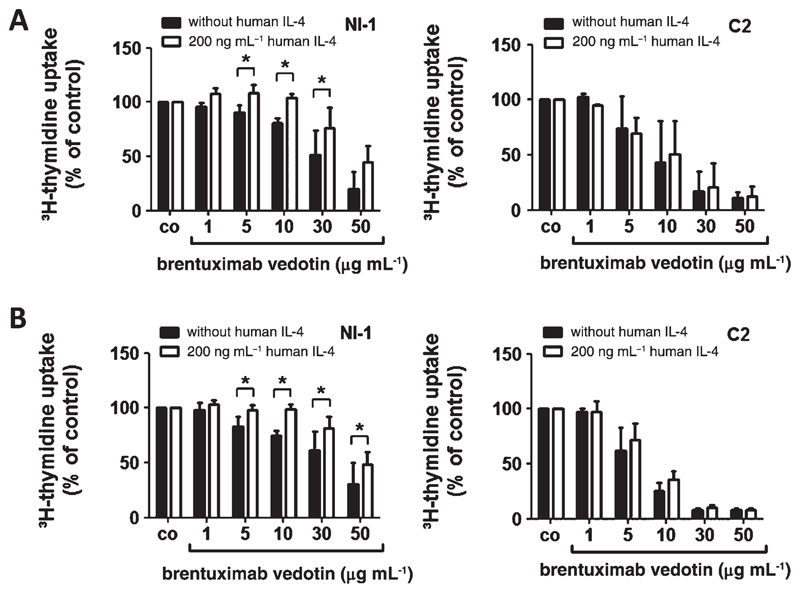
IL-4 decreases the responsiveness of the CD30+ canine MC line NI-1 against brentuximab vedotin
Based on our data described above, we asked whether canine mastocytoma cell lines might be less sensitive against brentuximab vedotin after IL-4 treatment due to decreased CD30 expression. To address this question, NI-1 and C2 cells were pre-incubated with control medium, rcIL-4 or rhIL-4, for 48 h before cells were exposed to brentuximab vedotin (96 h). In these experiments, IL-4-treated NI-1 cells were less responsive to brentuximab vedotin compared with untreated cells (Fig. 6A,B). In C2 cells expressing CD30 at a much lower level compared with NI-1 cells, IL-4 did not induce this effect (Fig. 6A,B).
Figure 6.
Effects of brentuximab vedotin on proliferation of IL-4-exposed NI-1 and C2 cells. (A, B) NI-1 and C2 cells were pre-incubated with 200 ng mL−1 recombinant canine IL-4 (A), recombinant human IL-4 (B) or control medium at 37 °C for 48 h. Then, cells were treated with various concentrations of brentuximab vedotin (plus 200 ng mL−1 IL-4) for 96 h. Thereafter, 3H-thymidine uptake was measured. Results are expressed as percent of control (co) and represent the mean ± SD of three independent experiments. Asterisk (*): P <0.05.
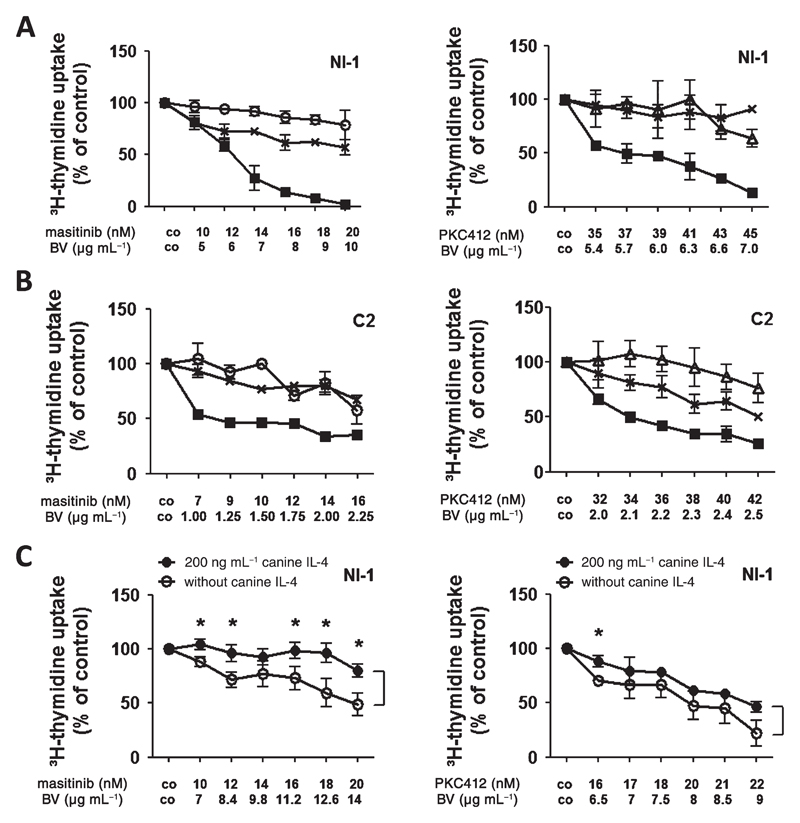
Brentuximab vedotin produces synergistic growth-inhibitory effects with masitinib and PKC412 in NI-1 and C2 cells
As currently available drugs are unable to induce long-lasting remissions in MCT patients; and several different mechanisms, including IL-4-induced downregulation of CD30, may lead to resistance, we applied drug combinations with the aim to achieve synergistic anti-neoplastic effects. Specifically, we applied brentuximab vedotin and masitinib or brentuximab vedotin and PKC412. As assessed by 3H-thymidine incorporation, brentuximab vedotin was found to synergize with masitinib and with PKC412 in producing growth inhibition in NI-1 and C2 cells (Fig. 7A,B). The synergistic drug interactions were confirmed by calculating CI values using Calcusyn software (Fig. S8). In a separate set of experiments, we tested drug combinations in the absence or presence of canine IL-4 in NI-1 cells. Pre-incubation with rcIL-4 for 48 h decreased responsiveness of NI-1 cells against brentuximab vedotin + masitinib or brentuximab vedotin + PKC412 treatment compared with NI-1 cells without rcIL-4 incubation (Fig. 7C).
Figure 7.
Effects of combinations of various targeted drugs on proliferation of IL-4-exposed NI-1 and C2 cells. NI-1 (A) and C2 cells (B) were incubated in various concentrations of brentuximab vedotin (⨉), masitinib (○), or PKC412 (△) alone (as single drug) or in various drug combinations (■) (at fixed ratio) at 37 °C for 96 h. Then, uptake of 3H-thymidine was determined. Results are expressed as percent of control (co) and represent the mean ± SD of triplicates from one typical experiment. (C) NI-1 cells were incubated with recombinant canine IL-4 (200 ng mL−1) or control medium for 48 h. Then, cells were treated with various drug combinations at fixed ratio [brentuximab vedotin (BV) + masitinib or brentuximab vedotin (BV) + PKC412] in the presence or absence of IL-4 for 96 h. Thereafter, 3H-thymidine uptake was measured. Results are expressed as percent of control (co) and represent the mean ± SD of triplicates from one typical experiment. Asterisk (*): P <0.05.
Discussion
During the last two decades, our increased understanding of the role of oncogenic kinases in the regulation of tumour cell growth in various cancers resulted in the development of specific TKI as new effective targeted drugs. Paradigmatic examples are canine and human MC neoplasms, both of which exhibit activating mutations in the Kit proto-oncogene that mediate growth-factor independent Kit activation and autonomous proliferation in neoplastic MC.5–7,41,42,46,50 Despite this knowledge and the consecutive development of various TKI for the treatment of advanced MC neoplasms, responses are mostly transient as advanced MCT often develop drug resistance.33
More recently, new insights into the molecular pathways involved in growth and survival of neoplastic MC has resulted in the identification of new drug targets. One emerging surface target aberrantly expressed on the surface of neoplastic MC is CD30, also known as the Ki-1 antigen.36,37,39,53 Recent data suggest that CD30 is expressed in human neoplastic MC and serves as a molecular target in advanced SM.39 Preliminary data from a small case series in human CD30-positive mastocytosis support this hypothesis although not all patients may respond to brentuximab.54 In this study, we show that CD30 is also expressed in neoplastic canine MC independent of the histopathological grade. In addition, we also show that CD30 serves as a cytokine-regulated target in canine MCT.
In a first step we examined MC in archival canine MCT samples for CD30 expression using IHC analysis. The results of these staining experiments suggest cytoplasmic and surface membrane expression of CD30 independent of the grade and stage of the MCT. Similar results were obtained in human MC disease. In fact, whereas initial studies described that neoplastic human MC display cytoplasmic CD30 in advanced SM rather than in indolent SM,36 subsequent studies revealed that CD30 is also expressed on the surface of human neoplastic MC and can be detected on these cells in both, indolent and aggressive mastocytosis.38 The staining reactions obtained in canine neoplastic MC (primary cells and cell lines) with an anti-CD30 mAb clearly indicate that CD30 is expressed in both the cytoplasm and on the surface of these cells.
Next, we investigated two established neoplastic canine MC lines, NI-1 and C2 cells, regarding surface and cytoplasmic expression of CD30. As assessed by ICC and flow cytometry, both cell lines expressed CD30 in a constitutive manner. In addition, we were able to confirm CD30 mRNA expression in all samples tested. These results are in line with recently published data on human neoplastic MC.36,37,39
To investigate the regulation of CD30 expression in neoplastic MC, we examined the effects of various cytokines, drugs targeting cytokine receptors and drugs targeting related downstream signalling molecules. In canine and human neoplastic MC, several different pro-oncogenic signalling pathways and molecules are triggered by the kinase activity of (mutated) Kit. Therefore, we first examined the role of Kit as a potential trigger of CD30 expression. Indeed, two Kit-blocking TKI, masitinib and PKC412, were found to downregulate the expression of CD30 in NI-1 and C2 cells. By contrast, however, no effect of SCF on CD30 expression was found, which may be explained by the fact that Kit is already expressed in neoplastic MC in an (maximally) activated form. Next, we examined Kit-downstream signalling pathways. In these experiments, we found that two different MEK inhibitors decrease the expression of CD30 in NI-1 and C2 cells. Moreover, PI3 kinase/mTOR inhibitors also reduced CD30 expression in the cell lines tested. These data suggest that both Kit-downstream pathways are involved in the regulation of expression of CD30 in neoplastic canine MC. By contrast, STAT5-inhibition did not result in a marked decrease of expression of CD30 in neoplastic canine MC.
We next asked whether other cytokine-interactions (apart from Kit) might be involved in the regulation of CD30 expression in neoplastic canine MC. Of all cytokines tested, IL-4 was found to downregulate the expression of CD30 in a dose-dependent manner in our flow cytometry and qPCR experiments. A number of different studies have shown that IL-4 is a major regulator of growth and differentiation of MC in humans as well as in mice.55–60 However, so far, no effects of IL-4 on canine MC have been reported. In this study, we were able to show that rcIL-4 promotes the proliferation of NI-1 and C2 cells. To the best of our knowledge this is the first report describing IL-4 as a MC growth factor in the canine system. An interesting aspect was that NI-1 and C2 cells responded well to canine IL-4, whereas no substantial effects of human IL-4 on growth of canine MC were seen, which may point to a species-specific cytokine effect. Nevertheless, human IL-4 was also found to suppress CD30 expression in NI-1 cells, although at higher concentrations when compared with canine IL-4. Alternatively, the differential response to canine and human IL-4 is a cell line-dependent phenomenon. Indeed, major growth-promoting effects were only seen in NI-1 cells, whereas in C2 cells, the growth-augmenting effect of IL-4 was rather weak.
In human CD30+ lymphomas, especially in drug-refractory Hodgkin’s disease, CD30 is an established target of therapy. Notably, several CD30-targeting drugs, including brentuximab vedotin, have been developed. In this study, we asked whether CD30 may also serve as a target of therapy in canine MCT, and applied the anti-CD30 antibody–drug conjugate brentuximab vetodin. Indeed, this drug was found to induce growth-inhibitory and cytotoxic effects on CD30+ canine neoplastic MC lines NI-1 and C2.
Recent data suggest that resistance of neoplastic (human) MC against TKI can be overcome by applying drug combinations.25,46 Therefore, we examined the cooperative effects of ‘brentuximab vedotin+PKC412’ and ‘brentuximab vedotin+masitinib’ on growth of neoplastic canine MC lines NI-1 and C2. Both drug combinations were found to produce synergistic growth-inhibitory effects in the absence and presence of IL-4. These results are encouraging and suggest that drug combinations may overcome IL-4-mediated partial resistance and may therefore induce prolonged responses in vivo.
In conclusion, we provide evidence that CD30 is expressed in canine MCT cells and may represent a potential marker and target. While IL-4 downregulates expression of CD30 and introduces partial resistance against brentuximab vedotin in our cell line experiments, we were able to overcome resistance by applying drug combinations. Based on our data, CD30 may serve as a novel therapeutic target in canine MCT but further studies investigating more patients, and finally clinical trials, are required to confirm our data generated in canine cell lines.
Supplementary Material
Additional Supporting Information may be found in the online version of this article:
Acknowledgements
This work was supported by the Austrian Science Fund (FWF) P 21173-B13 and F 4704-B20 as well as by a research grant of Millennium Takeda Oncology Company.
Footnotes
Conflict of interest
P. V. is consultant in a Novartis trial and received research funding from Novartis. The remaining authors declare no conflict of interest.
References
- 1.Metcalfe DD. Classification and diagnosis of mastocytosis: current status. Journal of Investigative Dermatology. 1991;96:2S–4S. [PubMed] [Google Scholar]
- 2.Valent P, Horny HP, Escribano L, Longley BJ, Li CY, Schwartz LB, et al. Diagnostic criteria and classification of mastocytosis: a consensus proposal. Leukemia Research. 2011;25:603–625. doi: 10.1016/s0145-2126(01)00038-8. [DOI] [PubMed] [Google Scholar]
- 3.Escribano L, Akin C, Castells M, Orfao A, Metcalfe DD. Mastocytosis: current concepts in diagnosis and treatment. Annals of Hematology. 2002;81:677–690. doi: 10.1007/s00277-002-0575-z. [DOI] [PubMed] [Google Scholar]
- 4.Metcalfe DD. Mast cells and mastocytosis. Blood. 2008;112:946–956. doi: 10.1182/blood-2007-11-078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.London CA, Galli SJ, Yuuki T, Hu ZQ, Helfand SC, Geissler EN. Spontaneous canine mast cell tumors express tandem duplications in the proto-oncogene c-kit. Experimental Hematology. 1999;27:689–697. doi: 10.1016/s0301-472x(98)00075-7. [DOI] [PubMed] [Google Scholar]
- 6.Zemke D, Yamini B, Yuzbasiyan-Gurkan V. Mutations in the juxtamembrane domain of c-KIT are associated with higher grade mast cell tumors in dogs. Veterinary Pathology. 2002;39:529–535. doi: 10.1354/vp.39-5-529. [DOI] [PubMed] [Google Scholar]
- 7.London CA, Seguin B. Mast cell tumors in the dog. Veterinary Clinics of North America: Small Animal Practice. 2003;33:473–489. doi: 10.1016/s0195-5616(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 8.Webster JD, Kiupel M, Kaneene JB, Miller R, Yuzbasiyan-Gurkan V. The use of KIT and tryptase expression patterns as prognostic tools for canine cutaneous mast cell tumors. Veterinary Patholology. 2004;41:371–377. doi: 10.1354/vp.41-4-371. [DOI] [PubMed] [Google Scholar]
- 9.Valent P, Akin C, Sperr WR, Escribano L, Arock M, Horny HP, et al. Aggressive systemic mastocytosis and related mast cell disorders: current treatment options and proposed response criteria. Leukemia Research. 2003;27:635–641. doi: 10.1016/s0145-2126(02)00168-6. [DOI] [PubMed] [Google Scholar]
- 10.Kluin-Nelemans HC, Jansen JH, Breukelman H, Wolthers BG, Kluin PM, Kroon HM, et al. Response to interferon alfa-2b in a patient with systemic mastocytosis. The New England Journal of Medicine. 1992;326:619–623. doi: 10.1056/NEJM199202273260907. [DOI] [PubMed] [Google Scholar]
- 11.Hauswirth AW, Simonitsch-Klupp I, Uffmann M, Koller E, Sperr WR, Lechner K, et al. Response to therapy with interferon alpha-2b and prednisolone in aggressive systemic mastocytosis: report of five cases and review of the literature. Leukemia Research. 2004;28:249–257. doi: 10.1016/s0145-2126(03)00259-5. [DOI] [PubMed] [Google Scholar]
- 12.Kluin-Nelemans HC, Oldhoff JM, Van Doormaal JJ, Van ’t Wout JW, Verhoef G, Gerrits WB, et al. Cladribine therapy for systemic mastocytosis. Blood. 2003;102:4270–4276. doi: 10.1182/blood-2003-05-1699. [DOI] [PubMed] [Google Scholar]
- 13.Böhm A, Sonneck K, Gleixner KV, Schuch K, Pickl WF, Blatt K, et al. In vitro and in vivo growth-inhibitory effects of cladribine on neoplastic mast cells exhibiting the imatinib-resistant KIT mutation D816V. Experimental Hematology. 2010;38:744–755. doi: 10.1016/j.exphem.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Gleixner KV, Mayerhofer M, Aichberger KJ, Derdak S, Sonneck K, Böhm A, et al. PKC412 inhibits in vitro growth of neoplastic human mast cells expressing the D816V-mutated variant of KIT: comparison with AMN107, imatinib, and cladribine (2CdA) and evaluation of cooperative drug effects. Blood. 2006;107:752–759. doi: 10.1182/blood-2005-07-3022. [DOI] [PubMed] [Google Scholar]
- 15.Shah NP, Lee FY, Luo R, Jiang Y, Donker M, Akin C. Dasatinib (BMS-354825) inhibits KITD816V, an imatinib-resistant activating mutation that triggers neoplastic growth in most patients with systemic mastocytosis. Blood. 2006;108:286–291. doi: 10.1182/blood-2005-10-3969. [DOI] [PubMed] [Google Scholar]
- 16.Gleixner KV, Mayerhofer M, Sonneck K, Gruze A, Samorapoompichit P, Baumgartner C, et al. Synergistic growth-inhibitory effects of two tyrosine kinase inhibitors, dasatinib and PKC412, on neoplastic mast cells expressing the D816V-mutated oncogenic variant of KIT. Haematologica. 2007;92:1451–1459. doi: 10.3324/haematol.11339. [DOI] [PubMed] [Google Scholar]
- 17.Hadzijusufovic E, Peter B, Rebuzzi L, Baumgartner C, Gleixner KV, Gruze A, et al. Growth-inhibitory effects of four tyrosine kinase inhibitors on neoplastic feline mast cells exhibiting a Kit exon 8 ITD mutation. Veterinary Immunology and Immunopathology. 2009;132:243–250. doi: 10.1016/j.vetimm.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akin C, Brockow K, D’Ambrosio C, Kirshenbaum AS, Ma Y, Longley BJ, et al. Effects of tyrosine kinase inhibitor STI571 on human mast cells bearing wild-type or mutated c-kit. Experimental Hematology. 2003;31:686–692. doi: 10.1016/s0301-472x(03)00112-7. [DOI] [PubMed] [Google Scholar]
- 19.Ma Y, Zeng S, Metcalfe DD, Akin C, Dimitrijevic S, Butterfield JH, et al. The c-KIT mutation causing human mastocytosis is resistant to STI571 and other KIT kinase inhibitors; kinases with enzymatic site mutations show different inhibitor sensitivity profiles than wild-type kinases and those with regulatory-type mutations. Blood. 2002;99:1741–1744. doi: 10.1182/blood.v99.5.1741. [DOI] [PubMed] [Google Scholar]
- 20.Gotlib J, Berube C, Growney JD, Chen CC, George TI, Williams C, et al. Activity of the tyrosine kinase inhibitor PKC412 in a patient with mast cell leukemia with the D816V KIT mutation. Blood. 2005;106:2865–2870. doi: 10.1182/blood-2005-04-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gotlib J. KIT mutations in mastocytosis and their potential as therapeutic targets. Immunology and Allergy Clinics of North America. 2006;26:575–592. doi: 10.1016/j.iac.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Verstovsek S, Tefferi A, Cortes J, O’Brien S, Garcia-Manero G, Pardanani A, et al. Phase II study of dasatinib in Philadelphia chromosome-negative acute and chronic myeloid diseases, including systemic mastocytosis. Clinical Cancer Research. 2008;14:3906–3915. doi: 10.1158/1078-0432.CCR-08-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Letard S, Yang Y, Hanssens K, Palmérini F, Leventhal PS, Guéry S, et al. Gain-of-function mutations in the extracellular domain of KIT are common in canine mast cell tumors. Molecular Cancer Research. 2008;6:1137–1145. doi: 10.1158/1541-7786.MCR-08-0067. [DOI] [PubMed] [Google Scholar]
- 24.Isotani M, Yamada O, Lachowicz JL, Tamura K, Yagihara H, Fujino Y, et al. Mutations in the fifth immunoglobulin-like domain of kit are common and potentially sensitive to imatinib mesylate in feline mast cell tumours. British Journal of Haematology. 2010;148:144–153. doi: 10.1111/j.1365-2141.2009.07926.x. [DOI] [PubMed] [Google Scholar]
- 25.Gleixner KV, Rebuzzi L, Mayerhofer M, Gruze A, Hadzijusufovic E, Sonneck K, et al. Synergistic antiproliferative effects of KIT tyrosine kinase inhibitors on neoplastic canine mast cells. Experimental Hematology. 2007;35:1510–1521. doi: 10.1016/j.exphem.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Isotani M, Ishida N, Tominaga M, Tamura K, Yagihara H, Ochi S, et al. Effect of tyrosine kinase inhibition by imatinib mesylate on mast cell tumors in dogs. Journal of Veterinary Internal Medicine. 2008;22:985–988. doi: 10.1111/j.1939-1676.2008.00132.x. [DOI] [PubMed] [Google Scholar]
- 27.London CA, Bear MD, McCleese J, Foley KP, Paalangara R, Inoue T, et al. Phase I evaluation of STA-1474, a prodrug of the novel HSP90 inhibitor ganetespib, in dogs with spontaneous cancer. PloS One. 2011;6:e27018. doi: 10.1371/journal.pone.0027018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.London CA, Bernabe LF, Barnard S, Kisseberth WC, Borgatti A, Henson M, et al. Preclinical evaluation of the novel, orally bioavailable Selective Inhibitor of Nuclear Export (SINE) KPT-335 in spontaneous canine cancer: results of a phase I study. PloS One. 2014;9:e87585. doi: 10.1371/journal.pone.0087585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dubreuil P, Letard S, Ciufolini M, Gros L, Humbert M, Castéran N, et al. Masitinib (AB1010), a potent and selective tyrosine kinase inhibitor targeting KIT. PloS One. 2009;4:e7258. doi: 10.1371/journal.pone.0007258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hahn KA, Ogilvie G, Rusk T, Devauchelle P, Leblanc A, Legendre A, et al. Masitinib is safe and effective for the treatment of canine mast cell tumors. Journal of Veterinary Internal Medicine. 2008;22:1301–1309. doi: 10.1111/j.1939-1676.2008.0190.x. [DOI] [PubMed] [Google Scholar]
- 31.Hahn KA, Legendre AM, Shaw NG, Phillips B, Ogilvie GK, Prescott DM, et al. Evaluation of 12- and 24-month survival rates after treatment with masitinib in dogs with nonresectable mast cell tumors. American Journal of Veterinary Research. 2010;71:1354–1361. doi: 10.2460/ajvr.71.11.1354. [DOI] [PubMed] [Google Scholar]
- 32.London CA. Tyrosine kinase inhibitors in veterinary medicine. Topics in Companion Animal Medicine. 2009;24:106–112. doi: 10.1053/j.tcam.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Halsey CH, Gustafson DL, Rose BJ, Wolf-Ringwall A, Burnett RC, Duval DL, et al. Development of an in vitro model of acquired resistance to toceranib phosphate (Palladia®) in canine mast cell tumor. BMC Veterinary Research. 2014;10:105. doi: 10.1186/1746-6148-10-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stein H, Mason DY, Gerdes J, O’Connor N, Wainscoat J, Pallesen G, et al. The expression of the Hodgkin’s disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985;66:848–858. [PubMed] [Google Scholar]
- 35.Chiarle R, Podda A, Prolla G, Gong J, Thorbecke GJ, Inghirami G. CD30 in normal and neoplastic cells. Clinical Immunology. 1999;90:157–164. doi: 10.1006/clim.1998.4636. [DOI] [PubMed] [Google Scholar]
- 36.Sotlar K, Cerny-Reiterer S, Petat-Dutter K, Hessel H, Berezowska S, Müllauer L. Aberrant expression of CD30 in neoplastic mast cells in high-grade mastocytosis. Modern Pathology. 2011;24:585–595. doi: 10.1038/modpathol.2010.224. [DOI] [PubMed] [Google Scholar]
- 37.Valent P, Sotlar K, Horny HP. Aberrant expression of CD30 in aggressive systemic mastocytosis and mast cell leukemia: a differential diagnosis to consider in aggressive hematopoietic CD30-positive neoplasms. Leukemia and Lymphoma. 2011;52:740–744. doi: 10.3109/10428194.2010.550072. [DOI] [PubMed] [Google Scholar]
- 38.Morgado JM, Perbellini O, Johnson RC, Teodósio C, Matito A, Álvarez-Twose I, et al. CD30 expression by bone marrow mast cells from different diagnostic variants of systemic mastocytosis. Histopathology. 2013;63:780–787. doi: 10.1111/his.12221. [DOI] [PubMed] [Google Scholar]
- 39.Blatt K, Cerny-Reiterer S, Schwaab J, Sotlar K, Eisenwort G, Stefanzl G, et al. Identification of the Ki-1 antigen (CD30) as a novel therapeutic target in systemic mastocytosis. Blood. 2015;24:2832–2841. doi: 10.1182/blood-2015-03-637728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DeVinney R, Gold WM. Establishment of two dog mastocytoma cell lines in continuous culture. American Journal of Respiratory Cell and Molecular Biology. 1990;3:413–420. doi: 10.1165/ajrcmb/3.5.413. [DOI] [PubMed] [Google Scholar]
- 41.Hadzijusufovic E, Peter B, Herrmann H, Rülicke T, Cerny-Reiterer S, Schuch K, et al. NI-1: a novel canine mastocytoma model for studying drug resistance and IgER-dependent mast cell activation. Allergy. 2012;67:858–868. doi: 10.1111/j.1398-9995.2012.02833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cerny-Reiterer S, Meyer RA, Herrmann H, Peter B, Gleixner KV, Stefanzl G, et al. Identification of heat shock protein 32 (Hsp32) as a novel target in acute lymphoblastic leukemia. Oncotarget. 2014;5:1198–1211. doi: 10.18632/oncotarget.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hohšteter M, Artuković B, Severin K, Kurilj AG, Beck A, Šoštarić-Zuckermann IC, et al. Canine testicular tumors: two types of seminomas can be differentiated by immunohistochemistry. BMC Veterinary Research. 2014;10:169. doi: 10.1186/s12917-014-0169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu CH, Hwang DN, Yhee JY, Kim JH, Im KS, Nho WG, et al. Comparative immunohistochemical characterization of canine seminomas and Sertoli cell tumors. Journal of Veterinary Science. 2009;10:1–7. doi: 10.4142/jvs.2009.10.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olivry T, Wofford J, Paps JS, Dunston SM. Stratum corneum removal facilitates experimental sensitization to mite allergens in atopic dogs. Veterinary Dermatology. 2011;22:188–196. doi: 10.1111/j.1365-3164.2010.00938.x. [DOI] [PubMed] [Google Scholar]
- 46.Gleixner KV, Ferenc V, Peter B, Gruze A, Meyer RA, Hadzijusufovic E, et al. Polo-like kinase 1 (Plk1) as a novel drug target in chronic myeloid leukemia: overriding imatinib resistance with the Plk1 inhibitor BI 2536. Cancer Research. 2010;70:1513–1523. doi: 10.1158/0008-5472.CAN-09-2181. [DOI] [PubMed] [Google Scholar]
- 47.Patnaik AK, Ehler WJ, MacEwen EG. Canine cutaneous mast cell tumor: morphologic grading and survival time in 83 dogs. Veterinary Patholology. 1984;21:469–474. doi: 10.1177/030098588402100503. [DOI] [PubMed] [Google Scholar]
- 48.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 49.van der Velden VH, Cazzaniga G, Schrauder A, Hancock J, Bader P, et al. European Study Group on MRD detection in ALL (ESG-MRD-ALL) Analysis of minimal residual disease by Ig/TCR gene rearrangements: guidelines for interpretation of real-time quantitative PCR data. Leukemia. 2007;21:604–611. doi: 10.1038/sj.leu.2404586. [DOI] [PubMed] [Google Scholar]
- 50.Furitsu T, Tsujimura T, Tono T, Ikeda H, Kitayama H, Koshimizu U, et al. Identification of mutations in the coding sequence of the proto-oncogene c-KIT in a human mast cell leukemia cell line causing ligand-independent activation of c-KIT product. Journal of Clinical Investigation. 1993;92:1736–1744. doi: 10.1172/JCI116761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nagata H, Worobec AS, Oh CK, Chowdhury BA, Tannenbaum S, Suzuki Y, et al. Identification of a point mutation in the catalytic domain of the protooncogene c-kit in peripheral blood mononuclear cells of patients who have mastocytosis with an associated hematologic disorder. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:10560–10564. doi: 10.1073/pnas.92.23.10560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sillaber C, Strobl H, Bevec D, Ashman LK, Butterfield JH, Lechner K, et al. IL-4 regulates c-kit proto-oncogene product expression in human mast and myeloid progenitor cells. Journal of Immunology. 1991;147:4224–4228. [PubMed] [Google Scholar]
- 53.van Anrooij B, Kluin PM, Oude Elberink JN, Kluin-Nelemans JC. CD30 in systemic mastocytosis. Immunology and Allergy Clinics of North America. 2014;34:341–355. doi: 10.1016/j.iac.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 54.Borate U, Mehta A, Reddy V, Tsai M, Josephson N, Schnadig I. Treatment of CD30-positive systemic mastocytosis with brentuximab vedotin. Leukemia Research. 2016;44:25–31. doi: 10.1016/j.leukres.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 55.Valent P, Bevec D, Maurer D, Besemer J, Di Padova F, Butterfield JH, et al. Interleukin 4 promotes expression of mast cell ICAM-1 antigen. Proceedings of the National Academy of Sciences of the United States of America. 1991;15:3339–3342. doi: 10.1073/pnas.88.8.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sillaber C, Bevec D, Butterfield JH, Heppner C, Valenta R, Scheiner O, et al. Tumor necrosis factor alpha and interleukin-1 beta mRNA expression in HMC-1 cells: differential regulation of gene product expression by recombinant interleukin-4. Experimental Hematology. 1993;21:1271–1275. [PubMed] [Google Scholar]
- 57.Koyasu S, Miyajima A, Arai K, Okajima F, Ui M, Yahara I. Growth regulation of multi-factor-dependent myeloid cell lines: IL-4, TGF-beta and pertussis toxin modulate IL-3- or GM-CSF-induced growth by controlling cell cycle length. Cell Structure and Function. 1989;14:459–471. doi: 10.1247/csf.14.459. [DOI] [PubMed] [Google Scholar]
- 58.Tsuji K, Nakahata T, Takagi M, Kobayashi T, Ishiguro A, Kikuchi T, et al. Effects of interleukin-3 and interleukin-4 on the development of “connective tissue-type” mast cells: interleukin-3 supports their survival and interleukin-4 triggers and supports their proliferation synergistically with interleukin-3. Blood. 1990;15:421–427. [PubMed] [Google Scholar]
- 59.Bischoff SC, Sellge G, Lorentz A, Sebald W, Raab R, Manns MP. IL-4 enhances proliferation and mediator release in mature human mast cells. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:8080–8085. doi: 10.1073/pnas.96.14.8080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buckley MG, Williams CM, Thompson J, Pryor P, Ray K, Butterfield JH, et al. IL-4 enhances IL-3 and IL-8 gene expression in a human leukemic mast cell line. Immunology. 1995;84:410–415. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.