Abstract
Leaf age alters the balance between the use of end-product of plastidic isoprenoid synthesis pathway, dimethylallyl diphosphate (DMADP), in prenyltransferase reactions leading to synthesis of pigments of photosynthetic machinery and in isoprene synthesis, but the implications of such changes on environmental responses of isoprene emission have not been studied. Because under light-limited conditions, isoprene emission rate is controlled by DMADP pool size (SDMADP), shifts in the share of different processes are expected to particularly strongly alter the light dependency of isoprene emission. We examined light responses of isoprene emission in young fully-expanded, mature and old non-senescent leaves of hybrid aspen (Populus tremula x P. tremuloides) and estimated in vivo SDMADP and isoprene synthase activity from postillumination isoprene release. Isoprene emission capacity was 1.5-fold larger in mature than in young and old leaves. The initial quantum yield of isoprene emission (αI) increased by 2.5-fold with increasing leaf age primarily as the result of increasing SDMADP. The saturating light intensity (QI90) decreased by 2.3-fold with increasing leaf age, and this mainly reflected limited light-dependent increase of SDMADP possibly due to feedback inhibition by DMADP. These major age-dependent changes in the shape of the light response need consideration in modeling canopy isoprene emission.
Keywords: dimethylallyl diphosphate, isoprene synthase, leaf ontogeny, light response curves
Introduction
Fast-growing early-successional tree species, especially when young, form leaves constantly at the top and lose senescent leaves at the bottom of the canopy. Highly dynamic age structure of these canopies can significantly alter the whole canopy physiological activity because of age-dependent differences in leaf physiological potentials (Anten & Werger 1996; Anten & Hirose 1998; Al Afas et al. 2005; Niinemets et al. 2015). In particular, physiological potentials increase with increasing leaf age in young leaves, level off in mature leaves and gradually decrease through leaf aging until induction of senescence that leads to very rapid reductions in leaf physiological potentials, and ultimately to leaf abscission (Garcia-Plazaola et al. 2003; Miyazawa et al. 2003; Jongebloed et al. 2004; Munné-Bosch 2007; Niinemets et al. 2012).
Once fully expanded, there is still a significant period of foliage mesophyll differentiation, including cellular expansion and formation of intercellular air spaces and plastid development, before the leaf attains the full physiological capacity. The period of mesophyll differentiation is associated with increases in the contents of ribulose-1,5-bisphophate carboxylase/oxygenase (Rubisco) and rate-limiting proteins of photosynthetic electron transport, and with increases in the contents of leaf photosynthetic pigments, carotenoids and chlorophylls (Shesták 1985a; Shesták 1985b; Niinemets et al. 2012; Tosens et al. 2012). Isoprene emission in emitting species also increases as the leaf matures, but the emission is characteristically induced somewhat later than positive values of photosynthesis are observed (Harley et al. 1994; Monson et al. 1994; Wiberley et al. 2005; Rasulov et al. 2014). In fact, as both the formation of photosynthetic pigments and isoprene rely on the same chloroplastic pool of one of the immediate isoprenoid precursors, dimethylallyl diphosphate (DMADP), there can be a competition between pigment synthesis and isoprene emission in developing leaves that constrains the rate of isoprene emission at given capacity of isoprene synthase reaction (Rasulov et al. 2014).
In mature leaves, there is a significant turnover of components of photosynthetic machinery, including photosynthetic pigments (Rundle & Zielinski 1991; Demmig-Adams & Adams 1993; Bertrand & Schoefs 1999; Beisel et al. 2010). Thus, even in fully-developed leaves, a certain substrate-level competition between pigment synthesis and isoprene emission can still be present, although it is operating at a low to moderate level because in mature leaves, the DMADP flux to larger isoprenoid synthesis is commonly much less than the flux going to isoprene formation (Ghirardo et al. 2014; Rasulov et al. 2014; Rasulov et al. 2015b). However, such a competition becomes increasingly unlikely with increasing leaf age as leaf physiological activity decreases. In older leaves, the rate of replacement of damaged proteins and pigments is expected to decrease because the nitrogen resorbed from non-functional proteins can be increasingly used to support the growth of new leaves or stored in woody tissues to support the growth of foliage in the next growing season.
In modeling isoprene emission, constant light and temperature responses are often used, and only the emission capacity is considered as a leaf-dependent parameter (Guenther et al. 1993; Guenther 1997; Monson et al. 2012; Grote et al. 2013). However, as DMADP pool size importantly controls responses of isoprene emission to environmental variables (Rasulov et al. 2009b; Rasulov et al. 2010; Li & Sharkey 2013b; Niinemets & Sun 2015), variation in the importance of substrate-level competition through leaf ontogeny can significantly modify the environmental responses of isoprene emission. The asymptotic light response of isoprene emission can be described by three parameters: the initial quantum yield, the light-saturated emission rate, the emission capacity (Imax), and the quantum flux density (Q) corresponding to Imax (saturation Q). While in the case of net assimilation rate, the initial quantum yield is relatively invariable and does not depend on photosynthetic capacity (Ehleringer & Björkman 1977), the quantum yield for isoprene emission is much more variable and is often correlated with Imax (Monson et al. 1992; Harley et al. 1996; Harley et al. 1997; Harley et al. 2004; Sun et al. 2012b; Rasulov et al. 2015a) due to reasons not yet fully understood.
Obviously, the share of ATP and NADPH produced in light among photosynthetic carbon metabolism and isoprenoid synthesis depends on the overall capacity of chloroplastic 2-C-methyl-D-erythritol 4-phosphate/1-deoxy-D-xylulose 5-phosphate pathway (MEP/DOXP pathway) of isoprenoid synthesis. However, once produced, the availability of DMADP for isoprene synthesis can depend on the capacity of its concurrent use in larger isoprenoid synthesis. Given that the Michaelis-Menten constant for DMADP of isoprene synthase is much larger than that for prenyltransferases, in particular, that of geranyl diphosphate synthases, the key enzymes responsible for the initial step of synthesis of larger isoprenoids (Orlova et al. 2009; Rajabi Memari et al. 2013; Rasulov et al. 2014), the enzymatic competition for DMADP by prenyltransferases and isoprene synthase is unequal. In particular, prenyltransferases could significantly draw down DMADP pool size in low light when the rate of DMADP synthesis is small and thereby reduce the rate of isoprene synthesis. Thus, a competition for DMADP among different DMADP-consuming reactions, might significantly alter the initial quantum yield for isoprene emission. With increasing the light level, DMADP becomes increasingly available, and the effect of such a competition on isoprene emission likely becomes gradually less. However, the competition could still shift the light-saturation point of isoprene emission, depending on how large the DMADP pool needs to become to saturate the prenyltransferase reactions, and also on the capacity of isoprene synthase relative to DMADP pool size.
On the other hand, it has been recently demonstrated that accumulation of DMADP can inhibit the overall flux through the MEP/DOXP pathway due to inhibition of deoxyxylulose 5-phosphate synthase, the first enzyme in the pathway (Banerjee et al. 2013; Ghirardo et al. 2014; Wright et al. 2014). Such a feedback inhibition could imply that rising DMADP pool size due to reduction of DMADP use in prenyltransferase reactions or with increasing light availability can inhibit the whole pathway flux, especially when isoprene synthase activity is limited as can occur in older leaves.
Here we studied light responses of isoprene emission in different-aged hybrid aspen (Populus tremula L. x P. tremuloides Michx.) leaves to test the hypothesis that age-dependent variations in DMADP pool size lead to changes in the initial quantum yield and light saturation of isoprene emission. In particular, because the consumption of DMADP for pigment synthesis is greater in young leaves, we hypothesized that (1) DMADP pool size limits the low-light isoprene emission more in young than in mature and old leaves, and that (2) greater light intensities are needed for saturation of isoprene emission rate in young and mature leaves than in old leaves. The results of this analysis demonstrate important variations in the initial quantum yield, emission capacity and saturation light for isoprene emission through leaf development, and suggest that age-dependent differences in DMADP available for isoprene emission play a major role in changes in the shape of the light response curve of isoprene emission.
Material and methods
Plant material
Two-year-old hybrid aspen (Populus tremula L. x. P. tremuloides Michx.) clone H200 (Rasulov et al. 2009a; Sun et al. 2012b; Niinemets & Sun 2015 for further information about the genotype) plants were used for the experiments. The saplings were planted in 4 L plastic pots filled with commercial garden soil including slow release NPK fertilizer with microelements (Biolan Oy, Finland) and grown in a Percival growth chamber (CLF Plant Climatics GmbH, Wertingen, Germany) at day/night temperatures of 25/20 °C for 14 h photoperiod and at a quantum flux density of 500 μmol m-2 s-1. The relative humidity was between 60-65% and the ambient CO2 concentration between 380-400 μmol mol-1.
By the time of the measurements, the plants had 20-24 leaves on the main stem. We used fully-expanded young (leaves 4-5 from the top, ca. 12 days old), young fully mature (leaves 10-12 from the top, ca. 30 days old) and old non-senescent fully mature (leaves 18-20 from the top, ca. 60 days old) leaves for these experiments.
Isoprene emission and photosynthesis measurements
We used a Walz GFS-3000 gas exchange system (Walz GmbH, Effeltrich, Germany) together with a proton transfer reaction quadrupole mass spectrometer (PTR-QMS, high sensitivity version, Ionicon, Innsbruck, Austria) for simultaneous measurements of foliage gas exchange and isoprene emission rates. The PTR-QMS system was calibrated frequently with a standard gas containing 4.47 ppm isoprene in N2 (Hills-Scientific, Boulder, CO, USA).
The measurements were conducted with attached leaves. After enclosure of the leaf in the cuvette, it was stabilized under the baseline conditions of photosynthetic quantum flux density (Q) of 500 μmol m-2 s-1 (growth light), leaf temperature of 30 °C, chamber humidity of 60%, and CO2 concentration of 380 μmol mol-1 until stomata opened and steady-state rates of net assimilation and isoprene emission were achieved, typically in 20 min. after leaf enclosure. Upon reaching the steady state, isoprene emission and net assimilation rates were recorded under the baseline conditions and dark release of isoprene emission was estimated (see the next section). Thereafter, the leaf was stabilized again in the baseline conditions, and Q (μmol m-2 s-1) was changed in the sequence of: 1850 → 0 (measurement of dark decay kinetics) → 1500→1000→ 750→ 500→ 250→ 0 (measurements of dark decay kinetics) → 100→ 50→ 20→ 0. At each new light level (except for transfer to darkness for dark decay kinetics measurements), isoprene emission and foliage gas exchange rates were recorded after a new steady state was established, characteristically in ca. 10 min. following the change of the light level. After the dark decay kinetics measurements (500 → 0, 1850 → 0, 250→ 0) and switching on the light, the leaf was stabilized at the new light intensity until a new steady state had been reached, typically for 20 min after switching on the light again. Leaf net assimilation rate (A), stomatal conductance and intercellular CO2 concentration (Ci) were computed according to von Caemmerer and Farquhar (1981), and leaf isoprene emission rate according to Niinemets et. al. (2011).
Estimation of in vivo dimethylallyl diphosphate (DMADP) pool size and isoprene synthase rate constant (k)
In vivo pool of DMADP responsible for isoprene synthesis and isoprene synthase rate constant were estimated according to the method of Rasulov et al. (2009a; 2010; 2011). This method is based on the assumption that the initial postillumination release of isoprene emission for 150-200 s after switching off the light relies on DMADP synthesized prior to switching off the light (Rasulov et al. 2009a; Li et al. 2011; Rasulov et al. 2011). Accordingly, the integral of isoprene emission during the initial postillumination burst provides the DMADP pool size corresponding to the steady-state isoprene emission rate in light (Rasulov et al. 2009a; Li et al. 2011; Rasulov et al. 2011). The postillumination isoprene emission burst can rely to some extent on isopentenyl diphosphate that is in equilibrium with DMADP (Li et al. 2011; Rasulov et al. 2011), but comparisons of the in vivo method and destructive chemical estimations of DMADP pool size have demonstrate a good correspondence between different methods (Rasulov et al. 2009a; Weise et al. 2013). As denoted in the previous section, dark decay kinetics corresponding to three different light intensities were taken during I vs. Q response curve measurements, and DMADP pool size was estimated in each case. Prior to integration, the decay kinetics were corrected for the leaf chamber effect as in Sun et al. (2012b). The leaf chamber effect was generally less than 10% of estimated DMADP pool size.
Given that the consumption of DMADP during postillumination isoprene release leads both to reduced DMADP pool size and isoprene emission rate, paired values of isoprene emission rate at any given moment of time t, I(t), and DMADP pool size supporting this rate, SDMADP(t), can be obtained. SDMADP(t) is given as the integral of isoprene emission rate from time t to the baseline value when all DMADP existing prior to switching off the light and remaining until time t has been consumed. Analogous integration for any value of t through the postillumination decay kinetics provides the kinetic curve of in vivo isoprene synthase. We define the initial slope of this curve as the rate constant of isoprene synthase (k, s-1) (Rasulov et al. 2011; Rasulov et al. 2015a).
Data analysis
Inverse modeling was used to calculate the rate of photosynthetic electron transport, J, needed to support the given rate of net assimilation (Farquhar & Sharkey 1982; Brooks & Farquhar 1985; Niinemets et al. 2002):
| (1) |
where Ci is the intercellular CO2 concentration, Rd is the dark respiration rate and Γ* is the CO2 compensation point in the absence of Rd (Laisk 1977) calculated according to Niinemets and Tenhunen (1997). Due to the existence of alternative electron sinks and lower CO2 concentration in the chloroplasts than in the intercellular air space, the actual photosynthetic electron transport rate can be larger than that calculated by Eq. 1. Nevertheless, compared with A, J estimated by Eq. 1 provides a measure of foliage photosynthetic activity that is independent of possible differences in stomatal openness. Inverse modeling was also used to calculate the minimum estimate of the apparent maximum carboxylase activity of Rubisco (Vcmax) that is needed to explain the measured light-saturated net assimilation rate (Niinemets & Tenhunen 1997; Niinemets et al. 1999a). Using these estimates of J and Vcmax, and Farquhar et al. (1980) photosynthesis model, the relative limitation of photosynthesis at light saturation, ΛS (%) was calculated as 100[1-A(Ci)/A(Ca)], where A(Ci) is the light-saturated net assimilation rate at the measured intercellular CO2 concentration, and A(Ca) the potential net assimilation rate when Ci equals the ambient CO2 concentration.
Dependencies of the rates of isoprene emission, net assimilation and photosynthetic electron transport on incident quantum flux density (Q) were fitted by the Smith equation (Niinemets & Tenhunen 1997):
| (2) |
where α is the initial quantum yield (αI for isoprene emission, αA for net assimilation and αJ for photosynthetic electron transport), and ymax is the capacity of the given process (Imax for isoprene emission, Amax for net assimilation and Jmax for photosynthetic electron transport), and y0 is the rate in darkness (y0 = 0 for isoprene emission and photosynthetic electron transport and y0 = Rd for net assimilation). Iterative minimization of the sum of error squares between measured and predicted values was used for data fitting.
Equation 2 predicts an asymptotic increase of the process rate with increasing Q, whereas for some combination of model parameters, the saturation Q can be predicted to occur at unrealistically high quantum flux densities (Leith & Reynolds 1987; Causton & Dale 1990). Thus, we define the saturating quantum flux density as the value of Q that is necessary to achieve 90% of the process rate at the quantum flux density of 2000 μmol m-2 s-1 (Q90). For isoprene emission, Q90 is given as:
| (3) |
where I2000 is the rate of isoprene emission at Q = 2000 μmol m-2 s-1. Q90 is given analogously for the photosynthetic electron transport rate (QJ90). For the net assimilation rate, Q90 equals:
| (4) |
where A2000 is the net assimilation rate at Q = 2000 μmol m-2 s-1. To further characterize the rate of asymptotic light saturation of the given process rate, the ratios of physiological capacities (Eq. 2, Imax, Amax, Jmax) to the process rates at Q = 1000 μmol m-2 s-1 (I1000, A1000, J1000) were also calculated.
To compare the light response curves with different Imax values, a modified Smith equation was used (Guenther et al. 1993; Monson et al. 2012). All emission rates were standardized with respect to I at Q = 1000 μmol m-2 s-1 (I1000) and the data were fitted again by a least squares method.
As DMADP pool size (SDMADP) estimates were available for three different Q values, we calculated the normalized light-dependent change of SDMADP (%) as:
| (5) |
where SDMADP(Q1) is the DMADP pool size at the light intensity of Q1 and SDMADP(Q2) that at the light intensity of Q2. The pool size change was normalized with respect to the average pool size to directly compare leaves with different absolute DMADP pool sizes.
The characteristics among leaf ages were compared by separate samples t-tests. The saturation light intensities (Q90) for different physiological processes (isoprene emission, net assimilation and photosynthetic electron transport) and the ratios of process capacities to the process rate at Q = 1000 μmol m-2 s-1 (Imax/I1000, Amax/A1000, Jmax/J1000) were compared among different leaf ages by paired samples t-tests. For each leaf age class, six replicate measurements were conducted with different plants.
Results
Basic structural and physiological differences among leaves of different age
All leaves included in the analysis were fully expanded with similar size, but young leaves had a lower leaf dry mass per unit area than mature and old leaves (Table 1). Incomplete foliage structural differentiation was also associated with lower capacities for isoprene emission, net assimilation rate and photosynthetic electron transport in young leaves compared with those characteristics in mature leaves (Table 1). However, these capacities were similar among young and old leaves (Table 1). Both intercellular CO2 concentration and relative stomatal limitation of photosynthesis were independent of leaf age (Table 1). Dark respiration rate was higher in young than in old leaves, and similar among young and mature and mature and old leaves (Table 1).
Table 1.
Average (± SE) values of structural and isoprene emission and photosynthetic characteristics in different-aged leaves of hybrid aspen (Populus tremula x P. tremuloides)
| Trait1 | Young | Mature | Old |
|---|---|---|---|
| Structural traits | |||
| Leaf area (cm2) | 48.7 ± 3.4a | 54.7 ± 4.4a | 48.8 ± 4.2a |
| Dry mass per unit area (g m-2) | 48 ± 7a | 65.2 ± 3.6b | 61.9 ± 1.5b |
| Dry to fresh mass ratio (g g-1) | 0.277 ± 0.018a | 0.298 ± 0.018a | 0.270 ± 0.013a |
| Isoprene emission traits | |||
| I1000 (nmol m-2 s-1) | 15.7 ± 2.9a | 28.7 ± 3.6b | 16.8 ± 2.0a |
| Imax (nmol m-2 s-1) | 19.0 ± 4.0a | 34.6 ± 5.0b | 17.3 ± 2.2a |
| Imax/I1000 | 1.18 ± 0.05a | 1.20 ± 0.05a | 1.027 ± 0.007b |
| αI (mmol mol-1) | 0.0323 ± 0.0033a | 0.0581 ± 0.011b | 0.081 ± 0.008c |
| QI90 (μmol m-2 s-1) | 970 ± 150a | 1020 ± 100a | 440 ± 60b |
| Assimilation traits | |||
| A1000 (μmol m-2 s-1) | 9.2 ± 1.1a | 14.0 ± 1.5b | 11.0 ± 1.5ab |
| Amax (μmol m-2 s-1) | 11.6 ± 1.1a | 16.2 ± 1.7b | 12.3 ± 1.5a |
| Amax/A1000 | 1.30 ± 0.08a | 1.192 ± 0.041ab | 1.129 ± 0.028b |
| Rd (μmol m-2 s-1) | 1.90 ± 0.47a | 1.14 ± 0.27ab | 0.66 ± 0.25b |
| Ci (μmol mol-1) | 230 ± 15a | 216 ± 16a | 233 ± 16a |
| (ΛS (%) | 33.3 ± 5.6a | 35.1 ± 4.7a | 28.0 ± 4.4a |
| αA (mol mol-1) | 0.0392 ± 0.0032a | 0.04031 ± 0.0019a | 0.04355 ± 0.0017a |
| QA90 (μmol m-2 s-1) | 650 ± 60ab | 730 ± 60b | 580 ± 70a |
| Photosynthetic electron transport traits | |||
| J1000 (μmol m-2 s-1) | 88 ± 8a | 121 ± 13b | 92 ± 7a |
| Jmax (μmol m-2 s-1) | 96 ± 10a | 136 ± 17b | 98 ± 8a |
| Jmax/J1000 | 1.076 ± 0.019ab | 1.118 ± 0.025b | 1.064 ± 0.013a |
| αJ (mol mol-1) | 0.257 ± 0.025a | 0.279 ± 0.016a | 0.282 ± 0.009a |
| QJ90 (μmol m-2 s-1) | 720 ± 80ab | 870 ± 70b | 670 ± 60a |
| Combined traits | |||
| Imax/Amax (mmol mol-1) | 1.77 ± 0.35a | 2.13 ± 0.38a | 1.63 ± 0.40a |
| Imax/Jmax (mmol mol-1) | 0.194 ± 0.030a | 0.228 ± 0.031a | 0.191 ± 0.037a |
| αI/αA (mmol mol-1) | 0.84 ± 0.09a | 1.20 ± 0.12b | 1.88 ± 0.21c |
| αI/αJ (mmol mol-1) | 0.128 ± 0.013a | 0.173 ± 0.014b | 0.288 ± 0.028c |
| QI90/QA90 | 1.56 ± 0.15a | 1.41 ± 0.07a | 0.85 ± 0.16b |
| QI90/QJ90 | 1.33 ± 0.11a | 1.181 ± 0.044a | 0.70 ± 0.11b |
Average values with the same lowercase letter are not significantly different (P > 0.05 according to separate samples t-tests)
Isoprene emission traits: I1000 - rate at a quantum flux density (Q) of 1000 μmol m-2 s-1, Imax - emission capacity (Eq. 2), αI - initial quantum (Eq. 2), QI90 - Q for 90% of I at Q = 2000 μmol m-2 s-1 (saturation Q, Eq. 3); net assimilation traits: A1000 - rate at a quantum flux density (Q) of 1000 μmol m-2 s-1, Amax - gross photosynthetic capacity (Eq. 2), Rd - dark respiration rate, Ci - average intercellular CO2 concentration for Q ≥ 200 μmol m-2 s-1, ΛS - relative stomatal limitation of photosynthesis at light saturation, αA - initial quantum (Eq. 2), QA90 - Q for 90% of A at Q = 2000 μmol m-2 s-1 (Eq. 4); photosynthetic electron transport traits: J1000 - rate at a quantum flux density (Q) of 1000 μmol m-2 s-1, Jmax - electron transport capacity (Eq. 2), αJ - initial quantum (Eq. 2), QJ90 - Q for 90% of J at Q = 2000 μmol m-2 s-1 (Eq. 3).
Leaf age effects on initial quantum yields and saturation light
The initial quantum yield for isoprene emission (αI) increased with increasing leaf age (Table 1 for average values and Fig. 1 for sample light response curves and for standardized light response curves including all data for given leaf age class). Quantum yields for net assimilation rate (αA) and photosynthetic electron transport rate (αJ) were independent of leaf age (Table 1). The saturation light (Q90) for isoprene emission was lower in old than in young and mature leaves (Table 1, Fig. 1), while the only significant contrasts among Q90 values for net assimilation and photosynthetic electron transport rates (QA90 and QJ90) were greater QA90 and QJ90 values in mature than in old leaves (Table 1). Age differences in the ratios Imax/I1000, Amax/A1000 and Jmax/J1000 typically reflected differences in corresponding Q90 values (Table 1).
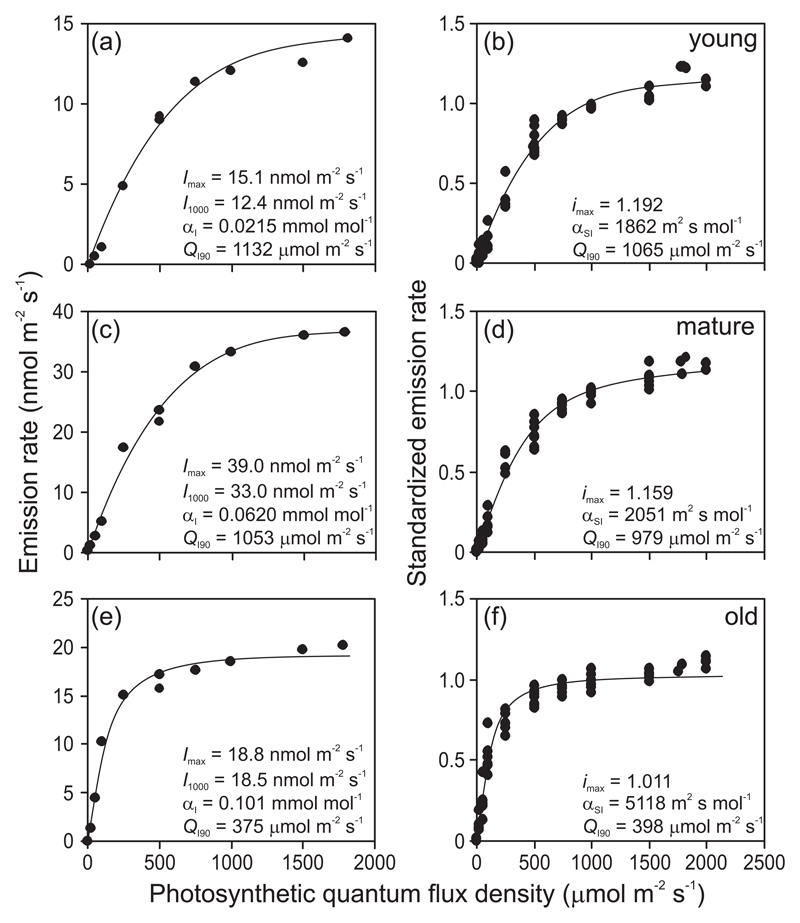
Fig. 1.
Representative light (Q) dependencies of isoprene emission rate for young (a), mature (c) and old non-senescent (e) leaves and comparison of standardized light dependencies of isoprene emission among young (b), mature (d) and old non-senescent (f) leaves of hybrid aspen (Populus tremula x P. tremuloides). Data in (a), (c) and (e) were fitted by Eq. 2 (r2 = 0.989 for the young, r2 = 0.991 for the mature and r2 = 0.982 for the old leaf) with the parameters defined as: Imax - the isoprene emission capacity, αI - the initial quantum yield of isoprene emission. In addition, the predicted isoprene emission rate at Q = 1000 μmol m-2 s-1, I1000, and the value of Q that is necessary to achieve 90% of the process rate at the quantum flux density of 2000 μmol m-2 s-1, QI90, (Eq. 3, saturating quantum flux density) are also shown. In (b), (d), and (f) all data (6 light response curves for each leaf age class pooled) were standardized with respect to I1000 and Eq. 2 was fitted to the data again (r2 = 0.981 for young, r2 = 0.983 for mature and r2 = 0.968 for old leaves). Standardized response curve parameters are denoted as imax (apparent capacity) and αSI (apparent quantum yield). Young leaves (4th-5th from the apex) were ca. 12 days old, young fully mature leaves (10th-12th from the apex) were ca. 30 days old and old non-senescent fully mature leaves (18th-20th from the apex) were ca. 60 days old.
Controls on isoprene emission rate by isoprene synthase activity and DMADP pool size in different-aged leaves
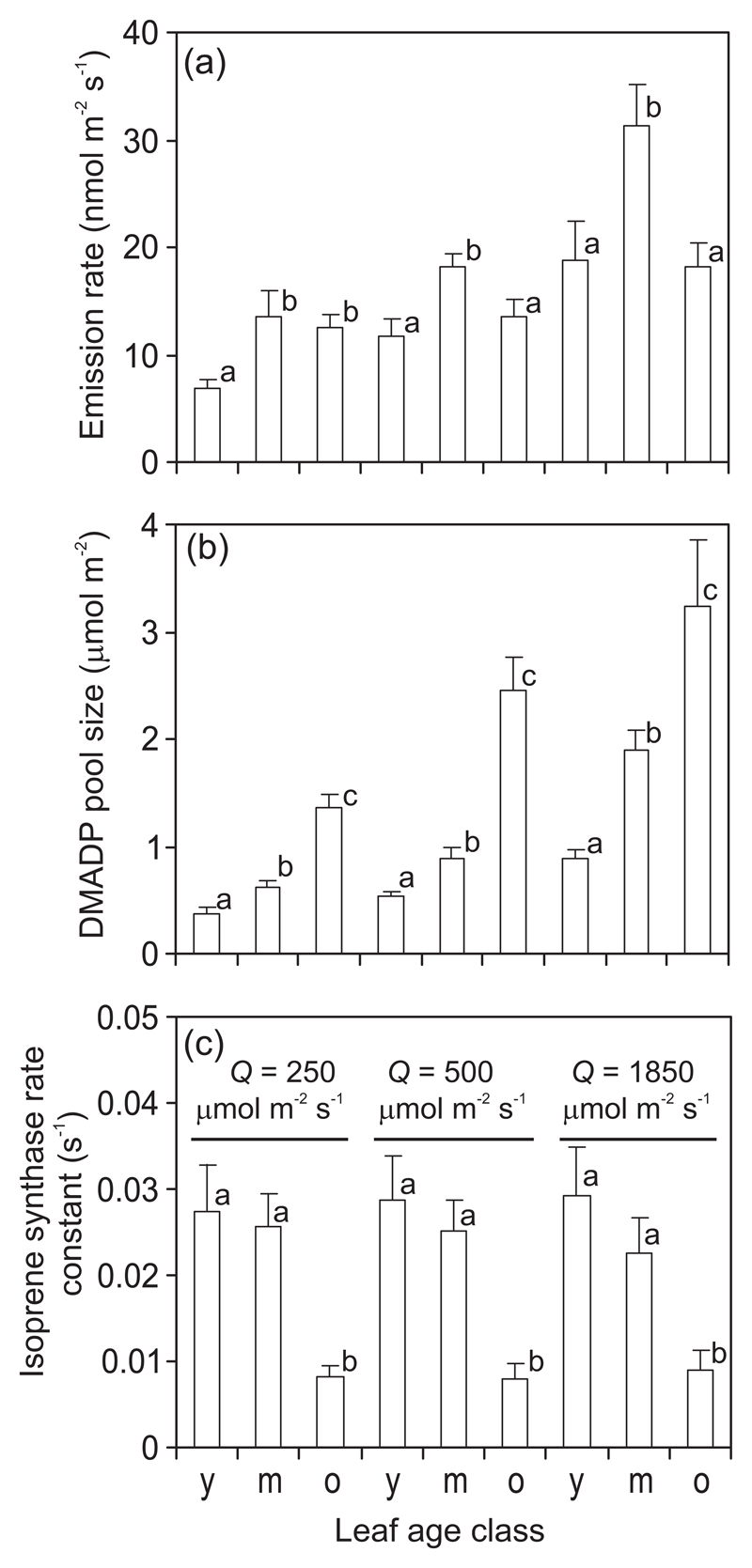
Differences in isoprene emission rate (I) among leaf ages (Fig. 2a) can result from differences either in DMADP pool size (SDMADP) or in isoprene synthase rate constant (k) or from differences in both controlling factors. Average DMADP pool size increased with increasing leaf age (Fig. 2b), but the isoprene synthase rate constant was similar among young and mature leaves and lower in old leaves (Fig. 3c). Despite higher isoprene synthase rate constant, in lower light, isoprene emission rate was lower in young than in old leaves (Fig. 2a), suggesting that SDMADP more strongly controlled the emission at limiting light. In contrast, at higher light, a similar average rate of isoprene emission for young and old leaves (Fig. 2a) resulted from different combinations of SDMADP and k in young and old leaves (Fig. 2b, c).
Fig. 2.
Leaf age dependent changes in isoprene emission rate (a), dimethylallyl diphosphate (DMADP) pool size (b) and in vivo isoprene synthase rate constant (c) at three different quantum flux densities (Q) in hybrid aspen (P. tremula x P. tremuloides) leaves. DMADP pool responsible for isoprene emission was measured from the postillumination release of isoprene emission (Rasulov et al. 2009a; Rasulov et al. 2010; Li et al. 2011), and the isoprene rate constant was taken as the initial slope of isoprene emission vs. DMADP pool size (Rasulov et al. 2011; Rasulov et al. 2014). Data are averages +SE (n = 6). Leaf age class as: y = young, m = mature, o = old (Fig. 1 for a detailed description of leaf age classes). Averages with the same lowercase letter at given light level are not significantly different among leaf age classes according to paired samples t-tests (P > 0.05).
Fig. 3.
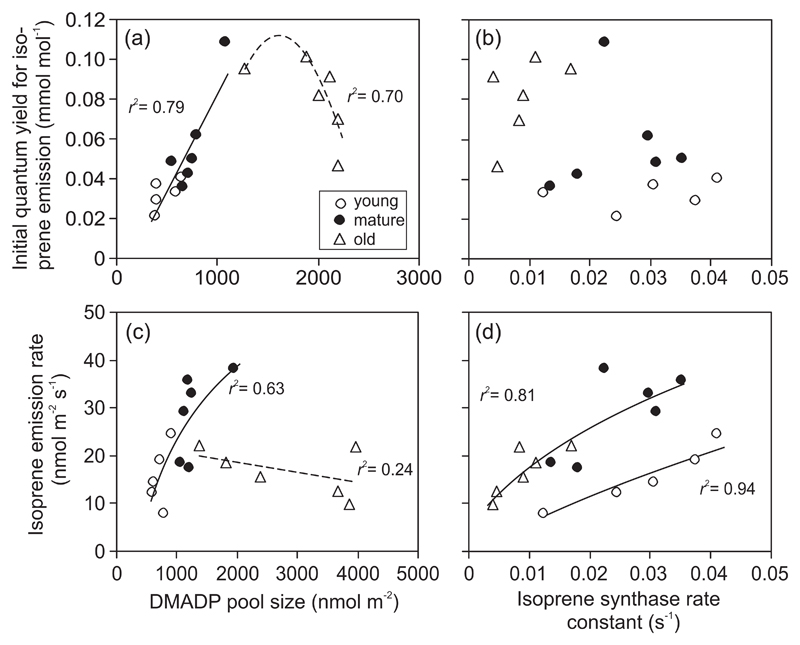
Correlations among the initial quantum yield of isoprene emission [αI in Eq. 2; (a, b)], and isoprene emission rate at a high light of Q = 1000 μmol m-2 s-1 (c, d) with DMADP pool size (a, c) and isoprene synthase rate constant (b, d) in different-aged leaves of hybrid aspen (P. tremula x P. tremuloides). Estimation of DMADP pool size (SDMADP) and isoprene synthase rate constant (k) as in Fig. 2. In (a), an average SDMADP for Q values of 250 and 500 μmol m-2 s-1 was used, while in (b), an average SDMADP for Q values of 500 and 1850 μmol m-2 s-1 was used. In (b) and (d), k is an average for all three light intensities because k did not vary with Q (Fig. 2). Data were fitted by linear and non-linear regressions (P < 0.01 for all regressions shown by solid lines, P < 0.1 for the regression shown by a dotted line in (a) and P > 0.2 for the regression shown by a dotted line in (c). In the regressions in (a) and (c), young and mature leaves were pooled, while in (d), old and mature leaves were pooled.
Initial quantum yield and saturating quantum flux density for isoprene emission as dependent on DMADP pool size and isoprene synthase rate constant
Across different leaves, αI increased with increasing SDMADP, but this relationship leveled off and αI even tended to decrease with increasing SDMADP in old leaves (Fig. 3a). No clear relationship between αI and k was observed (Fig. 3b). Isoprene emission rate at high light increased with increasing SDMADP in young and mature leaves, but not in old leaves (Fig. 3c). The emission rate was positively correlated with k, but the increase was less for young leaves with lower SDMADP than in mature and old leaves with higher SDMADP (Fig. 3d).
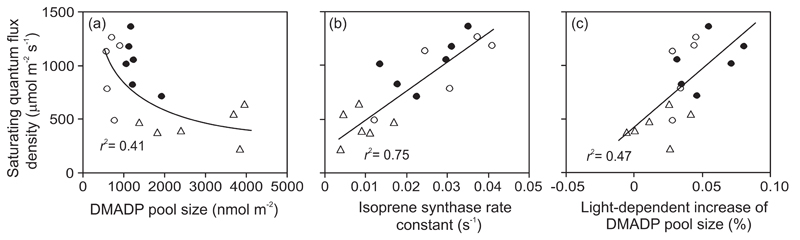
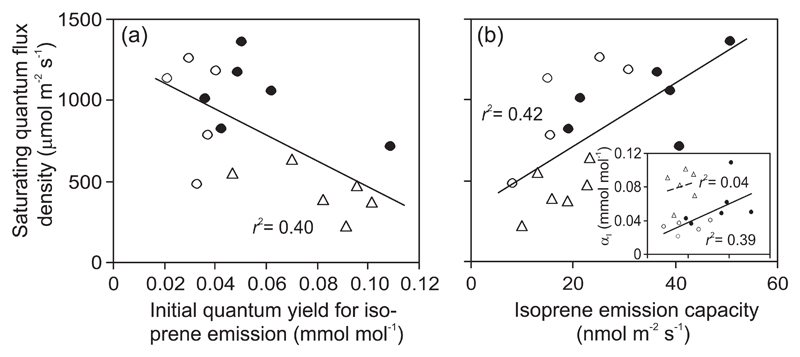
Across all leaves, the saturating quantum flux density (QI90, Eq. 3) was negatively correlated with SDMADP (Fig. 4a) and positively with k (Fig. 4b) and with the light-dependent increase of DMADP pool size (Eq. 5, Fig. 4c). QI90 and αI were negatively correlated (Fig. 5a), while QI90 and isoprene emission capacity (Imax, Eq. 2) were positively correlated (Fig. 5b). However, αI and Imax were positively correlated, except for old leaves (inset in Fig. 5b).
Fig. 4.
Correlations of saturating quantum flux density for isoprene emission (QI90, Eq. 3) with DMADP pool size (a), isoprene synthase rate constant (b) and normalized light-dependent increase of DMADP pool size [ΔS, Eq. 5; (c)] in hybrid aspen (P. tremula x P. tremuloides) young, mature and old leaves (symbols as in Fig. 3, and definition of leaf ages as in Fig. 1). The saturating quantum flux density is defined as the value of Q required to reach 90% of the emission rate at Q = 2000 μmol m-2 s-1. All data pooled were fitted by non-linear (a) and linear (b, c) regressions (P < 0.01). In (a), DMADP pool size is calculated as the average of measurements at high light intensities of 500 and 1850 μmol m-2 s-1, while in (b), the rate constant is the average value for all the three light intensities (250, 500 and 1850 μmol m-2 s-1) used for the measurements (Fig. 2 for the definitions and average values at different light intensities). ΔS is calculated for light intensities of 500 (Q1) and 1850 (Q2) μmol m-2 s-1 (Eq. 5).
Fig. 5.
Relationships of saturating quantum flux density for isoprene emission (Eq. 3) vs. (a) the initial quantum yield of isoprene emission [αI, (a)] and vs. the emission capacity [Imax, (b)], and the relationship of αI vs. Imax [inset in (b)] in different-aged leaves of hybrid aspen (P. tremula x P. tremuloides). The Smith model parameters αI and Imax are defined by Eq. 2. In the main panels, all data pooled (symbols as in Fig. 3 and leaf age classes as in Fig. 1) were fitted by linear regressions (P < 0.01). In the inset, separate fits were used for young and mature leaves pooled (solid lines, P < 0.05) and for old leaves (dashed line, P > 0.9).
Correlations among foliage isoprene emission and photosynthetic characteristics through leaf development
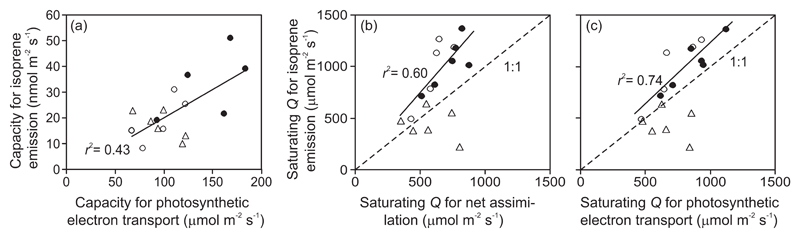
Across leaves with different ages, both the quantum yields for net assimilation (αA) and photosynthetic electron transport (αJ) and corresponding capacities (Amax and Jmax) were strongly correlated (r2 = 0.78 for the quantum yields and r2 = 0.72 for the capacities, P < 0.001 for both). The quantum yield for isoprene emission (αI) did not correlate with either αA (r2 = 0.08) or αJ (r2 = 0.09, P > 0.2 for both), while the capacity for isoprene emission was positively correlated with Jmax (Fig. 6a). Due to contrasting scaling of quantum yields for isoprene emission, net assimilation and photosynthetic electron transport with leaf age, the ratios of quantum yields, αI/αA and αI/αJ increased with increasing leaf age (Table 1).
Fig. 6.
Correlations between the capacities for isoprene emission (Imax) and photosynthetic electron transport [Jmax, (a)], and saturating quantum flux density (Q90) for isoprene emission in relation to Q90 for net assimilation (b) and photosynthetic electron transport (c) and in different-aged leaves of hybrid aspen (P. tremula x P. tremuloides). The process capacities are defined by Eq. 2 and the saturating quantum flux density by Eq. 3. Data for all leaf ages pooled in (a), and data for young and mature leaves pooled in (b) and (c) were fitted by linear regressions (symbols as in Fig. 3 and leaf age classes as in Fig. 1, all regressions are significant at P < 0.01). Dashed lines in (b) and (c) denote the 1:1 relationships.
The saturating light for isoprene emission was positively correlated with that for net assimilation (Fig. 6b) and photosynthetic electron transport (Fig. 6c). However, for young and mature leaves (Fig. 6b, Table 1), QI90 was larger than QA90 (for comparison of average values, P < 0.01 for young and P < 0.005 for mature leaves) and QJ90 (P < 0.001 for young and P < 0.01 for mature leaves). For old leaves, QI90 did not differ significantly from QA90 (P > 0.1) and QJ90 (P > 0.2).
Discussion
Leaf age effects on basic foliage physiological characteristics
Foliage expansion growth is typically completed earlier than foliage structural and physiological maturation (Miyazawa et al. 2003; Niinemets et al. 2012) as was also observed in our study (Table 1). While leaf size did not differ among young and mature leaves studied, young leaves had a lower dry mass per unit area and lower photosynthetic and isoprene emission capacities (Table 1), reflecting their incomplete structural and physiological maturation (s. Introduction for developmental changes during leaf ontogeny).
Although we have investigated non-senescent leaves, there typically is a certain reduction of foliage physiological activity with leaf aging before the rapid onset of decline of foliage physiological function after senescence has been triggered (Harley et al. 1994; Niinemets et al. 2004; Grassi & Magnani 2005; Sun et al. 2012a). This age-dependent decline in foliage physiological activity of non-senescent leaves is associated with remobilization of limiting nutrients such as nitrogen to support the growth of new leaves, especially in fast-growing canopies such as in young aspen plants (Noormets et al. 1996; Kull et al. 1998; Niinemets et al. 2015). A reduction in foliage photosynthetic and isoprene emission capacities in old leaves was also observed in our study such that young and old leaves had similar physiological capacities, ca. 0.5-0.8-fold less compared with mature leaves (Table 1, Fig. 1, 2a). Given that both leaf development and aging comprise significant periods of leaf life span, we argue that these differences play an important role in determining the whole canopy isoprene emission and carbon gain rates in fast-growing canopies of early-successional isoprene emitters such as woody Populus and Salix canopies or herbaceous Phragmites canopy (Niinemets et al. 2015 for a discussion of canopy development in dependence on plant functional type and successional status).
Controls of isoprene emission by DMADP pool size and isoprene synthase activity in relation to leaf age
In mature leaves, leaf-to-leaf variation in high-light isoprene emission rate is typically dependent on differences in isoprene synthase activity (Brüggemann & Schnitzler 2002a; Brüggemann & Schnitzler 2002b; Magel et al. 2006; Rasulov et al. 2010; Rasulov et al. 2011; Sun et al. 2012b) characterized in our study by the isoprene synthase rate constant (k). However, variation of the isoprene synthase substrate, dimethylallyl diphosphate, DMADP, pool size (SDMADP) can also alter the isoprene emission rate among leaves with given isoprene synthase activity (Sun et al. 2012b; Ghirardo et al. 2014; Rasulov et al. 2014). A control by SDMADP or a shared control by SDMADP and k is expected especially in conditions when isoprene synthase activity is high relative to SDMADP such that isoprene synthase operates much below its potential capacity, or in conditions when accumulation of SDMADP can lead to a feedback inhibition of MEP/DOXP pathway (s. Introduction and Banerjee et al. 2013; Wright et al. 2014). In vitro studies further suggest that under extremely high concentrations of dimethylallyl diphosphate, isoprene synthase could be directly inhibited by SDMADP (Silver & Fall 1991; Schnitzler et al. 2005), possibly due to inhibition of diphosphate release from the enzyme-product complex or inhibition of active site closure of substrate-enzyme complex (Köksal et al. 2010). The latter inhibition has been found at dimethylallyl diphosphate concentrations exceeding ca. 8 mM (Silver & Fall 1991; Schnitzler et al. 2005), i.e. at much greater concentrations than observed here (at most ca. 0.25 mM according to the conversion factors based on leaf structural characteristics derived in Rasulov et al. 2009a; Rasulov et al. 2014).
In our study, DMADP pool size increased with increasing leaf age (Fig. 2b), but the isoprene synthase rate constant was similar among young and mature leaves and lower in old leaves (Fig. 2c). Analysis of correlations of the light-saturated isoprene emission rate with k and SDMADP suggested that a similar light-saturated isoprene emission rate in young and old leaves (Fig. 2a) was achieved in completely different ways. Although the isoprene emission rate increased with increasing k in all cases, at a given k, the emission rate was greater in mature and old leaves (Fig. 3d). Thus, SDMADP limited high-light isoprene emission rate more in young leaves, while in old leaves, SDMADP was vastly over-dimensioned relative to k, and isoprene synthase seemed to operate in DMADP-saturated conditions (Fig. 3c).
Despite very high SDMADP values were observed in the current study in old leaves (Fig. 2b, Fig. 3c), the evidence of a feedback inhibition of isoprene emission by SDMADP in high light was somewhat limited. Indeed, the light-dependent increase of SDMADP was weaker in old leaves than in young and mature leaves, and it was even occasionally close to zero or slightly negative (Fig. 4c), consistent with the feedback inhibition. Nevertheless, in most cases, a lower isoprene emission rate in old leaves was primarily the result of reduced isoprene synthase rate constant, i.e., DMADP supply exceeded its consumption by isoprene synthase. The mismatch between DMADP supply and its consumption leading to a greater DMADP pool size does not rule out the possibility of a feedback control, but it suggests that such a control likely becomes operational at relatively large DMADP concentrations. Similarly to these observations, a previous study demonstrated that very high SDMADP pools in bisphosphonate-inhibited leaves led to only moderate feedback inhibition of MEP/DOXP pathway in hybrid aspen (Rasulov et al. 2015b).
What can be responsible for the large pool of DMADP in old leaves? DMADP pool size at any moment of time is the outcome of its production and consumption by prenyltransferases and isoprene synthase. Accordingly, a rise in the DMADP level suggests a certain imbalance between the activity of the MEP/DOXP pathway and DMADP consumption. Such an imbalance is consistent with the hypothesis that DMADP use in constitutive prenyltransferase reactions responsible for photosynthetic pigment synthesis becomes gradually inhibited with leaf aging. It is, however, unclear why MEP/DOXP pathway activity is not concurrently reduced to maintain a certain DMADP pool size in older leaves. Previous studies comparing isoprene-emitting and non-emitting species have suggested that isoprene emission keeps the MEP/DOXP pathway active and allows for a rapid induction of larger isoprenoid synthesis when needed, e.g., in stressed leaves (Rosenstiel et al. 2004; Owen & Peñuelas 2005; Fineschi et al. 2013). Analogously, in fully mature leaves where the constitutive prenyltransferase activity is reduced, a greater DMADP pool size could serve to rapidly respond to changes in the requirement for larger isoprenoids in fluctuating environments.
Furthermore, there is evidence that the rate of different processes does decrease with different time kinetics in aging leaves (Harley et al. 1994; Monson et al. 1994; Andersson et al. 2004; Niinemets et al. 2004; Keskitalo et al. 2005; Sun et al. 2012a). In particular, a decrease in foliage photosynthetic activity seems to precede reductions in foliage pigment content (Valjakka et al. 1999; Takeuchi et al. 2002; Niinemets et al. 2004) and isoprene emission capacity (Harley et al. 1994; Monson et al. 1994; Sun et al. 2012a). As a decrease in photosynthetic capacity can led to enhanced production of reactive oxygen species (ROS) due to imbalanced light energy interception and consumption (Huner et al. 1998; Foyer et al. 2012), maintenance of isoprene emission capacity in aging leaves could importantly contribute to quenching of ROS and preservation of the integrity of cellular metabolism that is necessary for safe dismantling of cellular structures upon senescence (Andersson et al. 2004; Keskitalo et al. 2005).
Changes in the initial quantum yield for isoprene emission in different-aged leaves
The light-limited isoprene emission rate is typically determined by DMADP pool size (Rasulov et al. 2009b; Li & Sharkey 2013a), and thus, any variation in SDMADP in low light should be associated with changes in the initial quantum yield of isoprene emission, αI. Indeed, in our study, leaf-age dependent variations in SDMADP (Fig. 2b, Table 1) were accompanied with major changes in αI that was the greatest in old leaves with the largest SDMADP, followed by mature and young leaves (Table 1, Fig. 1).
To our knowledge, such age-dependent variations in αI, 2.5-fold among old and young leaves (Table 1), and more than 5-fold across all leaves (Fig. 3a), and the connection of these changes to modifications in SDMADP have not been demonstrated so far. However, an analogous difference in αI was observed in aspen grown under elevated and ambient atmospheric [CO2] (Sun et al. 2012b), and in aspen grown under ambient and high temperatures (Rasulov et al. 2015a). In both studies, a lower αI in elevated-[CO2]-grown than in ambient-[CO2]-grown plants and in high-temperature-grown than in ambient-temperature-grown plants was associated with a lower SDMADP (although measured at high light in these studies). These previous observations together with the results of the current study underscore the important role of SDMADP in determining the initial quantum yield of isoprene emission.
Differently from young and mature leaves, the correlation between αI and SDMADP leveled off and even tended to be negative in old leaves (Fig. 3a). As discussed above, the decline of αI at higher SDMADP could be interpreted as evidence of a feedback inhibition. However, given that SDMADP did increase with further increases in light level in old leaves, such a reverse trend in αI more likely reflects the low isoprene synthase rate constant in old leaves (Fig. 3b), again emphasizing the importance of consideration of both the isoprene synthase activity and SDMADP to predict variations in isoprene emission rate across leaves with different MEP/DOXP pathway and isoprene synthase activities.
How saturating quantum flux density varies with leaf age
At current ambient CO2 concentrations, light-saturation of foliage net assimilation (QA90) rate occurs when the limitation of ribulose-1,5-bisphosphate (RuBP) carboxylation due to RuBP regeneration (photosynthetic electron transport limitation) crosses over to Rubisco (CO2) limitation. As electrons can be used to support both CO2 fixation and photorespiration and also alternative processes such as nitrate reduction, isoprene synthesis and cyclic and pseudocyclic electron flow (Stitt 1986; Bloom et al. 1989; Laisk et al. 2007; Laisk et al. 2010), photosynthetic electron transport rate typically saturates at higher quantum flux densities (QJ90) than net assimilation rate as was also confirmed in our study (Table 1). On the other hand, isoprene emission rate is more strongly related to the rate of photosynthetic electron transport than to the rate of net assimilation through the light response curve (Niinemets et al. 1999b; Rasulov et al. 2009b; Morfopoulos et al. 2014). In our study, the light-saturation of isoprene emission (QI90) tended to occur at higher light than that for photosynthetic electron transport, but we emphasize that the electron transport rate estimated here by Eq. 1 is the lowest estimate needed to explain the observed rate of gross CO2 assimilation and photorespiration and the true rate could be higher (Stitt 1986; Bloom et al. 1989).
In our study, a greater QI90 was observed for young and mature leaves, but not for old leaves (Table 1, Fig. 6b, c). In fact, in old leaves, QI90 did not differ from QA90 and QJ90 (Fig. 6b, c). Provided that isoprene synthase activity is independent of measurement light intensity (Rasulov et al. 2009b, Fig. 2c), for a given leaf, light-dependent increases of isoprene emission and saturation level should be primarily determined by the effects of light on DMADP pool size. Thus, as soon as additional DMADP becomes available with increasing light level, isoprene emission increases until the rate of DMADP production saturates due to reaching the maximum enzymatic capacities of MEP/DOXP pathway or due to limited supply or NADPH and/or ATP or due to feedback inhibition by DMADP. Given that the light-saturation of net assimilation rate is driven by the limited concentration of CO2 at carboxylation sites, a greater fraction of NADPH and ATP becomes available for DMADP formation at higher light, explaining the greater saturating light for isoprene emission than that for net assimilation.
Due to strong correlative patterns among the drivers of isoprene emission rate at different light intensities, a causal explanation of QI90 variation across leaves of different age is somewhat complicated. First, QA90 and QJ90 were also lower in old than in mature leaves (Table 1), suggesting that electron and carbon flow to isoprene emission increased less with increasing light intensity in old leaves, possibly explaining lower QI90 in old leaves. However, given that QA90 and QJ90 were similar among young and old leaves (Table 1), this suggestion does not explain lower QI90 in old compared with young leaves. Regarding the control by SDMADP, QI90 actually decreased with increasing SDMADP across leaves of different age, mainly reflecting the large SDMADP in old leaves (Fig. 4a). As stated above, such a negative relationship is consistent with the feedback inhibition of MEP/DOXP pathway by DMADP pool size, i.e. light-dependent increase in SDAMDP can be partly inhibited by its increasing concentration. Indeed, the light-dependent increase of SDAMDP pool was greater in young and mature leaves than in old leaves (Table 1), and this increase was strongly correlated with QI90 (Fig. 4c). Such a greater increase might reflect gradual DMADP-saturation of alternative DMADP-consuming reactions with increasing light level. Thus, age-dependent changes in saturation light are overall consistent with the hypothesis of a greater share of DMADP use by alternative DMADP sinks such as photosynthetic pigment synthesis in young and mature leaves relative to old leaves.
On the other hand, QI90 scaled positively with the isoprene synthase rate constant (Fig. 4b) and the emission capacity (Fig. 5b) across leaves of different age. While these correlations are not necessarily causal, a greater isoprene synthase activity relative to DMADP pool size allows for enhanced consumption of DMADP as soon as it becomes available with increasing quantum flux density. Such a greater capacity works against accumulation of DMADP and associated potential feedback-inhibition of emission at higher light intensities, although it still did not avoid excessive DMADP accumulation in old leaves in our study.
Conclusions
We have demonstrated that the initial quantum yield and the saturation light of isoprene emission importantly vary in leaves of different age and that these modifications are primarily triggered by changes in the pool size of DMADP, the substrate for isoprene synthase. To our knowledge, such age-dependent differences in the shape of the light response curve driven by variations in substrate availability have not been reported before. In highly dynamic canopies supporting foliage of different age, modifications in the shape of the light response curves of isoprene emission clearly importantly alter the whole canopy isoprene emission rate. Thus, we argue that instead of using constant emission algorithms as widely employed in the trace gas exchange community (s. Introduction), age-dependent modifications need to be taken into account in simulating canopy isoprene emissions. In the simplest manner, age-dependent changes in the light response can be incorporated in models using empirical relationships between leaf age and quantum yield for isoprene emission.
Furthermore, analysis of past evidence indicates that differences in the light response of isoprene emission due to changes in DMADP pool size can be extended to plants grown under different atmospheric [CO2] (Sun et al. 2012b) and under different temperatures (Rasulov et al. 2015a), suggesting that the substrate-level control of the shape of the light response is a general mechanism responsible for variations in the initial quantum yields. A mechanistic consideration of such changes is clearly difficult, because it would require information about DMADP pool size, which the models currently cannot predict. However, consideration of DMADP pool as an outcome of the competition by prenyltransferases and isoprene synthase might provide a promising opportunity, especially when an estimate of pigment content and turnover could be obtained by simple alternative techniques such as remote sensing (Lichtenthaler et al. 1996; Gamon & Surfus 1999; Sims & Gamon 2002; Peñuelas et al. 2013). We conclude that models linking all DMADP consuming processes, in particular, photosynthetic pigment synthesis and isoprene emission, could ultimately provide a way for fully mechanistic modeling of environmental responses of isoprene emission. Although changes in the DMADP pool size provided an explanation for modifications in the initial quantum yield for isoprene emission, future research is needed to explain the mismatch between the capacities of plastidic MEP/DOXP pathway and isoprene emission.
Summary statement.
Isoprene emission rate under light-limited conditions is driven by the availability of the end-product of plastidic isoprenoid synthesis pathway, dimethylallyl diphosphate (DMADP). Apart from isoprene emission, prenyltransferase reactions leading to synthesis of photosynthetic pigments can reduce DMADP pool size, but the implications of such a possible competition on the isoprene emission light response were unknown. We demonstrated that DMADP available for isoprene synthesis increased through leaf development and aging in aspen, and this was associated with increases in the quantum yield and reduced saturation light of isoprene emission, indicating that age-dependent changes in DMADP consumption by competing sinks importantly alter the light response of isoprene release.
Acknowledgements
This study has been funded by the Estonian Ministry of Science and Education (institutional grant IUT-8-3), the Estonian Science Foundation (grant 9253), the European Commission through the European Regional Fund (the Center of Excellence in Environmental Adaptation), the European Social Fund (Doctoral Studies and Internationalization Programme DoRa), and the European Research Council (advanced grant 322603, SIP-VOL+).
References
- Al Afas N, Pellis A, Niinemets Ü, Ceulemans R. Growth and production of a short rotation coppice culture of poplar. II. Clonal and year-to-year differences in leaf and petiole characteristics and stand leaf area index. Biomass and Bioenergy. 2005;28:536–547. [Google Scholar]
- Andersson A, Keskitalo J, Sjödin A, et al. A transcriptional timetable of autumn senescence. Genome Biology. 2004;5:R24. doi: 10.1186/gb-2004-5-4-r24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anten NPR, Hirose T. Biomass allocation and light partitioning among dominant and subordinate individuals in Xanthium canadense stands. Annals of Botany. 1998;82:665–673. [Google Scholar]
- Anten NPR, Werger MJA. Canopy structure and nitrogen distribution in dominant and subordinate plants in a dense stand of Amaranthus dubius L. with a size hierarchy of individuals. Oecologia. 1996;105:30–37. doi: 10.1007/BF00328788. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Wu Y, Banerjee R, Li Y, Yan H, Sharkey TD. Feedback inhibition of deoxy-D-xylulose 5-phosphate synthase regulates the methyl erythritol 4-phosphate pathway. The Journal of Biological Chemistry. 2013;288:16926–16936. doi: 10.1074/jbc.M113.464636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisel KG, Jahnke S, Hofmann D, Köppchen S, Schurr U, Matsubara S. Continuous turnover of carotenes and chlorophyll a in mature leaves of Arabidopsis revealed by 14CO2 pulse-chase labeling. Plant Physiology. 2010;152:2188–2199. doi: 10.1104/pp.109.151647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand M, Schoefs B. Photosynthetic pigment metabolism in plants during stress. In: Pessarakli M, editor. Handbook of plant and crop stress. Marcel Dekker; New York: 1999. p. 527. [Google Scholar]
- Bloom AJ, Caldwell RM, Finazzo J, Warner RL, Weissbart J. Oxygen and carbon dioxide fluxes from barley shoots depend on nitrate assimilation. Plant Physiology. 1989;91:352–356. doi: 10.1104/pp.91.1.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks A, Farquhar GD. Effects of temperature on the CO2/O2 specificity of ribulose-1,5-bisphosphate carboxylase/oxygenase and the rate of respiration in the light. Estimates from gas-exchange measurements on spinach. Planta. 1985;165:397–406. doi: 10.1007/BF00392238. [DOI] [PubMed] [Google Scholar]
- Brüggemann N, Schnitzler J-P. Diurnal variation of dimethylallyl diphosphate concentrations in oak (Quercus robur) leaves. Physiologia Plantarum. 2002a;115:190–196. doi: 10.1034/j.1399-3054.2002.1150203.x. [DOI] [PubMed] [Google Scholar]
- Brüggemann N, Schnitzler J-P. Relationship of isopentenyl diphosphate (IDP) isomerase activity to isoprene emission of oak leaves. Tree Physiology. 2002b;22:1011–1018. doi: 10.1093/treephys/22.14.1011. [DOI] [PubMed] [Google Scholar]
- Causton DR, Dale MP. The monomolecular and rectangular hyperbola as empirical models of the response of photosynthetic rate to photon flux density, with applications to three Veronica species. Annals of Botany. 1990;65:389–394. [Google Scholar]
- Demmig-Adams B, Adams WW., III The xanthophyll cycle, protein turnover, and the high light tolerance of sun-acclimated leaves. Plant Physiology. 1993;103:1413–1420. doi: 10.1104/pp.103.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehleringer J, Björkman O. Quantum yields for CO2 uptake in C3 and C4 plants. Dependence on temperature, CO2 and O2 concentration. Plant Physiology. 1977;59:86–90. doi: 10.1104/pp.59.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar GD, von Caemmerer S, Berry JA. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta. 1980;149:78–90. doi: 10.1007/BF00386231. [DOI] [PubMed] [Google Scholar]
- Farquhar GD, Sharkey TD. Stomatal conductance and photosynthesis. Annual Review of Plant Physiology. 1982;33:317–345. [Google Scholar]
- Fineschi S, Loreto F, Staudt M, Peñuelas J. Diversification of volatile isoprenoid emissions from trees: evolutionary and ecological perspectives. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013. pp. 1–20. [Google Scholar]
- Foyer CH, Neukermans J, Queval G, Noctor G, Harbinson J. Photosynthetic control of electron transport and the regulation of gene expression. Journal of Experimental Botany. 2012;63:1637–1661. doi: 10.1093/jxb/ers013. [DOI] [PubMed] [Google Scholar]
- Gamon JA, Surfus JS. Assessing leaf pigment content and activity with a reflectometer. The New Phytologist. 1999;143:105–117. [Google Scholar]
- Garcia-Plazaola JI, Hernández A, Becerill JM. Antioxidant and pigment composition during autumnal leaf senescence in woody deciduous species differing in their ecological traits. Plant Biology. 2003;5:557–566. [Google Scholar]
- Ghirardo A, Wright LP, Bi Z, Rosenkranz M, Pulido P, Rodríguez-Concepción M, Niinemets Ü, Brüggemann N, Gershenzon J, Schnitzler J-P. Metabolic flux analysis of plastidic isoprenoid biosynthesis in poplar leaves emitting and nonemitting isoprene. Plant Physiology. 2014;165:37–51. doi: 10.1104/pp.114.236018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi G, Magnani F. Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant, Cell and Environment. 2005;28:834–849. [Google Scholar]
- Grote R, Monson RK, Niinemets Ü. Leaf-level models of constitutive and stress-driven volatile organic compound emissions. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013. pp. 315–355. [Google Scholar]
- Guenther A. Seasonal and spatial variations in natural volatile organic compound emissions. Ecological Applications. 1997;7:34–45. [Google Scholar]
- Guenther AB, Zimmerman PR, Harley PC, Monson RK, Fall R. Isoprene and monoterpene emission rate variability: model evaluations and sensitivity analyses. Journal of Geophysical Research. 1993;98:12609–12617. [Google Scholar]
- Harley P, Guenther A, Zimmerman P. Effects of light, temperature and canopy position on net photosynthesis and isoprene emission from sweetgum (Liquidambar styraciflua) leaves. Tree Physiology. 1996;16:25–32. doi: 10.1093/treephys/16.1-2.25. [DOI] [PubMed] [Google Scholar]
- Harley P, Guenther A, Zimmerman P. Environmental controls over isoprene emission in deciduous oak canopies. Tree Physiology. 1997;17:705–714. doi: 10.1093/treephys/17.11.705. [DOI] [PubMed] [Google Scholar]
- Harley P, Vasconcellos P, Vierling L, De S Pinheiro C, Greenberg J, Guenther A, Klinger L, Soares De Almeida S, Neill D, Baker T, Phillips O, et al. Variation in potential for isoprene emissions among Neotropical forest sites. Global Change Biology. 2004;10:630–650. [Google Scholar]
- Harley PC, Litvak ME, Sharkey TD, Monson RK. Isoprene emission from velvet bean leaves. Interactions among nitrogen availability, growth photon flux density, and leaf development. Plant Physiology. 1994;105:279–285. doi: 10.1104/pp.105.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huner NPA, Öquist G, Sarhan F. Energy balance and acclimation to light and cold. Trends in Plant Science. 1998;3:224–230. [Google Scholar]
- Jongebloed U, Szederkényi J, Hartig K, Schobert C, Komor E. Sequence of morphological and physiological events during natural ageing and senescence of a castor bean leaf: sieve tube occlusion and carbohydrate back-up precede chlorophyll degradation. Physiologia Plantarum. 2004;120:338–346. doi: 10.1111/j.0031-9317.2004.0245.x. [DOI] [PubMed] [Google Scholar]
- Keskitalo J, Bergquist G, Gardeström P, Jansson S. A cellular timetable of autumn senescence. Plant Physiology. 2005;139:1635–1648. doi: 10.1104/pp.105.066845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köksal M, Zimmer I, Schnitzler J-P, Christianson DW. Structure of isoprene synthase illuminates the chemical mechanism of teragram atmospheric carbon emission. Journal of Molecular Biology. 2010;402:363–373. doi: 10.1016/j.jmb.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kull O, Koppel A, Noormets A. Seasonal changes in leaf nitrogen pools in two Salix species. Tree Physiology. 1998;18:45–51. doi: 10.1093/treephys/18.1.45. [DOI] [PubMed] [Google Scholar]
- Laisk A. Kinetika fotosinteza i fotodyhaniya C3-rastenii. (Kinetics of photosynthesis and photorespiration in C3-plants) Nauka; Moscow: 1977. [Google Scholar]
- Laisk A, Eichelmann H, Oja V, Talts E, Scheibe R. Rates and roles of cyclic and alternative electron flow in potato leaves. Plant and Cell Physiology. 2007;48:1575–1588. doi: 10.1093/pcp/pcm129. [DOI] [PubMed] [Google Scholar]
- Laisk A, Talts E, Oja V, Eichelmann H, Peterson R. Fast cyclic electron transport around photosystem I in leaves under far-red light: a proton-uncoupled pathway? Photosynthesis Research. 2010;103:79–95. doi: 10.1007/s11120-009-9513-4. [DOI] [PubMed] [Google Scholar]
- Leith JH, Reynolds JF. The nonrectangular hyperbola as a photosynthetic light response model: geometrical interpretation and estimation of the parameter. Photosynthetica. 1987;21:363–366. [Google Scholar]
- Li Z, Ratliff EA, Sharkey TD. Effect of temperature on postillumination isoprene emission in oak and poplar. Plant Physiology. 2011;155:1037–1046. doi: 10.1104/pp.110.167551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Sharkey TD. Metabolic profiling of the methylerythritol phosphate pathway reveals the source of post-illumination isoprene burst from leaves. Plant, Cell and Environment. 2013a;36:429–437. doi: 10.1111/j.1365-3040.2012.02584.x. [DOI] [PubMed] [Google Scholar]
- Li Z, Sharkey TD. Molecular and pathway controls on biogenic volatile organic compound emissions. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013b. pp. 119–151. [Google Scholar]
- Lichtenthaler HK, Gitelson A, Lang M. Non-destructive determination of chlorophyll content of leaves of a green and an aurea mutant of tobacco by reflectance measurements. Journal of Plant Physiology. 1996;148:483–493. [Google Scholar]
- Magel E, Mayrhofer S, Müller A, Zimmer I, Hampp R, Schnitzler J-P. Photosynthesis and substrate supply for isoprene biosynthesis in poplar leaves. Atmospheric Environment. 2006;40:S138–S151. [Google Scholar]
- Miyazawa SI, Makino A, Terashima I. Changes in mesophyll anatomy and sink-source relationships during leaf development in Quercus glauca, an evergreen tree showing delayed leaf greening. Plant, Cell and Environment. 2003;26:745–755. [Google Scholar]
- Monson RK, Grote R, Niinemets Ü, Schnitzler J-P. Tansley review. Modeling the isoprene emission rate from leaves. The New Phytologist. 2012;195:541–559. doi: 10.1111/j.1469-8137.2012.04204.x. [DOI] [PubMed] [Google Scholar]
- Monson RK, Harley PC, Litvak ME, Wildermuth M, Guenther AB, Zimmerman PR, Fall R. Environmental and developmental controls over the seasonal pattern of isoprene emission from aspen leaves. Oecologia. 1994;99:260–270. doi: 10.1007/BF00627738. [DOI] [PubMed] [Google Scholar]
- Monson RK, Jaeger CH, Adams WW, III, Driggers EM, Silver GM, Fall R. Relationships among isoprene emission rate, photosynthesis, and isoprene synthase activity as influenced by temperature. Plant Physiology. 1992;98:1175–1180. doi: 10.1104/pp.98.3.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfopoulos C, Sperlich D, Peñuelas J, Filella I, Llusià J, Medlyn BE, Niinemets Ü, Possell M, Sun Z, Prentice IC. A model of plant isoprene emission based on available reducing power captures responses to atmospheric CO2. The New Phytologist. 2014;203:125–139. doi: 10.1111/nph.12770. [DOI] [PubMed] [Google Scholar]
- Munné-Bosch S. Ageing in perennials. Critical Reviews in Plant Sciences. 2007;26:123–138. [Google Scholar]
- Niinemets Ü, García-Plazaola JI, Tosens T. Photosynthesis during leaf development and ageing. In: Flexas J, Loreto F, Medrano H, editors. Terrestrial photosynthesis in a changing environment. A molecular, physiological and ecological approach. Cambridge University Press; Cambridge: 2012. pp. 353–372. [Google Scholar]
- Niinemets Ü, Hauff K, Bertin N, Tenhunen JD, Steinbrecher R, Seufert G. Monoterpene emissions in relation to foliar photosynthetic and structural variables in Mediterranean evergreen Quercus species. The New Phytologist. 2002;153:243–256. [Google Scholar]
- Niinemets Ü, Keenan TF, Hallik L. Tansley review. A worldwide analysis of within-canopy variations in leaf structural, chemical and physiological traits across plant functional types. The New Phytologist. 2015;205:973–993. doi: 10.1111/nph.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinemets Ü, Kuhn U, Harley PC, Staudt M, Arneth A, Cescatti A, Ciccioli P, Copolovici L, Geron C, Guenther AB, Kesselmeier J, et al. Estimations of isoprenoid emission capacity from enclosure studies: measurements, data processing, quality and standardized measurement protocols. Biogeosciences. 2011;8:2209–2246. [Google Scholar]
- Niinemets Ü, Kull O, Tenhunen JD. Within canopy variation in the rate of development of photosynthetic capacity is proportional to integrated quantum flux density in temperate deciduous trees. Plant, Cell and Environment. 2004;27:293–313. [Google Scholar]
- Niinemets Ü, Sun Z. How light, temperature, and measurement and growth [CO2] interactively control isoprene emission in hybrid aspen. Journal of Experimental Botany. 2015;66:841–851. doi: 10.1093/jxb/eru443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinemets Ü, Tenhunen JD. A model separating leaf structural and physiological effects on carbon gain along light gradients for the shade-tolerant species Acer saccharum. Plant, Cell and Environment. 1997;20:845–866. [Google Scholar]
- Niinemets Ü, Tenhunen JD, Canta NR, Chaves MM, Faria T, Pereira JS, Reynolds JF. Interactive effects of nitrogen and phosphorus on the acclimation potential of foliage photosynthetic properties of cork oak, Quercus suber, to elevated atmospheric CO2 concentrations. Global Change Biology. 1999a;5:455–470. [Google Scholar]
- Niinemets Ü, Tenhunen JD, Harley PC, Steinbrecher R. A model of isoprene emission based on energetic requirements for isoprene synthesis and leaf photosynthetic properties for Liquidambar and Quercus. Plant, Cell and Environment. 1999b;22:1319–1336. [Google Scholar]
- Noormets A, Kull O, Koppel A. Nitrogen dynamics in Salix leaves during the first production year. In: Perttu K, Koppel A, editors. Short rotation willow coppice for renewable energy and improved environment. Proceedings of a joint Swedish-Estonian seminar on energy forestry and vegetation filters; Tartu. 24-26 September 1995; Uppsala: Swedish University of Agricultural Sciences; 1996. pp. 51–59. [Google Scholar]
- Orlova I, Nagegowda DA, Kish CM, Gutensohn M, Maeda H, Varbanova M, Fridman E, Yamaguchi S, Hanada A, Kamiya Y, Krichevsky A, et al. The small subunit of snapdragon geranyl diphosphate synthase modifies the chain length specificity of tobacco geranylgeranyl diphosphate synthase in planta. The Plant Cell. 2009;21:4002–4017. doi: 10.1105/tpc.109.071282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen SM, Peñuelas J. Opportunistic emissions of volatile isoprenoids. Trends in Plant Science. 2005;10:420–426. doi: 10.1016/j.tplants.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Peñuelas J, Marino G, Llusia J, Morfopoulos C, Farré-Armengol G, Filella I. Photochemical reflectance index as an indirect estimator of foliar isoprenoid emissions at the ecosystem level. Nature Communications. 2013;4:2604. doi: 10.1038/ncomms3604. [DOI] [PubMed] [Google Scholar]
- Rajabi Memari H, Pazouki L, Niinemets Ü. The biochemistry and molecular biology of volatile messengers in trees. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013. pp. 47–93. [Google Scholar]
- Rasulov B, Bichele I, Hüve K, Vislap V, Niinemets Ü. Acclimation of isoprene emission and photosynthesis to growth temperature in hybrid aspen: resolving structural and physiological controls. Plant, Cell & Environment. 2015a;38:751–766. doi: 10.1111/pce.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasulov B, Bichele I, Laisk A, Niinemets Ü. Competition between isoprene emission and pigment synthesis during leaf development in aspen. Plant, Cell and Environment. 2014;37:724–741. doi: 10.1111/pce.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasulov B, Copolovici L, Laisk A, Niinemets Ü. Postillumination isoprene emission: in vivo measurements of dimethylallyldiphosphate pool size and isoprene synthase kinetics in aspen leaves. Plant Physiology. 2009a;149:1609–1618. doi: 10.1104/pp.108.133512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasulov B, Hüve K, Bichele I, Laisk A, Niinemets Ü. Temperature response of isoprene emission in vivo reflects a combined effect of substrate limitations and isoprene synthase activity: a kinetic analysis. Plant Physiology. 2010;154:1558–1570. doi: 10.1104/pp.110.162081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasulov B, Hüve K, Laisk A, Niinemets Ü. Induction of a longer-term component of isoprene release in darkened aspen leaves: origin and regulation under different environmental conditions. Plant Physiology. 2011;156:816–831. doi: 10.1104/pp.111.176222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasulov B, Hüve K, Välbe M, Laisk A, Niinemets Ü. Evidence that light, carbon dioxide and oxygen dependencies of leaf isoprene emission are driven by energy status in hybrid aspen. Plant Physiology. 2009b;151:448–460. doi: 10.1104/pp.109.141978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasulov B, Talts E, Kännaste A, Niinemets Ü. Bisphosphonate inhibitors reveal a large elasticity of plastidic isoprenoid synthesis pathway in isoprene-emitting hybrid aspen. Plant Physiology. 2015b doi: 10.1104/pp.15.00470. xx, In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstiel TN, Ebbets AL, Khatri WC, Fall R, Monson RK. Induction of poplar leaf nitrate reductase: a test of extrachloroplastic control of isoprene emission rate. Plant Biology. 2004;6:12–21. doi: 10.1055/s-2003-44722. [DOI] [PubMed] [Google Scholar]
- Rundle SJ, Zielinski RE. Alterations in barley ribulose-1,5-bisphosphate carboxylase oxygenase activase gene-expression during development and in response to illumination. The Journal of Biological Chemistry. 1991;266:14802–14807. [PubMed] [Google Scholar]
- Schnitzler J-P, Zimmer I, Bachl A, Arend M, Fromm J, Fischbach R. Biochemical properties of isoprene synthase in poplar (Populus x canescens) Planta. 2005;222:777–786. doi: 10.1007/s00425-005-0022-1. [DOI] [PubMed] [Google Scholar]
- Shesták Z. Changes in electron transport chain composition, and activities of photosystems and photophosphorylation during leaf ontogeny. In: Shesták Z, editor. Photosynthesis during leaf development. Dr. W. Junk Publishers; Dordrecht - Boston - Lancaster: 1985a. pp. 128–144. [Google Scholar]
- Shesták Z. Chlorophylls and carotenoids during leaf ontogeny. In: Shesták Z, editor. Photosynthesis during leaf development. Dr. W. Junk Publishers; Dordrecht - Boston - Lancaster: 1985b. pp. 76–106. [Google Scholar]
- Silver GM, Fall R. Enzymatic synthesis of isoprene from dimethylallyl diphosphate in aspen leaf extracts. Plant Physiology. 1991;97:1588–1591. doi: 10.1104/pp.97.4.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims DA, Gamon JA. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sensing of Environment. 2002;81:337–354. [Google Scholar]
- Stitt M. Limitation of photosynthesis by carbon metabolism. I. Evidence for excess electron transport capacity in leaves carrying out photosynthesis in saturating light and CO2. Plant Physiology. 1986;81:1115–1122. doi: 10.1104/pp.81.4.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Copolovici L, Niinemets Ü. Can the capacity for isoprene emissions acclimate to environmental modifications during autumn senescence in temperate deciduous tree species Populus tremula? Journal of Plant Research. 2012a;125:263–274. doi: 10.1007/s10265-011-0429-7. [DOI] [PubMed] [Google Scholar]
- Sun Z, Niinemets Ü, Hüve K, Noe SM, Rasulov B, Copolovici L, Vislap V. Enhanced isoprene emission capacity and altered light responsiveness in aspen grown under elevated atmospheric CO2 concentration. Global Change Biology. 2012b;18:3423–3440. [Google Scholar]
- Takeuchi A, Yamaguchi T, Hidema J, Strid A, Kumagai T. Changes in synthesis and degradation of Rubisco and LHCII with leaf age in rice (Oryza sativa L.) growing under supplementary UV-B radiation. Plant, Cell and Environment. 2002;25:695–706. [Google Scholar]
- Tosens T, Niinemets Ü, Vislap V, Eichelmann H, Castro-Díez P. Developmental changes in mesophyll diffusion conductance and photosynthetic capacity under different light and water availabilities in Populus tremula: how structure constrains function. Plant, Cell and Environment. 2012;35:839–856. doi: 10.1111/j.1365-3040.2011.02457.x. [DOI] [PubMed] [Google Scholar]
- Valjakka M, Luomala E-M, Kangasjärvi J, Vapaavuori E. Expression of photosynthesis- and senescence-related genes during leaf development and senescence in silver birch (Betula pendula) seedlings. Physiologia Plantarum. 1999;106:302–310. [Google Scholar]
- von Caemmerer S, Farquhar GD. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta. 1981;153:376–387. doi: 10.1007/BF00384257. [DOI] [PubMed] [Google Scholar]
- Weise SE, Li Z, Sutter AE, Corrion A, Banerjee A, Sharkey TD. Measuring dimethylallyl diphosphate available for isoprene synthesis. Analytical Biochemistry. 2013;435:27–34. doi: 10.1016/j.ab.2012.11.031. [DOI] [PubMed] [Google Scholar]
- Wiberley AE, Linskey AR, Falbel TG, Sharkey TD. Development of the capacity for isoprene emission in kudzu. Plant, Cell and Environment. 2005;28:898–905. [Google Scholar]
- Wright LP, Rohwer JM, Ghirardo A, Hammerbacher A, Ortiz-Alcaide M, Raguschke B, Schnitzler J-P, Gershenzon J, Phillips MA. Deoxyxylulose 5-phosphate synthase controls flux through the methylerythritol 4-phosphate pathway in Arabidopsis. Plant Physiology. 2014;165:1488–1504. doi: 10.1104/pp.114.245191. [DOI] [PMC free article] [PubMed] [Google Scholar]