Summary
Carotenoids constitute a major target of chloroplastic photooxidative reactions, leading to the formation of several oxidized derivatives and cleavage products, some of which are volatile (VCCPs). Among them, β-cyclocitral (β-CC), at least, is a retrograde signaling molecule that modulates the activity of many key physiological processes. In the present work, we aimed to study whether β-CC and other VCCPs are released into the atmosphere from photosynthetic tissues. To overcome stomatal limitations, the foliose chlorolichen Lobaria pulmonaria was used as the model system, and the emissions of biogenic volatiles, induced by heat and wounding stresses, were monitored by proton-transfer reaction time-of-flight mass-spectrometry (PTR-TOF-MS) and gas-chromatography (GC-MS). Prior to stress treatments, VCCPs were emitted constitutively, accounting for 1.3 % of the total volatile release, with β-CC being the most abundant VCCP. Heat and wounding stresses induced a burst of volatile release, including VCCPs, and a loss of carotenoids. Under heat stress, the production of β-CC correlated positively with temperature. However the enhancement of production of VCCPs was the lowest among all the groups of volatiles analyzed. Given that the rates of carotenoid loss were three orders of magnitude higher than the release rates of VCCPs and that these compounds only represent a minor fraction in the blend of volatiles, it seems unlikely that VCCPs might represent a global stress signal capable of diffusing through the atmosphere to different neighboring individuals.
Keywords: β-cyclocitral, carotenoid degradation, heat stress, lichen, volatiles, wounding
1. Introduction
Chlorophyll (Chl) is a double-edged sword for plants. This molecule is capable of harvesting sunlight, initiating the process of photosynthesis, but it also involves an unavoidable risk of photooxidation. Thus, a certain proportion of the photons absorbed by Chl cause the generation of reactive oxygen species (ROS), which bring about oxidative damage in lipids, pigments and proteins (Mitler 2002). One of the most harmful ROS is the singlet oxygen (1O2), which is generated by the transfer of excitation energy from triplet excited chlorophyll (3Chl*) to ground triplet oxygen (3O2) (Krieger-Liszkay 2005). This process can be exacerbated by environmental factors that reduce photosynthetic efficiency and hence affect the balance between energy absorption and use, leading to the over-reduction of the electron transport chain and the accumulation of 3Chl* (Munné-Bosch et al. 2013).
To counteract ROS formation, plants possess a plethora of defense mechanisms. Among them, carotenoids play a pivotal role, being able to prevent 1O2 formation by direct quenching of 3Chl* and/or by deactivation of 1O2 (Triantaphylidès and Havaux 2009). Direct quenching of 3Chl* requires physical proximity and is unlikely to occur in the reaction center because of the distance between β-carotene (β-Car) and Chl. Thus, the main function of β-Car in reaction centers is to quench the 1O2generated by 3Chl* (Telfer 2002). Quenching of 1O2 by β-Car may occur through a physical or chemical interaction, the latter involving the oxidation of β-Car (Ramel et al. 2012a). Oxidation of β-Car by 1O2 generates an array of different cleavage products (apocarotenoids), most of them aldehydes of varying chain length (Ramel et al. 2012a). Among these compounds, those with short chain length are volatile (VCCPs): β-cyclocitral (β-CC, C10), β-ionone, α-ionone (both C13) and dihydroactinidiolide (dhA, C11) (Ramel et al. 2012a).
Two of the VCCPs, β-CC and dhA, have been identified recently as being involved in the transcriptional modulation of a large set of genes, most of them responsive to 1O2 (Ramel et al. 2012b; Shumbe et al. 2015). These changes in gene expression are associated with an enhancement of tolerance to photooxidative stress. This observation, together with the fact that the generation of volatile β–CC, dihydroactinidiolide and other VCCPs is faster in plants exposed to high light (Ramel et al. 2012b), has led researchers to propose their role as stress-signaling molecules. β-CC has been also considered as a candidate for retrograde (chloroplast control over nuclear gene expression) signaling, capable of crossing cell membranes and diffusing from its site of formation in chloroplasts to other organelles, thanks to its lipid-soluble and volatile character (Ramel et al. 2013, Estavillo et al. 2013). A likely mechanism for the regulatory effect of β-CC is through its reaction with thiol groups of proteins leading to regulation of gene expression through the activation of multiple transcription factors (Havaux et al. 2013).
When released into the atmosphere, all these volatile apocarotenoids add to the blend of inducible volatiles produced by plants (Holopainen 2004). Apart from their potential role in stress-signaling, β-CC and other VCCPs have been shown to play significant roles in biotic interactions as allelochemicals (Ikawa et al. 2001, Kato-Noguchi and Seki 2010) or chemical attractants to pollinators (Simkin et al. 2004a, Guédot et al. 2008), grazer repellents (Jüttner et al. 2010) and olfactory signals to birds with carotenoid-colored plumage (Senar et al. 2010). Recent studies also suggest that thanks to their ability to overcome restrictions imposed by vascular system, VCCPs may be involved in long-distance signaling (Carmody et al. 2016).
Overall, VCCPs, especially β-CC, are firm candidates for being involved in stress signaling and acclimation. Since these molecules are lipid-soluble and volatile, they may diffuse through membranes, escape the chloroplast and assist in communication between different organelles within the cell. For the same reason, they can leave the cell, and eventually the photosynthetic organ. Once in the atmosphere, uptake of these compounds by surrounding organisms is possible depending upon the physicochemical characteristics of the interaction (Niinemets et al. 2004, 2014). Considering the implications of the bidirectional exchange of signaling molecules such as β-CC, in the present study, we hypothesized that photosynthetic organisms under stress conditions can behave as emitters of β-CC and other VCCPs that can further be involved in long-distance signaling. To address this point we have used the foliose lichen Lobaria pulmonaria as the model system. We have chosen such a model and not a vascular plant to simplify the pathway between the thylakoids and the open atmosphere. Previous studies have detected the presence of β-CC in dried leaves (Nezhadali and Nezhadai Bagham 2011) or in leaf extracts (Priestap et al. 2003, Ramel et al 2012b, Shibamoto et al. 2007). In the present study, we demonstrate that β-CC and other VCCPs can be emitted in vivo from an intact photosynthetic organism.
2. Methods
2.1. Sampling and preservation of Lobaria pulmonaria thalli
Lobaria pulmonaria is a tripartite symbiosis formed by a fungus and two photobionts: a cyanobacterium Nostoc and a green alga Dyctiochloropsis. Among them, the second is quantitatively the dominant in terms of total biomass and it forms a continuous photosynthetic layer, while Nostoc only occurs in isolated cephalodia (Schofield et al. 2003; Cornejo and Scheidegger 2013). As a consequence, the photosynthetic responses observed in this species are mostly generated by the green algal layer. Lobaria pulmonaria is a threatened species in several western and central European countries, where is considered as an old-growth forest indicator. This is not the case of northern Spain where L. pulmonaria is frequent in most beech and oak forests. In the present study, to affect as less as possible sampled populations, the collection of thalli was limited to a small number of specimens and when possible, the specimens were collected from recently fallen trees or branches. The possibility of using pure algal cultures was rejected because of possible interactions of volatiles with the growth media, and their artificial character that impedes any extrapolation to intact photosynthetic tissues.
Thalli of L. pulmonaria were collected in an holm oak (Quercus ilex) forest in northern Spain (lat 42° 52' N long 3° 4' W, elevation 800 m a.s.l). Samples were dried under room conditions, and once dried, stored a maximum of two weeks at a relative humidity (RH) of less than 10 % and air temperature of 4 °C until use. No loss of vitality (measured as the dark-adapted maximum chlorophyll fluorescence yield Fv/Fm) was detected after this period in reactivated specimens. After storage, the thalli were re-moistened in contact with a moist paper tissue and preconditioned for 48 h at 100 % RH, 23 °C and dim light (12 h day/night), similarly to Gauslaa et al. (2012). For online volatile emissions, intact thalli were used, while for pigment determination, 12 mm discs were cut from the thalli.
2.2. Stress treatments
First, the emission of VCCPs was checked by qualitative assays by enclosing the intact thalli in a 10 x 10 cm ovenproof polyethylene terephthalate bag (Stewart-Jones and Poppy, 2006; Niinemets et al., 2011). Prior to trapping of volatiles, the bag with thalli was conditioned at 20 °C (control) and 33 °C (moderate heat stress treatment) for 20 min under a quantum flux density of 1000 μmol m−2 s−1.
For quantitative analyses, heat stress was induced by immersing each intact thallus into a bath of water for five minutes as described in Copolovici et al. (2012). Four temperature treatments were used: 23 °C (control), 37 °C, 46 °C and 51 °C (heat stress). These temperatures were chosen as 40 °C is the temperature threshold at which heat-induced damage, i.e. enhanced cellular ion leakage, is elicited in L. pulmonaria thalli (Shirazi et al. 1996). Immediately after the heat treatment, the thalli were incubated for 10 min beneath a sun simulation lamp (SOL 500, UV-A+VIS+IR (320-3000 nm), Dr. Hoenle, Germany) supplying a quantum flux density of ca. 350 µmol m-2 s-1. For pigment analysis, five discs were exposed at each temperature following the same protocol described before and collected after 10 min of light exposure. Samples were frozen in liquid nitrogen and stored at -80 °C until analysis. Additionally, and for comparative purposes, a parallel set of samples was subjected to an intensive wounding (approximately a total of 0.5 m of parallel linear cuts per sample performed with a razor blade).
2.3. Collection of volatiles in qualitative experiments
A solid-phase microextraction (SPME) fiber of 65 μm of polydimethyl siloxane/divinyl benzene (PDMS/DVB, Supelco, Bellefonte, PA, USA) was inserted in the bag for sampling the lichen volatiles for 20 min. After removing the SPME-fiber from the bag, it was immediately transferred to the injector port of the gas-chromatograph mass-spectrometer (GC-MS; 2010 Plus, Shimadzu Corporation, Kyoto, Japan). Emission of volatiles of an empty bag was considered when identifying the volatiles released by the thalli L. pulmonaria.
2.4. Incubation chamber for quantitative emission of volatiles
After heat shock treatments, excess water was gently removed from the thalli between two tissue papers and then transferred to an illuminated (light intensity of 350 µmol m-2 s-1 at the surface of the thalli) incubation glass chamber (0.5 L) in order to monitor the volatile emissions. Chamber flow was maintained at 1 L min-1 at lab conditions (25 °C, 101 kPa) with volatile-free air obtained by passing the air entering the cuvette through a catalytic scrubber heating the air at 350 °C and efficiently removing VOCs (GCU, Ionimed, Innsbruck, Austria). All the tubing was PTFE. The outlet of the chamber was diverted to a proton-transfer reaction time-of-flight mass-spectrometer (PTR-TOF-MS), which sampled the air exiting the chamber at a rate of 0.1 L min-1 to analyze the concentrations of volatiles. Prior to measurements, empty cuvette measurements were done and the signal of volatiles was subtracted from the data recorded during the experiment. The volatile emissions of thalli that did not undergo the heat shock treatment were measured and considered as pre-stress controls. Wound-induced volatile emission was also analyzed in the thalli subjected to the wounding treatment.
2.5. GC-MS analyses
Volatiles collected with the SPME fiber were analyzed with the Shimadzu GC-MS system equipped with a GC column of ZB5-MS (0.25 mm i.d. x 30 m, 0.25 μm film Zebron, Phenomenex, Torrance, CA, USA). Volatiles were separated with the following temperature program: 40 °C for 1 min, ramp of 10 °C min-1 to 220 °C followed by a 5 min hold. The mass spectrometer operated in electron-impact mode at 70 eV and in the m/z scan range of 30 to 400 amu. The temperature of the transfer line was set at 240 °C and the ion-source was operated at 150 °C.
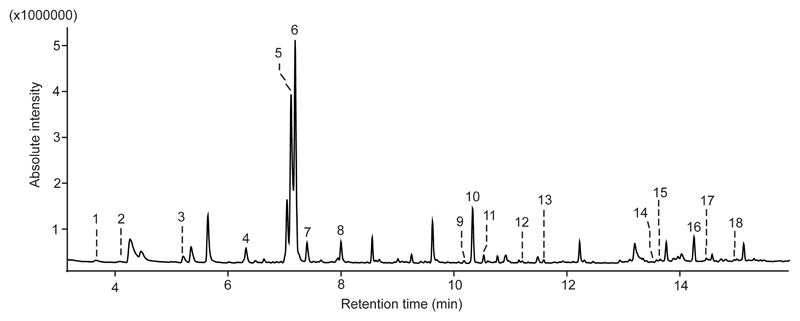
Volatiles were identified by analyzing the hydrocarbon standard of C8-C20 with GC-MS at the same conditions as SPME analyses. Finally, mass spectra and retention indices (RIs) of lichen volatiles on ZB5-MS were compared to the mass spectra and RIs of commercially available standards (Figure 1), and the spectra published in the NIST library (National Institute of Standards and Technology), or in the catalogue of essential oil components (Adams 2004) or in publications of other research groups (Gómez et al. 1993, Larsen and Frisvad 1995, Rana and Blazquez 2009, Rostad and Pereira 1986). All reference compounds were purchased from Sigma-Aldrich (St. Louis, MO, USA) at the highest purity available (> 98%).
Figure 1.
Volatiles of a heat-stressed Lobaria pulmonaria at 33 °C trapped with 65 µm polydimethyl siloxane / divinyl benzene (PDMS/DVB) fiber (Supelco, Bellefonte, PA, USA). 1 1-pentanol, 2 hexanal, 3 1-hexanol, 4 α-pinene, 5 β-pinene, 6 3-octanone, 7 3-octanol, 8 limonene, 9 iso-menthone, 10 1,3-dimethoxybenzene, 11 menthol, 12 β-cyclocitral, 13 carvone, 14 α-cubebene, 15 α-longipinene, 16 (E)- β-caryophyllene, 17 geranyl acetone, 18 γ-muurolene co-eluting with β-ionone. Volatiles were identified by comparing their mass spectra and retention indices (RIs) on ZB5-column to the ones of standards (Figure 2) and to the ones available in databases and literature (Supplementary Figure 1, Supplementary Table 1).
2.6. PTR-TOF-MS analytical procedures
The emission of volatile organic compounds was measured with a PTR-TOF-MS (Model, Ionicon, Austria) in real-time. Continuous measurements were performed during 2-3 minutes accounting for a total of 150 to 200 individual measurements. Principles of PTR-TOF-MS technique are described in Portillo-Estrada et al. (2015). The air sample underwent proton reactions with the hydronium ions (H3O+) produced within the discharge ion source. Subsequently, the resulting protonated ions passed through a drift tube operated at 600 V, 2.3 mbar pressure and 60 °C temperature, resulting in a field density ratio (E/N) of ≈130 Td. The ions were extracted from the drift tube and pulsed every 32 μs to the orthogonal time-of-flight chamber and separated by their m/z ratio. The ions were detected by a multi-channel-plate and a time-to-digital converter (Burle Industries Inc., Lancaster, PA, USA). The data was recorded at 1 s time resolution, being the average of 31250 spectra ranging from m/z of 0 to 316.
The raw spectra were acquired by TofDaq software (Tofwerk AG, Switzerland). The PTR-TOF-MS instrument has lower sensitivity for ions of smaller mass. The sensitivity of our PTR-TOF-MS instrument to the volatiles measured was derived from the reaction rate constant calculated from a gas mixture containing eight pure compounds ranging from m+/z 21 to 181 with known concentrations (Ionimed GmbH, Innsbruck, Austria).
The data post-processing by routine functions was done in PTR-MS Viewer v3.1 (Tofwerk AG, Switzerland). Mass calibration of the spectra was performed by identifying ions of known exact mass. Additionally, 1,3-diiodobenzene (protonated parent mass at m/z 330.85 and fragments at m/z 203.94 and 204.94) was continuously permeated into the air sample to improve the mass calibration. Then, the position of each protonated compound in the spectra was identified by their exact mass. The concentration of each protonated compound was calculated by correlating the integrated peak area and the concentration of primary ion H3O+ (using the isotopomer H318O+, m/z 21.0221) through the built-in software tools. A software built-in tool resolving multi-peaks in the spectrum was needed to accurately calculate the signal corresponding to β-CC and other lichen compounds. The presence of β–CC peak in the spectrum was identified by its time of flight from the pulser to the detector (proportional to the molecular mass) compared to the time performed by known standard compounds. Protonated β-CC (m/z 153.128) peak sits among (C8H8O3)H+ (m/z 153.055), (C12H8)H+ (m/z 153.070) and (C9H16N2)H+ (m/z 153.139), thus the tail of the peaks overlapped in some cases. The identification of other volatiles not present in the standard gas mixture was done similarly by inter- and extrapolation of the time of flight. Each individual peak of the multi-peak system was fitted to a Gaussian function by providing the exact mass of each peak center and the standard deviation of the Gaussian function. The fitting parameters could be tuned until the modeled multi-peak signal fitted to the measured signal. The software tool then derived the individual signal of each peak in every spectrum according to the parameterized model.
2.7. Pigment analysis by HPLC
The frozen samples, stored at -80 ºC until use, were homogenized with a mortar in pure acetone solution buffered with CaCO3. The extracts were centrifuged at 16100 g for 20 min, and supernatants were filtered with 0.2 μm PTFE filters (Teknokroma, Spain). Pigment separation was performed by HPLC with a reverse phase C18 column (Waters Spherisorb ODS1, 4.6 x 250 mm, Milford, MA, USA) and pigments were detected by a photodiode array (PDA) detector, following the method of (García-Plazaola and Becerril 1999) with the modifications included by Garcia-Plazaola and Esteban (2012). This method allows for separation and quantification of all major leaf carotenoids: neoxanthin (Neo), antheraxanthin (Ant), violaxanthin (Vio), lutein (Lut), zeaxanthin (Zea) and β-carotene (β-Car).
2.8. Statistical analyses
The relationships between (i) volatiles and temperature, (ii) β-CC and other volatiles, (iii) carotenoid degradation and VCCPs, and (iv) VCCPs and carotenoids (data not shown) were assessed by Pearson correlation coefficients, after checking for homocedascity of the data. When significant correlations were found, fit of data to a linear regression model was additionally tested.
Differences in pigment contents after temperature and light treatments were tested using one-way ANOVA with Duncan post hoc tests. All statistical analyses were performed with SPSS statistical package, v22, and significant differences considered at P<0.05.
3. Results
3.1. Identification of carotenoid oxidation products by GC-MS
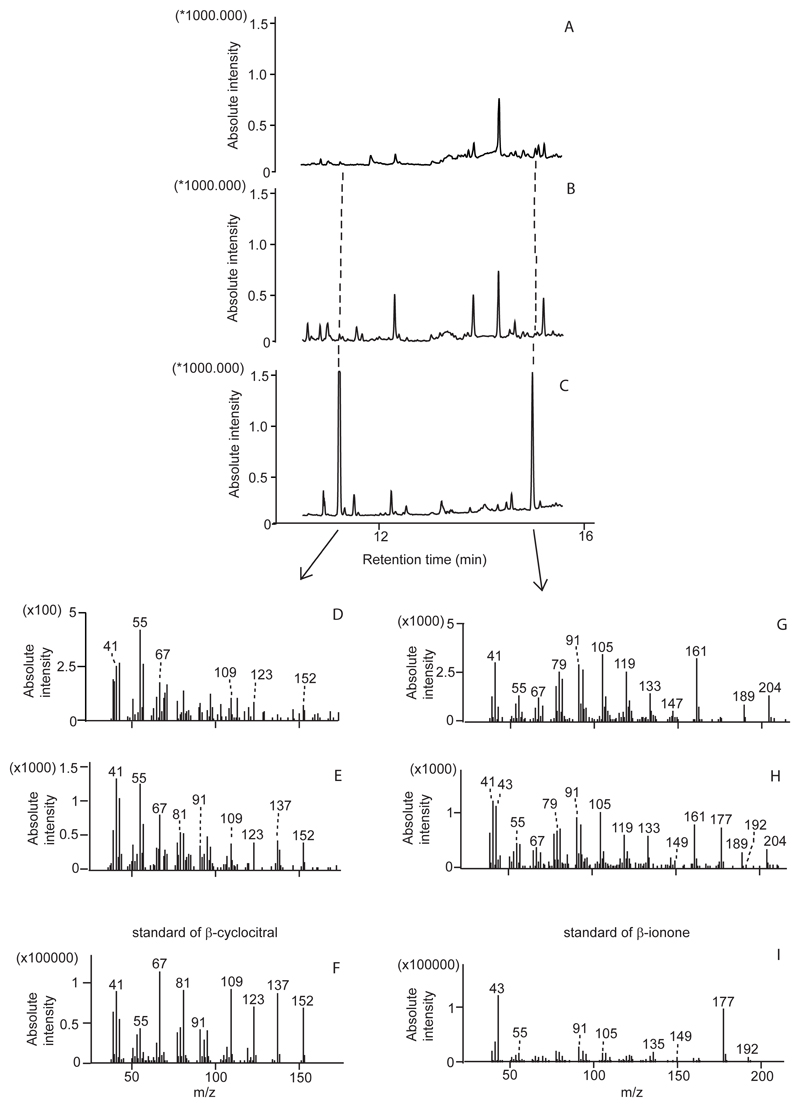
According to qualitative SPME analyses, the bouquet of a control and heat stressed thalli consisted of lipoxygenase chain oxidation products (LOXs) such as 1-pentanol, hexanal, 1-hexanol and (Z)-3-hexenol and isoprenoids including non-oxygenated monoterpenes (α-pinene, β-pinene, limonene, β-phellandrene) and sesquiterpenes (α-cubebene, α-longipinene, (E)-β-caryophyllene, γ-muurolene, α-amorphene and germacrene D) (Figure 1, Supplementary Table 1). SPME analyses also showed that non-stressed and stressed thalli released several oxygenated monoterpenes (iso-menthone, menthol, dihydrocarvone and carvone), benzenoids (methoxybenzene, phenol and 1,3-dimethoxybenzene), other volatiles including those of low molecular mass and finally VCCPs: β–CC and β-ionone (Figure 1, Supplementary Table 1). In fact, in the SPME analyses, β–CC and β-ionone were the only VCCPs identified and their occurrence was proven by comparing their mass spectra in lichen samples with the spectra of purchased standards (Figure 2).
Figure 2.
Identification of β-cyclocitral and β-ionone in the emission of L. pulmonaria by comparing the mass spectra of retention areas of β-cyclocitral (D) and β-ionone (G) in control analysis (A) or mass spectra of retention areas β-cyclocitral (E) and β-ionone (H) in heat stress analysis (B) to the ones (F and I) in a mixture of respective standards (C).
3.2. Quantitative characterization of volatiles in non-stressed thalli
The emission rates of volatiles identified by SPME analyses were quantified by PTR-TOF-MS. Qualitatively, PTR-TOF-MS confirmed the emission of 35 different volatiles from non-stressed thalli (Table 1). These compounds were grouped in five major categories: VCCPs, LOXs, isoprenoids, benzenoids, and lightweight oxygenated compounds (LOCs). PTR-TOF-MS detected also peaks with masses corresponding to those of β-CC, dihydroactinidiolide, hydroxycyclocitral, ionene, hydroxyionone and ionol (Table 1).
Table 1.
Emissions in pmol m-2 s-1 of selected biogenic volatile organic compounds (BVOCs) analysed by a proton-transfer-reaction time-of-flight mass spectrometer (PTR-TOF-MS) during heat shock and thallus wounding experiments in L. pulmonaria samples. MVK stands for methyl vinyl ketone, MACR stands for methacrolein. Values represent the average of 3 independent experiments ±SE.
| Most probable compound name | Protonated compound (MW) | Pre-stress level | Heat shock at 37 °C | Heat shock at 46 °C | Heat shock at 51 °C | Leaf wounding |
|---|---|---|---|---|---|---|
| VCCPs | ||||||
| β–CC + dihydrocarvone | (C10H16O)H+ (153.128) | 1.75±0.24 | 3.13±0.51 | 5.12±0.42 | 5.34±0.71 | 5.94±0.42 |
| Hydroxycyclocitral | (C10H16O2)H+ (169.122) | 0.81±0.10 | 1.56±0.19 | 1.95±0.10 | 3.12±0.43 | 3.30±0.28 |
| Ionene | (C13H18)H+ (175.148) | 0.70±0.11 | 0.68±0.03 | 0.80±0.10 | 0.73±0.15 | 0.67±0.08 |
| Dihydroactinidiolide | (C11H16O2)H+ (181.122) | 0.36±0.03 | 0.80±0.10 | 0.96±0.04 | 0.95±0.12 | 0.75±0.10 |
| Ionone | (C13H20O)H+ (193.159) | 0.26±0.05 | 2.73±0.16 | 2.99±0.08 | 2.42±0.36 | 1.54±0.13 |
| Geranyl acetone | (C13H22O)H+ (195.174) | 0.40±0.03 | 0.42±0.05 | 0.43±0.01 | 0.55±0.10 | 0.42±0.10 |
| Hydroxyionone | (C13H20O2)H+ (209.154) | 0.24±0.02 | 0.57±0.08 | 0.77±0.01 | 0.81±0.11 | 0.56±0.09 |
| Ionol | (C15H24O)H+ (211.190) | 0.57±0.03 | 0.53±0.08 | 0.48±0.04 | 0.41±0.06 | 0.45±0.04 |
| LOXs | ||||||
| Hexenols (frag) + hexanal (frag) | (C6H10)H+ (83.086) | 15.7±1.7 | 18.4±0.6 | 28.0±3.7 | 56.3±7.8 | 68.6±15.9 |
| Pentenone + pentenal | (C5H8O)H+ (85.065) | 6.00±0.64 | 9.74±1.83 | 19.5±1.5 | 63.7±8.4 | 61.7±12.2 |
| Hexanol (frag) | (C6H12)H+ (85.101) | 1.85±0.16 | 6.77±0.51 | 5.77±0.65 | 6.09±0.97 | 7.57±1.05 |
| Pentanol | (C5H12O)H+ (87.080) | 7.15±0.46 | 54.6±4.2 | 65.3±1.6 | 101.7±14.4 | 83.2±20.1 |
| Hexenal | (C6H10O)H+ (99.080) | 4.25±0.64 | 7.19±1.98 | 6.61±0.16 | 10.0±1.9 | 8.84±1.31 |
| Hexanal + hexenols | (C6H12O)H+ (101.096) | 3.52±0.65 | 6.26±0.36 | 7.22±0.64 | 18.4±2.3 | 18.9±3.4 |
| Hexanol | (C6H14O)H+ (103.112) | 0.27±0.04 | 0.27±0.6 | 0.33±0.03 | 0.52±0.06 | 0.46±0.05 |
| Hexenyl acetate | (C8H14O2)H+ (143.107) | 7.35±1.02 | 7.76±1.41 | 12.1±1.1 | 17.5±2.5 | 20.5±1.4 |
| Hexyl acetate | (C8H16O2)H+ (145.122) | 1.20±0.18 | 2.63±0.29 | 2.65±0.18 | 3.12±0.47 | 2.96±0.54 |
| Isoprenoids | ||||||
| MVK + MACR | (C4H6O)H+ (71.049) | 11.5±1.1 | 34.7±7.7 | 44.9±3.4 | 96.9±14.1 | 82.9±18.4 |
| Monoterpenes (frag) + hexenal (frag) | (C6H8)H+ (81.070) | 5.31±0.81 | 13.0±1.4 | 19.7±0.9 | 17.5±3.1 | 16.5±3.0 |
| Monoterpenes (frag) | (C7H10)H+ (95.086) | 20.4±2.4 | 304.0±45.3 | 218.2±20.8 | 114.8±20.0 | 94.1±4.5 |
| Monoterpenes | (C10H16)H+ (137.133) | 5.68±0.74 | 8.22±1.55 | 11.46±1.20 | 7.42±1.28 | 7.46±0.24 |
| Carvone | (C10H14O)H+ (151.112) | 0.91±0.09 | 0.64±0.05 | 0.77±0.08 | 1.05±0.16 | 0.54±0.11 |
| Menthone | (C10H18O)H+ (155.143) | 2.18±0.48 | 2.28±0.39 | 3.95±0.33 | 4.40±0.61 | 6.37±0.87 |
| Menthol+Decanal | (C10H20O)H+ (157.159) | 6.67±1.06 | 6.46±0.91 | 7.28±0.34 | 7.02±1.14 | 7.07±0.56 |
| Sesquiterpenes | (C15H24)H+ (205.195) | 2.21±0.19 | 2.58±0.39 | 4.22±0.51 | 2.18±0.29 | 2.31±0.12 |
| Benzenoids | ||||||
| Benzene | (C6H6)H+ (79.054) | 20.2±2.0 | 27.0±6.1 | 23.6±1.8 | 33.6±5.1 | 27.1±1.4 |
| Phenol | (C6H6O)H+ (95.049) | 140.6±22.4 | 1840±140 | 1198±120 | 554.7±91.8 | 383.4±31.4 |
| Benzaldehyde | (C7H6O)H+ (107.049) | 4.81±0.54 | 21.7±2.2 | 27.8±0.3 | 102.9±15.4 | 92.9±6.24 |
| Xylene | (C8H10)H+ (107.086) | 0.70±0.03 | 1.87±0.12 | 2.09±0.17 | 2.78±0.38 | 3.36±0.33 |
| Methoxybenzene | (C7H8O)H+ 109.065 | 4.32±0.37 | 21.9±5.4 | 17.8±1.2 | 17.3±2.1 | 15.3±0.8 |
| Benzoic acid | (C7H6O2)H+ (123.044) | 2.12±0.11 | 2.95±0.37 | 2.15±0.09 | 7.33±1.34 | 6.44±1.21 |
| Dimethoxybenzene | (C8H10O2)H+ (139.075) | 14.3±2.7 | 53.4±5.6 | 17.2±0.9 | 18.4±2.6 | 13.5±0.6 |
| Ethyl salicylate | (C9H10O3)H+ (167.070) | 0.78±0.05 | 2.12±0.55 | 2.82±0.27 | 8.25±1.01 | 9.16±1.63 |
| Other VOCs | ||||||
| Methanol | (CH4O)H+ (33.034) | 10.2±0.3 | 187.2±20.1 | 713.0±14.4 | 1261±179 | 1373±119 |
| Acetaldehyde | (C2H4O)H+ (45.034) | 124.6±25.0 | 1230±158 | 1697±39 | 5839±789 | 3883±697 |
| Ethanediol | (C2H6O2)H+ (63.044) | 46.6±7.0 | 471.4±28.8 | 429.6±17.3 | 1308±216 | 1022±146 |
| Propanediol | (C3H8O2)H+ (77.060) | 15.3±3.4 | 30.0±1.1 | 33.3±2.5 | 53.4±9.1 | 43.1±6.0 |
| Ethylene carbonate | (C3H4O3)H+ (89.023) | 19.4±1.7 | 36.4±3.7 | 33.2±1.6 | 46.1±6.2 | 37.7±3.1 |
| Octadiene | (C8H14)H+ (111.117) | 17.8±0.4 | 46.5±3.7 | 46.8±2.8 | 30.6±6.1 | 41.9±4.4 |
| Heptenal | (C7H12O)H+ (113.096) | 6.33±0.92 | 9.85±1.63 | 14.4±0.9 | 32.9±4.5 | 49.4±4.5 |
| Octane | (C8H18)H+ (115.148) | 0.79±0.16 | 0.82±0.11 | 0.67±0.06 | 0.99±0.11 | 1.17±0.11 |
| Methylpentanoate | (C6H12O2)H+ (117.091) | 3.60±0.45 | 2.99±0.43 | 3.36±0.33 | 4.92±0.81 | 2.13±0.61 |
| Cyclooctanone | (C8H14O)H+ (127.112) | 8.35±0.85 | 24.4±1.5 | 20.9±1.4 | 31.4±5.9 | 40.9±3.1 |
| Met heptanoate + octanol | (C8H16O)H+ (129.127) | 42.0±8.5 | 46.1±6.27 | 61.4±2.7 | 23.7±3.1 | 19.6±2.0 |
In non-stressed (untreated) thalli of Lobaria pulmonaria, total volatile emission amounted to approximately 530 pmol m-2 s-1 (Table 1), being dominated by low molecular weight oxygenated hydrocarbons (LOCs) that represented 44 % of the total. A minor fraction (1 %) of the total volatiles constitutively emitted by the lichen, was due to the emission of VCCPs. Among VCCPs, β-CC was the main component of VCCPs blend (34 %), followed by hydroxycitral (18 %).
3.3. Effects of heat stress and wounding on volatile release
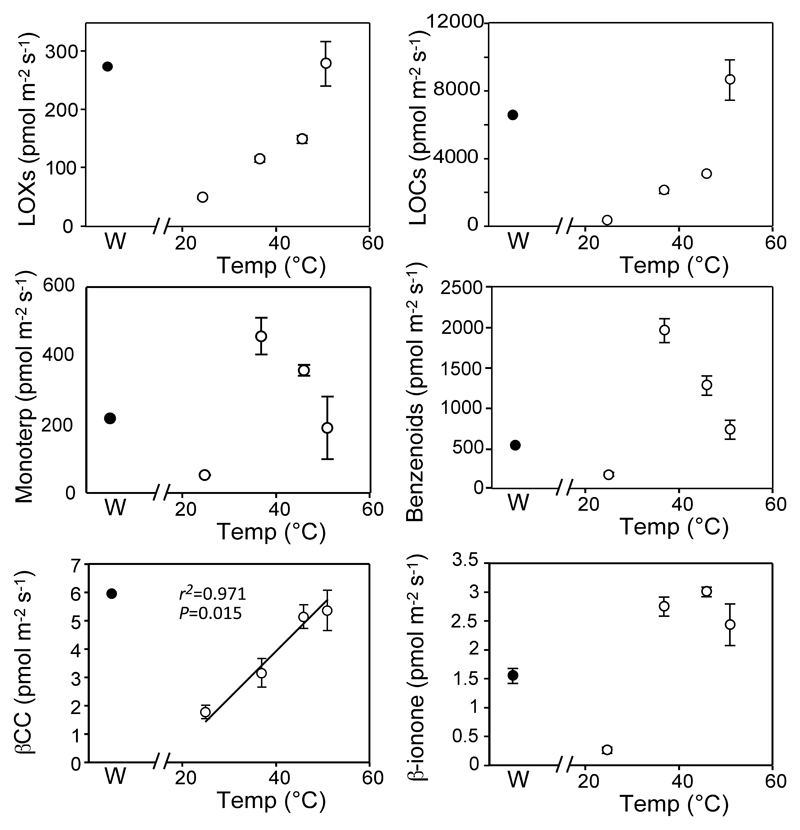
Heat shock resulted in a dramatic enhancement of the total volatiles emission (8 to 17-fold higher), which was particularly noticeable for acetaldehyde and methanol. The enhancement of volatile emission was temperature-dependent for acetaldehyde, methanol and some benzenoids such as the ethyl salicylate, but not for the other compounds analyzed (Table 1). Generally, the total emission of LOXs and LOCs increased linearly along with the stress temperature, while the emission of monoterpenoids increased abruptly after heat stress (Table 1, Figure 3). SPME analyses revealed that the increase of monoterpenoids was caused in part by the increased emission of α-pinene, limonene and especially of β-pinene (Figure 1 and Supplementary Table 1), which was present in trace amounts in the emissions of non-stressed thalli (Figure 1 and Supplementary Table 1). In addition, heating of thalli at 33 °C also increased the emission of β–CC (Figure 2) and induced the release of β-ionone (Figure 2), which began to co-elute together with γ-muurolene (Supplementary Figure 1, Supplementary Table 1). Increased emission of VCCPs after heat shock was also found in PTR-MS-TOF analyses (Figure 3). Yet, quantitatively, the enhancement of VCCPs was the lowest among all groups of volatiles analyzed (a maximum of 3-fold compared to 6-fold for LOXs, 7-fold for terpenoids, 7-fold for benzenoids or 29-fold for LOCs). Furthermore, it was basically independent of the severity of stress. As a consequence of the lower sensitivity of VCCPs emission rate to stress, its contribution to total volatiles released by the lichen, decreased to only 0.17 % when the thalli were exposed to the most acute heat stress.
Figure 3.
Temperature (empty circles) and wounding (solid circles) effects on the emission of the main groups of volatiles and volatile carotenoid derivatives identified by GC-MS (bottom row). Linear relationships between temperature and emissions were tested and calculated P values and regression lines are indicated on the figures whenever significant at P < 0.05. The values represent the averages of three independent replicates ± SE (error bars are shown when larger than symbols).
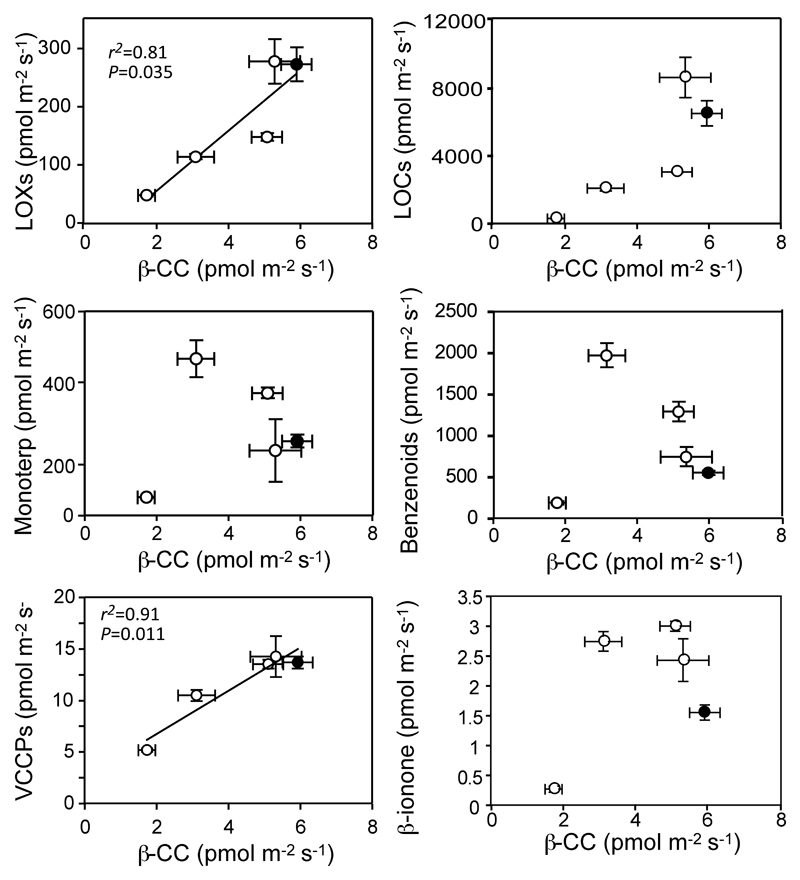
When each individual VCCP was considered separately, their emission also increased with stress, but this enhancement was basically independent of stress intensity for each compound detected. In the case of the most abundant, β-CC, its release correlated to a great extent with the total emissions of monoterpenes and VCCPs (Figure 4), but it did not relate to the release of other groups of volatiles.
Figure 4.
Relationship between the emission of β-CC and that of the main groups of volatiles. Linear relationships between temperature and emissions were tested and calculated P values and regression lines are indicated on the figures whenever significant at P < 0.05. Data presentation and statistics as in Figure 3. The values represent the averages of three independent replicates ± SE (error bars are shown when larger than symbols).
Volatile production after wounding, a well-characterized factor that induces massive volatile release, was also analyzed. The production of groups of volatiles increased after stress, acetaldehyde and methanol being the main compounds emitted under these conditions (increased by 31 and 135-fold, respectively) (Table 1). Increased emission of VCCPs after wounding, including β-CC, was also found in PTR-MS-TOF analyses (Figure 3).
3.4. Pigment changes
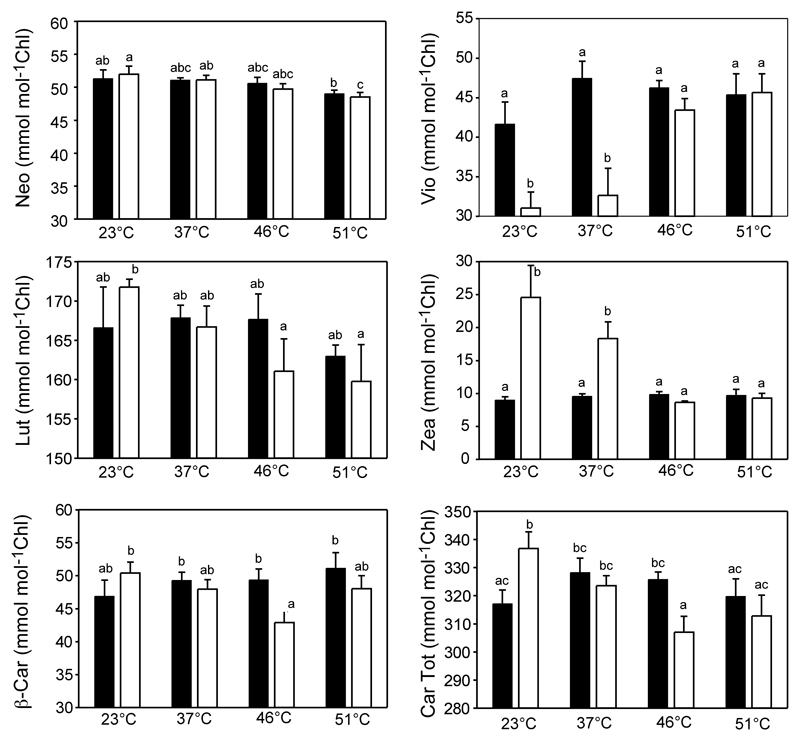
Heat shock caused carotenoid degradation, decreasing their content to a higher extent than that of chlorophylls. Besides, response patterns differed among carotenoids, with xanthophylls being more sensitive to heat shock than carotenes (Figure 5).
Figure 5.
Carotenoid contents (expressed on a chlorophyll basis) of thalli exposed to different temperatures before (solid bars) and after (empty bars) illumination. Average (± SE) pre-stress chlorophyll and carotenoid contents were 180.1 ± 6.0 µmol m-2 and 58.3 ± 1.6 µmol m-2, respectively. The bars indicate SE (n = 5). Letters above the bars indicate significant differences in the carotenoids content between temperature and light treatments (P<0.05).
At 23 °C and 37 °C, the xanthophyll cycle was active and Vio was enzymatically transformed into Zea upon illumination (Figure 5). However, at higher temperatures the reaction stopped. The other xanthophylls (Lut and Neo) also decreased progressively with temperature. In contrast, β-Car was more stable in non-illuminated thalli, decreasing with light exposition, particularly at 46 °C. At this temperature, 13 % of the β-Car pool was lost during 15 min of illumination, while at 51 °C, β-Car loss accounted for 6 % of the β-Car pool existing prior to illumination.
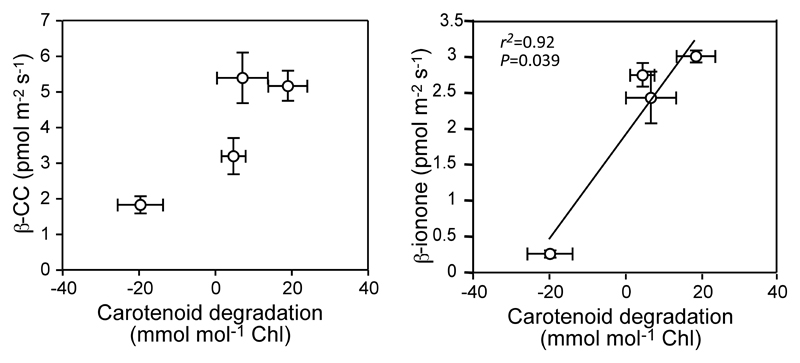
When VCCPs emission rates were compared with the total changes in carotenoid pool induced by the illumination period (Figure 6), the rate of carotenoid degradation and VCCPs release correlated positively for β–CC and the other confirmed VCCP (β-ionone). Besides, positive relationships were obtained between changes in the pools of individual carotenoid and release of VCCPs, in particular, between β–CC and Lut (r2=0.84) or β-Car (r2=0.83) (Suppl Table 2). No significant correlations were observed between any other VCCPs and carotenoid changes. Despite the correlation between carotenoid degradation and emission of VCCPs from stressed thalli, it should be noted here that when expressed on an area basis, the rates of carotenoid degradation were on average three orders of magnitude higher than those of emission of VCCPs.
Figure 6.
Relationship between total carotenoid degradation and the emission of main volatile carotene derivatives (VCCPs) confirmed by GC-MS. Negative values of carotenoid degradation indicate higher ratios of carotenoids per chlorophyll. Data presentation and statistics as in Figure 3.
4. Discussion
Biogenic emissions of VCCPs have rarely been considered a part of the blend of volatiles. However, it has been known for more than a decade that these volatile compounds can be formed enzymatically in fruits and flowers through the action of specific carotenoid cleavage dioxygenases (CCDs) (Simkin et al. 2004b), by exposure to UV light (Lamikanra et al. 2002) or by fungal pathogens (Zorn et al. 2003). More recently, it has been demonstrated that in photosynthetic tissues, generation of VCCPs can occur after oxidative damage to carotenoids (Havaux 2013) and it has been hypothesized that thanks to the capacity of volatiles to overcome vascular restrictions, VCCPs may be involved in long-distance communication (Carmody et al. 2016). The present study shows that several VCCPs, particularly β-CC, were constitutively released from lichen thalli, even under non-stressed conditions, together with other volatiles (Table 1). However, the actual emission of VCCPs only represents a minor fraction (1 %) of the total volatiles blend emitted by the lichen (Table 1).
Constitutive emission of VCCPs may be related to the continuous enzymatic degradation of carotenoids, which has been estimated to represent 15 % of the total carotenoid pool (in 24 hours) in Lemna plants treated with norflurazon (an inhibitor of phytoene desaturase that prevents carotenoid formation) (García-Plazaola et al. 2002). In another study with 14C labeling it has been shown that there is a rapid turnover of β-Car even under non-stressed conditions (Beisel et al. 2010), suggesting a continuous degradation of β-Car by chemical quenching or by the action CCDs that cleave the polyene chromophore of β-Car (Bouvier et al. 2005). Considering the estimated turnover rate (15 % in 24 h) and the fact that the carotenoid content of thalli is roughly 60 µmol m-2 (Figure 5), and assuming that all products of carotenoid degradation may potentially be transformed into volatile metabolites (1 or 2 VCCPs per carotenoid molecule) and released into the atmosphere, a maximum potential for generation of VCCPs can be estimated in the range of 100-200 pmol m-2 s-1. However, actual emission rates of VCCPs under steady-state conditions were approximately 20-fold lower than this maximum potential, a value that is, furthermore, far below the level of other volatiles (Table 1). It implies that degradation either occurs through pathways that produce non-volatile metabolites, therefore, not involving generation of VCCPs; or that the VCCPs produced are bound to volatiles of fungal cell walls or extracellular matrix (including lichenic compounds) that act as sinks for these compounds, decreasing their release into the atmosphere.
Heat shock and wounding usually involve extensive damage to cellular structures and the release of volatiles from the lipoxygenase pathway (LOX products) (Copolovici et al. 2012, Portillo-Estrada et al. 2015) as well as LOCs such as methanol and acetaldehyde (Loreto et al. 2006). In L. pulmonaria, both stress types resulted in enhanced emission rates of all volatiles, including VCCPs (Table 1). However, the increase in VCCP emissions was the lowest among all the groups of volatiles (2 to 3-fold enhancement compared to 3 to 29-fold in the other groups) and it was basically independent of stress severity and type (Figure 3).
The comparatively low rate of VCCP emissions is surprising, as temperatures above 40 °C trigger a progressive deactivation of PSII reaction centers (Marutani et al. 2012, Yamamoto et al. 2008), leading to a massive degradation of photosynthetic components. Furthermore, recent studies also show that heat stress itself triggers singlet oxygen production though a light-independent mechanism (Prasad et al. 2016, Mor et al. 2014). Thus, heat-induced photosynthetic damage should imply the oxidation of β-Car in the reaction centers, but also the release of antennae xanthophylls. Furthermore, oxidative damage should be exacerbated by the denaturation of the enzymes involved in the photoprotective xanthophyll cycle at temperatures higher than 36 °C, which is shown by the absence of Zea formation after 46 °C and 51 °C treatments (Figure 6). The absence of a massive release of VCCPs indicates that, xanthophylls and β-Car are either protected by other antioxidants such as tocopherols (Trebst 2003), or their oxidation does not generate VCCPs, or, once VCCPs are generated, they are not able to cross the different barriers between the thylakoid and the open atmosphere.
In the particular case of heat shock, enzymatic deactivation may also contribute to decreasing VCCP emissions by blocking the activity of carotenoid cleavage enzymes (Nisar et al. 2015), preventing the metabolic generation of VCCPs. This may justify why, in the experimental treatments reported here, the highest rates of VCCPs release were not observed at the highest temperature of 51 °C, but at 46 °C.
Despite their low rate of constitutive production of VCCPs, their generation cannot be neglected, and their well-proven role as infochemicals and allelopathics, should be considered. Thus, hydroxyl-β-ionone produced by the moss Rhynchostegium pallidifolium exerts allelopathic effects on the growth of vascular plants (Kato-Noguchi and Seki 2010) and β-ionone has a well-contrasted antimicrobial activity (Anzaldi et al. 1999) and it also shows lytic activity against cyanobacteria (Harada et al. 2009). In fact, the induction of VCCPs production has been proposed as an alternative to increasing disease resistance in crops (Lamikanra et al. 2002). On the other hand, ionone and β-CC are capable of inhibiting the growth of Chlorella (Ikawa et al. 2001) and the presence of β-CC around cyanobacteria colonies (Microcystis) has been identified as a repellent of the grazing behavior of Daphnia (Jüttner et al. 2010). As β-CC was generated basically by the damage to Microcystis mats, these authors hypothesized that β-CC may be an indicator of a low-quality food source. In terrestrial environments, the role of β-CC and α-ionone as repellents has also been identified for arthropods such as ticks (Lwande et al. 2009). Furthermore, the external application of VCCPs may be used to improve nutritional quality and stress resistance in crops. This is because carotenoid composition is under feedback control by carotenoid-derived compounds (Fanciullino et al. 2014). In fact, VCCPs may be the signal generated by photooxidative stress that is transmitted from leaves to nearby fruits (Poiroux-Gonord et al. 2013).
Overall, the present work shows that VCCPs are produced constitutively and released from the thalli of L. pulmonaria. However, although VCCP emissions from photosynthetic tissues might, potentially, serve similar functions as other well-recognized volatile infochemicals (LOX products, methyl salicylate, methyl jasmonate) that act as cues in biocommunication and plant signaling, low emission rates of VCCPs, and the absence of quantitative relationship with stress intensity, suggests that it is unlikely that these volatiles play such a role in the studied lichen species.
Supplementary Material
Acknowledgements
We acknowledge financial support by the EU through Marie Curie Action (FP7-PEOPLE-2012-IEF 328370 “MELISSA”), the European Research Council (advanced grant 322603, SIP-VOL+), the European Regional Development Fund (Centre of Excellence EcolChange, TK 131), the Basque Government (Research grant UPV/EHU-GV IT-624-13), the Spanish Ministry of Economy and Competitiveness (grant JdC-Incorporación IJCI-2014-22489 to BFM and research grant BFU 2010-15021 co-funded by Feder) and the Estonian Ministry of Science and Education (institutional grant IUT-8-3).
Abbreviations
- β-Car
β-carotene
- β-CC
β-cyclocitral
- Chl
chlorophyll
- dhA
dihydroactinidiolide
- GC-MS
gas-chromatograph mass-spectrometer
- LOCs
lightweight oxygenated compounds
- LOXs
lipoxygenase chain oxidation products
- Lut
lutein
- Neo
neoxanthin
- PTR-TOF-MS
proton-transfer reaction time-of-flight mass-spectrometer
- RH
relative humidity
- ROS
reactive oxygen species
- SPME
solid-phase microextraction
- VCCPs
volatile carotenoid cleavage products
- Vio
violaxanthin
- Zea
zeaxanthin
References
- Adams RP. Identification of essential oil components by gas chromatography / quadrupole mass spectroscopy. Allured Publishing; Carol Stream, IL: 2004. [Google Scholar]
- Anzaldi M, Sottofattori R, Granello di Casaleto B, Balbi A. Synthesis and antimicrobial activity of heterocyclic ionone-like derivatives. Eur J Med Chem. 1999;34:837–842. [Google Scholar]
- Beisel KG, Jahnke S, Hofmann D, Köppchen S, Schurr U, Matsubara S. Continuous turnover of carotenes and chlorophyll a in mature leaves of Arabidopsis by 14CO2 pulse-chase labeling. Plant Physiol. 2010;152:2188–2199. doi: 10.1104/pp.109.151647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier F, Isner JC, Dogbo O, Camara B. Oxidative tailoring of carotenoids: a prospect towards novel functions in plants. Trends Plant Sci. 2005;10:187–194. doi: 10.1016/j.tplants.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Carmody M, Crisp PA, d’Alessandro S, Ganguly D, Gordon M, Havaux M, Albrecht-Borth V, Pogson BJ. Uncoupling high light responses from singlet oxygen retrograde signaling and spatial-temporal systemic acquired acclimation. Plant Physiol. 2016;171:1734–1749. doi: 10.1104/pp.16.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copolovici L, Kännaste A, Pazouki L, Niinemets Ü. Emissions of green leaf volatiles and terpenoids from Solanum lycopersicum are quantitatively related to the severity of cold and heat shock treatments. J Plant Physiol. 2012;169:664–672. doi: 10.1016/j.jplph.2011.12.019. [DOI] [PubMed] [Google Scholar]
- Cornejo C, Scheidegger C. New morphological aspects of cephalodium formation in the lichen Lobaria pulmonaria (Lecanorales, Ascomycota) The Lichenologist. 2013;45:77–87. [Google Scholar]
- Estavillo GM, Chan KX, Phua SY, Pogson BJ. Reconsidering the nature and mode of action of metabolie retrograde signals from the chloroplast. Frontiers Plant Sci. 2012;3:300. doi: 10.3389/fpls.2012.00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanciullino AL, Bridel LPR, Urban L. Carotenoid responses to environmental stimuli: integrating redox and carbon controls into a fruit model. Plant Cell Environ. 2014;37:273–289. doi: 10.1111/pce.12153. [DOI] [PubMed] [Google Scholar]
- García-Plazaola JI, Hernández A, Artetxe U, Becerril JM. Regulation of the xanthophyll cycle pool size in duckweed (Lemna minor) plants. Physiol Plant. 2002;116:121–126. doi: 10.1034/j.1399-3054.2002.1160115.x. [DOI] [PubMed] [Google Scholar]
- García-Plazaola JI, Becerril JM. A rapid high-performance liquid chromatography method to measure lipophilic antioxidants in stressed plants: Simultaneous determination of carotenoids and tocopherols. Phytochem Anal. 1999;10:307–313. [Google Scholar]
- Garcia-Plazaola JI, Esteban R. Determination of chlorophylls and carotenoids by HPLC. [last visited May 03, 2016];PrometheusWiki. 2012 http://www.publish.csiro.au/prometheuswiki/tiki-pagehistory.php?page=Determination of chlorophylls and carotenoids by HPLC&preview=10.
- Gauslaa Y, Coxon DS, Solhaug KA. The paradox of higher light tolerance during desiccation in rare old forest cyanolichens than in more widespread co-occurring chloro- and cephalolichens. New Phytol. 2012;195:812–822. doi: 10.1111/j.1469-8137.2012.04221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez E, Ledbetter CA, Hartsell PL. Volatile compounds in apricot, plum, and their interspecific hybrids. J Agric Food Chem. 1993;41:1669–1676. [Google Scholar]
- Guédot C, Landolt PJ, Smithhisler CL. Odorants of the flowers of butterfly bush, Budleja davidii as possible attractants of pest species of moths. Florida Entomol. 2008;91:576–582. [Google Scholar]
- Harada KI, Ozaki K, Tsuzuki S, Kato H, Hasegawa M, Kuroda EK, Arii S, Tsuji K. Blue color formation of cyanobacteria with β-cyclocitral. J Chem Ecol. 2009;35:1295–1301. doi: 10.1007/s10886-009-9706-5. [DOI] [PubMed] [Google Scholar]
- Havaux M. Carotenoid oxidation products as stress signals in plants. Plant J. 2013;79:597–606. doi: 10.1111/tpj.12386. [DOI] [PubMed] [Google Scholar]
- Holopainen JK. Multiple functions of inducible plant volatiles. Trends Plant Sci. 2004;9:529–533. doi: 10.1016/j.tplants.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Ikawa M, Sasner JJ, Haney JF. Activity of cyanobacterial and algal odor compounds found in lake waters on green alga Chlorella pyrenoidosa growth. Hydrobiology. 2001;443:19–22. [Google Scholar]
- Jüttner F, Watson SB, von Elert E, Köster O. β-Cyclocitral, a grazer defence signal unique to the cyanobacterium Microcystis. J Chem Ecol. 2010;36:1387–1397. doi: 10.1007/s10886-010-9877-0. [DOI] [PubMed] [Google Scholar]
- Kato-Noguchi H, Seki T. Allelopathy of the moss Rhunchostegium pallidifolium and 3-hydroxy-β-ionone. Plant Signal Behav. 2010;5:702–704. doi: 10.4161/psb.5.6.11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger-Liszkay A. Singlet ocygen production in photosynthesis. J Exp Bot. 2005;56:337–346. doi: 10.1093/jxb/erh237. [DOI] [PubMed] [Google Scholar]
- Lamikanra O, Richard OA, Parker A. Ultraviolet induced stress response in fresh cut cantaloupe. Phytochem. 2002;60:27–32. doi: 10.1016/s0031-9422(02)00048-1. [DOI] [PubMed] [Google Scholar]
- Larsen TO, Frisvad JC. Characterization of volatile metabolites from 47 Penicillium taxa. Mycol Res. 1995;99(10):1153–1166. [Google Scholar]
- Loreto F, Barta C, Brilli F, Nogues I. On the induction of volatile organic compound emissions by plants as consequence of wounding or fluctuations of light and temperature. Plant Cell Environ. 2006;29:1820–1828. doi: 10.1111/j.1365-3040.2006.01561.x. [DOI] [PubMed] [Google Scholar]
- Lwande W, Ndakala AJ, Hassanali A, et al. Gynandropsis gynandra essential oil and its constituents as tick (Rhipicephalus appendiculatus) repellents. Phytochem. 1999;50:401–405. [Google Scholar]
- Marutani Y, Yamauchi Y, Kimura Y, Mizutani M, Sugimoto Y. Damage to photosystem II due to heat stress without light-driven electron flow: involvement of enhanced introduction of reducing power into thylakoid membrane. Planta. 2012;236:753–761. doi: 10.1007/s00425-012-1647-5. [DOI] [PubMed] [Google Scholar]
- Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Mor A, Koh E, Weiner L, Rosenwasser S, Sibony-Benyamini H, Fluhr R. Singlet oxygen signatures are detected independent of light or chloroplasts in response to multiple stresses. Plant Physiol. 2014;165:249–261. doi: 10.1104/pp.114.236380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munné-Bosch S, Queval G, Foyer CH. The impact of global change factors on redox signalling underpinning stress tolerance. Plant Physiology. 2013;161:5–19. doi: 10.1104/pp.112.205690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezhadali A, Nezhadali Bagham A. Study of the volatile compounds in Malabaila isfahanica from Iran using HS/SPME/GC/MS. Chem Sinica. 2011;2:38–42. [Google Scholar]
- Niinemets, Ü, Loreto F, Reichstein M. Physiological and physicochemical controls on foliar volatile organic compound emissions. Trends Plant Sci. 2004;9:180–186. doi: 10.1016/j.tplants.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü, Kuhn U, Harley PC, Staudt M, Arneth A, Cescatti A, Ciccioli P, Copolovici L, Geron C, Guenther AB, Kesselmeier J, et al. Estimations of isoprenoid emission capacity from enclosure studies: measurements, data processing, quality and standardized measurement protocols. Biogeosciences. 2011;8:2209–2246. [Google Scholar]
- Niinemets Ü, Fares S, Harley S, Jardine KJ. Bidirectional exchange of biogenic volatiles with vegetation: emission sources, reactions, breakdown and deposition. Plant Cell Environ. 2014;37:1790–1809. doi: 10.1111/pce.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisar N, Li L, Lu S, Khin NC, Pogson BJ. Carotenoid metabolism in plants. Molec Plant. 2015;8:68–82. doi: 10.1016/j.molp.2014.12.007. [DOI] [PubMed] [Google Scholar]
- Poiroux-Gonord F, Santini J, Fanciullino AL, Lopez-Lauri F, Giannettini J, Sallanon H, Berti L, Urban L. Metabolism in orange fruits is driven by photooxidative stress in the leaves. Physiol Plant. 2013;149:175–187. doi: 10.1111/ppl.12023. [DOI] [PubMed] [Google Scholar]
- Portillo-Estrada M, Kazantsev T, Talts E, Tosens T, Niinemets Ü. Emission timetable and quantitative patterns of wound-induced volatiles across different leaf damage treatments in aspen (Populus tremula) J Chem Ecol. 2015;41:1105–1117. doi: 10.1007/s10886-015-0646-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad A, Ferretti U, Sedlárová M, Pospisil P. Singlet oxygen production in Chlamydomonas reinhardtii under heat stress. Sci Rep. 2016;6:20094. doi: 10.1038/srep20094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priestap HA, van Baren CM, di Leo P, Coussio JD, Bandoni AL. Volatile constituents of Aristolochia argentina. Phytochem. 2003;63:221–225. doi: 10.1016/s0031-9422(02)00751-3. [DOI] [PubMed] [Google Scholar]
- Ramel F, Birtic S, Cuiné S, Triantaphylidès C, Ravanat JL, Havauz M. Chemical quenching of singlet oxygen by carotenoids in plants. Plant Physiol. 2012a;158:1267–1278. doi: 10.1104/pp.111.182394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramel F, Birtic S, Ginies C, Soubigou-Taconnat L, Triantaphylidès C, Havaux M. Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc Nat Acad Sci USA. 2012b;109:5535–5540. doi: 10.1073/pnas.1115982109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramel F, Mialoundama AS, Havaux M. Nonenzymic carotenoid oxidation and photooxidative stress signalling in plants. J Exp Bot. 2013;64:799–805. doi: 10.1093/jxb/ers223. [DOI] [PubMed] [Google Scholar]
- Rana VS, Blazquez M. Constituents of the essential oil of Meriandra bengalensis Benth. leaves from India. J Essent Oil Res. 2009;21(1):22–23. [Google Scholar]
- Rostad CE, Pereira WE. Kovats and Lee retention indices by gas chromatography/mass spectrometry for organic compounds of environmental interest. J High Resolut Chromatogr Chromatogr Commun. 1986;9(6):328–334. [Google Scholar]
- Senar JC, Moller AP, Negro JJ, Broggi J, Hohtola E. Specific appetite for carotenoids in a colourful bird. Plos One. 2010;5:e10716. doi: 10.1371/journal.pone.0010716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibamoto T, Horiuchi M, Umano K. Composition of the young green barley and wheat leaves. J Essent Oil Res. 2007;19:134–137. [Google Scholar]
- Shirazi AM, Muir PS, McCune B. Environmental factors influencing the distribution of the lichens Lobaria oregana and L. pulmonaria. The Bryologist. 1996;99:12–18. [Google Scholar]
- Schofield SC, Campbell DA, Funk C, MacKenzie TDB. Changes in macromolecular allocation in nondividing algal symbionts allow for photosynthetic acclimation in the lichen Lobaria pulmonaria. New Phytol. 2003;159:709–718. doi: 10.1046/j.1469-8137.2003.00857.x. [DOI] [PubMed] [Google Scholar]
- Shumbe L, Boot R, Havaux M. Dihydroactinidiolide, a high light-induced β-carotene derivative that can regulate gene expression and photoacclimation in Arabidopsis. Mol Plant. 2014;7:1248–1251. doi: 10.1093/mp/ssu028. [DOI] [PubMed] [Google Scholar]
- Simkin AJ, Underwood BA, Auldridge M. Circadian regulation of the PhCCD1 carotenoid cleavage dioxygenase controls emission of β-Ionone, a fragrance volatile of petunia flowers. Plant Physiol. 2004a;136:3504–3514. doi: 10.1104/pp.104.049718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkin AJ, Schwarz STH, Auldidge M, Taylor MG, Klee HJ. The tomato carotenoid cleavage dioxygenase 1 genes contribute to the formation of the flavour volatiles β-ionone, pseudoionone, and geranylacetone. Plant J. 2004b;40:882–892. doi: 10.1111/j.1365-313X.2004.02263.x. [DOI] [PubMed] [Google Scholar]
- Stewart-Jones A, Poppy GM. Comparison of glass vessels and plastic bags for enclosing living plant parts for headspace analysis. J Chem Ecol. 2006;32:845–864. doi: 10.1007/s10886-006-9039-6. [DOI] [PubMed] [Google Scholar]
- Telfer A. What is β-carotene doing in the photosystem II reaction centre? Phil Trans R Soc Lond B. 2002;357:1431–1439. doi: 10.1098/rstb.2002.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trebst A. Function of β-Carotene and tocopherol in Photosystem II. Z Naturforsch. 2003;58:609–620. doi: 10.1515/znc-2003-9-1001. [DOI] [PubMed] [Google Scholar]
- Triantaphylidès C, Havaux M. Singlet oxygen in plants: production, detoxification and signaling. Trends Plant Sci. 2009;14:219–228. doi: 10.1016/j.tplants.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Aminaka R, Yoshioka M, Khatoon M, Komayama K, Takenaka D, Yamashita A, Nijo N, Inagawa K, Morita N, Sasaki T, et al. Quality control of photosystem II: impact of light and heat stress. Photosynth Res. 2008;98:589–608. doi: 10.1007/s11120-008-9372-4. [DOI] [PubMed] [Google Scholar]
- Zorn H, Langhoff S, Scheibner M, Berger RG. Cleavage of β,β-carotene to flavor compounds by fungi. Appl Microbiol Biotechnol. 2003;62:331–336. doi: 10.1007/s00253-003-1309-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.