Abstract
A recent outbreak of Zika virus in Brazil has led to a simultaneous increase in reports of neonatal microcephaly. Zika targets cerebral neural precursors, a cell population essential for cortical development, but the cause of this neurotropism remains obscure. Here we report that the neural RNA-binding protein Musashi-1 (MSI1) interacts with the Zika genome and enables viral replication. Zika infection disrupts the binding of MSI1 to its endogenous targets, thereby deregulating expression of factors implicated in neural stem cell function. We further show that MSI1 is highly expressed in neural progenitors of the human embryonic brain, and is mutated in individuals with autosomal recessive primary microcephaly. Selective MSI1 expression in neural precursors could therefore explain the exceptional vulnerability of these cells to Zika infection.
Zika virus (ZIKV) recently emerged as a major public health risk because of its devastating effect on fetal neurodevelopment (1–3). ZIKV was first isolated in Uganda in 1947, and the virus subsequently spread through Asia, and from there to the Americas (4). A causal link between ZIKV infection and congenital brain malformations became apparent in 2016 following an outbreak in Brazil (1). Brazilian ZIKV is closely related to the Asian-lineage strain, which affected New Caledonia and French Polynesia, where cases of microcephaly were reported retrospectively (5).
Intrauterine infections can impair neurodevelopment (6), but ZIKV is highly neurotropic and interferes specifically with fetal brain development causing microcephaly, cortical malformations and intracranial calcifications (7–10). We hypothesized that the single-stranded RNA flavivirus ZIKV may hijack RNA-binding factors present in the developing central nervous system (11). Host RNA-binding proteins are known to interact with untranslated regions (UTRs) to regulate replication, translation and stabilization of viral genomes (11). In silico analysis of the genomic RNA of the Brazilian ZIKV strain, PE243, revealed three consensus binding sites in the 3’UTR for the highly conserved Musashi family of RNA binding proteins, Musashi-1 (MSI1) and Musashi-2 (MSI2), both important translational regulators in stem cells (12–15). Two sites were conserved between PE243 and the Ugandan MR766 strains (Sites 1, 2), whereas the third (Site 3) was found only in the Asian-lineage strains including PE243 (Fig. 1A; Fig. S1A, B). By mapping these sites onto a predicted secondary structure of ZIKV 3’UTR, we found all the three to be present on stem-loop structures, which are considered optimal for MSI binding (16, 17). Moreover, a recent study revealed nucleotide substitutions proximal to Sites 1 and 2 in the Asian-lineage strains, which could indicate positive selection for MSI1 binding during ZIKV evolution (18).
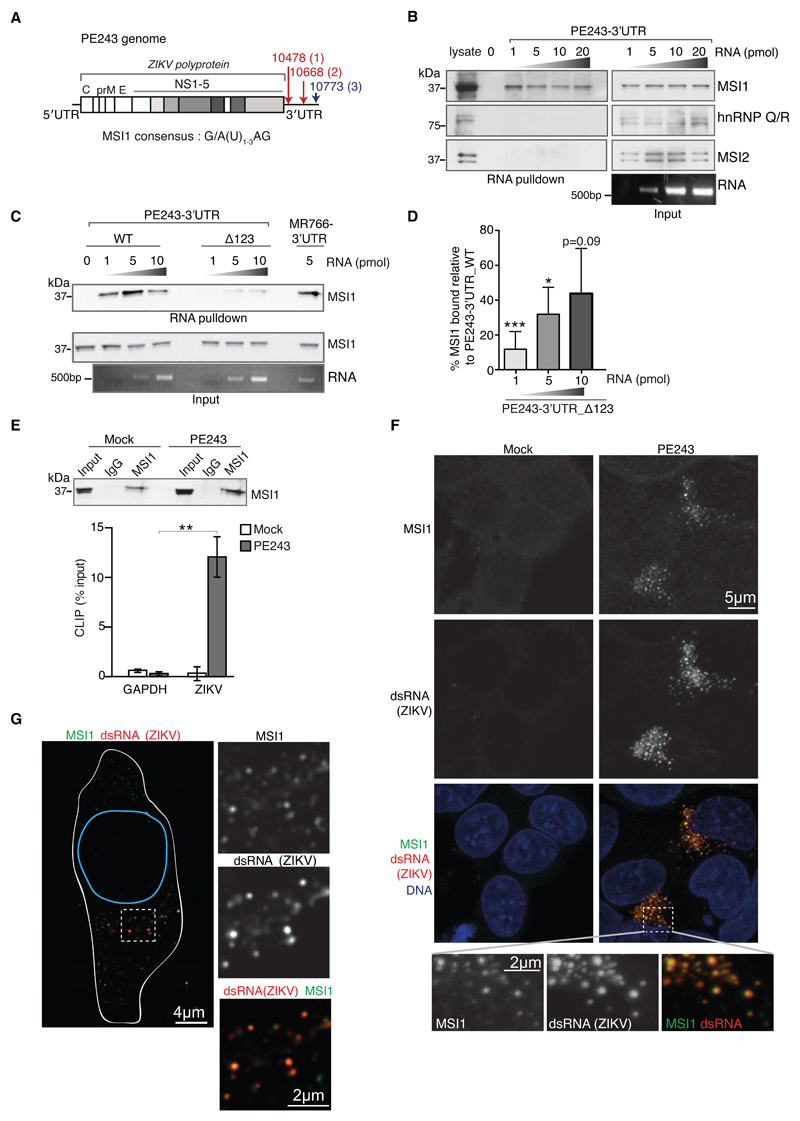
Fig. 1. MSI1 interacts directly with the ZIKV RNA genome.
A) Schematic diagram of PE243 ZIKV containing three putative MSI1 binding sites in its 3’UTR. Sites shared with MR766 are red, site unique to PE243 is blue.
B) RNA pull-down assays performed with the 3’UTR of PE243. Increasing concentrations of in vitro transcribed biotinylated PE243 RNA were incubated with U-251 cell extracts and RNA-protein complexes were captured on streptavidin beads. Representative western blots were probed with antibodies against MSI1 and the RNA binding proteins, Musashi-2 (MSI2) and hnRNP Q/R. Corresponding protein and RNA inputs are shown on right.
C) RNA pull-down assays performed with the wild type (WT) or triple mutant (Δ123) 3’UTR of PE243. Note that PE243-3’UTR_Δ123 lacks all three MSI1 binding sites depicted in Fig. 1A (see Fig. S1C for further details). Increasing concentrations of in vitro transcribed biotinylated RNA were incubated with U-251 cell extracts and RNA-protein complexes were captured on streptavidin beads. Representative western blots probed with antibody against MSI1 are shown together with corresponding protein and RNA inputs.
D) Densitometric analysis of MSI1 levels from western blots of RNA pull-down assays, an example for which is shown in Fig. 1C. The amount of MSI1 precipitated by PE243-3’UTR_Δ123 is expressed as a percentage of MSI1 precipitated by the same concentration of PE243-3’UTR_WT. n=3 biological replicates. P-values were obtained from Student’s t-test, unpaired, two-tailed: * p<0.05, *** p<0.0005. Bar charts depict mean±s.e.m.
E) CLIP analysis from mock- or ZIKV PE243-infected U-251 cells. Western blot shows immunoprecipitatations (IP) by rabbit IgG and MSI1 antibodies from mock and PE243-infected U-251 cells following UV crosslinking. Input (5%) represents whole cell extract. Western blot was probed with MSI1 antibody. Graph below shows qPCR performed on bound RNA from IP. CLIP values are presented as a percentage of input following subtraction of the IgG background. GAPDH serves as negative control. n=3 biological replicates. Note that a primer pair against 9519-9681 bp of ZIKV genome was used in these qPCRs (Table S4).
F) Immunofluorescence of mock- or PE243-infected U-251 cells. MSI1 (green) and dsRNA (red) signals are detected by confocal microscopy. DNA is detected by Hoechst stain (blue). Framed area is shown at higher magnification below.
G) Immunofluorescence of a PE243-infected U-251 cell. MSI1 (green) and dsRNA (red) signals are detected by STED super resolution microscopy. Outlines of the cell and nucleus are indicated in white and blue, respectively. Framed area is shown at higher magnification.
To address if the Musashi proteins interacted with ZIKV, we first tested their binding to ZIKV 3’UTR. RNA pull-downs identified binding of MSI1, but not MSI2, to the 3’UTR of PE243 (Fig. 1B) (15). Mutating the three consensus MSI1 sites in the 3’UTR of PE243 significantly weakened this interaction (Fig. 1C, D; Fig. S1C). We also confirmed binding between MSI1 and the 3’UTR of MR766 (Fig. 1C). To investigate whether MSI1 also binds ZIKV 3’UTR in vivo, ultraviolet (UV) crosslinking and RNA immunoprecipitation (CLIP) was performed from PE243-infected U-251 glioblastoma cells, revealing a robust direct interaction between MSI1 and PE243 ZIKV RNA (Fig. 1E). Consistently, in ZIKV-infected cells, MSI1 co-localised with double-stranded RNA, a viral replication intermediate, as visualised by confocal and STED super-resolution microscopy (Fig. 1F, G). These data confirm an interaction between MSI1 and ZIKV RNA, which is at least in part mediated by the 3’UTR of the virus.
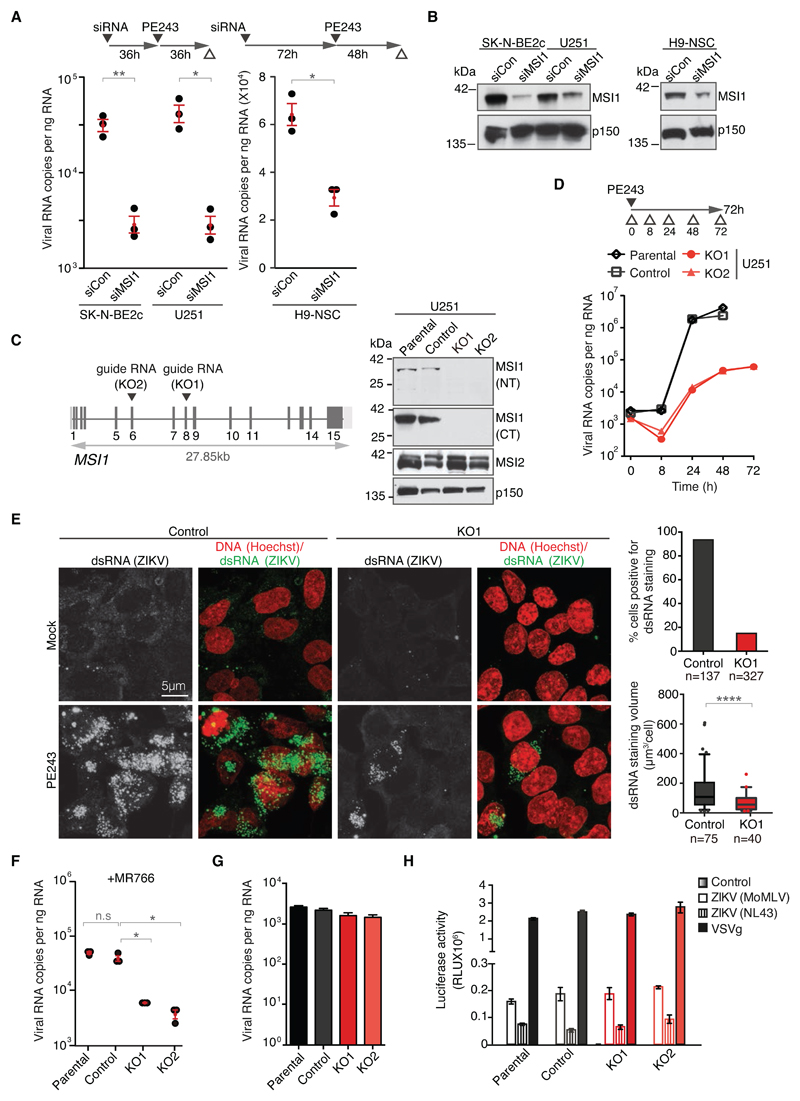
To investigate whether MSI1 had an impact on the life cycle of ZIKV, we used RNA interference to deplete the protein in U-251 glioblastoma, SK-N-BE2c neuroblastoma and H9 derived neural stem cells (NSC) and performed PE243 viral infections. In all three cell types MSI1 depletion led to a marked reduction in viral RNA levels (Fig. 2A, B). We then generated MSI1 knockouts (KO) in U-251 cells by CRISPR/Cas9-mediated targeting of exons 8 or 6 of MSI1 (KO1 and KO2, respectively; Fig. 2C; Fig. S2). Control cells were obtained through clonal expansion of cells transfected with Cas9 alone. By measuring viral RNA at different times following PE243 infection, a marked reduction of viral load was seen in KO1 and KO2 cells at 24 and 48 hours (Fig. 2D). Whereas extensive cell death precluded RNA analysis in the controls at 72 hours, viral RNA was comparable between the 48- and 72-hour timepoints in the KO cells. Consistently, levels of the viral dsRNA and flavivirus E protein as well as the infectious titre were reduced in the KOs (Fig. 2E; Fig S3). Because MSI2 levels were similar between control and KO cells (Fig. 2C), MSI1 and MSI2 are unlikely to have complete functional redundancy in ZIKV replication. Replication of the MR766 strain was also impaired in the KO cells (Fig. 2F). In summary, we identify MSI1 as an important factor for ZIKV replication, both in primary and transformed neural cell lines.
Fig. 2. MSI1 is required for effective replication of ZIKV.
A) Graph on left depicts viral RNA copies in control siRNA- (siCon) and MSI1 siRNA (siMSI1)-treated SK-N-BE2c and U-251 cells following infection with PE243 (MOI: 1 FFU/cell, 72h). Graph on right shows viral RNA copies in MSI1 siRNA-treated H9-derived neural stem cells (NSC) following infection with PE243 (MOI: 1 FFU/cell, 48h). Note that in all viral replication assays ZIKV was quantified by TaqMan assay as described in Materials and Methods. n=3 biological replicates. P-values were obtained from Student’s t-test, unpaired, two-tailed: * p<0.05, ** p<0.005, not significant (n.s.).
B) Representative western blots of cell lines treated with control and MSI1 siRNAs from Fig. 2A. Blots were probed with antibodies against MSI1 or p150 as loading control.
C) Location of the guide RNAs used for CRISPR/Cas9-mediated editing of the MSI1 locus in U-251 cells. For further details see text and Fig. S2. Western blots of parental, control, KO1 and KO2 cell lines probed with antibodies against N- and C-termini of MSI1 (NT or CT) and MSI2. p150 serves as loading control.
D) Kinetics of PE243 viral RNA copies following infection in U-251 cells of different genotypes at the indicated time points. Note that cell death precluded collection of RNA from parental and control cells at 72h (MOI: 3 FFU/cell, 72h).
E) Confocal microscopy images of PE243-infected control and KO1 U-251 cells immunostained with antibodies against dsRNA (green) and Hoechst DNA stain (red) following mock or PE243 infection (MOI: 3 FFU/cell, 48h). Graph on top shows percentage of cells containing dsRNA signal, whereas box plot on bottom depicts total dsRNA staining volume per cell. Note that only cells with detectable dsRNA signal were included in the latter analysis. Boxes: 25th to 75th percentile; whiskers: 5-95% range; line: median. P-value represents Student’s t-test, unpaired, two-tailed: **** p<0.0001.
F) Viral RNA copies in U-251 cells of different genotypes following infection with MR766 (MOI: 3 FFU/cell, 48 h). n=3 biological replicates. P-values were obtained from Student’s t-test, unpaired, two-tailed: * p<0.05, ** p<0.005, not significant (n.s.).
G) Virus-binding assays performed under conditions that prevent internalisation. PE243 infection was performed in U-251 cells of different genotypes (MOI: 3 FFU/cell, 1h). n=3 biological replicates. P-values were obtained from Student’s t-test, unpaired, two-tailed: * p<0.05, ** p<0.005, not significant (n.s.). Bar charts depict mean±s.e.m.
H) Pseudotyped particle infectivity assay in U-251 cells of different genotypes. HIV (pNL4-3.luc.R-E-) or MoMLV pseudotyped virus expressing a luciferase reporter, with either PE243 ZIKV, VSVg or a negative control envelope used to determine viral entry events. n=3 biological replicates. P-values were obtained from Student’s t-test, unpaired, two-tailed: * p<0.05, ** p<0.005, not significant (n.s.). Bar charts depict mean±s.e.m.
Since there was no discernible difference between ZIKV binding and entry into control and KO cells (Fig. 2G, H), we asked if MSI1 could regulate translation through ZIKV UTRs. To this end, luciferase RNA flanked by the 5’ and 3’UTRs of PE243, was transfected with increasing amounts of MSI1 into HEK293T cells, which do not normally express MSI1 (Fig. S4). We observed a modest MSI1-driven increase in luciferase expression. The ability of MSI1 to promote ZIKV UTR-driven translation in vitro raises the possibility that it performs a similar function in vivo. Alternatively, MSI1 might stabilise the viral RNA genome and/or regulate its cyclization or synthesis. In addition, given the pleiotropic roles of MSI1 in cellular pathways, it is plausible that MSI1-dependent regulation of gene expression contributes to the ZIKV life cycle (19). However, MSI1 is unlikely to act through general pro- or anti-viral pathways as infection with H1N1 influenza virus was unaffected by MSI1 expression levels (Fig. S5). In line with published work, we also find that MSI1 KO cells exhibited defective migration, increased doubling time and cell cycle delay (Fig. S6) (17, 20, 21). Because ZIKV replication requires cyclin-dependent kinase activity, such pro-proliferative effects exerted by MSI1 might contribute to virus production (22). Nevertheless, the direct interaction between MSI1 and the ZIKV genome is consistent with the hypothesis that the protein promotes some aspect of the viral life cycle.
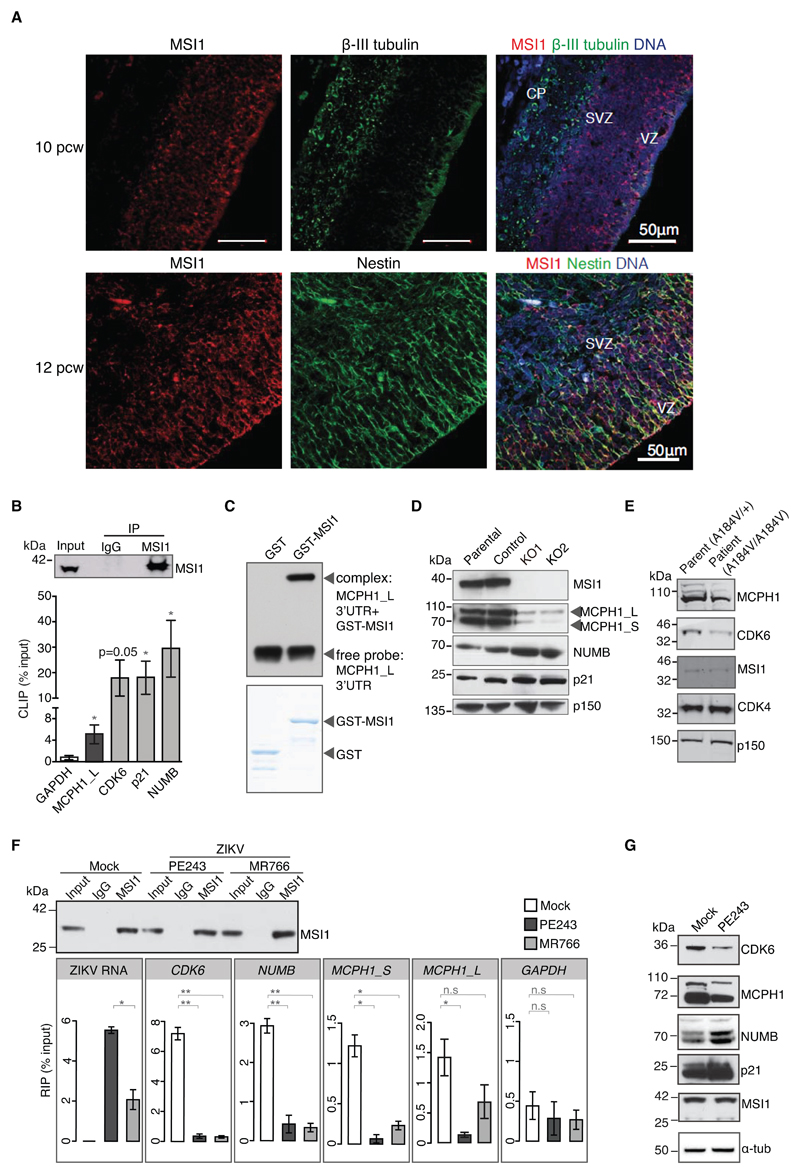
ZIKV predominantly infects neural progenitors in human fetal brain. We find MSI1 to be highly enriched in neural precursors of the ventricular and subventricular zones of the human embryonic brain, but absent from mature neurons (Fig. 3A; Fig. S7). Owing to its high levels in neural progenitors, and its ability to stimulate ZIKV replication, MSI1 could be instrumental to ZIKV-induced cytopathicity in the fetal brain. In addition, MSI1 is required for neurodevelopment in both invertebrates and vertebrates, with MSI1-depleted zebrafish displaying microcephaly, and mutant mice exhibiting a thin cerebral cortex and reduced number of mature neural cell types among other morphological brain abnormalities (14, 19, 23–25). We have identified a consanguineous Turkish family in which two siblings displayed clinical features suggestive of autosomal primary microcephaly (MCPH), a condition associated with a significant reduction in cerebral cortex size, but a structurally normal brain (Fig. S8A, B) (26, 27). Exome sequencing uncovered potentially deleterious homozygous mutations in MSI1, ACACB, DKK4 and DTX3L (Fig. S8C-F, Tables S1 and S2). Of these only MSI1 is known to have neural functions, but because mutations were present in four genes, the p. Ala184Val point mutation in MSI1 may not be the sole cause of MCPH in these individuals (referred to as MSI1A184V). Nevertheless, we have three lines of evidence showing that A184V mutant MSI1 is functionally impaired. First, MSI1A184V patient cells exhibit premature chromosome condensation (PCC), the same phenotype as MSI1-deficient glioblastoma cells. Second, we show that the A184V mutation impedes RNA binding of MSI1, leading to deregulated expression of its endogenous targets. Third, we find that the A184V mutant MSI1 is unable to support ZIKV replication.
Fig. 3. MSI1 is enriched in neural progenitors, regulates Microcephalin (MCPH1) expression and is mutated in MCPH patients.
A) Immunohistochemistry of human embryonic brain at post conception week (pcw) 10 and 12. Tissue sections stained with antibodies against MSI1 (red) combined with neuron-specific β-III tubulin (green), or the apical neural progenitor marker Nestin (green). DNA is detected by DAPI (blue). Note that MSI1 is enriched in neural progenitors at the ventricular and subventricular zones (VZ and SVZ), but is absent from the cortical plate (CP).
B) MSI1 CLIP from U-251 cells with genotypes as indicated. CLIP was performed with rabbit IgG or MSI1 antibodies. Graphs show qPCRs of bound transcripts. n=3 biological replicates. P-values were obtained from Student’s t-test, unpaired, two-tailed: * p<0.05, ** p<0.005, not significant (n.s).
C) RNA EMSA analysis to detect binding between MCPH1_L 3’UTR and purified GST-MSI1 recombinant protein. Coomassie staining of corresponding purified proteins is shown below.
D) Western blots of parental, control, KO1 and KO2 cell lines. Blots were probed with antibodies as indicated. p150 serves as loading control.
E) Western blots of whole cell lysates from parent-of-patient- and patient-derived primary lymphocytes. Blots were probed with antibodies as indicated. MSI1 levels are unchanged.
F) MSI1 RIP from mock- and ZIKV-infected U-251 cells (MOI: 1 FFU/cell). Rabbit IgG or MSI1 antibodies were used for immunoprecipitation. Input corresponds to 10% of whole cell extract. Western blot was probed with MSI1 antibody. Graphs below show amounts of bound RNAs including ZIKV genome and endogenous target transcripts. RIP values are presented as a percentage of input following subtraction of the IgG background. MR766 and PE243 were quantitated by TaqMan assay, whereas endogenous transcripts with SYBR qPCR. Bar charts depict mean±s.e.m. n=3 biological replicates. P-values were obtained from Student’s t-test, unpaired, two-tailed: * p<0.05, ** p<0.005, not significant (n.s.).
G) Western blots of PE243-infected U-251 cells. (MOI: 1 FFU/cell, 72h). Blots were probed with antibodies as indicated. α-tubulin serves as loading control. Note that MSI1 positively regulates MCPH1 and CDK6, and negatively regulates NUMB and p21 protein levels.
The PCC phenotype seen in MSI1A184V patient cells has been previously described in cells deficient of the MCPH-associated protein, Microcephalin (MCPH1) (Fig. S8G) (28, 29). Since the MCPH1 locus is unaffected in MSI1A184V patients, we speculated that MSI1 could control chromosome condensation by regulating MCPH1 expression. To determine if MCPH1 was a MSI1 target, we performed RNA immunoprecipitation under native conditions. In addition to its known targets NUMB, p21WAF and the MCPH-associated gene, CDK6/MCPH12, MSI1 co-precipitated with two isoforms of MCPH1 (MCPH1_S and MCPH_L) despite their divergent 3’ UTRs, but did not interact with other MCPH genes tested (Fig. S9A, B) (12, 30, 31). CLIP and RNA mobility shift assays suggest a direct interaction between MSI1 and MCPH1_L (Fig. 3B, C). MSI1 can act as a translational suppressor (i.e. for NUMB and p21WAF1) or activator (i.e. for CDK6) (12, 21, 30). Consistent with a role for MSI1 in translational activation of MCPH1, we observed low MCPH1 protein levels in MSI1A184V patient and MSI1-deficient U-251 cells and a reduction in polysome-associated MCPH1 transcripts in KO cells (Fig. 3D, E; Figs. S9C, S10). Given that MSI1 interacts with ZIKV RNA, and viral RNA is abundant in the infected cell, it could compete with endogenous targets for binding MSI1. Indeed, upon ZIKV infection of U-251 cells, we observed a marked reduction in the interaction between MSI1 and its target RNAs including MCPH1 and NUMB, accompanied by changes in their protein levels that mirrored those of MSI1 KO cells (Fig. 3F,G).
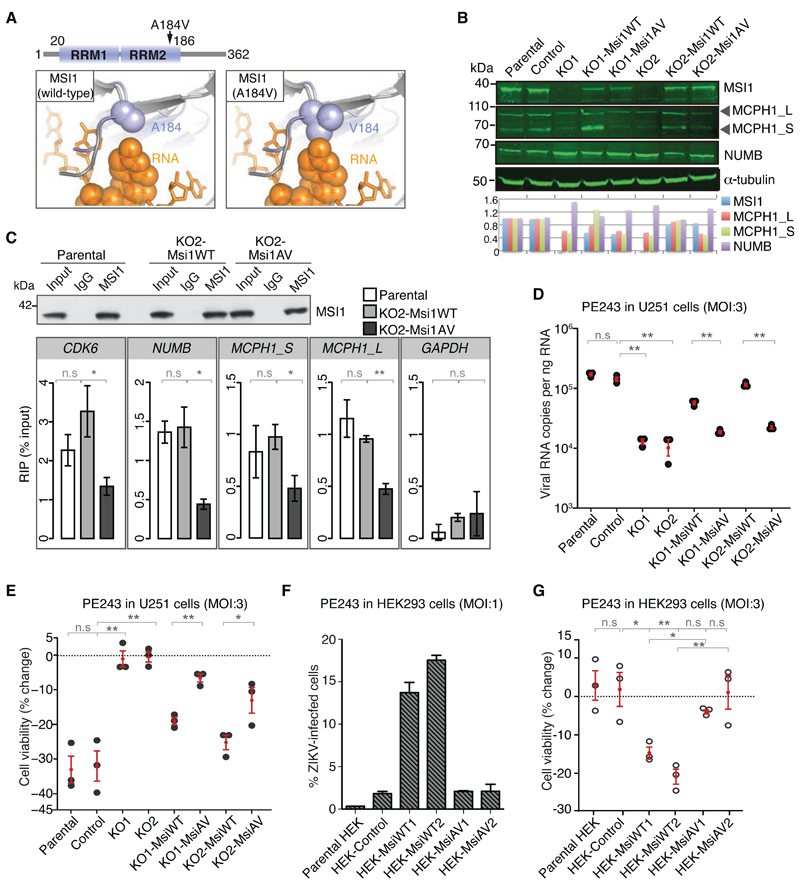
MSI1 interacts with target transcripts via its two RNA recognition motifs (RRM) (19, 30, 32). NMR studies show that the conserved Ala184 within the RRM2 is an RNA-binding residue (33). Modeling based on the crystal structure of the RNA-binding protein HRP1 with RNA, indicates that the A184V mutation impairs the MSI1-RNA interaction (Fig. 4A). To evaluate the effect of A184V on MSI1 function in cells, transgenes encoding wild-type and A184V MSI1 (MsiWT and MsiAV, respectively) were randomly integrated into KO1 or KO2 U-251 cells and single clones were isolated (KO1/2-MsiWT or KO1/2-MsiAV). MSI1 protein expression in these clones was quantified with respect to endogenous protein levels in parental cells (Fig. 4B). When compared to KO2-MsiWT cells, MSI1 RIP recovered 2-4 fold fewer target transcripts from KO2-MsiAV, indicative of reduced RNA binding by the A184V mutant (Fig. 4C). NUMB and MCPH1 protein levels changed accordingly (Fig. 4B). These results were recapitulated in HEK293T cells expressing Msi1WT or Msi1AV (Fig. S11). MSI1A184V, MSI1 KO, KO1/2-MsiAV and MSI1-depleted cells all exhibited suboptimal MCPH1 protein levels and PCC, prompting us to investigate if a functional link existed between these phenotypes (Fig. S9C-F). MCPH1 overexpression reduced PCC frequency in MSI1-depleted cells, thereby confirming a role for MSI1 in chromosome condensation via translational control of MCPH1 (Fig. S8G, H). Therefore, the A184V mutation impairs binding of MSI1 to RNA, which leads to reduced MCPH1 expression and a concomitant increase in PCC frequency. Remarkably, defective chromosome condensation has been recently found to cause MCPH (34).
Fig. 4. The A184V MCPH mutation disrupts RNA binding by MSI1 and impairs the ability of MSI1 to drive ZIKV replication.
A) Structural model of MSI1 (blue) and HRP1/RNA complex (grey/orange) predicts that the A184V mutation impairs the interaction with RNA due to a steric clash.
B) Western blots of cell lines stably expressing WT or A184V MSI1 transgenes. Note that cell lines were derived from either KO1 or KO2 cells as specified. Blots were probed with antibodies as indicated. p150 serves as loading control. Graph shows signal intensities of each protein normalized to parental cells.
C) MSI1 RIP from U-251 cells with genotypes as indicated. Rabbit IgG or MSI1 antibodies were used for immunoprecipitation. Input corresponds to 10% of whole cell extract. Western blot was probed with MSI1 antibody. Graphs below show qPCRs of bound transcripts. n=3 biological replicates. P-values were obtained from Student’s t-test, unpaired, two-tailed: * p<0.05, ** p<0.005, not significant (n.s).
D) Quantification of viral RNA copies in U-251 cells with the indicated genotypes following infection with PE243 (MOI: 3 FFU/cell, 48h). n=3 biological replicates. P-values were obtained from Student’s t-test, unpaired, two-tailed: ** p<0.005, not significant (n.s).
E) Changes in survival of U-251 cells with different genotypes following infection with PE243 (MOI: 3 FFU/cell, 48h). n=3 biological replicates. P-values were obtained from Student’s t-test, unpaired, two-tailed: * p<0.05, ** p<0.005, not significant (n.s).
F) ZIKV infection of HEK293T cells as measured by FACS analysis of flavivirus protein E. FACS was performed on control, MsiWT or MsiAV transgene-expressing cells following infection with PE243 (MOI: 1 FFU/cell, 48h). n=2 biological replicates. Bar charts depict mean±s.e.m.
G) Changes in survival of control, MsiWT or MsiAV transgene-expressing HEK293T cells following infection with PE243 (MOI: 3 FFU/cell, 48h). n=3 biological replicates, and P-values were obtained from Student’s t-test, unpaired, two-tailed: * p<0.05, ** p<0.005, not significant (n.s).
Given that the A184V mutation impedes binding of MSI1 to RNA, we next probed the effect of A184V mutant MSI1 on ZIKV replication. To this end, viral RNA levels and cell viability were assayed in KO1/2, KO1/2-MsiWT and KO1/2-MsiAV U-251 cells infected with PE243. Complementation of KO1/2 cells with MsiWT increased both ZIKV RNA levels and cell death (Fig. 4D, E). By contrast, MsiAV was unable to support ZIKV replication, and showed minimal impact on cell viability. Additionally, in HEK293T cells, expression of MsiWT, but not MsiAV, increased viral RNA and cell death upon infection (Fig. 4F, G; Fig. S12). These findings also imply that MSI1 expression increases susceptibility of HEK293T cells to ZIKV infection (35). Furthermore, we noted an apparent dose-dependent effect of MSI1 on viral replication; those U-251 and HEK293T clones that express higher levels of MSI1 displayed greater viral RNA levels and increased cell death (Fig. 4B, D-G; Figs. S11 and S12).
Our study raises the question whether MSI1 could have functions in other flaviviruses. We have surveyed putative MSI1 binding sites in a number of flaviviruses by mapping the consensus (A/GU(1-3)AG) onto predicted secondary structures of flaviviridae 3’UTRs obtained from a recent publication (Table S3) (16). Although several flaviviruses harbour consensus MSI1 sites within appropriate structural landscapes, whether MSI1 is relevant to the biology of these viruses remains to be established. Furthermore, while our data are consistent with a role for MSI1 in ZKV neurotropism and pathology, multiple factors must collude in ZIKV infection of the fetal brain, not least viral entry receptors that allow the virus to cross the placental barrier (36). Viruses such as human cytomegalovirus and Rubella can also access the developing fetal brain, but whether MSI1 contributes to their replication or pathogenesis is unknown and would require further study.
This work suggests that high MSI1 expression levels in neural precursors could be a key contributor to the fetal neurotropism exhibited by ZIKV (2, 10, 37) (Fig. S13). Intriguingly, MSI1 is also highly expressed in the retina and testis, other tissues deemed vulnerable to ZIKV infection (38–41). While our study provides new insight into the potential pathogenic mechanisms of ZIKV, further work will be required to determine if the modification or interference of the MSI1-ZIKV interaction results in neuronal attenuation of ZIKV.
Supplementary Material
Acknowledgements
The authors are indebted to Alain Kohl (Centre for Virus Research, University of Glasgow) and Lindomar J. Pena and Rafael Oliveira de Freitas França, Fiocruz Recife, Pernambuco, Brazil, for the provision of PE243 ZIKV RNA used to generate the virus stock. We would like to thank and acknowledge Steve Lisgo for the expert provision of human embryonic histology sections through the Human Developmental Biology Resource (HDBR) at the University of Newcastle funded by a joint UK MRC/Wellcome Trust grant (099175/Z/12/Z). We would like to thank Leanna Smith for her assistance with homology modeling, Guillaume van Zande for his help and the patients’ families for their participation. The National Research Ethics Service Committee, East of England - Cambridge Central, UK (C.G. Woods, REC 05/Q0108/402) approved the informed consent to enter the study. We would like to further thank Tanweer Hussain for help with polysome fractionations, and John Sinclair and Anna Git for technical advice. We thank KJ Patel and members of the Gergely lab for useful discussions and comments. We are grateful for expert help by the CRUK CI Core Facilities. I.G. and A. E. F are Wellcome Trust Senior Fellows. I.G. was supported by research grants 097997/Z/11/A and 097997/Z/11/Z, whereas A. E. F by grant 106207. M.S.N was funded by the Wellcome Trust (200183/Z/15/Z) and T. R. S is a Wellcome Trust Henry Dale Fellow (202471/Z/16/Z). This work was made possible by funding from Cancer Research UK C14303/A17197 to FG and C24461/A12772 to R.B. F.G. and C.G.W. acknowledge support from NIHR Cambridge Biomedical Research Centre, the University of Cambridge and Hutchison Whampoa Ltd. All data to understand and assess the conclusions of this research are available in the main paper and supplementary materials.
References and notes
- 1.Mlakar J, et al. Zika Virus Associated with Microcephaly. The New England journal of medicine. 2016;374:951–958. doi: 10.1056/NEJMoa1600651. [DOI] [PubMed] [Google Scholar]
- 2.Oliveira Melo AS, et al. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2016;47:6–7. doi: 10.1002/uog.15831. [DOI] [PubMed] [Google Scholar]
- 3.Cugola FR, et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature. 2016;534:267–271. doi: 10.1038/nature18296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slavov SN, Otaguiri KK, Kashima S, Covas DT. Overview of Zika virus (ZIKV) infection in regards to the Brazilian epidemic. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas / Sociedade Brasileira de Biofisica … [et al.] 2016;49:e5420. doi: 10.1590/1414-431X20165420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cauchemez S, et al. Association between Zika virus and microcephaly in French Polynesia, 2013-15: a retrospective study. Lancet. 2016;387:2125–2132. doi: 10.1016/S0140-6736(16)00651-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams Waldorf KM, McAdams RM. Influence of infection during pregnancy on fetal development. Reproduction. 2013;146:R151–162. doi: 10.1530/REP-13-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li C, et al. Zika Virus Disrupts Neural Progenitor Development and Leads to Microcephaly in Mice. Cell stem cell. 2016;19:120–126. doi: 10.1016/j.stem.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 8.Li H, et al. Zika Virus Infects Neural Progenitors in the Adult Mouse Brain and Alters Proliferation. Cell stem cell. 2016 doi: 10.1016/j.stem.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miner JJ, Diamond MS. Understanding How Zika Virus Enters and Infects Neural Target Cells. Cell stem cell. 2016;18:559–560. doi: 10.1016/j.stem.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 10.Dang J, et al. Zika Virus Depletes Neural Progenitors in Human Cerebral Organoids through Activation of the Innate Immune Receptor TLR3. Cell stem cell. 2016;19:258–265. doi: 10.1016/j.stem.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Z, Nagy PD. Diverse roles of host RNA binding proteins in RNA virus replication. RNA biology. 2011;8:305–315. doi: 10.4161/rna.8.2.15391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imai T, et al. The neural RNA-binding protein Musashi1 translationally regulates mammalian numb gene expression by interacting with its mRNA. Molecular and cellular biology. 2001;21:3888–3900. doi: 10.1128/MCB.21.12.3888-3900.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donald CL, et al. Full Genome Sequence and sfRNA Interferon Antagonist Activity of Zika Virus from Recife, Brazil. PLoS neglected tropical diseases. 2016;10:e0005048. doi: 10.1371/journal.pntd.0005048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakakibara S, et al. Mouse-Musashi-1, a neural RNA-binding protein highly enriched in the mammalian CNS stem cell. Developmental biology. 1996;176:230–242. doi: 10.1006/dbio.1996.0130. [DOI] [PubMed] [Google Scholar]
- 15.Sakakibara S, Nakamura Y, Satoh H, Okano H. Rna-binding protein Musashi2: developmentally regulated expression in neural precursor cells and subpopulations of neurons in mammalian CNS. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:8091–8107. doi: 10.1523/JNEUROSCI.21-20-08091.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Villordo SM, Filomatori CV, Sanchez-Vargas I, Blair CD, Gamarnik AV. Dengue virus RNA structure specialization facilitates host adaptation. PLoS Pathog. 2015;11:e1004604. doi: 10.1371/journal.ppat.1004604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uren PJ, et al. RNA-Binding Protein Musashi1 Is a Central Regulator of Adhesion Pathways in Glioblastoma. Molecular and cellular biology. 2015;35:2965–2978. doi: 10.1128/MCB.00410-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klase ZA, et al. Zika Fetal Neuropathogenesis: Etiology of a Viral Syndrome. PLoS neglected tropical diseases. 2016;10:e0004877. doi: 10.1371/journal.pntd.0004877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox RG, Park FD, Koechlein CS, Kritzik M, Reya T. Musashi signaling in stem cells and cancer. Annual review of cell and developmental biology. 2015;31:249–267. doi: 10.1146/annurev-cellbio-100814-125446. [DOI] [PubMed] [Google Scholar]
- 20.de Araujo PR, et al. Musashi1 Impacts Radio-Resistance in Glioblastoma by Controlling DNA-Protein Kinase Catalytic Subunit. The American journal of pathology. 2016;186:2271–2278. doi: 10.1016/j.ajpath.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Battelli C, Nikopoulos GN, Mitchell JG, Verdi JM. The RNA-binding protein Musashi-1 regulates neural development through the translational repression of p21WAF-1. Molecular and cellular neurosciences. 2006;31:85–96. doi: 10.1016/j.mcn.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Xu M, et al. Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nature medicine. 2016;22:1101–1107. doi: 10.1038/nm.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaneko Y, et al. Musashi1: an evolutionally conserved marker for CNS progenitor cells including neural stem cells. Dev Neurosci. 2000;22:139–153. doi: 10.1159/000017435. [DOI] [PubMed] [Google Scholar]
- 24.Sakakibara S, et al. RNA-binding protein Musashi family: roles for CNS stem cells and a subpopulation of ependymal cells revealed by targeted disruption and antisense ablation. Proc Natl Acad Sci U S A. 2002;99:15194–15199. doi: 10.1073/pnas.232087499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eppig JT, et al. The Mouse Genome Database (MGD): facilitating mouse as a model for human biology and disease. Nucleic acids research. 2015;43:D726–736. doi: 10.1093/nar/gku967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilmore EC, Walsh CA. Genetic causes of microcephaly and lessons for neuronal development. Wiley Interdiscip Rev Dev Biol. 2013;2:461–478. doi: 10.1002/wdev.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chavali PL, Putz M, Gergely F. Small organelle, big responsibility: the role of centrosomes in development and disease. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2014;369 doi: 10.1098/rstb.2013.0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trimborn M, et al. Mutations in microcephalin cause aberrant regulation of chromosome condensation. American journal of human genetics. 2004;75:261–266. doi: 10.1086/422855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson AP, et al. Identification of microcephalin, a protein implicated in determining the size of the human brain. American journal of human genetics. 2002;71:136–142. doi: 10.1086/341283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cambuli FM, et al. A Mouse Model of Targeted Musashi1 Expression in Whole Intestinal Epithelium Suggests Regulatory Roles in Cell Cycle and Stemness. Stem Cells. 2015;33:3621–3634. doi: 10.1002/stem.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hussain MS, et al. CDK6 associates with the centrosome during mitosis and is mutated in a large Pakistani family with primary microcephaly. Human molecular genetics. 2013;22:5199–5214. doi: 10.1093/hmg/ddt374. [DOI] [PubMed] [Google Scholar]
- 32.Kawahara H, et al. Neural RNA-binding protein Musashi1 inhibits translation initiation by competing with eIF4G for PABP. The Journal of cell biology. 2008;181:639–653. doi: 10.1083/jcb.200708004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagata T, et al. Structure, backbone dynamics and interactions with RNA of the C-terminal RNA-binding domain of a mouse neural RNA-binding protein, Musashi1. J Mol Biol. 1999;287:315–330. doi: 10.1006/jmbi.1999.2596. [DOI] [PubMed] [Google Scholar]
- 34.Martin CA, et al. Mutations in genes encoding condensin complex proteins cause microcephaly through decatenation failure at mitosis. Genes & development. 2016;30:2158–2172. doi: 10.1101/gad.286351.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamel R, et al. Biology of Zika Virus Infection in Human Skin Cells. Journal of virology. 2015;89:8880–8896. doi: 10.1128/JVI.00354-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richard AS, et al. AXL-dependent infection of human fetal endothelial cells distinguishes Zika virus from other pathogenic flaviviruses. Proc Natl Acad Sci U S A. 2017;114:2024–2029. doi: 10.1073/pnas.1620558114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brault JB, et al. Comparative Analysis Between Flaviviruses Reveals Specific Neural Stem Cell Tropism for Zika Virus in the Mouse Developing Neocortex. EBioMedicine. 2016;10:71–76. doi: 10.1016/j.ebiom.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lazear HM, et al. A Mouse Model of Zika Virus Pathogenesis. Cell Host Microbe. 2016;19:720–730. doi: 10.1016/j.chom.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salinas S, et al. Zika Virus Efficiently Replicates in Human Retinal Epithelium and Disturbs Its Permeability. Journal of virology. 2017;91 doi: 10.1128/JVI.02144-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Susaki K, et al. Musashi-1, an RNA-binding protein, is indispensable for survival of photoreceptors. Experimental eye research. 2009;88:347–355. doi: 10.1016/j.exer.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 41.Ma W, et al. Zika Virus Causes Testis Damage and Leads to Male Infertility in Mice. Cell. 2016;167:1511–1524 e1510. doi: 10.1016/j.cell.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 42.Woods CG, Valente EM, Bond J, Roberts E. A new method for autozygosity mapping using single nucleotide polymorphisms (SNPs) and EXCLUDEAR. J Med Genet. 2004;41:e101. doi: 10.1136/jmg.2003.016873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lander ES, Botstein D. Homozygosity mapping: a way to map human recessive traits with the DNA of inbred children. Science. 1987;236:1567–1570. doi: 10.1126/science.2884728. [DOI] [PubMed] [Google Scholar]
- 44.Mueller RF, Bishop DT. Autozygosity mapping, complex consanguinity, and autosomal recessive disorders. J Med Genet. 1993;30:798–799. doi: 10.1136/jmg.30.9.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ogawa S, Nanya Y, Yamamoto G. Genome-wide copy number analysis on GeneChip platform using copy number analyzer for affymetrix GeneChip 2.0 software. Methods Mol Biol. 2007;396:185–206. doi: 10.1007/978-1-59745-515-2_13. [DOI] [PubMed] [Google Scholar]
- 46.Woods CG, et al. Quantification of homozygosity in consanguineous individuals with autosomal recessive disease. American journal of human genetics. 2006;78:889–896. doi: 10.1086/503875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bochukova E, et al. A mutation in the thyroid hormone receptor alpha gene. The New England journal of medicine. 2012;366:243–249. doi: 10.1056/NEJMoa1110296. [DOI] [PubMed] [Google Scholar]
- 48.Miller JA, et al. Transcriptional landscape of the prenatal human brain. Nature. 2014;508:199–206. doi: 10.1038/nature13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.C Genomes Project et al. A global reference for human genetic variation. Nature. 2015;526:68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fu W, et al. Analysis of 6,515 exomes reveals the recent origin of most human protein-coding variants. Nature. 2013;493:216–220. doi: 10.1038/nature11690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gandin V, et al. Polysome fractionation and analysis of mammalian translatomes on a genome-wide scale. Journal of visualized experiments : JoVE. 2014 doi: 10.3791/51455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Urbanowicz RA, et al. Human Adaptation of Ebola Virus during the West African Outbreak. Cell. 2016;167:1079–1087 e1075. doi: 10.1016/j.cell.2016.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stojic L, et al. Transcriptional silencing of long noncoding RNA GNG12-AS1 uncouples its transcriptional and product-related functions. Nat Commun. 2016;7 doi: 10.1038/ncomms10406. 10406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoon JH, Gorospe M. Cross-Linking Immunoprecipitation and qPCR (CLIP-qPCR) Analysis to Map Interactions Between Long Noncoding RNAs and RNA-Binding Proteins. Methods Mol Biol. 2016;1402:11–17. doi: 10.1007/978-1-4939-3378-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perez-Canadillas JM. Grabbing the message: structural basis of mRNA 3'UTR recognition by Hrp1. The EMBO journal. 2006;25:3167–3178. doi: 10.1038/sj.emboj.7601190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.