Abstract
Background
The West African outbreak of Ebola virus disease that peaked in 2014 has caused more than 11,000 deaths. The development of an effective Ebola vaccine is a priority for control of a future outbreak.
Methods
In this phase 1 study, we administered a single dose of the chimpanzee adenovirus 3 (ChAd3) vaccine encoding the surface glycoprotein of Zaire ebolavirus (ZEBOV) to 60 healthy adult volunteers in Oxford, United Kingdom. The vaccine was administered in three dose levels — 1×1010 viral particles, 2.5×1010 viral particles, and 5×1010 viral particles — with 20 participants in each group. We then assessed the effect of adding a booster dose of a modified vaccinia Ankara (MVA) strain, encoding the same Ebola virus glycoprotein, in 30 of the 60 participants and evaluated a reduced prime–boost interval in another 16 participants. We also compared antibody responses to inactivated whole Ebola virus virions and neutralizing antibody activity with those observed in phase 1 studies of a recombinant vesicular stomatitis virus–based vaccine expressing a ZEBOV glycoprotein (rVSV-ZEBOV) to determine relative potency and assess durability.
Results
No safety concerns were identified at any of the dose levels studied. Four weeks after immunization with the ChAd3 vaccine, ZEBOV-specific antibody responses were similar to those induced by rVSV-ZEBOV vaccination, with a geometric mean titer of 752 and 921, respectively. ZEBOV neutralization activity was also similar with the two vaccines (geometric mean titer, 14.9 and 22.2, respectively). Boosting with the MVA vector increased virus-specific antibodies by a factor of 12 (geometric mean titer, 9007) and increased glycoprotein-specific CD8+ T cells by a factor of 5. Significant increases in neutralizing antibodies were seen after boosting in all 30 participants (geometric mean titer, 139; P<0.001). Virus-specific antibody responses in participants primed with ChAd3 remained positive 6 months after vaccination (geometric mean titer, 758) but were significantly higher in those who had received the MVA booster (geometric mean titer, 1750; P<0.001).
Conclusions
The ChAd3 vaccine boosted with MVA elicited B-cell and T-cell immune responses to ZEBOV that were superior to those induced by the ChAd3 vaccine alone. (Funded by the Wellcome Trust and others; ClinicalTrials.gov number, NCT02240875.)
The recent outbreak of Ebola virus disease (EVD) in West Africa has led to more than 11,000 deaths, with a peak in mortality from August through December of 2014 and a subsequent decline in the number of new cases. The development of a durable and effective Ebola vaccine is a priority both to eliminate the remnants of the outbreak and to prevent and control future epidemics. Several candidate vaccines have shown promising results in phase 1 trials,1–6 and a recombinant vesicular stomatitis virus–based vaccine expressing the surface glycoprotein of Zaire ebolavirus (rVSV-ZEBOV) showed efficacy in an interim analysis of a phase 3 trial in Guinea (ring vaccination trial).7 More data will be required before the rVSV-ZEBOV vaccine can be licensed. However, the use of this vaccine could contribute to ending the current outbreak in West Africa by limiting the spread of infection among close contacts of persons with EVD. In this context, the duration of vaccine efficacy can be relatively short, since the time since exposure is typically known and protection is conferred within the time frame necessary to prevent clinical disease and transmission. In a different context, during the earlier, uncontrolled phase of an outbreak in which most transmission is undetected and new cases appear in geographically disparate locations, an effective vaccine would need to have longer durability. For this earlier phase of the outbreak, longer-lasting vaccine efficacy would be required to provide sufficient protection to the entire population within an affected area to interrupt transmission, particularly where transmission is unpredictable.
The demonstration in humans of vaccine efficacy against EVD with the rVSV-ZEBOV vaccine has facilitated the development of an Ebola virus vaccine by adding to our knowledge of immunity associated with protection, data that were previously derived only from rodent and primate challenge models. Before the current outbreak and the subsequent trial of rVSV-ZEBOV, licensure of an Ebola vaccine was dependent on the demonstration of adequate immunogenicity and safety in humans, along with linkage to immunogenicity and efficacy data in challenge studies conducted in nonhuman primates.8 Now we can compare cellular and humoral immune responses induced by various candidate vaccines in phase 1 studies with responses observed in rVSV-ZEBOV trials, in which various measures of humoral immunity (e.g., ZEBOV glycoprotein–specific antibody responses and neutralizing antibody titers) have been described in African and European cohorts.4 In contrast, substantial cellular immunogenicity induced by rVSV-ZEBOV immunization has not been shown in nonhuman primate models or in recent human phase 1 trials.3,4,9,10
The induction of both antibodies and CD8+ T-cell responses is potentially protective against EVD. Antibody levels as measured on an enzyme-linked immunosorbent assay (ELISA) against the Mayinga strain glycoprotein of ZEBOV had broad correlation with protection across a range of studies of vectored vaccination conducted in cynomolgus macaques, with a reciprocal titer of 3700 correlating with complete protection against challenge.11,12 However, after immunization of macaques with a protective vaccine dose of human serotype 5 adenovirus (AdHu5), antibodies did not adoptively transfer protection to other macaques, and depletion of CD8+ T cells largely ablated protection.13 This finding indicates a potential role for induced CD8+ T cells in vaccine efficacy and the likelihood that the observed antibody correlate is not a causal mechanism. Such immune activity may reflect a constellation of induced T-cell and antibody responses, both of which may contribute to protection. In cynomolgus macaques, the addition of a booster vaccination with a modified vaccinia Ankara (MVA) strain to priming immunization with the chimpanzee adenovirus 3 (ChAd3) vaccine encoding the ZEBOV surface glycoprotein increased immunogenicity by a factor of at least 10 and increased the duration of protective efficacy against Ebola virus challenge from 5 weeks to 10 months after vaccination,11 which indicates that boosting improves both immunogenicity and durability of protection.
Methods
Study Participants
The study was conducted at the Centre for Clinical Vaccinology and Tropical Medicine at the University of Oxford. Participants were healthy adults between the ages of 18 and 50 years who provided written informed consent (Table 1).
Table 1. Characteristics of the Participants at Baseline.*.
| Characteristic | Group 1† | Group 2† | Group 3† | Group 4 (N = 8)‡ |
Group 5 (N = 8)‡ |
All Participants (N = 76) |
|||
|---|---|---|---|---|---|---|---|---|---|
| Unboosted (N = 10) |
Boosted (N = 10) |
Unboosted (N = 10) |
Boosted (N = 10) |
Unboosted (N = 10) |
Boosted (N = 10) |
||||
| Sex — no. (%) | |||||||||
| Male | 1 (10) | 5 (50) | 8 (80) | 5 (50) | 7 (70) | 6 (60) | 3 (38) | 4 (50) | 39 (51) |
| Female | 9 (90) | 5 (50) | 2 (20) | 5 (50) | 3 (30) | 4 (40) | 5 (62) | 4 (50) | 37 (49) |
| Age | |||||||||
| Distribution — no. (%) | |||||||||
| 18–20 yr | 0 | 0 | 0 | 0 | 3 (30) | 0 | 0 | 1 (12) | 4 (5) |
| 21–30 yr | 4 (40) | 5 (50) | 4 (40) | 6 (60) | 2 (20) | 7 (70) | 6 (75) | 5 (62) | 39 (51) |
| 31–40 yr | 4 (40) | 3 (30) | 3 (30) | 3 (30) | 2 (20) | 1 (10) | 1 (12) | 1 (12) | 18 (24) |
| 41–50 yr | 2 (20) | 2 (20) | 3 (30) | 1 (10) | 3 (30) | 2 (20) | 1 (12) | 1 (12) | 15 (20) |
| Mean — yr | 32.2±10.0 | 30.4±8.2 | 35.2±8.2 | 30.8±7.4 | 31.9±11.2 | 39.9±7.6 | 28.0±10.0 | 27.8±8.1 | 30.9±8.8 |
| Range — yr | 21–48 | 22–42 | 24–48 | 22–48 | 18–48 | 22–42 | 21–50 | 20–43 | 18–50 |
| Race — no. (%)§ | |||||||||
| White | 9 (90) | 10 (100) | 10 (100) | 9 (90) | 9 (90) | 9 (90) | 7 (88) | 7 (88) | 70 (92) |
| Black | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Asian | 0 | 0 | 0 | 1 (10) | 1 (10) | 0 | 1 (12) | 1 (12) | 4 (5) |
| Mixed | 1 (10) | 0 | 0 | 0 | 0 | 1 (10) | 0 | 0 | 2 (3) |
| Body-mass index¶ | |||||||||
| Distribution — no. (%) | |||||||||
| <18.5 | 0 | 0 | 1 (10) | 0 | 0 | 1 (10) | 0 | 0 | 2 (3) |
| 18.5–24.9 | 6 (60) | 5 (50) | 5 (50) | 6 (60) | 8 (80) | 8 (80) | 6 (75) | 6 (75) | 50 (66) |
| 25–29.9 | 3 (30) | 5 (50) | 3 (30) | 3 (30) | 2 (20) | 1 (10) | 2 (25) | 2 (25) | 21 (28) |
| ≥30 | 1 (10) | 0 | 1 (10) | 1 (10) | 0 | 0 | 0 | 0 | 3 (4) |
| Mean | 23.8±4.3 | 25.2±1.9 | 24.5±4.0 | 24.7±2.9 | 22.7±2.0 | 22.8±2.7 | 22.2±3.7 | 23.4±3.1 | 23.7±3.2 |
| Range | 18.5–30.5 | 22.0–28.5 | 17.4–33.0 | 21.4–30.7 | 20.4–26.6 | 17.1–27.4 | 18.6–27.8 | 18.5–29.0 | 17.1–33.0 |
Plus–minus values are means ±SD. There were no significant differences between the study groups. Percentages may not total 100 because of rounding.
Among the 30 participants who received a booster dose of the MVA vaccine, 18 received a dose of 1.5×108 plaque-forming units (PFU) and 12 received a dose of 3×108 PFU, with stratification according to priming-dose group.
Two additional groups of 8 participants each were recruited to assess the effect of reducing the interval between priming and boosting to either 1 week (group 4) or 2 weeks (group 5). The 16 participants in these two groups received a priming dose of 2.5×1010 viral particles of ChAd3 and a boosting dose of 1.5×108 PFU of MVA.
Race was self-reported.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Study Oversight
The study was reviewed and approved by the United Kingdom National Research Ethics Service, the Committee South Central–Oxford A, the Medicines and Healthcare Products Regulatory Agency, and the Oxford University Clinical Trials and Research Governance team, who monitored compliance with Good Clinical Practice guidelines. An independent data and safety monitoring board provided safety oversight.
The ChAd3 vaccine was provided by the Vaccine Research Center of the National Institute of Allergy and Infectious Diseases (NIAID) and GlaxoSmithKline, which manufactured the vaccine. The MVA vaccine was produced under a contract between NIAID and Fisher BioServices.
Study Design
In this phase 1 study, we administered the ChAd3 vaccine to 60 participants; 20 participants received the vaccine in a dose of 1×1010 viral particles (group 1), 20 received the vaccine in a dose of 2.5×1010 viral particles (group 2), and 20 received the vaccine in a dose of 5×1010 viral particles (group 3). In addition, in an attempt to improve immune responses, we invited 10 participants from each of the three groups to receive a single booster dose of MVA (called MVA-BN Filo), which encodes the same Mayinga strain glycoprotein antigen as that encoded by the ChAd3 vaccine, along with glycoproteins of the Sudan Ebola virus species and Marburg virus and the nucleoprotein of Taï Forest Ebola virus.
From late November to early December 2014 (at 3 to 10 weeks after the priming immunization), we administered the MVA vaccine at a dose of 1.5×108 plaque-forming units (PFU) to 18 participants and at a dose of 3×108 PFU to 12 participants, with stratification according to priming-dose group. We then recruited and immunized two additional groups of 8 participants each to assess the effect of reducing the interval between priming and boosting to either 1 week (group 4) or 2 weeks (group 5). In this analysis, all the participants received a priming dose of 2.5×1010 viral particles of ChAd3 and a boosting dose of 1.5×108 PFU of MVA.
Details regarding the study design and participants are provided in Figure S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org. Additional data on vaccines, safety-assessment techniques, and study design are provided in the study protocol, also available at NEJM.org.
Assessment of Humoral Immunity
We assessed antibody responses using four separate types of IgG ELISA: an in-house standardized ELISA that was developed at the Jenner Institute and uses a recombinant ZEBOV glycoprotein, a commercially available ZEBOV glycoprotein ELISA kit (Alpha Diagnostic International), an end-point ELISA performed at the National Institutes of Health with a readout for the EC90 assay (the concentration at which there is a 90% decrease in antigen binding), and a whole-virion ELISA that uses inactivated ZEBOV Makona (the current outbreak strain). Two assays were used to measure neutralizing antibodies. The first measured direct neutralization of live ZEBOV (Mayinga strain) from all participants who received the booster dose at 28 days after the dose of ChAd3 vaccine and 14 days after the dose of the MVA vaccine. The second measured the blocking ability of vaccine-induced antibodies with the use of a pseudotyped lentivirus expressing the glycoprotein from the Mayinga strain, with a readout of the 50% inhibitory concentration (IC50) assay. A competitive ELISA-based assay was also used to detect blocking of a neutralizing monoclonal antibody (4G7)14 by serum after boosting with MVA. (A detailed description of the immunologic analyses is provided in the Supplementary Appendix.)
T-cell Assays
We measured T-cell responses to vaccination using ex vivo interferon-γ enzyme-linked immunosorbent spot (ELISPOT) assays at all time points and flow cytometry with intracellular cytokine staining at the peak of the immune response after each vaccination. T-cell assays were performed on freshly isolated peripheral-blood mononuclear cells (PBMCs).
Results
Study Population
A total of 76 of the 123 volunteers who were screened for eligibility were vaccinated (Fig. S1 in the Supplementary Appendix). Of the 60 participants who were included in the original analysis of the ChAd3 vaccine, 59 completed at least 28 days of follow-up. One participant in group 1 withdrew on day 1 after vaccination owing to an aversion to venipuncture. Communication with the participant on day 10 after vaccination confirmed that the participant remained well, with no symptoms to report. Among the participants who were followed for 180 days were all 30 who received the MVA booster and 28 who did not receive the booster.
Safety
A complete list of the frequency and maximum severity of solicited, unsolicited, and laboratory adverse events, according to dose group, is provided in Tables S1 through S8 in the Supplementary Appendix. The majority of adverse events that were reported in all dose groups were mild in severity, with no unexpected serious adverse reactions or serious adverse events. Local reactogenicity appeared to be more pronounced after boosting vaccination than after priming vaccination, a finding that is consistent with the results of other studies of heterologous prime–boost vaccine schedules incorporating a ChAd prime and MVA booster. In contrast, fewer systemic adverse events were reported with boosting vaccination than with priming vaccination.
The majority of adverse events were self-limited and mild. Local pain was the most common local event (with one case reported as severe). Moderate systemic adverse events were fever, myalgia, arthralgia, headache, fatigue, nausea, and malaise. No severe systemic solicited adverse events were reported. Four episodes of mild fever (37.6 to 38.0°C in 4 participants) were reported. No fever persisted for more than 24 hours.
A prolonged activated partial-thromboplastin time was observed in four participants during the first 2 weeks after vaccination (three with a grade 1 elevation and one with a grade 2 elevation). None of the prolongations were associated with symptoms or clinical features of coagulopathy. The elevations fully resolved in all participants by 10 weeks after vaccination. No further abnormality was found on extended hematologic and coagulation evaluation.
A transient induction of an antiphospholipid antibody causing an in vitro artifact on the laboratory assay for activated partial-thromboplastin time after the administration of adenovirus vectors has been reported previously.15,16 Transient mild lymphocytopenia was noted on day 1 after vaccination in five participants in group 1, four in group 2, and eight in group 3; moderate lymphocytopenia was noted in two participants each in group 2 and group 3 on day 1. Transient mild or moderate elevations in bilirubin were recorded in three participants in group 2 and three in group 3. Transient hyperbilirubinemia in the severe range was recorded in two participants (one in group 2 and one in group 3) who had a prevaccination diagnosis of Gilbert’s syndrome.
Antibody Responses
Antibody responses as measured by means of standardized glycoprotein ELISA increased significantly by 7 days after the MVA dose and peaked at day 14 after boosting and then decreased slightly by day 28 (P<0.01 by the Friedman test for all comparisons) (Fig. 1A). Responses remained significantly above pre-boost levels at 180 days after MVA vaccination and were four times as high as titers measured at 180 days after priming with the ChAd3 vaccine alone (P<0.001 by the Mann–Whitney test) (Fig. 1B); in addition, 100% of the participants who received the MVA vaccine remained seropositive, as compared with less than half of those who received the priming vaccination alone.
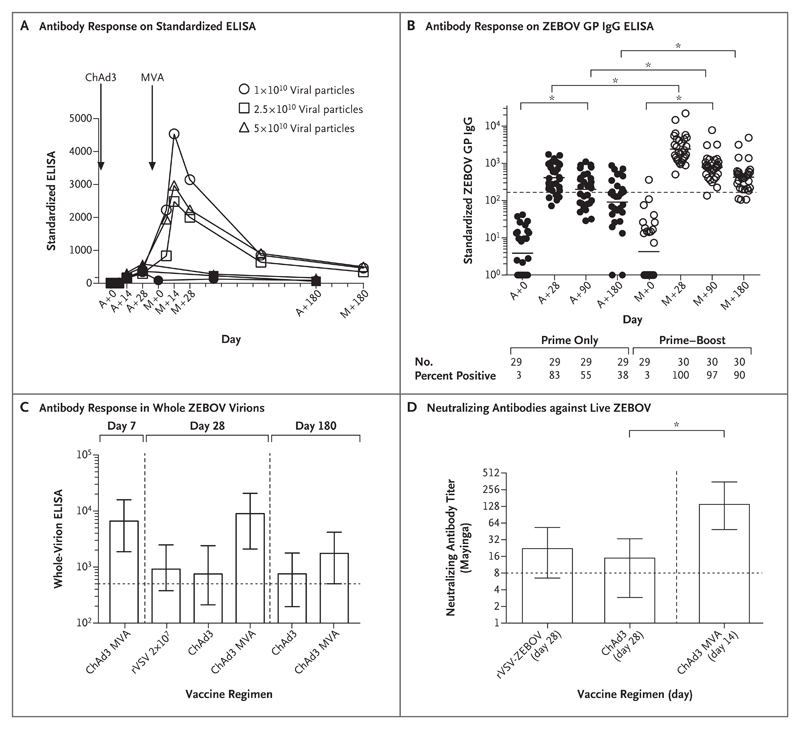
Figure 1. Antibody Responses to the Zaire ebolavirus (ZEBOV) Glycoprotein.
Panel A shows the geometric mean titer of antibody responses to increasing doses of the chimpanzee adenovirus 3 (ChAd3) vaccine encoding the surface glycoprotein of ZEBOV, followed by a booster dose of a modified vaccinia Ankara (MVA) strain. Antibody responses are shown according to measurements on a standardized enzyme-linked immunosorbent assay (ELISA) for doses of 1×1010 viral particles (in 19 participants), 2.5×1010 viral particles (in 20 participants), and 5×1010 viral particles (in 20 participants). Solid symbols indicate that participants received only the ChAd3 vaccine, and open symbols, that participants received the ChAd3 vaccine followed by booster MVA. Antibody responses increased significantly by 7 days after the MVA dose and peaked at day 14 after boosting and then decreased slightly by day 28. There were no significant differences in responses among the dose groups in the cohort that received the MVA booster at any time point after vaccination. The days of the analysis are indicated by a plus sign after administration of the ChAd3 vaccine (A) and the MVA vaccine (M). Panel B shows the responses of participants after administration of the prime and booster vaccines, according to results on anti-ZEBOV glycoprotein (GP) IgG ELISA. The solid horizontal lines represent the geometric mean titer. Percentages of vaccinees with positive antibody responses at each time point are indicated below the graph. The horizontal dashed line represents the threshold for a positive result (arbitrary ELISA units, +0.561), calculated as the mean plus 3 standard deviations of the response on day 0 for all participants. Panel C shows antibody titers to inactivated whole ZEBOV virions (Makona strain) as measured on ELISA. The data show that immunogenicity at 4 weeks after priming with ChAd3 was similar to that measured after immunization with a recombinant vesicular stomatitis virus–based vaccine expressing ZEBOV glycoprotein (rVSV-ZEBOV) in 10 vaccinees in Hamburg, Germany. Panel D shows titers of neutralizing antibodies against live ZEBOV (Mayinga strain) from all participants who received the MVA booster, as measured at 28 days after the ChAd3 dose and 14 days after the MVA dose. Low levels of neutralizing antibodies that were detected in participants at 28 days were similar to levels reported after the administration of the rVSV-ZEBOV vaccine. By 14 days after MVA vaccination, the levels had increased by a factor of 9. In Panels C and D, the columns represent the geometric mean titer, the I bars represent 95% confidence intervals, and the horizontal dashed lines represent the positive threshold. In Panels B and D, the asterisk denotes P<0.001 by the two-tailed Mann–Whitney test.
Titers on whole-virion ELISA showed that immunogenicity at 4 weeks after priming with ChAd3 was similar to that measured after immunization with rVSV-ZEBOV in 10 vaccinees in Hamburg, Germany, at the dose administered in the ring vaccination study (geometric mean titer with ChAd3, 752.4; 95% confidence interval [CI], 541 to 1647; geometric mean titer with rVSV-ZEBOV, 920.7; 95% CI, 541 to 1566). In our study, after boosting with MVA, titers increased by a factor of 9 (geometric mean titer, 6625; 95% CI, 4748 to 9245) at 1 week and by a factor of 12 (geometric mean titer, 9007; 95% CI, 6909 to 11741) at 4 weeks (Fig. 1C).
Six months after vaccination, titers in the group primed only with ChAd3 were similar to those detected 1 month after vaccination (geometric mean titer, 758; 95% CI, 561 to 1023; P = 0.90 by the two-tailed Wilcoxon matchedpairs test). Titers remained significantly higher in the group that received the MVA booster (geometric mean titer, 1750; 95% CI, 1247 to 2456) than in the ChAd3 prime-only group (P<0.001 by the two-tailed Mann–Whitney test). At that time, 77% of vaccinees in the group that received the MVA booster remained seropositive, as compared with 25% of those in the prime-only group. (Summary data are provided in Tables S9 through S12 in the Supplementary Appendix.)
Neutralizing antibody titers to live ZEBOV (Mayinga strain) from all participants who received the MVA booster were measured at 28 days after the ChAd3 dose and at 14 days after the MVA dose (Fig. 1D). Low levels of neutralizing antibodies were detected in participants at 28 days (geometric mean titer, 14.9; 95% CI, 12 to 18.5) — levels that were similar to those reported after the rVSV-ZEBOV vaccine4 (geometric mean titer, 22.2; 95% CI, 15.7 to 31.4); by 14 days after the MVA vaccine, the levels had increased by a factor of 9 (geometric mean titer, 139; 95% CI, 90 to 215) and all participants were seropositive (geometric mean titer, >8). Boosting with the high dose of MVA elicited neutralizing antibody titers that were higher than those with the low dose (geometric mean titer in the high-dose group, 243.9; 95% CI, 96 to 628; geometric mean titer in the low-dose group, 95.7; 95% CI, 65 to 142; P=0.03 by the two-tailed Mann–Whitney test) (Fig. 1D). (Additional details regarding neutralizing antibodies and IgG antibodies are provided in Fig. S2 and S3 in the Supplementary Appendix.)
The dose of ChAd3 or MVA vaccine had no significant effect on post-boost IgG titers, nor did the interval between priming and boosting vaccinations affect the magnitude of the antibody response (r=0.20, P=0.30) (Fig. S3C in the Supplementary Appendix). However, there was a significant positive correlation between the prime–boost interval and the neutralizing antibody titer, regardless of the MVA dose (r=0.72, P<0.001) (Fig. S3D in the Supplementary Appendix). Antibody induction to the Sudan Ebola virus glycoprotein was assessed, but as expected in the absence of priming with this antigen, antibody titers against the Sudan virus glycoprotein were not detected after administration of the prime vaccine, which suggests a lack of cross-reactivity to the Sudan strain with antibodies raised against the ZEBOV glycoprotein. However, after boosting with the MVA vaccine (which expresses a Sudan Ebola virus glycoprotein), IgG titers increased significantly (geometric mean titer before boosting, 0.1; 95% CI, 0.07 to 0.2; geometric mean titer 14 days after boosting, 1.5; 95% CI, 1.1 to 2.0; P<0.001 by the Friedman test) (Fig. S3F in the Supplementary Appendix).
Cell-Mediated Immunity Induced by Vaccination
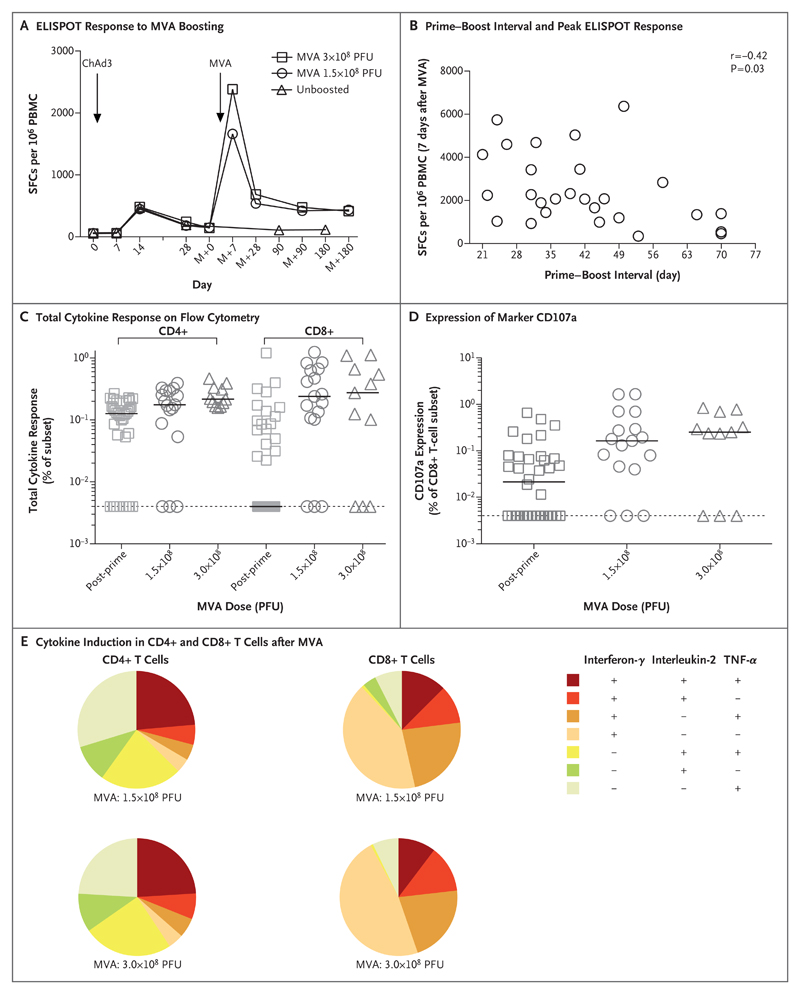
ELISPOT responses peaked 7 days after boosting with MVA at a median of 2068 spot-forming cells (SFCs) (interquartile range, 1197 to 3447) per million PBMCs and were significantly higher than peak responses after prime vaccination at 14 days (SFCs, 633; interquartile range, 274 to 820; P<0.001 by the two-tailed Wilcoxon matched-pairs test). Responses were maintained at 180 days after boosting (SFCs, 498; interquartile range, 207 to 905) and were significantly higher than non-boosted responses (SFCs, 84; interquartile range, 50 to 192; P<0.001 by the Mann–Whitney test) (Fig. 2A). There was a modest negative correlation between the prime–boost interval and the peak ELISPOT response (r=−0.42, P=0.03 by two-tailed Pearson’s correlation coefficient) (Fig. 2B). However, there was no significant relationship between the magnitude of the antibody response and the T-cell response (Spearman’s correlation coefficient, 0.17; P=0.39).
Figure 2. T-Cell Responses and Induction of Cytokines after Boosting with MVA.
Panel A shows the median T-cell responses to ChAd3 vaccination and MVA boosting on enzyme-linked immunosorbent spot (ELISPOT) assay at all time points, as measured in spot-forming cells (SFCs) per million peripheral-blood mononuclear cells (PBMCs). The dose of MVA is indicated in plaque-forming units (PFU). Panel B shows the relationship between the prime–boost interval and the peak ELISPOT response 7 days after MVA vaccination, as calculated by means of a two-tailed Spearman’s test. Panel C shows the total cytokine response on flow cytometry with intracellular cytokine staining at 28 days after priming (post-prime) or 7 days after boosting, according to the MVA dose. The secretion of interferon-γ, interleukin-2, and tumor necrosis factor α (TNF-α) by CD4+ and CD8+ T cells was quantified for each booster-dose group and expressed as the frequency of cells expressing any one of the three cytokines. Panel D shows the expression of the degranulation marker CD107a 28 days after priming or 7 days after boosting. In Panels C and D, the solid horizontal lines indicate median values, and the dashed horizontal lines indicate the positive threshold. Panel E shows the proportions of CD4+ and CD8+ T cells that secreted any combination of interferon-γ, interleukin-2, and TNF-α after stimulation with two different MVA doses.
Intracellular cytokine staining revealed that all participants had positive CD4+ and CD8+ interferon-γ T-cell responses after boosting. The median frequency of CD4+ T cells secreting interferon-γ, interleukin-2, or tumor necrosis factor α (TNF-α) increased from 0.13% (interquartile range, 0.004 to 0.19) at 14 days after prime vaccination to 0.20% (interquartile range, 0.15 to 0.31) at 7 days after MVA boosting (P<0.001 by the Kruskal–Wallis test) (Fig. 2C). The increase in the median frequency of cytokine-secreting CD8+ T cells was even more pronounced, from 0.004% (interquartile range, 0.004 to 0.09) at 14 days after prime vaccination to 0.25% (interquartile range, 0.10 to 0.65) at 7 days after MVA boosting (P<0.001 by the Kruskal–Wallis test).
The expression of the degranulation marker CD107a on CD8+ T cells increased by a factor of 5 after boosting (P=0.003 by the Kruskal–Wallis test) (Fig. 2D). The MVA dose had no significant effect on the magnitude of the T-cell response as measured by means of ELISPOT (Fig. 2A) or intracellular cytokine staining (Fig. 2C and 2D). Analysis of polyfunctionality confirmed the dominance of TNF-α–secreting CD4+ T cells over cells secreting either interferon-γ or interleukin-2 (Fig. 2E). Cells that were positive only for interferon-γ and double-positive cells secreting interferon-γ and TNF-α (with the latter being associated with protection in macaques17) were the largest subgroups in the CD8+ T-cell response (Fig. 2E).
Short-Interval Boosting
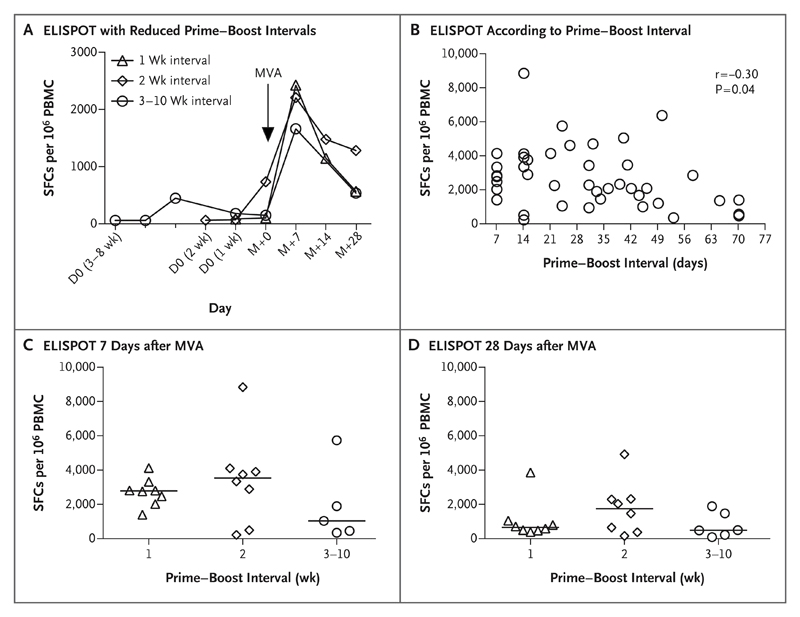
Given the pivotal role that has been shown for T cells in preclinical efficacy studies in macaques and the finding of high T-cell and antibody immunogenicity even with the shortest prime–boost intervals, we assessed the effect of reducing the prime–boost interval further to either 1 week or 2 weeks in two groups of eight participants each. ELISPOT responses in the two groups still peaked at 7 days after boosting (Fig. 3A). We observed a modest negative correlation between the prime–boost interval and peak T-cell immunogenicity among all participants (r=−0.30, P=0.04 by two-tailed Spearman’s correlation coefficient) (Fig. 3B). In a comparison of the median ELISPOT response in groups that received the MVA booster dose at an interval of 1 week, 2 weeks, or 3 to 10 weeks after the priming vaccination, there were no significant between-group differences at either 7 days or 28 days after the MVA dose (Fig. 3C and 3D).
Figure 3. Effect of Reduced Prime–Boost Intervals on Cellular Immunogenicity.
Panel A shows the results of reducing the interval between prime vaccination with ChAd3 and booster vaccination with MVA to either 1 week or 2 weeks (as compared with 3 to 10 weeks) in two groups of eight participants each. The results are shown as median T-cell responses on ELISPOT assay, as measured in SFCs per million PBMCs. D0 indicates the beginning of the prime–boost interval for each group. Responses in all three groups peaked at 7 days after boosting, regardless of the prime–boost interval. Panel B shows the relationship between the prime–boost interval and the peak ELISPOT response 7 days after boosting, with a modest negative correlation between the prime–boost interval and peak T-cell immunogenicity. Also shown are individual ELISPOT responses to summed glycoprotein peptide pools at 7 days (Panel C) and 28 days (Panel D) after boosting; no significant differences between the groups were seen at either 7 days or 28 days. The black horizontal lines indicate median values. In these analyses, all the participants received 2.5×1010 viral particles of the ChAd3 vaccine and a booster dose of 1.5×108 PFU of MVA.
An analysis of antibody responses showed that reducing the prime–boost interval resulted in a decrease in the peak IgG titer after the MVA dose (P<0.05 for all comparisons) (Fig. S4A in the Supplementary Appendix). Additional cellular and humoral immunologic analyses are described in the Supplementary Appendix.
Discussion
The boosting ability of MVA, particularly to enhance T-cell responses, has been described pre-clinically and clinically for vaccine candidates targeting several diseases18–22; however, data on boosting of virus-specific human neutralizing antibodies are lacking. In our study, we assessed such boosting ability with respect to the ChAd3 vaccine, a relatively immunogenic priming agent, and report large enhancements of antibody and T-cell immunogenicity. We found induction of human neutralizing antibodies to Ebola virus at substantial titers by boosting, which correlated with the overall increased magnitude of antibody titers on IgG ELISA assays. Antibodies to the Sudan strain of Ebola virus glycoprotein were also detected after boosting, albeit at a low level. The induction of a response to the Sudan strain is an important consideration for future outbreak control. We also found an acceptable safety profile for MVA at the two doses and at all intervals that we evaluated. We found that boosting can be immunogenic for antibodies and T cells at prime–boost intervals as short as 1 week. Such short-interval regimens may facilitate vaccine deployment in outbreak settings where both rapid onset and durable vaccine efficacy are required.
A single dose of the ChAd3 vaccine induced uniform protection shortly after vaccination and partial longer-term protection in macaques.11 In humans, the ChAd3 vaccine induced levels of anti-ZEBOV IgG and virus-neutralizing antibodies that were similar to the levels in the rVSV-ZEBOV ring vaccination study. Since no evidence of cellular immunogenicity has yet been reported for the rVSV-ZEBOV vaccine, these vectors probably induce different immune responses. The induction of more CD4+ T cells than CD8+ T cells after the administration of the ChAd3 vaccine was unexpected on the basis of preclinical studies of these vaccine vectors, but we found that the T-cell balance was reversed to greater levels of CD8+ T cells after MVA boosting in humans. This increase in CD8+ T-cell levels may enhance the vaccine efficacy, since CD8+ T-cell depletion was found to reduce the efficacy of an adenovirus vaccine in macaques.13 The durability in protection that we observed with this regimen may result from help provided by CD4+ T cells. The cellular immunogenicity induced by the ChAd3 vaccine provides an additional potential mechanism to provide greater vaccine efficacy and durability than that provided by the rVSV-ZEBOV vaccine, although this hypothesis cannot be confirmed without an efficacy trial.
The ChAd3 and MVA viral vectors have a number of other practical advantages in that large-scale manufacturing processes concordant with Good Manufacturing Practice standards have been established and both vectors have been assessed in large numbers of vaccinees for a range of indications without reports of any substantial safety concerns to date.18,23–30 Nonreplicating viral vectors have shown a reasonable safety profile and may be preferred to replication-competent vectors for widespread use in populations at risk for undetected immunodeficiencies.
We also found antibody responses that remained positive 6 months after vaccination above a threshold associated with efficacy in humans. High-level durable efficacy is desirable for protecting populations against future epidemics and may be particularly important for high-risk populations such as health care workers. Single-dose vaccines may prove to be preferable for logistic simplicity if just short-term efficacy is required in outbreak settings. However, we found that a 1-week interval between the administration of the prime vaccine and the booster vaccine provided CD8+ T-cell immunogenicity just 2 weeks after the prime dose. We also found higher antibody responses than with single-dose vaccination, even though such responses were lower than with longer prime–boost intervals. Taken together, these data provide a basis for consideration of particular vectored vaccine regimens for use in either prevention or control of an outbreak.
Supplementary Material
A preliminary version of this article was published on January 28, 2015, at NEJM.org.
Acknowledgments
Supported by the Wellcome Trust, the United Kingdom Medical Research Council, the United Kingdom Department for International Development, and the United Kingdom National Institute for Health Research Oxford Biomedical Research Centre. The National Health Service Blood and Transplant and Public Health England provided funding for the competition ELISA. The ChAd3 vaccine was provided by the Vaccine Research Center of the National Institute of Allergy and Infectious Diseases (NIAID) and GlaxoSmithKline. MVA-BN Filo was produced under a contract (FBS-004-009) between the NIAID and Fisher BioServices and a contract (HHSN272200800044C) between the National Institutes of Health and Fisher Bioservices.
Appendix
The authors’ full names and academic degrees are as follows: Katie Ewer, Ph.D., Tommy Rampling, M.R.C.P., Navin Venkatraman, M.R.C.P., Georgina Bowyer, B.A., Danny Wright, M.Sc., Teresa Lambe, Ph.D., Egeruan B. Imoukhuede, M.D., Ruth Payne, M.R.C.P., Sarah Katharina Fehling, Ph.D., Thomas Strecker, Ph.D., Nadine Biedenkopf, Ph.D., Verena Krähling, Ph.D., Claire M. Tully, B.A., Nick J. Edwards, B.Sc., Emma M. Bentley, B.Sc., Dhanraj Samuel, Ph.D., Geneviève Labbé, Ph.D., Jing Jin, Ph.D., Malick Gibani, M.R.C.P., Alice Minhinnick, M.B., Ch.B., Morven Wilkie, M.R.C.P., Ian Poulton, Dip.H.E., Natalie Lella, B.A., Rachel Roberts, M.Sc., Felicity Hartnell, M.B., B.S., Carly Bliss, B.A., Kailan Sierra-Davidson, B.A., Jonathan Powlson, B.Sc., Eleanor Berrie, Ph.D., Richard Tedder, M.B., B.Chir., Francois Roman, M.D., Iris De Ryck, Ph.D., Alfredo Nicosia, Ph.D., Nancy J. Sullivan, Ph.D., Daphne A. Stanley, M.S., Olivier T. Mbaya, M.D., Julie E. Ledgerwood, D.O., Richard M. Schwartz, Ph.D., Loredana Siani, Ph.D., Stefano Colloca, Ph.D., Antonella Folgori, Ph.D., Stefania Di Marco, Ph.D., Riccardo Cortese, M.D., Edward Wright, Ph.D., Stephan Becker, Ph.D., Barney S. Graham, M.D., Richard A. Koup, M.D., Myron M. Levine, M.D., Ariane Volkmann, Ph.D., Paul Chaplin, Ph.D., Andrew J. Pollard, Ph.D., Simon J. Draper, D.Phil., W. Ripley Ballou, M.D., Alison Lawrie, Ph.D., Sarah C. Gilbert, Ph.D., and Adrian V.S. Hill, D.M.
The authors’ affiliations are as follows: the Jenner Institute and Centre for Clinical Vaccinology and Tropical Medicine, University of Oxford, and the National Institute for Health Research Oxford Biomedical Research Centre, Oxford (K.E., T.R., N.V., G.B., D.W., T.L., E.B.I., R.P., C.M.T., N.J.E., G.L., J.J., M.G., A.M., M.W., I.P., N.L., R.R., F.H., C.B., K.S.-D., J.P., E.B., A.J.P., S.J.D., A.L., S.C.G., A.V.S.H.), and Viral Pseudotype Unit, Faculty of Science and Technology, University of Westminster (E.M.B., E.W.), and Virus Reference Department, Public Health Agency (D.S., R.T.), London — all in the United Kingdom; the Institute of Virology, Philipps University Marburg (S.K.F., T.S., N.B., V.K., S.B.), and German Center for Infection Research, Partner Site Giessen–Marburg–Langen (S.B.), Marburg, and Bavarian Nordic, Martinsried (A.V., P.C.) — all in Germany; GlaxoSmithKline Biologicals, Rixensart, Belgium (F.R., I.D.R., W.R.B.); ReiThera, Rome (A.N., L.S., S.C., A.F., S.D.M.), and CEINGE and the Department of Molecular Medicine and Medical Biotechnology, University of Naples Federico II, Naples (A.N.) — both in Italy; Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda (N.J.S., D.A.S., O.T.M., J.E.L., R.M.S., B.S.G., R.A.K.), and the Center for Vaccine Development, University of Maryland School of Medicine, Baltimore (M.M.L.) — both in Maryland; and Keires, Basel, Switzerland (R.C.).
Footnotes
The views expressed in this article are those of the authors and do not necessarily represent the position or policies of the World Health Organization (WHO).
We thank the members of the data and safety monitoring board: Peter Smith (chair), George Griffin, Saul Faust, Brian Angus, Kwadwo Koram, Kalifa Bojang, and Mahamodou Thera; the Wellcome Trust award scientific advisory board: Brian Greenwood (chair), Peter Piot, Pontiano Kaleebu, Allan Saul, Paul Kaye, Charlie Weller, and Morven Roberts; John Mascola of the National Institutes of Health, Marie-Paul Kieny of the WHO, Samba Sow of the Center for Vaccine Development of Mali, and Umberto Dalessandro of the Medical Research Council of Gambia for their advice; Carly Banner and Geoff Lees for arranging contracts; Xiangguo Qiu and Gary Kobinger of the Special Pathogens Program, National Microbiology Laboratory, Public Health Agency of Canada, for provision of anti-4G7 purified IgG used in the competition ELISA; Steve Dicks and Lisa Hill for technical assistance with the competition ELISA; Michael Schmidt and Gotthard Ludwig, University of Marburg, for technical support in biosafety level 4; the Medicines and Healthcare Products Regulatory Agency and the Oxfordshire research ethics committee for exceptionally rapid review; and Vasee Moorthy for serving as the study WHO liaison.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Zhu FC, Hou LH, Li JX, et al. Safety and immunogenicity of a novel recombinant adenovirus type-5 vector-based Ebola vaccine in healthy adults in China: preliminary report of a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet. 2015;385:2272–9. doi: 10.1016/S0140-6736(15)60553-0. [DOI] [PubMed] [Google Scholar]
- 2.Kibuuka H, Berkowitz NM, Millard M, et al. Safety and immunogenicity of Ebola virus and Marburg virus glycoprotein DNA vaccines assessed separately and concomitantly in healthy Ugandan adults: a phase 1b, randomised, double-blind, placebo-controlled clinical trial. Lancet. 2015;385:1545–54. doi: 10.1016/S0140-6736(14)62385-0. [DOI] [PubMed] [Google Scholar]
- 3.Regules JA, Beigel JH, Paolino KM, et al. A recombinant vesicular stomatitis virus Ebola vaccine — preliminary report. N Engl J Med. doi: 10.1056/NEJMoa1414216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agnandji ST, Huttner A, Zinser ME, et al. Phase 1 trials of rVSV Ebola vaccine in Africa and Europe. N Engl J Med. 2016;374:1647–60. doi: 10.1056/NEJMoa1502924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ledgerwood JE, DeZure AD, Stanley DA, et al. Chimpanzee adenovirus vector Ebola vaccine — preliminary report. N Engl J Med. doi: 10.1056/NEJMoa1410863. [DOI] [PubMed] [Google Scholar]
- 6.Rampling T, Ewer K, Bowyer G, et al. A monovalent chimpanzee adenovirus Ebola vaccine — preliminary report. N Engl J Med. doi: 10.1056/NEJMoa1411627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henao-Restrepo AM, Longini IM, Egger M, et al. Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomised trial. Lancet. 2015;386:857–66. doi: 10.1016/S0140-6736(15)61117-5. [DOI] [PubMed] [Google Scholar]
- 8.Sullivan NJ, Martin JE, Graham BS, Nabel GJ. Correlates of protective immunity for Ebola vaccines: implications for regulatory approval by the animal rule. Nat Rev Microbiol. 2009;7:393–400. doi: 10.1038/nrmicro2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones SM, Feldmann H, Ströher U, et al. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat Med. 2005;11:786–90. doi: 10.1038/nm1258. [DOI] [PubMed] [Google Scholar]
- 10.Geisbert TW, Geisbert JB, Leung A, et al. Single-injection vaccine protects nonhuman primates against infection with Marburg virus and three species of Ebola virus. J Virol. 2009;83:7296–304. doi: 10.1128/JVI.00561-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stanley DA, Honko AN, Asiedu C, et al. Chimpanzee adenovirus vaccine generates acute and durable protective immunity against ebolavirus challenge. Nat Med. 2014;20:1126–9. doi: 10.1038/nm.3702. [DOI] [PubMed] [Google Scholar]
- 12.Fausther-Bovendo H, Mulangu S, Sullivan NJ. Ebolavirus vaccines for humans and apes. Curr Opin Virol. 2012;2:324–9. doi: 10.1016/j.coviro.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sullivan NJ, Hensley L, Asiedu C, et al. CD8+ cellular immunity mediates rAd5 vaccine protection against Ebola virus infection of nonhuman primates. Nat Med. 2011;17:1128–31. doi: 10.1038/nm.2447. [DOI] [PubMed] [Google Scholar]
- 14.Qiu X, Alimonti JB, Melito PL, Fernando L, Ströher U, Jones SM. Characterization of Zaire ebolavirus glycoprotein-specific monoclonal antibodies. Clin Immunol. 2011;141:218–27. doi: 10.1016/j.clim.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Enama ME, Novik L, Hendel CS, Sheets R, Ledgerwood JE, Graham BS. Induction of false-positive PTT elevations by investigational adenoviral vector vaccines. Presented at the 14th Annual Conference on Vaccine Research; Baltimore. May 16–18, 2011. [Google Scholar]
- 16.Malaeb BS, Gardner TA, Margulis V, et al. Elevated activated partial thromboplastin time during administration of first-generation adenoviral vectors for gene therapy for prostate cancer: identification of lupus anticoagulants. Urology. 2005;66:830–4. doi: 10.1016/j.urology.2005.04.041. [DOI] [PubMed] [Google Scholar]
- 17.Zhou Y, Sullivan NJ. Immunology and evolvement of the adenovirus prime, MVA boost Ebola virus vaccine. Curr Opin Immunol. 2015;35:131–6. doi: 10.1016/j.coi.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Ewer KJ, O’Hara GA, Duncan CJ, et al. Protective CD8+ T-cell immunity to human malaria induced by chimpanzee adenovirus-MVA immunisation. Nat Commun. 2013;4:2836. doi: 10.1038/ncomms3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swadling L, Capone S, Antrobus RD, et al. A human vaccine strategy based on chimpanzee adenoviral and MVA vectors that primes, boosts, and sustains functional HCV-specific T cell memory. Sci Transl Med. 2014;6:261ra153. doi: 10.1126/scitranslmed.3009185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antrobus RD, Lillie PJ, Berthoud TK, et al. A T cell-inducing influenza vaccine for the elderly: safety and immunogenicity of MVA-NP+M1 in adults aged over 50 years. PLoS One. 2012;7(10):e48322. doi: 10.1371/journal.pone.0048322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borthwick NJ, Rosario M, Schiffner T, et al. Humoral responses to HIVconsv induced by heterologous vaccine modalities in rhesus macaques. Immun Inflamm Dis. 2015;3:82–93. doi: 10.1002/iid3.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tapia MD, Sow SO, Lyke KE, et al. Use of ChAd3-EBO-Z Ebola virus vaccine in Malian and US adults, and boosting of Malian adults with MVA-BN-Filo: a phase 1, single-blind, randomised trial, a phase 1b, open-label and double-blind, dose-escalation trial, and a nested, randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2016;16:31–42. doi: 10.1016/S1473-3099(15)00362-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borthwick N, Ahmed T, Ondondo B, et al. Vaccine-elicited human T cells recognizing conserved protein regions inhibit HIV-1. Mol Ther. 2014;22:464–75. doi: 10.1038/mt.2013.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barnes E, Folgori A, Capone S, et al. Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Sci Transl Med. 2012;4:115ra1. doi: 10.1126/scitranslmed.3003155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Hara GA, Duncan CJ, Ewer KJ, et al. Clinical assessment of a recombinant simian adenovirus ChAd63: a potent new vaccine vector. J Infect Dis. 2012;205:772–81. doi: 10.1093/infdis/jir850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheehy SH, Duncan CJ, Elias SC, et al. Phase Ia clinical evaluation of the safety and immunogenicity of the Plasmodium falciparum blood-stage antigen AMA1 in ChAd63 and MVA vaccine vectors. PLoS One. 2012;7(2):e31208. doi: 10.1371/journal.pone.0031208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheehy SH, Duncan CJ, Elias SC, et al. ChAd63-MVA-vectored blood-stage malaria vaccines targeting MSP1 and AMA1: assessment of efficacy against mosquito bite challenge in humans. Mol Ther. 2012;20:2355–68. doi: 10.1038/mt.2012.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogwang C, Afolabi M, Kimani D, et al. Safety and immunogenicity of heterologous prime-boost immunisation with Plasmodium falciparum malaria candidate vaccines, ChAd63 ME-TRAP and MVA ME-TRAP, in healthy Gambian and Kenyan adults. PLoS One. 2013;8(3):e57726. doi: 10.1371/journal.pone.0057726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Barra E, Hodgson SH, Ewer KJ, et al. A phase Ia study to assess the safety and immunogenicity of new malaria vaccine candidates ChAd63 CS administered alone and with MVA CS. PLoS One. 2014;9(12):e115161. doi: 10.1371/journal.pone.0115161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogwang C, Kimani D, Edwards NJ, et al. Prime-boost vaccination with chimpanzee adenovirus and modified vaccinia Ankara encoding TRAP provides partial protection against Plasmodium falciparum infection in Kenyan adults. Sci Transl Med. 2015;7(286):286re5. doi: 10.1126/scitranslmed.aaa2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.