Abstract
Extended daytime and nighttime activities are major contributors to the growing sleep deficiency epidemic1,2, as is the high prevalence of sleep disorders like insomnia. The consequences of chronic insufficient sleep for health remain uncertain3. Sleep quality and duration predict presence of pain the next day in healthy subjects4–7, suggesting that sleep disturbances alone may worsen pain, and experimental sleep deprivation in humans supports this claim8,9. We demonstrate that sleep loss, but not sleep fragmentation, in healthy mice increases sensitivity to noxious stimuli (referred to as ‘pain’) without general sensory hyper-responsiveness. Moderate daily repeated sleep loss leads to a progressive accumulation of sleep debt and also to exaggerated pain responses, both of which are rescued after restoration of normal sleep. Caffeine and modafinil, two wake-promoting agents that have no analgesic activity in rested mice, immediately normalize pain sensitivity in sleep-deprived animals, without affecting sleep debt. The reversibility of mild sleep-loss-induced pain by wake-promoting agents reveals an unsuspected role for alertness in setting pain sensitivity. Clinically, insufficient or poor-quality sleep may worsen pain and this enhanced pain may be reduced not by analgesics, whose effectiveness is reduced, but by increasing alertness or providing better sleep.
At least 20% of the population in Europe and the United States suffers from chronic pain10. Unalleviated pain disrupts sleep, and, as clinical data suggest that sleep and pain interact8,11, this loss of sleep may worsen pain. In individuals with burn injuries, insomnia at the time of discharge is a predictor for the development of chronic pain12. In individuals with fibromyalgia, self-reported nonrestorative sleep is associated with more pain whereas restorative sleep resolves pain13. Both sleep–wake patterns and pain sensitivity are influenced by heritable traits14,15, stress16,17 and the environment18,19, making human experimental protocols that control for these factors almost impossible to achieve. To circumvent this, we studied the relationship between sleep loss and pain in inbred C57BL/6J mice instrumented for polysomnographic recordings (electroencephalogram, EEG; electromyogram, EMG) in a controlled environment.
Protocols previously used to chronically sleep deprive rodents typically involve either prolonged forced activity and limited sleep opportunity20,21 or selective rapid-eye-movement sleep (REMS) deprivation22,23. Although these proceures change pain thresholds22,23, the confounding effects of stress make interpretation of the results difficult24.
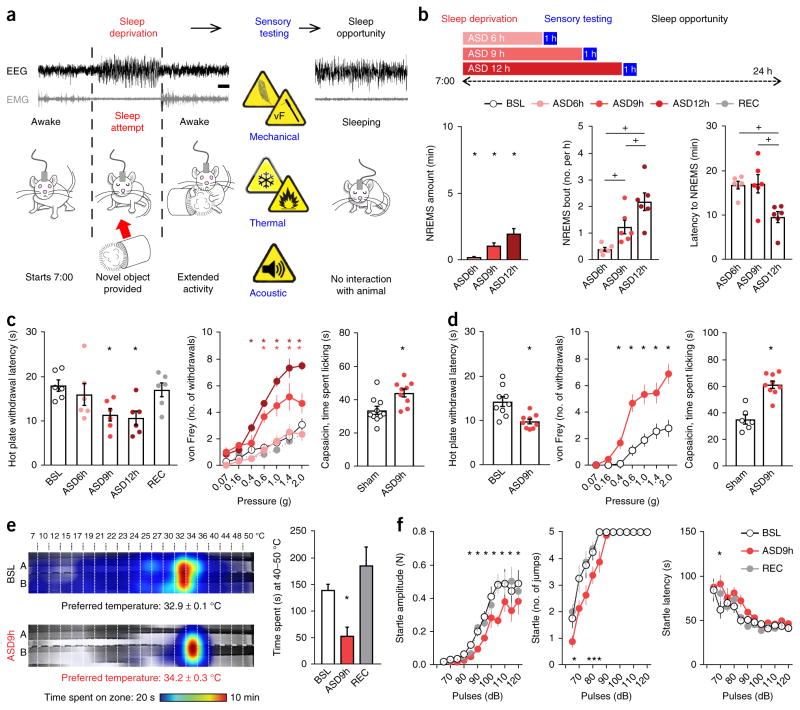
To deprive mice of sleep in a nonstressful way, we continuously monitored the EEGs and EMGs of the mice and, just as non-REMS (NREMS) began (a ‘sleep attempt’), we placed a single novel object in the cage without touching the animal (Fig. 1a, Online Methods and Supplementary Fig. 1a). Using this protocol, the average total time in NREMS was reduced by 98 ± 0.4% (Fig. 1b) and REMS was eliminated in all mice. With longer periods of sleep deprivation (12 h), sleep pressure was higher, and mice made more attempts to enter NREMS and had a shorter latency period before sleep when allowed to sleep undisturbed (Fig. 1b and Supplementary Fig. 1b). We performed a ‘dose-response’ analysis of this acute sleep deprivation (ASD), testing pain sensitivity immediately after 6, 9 or 12 h of deprivation, using a range of standard thermal and mechanical stimuli (Fig. 1b).
Figure 1.
Acute sleep deprivation progressively increases sleepiness and sensitivity to pain. (a) Schematic representation of the sleep deprivation protocol: an experimenter continuously monitored EEG and EMG of the mice and placed a novel object in their cage to promote arousal whenever an NREMS entry (sleep attempt) was detected. Sensory testing (in blue) took place immediately after the sleep deprivation period; mice were then allowed to sleep ad libitum (sleep opportunity). Scale bar, 5 s. (b) Top, ASD was carried out for 6, 9 or 12 h starting at 7:00. Left, NREMS amount expressed as percentage of corresponding baseline values. Middle, the number of NREMS entries (bouts lasting at least 5 s). Right, latency to entering NREMS upon return of the mice to their home cage (n = 6 mice). BSL, assessment at baseline; REC, assessment after a night of recovery sleep. (c) Sensory assessment in male mice. Left, latency to withdrawal from contact with a 52 °C hot plate (n = 6 mice). Middle, mechanical sensitivity measured as the number of brisk withdrawals from ten stimulations with von Frey filaments of different forces applied to the plantar surface of the hindpaw (n = 6 mice). Right, nocifensive response (time spent licking) after intraplantar injection of capsaicin in male mice (1 μg diluted in 20 μl of saline with 1% DMSO; n = 10 sham-treated mice and 9 ASD mice). (d) Sensory assessment in female mice. Left, latency to withdrawal from contact with a 52 °C hot plate (n = 9 mice). Middle, mechanical sensitivity (n = 9 mice). Right, nocifensive response after intraplantar injection of capsaicin (1 μg diluted in 20 μl; n = 6 sham mice and 9 ASD mice). (e) Left, heat map of the time spent in the zones of a thermal gradient at baseline and after 9 h of ASD and the preferred temperature (n = 6 mice). When sleep deprived, mice preferred a slightly warmer temperature. Right, time spent in the noxious heat range (40–50 °C) at baseline, after 9 h of ASD and after recovery (n = 6 mice). (f) Left, intensity, measured as the force produced by a mouse upon jumping; middle, number of jumps; right, latency to jumping. Mice underwent the acoustic startle reflex test at baseline, after 9 h of ASD and after recovery (n = 8 mice). All data are presented as means ± s.e.m. Circles overlaid upon the bar in histograms represent data from each individual animal. *P < 0.05, in comparison to baseline; +P < 0.05, in multiple-group comparisons. For complete statistical analyses (post hoc test, within-subjects or inter-subjects comparison, effect size), please refer to Supplementary Table 1.
ASD in male mice for either 9 or 12 h increased the behavioral response to noxious heat and mechanical stimulation when compared to both the same mice at baseline and sham mice (instrumented but non-sleep-deprived animals), with a return to baseline after 24 h of recovery sleep (Fig. 1c and Supplementary Fig. 1c; for statistics, see Supplementary Table 1). In addition, 9 h of sleep deprivation increased nocifensive behavior (paw licking) after intraplantar injection of capsaicin, a TRPV1 agonist (Fig. 1c). Female C57BL/6J mice phenocopied males after 9 h of sleep deprivation (Fig. 1d and Supplementary Fig. 1d). In both sexes, ASD (9 and 12 h) increased the response only to relatively high mechanical force from von Frey filaments (0.4 to 2 g), which activate nociceptors (pain sensory neurons) in mice25; there was no change in the response to innocuous, low-force mechanical stimuli (0.07 and 0.16 g), which activate low-mechanothreshold fibers in mice (Fig. 1c)26. Responses to a gentle brush stimulus or cooling from an acetone drop were unaffected by sleep deprivation (Supplementary Fig. 1e). ASD for 9 h resulted in avoidance of temperature zones in the noxious heat range (40–50 °C) in an operant thermal gradient assay, with this activity resolving after one night of sleep recovery (Fig. 1e and Supplementary Fig. 1f).
To test whether the increased pain sensitivity produced by ASD reflected a general state of hyper-responsiveness, we measured the startle reflex evoked by a series of sounds of increasing power (Fig. 1f). Sleep deprivation for 9 h reduced startle reflex amplitudes (especially for high-intensity pulses) without altering the probability of or latency to reaction, and amplitudes were restored to baseline levels after sleep recovery (Fig. 1f).
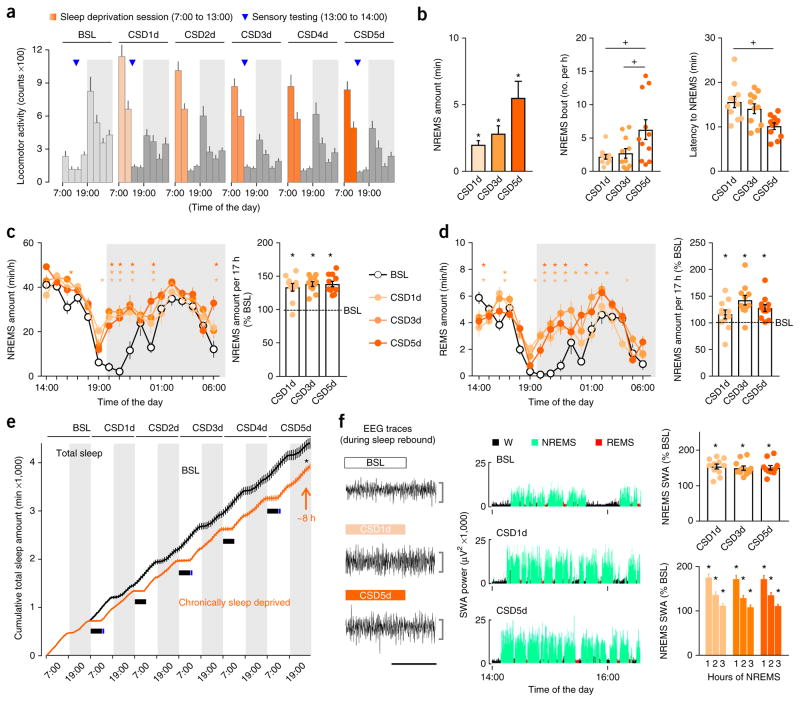
To extend these findings to persistent sleep deficiency, we developed a model of moderate chronic sleep deprivation (CSD). Mice were sleep deprived for 6 h a day, a duration that did not produce hyperalgesia with acute sleep deprivation (Fig. 1), by providing novel objects as before, but now repeating this over five consecutive days and beginning the protocol each day at light onset (7:00). This protocol extended the active period of the mice (Fig. 2a), yet plasma corticosterone levels remained normal, indicating that the mice experienced no stress (Supplementary Fig. 2a). Locomotor activity was greater during each sleep deprivation session than at baseline owing to exploration of the novel objects, but the overall daily activity pattern was conserved between periods of sleep deprivation and the baseline (Fig. 2a). CSD caused a marked decrease in the NREMS amount as compared to the baseline (98% less NREMS with 1 d of CSD and 95% less NREMS with 5 d of CSD; Fig. 2b) and resulted in no REMS. Over the 5 d of sleep deprivation, sleep pressure gradually increased, as indicated by more attempts to enter NREMS during the period of sleep deprivation and a shorter latency to fall asleep at the onset of the sleep opportunity period (at 14:00; Fig. 2b and Supplementary Fig. 2b; for statistics, see Supplementary Table 1).
Figure 2.
Chronic sleep deprivation progressively increases sleepiness. (a) Locomotor activity of mice (3-h intervals) recorded with telemetry throughout CSD. On each day of CSD (CSD1d–CSD5d), mice underwent sleep deprivation (7:00–13:00; orange shading) followed by sleep opportunity periods (14:00–7:00, 17 h total; gray). Sensory testing (13:00; blue triangle) was performed for 1 h at baseline, CSD1d, CSD3d and CSD5d. Periods of darkness (19:00–7:00) are represented as boxes shaded in light gray. (b) Left, NREMS amount expressed as percentage of corresponding baseline values. Middle, number of NREMS entries during sleep deprivation sessions. Right, latency to NREMS onset at the beginning of the sleep opportunity period. (c) Hourly amount of NREMS and rebound sleep computed over the entire sleep opportunity period from 14:00 to 7:00, expressed as percentage of the time-matched baseline amount of NREMS. (d) Hourly amount of REMS and rebound sleep computed over the entire sleep opportunity period from 14:00 to 7:00, expressed as percentage of the time-matched baseline amount of REMS. (e) Cumulative time course of the total sleep amount (summed in 1-h bins) at baseline (black) and on CSD5d (orange). The control curve was generated by cumulatively adding the individual curve obtained at baseline six times. The absence of sleep during each 6-h sleep deprivation session is represented by a solid black rectangle. The net estimated total sleep loss at the end of 5 h of CSD was 7.9 ± 1.5 h. (f) Left, example EEGs of NREMS. Middle, time course of SWA (spectral EEG power in the 0.5- to 4.0-Hz range) during the first 2.5 h of sleep opportunity at baseline, CSD1d and CSD5d in one representative mouse. W, wake. Top right, NREMS SWA computed over 5 h (14:00–19:00) expressed as percentage of the 24-h baseline mean. Bottom right, time course of NREMS SWA during the first 3 h of NREMS after sleep deprivation. Horizontal scale bar, 10 s; vertical scale bar, 500 μV. Data are presented as means ± s.e.m. For all panels, n = 11 mice. Circles overlaid upon the bar in histograms represent data from each individual animal. *P < 0.05, in comparison to baseline; +P < 0.05, in multiple-group comparisons. For complete statistical analyses (post hoc test, within-subjects or inter-subjects comparison, effect size), please refer to Supplementary Table 1.
Prolonged wakefulness is followed by rebound sleep, a homeostatic increase in both NREMS and REMS27. Despite the cumulative increase in sleep pressure produced by CSD (Fig. 2b), the mice displayed a similar degree of rebound NREMS and REMS sleep during the sleep opportunity period (14:00–7:00) on days 1, 3 and 5 (Fig. 2c,d), such that the amount of sleep each day did not fully recover to baseline, leading to a cumulative net sleep deficit of 7.9 ± 1.5 h by the end of the 5 d (Fig. 2e and Supplementary Fig. 2c–e). Another marker of sleep homeostasis is increased slow-wave (0.5- to 4.0-Hz) EEG activity (SWA) during NREMS, an indicator of sleep debt and intensity28. Spectral analysis showed that NREMS SWA increased by about ~50% over baseline (measured from 14:00–19:00), with an equivalent buildup and resolution within NREMS episodes on days 1 and 5 of CSD (Fig. 2f and Supplementary Fig. 2f), indicating similar transitions into deeper sleep in the sleep-deprived mice during sleep opportunities.
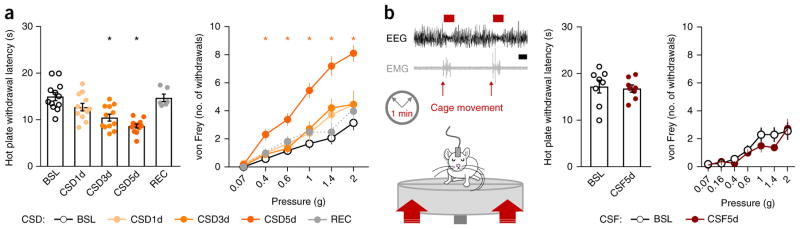
The 5 d of sleep deprivation gradually increased heat-induced pain sensitivity in comparison to mice at baseline or sham mice, with maximal effect on day 5 (Fig. 3a and Supplementary Fig. 3a; for statistics, see Supplementary Table 1). By the fifth day of sleep deprivation, mechanical sensitivity to intense von Frey stimuli also increased (Fig. 3a), whereas responses to non-noxious mechanical stimuli (gentle brush or low intensity von Frey filaments) were unaffected (Supplementary Fig. 3b). Because repeated sensory testing in control mice did not change their pain sensitivity (Supplementary Fig. 3c), and both heat and mechanical pain responses returned to baseline after a recovery period (ad libitum sleep) of 2 d (Fig. 3a), we conclude that cumulative insufficient sleep caused the buildup in pain hypersensitivity observed in the sleep-deprived mice.
Figure 3.
Chronic sleep deprivation but not chronic sleep fragmentation progressively increases pain sensitivity. (a) Left, latency to withdrawal from noxious heat contact (52 °C hot plate). Right, mechanical sensitivity measured as the number of brisk withdrawals from ten stimulations with von Frey filaments of different forces that were applied to the plantar surface of the hindpaw. Mice were tested at baseline, CSD1d, CSD3d, CSD5d and on recovery (n = 12 mice). (b) Left, schematic representation of the CSF protocol and a representative EEG trace of fragmented sleep. A platform below the cage briefly (10–20 ms) moved upward at a random intensity every minute from 7:00 to 19:00 over five consecutive days. Mice were tested for sensory responsiveness at baseline and CSF5d. Middle, latency to withdrawal from heat contact (52 °C hot plate). Right, mechanical sensitivity. n = 8 mice; nonsignificant Student’s t-test. Scale bar, 10 s. Data are presented as means ± s.e.m. Circles overlaid upon the bar in histograms represent data from each individual animal. *P < 0.05, in comparison to baseline. For complete statistical analyses (post hoc test, within-subjects or inter-subjects comparison, effect size), please refer to Supplementary Table 1.
Arousal from sleep can reduce sleep quality. To determine whether chronic (over 5 d) sleep fragmentation altered pain sensitivity in the mice, we used a device that produces a fast but moderate upward motion of the home cage every minute, waking the animal with limited stress and no forced locomotor activity (Supplementary Fig. 3d). This intervention reduced the mean duration of NREMS episodes by ~60% and the total REMS amount by ~65% but did not change sensitivity to noxious thermal or mechanical stimuli (Fig. 3b and Supplementary Fig. 3c; for statistics, see Supplementary Table 1).
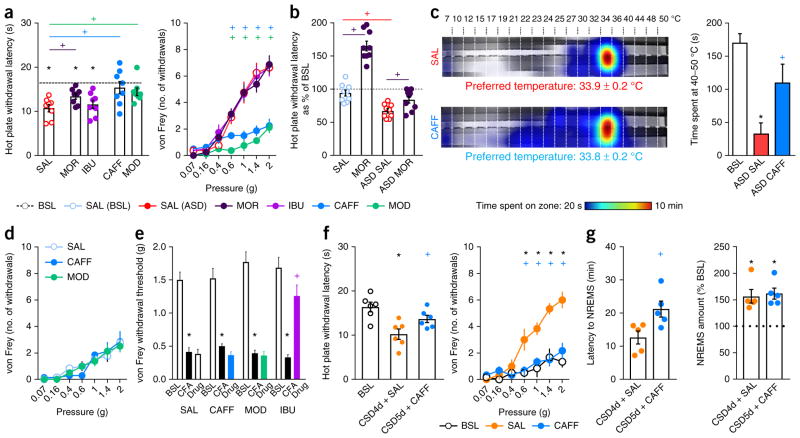
To investigate the mechanisms responsible for this sleep-loss-induced hyperalgesia, we first tested whether cyclooxygenases (COXs) contributed; prostanoids produced from arachidonic acid by these enzymes during inflammation are sensitizers of nociceptors and are linked to spontaneous pain after sleep restriction29. We found that administration of the nonselective COX inhibitor ibuprofen (30 mg per kg bodyweight, subcutaneously (s.c.)) 30 min before the end of a 9-h period of ASD did not alter heat or mechanical hypersensitivity in sleep-deprived mice (Fig. 4a). Next, we administered morphine (5 mg per kg bodyweight, s.c.; a dose that causes analgesia to heat but not to mechanical stimuli in naive mice; Supplementary Fig. 4a) after 9 h of sleep deprivation and found only a slight reduction in the heat hypersensitivity caused by the sleep loss but no change in hypersensitivity to mechanically induced pain (Fig. 4a). Overall, the analgesic effects of morphine were strongly reduced in sleep-deprived mice (Fig. 4b).
Figure 4.
Caffeine and modafinil, but not ibuprofen or morphine, prevent sleep-deprivation-induced hypersensitivity to pain. (a) Left, latency to withdrawal from noxious heat contact (52 °C hot plate). Right, mechanical sensitivity measured as the number of brisk withdrawals from ten stimulations with von Frey filaments of different forces that were applied to the plantar surface of the hindpaw. Mice were sleep-deprived for 9 h (ASD9h) and injected with saline (SAL, i.p.; red), morphine (5 mg per kg bodyweight, s.c.; dark purple), ibuprofen (IBU, 30 mg per kg bodyweight, s.c.; light purple), caffeine (CAFF, 20 mg per kg bodyweight, i.p.; blue) or modafinil (MOD, 45 mg per kg bodyweight, i.p.; green). Morphine and ibuprofen were administered 30 min before the end of the 9-h ASD period, and caffeine and modafinil were injected 2 h before the end of the 9-h ASD period. n = 8 mice per group. (b) Withdrawal latencies to noxious heat (expressed as percentage of the baseline) of mice treated with saline or morphine (5 mg per kg bodyweight, s.c.) after ad libitum undisturbed sleep and 9 h of sleep deprivation (n = 8 mice per group). (c) Left, heat map of the time spent in the zones of a thermal gradient showing thermal place preference and the calculated preferred temperature. Right, time spent in the noxious heat range (40–50 °C). Sleep-deprived mice were injected with either caffeine (20 mg per kg bodyweight, i.p.) or saline (i.p.) 2 h before the end of the 9-h ASD period (n = 6 mice per group). (d) Mechanical sensitivity in naive animals (ad libitum sleep) 2 h after caffeine (20 mg per kg bodyweight, i.p.), modafinil (45 mg per kg bodyweight, i.p.) or saline (i.p.) injection (n = 13 SAL-, 7 CAFF- and 8 MOD-injected mice). (e) Effects of caffeine, modafinil or ibuprofen on hypersensitivity to mechanically induced pain resulting from peripheral inflammation. Thresholds were assessed at baseline (pre-CFA), the day after intraplantar injection of CFA and 2 h after administration of saline, caffeine or modafinil (n = 8 SAL-, 8 CAFF- and 7 MOD-injected mice) and 30 min after administration of ibuprofen (n = 7 mice). (f) Left, withdrawal latency to contact with heat. Right, mechanical sensitivity. Mice undergoing CSD (6 h daily) were injected with saline (i.p.) or caffeine (20 mg per kg bodyweight, i.p.) on day 4 of CSD (n = 6 mice). (g) Latency to NREMS onset at the beginning of the sleep opportunity period (left) and NREMS rebound (right) following administration of saline on CSD4d and caffeine (20 mg per kg bodyweight, i.p.) on CSD5d computed over the entire sleep opportunity period from 14:00 to 7:00, expressed as percentage of corresponding baseline values (n = 5 mice). Data are presented as means ± s.e.m. Circles overlaid upon the bar in histograms represent data from each individual animal. *P < 0.05, in comparison to baseline; +P < 0.05, in multiple-group comparisons. For complete statistical analyses (post hoc test, within-subjects or inter-subjects comparison, effect size), please refer to Supplementary Table 1.
To test whether the hyperalgesia produced by sleep loss is related to decreased alertness, we examined the effects of two wake-promoting agents: the A1 and A2A adenosine receptor antagonist caffeine30 and modafinil, which promotes dopaminergic transmission31. The drugs were administered 2 h before the end of the 9-h ASD period at doses that promote wakefulness for about 3 h32 (caffeine, 20 mg per kg body weight; modafinil, 45 mg per kg bodyweight; intraperitoneally (i.p)). Both compounds increased alertness, as indicated by a decrease in the number of sleep attempts and an increased latency to NREMS upon return to the home cage (Supplementary Fig. 4b). During this period of alertness, both caffeine and modafinil blocked the sleep-deprivation- induced mechanical pain hypersensitivity and partially reduced heat induced pain hypersensitivity (Fig. 4a). Avoidance of noxious heat temperatures in the thermal gradient assay was also reduced by caffeine (Fig. 4c and Supplementary Fig. 4c). Caffeine did not normalize the decreased acoustic startle reflex in sleep-deprived mice, but did prevent the prepulse inhibition startle deficit that normally occurs after sleep deprivation33, suggesting an effect on sensorimotor gating (Supplementary Fig. 4d). In sham animals, neither caffeine nor modafinil affected nociceptive heat or mechanical sensitivity (Fig. 4d and Supplementary Fig. 4e) or inflammatory pain hypersensitivity after intraplantar injection of Complete Freund’s Adjuvant (CFA) (Fig. 4e and Supplementary Fig. 4f), indicating that the reduction in sleep-loss-induced hyperalgesia was not due to any intrinsic analgesic property of these compounds.
To test whether transiently increasing alertness is sufficient to reverse pain hypersensitivity caused by chronic (5-d) sleep deprivation, we administered a single dose of caffeine (20 mg per kg bodyweight, i.p.) at 11:00 on the last day of CSD. This treatment blocked both sleepiness and pain hypersensitivity (Fig. 4f and Supplementary Fig. 4g), but it did not change the sleep debt, as indicated by maintenance of a robust NREMS rebound during the sleep opportunity period (Fig. 4g). Therefore, transient restoration of alertness is sufficient to also counteract the manifestation of pain sensitivity in response to CSD.
Nonstressful sleep deprivation increases sensitivity to the pain caused by thermal, mechanical and chemical stimuli in mice of both sexes, similar to changes observed in humans after one or two nights of sleep deprivation9,34–36. The analgesia resulting from caffeine and modafinil administration in sleep-deprived mice suggest that the decline in alertness resulting from the sleep deprivation is a major contributor to the pain hypersensitivity in these animals. Although these compounds act on different molecular targets, they both facilitate dopaminergic transmission37–39. Sleep deprivation downregulates D2 and D3 receptors—caffeine can overcome this by upregulating their expression40,41 and by increasing D2R binding42, and modafinil inhibits the dopamine transporter39. Dopamine in the mesolimbic system modulates the salience of pain stimuli43, and reduced dopaminergic transmission is associated with increased pain in healthy subjects44 and individuals with fibromyalgia45. A reduction in dopamine signaling produced by sleep deprivation may therefore be a driver of pain hypersensitivity.
The amplification of pain in response to sleep deprivation, and its reversal by increasing alertness, reveals a new class of ‘state-dependent analgesics’: drugs that do not target pain but instead target biological processes that exacerbate it. Caffeine is an additive in many analgesic preparations but, to our knowledge, there was no mechanistic basis for its inclusion until now. However, we predict that caffeine will contribute to analgesia only in sleep-deprived individuals. Although wake-promoting agents reduce hyperalgesia caused by sleep loss, they do not erase the actual sleep debt that accumulates over time. The level of pain in an individual at any given time will reflect, in part, recent sleep history and exposure to caffeine, and these influences could confound clinical analgesic trials, contributing to their high variance46. For example, sleep deprivation reduces the analgesic activity of morphine47,48, raising the concern that insufficient sleep may spur patients to use higher doses. Because sleep itself lessens the hyperalgesic effects of sleep deprivation in our mouse model, and extending sleep in healthy volunteers after sleep restriction49,50 reduces pain hypersensitivity, improving sleep in individuals with insufficient sleep might represent an effective strategy to interrupt the sleep deprivation–enhanced pain cycle and to preserve the maximum efficacy of analgesics.
URLs
National Sleep Foundation 2015 poll, http://sleepfoundation.org/sleep-polls-data/2015-sleep-and-pain.
ONLINE METHODS
Mice
Adult male and female C57BL/6J mice (aged 3–6 months) were purchased from Jackson Laboratory (Bar Harbor, ME). All procedures were approved in advance by the Institutional Animal Care and Use Committees (IACUC) of Beth Israel Deaconess Medical Center, Boston Children’s Hospital and Harvard Medical School and were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. During all experiments, mice were kept under a 12-h light/12-h dark cycle (lights on at 7:00) at constant temperature (22–24 °C) and received food and water ad libitum.
Sleep recording surgery
Mice were anesthetized with ketamine–xylazine (100 mg and 10 mg per kg bodyweight, i.p.) and placed in a stereotaxic apparatus (model 1900, David Kopf Instruments). Two stainless steel screws were implanted for ipsilateral frontoparietal EEG recordings (1.5 mm lateral to the right of the sagittal suture, 1 mm anterior to the bregma and 2 mm anterior to the lambda). Two flexible EMG electrodes (multistranded stainless steel wire; AS131, Cooner Wire) were inserted into the neck extensor muscles. All electrodes were attached to a 2 × 2 microstrip connector affixed to the animal’s head with dental cement, and the scalp wound was closed with surgical sutures. Mice were given meloxicam (5 mg per kg bodyweight, s.c.) before they regained consciousness and then daily for 2 d and were housed singly after surgery. Ten days later, they were transferred to individual recording cages in a sound-attenuated chamber and connected by a flexible tether to a commutator (Crist Instrument Co.) for 5 d before their baseline sleep–wake and sensory behaviors were measured.
Sensory testing
All sensory testing was performed in the same procedure room from 13:00 to 14:00 (6-h ASD, CSD and CSF), 16:00 to 17:00 (9-h sleep deprivation) or 18:00 to 19:00 (12-h sleep deprivation). Sensory responsivity was similar when tested at various times in the afternoon, indicating minimal influence of circadian variation in these tests (Supplementary Fig. 1c). For each sleep disruption experiment, mice were randomly assigned to an experimental group (non-sleep deprived (sham) or sleep deprived/fragmented; saline or drugs). The experimenter was always blinded to the experimental groups. One sleep deprivation animal was excluded from sleep analysis because the EEG and EMG traces were not reliable. One control animal was excluded for health-related issues. When testing several sensory modalities in one session, the following sequence of behavioral tests was used: von Frey test, acetone test, brush test and then hot plate or capsaicin. Some experiments only assessed one modality. For all experiments, mice were always acclimated to their environment (room, cage and experimenter) before sensory testing. The experimenter was in the room with the animals while they habituated to reduce the risk of analgesia related to male experimenter–induced stress51. Sleep disruption experiments started only after sleep analysis and sensory testing displayed consistent baseline responses (i.e., at least two consecutive sessions with similar values). Mice undergoing sleep disruption protocols were always tested together with non-sleep-deprived (sham) animals, with all animals implanted for EEG and EMG and housed singly with blinding to treatment. Pharmacological studies were carried out with the experimenter blinded to the compound administered. For CSD studies, we tested the effects of daily repeated testing to rule out possible habituation or sensitization. For all sensory tests, we found that, once habituated, mice displayed stable sensitivity when tested daily for five consecutive days (Supplementary Fig. 3a,c).
Von Frey filaments (static punctate mechanical stimuli)
Mice were placed on a mesh grid (5 × 5 mm) under an upside-down 500-ml beaker, and mechanical sensitivity was determined with a graded series of seven von Frey filaments that produced a bending force of 0.07, 0.16, 0.4, 0.6, 1, 1.4 and 2 g. The stimuli were applied within the sciatic nerve territory of the mice for 1–3 s. Each filament was tested ten times in order of increasing force, starting with the filament producing the lowest force. Successive von Frey filament applications were separated by at least 5 s after the mice had returned to their initial resting state52. The same male experimenter tested all conditions.
Acetone test (response to cold)
Mice were placed onto a mesh grid (5 × 5 mm) under an upside-down 500-ml beaker, and a small volume of acetone (5 μl) was applied to the plantar surface of the hindpaw using a 1-ml syringe. The time spent flinching or licking the paw was recorded for 1 min with a stopwatch53, 54. Mice were tested twice per session. The same male experimenter tested all conditions.
Brush test (dynamic mechanical stimuli)
Mice were placed onto a mesh grid (5 × 5 mm) under an upside-down 500-ml beaker, and three successive gentle touch stimuli were applied with a round-head paintbrush with a diameter of 2 mm (Princeton Brush Co.) onto the sural territory of the paw. Each stimulation was applied from the distal part of the paw to its middle and lasted less than 1 s. The total time spent flinching or licking the paw was measured with a stopwatch for three stimulations55. The same male experimenter tested all conditions.
Contact heat pain (hot plate test)
Mice were placed on a metallic plate heated at 52 °C within an acrylic container (Bioseb, France), and the latency to flinching, licking one of the hindpaws or jumping was measured56, 57. Mice were habituated to the procedure by placing them on a plate set at 30 °C, and several baseline measurements were taken to reduce the risk of habituation or sensitization58,59. Only one measurement was performed per day. The same experimenter (male) tested all conditions.
Thermal gradient assay
A continuous temperature gradient (7–50 °C) was established along a metallic base plate on which the mice walked freely while being video-recorded from above (Bioseb, France). After an exploration period, the mouse showed a distinct preference, indicating the most comfortable temperature range. Data are presented by time spent on zones set at specific temperatures60. For each condition, the best-fitted Gaussian curve was applied and the preferred temperature was determined as the peak. Statistical comparisons were carried out on the calculated preferred temperatures. Each run lasted 1.5 h, and two mice were simultaneously recorded in separate corridors (line A and line B).
Capsaicin
After injection of 20 μl of capsaicin (1 μg diluted in saline with 1% DMSO; M2028, Sigma-Aldrich) into the plantar surface of the hindpaw, the mouse was placed onto a surface set at 30 °C within an acrylic container, and the time spent licking, flinching or biting the paw was measured61. Both male and female experimenters performed this test (each tested sleep-deprived and non-sleep-deprived (sham) mice; experimenters were blinded to experimental condition).
Startle reflex
Mice were placed in a cubical Plexiglas recording chamber (27 cm × 10 cm × 12.5 cm) fixed on a piezo/Plexiglass sensing assembly and allowed to acclimate for 5 min with background white noise at 60 dB. This noise continued throughout the entire session, which consisted of exposure to 60 pulses of 12 increasing intensities (from 65 to 120 dB, by increment of 5 dB) for a total duration of 35 min. Each pulse was repeated five times in a pseudorandom order, with an average 30-s (range 25–35 s) intertrial interval. The recording window for a startle response was 250 ms after the startle pulse. After each pulse, the maximum intensity of the startle and its latency were recorded and used for analysis62. To determine the number of jumps after each pulse, the average force recorded under the baseline condition for each mouse (for example, with only white noise) was defined as a threshold. For each pulse, if the startle produced a force superior to the threshold, it was considered a jump.
Prepulse inhibition startle
Mice were placed as for the startle reflex test. After a 5-min habituation period, mice were exposed to a prepulse of 62, 72 or 84 dB followed by a 105-dB pulse 20 ms later. Prepulse inhibition was calculated with the following formula: 100 − ((startle response for prepulse + startle response for pulse)/(startle response for pulse alone) × 100)63.
EEG and EMG acquisition and analysis
EEG and EMG signals were amplified (×5,000) using a Grass Instruments model 12 amplifier (West Warwick, RI) and were filtered as follows: high-pass filter at 0.3 Hz and low-pass filter at 1,000 Hz. Signals were sampled and stored at 128 Hz, digitally filtered (EEG, 0.3–30 Hz; EMG, 10–100 Hz), and semiautomatically scored in 10-s epochs as wake, NREMS or REMS using SleepSign for Animal (Kissei Comtec). This preliminary scoring was visually inspected by a trained experimenter and corrected when appropriate64. The percentage of time spent in wake, NREMS and REMS, as well as the mean duration and number of behavioral state bouts, was calculated for each condition. NREMS sleep attempts were defined as NREMS bouts of <5 s. NREMS latency was defined as the time elapsed between the beginning of the recovery period (immediately after the mice returned to their home cages and were reconnected to their tethers) and the first NREMS episode lasting 30 s.
EEG power spectra were computed by a fast Fourier transformation routine (using a Hanning window) for each 10-s epoch between 0.5 and 64.0 Hz with 0.25-Hz resolution. Epochs containing movement artifacts, predominantly during active wake, were excluded from spectral analysis. SWA (EEG power in the band at 0.5–4.0 Hz) during NREMS was computed over the first 5 h of the sleep opportunity period (14:00–19:00) for each day of CSD (CSD1d–CSD5d) in absolute values and expressed as the percentage of the baseline SWA mean value during NREMS measured over 24 h for each mouse. This transformation allowed us to correct for individual differences in absolute power. Next, we calculated SWA over 360 NREMS epoch intervals (first, second and third hours of NREMS) to plot the time course of SWA decay expressed as the percentage of the baseline SWA mean value during NREMS measured over 24 h. We analyzed changes in SWA within an individual NREMS episode by selecting all NREMS bouts lasting at least 60 s and preceded by at least 10 s of wake that occurred during the first 3 h of the sleep opportunity period and the corresponding baseline time interval for each animal. For normalization, all 10-s epochs were expressed relative to the baseline SWA mean value during NREMS measured over 24 h.
Signals from telemetry transmitters were received by antennas (RPC-1, Data Sciences International) below each recording cage and digitally acquired (Dataquest, Data Sciences International). Locomotor activity (LMA) was measured as movements around the cage and tallied in 5-min bins.
Sleep disruptions
Sleep deprivation protocol
For all sleep deprivation experiments, we kept the mice awake by providing new nesting material or novel objects in their home cage, tapping the cage only when necessary. During this period, EEG and EMG signals were closely monitored to be certain that each mouse was fully awake. Mice were not disturbed when they were spontaneously awake. When a mouse was idle for more than 1 min or the EEG signals displayed slow waves (indicating that an entry to NREMS was imminent), a novel object was provided without touching the mouse. The first item provided was always nesting material. Additional toys were designed to incite chewing, a nonstressful innate behavior65, and were given one at a time to promote wakefulness or activity. Examples of custom-made toys are shown in Supplementary Figure 1a. For all experiments, sham mice (instrumented but undisturbed; for example, non-sleep deprived) were similarly housed singly and tested for nociceptive responses as the sleep-deprived animals by an experimenter blinded to the sleep condition.
Acute sleep deprivation
To test the effects of 6, 9 and 12 h of ASD on nociceptive thresholds, we sleep deprived mice for 6 and 9 h starting at light onset (7:00) and for 12 h starting at 6:00, which allowed all behavioral tests to be performed during the light cycle, immediately at the end of the ASD (at 13:00, 16:00, and 18:30, respectively). A cage change was performed at 13:00 during 9- and 12-h ASD to promote arousal through exploratory behavior. ASD sessions were separated by at least 8 d.
Chronic sleep deprivation
We kept mice awake for the first 6 h of the light period (7:00–13:00) for five consecutive days using the same protocol as for ASD. We performed sensory testing at baseline and at 1, 3 and 5 d of CSD, immediately at the end of the session (13:00–14:00). Mice were then reconnected to their tethers (at 14:00) and allowed to sleep ad libitum for the next 17 h until the next sleep deprivation day. Sleep–wake behaviors were recorded at baseline and on each day of sleep restriction (days 1–5) for all animals and during 2 d of recovery for a subset of mice.
Chronic sleep fragmentation
Mice were submitted to CSF using a shaker apparatus (PVC cylinder, diameter 30 cm, height 45 cm; Viewpoint, France) that prevents sleep by transient vertical movements (10–20 ms, height 1 cm)66. This system allows sleep disruptions to be achieved without forced locomotion or ambulation. The number of stimulations (2–4) and the delay between two stimulations (100–200 ms) were randomized and controlled by Shaker software (Viewpoint, France). Sequences of stimulations were administered every minute for 12 h starting at light onset (entire light period: 7:00–19:00) for five consecutive days (days 1–5 of CSF).
Drug injections
Caffeine (20 mg per kg bodyweight, i.p.; Sigma-Aldrich), modafinil (45 mg per kg bodyweight, i.p.; Sigma-Aldrich) or vehicle (0.9% saline) was administered 2 h before the end of sleep deprivation (i.e., at 14:00 for 9-h ASD and 11:00 for CSD), and sensory testing was carried out immediately at the end of the session, with the experimenter blinded to the sleep condition. Ibuprofen (30 mg per kg bodyweight, s.c.; Sigma-Aldrich), morphine (5 mg per kg bodyweight, s.c.; BCH Pharmacy) or vehicle (0.9% saline) was administered 30 min before the end of sleep deprivation.
Plasma corticosterone measurement
We measured plasma corticosterone levels to assess potential stress under the CSD and CSF protocols. Animals were distributed to the baseline (BSL; non-sleep deprived, group housed), sham (non-sleep deprived; singly housed), 1-d CSD (6 h of sleep deprivation for 1 d; singly housed), 5-d CSD (6 h of sleep deprivation for 5 d; singly housed) and 5-d CSF (12 h of sleep fragmentation for 5 d; singly housed) groups. Mice were euthanized between 13:00 and 14:00, and trunk blood was collected in tubes supplemented with EDTA kept on ice. Samples were then centrifuged at 20,000g for 15 min at 4 °C, and plasma was extracted from the samples and frozen at −80 °C until processing. Corticosterone levels were determined using the ImmuChem Double Antibody 125I RIA Kit (MP Biomedicals, LLC, USA, 07-120113). Samples and standards were assayed in duplicate at a 1:200 dilution according to the manufacturer’s protocol. No differences were found between group-housed and singly housed mice.
Statistical analysis
Statistical analysis was performed using Prism version 7.00 for Windows, GraphPad Software (La Jolla, CA, USA). All results are expressed as means ± s.e.m. Normality was assessed using the Shapiro–Wilk test. A Student’s t-test was used to determine significance between two groups, and F-tests for equality of variance were used for all t-tests to compare variances. Comparison between more than two groups, i.e., for sleep (6- to 12-h ASD, 1- to 5-d CSD) or drug (saline, ibuprofen, caffeine, modafinil or morphine) conditions, were analyzed by one- or two-way ANOVA with repeated measures when appropriate. Bartlett’s test was used to test variance for all one-way ANOVA. For experiments that included sham animals, we first performed between-subject analyses to compare sham to sleep-disrupted mice. Where appropriate, we then performed within-subject analyses to compare baseline and sleep intervention (in this case, animals served as their own control). In the case of significance, ANOVA analysis was followed by the appropriate multiple-comparisons tests. P ≤ 0.05 was considered significant. We calculated the effect size for each data set: (partial) η2 or η2p for factorial analyses (ANOVA) and Cohen’s d for t-tests. Sample size was determined on the basis of previous studies carried out in our laboratory. For complete statistical analyses, please refer to Supplementary Table 1.
Data availability
The data from this study are available from the corresponding author upon reasonable request.
Supplementary Material
Acknowledgments
This work was supported by NIH grants DE022912 (C.J.W. and T.E.S.), NS038253-11S1 (C.J.W.) and HL095491 (T.E.S.), the IDDRC of Boston Children’s Hospital (U54 HD090255) and the Metabolic Physiology Core (P30 DK057521). We are grateful to N. Andrews, O. Peroni, F. Latremoliere, T. Mochizuki, P.-A. Libourel and R. Hersher for advice and technical assistance and O. Mazor of the HMS Research Instrumentation Core for instrument design and fabrication.
Footnotes
AUTHOR CONTRIBUTIONS
C.A., A.L., T.E.S. and C.J.W. conceived and designed experiments, interpreted the results and wrote the manuscript. C.A., A.L., A.F. and G.M. performed sleep studies and analysis. C.A., A.L., A.F., G.M. and M.Y. performed sleep deprivation experiments. C.A., A.L. and A.F. performed behavioral experiments and analysis.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Van den Bulck J. Television viewing, computer game playing, and Internet use and self-reported time to bed and time out of bed in secondary-school children. Sleep. 2004;27:101–104. doi: 10.1093/sleep/27.1.101. [DOI] [PubMed] [Google Scholar]
- 2.Fossum IN, Nordnes LT, Storemark SS, Bjorvatn B, Pallesen S. The association between use of electronic media in bed before going to sleep and insomnia symptoms, daytime sleepiness, morningness, and chronotype. Behav Sleep Med. 2014;12:343–357. doi: 10.1080/15402002.2013.819468. [DOI] [PubMed] [Google Scholar]
- 3.Luyster FS, Strollo PJ, Jr, Zee PC, Walsh JK. Sleep: a health imperative. Sleep. 2012;35:727–734. doi: 10.5665/sleep.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards RR, Almeida DM, Klick B, Haythornthwaite JA, Smith MT. Duration of sleep contributes to next-day pain report in the general population. Pain. 2008;137:202–207. doi: 10.1016/j.pain.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell CM, et al. Self-reported sleep duration associated with distraction analgesia, hyperemia, and secondary hyperalgesia in the heat–capsaicin nociceptive model. Eur J Pain. 2011;15:561–567. doi: 10.1016/j.ejpain.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haack M, et al. Pain sensitivity and modulation in primary insomnia. Eur J Pain. 2012;16:522–533. doi: 10.1016/j.ejpain.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith MT, Edwards RR, Stonerock GL, McCann UD. Individual variation in rapid eye movement sleep is associated with pain perception in healthy women: preliminary data. Sleep. 2005;28:809–812. doi: 10.1093/sleep/28.7.809. [DOI] [PubMed] [Google Scholar]
- 8.Lautenbacher S, Kundermann B, Krieg JC. Sleep deprivation and pain perception. Sleep Med Rev. 2006;10:357–369. doi: 10.1016/j.smrv.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Roehrs T, Hyde M, Blaisdell B, Greenwald M, Roth T. Sleep loss and REM sleep loss are hyperalgesic. Sleep. 2006;29:145–151. doi: 10.1093/sleep/29.2.145. [DOI] [PubMed] [Google Scholar]
- 10.Mansfield KE, Sim J, Jordan JL, Jordan KP. A systematic review and meta-analysis of the prevalence of chronic widespread pain in the general population. Pain. 2016;157:55–64. doi: 10.1097/j.pain.0000000000000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14:1539–1552. doi: 10.1016/j.jpain.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith MT, et al. Sleep onset insomnia symptoms during hospitalization for major burn injury predict chronic pain. Pain. 2008;138:497–506. doi: 10.1016/j.pain.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies KA, et al. Restorative sleep predicts the resolution of chronic widespread pain: results from the EPIFUND study. Rheumatology (Oxford) 2008;47:1809–1813. doi: 10.1093/rheumatology/ken389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim H, et al. Genetic influence on variability in human acute experimental pain sensitivity associated with gender, ethnicity and psychological temperament. Pain. 2004;109:488–496. doi: 10.1016/j.pain.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 15.Kuna ST, et al. Heritability of performance deficit accumulation during acute sleep deprivation in twins. Sleep. 2012;35:1223–1233. doi: 10.5665/sleep.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meerlo P, Pragt BJ, Daan S. Social stress induces high intensity sleep in rats. Neurosci Lett. 1997;225:41–44. doi: 10.1016/s0304-3940(97)00180-8. [DOI] [PubMed] [Google Scholar]
- 17.Rhudy JL, Meagher MW. Fear and anxiety: divergent effects on human pain thresholds. Pain. 2000;84:65–75. doi: 10.1016/S0304-3959(99)00183-9. [DOI] [PubMed] [Google Scholar]
- 18.Kaprio J, Koskenvuo M. Genetic and environmental factors in complex diseases: the older Finnish Twin Cohort. Twin Res. 2002;5:358–365. doi: 10.1375/136905202320906093. [DOI] [PubMed] [Google Scholar]
- 19.Nielsen CS, et al. Individual differences in pain sensitivity: genetic and environmental contributions. Pain. 2008;136:21–29. doi: 10.1016/j.pain.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 20.Leemburg S, et al. Sleep homeostasis in the rat is preserved during chronic sleep restriction. Proc Natl Acad Sci USA. 2010;107:15939–15944. doi: 10.1073/pnas.1002570107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clasadonte J, McIver SR, Schmitt LI, Halassa MM, Haydon PG. Chronic sleep restriction disrupts sleep homeostasis and behavioral sensitivity to alcohol by reducing the extracellular accumulation of adenosine. J Neurosci. 2014;34:1879–1891. doi: 10.1523/JNEUROSCI.2870-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hakki Onen S, Alloui A, Jourdan D, Eschalier A, Dubray C. Effects of rapid eye movement (REM) sleep deprivation on pain sensitivity in the rat. Brain Res. 2001;900:261–267. doi: 10.1016/s0006-8993(01)02320-4. [DOI] [PubMed] [Google Scholar]
- 23.Tomim DH, et al. The pronociceptive effect of paradoxical sleep deprivation in rats: evidence for a role of descending pain modulation mechanisms. Mol Neurobiol. 2016;53:1706–1717. doi: 10.1007/s12035-014-9059-0. [DOI] [PubMed] [Google Scholar]
- 24.Wang PK, et al. Short-term sleep disturbance–induced stress does not affect basal pain perception, but does delay postsurgical pain recovery. J Pain. 2015;16:1186–1199. doi: 10.1016/j.jpain.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boada MD, Woodbury CJ. Physiological properties of mouse skin sensory neurons recorded intracellularly in vivo: temperature effects on somal membrane properties. J Neurophysiol. 2007;98:668–680. doi: 10.1152/jn.00264.2007. [DOI] [PubMed] [Google Scholar]
- 26.Boada MD, Woodbury CJ. Myelinated skin sensory neurons project extensively throughout adult mouse substantia gelatinosa. J Neurosci. 2008;28:2006–2014. doi: 10.1523/JNEUROSCI.5609-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borbély AA, Achermann P. Sleep homeostasis and models of sleep regulation. J Biol Rhythms. 1999;14:557–568. doi: 10.1177/074873099129000894. [DOI] [PubMed] [Google Scholar]
- 28.Mignot E. Why we sleep: the temporal organization of recovery. PLoS Biol. 2008;6:e106. doi: 10.1371/journal.pbio.0060106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haack M, Lee E, Cohen DA, Mullington JM. Activation of the prostaglandin system in response to sleep loss in healthy humans: potential mediator of increased spontaneous pain. Pain. 2009;145:136–141. doi: 10.1016/j.pain.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fredholm BB, Bättig K, Holmén J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- 31.Qu WM, Huang ZL, Xu XH, Matsumoto N, Urade Y. Dopaminergic D1 and D2 receptors are essential for the arousal effect of modafinil. J Neurosci. 2008;28:8462–8469. doi: 10.1523/JNEUROSCI.1819-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang ZL, et al. Adenosine A2A, but not A1, receptors mediate the arousal effect of caffeine. Nat Neurosci. 2005;8:858–859. doi: 10.1038/nn1491. [DOI] [PubMed] [Google Scholar]
- 33.Petrovsky N, et al. Sleep deprivation disrupts prepulse inhibition and induces psychosis-like symptoms in healthy humans. J Neurosci. 2014;34:9134–9140. doi: 10.1523/JNEUROSCI.0904-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schuh-Hofer S, et al. One night of total sleep deprivation promotes a state of generalized hyperalgesia: a surrogate pain model to study the relationship of insomnia and pain. Pain. 2013;154:1613–1621. doi: 10.1016/j.pain.2013.04.046. [DOI] [PubMed] [Google Scholar]
- 35.Ødegård SS, et al. The effect of sleep restriction on laser evoked potentials, thermal sensory and pain thresholds and suprathreshold pain in healthy subjects. Clin Neurophysiol. 2015;126:1979–1987. doi: 10.1016/j.clinph.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 36.Tiede W, et al. Sleep restriction attenuates amplitudes and attentional modulation of pain-related evoked potentials, but augments pain ratings in healthy volunteers. Pain. 2010;148:36–42. doi: 10.1016/j.pain.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 37.Ferraro L, et al. The vigilance promoting drug modafinil increases dopamine release in the rat nucleus accumbens via the involvement of a local GABAergic mechanism. Eur J Pharmacol. 1996;306:33–39. doi: 10.1016/0014-2999(96)00182-3. [DOI] [PubMed] [Google Scholar]
- 38.Solinas M, et al. Caffeine induces dopamine and glutamate release in the shell of the nucleus accumbens. J Neurosci. 2002;22:6321–6324. doi: 10.1523/JNEUROSCI.22-15-06321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Volkow ND, et al. Effects of modafinil on dopamine and dopamine transporters in the male human brain: clinical implications. J Am Med Assoc. 2009;301:1148–1154. doi: 10.1001/jama.2009.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Volkow ND, et al. Evidence that sleep deprivation downregulates dopamine D2R in ventral striatum in the human brain. J Neurosci. 2012;32:6711–6717. doi: 10.1523/JNEUROSCI.0045-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Volkow ND, et al. Caffeine increases striatal dopamine D2/D3 receptor availability in the human brain. Transl Psychiatry. 2015;5:e549. doi: 10.1038/tp.2015.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonaventura J, et al. Allosteric interactions between agonists and antagonists within the adenosine A2A receptor–dopamine D2 receptor heterotetramer. Proc Natl Acad Sci USA. 2015;112:E3609–E3618. doi: 10.1073/pnas.1507704112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor AM, Becker S, Schweinhardt P, Cahill C. Mesolimbic dopamine signaling in acute and chronic pain: implications for motivation, analgesia, and addiction. Pain. 2016;157:1194–1198. doi: 10.1097/j.pain.0000000000000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Treister R, et al. Associations between polymorphisms in dopamine neurotransmitter pathway genes and pain response in healthy humans. Pain. 2009;147:187–193. doi: 10.1016/j.pain.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 45.Wood PB, et al. Fibromyalgia patients show an abnormal dopamine response to pain. Eur J Neurosci. 2007;25:3576–3582. doi: 10.1111/j.1460-9568.2007.05623.x. [DOI] [PubMed] [Google Scholar]
- 46.Edwards RR, et al. Patient phenotyping in clinical trials of chronic pain treatments: IMMPACT recommendations. Pain. 2016;157:1851–1871. doi: 10.1097/j.pain.0000000000000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Skinner GO, Damasceno F, Gomes A, de Almeida OM. Increased pain perception and attenuated opioid antinociception in paradoxical sleep-deprived rats are associated with reduced tyrosine hydroxylase staining in the periaqueductal gray matter and are reversed by l-DOPA. Pharmacol Biochem Behav. 2011;99:94–99. doi: 10.1016/j.pbb.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 48.Steinmiller CL, et al. Differential effect of codeine on thermal nociceptive sensitivity in sleepy versus nonsleepy healthy subjects. Exp Clin Psychopharmacol. 2010;18:277–283. doi: 10.1037/a0018899. [DOI] [PubMed] [Google Scholar]
- 49.Faraut B, et al. Napping reverses increased pain sensitivity due to sleep restriction. PLoS One. 2015;10:e0117425. doi: 10.1371/journal.pone.0117425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roehrs TA, Harris E, Randall S, Roth T. Pain sensitivity and recovery from mild chronic sleep loss. Sleep. 2012;35:1667–1672. doi: 10.5665/sleep.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sorge RE, et al. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat Methods. 2014;11:629–632. doi: 10.1038/nmeth.2935. [DOI] [PubMed] [Google Scholar]
- 52.Latremoliere A, et al. Reduction of neuropathic and inflammatory pain through inhibition of the tetrahydrobiopterin pathway. Neuron. 2015;86:1393–1406. doi: 10.1016/j.neuron.2015.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 1994;59:369–376. doi: 10.1016/0304-3959(94)90023-X. [DOI] [PubMed] [Google Scholar]
- 54.Smith SB, Crager SE, Mogil JS. Paclitaxel-induced neuropathic hypersensitivity in mice: responses in 10 inbred mouse strains. Life Sci. 2004;74:2593–2604. doi: 10.1016/j.lfs.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 55.Vicuña L, et al. The serine protease inhibitor SerpinA3N attenuates neuropathic pain by inhibiting T cell–derived leukocyte elastase. Nat Med. 2015;21:518–523. doi: 10.1038/nm.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol Rev. 2001;53:597–652. [PubMed] [Google Scholar]
- 57.Mogil JS, et al. Heritability of nociception I: responses of 11 inbred mouse strains on 12 measures of nociception. Pain. 1999;80:67–82. doi: 10.1016/s0304-3959(98)00197-3. [DOI] [PubMed] [Google Scholar]
- 58.Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci. 2009;10:283–294. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- 59.Sandkühler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev. 2009;89:707–758. doi: 10.1152/physrev.00025.2008. [DOI] [PubMed] [Google Scholar]
- 60.Moqrich A, et al. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science. 2005;307:1468–1472. doi: 10.1126/science.1108609. [DOI] [PubMed] [Google Scholar]
- 61.Caterina MJ, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 62.Davis M. Neurochemical modulation of sensory-motor reactivity: acoustic and tactile startle reflexes. Neurosci Biobehav Rev. 1980;4:241–263. doi: 10.1016/0149-7634(80)90016-0. [DOI] [PubMed] [Google Scholar]
- 63.Geyer MA, McIlwain KL, Paylor R. Mouse genetic models for prepulse inhibition: an early review. Mol Psychiatry. 2002;7:1039–1053. doi: 10.1038/sj.mp.4001159. [DOI] [PubMed] [Google Scholar]
- 64.Alexandre C, et al. Sleep-stabilizing effects of E-6199, compared to zopiclone, zolpidem and THIP in mice. Sleep. 2008;31:259–270. doi: 10.1093/sleep/31.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hennessy MB, Foy T. Nonedible material elicits chewing and reduces the plasma corticosterone response during novelty exposure in mice. Behav Neurosci. 1987;101:237–245. doi: 10.1037//0735-7044.101.2.237. [DOI] [PubMed] [Google Scholar]
- 66.Libourel PA, Corneyllie A, Luppi PH, Chouvet G, Gervasoni D. Unsupervised online classifier in sleep scoring for sleep deprivation studies. Sleep. 2015;38:815–828. doi: 10.5665/sleep.4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data from this study are available from the corresponding author upon reasonable request.