Abstract
Cross-sectional studies show that elevated cerebral amyloid is associated with greater white-matter hyperintensity (WMH) burden in cognitively normal (CN) older adults. However, the relative time courses of amyloid and WMH accrual are unclear. To address this, we tested the associations between known WMH correlates—age, hypertension, and amyloid—with WMH accrual rate. We used brain magnetic resonance imaging to measure WMH change in 112 CN Alzheimer’s Disease Neuroimaging Initiative (GO/2) participants over a 2-year period. A linear mixed effects model assessed baseline cerebrospinal fluid amyloid beta (Aβ) 1–42, hypertension, age, and their interactions, as predictors of greater WMH accrual. Greater amyloid burden was associated with greater WMH accrual over time. Those with hypertension showed a stronger association between greater amyloid burden and WMH accrual rate. Greater age was not significantly associated with greater WMH accrual in this model. Although the direction of the relationship cannot be tested in this model, CN individuals harboring cerebral amyloid had greater accrual of WMH over a 2-year period after accounting for hypertension and age. Impaired amyloid clearance and cerebral small vessel disease may both underlie the more rapid emergence of WM lesions. The role of cerebral amyloid burden in white-matter injury should thus be considered as a relevant factor when WMHs are detected clinically.
Keywords: Amyloid, FLAIR, MRI, Normal aging, Hypertension, ADNI
1. Introduction
White-matter hyperintensities (WMHs), imaging markers of gross white-matter lesions in the brain, are highly prevalent in cognitively normal (CN) older adults and are associated with increased risk of adverse outcomes including dementia, early mortality, frailty, and depression (Abraham et al., 2016; Prins and Scheltens, 2015; Provenzano et al., 2013). Recognized vascular risk factors, such as hypertension, are associated with greater WMH burden (Gouw et al., 2011; Kertesz et al., 1988). Impaired blood cerebral blood flow linked to systemic hypertension is thought to be the primary mechanism behind ischemic tissue damage that appears as WMH (Hughes and Sink, 2016). However, cerebral amyloidosis can also interfere with perfusion of the brain by damaging perivascular spaces among other processes (Thomas et al., 1996). Conversely, hypertension may increase vascular resistance thereby reducing clearance of amyloid (Hughes et al., 2013, 2014). It is unclear whether systemic vascular risk factors and cerebral amyloidosis are associated with white matter damage due to parallel processes or converging pathologies (Prins and Scheltens, 2015).
In CN older adults, hypertension and elevated cerebral amyloid are common, but few studies have questioned the independent and interacting associations of each with WMH burden (Toledo et al., 2013). We and others previously demonstrated that in CN, elevated cerebral amyloid is associated with greater WMH burden and broadly reduced WM integrity, measured by DTI (Gold et al., 2014; Marnane et al., 2016; Scott et al., 2015; Wolf et al., 2015). However, these cross-sectional studies fell short of providing information about temporal relationships between amyloid burden and WMH accrual, and in particular, whether the presence of cerebral amyloid is associated with greater subsequent accrual of WMH—as would be predicted by current data on the neurobiological consequences of amyloid (Brickman, 2013; Saito and Ihara, 2016).
Characterization of the temporal relationships between amyloid and WMH is relevant to the guidance of clinical practice for interventions to reduce dementia risk. The current state of care associated with WMH is to manage vascular risk factors to reduce growth of white-matter lesions (Mok and Kim, 2015). However, even when vascular risk is low or vascular factors, such as hypertension, are treated, WMH load remains elevated (Scott et al., 2015). This prompts the investigation of additional factors, like cerebral amyloid, that could be modulated to lessen the accumulation of aging-associated white-matter damage. Targeting amyloid is a promising approach demonstrated by recent advances in the development of anti-amyloid therapeutics (D’Avanzo et al., 2015; Liu et al., 2015; Lovell et al., 2016). Asymptomatic CN older adults at risk of dementia based on biomarkers—WMH and amyloid burden—identify a possible target group for anti-amyloid therapies for the prevention, rather than treatment of, Alzheimer’s disease.
In this study, we harnessed the longitudinal imaging data from 112 participants of the Alzheimer’s Disease Neuroimaging Initiative (ADNI) to test predictors of WMH accrual over a 2-year period who also had cerebrospinal fluid (CSF) biomarkers assays completed. Specifically, we modeled the effects of CSF amyloid level, clinical hypertension history, and age on WMH volume at baseline and the rate of WMH accrual in CN. We show that amyloid level predicts WMH at baseline and modulates the rate of WMH accrual in CN older adults with hypertension.
2. Methods
2.1. Participants
Data used in preparing this article were obtained from the ADNI-GO and ADNI-2 studies (adni.loni.usc.edu) as described earlier (Scott et al., 2015). These CN participants are a subset of the sample reported in Scott et al. (2015) that had 1 or more follow-up scans. Of these, 112 participants had data for all variables analyzed at baseline. Data used in the preparation of this article were obtained from the ADNI database (adni.loni.usc.edu). The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography, and other biological markers and clinical and neuropsychological assessment can be combined to measure the progression of mild cognitive impairment and early Alzheimer’s disease (AD). Inclusion criterion of a modified Hachniski score of 4 or lower excluded people with a clinical diagnosis of vascular dementia. Cognitively normal older adults and patients clinically diagnosed with early or late mild cognitive impairment, or AD were recruited from over 50 sites across the United States and Canada. Participants recruited for ADNI-1 and ADNI-GO had the option to be followed in ADNI-2. Group clinical and demographic data are reported in Table 1. Data were extracted in November 2015, and all available associated data was used.
Table 1.
Study demographics, vascular parameters, CSF immunoassays, and imaging metrics at baseline
| Participants at baseline | 112 |
| 3 mo | 91% |
| 6 mo | 93% |
| 12 mo | 90% |
| 24 mo | 79% |
| Sex (male) | 59.1% |
| Age (y) | 73.2 (6.6) |
| Education (y) | 17 (2) |
| White—not Hispanic/Latino | 86.6% |
| APOE ε4 genotype | 27.9% |
| White matter hyperintensity volume at baseline | 6.30 (11.46) |
| 3 mo | 6.46 (10.94) |
| 6 mo | 6.21 (10.46) |
| 12 mo | 6.42 (12.02) |
| 24 mo | 6.78 (12.99) |
| Intracranial volume (ICV) (cm3) | 1504 (152) |
| CSF Aβ1–42 (pg/mL) at baseline | 202.6 (52.8) |
| 24 mo | 190.7 (51.3) |
| CSF t-tau (pg/mL) | 70.9 (38.8) |
| CSF p-tau181 (pg/mL) | 35.4 (20.8) |
| Current vascular status | |
| Body mass index | 28 (5) |
| Systolic blood pressure (mm Hg) | 136 (16) |
| Diastolic blood pressure (mm Hg) | 75 (10) |
| Fasting blood glucose (mg/dL) | 100 (18) |
| Total serum cholesterol (mg/dL) | 190 (35) |
| Vascular history | |
| Hypertension | 52.7% |
| Dyslipidemia | 40.2% |
| Diabetes mellitus | 8.0% |
| Smoking | 13.4% |
| MI/CVA/AF | 2.7% |
| Micro-hemorrhage | 14.3% |
All numerical entries are mean (standard deviation).
Key: AF, atrial fibrillation; CVA, stroke; MI, myocardial infarction.
2.2. MR imaging protocol and WMH measurement
MRI acquisition: 3D Axial T2-weighted fluid attenuated inversion recovery (FLAIR) and T1-weighted MRI sequences were collected at each site. Exact protocols varied by scanner type and strength (1.5 T or 3.0 T). General FLAIR sequence characteristics were as follows: field of view read 280 mm; field of view phase 100.0%; slice thickness 8.0 mm; TR 20.0 ms; TE 5.00 ms; flip angle, 40°.
The WMH measurement approach is detailed on the ADNI site (adni.loni.usc.edu, “4-Tissue Segmentation Methods for ADNI MR Scans.pdf”) (Carmichael et al., 2012). Images were processed to (1) remove nonbrain tissues from T1-weighted and FLAIR images; (2) spatially align the image pair; and (3) remove MRI field artifacts. Next images were warped to a standard template space in which the prior probability of WMH occurrence and the FLAIR signal characteristics of WMHs were modeled at every location in the cerebral white matter. This prior information, together with the signal intensities of the FLAIR image in question, was used to identify WMHs. Total WMH volumes were calculated for 507 MRIs. Participants were imaged at study entry (n = 112), and at 3- (n = 102), 6- (n = 104), 12- (n = 101), and 24-month (n = 88) follow-ups.
2.3. CSF immunoassay
CSF immunoassay of amyloid was chosen because CSF amyloid levels are more dynamic in the earlier phases of amyloid accumulation that would be occurring before cognitive impairment (Landau et al., 2010). CSF collection procedures can be downloaded from the ADNI database (ADNI_Methods_UPENN_Biomarker_20120710.pdf; Shaw et al., 2011). The xMAP Luminex platform and Innogenetics/Fujirebio AlzBio3 immunoassay kits were used following procedures in place at the UPenn/ADNI Biomarker Laboratory, according to the kit manufacturer’s instructions and as described in previous publications (Shaw et al., 2011). In this study, we report CSF Aβ1–42, total tau, and phosphorylated-tau181.
2.4. Vascular history
We documented vascular history (Table 1) by screening the database of participant medical histories (Provenzano et al., 2013), as well as medication records. We report histories of hypertension, dyslipidemia (high lipids or cholesterol), smoking, type II diabetes mellitus, atrial fibrillation, myocardial infarction, or stroke.
2.5. Statistical analyses
A linear mixed effects model identified trends in change in total WMH volume (ln transformed) over time. First, a model with only time (years) from baseline scan was run to determine whether there was a general trend of linear WMH volume change over the 2-year period. To address whether baseline covariates of WMH predicted WMH accrual, we selected significant predictors from our initial cross-sectional analysis of WMH (Scott et al., 2015) as predictors of change in WMH. Significant predictors of baseline WMH in the previous report were CSF Aβ1–42, hypertension, and age, and thus these same predictors plus the interaction of CSF Aβ1–42 and hypertension and the interaction of Aβ1–42 and age were predictors of WMH change in the current model. Predictors of baseline WMH included CSF Aβ1–42, hypertension, and age, as well as adjusting for intracranial volume, history of hyperlipidemia, systolic blood pressure at baseline, APOE 34 genotype status (≥1 allele), education (centered at 12 years), sex (male = 0), and the interaction of hypertension and systolic blood pressure. Continuous variables were converted to z-scores for statistical analyses so that parameters estimates were comparable.
3. Results
3.1. Cohort characteristics
Group clinical and demographic data in this subset (Table 1) were similar to that of the sample reported previously (Scott et al., 2015), with the exception of hyperlipidemia (40% presently vs. 14% previously). The difference in this variable was due to the additional criterion of lipid-related medication included presently. The mean age was 73 years, and nearly 60% of the sample was male. Roughly one-third of the CN group carried 1 or 2 APOE ε4 alleles, and the mean amyloid level was 202 pg/mL (±52.8 pg/mL). Hypertension prevalence was common, with more than half the sample having a history of hypertension exposure.
3.2. WMH accrual
The average raw WMH volume at baseline was 6.30 mL (±11.47 mL) and the average rate of WMH accrual was 0.32 mL/y (±0.09 mL/y) (p < 0.001), which shows that WMH accrual is detectable over a 2-year period in the CN group.
Table 2 reports the parameter estimates for the model of WMH accrual using log transformed WMH. CSF Aβ1–42 had significant effects on WMH baseline load and accrual rate that had an interaction with hypertension history. Lower CSF Aβ1–42 levels (i.e., greater cerebral Aβ1–42 burden, p < 0.001) and greater age (p = 0.012) were associated with greater WMH at baseline. Lower CSF Aβ1–42 level at baseline was also associated with greater WMH accrual (p = 0.002). Further in those with a clinical history of hypertension, lesser CSF Aβ1–42 was associated with a faster rate of WMH accrual than in those lacking a clinical history of hypertension (p = 0.034).
Table 2.
Linear mixed model for white-matter hyperintensity change over 2 years
| Independent variable | Beta | Standard error | p-value |
|---|---|---|---|
| Intercept | 1.265 | 0.352 | <0.001 |
| Time (y) | 0.036 | 0.019 | 0.064 |
| Time × CSF Aβ1–42 | −0.054 | 0.018 | 0.002 |
| Time × hypertension | −0.004 | 0.027 | 0.892 |
| Time × age | −0.003 | 0.002 | 0.212 |
| Time × CSF Aβ1–42 × Hypertension | 0.057 | 0.027 | 0.034 |
| Time × CSF Aβ1–42 × Age | 0.004 | 0.002 | 0.073 |
| CSF Aβ1–42 | −0.447 | 0.118 | <0.001 |
| Hypertension | 0.226 | 0.357 | 0.299 |
| Systolic blood pressure | 0.234 | 0.284 | 0.084 |
| Hypertension × systolic blood pressure | −0.198 | 0.431 | 0.343 |
| Age | 0.043 | 0.017 | 0.012 |
| Intracranial volume | −0.110 | 0.071 | 0.123 |
| Sex | −0.350 | 0.235 | 0.137 |
| Education | −0.022 | 0.044 | 0.617 |
| Hyperlipidemia | 0.075 | 0.212 | 0.722 |
| APOE ε4 genotype | 0.068 | 0.242 | 0.777 |
For illustrative purposes, we calculated WMH accrual trajectories for prototypical normotensive and hypertensive individuals with average amyloid levels above (negative) and below (positive) the established threshold for cerebral amyloidosis (192 pg/mL) (Shaw et al., 2009) (Fig. 1). Within normotensives, amyloid status at baseline did not greatly impact the rate of WMH accrual over this brief 2-year follow-up period (negative: 0.041 ln-mL/y, positive: 0.036 ln-mL/y), although WMH baseline levels were estimated to be higher in amyloid positive normotensives. On the other hand, WMH volume appeared to remain stable in hypertensives with negative amyloid status (−0.001 ln-mL/y). The interaction between CSF Aβ1–42 and hypertension is shown by the final estimated trajectory in which WMH accrual rate was greatest in amyloid positive, hypertensive individuals (0.095 ln-mL/y). These sample trajectories illustrate the statistical effects in the overall model.
Fig. 1.
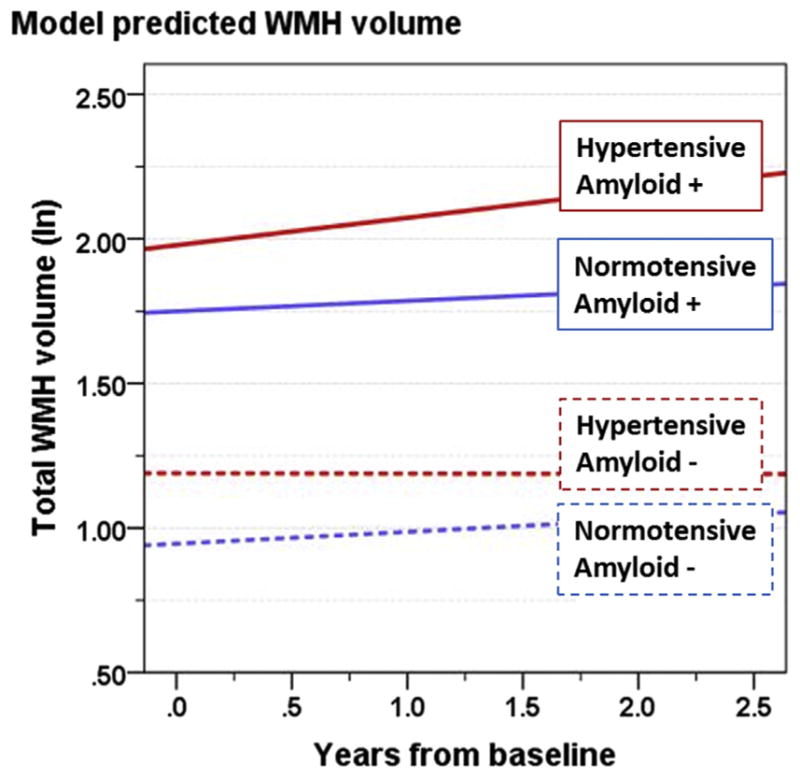
Using parameter estimates from Table 2, estimated trends of WMH volume as a function of years from baseline, are shown for prototype individuals for the following categories: normotensive/negative amyloid (blue, dashed), normotensive/positive amyloid (blue, solid), hypertensive/negative amyloid (red, dashed), and hypertensive/positive amyloid (red, solid). Amyloid positive threshold was CSF Aβ1–42 < 192 pg/mL; the amyloid level used in these calculations is the mean for each category. For each, the intercept represents a 74-year-old, APOE ε4 negative male with a high school education, no hyperlipidemia, and average intracranial volume. Abbreviation: WMH, white-matter hyperintensity. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
Our longitudinal analysis of correlates of WMH accrual in a population with low-to-moderate vascular risk factors and elevated cerebral amyloid burden shows that the combined effects of hypertension and cerebral amyloidosis predict greater contemporaneous WMH volume and accelerated accrual of WMH. Given that over 90% of participants were actively treated for hypertension, our data suggest that this approach is effective in reducing the accrual of WMH, which is in agreement with past studies (Godin et al., 2011). However, we extended this finding to show that the presence of cerebral amyloidosis mitigates this therapeutic effect and results in the greatest amount of WMH accrual over the 2-year period, despite highly compliant treatment of hypertension (Lee et al., 2009).
Although these data are not adequate to determine causal relationships between amyloid and WMH, it is consistent with the hypothesis that vascular injury mechanisms and cerebral amyloidosis may exacerbate each other, leading to accelerated white-matter injury (van Norden et al., 2012). Impaired functioning of cerebral small vessels, driven by systemic vascular risk factors, is 1 mechanism that may both promote development of WMH and hinder cerebral amyloid clearance via perivascular spaces, thus leading to an observed link between the 2 pathologies (Hughes and Sink, 2016). In addition, elevated cerebral amyloid (associated with lower CSF Aβ1–42) may damage vessel walls and promote vasoconstriction, and thus promote WMH irrespective of systemic vascular factors (Thomas et al., 1996). Because WMH in older adults is thought to be primarily a consequence of ischemic tissue damage (Prins and Scheltens, 2015), the contribution of amyloid accumulation to hypoperfusion could be a major factor in WMH accrual.
Although hypertension is traditionally viewed as a predominant vascular determinant of WMH, our data show that greater WMH accrual was more strongly associated with amyloid than it was with hypertension in CN with overall low vascular burden. This finding is consistent with those of the prior cross-sectional study of this population in that greater amyloid burden had a similar effect on total WMH load as hypertension history (Scott et al., 2015). Together, these data support the hypothesis that cerebral amyloidosis and white-matter damage are inter-related and whose relationship could be better understood through larger epidemiological studies that assess cerebral amyloid, vascular risk factors, and WMH.
Although this study demonstrates the relevance of cerebral amyloidosis to white-matter integrity in healthy older adults, the generalizations are limited. First, we are assessing total WMH rather than periventricular WMH exclusively; periventricular WMH has been more strongly associated with amyloid (Gordon et al., 2015; Marnane et al., 2016) and cerebrovascular disease (Black et al., 2009). Thus, the correlation between WMH and hypertension measured presently may be weaker than if periventricular WMH was used. Second, our follow-up period is limited to 2 years. Typically, longitudinal WMH studies use follow-up periods of 3 to 4 years since WMH accrue slowly (e.g., Maillard et al., 2014). Thus, we are modeling subtle expansion of white-matter injury, which is highlighted by the small annual rate of change. On the other hand, we have the benefit of dense sampling within those 2 years with up to 5 data points per individual and therefore have confidence in the significance of the model.
The relationship observed presently may not be found in a typical community-based epidemiological cohort because of the select sample characteristics. Because the ADNI mission is to study Alzheimer’s disease, participant recruitment is biased toward those at greater risk of dementia, and this is supported by the higher than population normal incidence of APOE ε4 carriers in CN (Table 1). Further, the ADNI exclusion criterion of a modified Hachinski score less than 4 prevents enrollment of individuals with severe cerebrovascular disease. Consequently, the contributions of amyloidosis to white-matter damage may be more clearly elucidated in this particular population.
Although a logical next step would be to model modifiers of the accrual rates of amyloid, a 2-year follow-up period was insufficient to detect reliable change in CSF Aβ1–42 in CN individuals. Longer follow up or investigation of cognitively impaired groups may be better suited to address questions regarding amyloid burden risk factors (Gomar et al., 2016).
5. Conclusions
In cognitively normal older adults with low-to-moderate vascular risk factor burden, greater cerebral amyloid burden at baseline is associated with greater WMH accrual over a subsequent 2-year period. Our findings point to the importance of better understanding the roles that vascular risk factor reduction and anti-amyloid therapies can play in the prevention and treatment of asymptomatic brain injury (Black et al., 2009; Hughes and Sink, 2016; Saito and Ihara, 2016).
Acknowledgments
The authors thank Shannon Risacher for providing hypertension medication data tables for ADNI. University of California Davis Alzheimer’s Disease Center P30 AG010129 (Charles DeCarli); California Department of Public Health 13-12004 (Owen T Carmichael, Charles DeCarli). Paul Thompson is also supported by NIH grant U54 EB 020403 (Big Data to Knowledge Centers of Excellence, or the BD2K Program), which is funded by a cross-NIH partnership. Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
Footnotes
Disclosure statement
The authors have no conflicts of interest to disclose.
References
- Abraham HM, Wolfson L, Moscufo N, Guttmann CR, Kaplan RF, White WB. Cardiovascular risk factors and small vessel disease of the brain: blood pressure, white matter lesions, and functional decline in older persons. J Cereb Blood Flow Metab. 2016;36:132–142. doi: 10.1038/jcbfm.2015.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black S, Gao F, Bilbao J. Understanding white matter disease: imaging-pathological correlations in vascular cognitive impairment. Stroke. 2009;40:S48–S52. doi: 10.1161/STROKEAHA.108.537704. [DOI] [PubMed] [Google Scholar]
- Brickman AM. Contemplating Alzheimer’s disease and the contribution of white matter hyperintensities. Curr Neurol Neurosci Rep. 2013;13:415. doi: 10.1007/s11910-013-0415-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael O, Mungas D, Beckett L, Harvey D, Tomaszewski Farias S, Reed B, Olichney J, Miller J, Decarli C. MRI predictors of cognitive change in a diverse and carefully characterized elderly population. Neurobiol Aging. 2012;33:83–95. doi: 10.1016/j.neurobiolaging.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Avanzo C, Sliwinski C, Wagner SL, Tanzi RE, Kim DY, Kovacs DM. Gamma-secretase modulators reduce endogenous amyloid beta42 levels in human neural progenitor cells without altering neuronal differentiation. FASEB J. 2015;29:3335–3341. doi: 10.1096/fj.15-271015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin O, Tzourio C, Maillard P, Mazoyer B, Dufouil C. Antihypertensive treatment and change in blood pressure are associated with the progression of white matter lesion volumes: the Three-City (3C)-Dijon Magnetic Resonance Imaging Study. Circulation. 2011;123:266–273. doi: 10.1161/CIRCULATIONAHA.110.961052. [DOI] [PubMed] [Google Scholar]
- Gold BT, Zhu Z, Brown CA, Andersen AH, LaDu MJ, Tai L, Jicha GA, Kryscio RJ, Estus S, Nelson PT, Scheff SW, Abner E, Schmitt FA, Van Eldik LJ, Smith CD. White matter integrity is associated with cerebrospinal fluid markers of Alzheimer’s disease in normal adults. Neurobiol Aging. 2014;35:2263–2271. doi: 10.1016/j.neurobiolaging.2014.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomar JJ, Conejero-Goldberg C, Davies P, Goldberg TE Alzheimer’s Disease Neuroimaging Initiative. Anti-correlated cerebrospinal fluid biomarker trajectories in preclinical Alzheimer’s disease. J Alzheimers Dis. 2016;51:1085–1097. doi: 10.3233/JAD-150937. [DOI] [PubMed] [Google Scholar]
- Gordon BA, Najmi S, Hsu P, Roe CM, Morris JC, Benzinger TL. The effects of white matter hyperintensities and amyloid deposition on Alzheimer dementia. Neuroimage Clin. 2015;8:246–252. doi: 10.1016/j.nicl.2015.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouw AA, Seewann A, van der Flier WM, Barkhof F, Rozemuller AM, Scheltens P, Geurts JJ. Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. J Neurol Neurosurg Psychiatry. 2011;82:126–135. doi: 10.1136/jnnp.2009.204685. [DOI] [PubMed] [Google Scholar]
- Hughes TM, Kuller LH, Barinas-Mitchell EJ, Mackey RH, McDade EM, Klunk WE, Aizenstein HJ, Cohen AD, Snitz BE, Mathis CA, Dekosky ST, Lopez OL. Pulse wave velocity is associated with beta-amyloid deposition in the brains of very elderly adults. Neurology. 2013;81:1711–1718. doi: 10.1212/01.wnl.0000435301.64776.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes TM, Kuller LH, Barinas-Mitchell EJ, McDade EM, Klunk WE, Cohen AD, Mathis CA, Dekosky ST, Price JC, Lopez OL. Arterial stiffness and beta-amyloid progression in nondemented elderly adults. JAMA Neurol. 2014;71:562–568. doi: 10.1001/jamaneurol.2014.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes TM, Sink KM. Hypertension and its role in cognitive function: current evidence and challenges for the Future. Am J Hypertens. 2016;29:149–157. doi: 10.1093/ajh/hpv180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A, Black SE, Tokar G, Benke T, Carr T, Nicholson L. Periventricular and subcortical hyperintensities on magnetic resonance imaging. ‘Rims, caps, and unidentified bright objects’. Arch Neurol. 1988;45:404–408. doi: 10.1001/archneur.1988.00520280050015. [DOI] [PubMed] [Google Scholar]
- Landau SM, Harvey D, Madison CM, Reiman EM, Foster NL, Aisen PS, Petersen RC, Shaw LM, Trojanowski JQ, Jack CR, Jr, Weiner MW, Jagust WJ Alzheimer’s Disease Neuroimaging Initiative. Comparing predictors of conversion and decline in mild cognitive impairment. Neurology. 2010;75:230–238. doi: 10.1212/WNL.0b013e3181e8e8b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DY, Fletcher E, Martinez O, Ortega M, Zozulya N, Kim J, Tran J, Buonocore M, Carmichael O, DeCarli C. Regional pattern of white matter microstructural changes in normal aging, MCI, and AD. Neurology. 2009;73:1722–1728. doi: 10.1212/WNL.0b013e3181c33afb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu E, Schmidt ME, Margolin R, Sperling R, Koeppe R, Mason NS, Klunk WE, Mathis CA, Salloway S, Fox NC, Hill DL, Les AS, Collins P, Gregg KM, Di J, Lu Y, Tudor IC, Wyman BT, Booth K, Broome S, Yuen E, Grundman M, Brashear HR Bapineuzumab 301 and 302 Clinical Trial Investigators. Amyloid-beta 11C-PiB-PET imaging results from 2 randomized bapineuzumab phase 3 AD trials. Neurology. 2015;85:692–700. doi: 10.1212/WNL.0000000000001877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell MA, Lynn BC, Fister S, Bradley-Whitman M, Murphy MP, Beckett TL, Norris CM. A novel small molecule modulator of amyloid pathology. J Alzheimers Dis. 2016;53:273–287. doi: 10.3233/JAD-151160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillard P, Fletcher E, Lockhart SN, Roach AE, Reed B, Mungas D, DeCarli C, Carmichael OT. White matter hyperintensities and their penumbra lie along a continuum of injury in the aging brain. Stroke. 2014;45:1721–1726. doi: 10.1161/STROKEAHA.113.004084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marnane M, Al-Jawadi OO, Mortazavi S, Pogorzelec KJ, Wang BW, Feldman HH, Hsiung GY Alzheimer’s Disease Neuroimaging Initiative. Periventricular hyperintensities are associated with elevated cerebral amyloid. Neurology. 2016;86:535–543. doi: 10.1212/WNL.0000000000002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok V, Kim JS. Prevention and management of cerebral small vessel disease. J Stroke. 2015;17:111–122. doi: 10.5853/jos.2015.17.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Norden AG, van Dijk EJ, de Laat KF, Scheltens P, Olderikkert MG, de Leeuw FE. Dementia: Alzheimer pathology and vascular factors: from mutually exclusive to interaction. Biochim Biophys Acta. 2012;1822:340–349. doi: 10.1016/j.bbadis.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Prins ND, Scheltens P. White matter hyperintensities, cognitive impairment and dementia: an update. Nat Rev Neurol. 2015;11:157–165. doi: 10.1038/nrneurol.2015.10. [DOI] [PubMed] [Google Scholar]
- Provenzano FA, Muraskin J, Tosto G, Narkhede A, Wasserman BT, Griffith EY, Guzman VA, Meier IB, Zimmerman ME, Brickman AM Alzheimer’s Disease Neuroimaging Initiative. White matter hyperintensities and cerebral amyloidosis: necessary and sufficient for clinical expression of Alzheimer disease? JAMA Neurol. 2013;70:455–461. doi: 10.1001/jamaneurol.2013.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S, Ihara M. Interaction between cerebrovascular disease and Alzheimer pathology. Curr Opin Psychiatry. 2016;29:168–173. doi: 10.1097/YCO.0000000000000239. [DOI] [PubMed] [Google Scholar]
- Scott JA, Braskie MN, Tosun D, Thompson PM, Weiner M, DeCarli C, Carmichael OT Alzheimer’s Disease Neuroimaging Initiative. Cerebral amyloid and hypertension are independently associated with white matter lesions in elderly. Front Aging Neurosci. 2015;7:221. doi: 10.3389/fnagi.2015.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, Dean R, Siemers E, Potter W, Lee VM, Trojanowski JQ Alzheimer’s Disease Neuro-imaging Initiative. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw LM, Vanderstichele H, Knapik-Czajka M, Figurski M, Coart E, Blennow K, Soares H, Simon AJ, Lewczuk P, Dean RA, Siemers E, Potter W, Lee VM, Trojanowski JQ Alzheimer’s Disease Neuroimaging Initiative. Qualification of the analytical and clinical performance of CSF biomarker analyses in ADNI. Acta Neuropathol. 2011;121:597–609. doi: 10.1007/s00401-011-0808-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T, Thomas G, McLendon C, Sutton T, Mullan M. Beta-amyloid-mediated vasoactivity and vascular endothelial damage. Nature. 1996;380:168–171. doi: 10.1038/380168a0. [DOI] [PubMed] [Google Scholar]
- Toledo JB, Xie SX, Trojanowski JQ, Shaw LM. Longitudinal change in CSF tau and Abeta biomarkers for upto 48 months in adni. Acta Neuropathol. 2013;126:659–670. doi: 10.1007/s00401-013-1151-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf D, Fischer FU, Scheurich A, Fellgiebel A Alzheimer’s Disease Neuroimaging Initiative. Non-linear association between cerebral amyloid deposition and white matter microstructure in cognitively healthy older adults. J Alzheimers Dis. 2015;47:117–127. doi: 10.3233/JAD-150049. [DOI] [PubMed] [Google Scholar]


