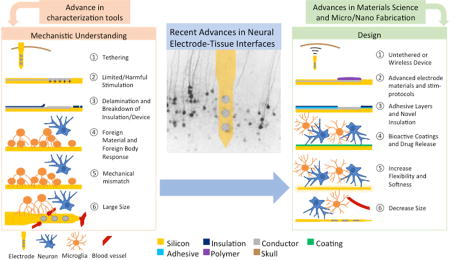
Abstract
Neurotechnology is facing an exponential growth in the recent decades. Neural electrode-tissue interface research has been well recognized as an instrumental component of neurotechnology development. While satisfactory long-term performance was demonstrated in some applications, such as cochlear implants and deep brain stimulators, more advanced neural electrode devices requiring higher resolution for single unit recording or microstimulation still face significant challenges in reliability and longevity. In this article, we review the most recent findings that contribute to our current understanding of the sources of poor reliability and longevity in neural recording or stimulation, including the material failure, biological tissue response and the interplay between the two. The newly developed characterization tools are introduced from electrophysiology models, molecular and biochemical analysis, material characterization to live imaging. The effective strategies that have been applied to improve the interface are also highlighted. Finally, we discuss the challenges and opportunities in improving the interface and achieving seamless integration between the implanted electrodes and neural tissue both anatomically and functionally.
Graphical abstract
1. Introduction
Neurotechnology is facing an exponential growth in the recent decades thanks to the advances demonstrated by brain machine interface human trials and clinical successes in neuromodulation therapies. A core component of neurotechnology involves invasive electrode devices interfacing directly with neural tissue for recording and/or stimulation. While satisfactory long-term performance was demonstrated in some applications, such as cochlear implants and deep brain stimulators, more advanced neural interfacing devices requiring higher resolution for single unit recording or microstimulation still face significant challenges in reliability and longevity. The most significant challenge lies in the neural electrode-tissue interface, where a man-made device is brought in contact with biological neural tissue and electrical voltages or currents are being transmitted across the electrode-tissue interface. Like any implantable devices, the highly corrosive and dynamic environment of the host tissue is hostile to implants, among which micro-electronic devices are especially vulnerable. Although an old topic, the material and mechanical reliability of neural electrode arrays continue to be a critical area of research, and in our opinion, deserves more attention especially in the development of newer and more advanced devices. Conversely, the implantation and presence of an artificial device elicits acute injury and chronic inflammatory reactions that lead to tissue remodeling, degeneration and regeneration that alter the microenvironment with which the device is interfacing. Dynamic changes in the neural tissue around the implants affect the quality and stability of the neural electrode recording and/or stimulation performance, and this has been a hot area of research in recent years. Advanced electrodes are being designed to mitigate the issues faced when chronically interfacing with traditional electrodes by changing the geometry, increasing flexibility, and incorporating bioactive coatings and drugs. This article intends to 1) review the most recent findings that contribute to our current understanding of the unsatisfactory quality, stability and longevity of neural recording or stimulation, 2) highlight the development of characterization tools for the study of neural electrode-tissue interface, 3) summarize the strategies that have been applied to improve the interface, and 4) finally discuss the challenges and opportunities in improving the interface and achieving seamless integration between the implanted electrodes and neural tissue both anatomically and functionally. This article provides an overview of nervous system electrodes, but puts emphasis on central nervous system recording electrodes. For more detailed discussion on peripheral nervous system devices and stimulating electrodes, we would like to kindly direct the reader towards the complementary articles found in this issue of “Current Opinion in Biomedical Engineering” (titled “Peripheral Nerve Interfaces for Limb Prosthetics” and “Central Nervous System Microstimulation”. respectively).
2. Current Understanding of Failure Mechanisms
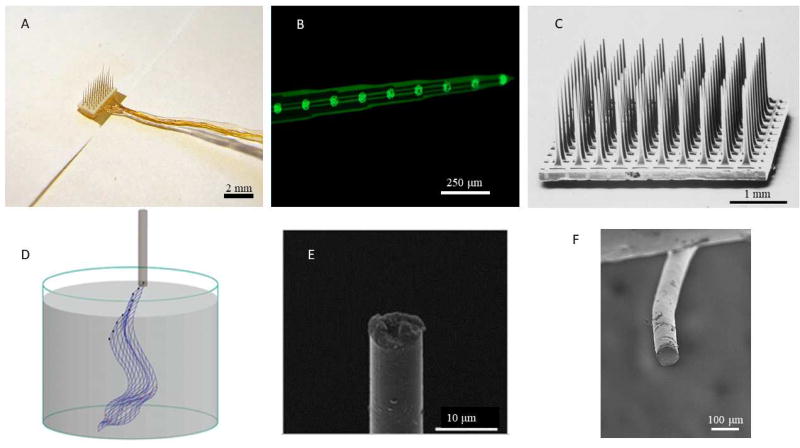
Recording the highest quality single neuron activity in the brain requires penetrating electrodes [1]. While many designs have been utilized for electrode devices implanted in the brain parenchyma, almost all electrodes have certain basic features: electrode sites of a conductive material, conducting leads connecting the electrode sites and external electrical components, and an insulating layer defining the electrode site areas and protecting the connection from electrical shunting. While microwire arrays (bundles of insulated metal wires), Michigan electrodes (planar arrays), and Utah Arrays (bed of needles) are some of the more well-known and studied designs, many new designs and materials have been proposed for neural interfacing (Fig 1). The multitude of device designs and materials is well covered by Patil and Thakor in their review [2]. While neural electrodes have advanced greatly over the past few decades, recording and stimulation performance is highly variable and most devices show failure after chronic implantation ranging from weeks to months and a few years. A summary of device performance from selected studies is shown in Table 1. The causes of variability and long-term failure have been attributed to mechanical/material and biological factors.
Figure 1.
Comparison of selected traditional and advanced electrodes. (A) Microwire array, reproduced with permission from Microprobes for Life Sciences Inc. (B) Planar (Michigan) electrode. (C) Bed of needles (Utah) array [112], copyright 1998 Elsevier. (D) Syringe injectable mesh electrode [80], copyright 2017 National Academy of Sciences. (E) Carbon fiber electrode [47], copyright 2012 Nature Publishing. (F) Conducting elastomer microwire electrode [29].
Table 1.
Summary of selected studies of device failure for traditional electrode designs. For each study, samples which did not undergo the entire chronic implantation were removed.
| Electrode Type |
Animal | Number of arrays (Electrodes) |
Time course of experiment (Days) |
Yield at end of experiment (%) |
Total Failure (%) |
Ref. |
|---|---|---|---|---|---|---|
| Utah 10×10 | Monkey | 69 | 2104 | N/A | 79 | [3] |
| Michigan Single Shank | Mouse | 3 (36) | 133–189 | N/A | N/A | [5] |
| Tungsten Microwire | Rat | 12 (192) | 260 | 24.6* | 75.4 | [4] |
| Pt/Ir Microwire | Rat | 6 (96) | 71–180 | 33** | N/A | [6] |
Average yield on the last day of the implantation
Average yield for the entirety of the chronic phase of implantation.
2.1 Mechanical and Material Failure
Despite decades of research and development, mechanical and material failures are still a major contributor of performance failure for neural electrode devices. In a non-human primate study examining chronic recording performance of the Utah array (Blackrock Microsystems), mechanical and material failure have been reported to be the greatest cause of failure, accounting for upwards of 48% of all failure in the first year [3]. While many failures occurred at the percutaneous connectors and wire bundles, further difficulties arise with de-insulation, corrosion, and cracking of the electrodes directly interfacing with the neural tissue.
Several recent studies characterized different types of material failures and their recording/stimulation consequences [4, 5]. One potential cause of material failure is de-insulation along the electrode or at the electrode tip. De-insulation at the electrode site increases the surface area exposed, decreasing the impedance of the electrode but also decreasing the recording quality [6]. Another notable observation is that failure is variable from animal to animal, with some electrode arrays recording on 80% of channels while others produced no recording [4]. One interesting study focused on the mechanical and material failure mode analysis on chronically implanted planar microelectrode arrays (Michigan probes, NeuroNexus) with multiple electrode sites along the shanks [5]. By correlating neural recordings, electrochemical impedance spectroscopy, and scanning electron microscopy of the explanted probes with Finite Element Modeling (FEM), several modes of material failure were identified that resulted in degradation and/or loss of recording, including loss of the metal site, delamination and cracking. Interestingly, cracking and delamination of conducting traces in vivo after long term implantation were most frequently observed near the electrode sites where the strain is most concentrated as determined by FEM, due to the mechanical mismatch between the iridium and silicon layers. This study points out the regions of the planar devices most vulnerable to mechanical stress induced failure, and can be used as a guide to design more robust planar arrays in the future. While this study focused on planar brain electrodes, the results are likely applicable to any electrode in the central or peripheral nervous system whose components have clashing mechanical properties.
Material corrosion/degradation can occur naturally, or be accelerated through electrical stimulation. Corrosion products have concerns of toxicity [7], but the greatest difficulty lies in loss of the structural stability and electrical functionality of the device. Implanted tungsten recording electrode exhibited a high degree of corrosion and subsequent delamination of their insulation [4]. The common electrode material silicon dioxide may dissolve away in aqueous environments at the rate of 3.7–43.5 pm h−1 [8, 9]. Smaller devices are more susceptible to degradation, as such nanowires fabricated from silicon or assorted semi-conductors will degrade away in a few weeks unless protected [10].
Stimulations pose additional harsh conditions to the electrodes. Improper stimulation can lead to electrode damage (metal corrosion, metal site detachment and insulation failure) and tissue inflammation due to electrolysis of water [11], local pH shifts [11], generation of free radicals, and release of metal ions [12]. Even some of the more electrochemically stable carbon electrodes can be oxidatively etched by stimulation [13]. As such, materials capable of withstanding high degrees of repetitive electrical stimulation and have a large charge injection capacity continue to be investigated [14, 15]. Using a combination of experimental data and theoretical analysis, Cogan et al. demonstrated that for microstimulation utilizing microelectrodes, the previously defined tissue damage limit using charge density and charge per phase by Shannon’s equation, no longer applies [12, 16–18]. This study calls for new considerations and tests in order to establish and validate safe stimulation limit for microelectrodes.
2.2 Biological Tissue Response
Regardless of implant location, biological tissue response against the implants is a major cause of electrode failure. On the macro scale, meningeal fibroblasts may migrate down the electrode shanks from the brain surface, contribute to the scar formation [19]. In more severe cases, the dural overgrowth may even encapsulate the whole device, resulting in ejection of the probes and signal loss. On the micro scale, several types of cells are involved in the inflammatory response to the implants, known as the foreign body response (FBR). For comprehensive review of the cellular responses, see review [20–22]. Briefly, microglial cells were immediately activated upon implantation [23] and release various inflammatory factors to recruit monocytes and astrocytes [24]. These activated microglia/macrophages remain at the vicinity of the implants over long-term implantations, and are surrounded by a dense layer of astrocytes, often referred to as glial scar. Glial encapsulation insulates electrodes from nearby neurons, increasing the impedance and the distance between electrodes and viable neurons [25]. Meanwhile, neurons (cell body and processes) may be damaged during insertion, pushed away by the glia scar, or degenerated by reactive oxygen species and proinflammatory or cytotoxic factors released from the chronic inflammation and/or become less active due to mechanical strain or disconnect from the rest of the network. It is assumed that these biological effects will result in recording or stimulation failure, but the contribution of each mechanism to device function has not been clearly understood.
One trigger of cellular responses and high degree of variability is vascular damage or breach of blood brain barrier (BBB). Electrode implantations inevitably break vasculature to various degrees. BBB leakage leads to release of blood cells, clotting factors and neurotoxic plasma proteins, and monocytes recruitment. A two-photon imaging study demonstrated that inflammatory tissue response may be minimized by reducing the vascular damage during insertion [26]. BBB damage does not stop at the insertion injury, more data have shown persistent BBB leakage during chronic implantation time, which is inversely related to electrode performance [27].
Mechanical mismatch between the biological tissue and electrode devices may be of concern for chronic implantation. Conventional neural electrode devices are made of metal and silicon having mechanical modulus several orders of magnitude higher than the brain or peripheral nervous tissue. During breathing or movement of the animal, micromotion between the brain tissue and the inserted electrodes pose strain to the surrounding tissue, which lead to neural apoptosis [28]. Based on this, various mechanically soft and flexible electrode devices have been developed and when compared to the stiff devices, reduced inflammatory tissue responses have indeed been observed [29–31]. The mechanical strain may be aggravated by tethering the electrodes on the skull as opposed to free floating ones. Tethering increases the relative movement between implanted electrodes and the brain tissue and prevents the healing of the BBB, both of which may worsen the inflammation and cause neuronal degeneration or demyelination [32, 33].
Another mechanism for the implant to cause persistent inflammation is thought to be the accumulation of inflammatory cells and their inflammatory products. This is supported by two studies. In the first study, lattice probes were compared to solid probes of the same dimension and materials [34]. The lattice probes were designed with a low surface area and an open architecture to allow inflammatory products to freely diffuse away from the electrode. The study found that the lattice devices resulted in reduced inflammatory response when compared to solid probes. In the second study, diffusion sinks were created on the electrode using a thick hydrogel coating, and significantly reduced foreign body response was also observed [35].
Surface chemistry of the implant has been hypothesized to play a role on host tissue response, because plasma protein adsorption and inflammatory cell attachment occur on the surface are the earliest events after implantation. No differences in glial and neuronal responses have been found between Parylene C and silicon dioxide surfaces, two commonly used insulation materials [33], and polyethylene glycol based hydrogel coatings did not shown benefit [36]. These results suggest that physical chemistry alone may not be sufficient to alter the host tissue response. Bioactive coatings [37, 38] that interact with the host tissue via biologics or therapeutics may be necessary to actively modulate the tissue response.
In order to find the right bioactive intervention, it is important to identify the molecular pathways critical to the host tissue responses. Several nice studies utilizing knockout animals or RNA array analysis have shed new light on the relevant molecular pathways. For example, Caspase-1 knock out (KO) mice implanted with the Michigan probes have shown improved electrophysiological recording performance, indicating that caspase-1 mediated inflammation and/or apoptosis pathways are playing an important role [39]. In another study, foreign body response to intracortical silicon implants in MCP-1(also CCL-2) KO mice was reduced [40], possibly through inhibiting the TNF induction and NFκB pathway [41].
In sum, the biological tissue response to the implants involves a cascade of reactions in multiple types of cells. While it is clear that the biological response to implants contributes to the devices performance, the contributions of different mechanisms (glial scar, neuronal degeneration and loss, inflammation etc.) to the failure of implanted devices remains to be determined. Furthermore, multiple triggers of these biological responses have been proposed but the challenge is to separate the impact of many biological factors and physicochemical triggers. To date, no single mechanism or factor has been found to predominate the performance of electrodes.
2.3 Interplay Between Material Response and Biological Response
As mentioned before, implantations of artificial devices cause tissue injury and inflammation, which may lead to release of free radicals, reactive oxygen and nitrogen species. These species may not only damage the tissue, but also accelerate the degradation of the electrode materials. In an in vitro study, tungsten electrodes exhibit a heightened degree of corrosion when exposed to common reactive oxygen species and H2O2 [42, 43]. This correlates with in vivo findings, where tungsten wires exhibit the greatest degree of degeneration immediately after implantation, likely due to the increased free radical concentration and comparatively harsh environment around the implant post-surgery [4]. In addition to tungsten, reactive oxygen species showed corrosive effects on Pt/Ir, Pt, Ir, Au, Silicon Nitride, Polyimide, and Parylene-C [43]. Depending on the type of degrading material, degradation products may worsen the inflammation. This leads to more reactive species, which in turn accelerates the material failure. Therefore, in vitro testing protocols and in vivo evaluations need to take into account these important interplays.
3. Methodology Development for Characterizing the Interface
Evaluation and characterization of the interface are of crucial importance for understanding the interaction between host tissue and neural implants and for guiding the device designs.
First, to evaluate the functionality of electrodes, electrophysiological recording is often necessary. Additionally, electrophysiological recording can be used as a characterization tool to probe neuronal health and activity at the interface. Careful consideration needs to be taken when selecting a recording model. For generic studies focusing on understanding the electrode-tissue interface, a model that is most reliable and reproducible would be preferred. For studies intended to evaluate functionality of devices in a specific application, the model should mimic the end use situation. Rodent models are the most common for generic studies due to low cost, well-established recording paradigms and broad availability of antibodies and genetic tools. While anesthetized recording are often criticized for being heavily influenced by the types and levels of anesthetics, awake recordings are often contaminated by electromagnetic motion artifact as the animal moves, making it difficult to distinguish electromechanical noise and neural activity [44]. A visually evoked rodent neural recording model was reported with detailed description of comprehensive evaluations [45]. It was demonstrated that compared to spontaneous recording, evoked response is more reproducible, elicits more single unit firing, and allows the quantification of multiunit and local field potential recordings. In this work, it was clearly demonstrated that single unit and multi-unit recording performance varies significantly in different anatomical layers, highlighting the need for layer dependent analysis taking into account the heterogeneous architecture of the brain. Some published studies compared the recording yield (% of channels detecting single units) of MEAs with all electrode sites located at layer 4 (where the activity is the highest by default) to MEAs that have electrode sites spanning multiple layers of the brain [46, 47]. Such comparison puts linear arrays at significant disadvantage.
Postmortem histology and immunohistochemical staining has been the most common way to measure cellular tissue response. Commonly used makers are GFAP for astrocytes, Iba-1 or ED-1 for microglial/macrophages, NF for neurofilament, NeuN for neuron nuclei, and IgG, MSA or ferritin for BBB leakage. More recently, new markers such as PDGFRβ+ for pericytes, APC for oligodendrocytes, caspase 3 for apoptosis [39], CD80 for M1 type microglia, CD 163, CD 206, and Arg-1 for M2 type microglia [40], have been applied to indicate previously unexplored cell types, different phenotypes or health state of the cells. To take a further look into the molecular mechanism underlying the cellular reactions, RNA could be extracted specifically from the region of implantation by laser capture microdissection, followed by RT-PCR arrays to quantify the expression of various molecules in the inflammation or apoptosis pathways. Using this method, upregulation of IL-36Ra (≈ 20-fold) and IL-1Ra (≈ 1500-fold) was found at the vicinity of implanted microwire arrays 3 days post implantation. Using stretched microglia and astrocyte cultures, upregulation of interleukin receptor antagonist IL-36R1 was found to be strain induced and may negatively impact neuronal health upon electrode implantation [28].
Postmortem histology is naturally low in temporal resolution. To catch cellular and vascular dynamics, and correlates changes of tissue characteristics to recording performance in real time, various in vivo live imaging approaches have been developed. Two-photon microscopy (TPM) has been used to visualize vascular damage and remodeling, microglial polarization and migration [48, 49], or shape and activity of the neurons around electrode devices [20, 50] before and after electrode array insertion and over extended periods of time. Compared to technologies like MRI and microCT, multi-photon microscopy offers high spatial resolution for resolving cellular processes, sufficient temporal resolution for tracking calcium activity, and ample cell type specific labeling (Capabilities of various imaging modality is summarized in Table 2). Another optical live imaging method, optical coherence tomography (OCT), has been used to characterize tissue displacement and changes in capillary perfusion and flow velocity during probe implantation [51], as well as vascular remodeling over extended implantation [52]. Although having lower spatial resolution, OCT’s higher depth penetration and angiography capability may complement TPM [53]. As a number of technical challenges (such as maintaining stable chronic imaging window and simultaneous imaging and recording) are overcome, in vivo microscopy is poised to shed many new insights on neural electrode-tissue interface dynamics.
Table 2.
Common imaging modalities and their resolutions, depth of penetration, typical usages, and in vivo compatibility.
| Spatial Resolution |
Temporal Resolution |
Penetration Depth |
Level of Visualization |
In Vivo Compatible |
Sample papers |
|
|---|---|---|---|---|---|---|
| Fluorescence microscopy | ~0.2 µm | N/A | ~100 µm | Molecule, Cell | No | [58] |
| Two-photon microscopy (TPM) | ~0.2 µm | ~10 ms | ~1 mm | Molecule, Cell | Yes | [48, 49] |
| MRI | ~0.1 mm | ~10 ms | No limit | Blood oxygenation | Yes | [113] |
| MicroCT | ~10 µm | ~10 ms | ~50 mm | Bone, soft tissue | Yes | [114] |
| Optical coherence tomography (OCT) | ~5 µm | ~5 ms | ~2 mm | Vasculature, Tissue Architecture | Yes | [51–53] |
| Scanning Electron Microscopy (SEM) | ~nm | N/A | N/A | Material Surface, Large Molecule, Cell | No | [56] |
Another non-invasive way of probing neural electrode-tissue interface is via electrochemical impedance spectroscopy (EIS). EIS can be used to evaluate the electrochemical property of the electrode sites, electrical connectivity of the conducting leads, as well as the quality of the insulation. Additionally, tissue response such as protein adsorption, edema, array ejection and glial encapsulation can also affect the characteristics of the EIS. Equivalent circuit models may be used to relate different aspects of the electrode-tissue interface to circuit model components, which would provide more information than single frequency impedance modulus [54, 55]. However, care needs to be taken to draw conclusions using these models, as many biotic and abiotic changes are not easily distinguishable solely based on EIS.
Explant analysis is indispensable in uncovering the mechanical and material failure of the electrodes integrity by examining the device at high magnification using scanning electron microscopy (SEM) [56]. The cellular species attached to the explanted probes can also be immunostained to identify cell types [57] or even cultured to interrogate the secreted products [58].
Because of the highly variable dynamic nature of both the electrode and biological tissue and their interplay, understanding the neural electrode tissue interface requires more advanced molecular and imaging tools, more comprehensive examination that look at both biotic and abiotic features, and moving away from bulk averaging methodology and moving toward depth and region specific or even individualized diagnosis.
4. Strategies
Strategies to improve the chronic performance of the electrodes are widely varied in the field including altering implant dimensions and mechanical properties, improving material stability and functionalities, surface coatings, and drug delivery to minimize the FBR.
As discussed before, size of implants contributes to FBR via multiple mechanisms. Smaller sizes reduce the magnitude of BBB damage and tissue displacement, reduce surface area and may also decrease the device stiffness by decreasing the cross-sectional area. For these reasons, reducing the size of implanted electrode devices to subcellular level i.e. below several microns [10, 59–65] has been actively pursued by many groups. One study pioneered the use of carbon fiber (7 µm in diameter) for chronic neural recording and demonstrated negligible gliosis and neuron loss [47]. This study motivated several groups to fabricate arrays of carbon fiber for multisite recording/stimulation [66, 67]. However, making carbon fiber arrays is a labor intensive and largely manual operation, and the array configuration is limited to one electrode site per fiber. Lieber’s lab has been successful in developing SU-8 based 3D macroporous mesh electrode arrays using micro/nanofabrication technology with features sizes less than 10 µm and exceptionally low bending forces, resulting in minimum foreign body responses and intimate neuron-device interaction [63]. Chronic recording with such mesh electrodes was demonstrated for at least 8 months [68].
In efforts to reduce mechanical mismatch between the electrode and the nervous tissue, electrodes constructed to be soft and flexible [63, 69–71] or stretchable [29, 57, 72, 73] continues to be a hot topic of research. Increasing flexibility can be achieved via reducing the cross sectional area [50] or increasing the length of the device, without changing the material properties [74]. Alternatively, novel soft and/or elastic conducting materials may be developed. Many of these flexible and elastic electrodes have demonstrated reduced FBR chronically, but rigorous and direct comparisons in chronic recording performance between soft electrode and traditional silicon/metal electrodes remain to be seen.
Creating soft and flexible electrodes presented difficulties with insertion. Many of these difficulties are handled by coatings such as silk [75, 76] and carboxymethyl cellulose [77], or shuttles [69, 78, 79], syringe injection [80] and magnetic insertion [81]. Materials that are stiff at room temperature and soften after implantation in the brain are another interesting strategy [82, 83], however, these materials tend to absorb water in vivo, which challenges the insulation. Functional devices with these mechanically adaptive materials have yet to be demonstrated.
Mechanical mismatch can also be minimized by removing the tether. Note “tether” here means the interconnect between the device that is in the brain tissue and the connector that is usually mounted on the skull. This may be accomplished by implanting the entire device below the meninges [84], and using ultrasonic [85, 86] or induction [87] based power supplies for signal and/or power communication. While currently limited in ability to gather and transmit high quality information, the potential benefits of wireless devices are tremendous.
Bioactive coatings work by directly modulating the cellular activity around the electrode via bioactive molecules immobilized on implant surface or released from coatings [37, 88–90]. One strategy is to promote neuron-electrode integration by surface-immobilizing biomolecules that encourage neuronal attachment and growth. Extracellular matrix protein laminin has been applied to the surface of neural probes as “neuro-integrative” coating. Interestingly, no improvement on neuronal growth was found but the coating appears to increase the initial inflammatory response, while attenuating the activation of microglia and astrocytes after 4 weeks [91]. This burst in proinflammatory signaling may aid in acute wound healing and minimize long term tissue damage around the electrode. On the other hand, use of L1, a neuron specific cell adhesion molecule, has resulted in increased neuronal attachment along with decreased gliosis around the implants [38, 49, 92, 93]. More recently an in vivo TPM study revealed that microglia cells send processes to probes coated with L1 immediately after implantation at a similar speed as they do to uncoated controls. However upon arriving at the probe surface, the spreading of microglia processes was significantly reduced by the surface immobilized L1 [49]. This study suggests that there is a window of opportunity to modulate the initial cellular behavior via bioactive surface cues.
Alternatively, drug delivery can be effective at reducing the implantation trauma and decreasing inflammation and degeneration around the site of implantation [88, 94]. While systemic administration bears the risk of side effects [95], local delivery from coatings faces the challenge of drug exhaustion after long-term implantation [88–90]. For sustained release, drug delivery channels may be incorporated into the electrode device [96–98]. Due to the electrical nature on neural implants, electrically driven drug release may be beneficial in delivering drug locally on demand [89, 99, 100]. While the effects of drug delivery on neural degeneration and glial encapsulation are quite pronounced, the long-term effects on neural recording quality are yet to be established.
As devices are made smaller and more flexible, more advanced and robust materials are required. Ultrasmall and flexible devices are more prone to mechanical and material failure by the nature of their geometry. In addition, these devices often require very thin insulation. However, almost all thin (<1 um) insulating materials have problems with long term stability and reliability [101], indicating the need for greatly improved insulation materials/methods. Xie et al. has improved the insulation by first depositing a 52 nm adhesive layer of Al2O3 via atomic layer deposition before the Parylene C coating [102], however, results seem to vary between positive results for planar electrodes [103] and potentially detrimental results for bed of needle style electrodes [104]. Thermally deposited silicon dioxide (100 nm) on silicon wafers has shown exceptionally good resistance to water penetration as well as highly uniform corrosion [101]. For flexible electronics, materials not only need to present sufficient flexibility, but also need to maintain the conductivity upon flexing/stretching. A notable development in elastic conductors has been produced through nanoconfinement effect [105]. These promising materials have yet to be introduced into neural devices.
Device size reduction also leads to necessary decrease in the size of the electrode sites, increasing the site impedance. While the small size of electrode sites may improve single neuron discrimination, very high impedances can increase the noise of recording or prevent effective stimulation. A potential solution may be found in conducting polymers. Conducting polymer coatings are known to dramatically decrease electrode impedance and increase charge injection limit [18, 106], and have been used to enable recording from ultrasmall electrode sites [47, 107]. New advances in conducting polymers may be found in novel dopants [61, 89], drug delivery [89], and novel monomers and crosslinkers for ease of functionalization or improved stability [108–111]. These novel polymer coatings may allow for further decreased size of both recording and stimulating electrodes for central and peripheral nervous system applications.
5. Conclusions and Outlook
Neural electrode-tissue interface research has been well recognized as an instrumental component of neurotechnology development. Multiple funding initiatives of the recent years have attracted many research groups to join force in understanding the interactions between implanted devices and neural tissue, and how these interactions affect neural electrode performances. The advances in molecular, biochemical and imagining tools have brought new insights. Combining high resolution, real time tracking of the interface in conjunction with electrophysiology may more definitively identify various modes of recording failure. Based on the current understanding, the trend of novel neural interfacing devices is to go smaller, softer and more flexible, and wireless. While certainly attractive, such devices present additional challenges on material stability and device durability. Revolutionary advances in material science and fabrication technologies may be needed to achieve required long-term stability for these devices. Meanwhile, numerous reports have shown that the biological tissue responses can be modulated using bioactive and genetic approaches. The next generation device design should take advantage of these biological approaches to actively modulate the host tissue. It is our opinion that the ideal neural electrode will require a combinatorial approach, incorporating biomimetics and advanced materials and fabrication to seamlessly interface with the nervous system.
Highlight.
Material failure is still a significant factor in neural recording/stimulation and stimulation electrodes
More comprehensive understanding of the vascular and cellular responses to the insertion injury and foreign body may be gained via molecular approach and novel markers
Characterization of the neural electrode-tissue interface needs to take a comprehensive approach to examine both abiotic and biotic factors and the interplay between them.
Advanced live imaging technologies have begun to reveal new dynamic insight at the neural electrode-tissue interface with real potential to correlate cellular and vascular characteristics to recording performance
Smaller, softer, more flexible and wireless devices have showed great promise in seamless neural tissue integration, yet these devices face challenges with material stability and device reliability
Bioactive approaches via biomimetic coatings and drug delivery to actively modulate the host tissue response is another promising strategy to improve the interface
Acknowledgments
The authors would like to thank the financial support from NIH NINDS (Grant 5R01NS062019, 1R01NS089688).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schwartz AB, et al. Brain-Controlled Interfaces: Movement Restoration with Neural Prosthetics. Neuron. 2006;52(1):205–220. doi: 10.1016/j.neuron.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 2.Patil AC, Thakor NV. Implantable neurotechnologies: a review of micro- and nanoelectrodes for neural recording. Medical & Biological Engineering & Computing. 2016;54(1):23–44. doi: 10.1007/s11517-015-1430-4. [DOI] [PubMed] [Google Scholar]
- 3.Barrese JC, et al. Failure mode analysis of silicon-based intracortical microelectrode arrays in non-human primates. Journal of neural engineering. 2013;10(6):066014–066014. doi: 10.1088/1741-2560/10/6/066014. This work analyses the forms of failure in bed of needles style utah arrays. Barrese et al. give a comprehesive evaluation of mechanical, material, and bilogical causes of electrode failure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abhishek P, et al. Comprehensive characterization and failure modes of tungsten microwire arrays in chronic neural implants. Journal of Neural Engineering. 2012;9(5):056015. doi: 10.1088/1741-2560/9/5/056015. [DOI] [PubMed] [Google Scholar]
- 5.Kozai TDY, et al. Mechanical failure modes of chronically implanted planar silicon-based neural probes for laminar recording. Biomaterials. 2015;37:25–39. doi: 10.1016/j.biomaterials.2014.10.040. This work utalises in vivo studies and finite element analysis to examine the causes of mechanical failure of chronically implanted michigan style shank electrodes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prasad A, et al. Abiotic-biotic characterization of Pt/Ir microelectrode arrays in chronic implants. Frontiers in Neuroengineering. 2014;7:2. doi: 10.3389/fneng.2014.00002. This work analyses both the acute and chronic degredation of platinum iridium microwire electrodes. Notable changes occurred in electrochemical properties which corelated to the physical characteristics after explantation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao S, et al. Graphene Encapsulated Copper Microwires as Highly MRI Compatible Neural Electrodes. Nano Letters. 2016;16(12):7731–7738. doi: 10.1021/acs.nanolett.6b03829. [DOI] [PubMed] [Google Scholar]
- 8.Ahn S, et al. Quantification of surface etching by common buffers and implications on the accuracy of label-free biological assays. Biosensors and Bioelectronics. 2012;36(1):222–229. doi: 10.1016/j.bios.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 9.Brady PV, Walther JV. Kinetics of quartz dissolution at low temperatures. Chemical Geology. 1990;82:253–264. [Google Scholar]
- 10.Zhou W, et al. Long Term Stability of Nanowire Nanoelectronics in Physiological Environments. Nano Letters. 2014;14(3):1614–1619. doi: 10.1021/nl500070h. Zhou et al. examine 2 nanowires, one of bare silicon and one of silicon coated in an insulating layer of Al2O3. While the bare silicon electrode degraded over the several days, the insulated wire lasted past 100 days. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brummer SB, Robblee LS, Hambrecht FT. CRITERIA FOR SELECTING ELECTRODES FOR ELECTRICAL STIMULATION: THEORETICAL AND PRACTICAL CONSIDERATIONS. Annals of the New York Academy of Sciences. 1983;405(1):159–171. doi: 10.1111/j.1749-6632.1983.tb31628.x. [DOI] [PubMed] [Google Scholar]
- 12.Merrill DR, Bikson M, Jefferys JGR. Electrical stimulation of excitable tissue: design of efficacious and safe protocols. Journal of Neuroscience Methods. 2005;141(2):171–198. doi: 10.1016/j.jneumeth.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 13.Takmakov P, et al. Carbon Microelectrodes with a Renewable Surface. Analytical Chemistry. 2010;82(5):2020–2028. doi: 10.1021/ac902753x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arreaga-Salas DE, et al. Integration of High-Charge-Injection-Capacity Electrodes onto Polymer Softening Neural Interfaces. ACS Applied Materials & Interfaces. 2015;7(48):26614–26623. doi: 10.1021/acsami.5b08139. [DOI] [PubMed] [Google Scholar]
- 15.Lu Y, et al. Flexible Neural Electrode Array Based-on Porous Graphene for Cortical Microstimulation and Sensing. Scientific Reports. 2016;6:33526. doi: 10.1038/srep33526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brummer SB, Turner MJ. Electrochemical Considerations for Safe Electrical Stimulation of the Nervous System with Platinum Electrodes. IEEE Transactions on Biomedical Engineering. 1977;BME-24(1):59–63. doi: 10.1109/TBME.1977.326218. [DOI] [PubMed] [Google Scholar]
- 17.Cogan SF, et al. Tissue damage thresholds during therapeutic electrical stimulation. Journal of Neural Engineering. 2016;13(2):021001. doi: 10.1088/1741-2560/13/2/021001. Previous works have established the shanon equation for macroelectrodes and theoretical safe stimulations. Cogan et al. examine the levels of stimulation which are safe for microelectrodes, finding that microelectrode do not exhitit the same charge per phase and charge density relationships found in the shannon equation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cogan SF. Neural Stimulation and Recording Electrodes. Annual Review of Biomedical Engineering. 2008;10:275–309. doi: 10.1146/annurev.bioeng.10.061807.160518. [DOI] [PubMed] [Google Scholar]
- 19.Cui X, et al. In vivo studies of polypyrrole/peptide coated neural probes. Biomaterials. 2003;24(5):777–787. doi: 10.1016/s0142-9612(02)00415-5. [DOI] [PubMed] [Google Scholar]
- 20.Kozai TD, et al. Brain tissue responses to neural implants impact signal sensitivity and intervention strategies. ACS chemical neuroscience. 2015;6(1):48–67. doi: 10.1021/cn500256e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tresco PA, Winslow BD. The challenge of integrating devices into the central nervous system. Crit Rev Biomed Eng. 2011;39(1):29–44. doi: 10.1615/critrevbiomedeng.v39.i1.30. [DOI] [PubMed] [Google Scholar]
- 22.Polikov VS, Tresco PA, Reichert WM. Response of brain tissue to chronically implanted neural electrodes. J Neurosci Methods. 2005;148(1):1–18. doi: 10.1016/j.jneumeth.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 23.Kozai TDY, et al. In vivo two-photon microscopy reveals immediate microglial reaction to implantation of microelectrode through extension of processes. Journal of neural engineering. 2012;9(6):066001. doi: 10.1088/1741-2560/9/6/066001. This work observed immediate processes extension from microglia after probe insertion, but minimun cell body movement in the first 6 hours by in vivo two-photon microscopy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakajima K, et al. Neurotrophin secretion from cultured microglia. Journal of neuroscience research. 2001;65(4):322–331. doi: 10.1002/jnr.1157. [DOI] [PubMed] [Google Scholar]
- 25.Turner J, et al. Cerebral astrocyte response to micromachined silicon implants. Experimental neurology. 1999;156(1):33–49. doi: 10.1006/exnr.1998.6983. [DOI] [PubMed] [Google Scholar]
- 26.Kozai T, et al. Reduction of neurovascular damage resulting from microelectrode insertion into the cerebral cortex using in vivo two-photon mapping. Journal of neural engineering. 2010;7(4):046011. doi: 10.1088/1741-2560/7/4/046011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saxena T, et al. The impact of chronic blood–brain barrier breach on intracortical electrode function. Biomaterials. 2013;34(20):4703–4713. doi: 10.1016/j.biomaterials.2013.03.007. Intracortical electrode causes chronic BBB leakage. The extent of BBB breach varies in different animals, different types of electrodes (Michigan probe and microwire), and different shanks; BBB permeability strongly correlates with electrodes recording performance. [DOI] [PubMed] [Google Scholar]
- 28.Karumbaiah L, et al. The upregulation of specific interleukin (IL) receptor antagonists and paradoxical enhancement of neuronal apoptosis due to electrode induced strain and brain micromotion. Biomaterials. 2012;33(26):5983–96. doi: 10.1016/j.biomaterials.2012.05.021. In vitro study simulating brain micromotion at brain-electrode interface showed that low-magnitude cyclical strain result in overexpression of IL-36Ra in subjecting astrocytes and microglias, which initially enhances anti-inflammatory cytokine responses but also initiates neuronal apoptosis. [DOI] [PubMed] [Google Scholar]
- 29.Du ZJ, Kolarcik CL, Kozai TD, Luebben SD, Sapp SA, Zheng XS, Nabity JA, Cui XT. Ultrasoft microwire neural electrodes improve chronic tissue integration. Acta Biomater. 2017;53:46–58. doi: 10.1016/j.actbio.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sohal HS, et al. Mechanical flexibility reduces the foreign body response to long-term implanted microelectrodes in rabbit cortex. PLoS One. 2016;11(10):e0165606. doi: 10.1371/journal.pone.0165606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen JK, et al. Mechanically-compliant intracortical implants reduce the neuroinflammatory response. Journal of neural engineering. 2014;11(5):056014. doi: 10.1088/1741-2560/11/5/056014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karumbaiah L, Saxena T, Carlson D, Patil K, Patkar R, Gaupp EA, Bellamkonda RV. Relationship between intracortical electrode design and chronic recording function. Biomaterials. 2013;34(33):8061–8074. doi: 10.1016/j.biomaterials.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 33.Winslow BD, et al. A comparison of the tissue response to chronically implanted Parylene-C-coated and uncoated planar silicon microelectrode arrays in rat cortex. Biomaterials. 2010;31(35):9163–72. doi: 10.1016/j.biomaterials.2010.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skousen JL, et al. Reducing surface area while maintaining implant penetrating profile lowers the brain foreign body response to chronically implanted planar silicon microelectrode arrays. Prog Brain Res. 2011;194:167–80. doi: 10.1016/B978-0-444-53815-4.00009-1. [DOI] [PubMed] [Google Scholar]
- 35.Skousen JL, Bridge MJ, Tresco PA. A strategy to passively reduce neuroinflammation surrounding devices implanted chronically in brain tissue by manipulating device surface permeability. Biomaterials. 2015;36:33–43. doi: 10.1016/j.biomaterials.2014.08.039. [DOI] [PubMed] [Google Scholar]
- 36.Gutowski SM, et al. Host response to microgel coatings on neural electrodes implanted in the brain. J Biomed Mater Res A. 2014;102(5):1486–99. doi: 10.1002/jbm.a.34799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gutowski SM, et al. Protease-degradable PEG-maleimide coating with on-demand release of IL-1Ra to improve tissue response to neural electrodes. Biomaterials. 2015;44:55–70. doi: 10.1016/j.biomaterials.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Azemi E, Lagenaur CF, Cui XT. The surface immobilization of the neural adhesion molecule L1 on neural probes and its effect on neuronal density and gliosis at the probe/tissue interface. Biomaterials. 2011;32(3):681–692. doi: 10.1016/j.biomaterials.2010.09.033. This study demonstrated that covalent L1 coating on silicon electrode arrays resulted in decreased glia reaction and promoted neuronal density and axonal regeneration in close region to the probe, indicating L1 coating to be a promissing strategy to improve biocompatibility of neural probes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kozai TD, et al. Effects of caspase-1 knockout on chronic neural recording quality and longevity: insight into cellular and molecular mechanisms of the reactive tissue response. Biomaterials. 2014;35(36):9620–9634. doi: 10.1016/j.biomaterials.2014.08.006. Caspase-1 knockout mice showed increased neuronal survival and improved single-unit recording performance (yield and signal to noise ratio) of Michigan probes over 6 months after implantation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sawyer AJ, et al. The effect of inflammatory cell-derived MCP-1 loss on neuronal survival during chronic neuroinflammation. Biomaterials. 2014;35(25):6698–6706. doi: 10.1016/j.biomaterials.2014.05.008. Monocyte chemoattractant protein 1 (MCP-1, also CCL2) knockout mice were examed at 1, 2, and 8 weeks after implantation, showing reductions in BBB leakage, macrophage/microglia accumulation, and astrogliosis, and an increased neuronal density. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moore LB, et al. Loss of monocyte chemoattractant protein-1 alters macrophage polarization and reduces NFκB activation in the foreign body response. Acta biomaterialia. 2015;11:37–47. doi: 10.1016/j.actbio.2014.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patrick E, et al. Corrosion of Tungsten Microelectrodes used in Neural Recording Applications. Journal of neuroscience methods. 2011;198(2):158–171. doi: 10.1016/j.jneumeth.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pavel T, et al. Rapid evaluation of the durability of cortical neural implants using accelerated aging with reactive oxygen species. Journal of Neural Engineering. 2015;12(2):026003. doi: 10.1088/1741-2560/12/2/026003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ludwig KA, et al. Using a common average reference to improve cortical neuron recordings from microelectrode arrays. Journal of neurophysiology. 2009;101(3):1679–1689. doi: 10.1152/jn.90989.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kozai TD, et al. Comprehensive chronic laminar single-unit, multi-unit, and local field potential recording performance with planar single shank electrode arrays. Journal of neuroscience methods. 2015;242:15–40. doi: 10.1016/j.jneumeth.2014.12.010. This work demonstrated that compared to spontaneous recording, visually evoked stimulation detectes more single unit activities, and enables multi-unit and local field potential characterization. Single unit and multi-unit recording performed differently in different anatomical layers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karumbaiah L, et al. Relationship between intracortical electrode design and chronic recording function. Biomaterials. 2013;34(33):8061–74. doi: 10.1016/j.biomaterials.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 47.Kozai TD, et al. Ultrasmall implantable composite microelectrodes with bioactive surfaces for chronic neural interfaces. Nat Mater. 2012;11(12):1065–73. doi: 10.1038/nmat3468. Ultramicroelectrode consisting of a carbon-fibre core, a poly(p-xylylene)-based thin-film coating, and a poly(thiophene)-based recording pad was developed, eliciting much reduced chronic foreign body responses and enabling single neuron recording in rats. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kozai TD, et al. Two-photon imaging of chronically implanted neural electrodes: Sealing methods and new insights. Journal of neuroscience methods. 2016;258:46–55. doi: 10.1016/j.jneumeth.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eles JR, et al. Neuroadhesive L1 coating attenuates acute microglial attachment to neural electrodes as revealed by live two-photon microscopy. Biomaterials. 2017;113:279–292. doi: 10.1016/j.biomaterials.2016.10.054. This study tracked microglia response to L1 coated neural electrodes with in vivo two-photon microscopy, showing significant decreased microglia coverage and deminished radius of microglia activation compared to non-coated probes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luan L, et al. Ultraflexible nanoelectronic probes form reliable, glial scar-free neural integration. Sci Adv. 2017;3(2):e1601966. doi: 10.1126/sciadv.1601966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hammer DX, et al. Acute insertion effects of penetrating cortical microelectrodes imaged with quantitative optical coherence angiography. Neurophotonics. 2016;3(2) doi: 10.1117/1.NPh.3.2.025002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hammer DX, et al. Longitudinal vascular dynamics following cranial window and electrode implantation measured with speckle variance optical coherence angiography. Biomedical Optics Express. 2014;5(8):2823–2836. doi: 10.1364/BOE.5.002823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hammer DX, et al. Concurrent electrophysiology and TPM/OCT imaging of long-term implanted electrodes (Conference Presentation). SPIE BiOS; International Society for Optics and Photonics.2017. [Google Scholar]
- 54.Williams JC, et al. Complex impedance spectroscopy for monitoring tissue responses to inserted neural implants. Journal of neural engineering. 2007;4(4):410. doi: 10.1088/1741-2560/4/4/007. [DOI] [PubMed] [Google Scholar]
- 55.Badstuebner K, et al. Impedance detection of the electrical resistivity of the wound tissue around deep brain stimulation electrodes permits registration of the encapsulation process in a rat model. Journal of Electrical Bioimpedance. 2017;8(1):11–24. [Google Scholar]
- 56.Alba NA, et al. In vivo electrochemical analysis of a PEDOT/MWCNT neural electrode coating. Biosensors. 2015;5(4):618–646. doi: 10.3390/bios5040618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Du ZJ, et al. Ultrasoft microwire neural electrodes improve chronic tissue integration. Acta Biomater. 2017 doi: 10.1016/j.actbio.2017.02.010. Du et al. examine the effects of chronic implantation of electrodes with young’s moduli close to that of neural tissues. Implanted soft wire electrodes evoked lower degrees of inflamation and exhibited higher neural density near the surface of the electrode when compared to stiff tungsten electrodes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Biran R, Martin DC, Tresco PA. Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Exp Neurol. 2005;195(1):115–26. doi: 10.1016/j.expneurol.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 59.Paras RP, et al. Chronic in vivo stability assessment of carbon fiber microelectrode arrays. Journal of Neural Engineering. 2016;13(6):066002. doi: 10.1088/1741-2560/13/6/066002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paras RP, et al. Insertion of linear 8.4 µ m diameter 16 channel carbon fiber electrode arrays for single unit recordings. Journal of Neural Engineering. 2015;12(4):046009. doi: 10.1088/1741-2560/12/4/046009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kozai TDY, et al. Chronic in vivo evaluation of PEDOT/CNT for stable neural recordings. IEEE transactions on bio-medical engineering. 2016;63(1):111–119. doi: 10.1109/TBME.2015.2445713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luan L, et al. Ultraflexible nanoelectronic probes form reliable, glial scar–free neural integration. Science Advances. 2017;3(2):e1601966. doi: 10.1126/sciadv.1601966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xie C, et al. Three-dimensional macroporous nanoelectronic networks as minimally invasive brain probes. Nat Mater. 2015;14(12):1286–1292. doi: 10.1038/nmat4427. This work examines the effects of round flexible and macroporous mesh electrodes on recording and foreign body response. The mesh electrode was capable of recording single unit action potentials and local field potentials, and was exceptionaly well integrated into the nervous tissue. [DOI] [PubMed] [Google Scholar]
- 64.Xie C, et al. Intracellular Recording of Action Potentials by Nanopillar Electroporation. Nature nanotechnology. 2012;7(3):185–190. doi: 10.1038/nnano.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kang M, et al. Subcellular Neural Probes from Single-Crystal Gold Nanowires. ACS Nano. 2014;8(8):8182–8189. doi: 10.1021/nn5024522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Patel PR, et al. Chronic in vivo stability assessment of carbon fiber microelectrode arrays. J Neural Eng. 2016;13(6):066002. doi: 10.1088/1741-2560/13/6/066002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guitchounts G, et al. A carbon-fiber electrode array for long-term neural recording. J Neural Eng. 2013;10(4):046016. doi: 10.1088/1741-2560/10/4/046016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fu TM, et al. Stable long-term chronic brain mapping at the single-neuron level. Nature Methods. 2016;13(10):875-+. doi: 10.1038/nmeth.3969. [DOI] [PubMed] [Google Scholar]
- 69.BJKim J, et al. 3D Parylene sheath neural probe for chronic recordings. JOURNAL OF NEURAL ENGINEERING. 2013;10 doi: 10.1088/1741-2560/10/4/045002. [DOI] [PubMed] [Google Scholar]
- 70.David-Pur M, et al. All-carbon-nanotube flexible multi-electrode array for neuronal recording and stimulation. Biomed Microdevices. 2014;16:43–53. doi: 10.1007/s10544-013-9804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vitale F, et al. Neural Stimulation and Recording with Bidirectional, Soft Carbon Nanotube Fiber Microelectrodes. ACS Nano. 2015;9(4):4465–4474. doi: 10.1021/acsnano.5b01060. [DOI] [PubMed] [Google Scholar]
- 72.Guo L, et al. A PDMS-Based Integrated Stretchable Microelectrode Array (isMEA) for Neural and Muscular Surface Interfacing. IEEE TRANSACTIONS ON BIOMEDICAL CIRCUITS AND SYSTEMS. 2013;7 doi: 10.1109/TBCAS.2012.2192932. [DOI] [PubMed] [Google Scholar]
- 73.Guo L, et al. Stretchable Polymeric Multielectrode Array for Conformal Neural Interfacing. Advanced Materials. 2013;26:1427–1433. doi: 10.1002/adma.201304140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Khilwani R, et al. Ultra-miniature ultra-compliant neural probes with dissolvable delivery needles: design, fabrication and characterization. Biomed Microdevices. 2016;18(6):97. doi: 10.1007/s10544-016-0125-4. [DOI] [PubMed] [Google Scholar]
- 75.Wu F, et al. Silk-Backed Structural Optimization of High-Density Flexible Intracortical Neural Probes. Journal of microelectromechanical systems. 2015;24:62–69. [Google Scholar]
- 76.Fan Wu MI, Yoon Euisik. A flexible fish-bone-shaped neural probe strengthened by biodegradable silk coating for enhanced biocompatibility. Transducers. 2011;11:966–969. [Google Scholar]
- 77.Kozai TDY, et al. Chronic tissue response to carboxymethyl cellulose based dissolvable insertion needle for ultra-small neural probes. Biomaterials. 2014;35(34):9255–9268. doi: 10.1016/j.biomaterials.2014.07.039. [DOI] [PubMed] [Google Scholar]
- 78.Liu J, et al. Syringe injectable electronics. Nature nanotechnology. 2015;10(7):629–636. doi: 10.1038/nnano.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Felix SH, et al. Neural Probes Using Rigid Stiffeners Attached with Biodissolvable Adhesive. J. Vis. Exp. 2013;79 doi: 10.3791/50609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou T, et al. Syringe-injectable mesh electronics integrate seamlessly with minimal chronic immune response in the brain. Proc Natl Acad Sci U S A. 2017;114(23):5894–5899. doi: 10.1073/pnas.1705509114. Flexible electronics are notouriously difficult to insert into the nervous tissues. Zhou et al. have developed a mesh style electrode which is capable of being injected into the brain tissues by a syringe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dryg ID, et al. Magnetically Inserted Neural Electrodes: Tissue Response and Functional Lifetime. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2015;23(4):562–571. doi: 10.1109/TNSRE.2015.2399856. [DOI] [PubMed] [Google Scholar]
- 82.Jorfi M, et al. Progress Towards Biocompatible Intracortical Microelectrodes for Neural Interfacing Applications. Journal of neural engineering. 2015;12(1):011001–011001. doi: 10.1088/1741-2560/12/1/011001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ware T, et al. Smart Polymers for Neural Interfaces. Polymer Reviews. 2013;53(1):108–129. [Google Scholar]
- 84.Markwardt NT, et al. Sub-meninges Implantation Reduces Immune Response to Neural Implants. J Neurosci Method. 2013;214(2):119–125. doi: 10.1016/j.jneumeth.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Seo D, et al. Neural Dust: An Ultrasonic, Low Power Solution for Chronic Brain-Machine Interfaces. 2013 [Google Scholar]
- 86.Seo D, et al. Model validation of untethered, ultrasonic neural dust motes for cortical recording. Journal of Neuroscience Methods. 2015;244:114–122. doi: 10.1016/j.jneumeth.2014.07.025. This work describes a method of transmitting data and receiveing power without a transcutanous wire bundle by using ultrasound and piezoelectrics. [DOI] [PubMed] [Google Scholar]
- 87.Yeon P, et al. Fabrication and Microassembly of a mm-Sized Floating Probe for a Distributed Wireless Neural Interface. Micromachines. 2016;7(9):154. doi: 10.3390/mi7090154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Potter KA, et al. Curcumin-releasing mechanically adaptive intracortical implants improve the proximal neuronal density and blood–brain barrier stability. Acta Biomaterialia. 2014;10(5):2209–2222. doi: 10.1016/j.actbio.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 89.Weaver CL, et al. Electrically Controlled Drug Delivery from Graphene Oxide Nanocomposite Films. ACS Nano. 2014;8(2):1834–1843. doi: 10.1021/nn406223e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhong Y, Bellamkonda RV. Controlled release of anti-inflammatory agent α-MSH from neural implants. Journal of Controlled Release. 2005;106(3):309–318. doi: 10.1016/j.jconrel.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 91.Wei H, George CM, Ravi VB. Nanoscale laminin coating modulates cortical scarring response around implanted silicon microelectrode arrays. Journal of Neural Engineering. 2006;3(4):316. doi: 10.1088/1741-2560/3/4/009. [DOI] [PubMed] [Google Scholar]
- 92.Bo Zhu S-CL, et al. Large enhancement in neurite outgrowth on a cell membrane-mimicking conducting polymer. Nature Communications. 2014 doi: 10.1038/ncomms5523. [DOI] [PubMed] [Google Scholar]
- 93.Kolarcik CL, et al. In vivo effects of L1 coating on inflammation and neuronal health at the electrode/tissue interface in rat spinal cord and dorsal root ganglion. Acta biomaterialia. 2012;8(10):3561–3575. doi: 10.1016/j.actbio.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kozai TDY, et al. Dexamethasone retrodialysis attenuates microglial response to implanted probes in vivo. Biomaterials. 2016;87:157–169. doi: 10.1016/j.biomaterials.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Potter-Baker KA, et al. Implications of Chronic Daily Anti-Oxidant Administration on the Inflammatory Response to Intracortical Microelectrodes. Journal of neural engineering. 2015;12(4):046002–046002. doi: 10.1088/1741-2560/12/4/046002. This work examines the effects of chronic antioxidant administration on the brain and end organs. While the administration shows promising signs for neural electrodes, there is concerns raised about liver toxicity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Spieth S, et al. Approaches for drug delivery with intracortical probes. Biomedical Engineering / Biomedizinische Technik. 2014:291. doi: 10.1515/bmt-2012-0096. [DOI] [PubMed] [Google Scholar]
- 97.Minev IR, et al. Electronic dura mater for long-term multimodal neural interfaces. Science. 2015;347(6218):159–163. doi: 10.1126/science.1260318. [DOI] [PubMed] [Google Scholar]
- 98.Canales A, et al. Multifunctional fibers for simultaneous optical, electrical and chemical interrogation of neural circuits in vivo. Nat Biotechnol. 2015;33(3):277–84. doi: 10.1038/nbt.3093. [DOI] [PubMed] [Google Scholar]
- 99.Luo X, et al. Carbon Nanotube Nanoreservior for Controlled Release of Anti-inflammatory Dexamethasone. Biomaterials. 2011;32(26):6316–6323. doi: 10.1016/j.biomaterials.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kolarcik CL, et al. Evaluation of poly(3,4-ethylenedioxythiophene)/carbon nanotube neural electrode coatings for stimulation in the dorsal root ganglion. J Neural Eng. 2015;12(1):016008. doi: 10.1088/1741-2560/12/1/016008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fang H, et al. Ultrathin, transferred layers of thermally grown silicon dioxide as biofluid barriers for biointegrated flexible electronic systems. Proceedings of the National Academy of Sciences. 2016;113(42):11682–11687. doi: 10.1073/pnas.1605269113. This work examines the durability of several insulative materials when deposited in highly thin layers. Thermally grown silicon dioxide has shown exceptionaly promising results for insulation of increasingly small devices. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xie X, et al. Long-term reliability of Al2O3 and Parylene C bilayer encapsulated Utah electrode array based neural interfaces for chronic implantation. J Neural Eng. 2014;11(2):026016. doi: 10.1088/1741-2560/11/2/026016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xie X, et al. Long-Term Bilayer Encapsulation Performance of Atomic Layer Deposited Al and Parylene C for Biomedical Implantable Devices. IEEE Transactions on Biomedical Engineering. 2013;60(10):2943–2951. doi: 10.1109/TBME.2013.2266542. [DOI] [PubMed] [Google Scholar]
- 104.Ryan C, et al. Analysis of Al 2 O 3 —parylene C bilayer coatings and impact of microelectrode topography on long term stability of implantable neural arrays. Journal of Neural Engineering. 2017;14(4):046011. doi: 10.1088/1741-2552/aa69d3. [DOI] [PubMed] [Google Scholar]
- 105.Xu J, et al. Highly stretchable polymer semiconductor films through the nanoconfinement effect. Science. 2017;355(6320):59. doi: 10.1126/science.aah4496. Xu et al. have created and extremely conductive and highly flexable conducting polymer film by codeposition of SEBS with DPPT-TT. While this material has not been applied to neural electrodes, the extremely favorable properties of the polymer film are promising. [DOI] [PubMed] [Google Scholar]
- 106.Cui X, et al. Surface modification of neural recording electrodes with conducting polymer/biomolecule blends. J. Biomed. Mater. Res. 2001;56:261–272. doi: 10.1002/1097-4636(200108)56:2<261::aid-jbm1094>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 107.Ludwig KA, et al. Chronic neural recordings using silicon microelectrode arrays electrochemically deposited with a poly(3,4-ethylenedioxythiophene) (PEDOT) film. J Neural Eng. 2006;3(1):59–70. doi: 10.1088/1741-2560/3/1/007. [DOI] [PubMed] [Google Scholar]
- 108.Ouyang L, et al. Poly[3,4-ethylene dioxythiophene (EDOT)-co-1,3,5-tri[2-(3,4-ethylene dioxythienyl)]-benzene (EPh)] copolymers (PEDOT-co-EPh): optical, electrochemical and mechanical properties. Journal of Materials Chemistry B. 2015;3(25):5010–5020. doi: 10.1039/C5TB00053J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wei B, et al. Post-polymerization functionalization of poly(3,4-propylenedioxythiophene) (PProDOT) via thiol-ene "click" chemistry. Journal of Materials Chemistry B. 2015;3(25):5028–5034. doi: 10.1039/c4tb02033b. This work shows the capabilities to polymerize a post funtionalizable conduction monomer “ProDOT”. The chemical structure is highly similar to EDOT and allows for surface addition of many thiol containing compounds. [DOI] [PubMed] [Google Scholar]
- 110.Bhagwat N, Kiick KL, Martin DC. Electrochemical deposition and characterization of carboxylic acid functionalized PEDOT copolymers. Journal of Materials Research. 2014;29(23):2835–2844. [Google Scholar]
- 111.Povlich LK, et al. Synthesis, copolymerization and peptide-modification of carboxylic acid-functionalized 3,4-ethylenedioxythiophene (EDOTacid) for neural electrode interfaces. Biochimica et Biophysica Acta (BBA) - General Subjects. 2013;1830(9):4288–4293. doi: 10.1016/j.bbagen.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 112.Rousche PJ, Normann RA. Chronic recording capability of the Utah Intracortical Electrode Array in cat sensory cortex. Journal of Neuroscience Methods. 1998;82(1):1–15. doi: 10.1016/s0165-0270(98)00031-4. [DOI] [PubMed] [Google Scholar]
- 113.Studholme C, et al. Estimating tissue deformation between functional images induced by intracranial electrode implantation using anatomical MRI. Neuroimage. 2001;13(4):561–576. doi: 10.1006/nimg.2000.0692. [DOI] [PubMed] [Google Scholar]
- 114.Opie NL, et al. Micro-CT and Histological Evaluation of an Neural Interface Implanted Within a Blood Vessel. IEEE Transactions on Biomedical Engineering. 2017;64(4):928–934. doi: 10.1109/TBME.2016.2552226. [DOI] [PubMed] [Google Scholar]