Abstract
Objective
To assess the relative risk of oral clefts associated with maternal use of high and low doses of topiramate during the first trimester for epilepsy and nonepilepsy indications.
Methods
This population-based study nested in the US 2000–2010 Medicaid Analytic eXtract included a cohort of 1,360,101 pregnant women with a live-born infant enrolled in Medicaid from 3 months before conception through 1 month after delivery. Oral clefts were defined as the presence of a recorded diagnosis in claims during the first 90 days after birth. Women with a topiramate dispensing during the first trimester were compared with those without any dispensing and with an active reference group of women with a lamotrigine dispensing during the first trimester. Risk ratios (RRs) were estimated with generalized linear models with fine stratification on the propensity score of treatment to control for potential confounders. Stratified analyses by indication of use and dose were conducted.
Results
The risk of oral clefts at birth was 4.1 per 1,000 in the 2,425 infants born to women exposed to topiramate compared with 1.1 per 1,000 in the unexposed group (RR 2.90, 95% confidence interval [CI] 1.56–5.40). The RR among women with epilepsy was 8.30 (95% CI 2.65–26.07); among women with other indications such as bipolar disorder, it was 1.45 (95% CI 0.54–3.86). The median daily dose for the first prescription filled during the first trimester was 200 mg for women with epilepsy and 100 mg for women without epilepsy. For topiramate monotherapy, the RR for oral clefts associated with doses ≤100 mg was 1.64 (95% CI 0.53–5.07) and for doses >100 mg it was 5.16 (95% CI 1.94–13.73). Results were similar when lamotrigine was used as a reference group.
Conclusion
The increased risk of oral clefts associated with use of topiramate early in pregnancy was more pronounced in women with epilepsy, who used higher doses.
Topiramate is an anticonvulsant drug commonly used for indications other than epilepsy such as bipolar disorder and migraine. Multiple studies have shown a 2- to 5-fold increased risk of oral clefts in the infant after maternal use of topiramate early in pregnancy.1–4 Topiramate has also been associated with low birth weight.5 However, most participants in these studies used topiramate to prevent seizures, and questions remain as to whether the lower doses used for nonepilepsy indications also confer risk.2 The potential dose-dependent teratogenic effect of topiramate is also relevant for the low-dose topiramate-containing (23–92 mg/d) weight loss product (phentermine/topiramate) recently approved by the US Food and Drug Administration.6–8 Although this product is not indicated for pregnant women, given that half of pregnancies are unplanned,9 there may be more low-dose exposures in future pregnancies.
Given the relatively low incidence of oral clefts, evidence must necessarily come from large observational studies. The lack of randomization in these studies has to be compensated for by methods that maximize the comparability between the exposed and reference groups. These methods include adjusting for crucial confounders such as the indication for the drug when topiramate users are compared with a nonuser group and using comparative safety approaches where topiramate is compared to a standard therapy. Lamotrigine is a valid active comparator for topiramate given the overlap of some indications (epilepsy and bipolar disorder) and the amount of information supporting its safety for fetal development.2,10–13
Therefore, we conducted a population-based, propensity score (PS)–stratified cohort study nested in the nationwide Medicaid Analytic eXtract (MAX) to evaluate whether first-trimester exposure to topiramate increases the risk of oral clefts in a population of pregnant women exposed to high and low doses for epilepsy and nonepilepsy indications relative to both untreated women and women treated with lamotrigine.
Methods
Data source
We used data from the MAX from 2000 to 2010. MAX captures demographic and Medicaid enrollment information, health care use, all diagnoses and procedures received as an inpatient or outpatient, and all prescriptions filled outside of a hospital. The creation of the pregnancy cohort within MAX has been described in detail elsewhere.14 Diagnoses and procedures are identified by the ICD-9-CM and Current Procedural Terminology codes. Use of medications is identified by drug-dispensing claims that include National Drug Codes, filling date, quantity dispensed, and days' supply.
Study design
The cohort consisted of all pregnancies in women 12 to 55 years old that resulted in a live birth. The date of the last menstrual period (LMP) was estimated based on a validated algorithm.15 Women were required to be enrolled in Medicaid, without supplementary private insurance or restricted benefits, from 3 months before the date of the LMP to 1 month after delivery. Infants were required to be enrolled in Medicaid for the first 3 months of life unless they died sooner. Pregnancies exposed to a known teratogenic medication during the first trimester other than an antiepileptic drug (e.g., warfarin, antineoplastic agents, lithium, isotretinoin, misoprostol, thalidomide) and pregnancies with a documented chromosomal abnormality (ICD-9 code 758.xx or 759.81–759.83) were excluded (figure e-1, http://links.lww.com/WNL/A54).
Exposure definition
Exposure to topiramate was defined as filling ≥1 prescriptions during the first trimester of pregnancy (i.e., the first 90 days). The primary reference group of unexposed pregnancies included women without any fill for a topiramate or other anticonvulsant prescription from 3 months before the LMP to the end of the first trimester. No prescription fill during the 3 months before the start of pregnancy was required to avoid misclassifying as unexposed women who were still using the medication from an earlier filling. We also identified a secondary reference group of pregnancies exposed to lamotrigine, defined as at least 1 filled prescription during the first trimester, and no prescription of topiramate or other anticonvulsant during the 90 days before the LMP through the end of the first trimester.2,10–13
Outcome definition
The presence of oral clefts was ascertained from inpatient or outpatient ICD-9 diagnoses and related procedure codes in the infant records within the first 3 months after the date of birth. Maternal records during the first month after delivery were also considered because claims are sometimes recorded under the mother while the infant's Medicaid enrollment is being processed. We considered an oral cleft (including cleft lip and cleft palate) to be present in the infant if there was an ICD-9 diagnosis code of 749.xx recorded on >1 date, because the presence of a single code may indicate a rule-out diagnosis, or if there was a diagnosis code recorded on only 1 date but there was also a related surgery or procedure code.16 A validation study in Medicaid data showed a positive predictive value for oral clefts of 91%.17
Covariates
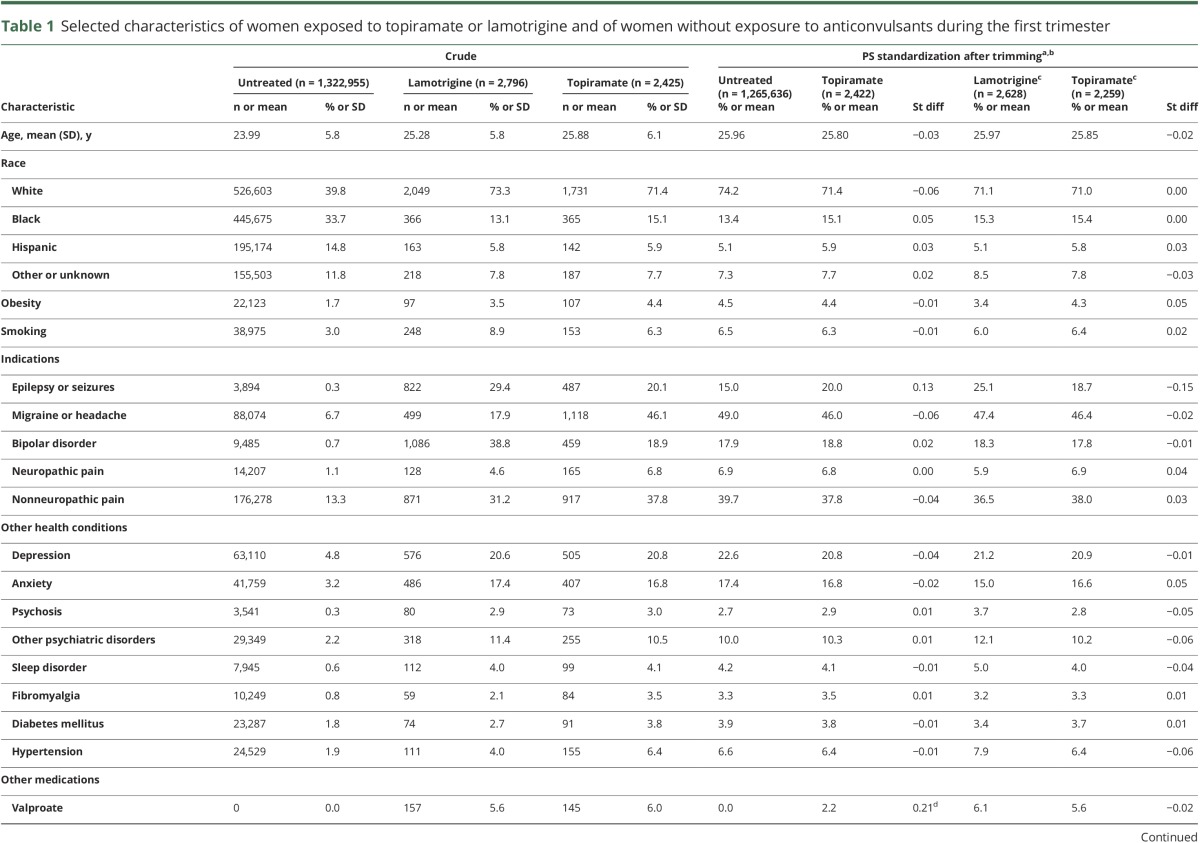
Potential confounders considered included maternal age, race, topiramate indications (i.e., epilepsy or seizures, migraine, bipolar disorder, pain conditions), obesity, smoking, comorbidities (e.g., diabetes mellitus, hypertension), concomitant medications (e.g., antipsychotics, antidepressants, antidiabetic and antihypertensive medications), and proxies for overall health status (e.g., Obstetric Comorbidity Index,18 number of hospitalizations).19 Chronic maternal illnesses were ascertained during the time period from 3 months before the LMP to the end of the first trimester. Concomitant medication use was assessed during the first trimester. Proxies for health care use were assessed during the 3 months before pregnancy (table 1).
Table 1.
Selected characteristics of women exposed to topiramate or lamotrigine and of women without exposure to anticonvulsants during the first trimester
Primary data analysis
Baseline characteristics were compared between women treated with topiramate, with or without other anticonvulsants, and the 2 reference groups. The risk of oral clefts and the unadjusted risk ratio (RR) with its 95% confidence interval (CI) were calculated. Then, PSs were estimated as the predicted probability of topiramate exposure during the first trimester with logistic regression models including all covariates specified above without further selection. After the exclusion of pregnancies from the nonoverlapping regions of the PS distributions, 50 equally sized strata based on the PS distribution among exposed women were created. Adjusted RRs were estimated with generalized linear models in which the unexposed pregnancies were weighted by the PS distribution of the topiramate-exposed pregnancies (SAS PROC GENMOD with weight statement and loglink function). To account for correlations within women with multiple pregnancies, we used a robust variance estimator. However, because results were almost identical, the primary analysis omitted correlation structures.
Secondary data analysis
To evaluate the effect of topiramate as used for different indications, we repeated the primary analyses within women with epilepsy or seizures and women without epilepsy or seizures claims from 3 months before the LMP to the end of the first trimester. That is, if a woman had epilepsy and migraine, she would be included only in the first group.
To evaluate the effect of concomitant exposure to other anticonvulsants, some of which may be teratogenic, we stratified the topiramate-exposed group into monotherapy and polytherapy. The monotherapy category included women exposed to topiramate but without any dispensing for other anticonvulsant drugs during the 3 months before the start of pregnancy or during the first trimester. The polytherapy category comprised women with at least 1 dispensing for other anticonvulsants in addition to topiramate during the first trimester, including those who added or switched to a different anticonvulsant. The RR was estimated for the monotherapy and polytherapy exposure categories in relation to the same unexposed reference group.
For topiramate use in monotherapy, we evaluated the effect of dose using strata based on daily dose ≤100 and >100 mg for the first topiramate prescription filled during the first trimester. This threshold corresponded to the doses usually recommended for epilepsy and nonepilepsy indications. Within the epilepsy indication, the range of doses recommended is larger, while recommended doses are lower for bipolar disorder or migraine. The RR was estimated for each dose category in relation to the same unexposed reference group.
Sensitivity analyses
A number of prespecified sensitivity analyses were conducted to assess the robustness of the primary analysis estimates. To evaluate the effect of exposure misclassification, we redefined exposure as having at least 2 pharmacy dispensing records for topiramate during the first trimester under the assumption that women with ≥2 prescriptions filled in 3 months are more likely to have adhered to at least the first one. We also repeated the dose-response analysis using the highest dose during the first trimester because topiramate doses may be increased as pregnancy progresses to prevent seizures. To evaluate the effect of outcome misclassification, we redefined the outcome on the basis of infant claims only and extended infant follow-up to 1 year. To evaluate the sensitivity of the results to the timing of topiramate exposure during the etiologically relevant gestational window, we assessed the RR associated with topiramate dispensing during the 3 months before pregnancy and not during the first trimester.
Finally, we added the current study to prior evidence on the association between use of topiramate early in pregnancy and oral clefts, which consisted of 6 nonrandomized studies, and estimated a pooled RR using a random-effects model. We assessed between-study heterogeneity with the χ2 statistic and the I2 statistic and pooled estimates using the DerSimonian and Laird random-effects model.
All analyses were conducted with SAS software, version 9.3 of the SAS System for Unix (SAS Institute Inc, Cary, NC).
Standard protocol approvals, registrations, and patient consents
The research was approved by the Institutional Review Board of Brigham and Women's Hospital.
Results
The number of pregnancies eligible for analyses was 2,425 in the topiramate-exposed group, 1,322,955 in the unexposed group, and 2,796 in the lamotrigine-exposed reference group (figure e-1, http://links.lww.com/WNL/A54). Compared to the unexposed women, women on topiramate were older and more likely to be white and obese; a higher proportion smoked, had chronic illnesses, and used concomitant medications; and they had greater health care utilization (table 1). Baseline characteristics were more homogeneous between the topiramate- and the lamotrigine-exposed groups. After standardization by PS, the 2 reference cohorts were very similar to the topiramate users (table 1).
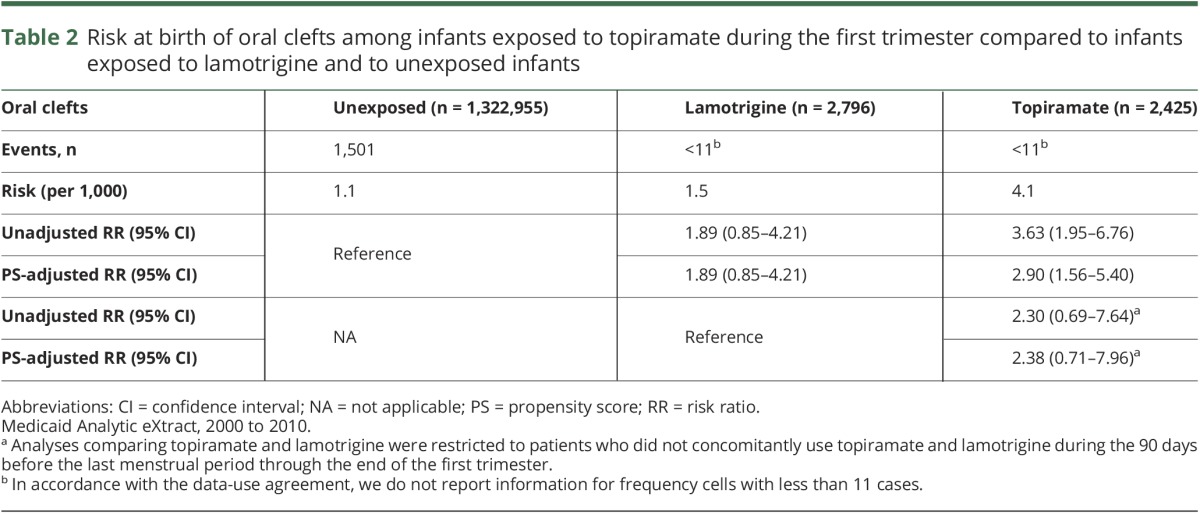
The risk of oral clefts was 4.1 per 1,000 live births in the topiramate group, 1.1 per 1,000 in the unexposed group, and 1.5 per 1,000 in the lamotrigine group (table 2). The adjusted RR for oral clefts associated with topiramate exposure was 2.90 (95% CI 1.56–5.40) compared to the unexposed and 2.38 (95% CI 0.71–7.96) compared to the lamotrigine-exposed reference groups. Because the results for comparisons with lamotrigine were similar, the analyses below present estimates based on the unexposed reference group only. The risk of malformations overall was not increased in the topiramate-exposed compared to the reference groups.
Table 2.
Risk at birth of oral clefts among infants exposed to topiramate during the first trimester compared to infants exposed to lamotrigine and to unexposed infants
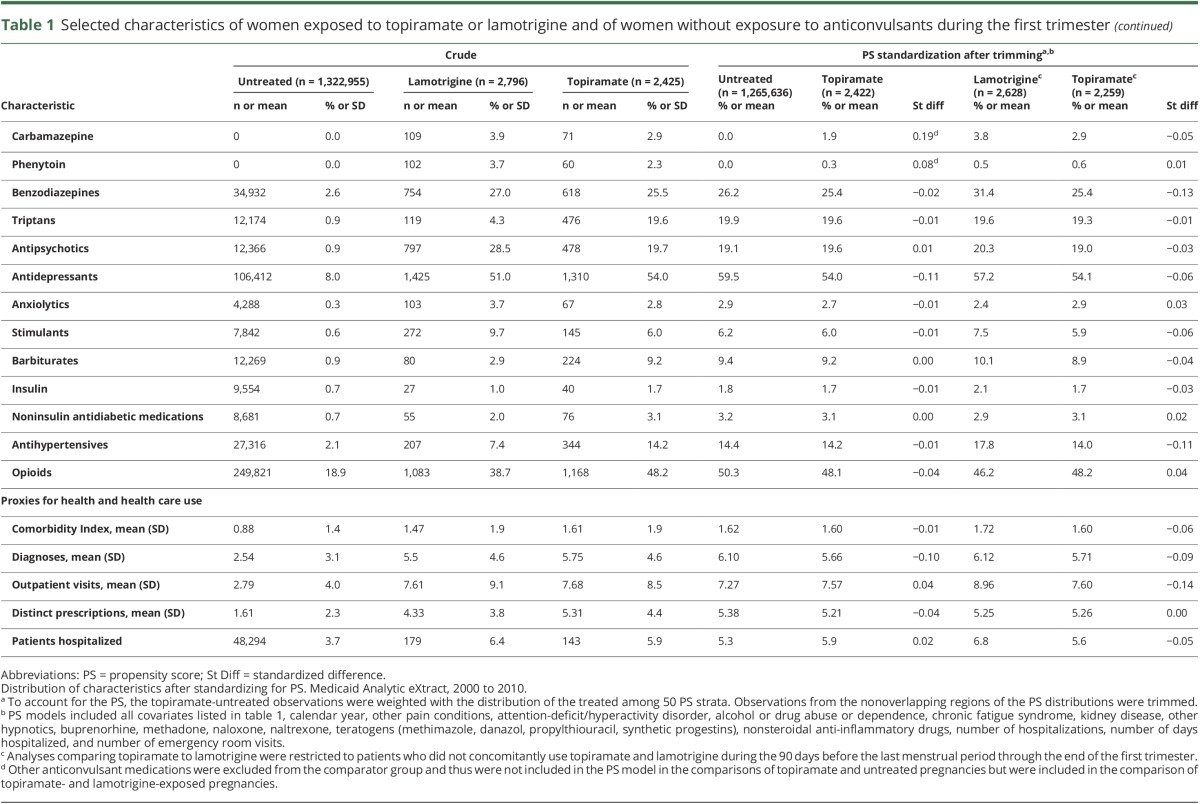
Restriction to women with epilepsy resulted in a risk of oral clefts at birth of 12.3 per 1,000 topiramate-exposed infants and an adjusted RR of 8.30 (95% CI 2.65–26.07); for women without a recorded epilepsy diagnosis, the risk of oral clefts was 2.1 per 1,000 exposed infants and the adjusted RR was 1.45 (95% CI 0.54–3.86) (figures 1 and 2). Considering any indication, the adjusted RR for topiramate in monotherapy was 2.69 (95% CI 1.28–5.64) and for polytherapy was 2.22 (95% CI 0.72–6.87). Within first-trimester monotherapy, the risk of oral clefts for daily topiramate doses ≤100 mg was 2.4 per 1,000 live births, and for daily doses >100 mg, it was 7.3 per 1,000 (figure 1); the corresponding adjusted RRs for daily doses ≤100 and >100 mg were 1.64 (95% CI 0.53–5.07) and 5.16 (95% CI 1.94–13.73), respectively (figure 2). The median daily dose was 200 mg (interquartile range 100–250 mg) for women with epilepsy and 100 mg (interquartile range 50–150 mg) for women without epilepsy. The sample size was insufficient to assess a dose response within indications.
Figure 1. Risk of oral clefts in the exposed and reference groups.
Prevalence of oral clefts at birth (dots) and 95% confident intervals (lines) in infants after maternal exposure to topiramate and in the unexposed and lamotrigine-exposed reference groups. The topiramate-exposed group was further divided according to indication and, within monotherapy, by dose. Medicaid Analytic eXtract, 2000 to 2010.
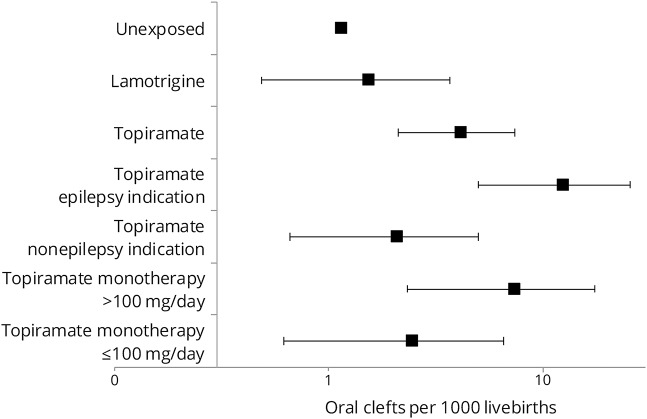
Figure 2. Secondary and sensitivity analyses with the unexposed group as reference.
Adjusted risk ratio (RR) of oral clefts (dots) and 95% confident intervals (CIs, lines). Medicaid Analytic eXtract, 2000 to 2010. Relative risk estimate for the primary analysis is referred to as primary RR (2.90, 95% CI 1.56–5.40). Rx = prescription; T1 = first trimester.
Results from sensitivity analyses were robust; the RR remained around the 3-fold increased risk (figure 2). The RR for ≥2 prescriptions during the first trimester was 3.50 (95% CI 1.46–8.41). Exposed women with epilepsy were more likely to have ≥2 prescriptions (59%) than women without epilepsy (32%). The sample size was insufficient to assess the effect of the number of prescriptions within indications. The RR for topiramate dispensing during the 3 months before the LMP but not during the first trimester was 1.16 (95% CI 0.44–3.10) and for dispensing during the most etiologically relevant period for oral clefts (6–12 weeks after LMP) was 3.36 (95% CI 1.09–10.40).
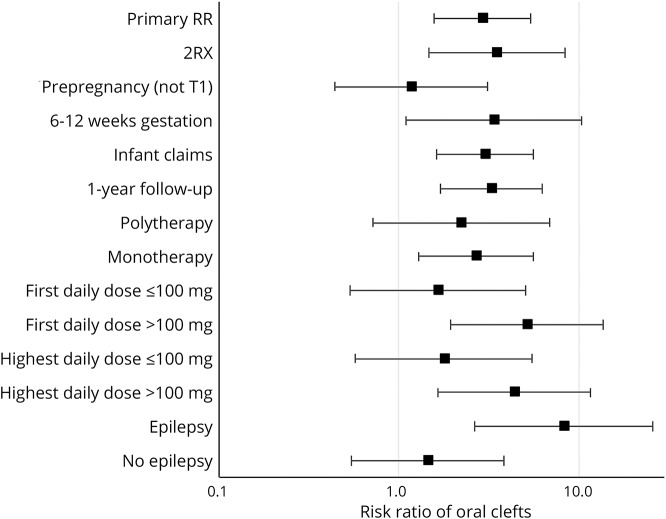
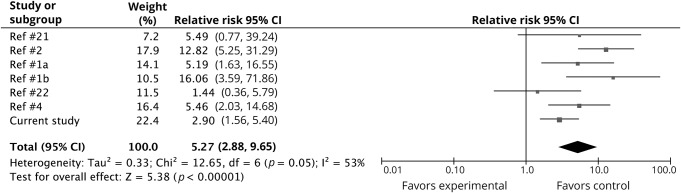
The estimated pooled primary RR of this study with the previous 6 studies was 5.27 (95% CI 2.88–9.65) (figure 3). The I2 was 53%, suggesting moderate heterogeneity between studies, and the p value for the χ2 statistic was 0.05.
Figure 3. Topiramate in early pregnancy and risk of oral clefts: Meta-analysis.
Meta-analysis includes the relative risk estimate for the primary analysis from the current study (risk ratio = 2.90, 95% confidence interval [CI] 1.56–5.40). Size of the markers reflects the weight of the studies.
Discussion
In a cohort of >1.3 million pregnancies, we found that maternal use of topiramate during the first trimester was associated with an ≈3-fold increased risk of oral clefts after accounting for confounding by clinical characteristics. The RR was larger at the higher doses used for epilepsy. The association remained in sensitivity analyses that varied the exposure and outcome definitions.
The use of topiramate increased during the last decade.20 In contemporary practice, it is commonly used to prevent migraine headaches and as a mood stabilizer to treat bipolar disorders. However, most of the evidence on its safety during pregnancy comes from the experience treating women with epilepsy. The pregnancy registries in particular included mainly women with epilepsy, with a median daily dose of 200 mg during the first trimester, and could not assess the effect of the lower doses recommended for nonepilepsy indications.2,3 The 2 studies that included a larger proportion of topiramate users for nonepilepsy indications identified oral cleft cases at daily doses as low as 25 mg,1,4 but the sample sizes were insufficient for formal dose-response analyses. The current larger cohort study found a substantially weaker association between topiramate and oral clefts for the lower doses used to treat nonepilepsy indications. However, the finding of lower risk at doses ≤100 mg needs to be interpreted with caution because this estimate is less precise due to the smaller sample size of women treated at this dose. Moreover, the indication may modify the RR for topiramate for multiple reasons, including different doses, differential compliance with the treatment, or an interaction between the underlying indication and the drug. We explored these possibilities with secondary analyses; however, the sample size was insufficient to completely disentangle them.
Overall, at least 6 studies have reported a risk for oral clefts among prenatally exposed live births >5 times larger than in the reference populations.1–4,21 The study that did not find an association was based on 2 topiramate-exposed cases, and the 95% CI for the RR included a 5-fold increased risk; therefore, it was not inconsistent with the other findings (figure 3).22 The larger proportion of women on lower topiramate doses for nonepilepsy indications may explain the more modest primary RR magnitude in the current study; however, the RR was 5.2 for daily doses >100 mg. Studies also differed in their outcome definition, with most including oral clefts (both cleft lip and palate) but some focusing on cleft lip.2 (Our sample size was insufficient for us to explore these 2 malformations separately.) Another distinguishing characteristic is that our study population included Medicaid-eligible pregnant women, a young, racially diverse, and socioeconomically disadvantaged group. However, although these factors may affect baseline risks, they are not believed to affect the effect of teratogens.
Strengths of our study include its very large population-based cohort, systematic prospective assessment of drug exposure, an outcome with high positive predictive value in validation studies, and the availability of information on a wide range of covariates used for careful control for potential confounders. The study limitations include the potential for residual confounding, misclassification, and selection biases.
The reasons for treatment are the most concerning source of confounding in the evaluation of the safety of topiramate.4 It was initially proposed that epilepsy itself could increase the risk of congenital malformations, although most studies have refuted this hypothesis.23–28 The same could be speculated for nonepilepsy indications such as migraine or bipolar disorders, together with associated lifestyle factors. Comparative safety methods minimize this potential channeling bias by comparing different drugs among women with balanced indications. We were able to study topiramate when used for a range of indications and to compare topiramate with lamotrigine after balancing indications of use. In addition, we adjusted for a large number of predefined potential confounders through the use of PS, which resulted in exposure groups with balanced measured characteristics and tended to move the RR estimates downward. However, this approach does not eliminate potential confounding by unmeasured or poorly measured characteristics such as obesity or smoking.
Smoking was more common in topiramate users than in the unexposed reference group, which is consistent with previous studies.2 Obesity was also more frequently recorded in topiramate users in our data, albeit based on incomplete ascertainment. According to the US National Health and Nutrition Examination Survey,29 young women on topiramate are more frequently overweight than those on other anticonvulsants, probably because topiramate has been preferentially prescribed to overweight women seeking its weight loss effect. Thus, our RR estimates could be affected by unbalanced maternal smoking and body weight in the 2 groups. However, given the modest positive association between smoking or obesity and oral clefts, we would be only slightly overestimating the effect of topiramate.1,5 Adjustment for smoking or obesity in previous studies did not move the effect estimates.1 Moreover, we used PS to adjust for a variety of characteristics that are correlated with smoking or obesity, including psychiatric conditions, diabetes mellitus, and diabetes treatments, and we compared topiramate users with a group of lamotrigine users with presumably more balanced unmeasured characteristics.
An important concern of claims databases is the misclassification of both exposure and outcome. Nondifferential misclassification tends to bias results toward the null. Filling a prescription does not guarantee that the anticonvulsant was actually taken during the days supplied. While women with epilepsy are more likely to adhere to therapy, women with migraine may often discontinue treatment or use the drug intermittently. To address this concern, in secondary analyses, we required women to have filled a minimum of 2 prescriptions during the first trimester; the RR moved to 3.5 with wide CIs.
Regarding the outcome, the risk among the unexposed (1.1 oral clefts per 1,000 live-born infants) is in line with expectations given prior publications. Moreover, the positive predictive value for oral clefts in health care databases has been estimated to be between 91% and 97%.4,17 This nondifferential modest misclassification would minimally bias the RR toward the null.
Lastly, our cohort was restricted to live births. Therefore, severe congenital malformations that result in pregnancy losses or terminations will be missed. Although this selection could theoretically result in bias, nonsyndromic oral clefts do not result in fetal deaths and are rarely a reason for terminations.30 Thus, it is highly unlikely that differential terminations among topiramate users vs the reference groups could account for our findings.
The accumulated evidence consistently suggests that first-trimester use of topiramate increases the risk of oral clefts. Our results suggest that the increased risk may be more pronounced in women with epilepsy, likely because of the higher doses, and thus further support avoiding high doses of topiramate in women of childbearing age to prevent exposures early in pregnancy unless the benefits clearly outweigh these risks. Approximately 1 in 1,000 infants is born with an oral cleft31; assuming that the association is causal, the observed RR would translate to a risk on the order of 5 cases of oral clefts per 1,000 pregnancies exposed to topiramate at daily doses >100 mg in the first trimester.
Glossary
- CI
confidence interval
- ICD-9-CM
International Classification of Diseases, 9th Revision, Clinical Modification
- LMP
last menstrual period
- MAX
Medicaid Analytic eXtract
- PS
propensity score
- RR
risk ratio
Author contributions
Study concept and design: Hernandez-Diaz, Patorno, Bateman, Huybrechts. Acquisition, analysis, or interpretation of data: all authors. Drafting of the manuscript: Hernandez-Diaz, Bateman, Huybrechts, Patorno. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: Hernandez-Diaz, Patorno, Huybrechts, Desai, Mogun. Obtained funding: Hernandez-Diaz, Huybrechts. Administrative, technical, or material support: Mogun, Cohen. Study supervision: Hernandez-Diaz, Bateman, Huybrechts, Patorno.
Study funding
This study was supported by an R01 grant (R01 MH100216) from the National Institute of Mental Health. Brian T. Bateman was supported by a career development grant K08HD075831 from the National Institute of Child Health & Human Development. Krista F. Huybrechts was supported by a career development grant K01MH099141 from the National Institute of Mental Health. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Disclosure
S. Hernandez-Diaz received salary support from the North American AED Pregnancy Registry; has received research funding from GSK, Lilly, and Pfizer; and consulted for UCB, Teva, and Boehringer-Ingelheim. Her institution received training grants from Pfizer, Takeda, Bayer, and Asisa. K. Huybrechts has received research funding from GSK, Lilly, and Pfizer. R. Desai reports receiving a research grant from Merck. J. Cohen and H. Mogun report no disclosures relevant to the manuscript. P. Pennell was supported by a grant from the Epilepsy Foundation and grant U01 NS038455 from the NIH, National Institute for Neurologic Disorders and Stroke, and National Institute of Child Health and Human Development. B. Bateman has received research funding from GSK, Lilly, Pfizer, Pacira, and Baxalta. E. Patorno reports receiving research funding from GSK and Boehringer-Ingelheim. Go to Neurology.org/N for full disclosures.
References
- 1.Margulis AV, Mitchell AA, Gilboa SM, et al. Use of topiramate in pregnancy and risk of oral clefts. Am J Obstet Gynecol 2012;207:405.e1–407.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hernandez-Diaz S, Smith CR, Shen A, et al. Comparative safety of antiepileptic drugs during pregnancy. Neurology 2012;78:1692–1699. [DOI] [PubMed] [Google Scholar]
- 3.Hunt S, Russell A, Smithson W, et al. Topiramate in pregnancy: preliminary experience from the UK Epilepsy and Pregnancy Register. Neurology 2008;71:272–276. [DOI] [PubMed] [Google Scholar]
- 4.Mines D, Tennis P, Curkendall S, et al. Topiramate use in pregnancy and the birth prevalence of oral clefts. Pharmacoepidemiol Drug Saf 2014;23:1017–1025. [DOI] [PubMed] [Google Scholar]
- 5.Hernandez-Diaz S, Mittendorf R, Smith CR, Hauser WA, Yerby M, Holmes LB. Association between topiramate and zonisamide use during pregnancy and low birth weight. Obstet Gynecol 2014;123:21–28. [DOI] [PubMed] [Google Scholar]
- 6.Bays H. Phentermine, topiramate and their combination for the treatment of adiposopathy (“sick fat”) and metabolic disease. Expert Rev Cardiovasc Ther 2010;8:1777–1801. [DOI] [PubMed] [Google Scholar]
- 7.Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr 2012;95:297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.VIVUS I. VI-0521 (Qnexa®) advisory committee briefing document. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM292317.pdf. Accessed August 20, 2012.
- 9.Finer LB, Henshaw SK. Disparities in rates of unintended pregnancy in the United States, 1994 and 2001. Perspect Sex Reprod Health 2006;38:90–96. [DOI] [PubMed] [Google Scholar]
- 10.Sabers A, Dam M, A-Rogvi-Hansen B, et al. Epilepsy and pregnancy: lamotrigine as main drug used. Acta Neurol Scand 2004;109:9–13. [DOI] [PubMed] [Google Scholar]
- 11.Vajda F, Hitchcock A, Graham J, O'Brien T, Lander C, Eadie M. The Australian register of antiepileptic drugs in pregnancy: the first 1002 pregnancies. Aust N Z J Obstet Gynaecol 2007;47:468–474. [DOI] [PubMed] [Google Scholar]
- 12.Morrow J, Russell A, Guthrie B, et al. Malformation risks of antiepileptic drugs in pregnancy: a prospective study from the UK Epilepsy and Pregnancy Register. J Neurol Neurosurg Psychiatry 2006;77:193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cunnington M, Ferber S, Quartey G; The International Lamotrigine Pregnancy Registry Scientific Advisory Committee. Effect of dose on the frequency of major birth defects following fetal exposure to lamotrigine monotherapy in an international observational study. Epilepsia 2007;48:1207–1210. [DOI] [PubMed] [Google Scholar]
- 14.Palmsten K, Huybrechts KF, Mogun H, et al. Harnessing the Medicaid Analytic extract (MAX) to evaluate medications in pregnancy: design considerations. PLoS One 2013;8:e67405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Margulis AV, Setoguchi S, Mittleman MA, Glynn RJ, Dormuth CR, Hernandez-Diaz S. Algorithms to estimate the beginning of pregnancy in administrative databases. Pharmacoepidemiol Drug Saf 2012;22:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tikkanen J, Heinonen OP. Risk factors for ventricular septal defect in Finland. Public Health 1991;105:99–112. [DOI] [PubMed] [Google Scholar]
- 17.Cooper WO, Hernandez-Diaz S, Gideon P, et al. Positive predictive value of computerized records for major congenital malformations. Pharmacoepidemiol Drug Saf 2008;17:455–460. [DOI] [PubMed] [Google Scholar]
- 18.Bateman BT, Mhyre JM, Hernandez-Diaz S, et al. Development of a comorbidity index for use in obstetric patients. Obstet Gynecol 2013;122:957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneeweiss S, Seeger J, Maclure M, Wang P, Avorn J, Glynn R. Performance of comorbidity scores to control for confounding in epidemiologic studies using claims data. Am J Epidemiol 2001;154:854–864. [DOI] [PubMed] [Google Scholar]
- 20.Landmark CJ, Fossmark H, Larsson PG, Rytter E, Johannessen SI. Prescription patterns of antiepileptic drugs in patients with epilepsy in a nation-wide population. Epilepsy Res 2011;95:51–59. [DOI] [PubMed] [Google Scholar]
- 21.Molgaard-Nielsen D, Hviid A. Newer-generation antiepileptic drugs and the risk of major birth defects. JAMA 2011;305:1996–2002. [DOI] [PubMed] [Google Scholar]
- 22.Green M, Seeger J, Peterson C, Bhattacharyya A. Utilization of topiramate during pregnancy and risk of birth defects. Headache 2012;52:1070–1084. [DOI] [PubMed] [Google Scholar]
- 23.Shapiro S, Hartz SC, Siskind V, et al. Anticonvulsants and parental epilepsy in the development of birth defects. Lancet 1976;1:272–275. [DOI] [PubMed] [Google Scholar]
- 24.Finnell RH, Bielec B, Nau H. Anticonvulsant drugs: mechanisms and pathogenesis of teratogenicity. In: Kavlock RJ, Daston GP, editors. Drug Toxicity in Embryonic Development II. Berlin: Springer; 1997:121–159. [Google Scholar]
- 25.Dansky LV, Finnell RH. Parental epilepsy, anticonvulsant drugs, and reproductive outcome: epidemiologic and experimental findings spanning three decades, 2: human studies. Reprod Toxicol 1991;5:301–335. [DOI] [PubMed] [Google Scholar]
- 26.Bromfield E, Dworetsky B, Wyszynski D, Smith C, Baldwin E, Holmes L. Valproate teratogenicity and epilepsy syndrome. Epilepsia 2008;49:2122–2124. [DOI] [PubMed] [Google Scholar]
- 27.Fried S, Kozer E, Nulman I, Einarson TR, Koren G. Malformation rates in children of women with untreated epilepsy: a meta-analysis. Drug Saf 2004;27:197–202. [DOI] [PubMed] [Google Scholar]
- 28.Friis ML, Holm NV, Sindrup EH, Fogh-Andersen P, Hauge M. Facial clefts in sibs and children of epileptic patients. Neurology 1986;36:346–350. [DOI] [PubMed] [Google Scholar]
- 29.Dugan K, Margulis A, Hernandez-Diaz S. Topiramate for weight loss. Pharmacoepidemiol Drug Saf 2011;20:S1–S382.21837638 [Google Scholar]
- 30.Stephansson O, Kieler H, Haglund B, et al. Selective serotonin reuptake inhibitors during pregnancy and risk of stillbirth and infant mortality. JAMA 2013;309:48–54. [DOI] [PubMed] [Google Scholar]
- 31.Parker SE, Mai CT, Canfield MA, et al. Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res A Clin Mol Teratol 2010;88:1008–1016. [DOI] [PubMed] [Google Scholar]