Abstract
Midlife obesity is associated with increased risk of dementia, whereas late-life obesity is commonly associated with a lower risk of dementia. Although methodologic issues are often discussed in this apparent risk reversal, chronic exposure to low-dose organochlorine pesticides (OCPs), an emerging risk factor for dementia in general populations, may contribute to a direct explanation for these differences. OCPs are strong lipophilic chemicals with very long half-lives (several years), primarily stored in adipose tissue and very slowly released and metabolized over years. As serum concentrations of neurotoxic OCPs strongly correlate with brain OCPs (r = 0.95), any condition enhancing the release of OCPs from the adipose tissue into circulation would increase the risk of dementia. Increased release of OCPs from adipose tissue typically occurs in (1) dysfunctional adipocytes accompanied by uncontrolled lipolysis and (2) weight loss. Weight gain may help sequester circulating OCPs in adipose tissue. As obesity is the most common reason that adipocytes become dysfunctional, midlife obesity can increase dementia risk through the chronic release of OCPs into circulation. However, late-life obesity potentially decreases dementia risk because weight loss after midlife will increase the release of OCPs while weight gain may actually decrease the release. These countervailing forces may underlie paradoxical associations with dementia of obesity in midlife vs late life which is influenced by weight change after midlife. This hypothesis should be tested in future experimental and human studies on obesity and dementia.
There are conflicting findings regarding possible links between obesity and dementia. In systematic reviews and meta-analyses of epidemiologic studies, midlife obesity was consistently associated with increased risk of dementia in old age, while late-life obesity was often associated with a lower risk of dementia.1
This reverse association observed in late life has been attributed to unintentional weight loss, which is related to other comorbidities or a sign of subclinical dementia, as well as to several methodologic issues such as using body mass index, which is an imperfect measure of body composition in the elderly.2 However, the adverse neurologic effects of organochlorine pesticides (OCPs), which contaminate contemporary adipose tissue,3 may help explain the puzzling findings on obesity and dementia.
Common neurotoxic chemicals in adipose tissue
As typical man-made chemicals, OCPs were widely used from the 1940s through the 1960s for vector control and agriculture. Unlike present common pesticides, OCPs have strong lipophilicity, poor biodegradation, and long half-lives (several years to decades). As a result of these features, their concentrations in the tissues of living organisms are higher at successively higher levels in the food chain, a process called biomagnification.
Although most OCPs have been banned worldwide, humans remain exposed to these lipophilic chemicals. There are 2 main exposure routes for the general population.4 First, the consumption of OCP-contaminated food is a major external source. Even though OCPs were introduced as agricultural pesticides, the main food groups contaminated by OCPs today are animal products such as fish, meat, milk (including breast milk), and eggs; this is due to their biomagnification in the food chain. Second, OCP-contaminated adipose tissue in human bodies3 is an internal source of exposure. When OCPs enter the body through food, they are primarily stored in adipose tissue. Therefore, even if there were no external exposure source, OCPs that had previously accumulated in adipose tissue would be continuously released to circulation during fat mobilization to maintain equilibrium.5
Serum concentrations of OCPs were recently linked to cognitive impairment and dementia in general populations, including one prospective cohort study.6–8 For example, the future risk of cognitive impairment or dementia in elderly persons with higher serum concentrations of OCPs was about 3 times higher than in elders with low concentrations, after extensive covariate adjustment.7 The findings linking serum concentrations of OCPs to cognition are plausible because OCPs reach the brain. In fact, serum concentrations of these chemicals drawn before death in patients with Alzheimer disease were highly correlated with concentrations in their postmortem brain samples (r = +0.95).8
Notably, human studies suggest that OCPs may modify the well-known associations of established risk factors, such as APOE genotype, age, and hypertension, with cognitive impairment. For example, OCPs were more strongly associated with decreased cognitive function among elders carrying an APOE ε4 allele, compared with those without an APOE ε4 allele.8 In addition, the risk of aging- or hypertension-related cognitive impairment was more prominent among elders with higher serum concentrations of OCPs in general populations.9,10
However, the specific OCPs showing significant associations varied across human studies.6–8 Although this kind of inconsistency is commonly regarded as lack of human evidence, any interpretation focusing on single compounds is problematic for strong lipophilic chemicals such as OCPs, because humans are exposed to OCPs as a mixture and serum concentrations of individual OCPs are highly correlated in general populations.4 Specific OCPs measured in human studies act as surrogate markers for OCP mixtures and the findings from human studies should be interpreted as results from OCP mixtures rather than from one or several individual compounds.
Even though the neurotoxicity of high-dose OCPs is well-known,11 these recent findings linking OCPs to cognition and dementia are remarkable because the concentrations of OCPs in current general populations are very low in absolute terms. Therefore, biological mechanisms linking chronic exposure to low-dose OCPs to cognition might differ from those of direct neurotoxicity of high-dose OCPs. Low-dose chronic exposure to OCPs may be more harmful to the brain than was previously appreciated. Indeed, today's elders are the first generation with almost lifetime exposure to these chemicals because OCPs were first introduced in the 20th century.
Direct evidence about possible molecular mechanisms is largely unavailable because possible harmful effects of chronic exposure to low-dose OCPs have been observed only recently in humans. One in vitro study has demonstrated that the exposure for 48 hours to 1 μM dichlorodiphenyltrichloroethane (DDT, a typical OCP) on human neuroblastoma cells increased levels of amyloid precursor protein8; this study did not include further work to describe molecular mechanisms.
On the other hand, there is overwhelming evidence of glutathione deficiency and mitochondrial dysfunction as a causal mechanism in most neurodegenerative diseases.12,13 It is noteworthy that chronic exposure to low-dose strong lipophilic chemical mixtures such as OCPs can impair mitochondrial function.14,15 Also, this type of exposure can lead to glutathione depletion as glutathione is consumed during normal metabolism of these chemicals.16,17
How are these chemicals related to the dynamics of adipose tissue?
In chemically contaminated modern societies, adipose tissue of living organisms cannot be considered a pure organ. OCPs stored in adipose tissue should be considered for a possible role in the associations among obesity, weight change, and dementia in humans because the toxicodynamics of these chemicals is mechanistically linked to the dynamics of adipose tissue.
In fact, adipose tissue may play a protective role against lipophilic chemicals like OCPs as a relatively safe storage site.18 OCPs harbored in other organs such as the brain may be more dangerous. Therefore, in the short term, the sequestration of these chemicals in adipose tissue may conceivably minimize their toxic effects in the brain and other critical organs.4,18
However, there may be enhanced release of OCPs from adipose tissue to circulation, beyond the usual release rate that occurs with normal fat mobilization. First is the case of the dysfunctional adipocyte because uncontrolled lipolysis is one common phenotype of dysfunctional adipocytes.19 In particular, adipose tissue with insulin resistance increases basal lipolysis because insulin is the main factor in the inhibition of lipolysis within adipocytes.20 Second is what happens during weight loss, which dumps OCPs into blood as adipocytes shrink.21 It is important to note that the increase in serum OCP concentrations after weight loss is proportional to the magnitude of weight loss. In contrast, weight gain can decrease blood concentrations through sequestration to adipose tissue.22 One animal experimental study demonstrated that weight loss redistributed hexachlorobenzene (an OCP) from adipose tissue to the brain and vice versa23; continued weight loss resulted in a threefold increase of hexachlorobenzene concentration in the brain, but decreased after weight regain.
Compared to weight loss, the uncontrolled release of OCPs from adipose tissue among persons with dysfunctional adipocytes may occur more subtly and may be chronic. Thus, as the most common reason for dysfunctional adipocytes, obesity in middle age can increase dementia risk in late life because this pathway may require a long time to increase the risk of dementia. However, if the uncontrolled release of OCPs does not develop in obesity, as in metabolically healthy obese persons, even midlife obesity may play a protective role against dementia due to the efficient storage of OCPs in adipocytes. This protection would be greater for subcutaneous adipose tissue than for visceral adipose tissue because visceral adipose tissue seems to be a less stable storage site for OCPs than subcutaneous adipose tissue,24 possibly due to the high lipolytic activity of the visceral adipocyte.25
However, the role of obesity may change from midlife to late life. Among persons with the same body weight in their middle age, there may be substantial variation of subsequent weight changes into late life. Importantly, the toxicodynamics of OCPs related to weight change work oppositely from the action in obesity with dysfunctional adipocytes: weight loss increases the release of OCPs into circulation while weight gain reverses this relation. Thus, although it may seem paradoxical, countervailing forces of obesity in midlife and weight gain after midlife can lead to decreased risk of dementia among obese persons in late life. In a recent cohort study, which evaluated the association between weight change for about 9 years during midlife and the dementia-related mortality risk more than 3 decades later,26 weight loss during midlife was associated with increased risk, whereas weight gain was associated with reduced risk. Although this study was limited by not having body weight measurement after midlife, the findings generally support our speculation on the risk of dementia related to weight change.
In figure 1, we present 4 scenarios, which show how the increased risk of dementia among obese persons at midlife can convert to decreased risk of dementia among obese elderly.
Figure 1. Four hypothetical scenarios of relations between obesity and dementia regarding neurotoxic organochlorine pesticides (OCPs).
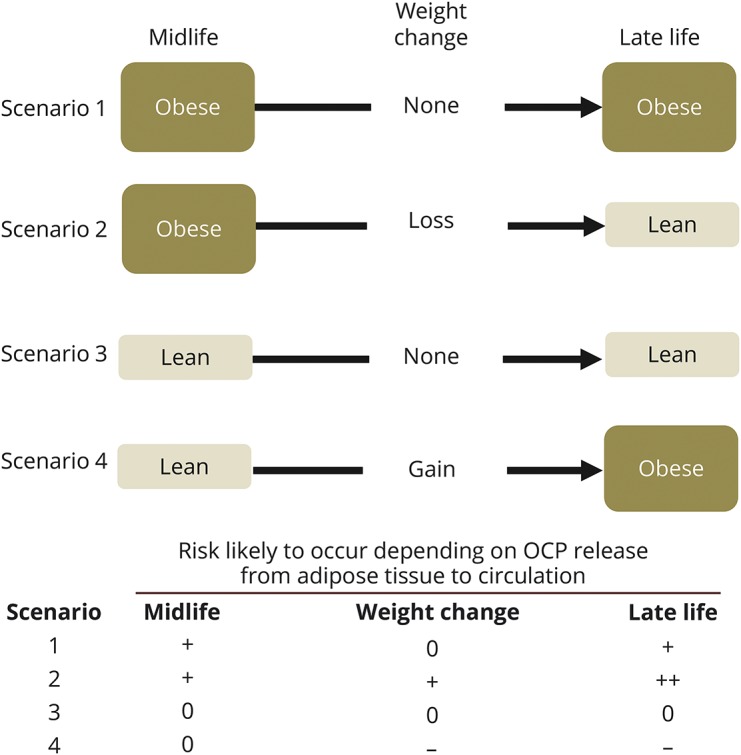
There are 2 ways of releasing OCPs from adipose tissue to circulation, which in turn lead to increased brain concentrations: obesity-related dysfunctional adipocytes and weight loss. Meanwhile, weight gain decreases serum concentrations of OCPs through sequestration to adipose tissue. Risks of dementia likely to occur under our hypothesis according to 4 scenarios of weight and weight change from midlife to late life are assumed to be + for obese persons and 0 for lean persons at midlife, while risk during weight change are assumed to be + for weight loss, 0 for no change, and − for weight gain. As body weight in late life results from body weight in midlife and its subsequent change from midlife to late life, a mean risk for 2 obese elderly is 0 (mean of + and −) while this figure for 2 lean elderly is + (mean of ++ and 0). Thus, the increased risk of dementia observed among obese persons at midlife may paradoxically become protective for dementia, given the observed changes in body mass index trajectory over the life course.
Unintentional weight loss among patients with subclinical dementia
Unintentional weight loss is considered to be one important predictor of accelerated progression from mild cognitive impairment to dementia.27 This unintentional weight loss preceding the diagnosis of dementia can be caused by many factors, which usually coexist with progressive loss of cognitive function. Possible mechanisms include decreased olfaction and hence decreased appetite, declining ability for self-care, disturbance of fat-brain axis homeostasis, and changes in the hormonal regulation of energy metabolism.27,28 In this interpretation, unintentional weight loss is not deemed itself a factor that contributes to increase the risk of dementia. Nevertheless, unintentional weight loss can be a direct risk factor for dementia through the release of OCPs.
Can OCPs explain a high risk of dementia among patients with type 2 diabetes?
Type 2 diabetes (T2D) has been known as an important risk factor for cognitive impairment and dementia.29 Even though abnormalities in glucose regulation among patients with T2D have been suspected as a mechanism linking T2D and dementia, the strength of this association is generally weak; e.g., HbA1C values accounted for less than 10% of the variance in cognition.30
Although obesity is a well-established risk factor for T2D, a rich body of evidence from human and animal studies suggests that chronic exposure to persistent organic pollutants (POPs), strong lipophilic chemical mixtures including OCPs, plays a key role in the development of T2D.4 In fact, serum concentrations of OCPs and other POPs in patients with T2D are higher than those of persons without T2D in many human studies on POPs and T2D.4 Therefore, OCPs increasing the risk of dementia could explain why patients with T2D have a higher risk of dementia than persons without T2D. Particularly, as there is a substantial variation of concentrations of OCPs even among patients with T2D, patients with T2D with high concentrations of OCPs can have a higher risk of dementia than patients with T2D with relatively low concentrations of OCPs, regardless of glucose control. Lifetime exposure to POPs may be an underlying common risk factor for both T2D and dementia; this hypothesis needs to be tested in future studies.
Can OCPs explain the decreasing trend in the incidence rate of dementia?
Time trend studies from developed countries including the Framingham Heart Study in the United States have suggested that the age-specific incidence of dementia may be declining, even though the prevalence of dementia is soaring due to an expanding elderly population.31 Improved management of cardiovascular risk factors such as hypertension and changes in health behaviors, including smoking cessation, are likely important contributing factors to lowering the incidence rate of dementia. However, none of these trends completely explains the decrease in the incidence of dementia.32 We here propose that the decreasing trend of human exposure to OCPs might be one further possible contributory explanation for the decreasing time trend of dementia.
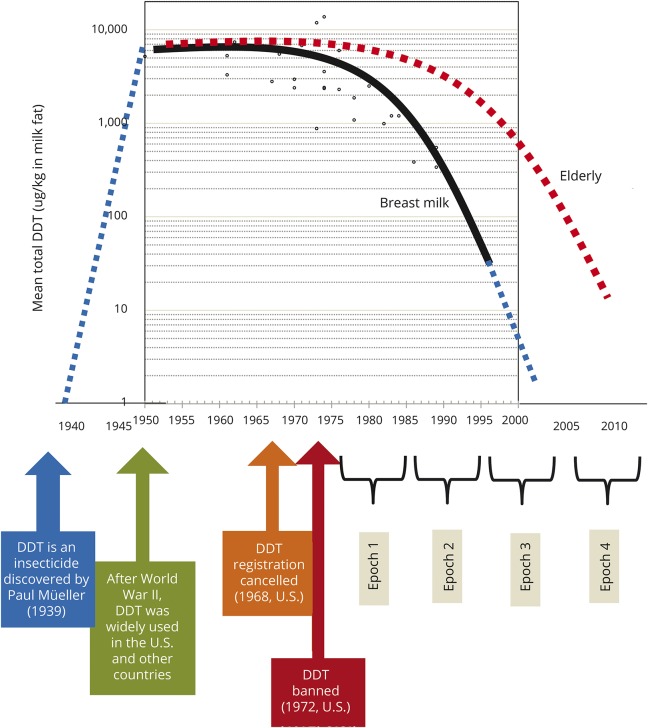
Figure 2 shows the time trend in the United States and Canada of concentrations of breast milk DDT, one typical OCP compound. As breast milk is a practical medium for biomonitoring lipophilic chemicals like OCPs,33 DDT measured in breast milk can be interpreted to reflect DDT in adipose tissue. In the United States, even though DDT was banned in 1972, DDT concentration in breast milk only started to decrease from the late 1970s. Body burden in humans has decreased very slowly because of the long half-life of DDT; thus it is detected in humans today.
Figure 2. Ecological similarity of secular trend between body burden of dichlorodiphenyltrichloroethane (DDT) and age-specific dementia incidence rates in developed countries.
Time trend (solid black line) of concentrations of DDT in breast milk in studies from the United States and Canada, with spline fit, with added explanatory notes.33 Breast milk, rather than adipose tissue, was used as a practical way to biomonitor body burden of lipophilic chemicals like DDT. The blue dotted line was added to display the whole period from the development of DDT to the present. Compared to that of young women in their 20s or 30s (providing breast milk), decreasing time trend of body burden of DDT in the elderly, who typically have higher levels due to exposure throughout their lifetimes, may be assumed to be slower (red dotted line). In the Framingham cohort, dementia incidence rates were reported to have declined over recent decades, as defined by epochs in the figure.32 Exposure levels to DDT in elderly at high risk of dementia have gradually decreased from Framingham epoch 1 to epoch 4. A birth cohort effect related to this exposure pattern to organochlorine pesticides may have contributed to the decreasing trend of dementia reported in the Framingham study.
In the Framingham cohort,32 absolute levels of DDT in the elderly would be estimated to be higher compared to young women who provided breast milk. Elderly would have had higher lifetime exposures including the period of active DDT use. However, we also expect that exposure to DDT in elderly has also gradually decreased over time. Therefore, there is an anticipated birth cohort effect based on exposure to 20th-century chemicals. This speculation based on ecologic data of generally declining population-wide exposure to OCP may have contributed to the decreasing trend of dementia reported in the Framingham study.
Why have OCPs mostly been neglected in the area of neurodegenerative diseases?
Although researchers have had an interest in the role of neurotoxic chemicals like pesticides as a risk factor for neurodegenerative diseases including dementia and Parkinson disease, OCPs have not drawn a lot of attention. First, these banned chemicals may not be considered to be of high scientific interest because many researchers have not recognized how the unique chemical/physical characteristics of OCPs can adversely affect human health including neurodegenerative diseases even after banning. Second, generally researchers have more interest in doses than in duration of exposure. Thus, very low dose OCPs in current general populations may have been assumed to be safe even though the exposure duration is lifetime. Third, practical difficulties in measuring OCPs in human biospecimens (e.g., need for a large volume of serum or plasma, relatively high costs, complex assays) have hindered investigations.
Yet, despite the decreasing trend of many of these chemicals in the environment and in humans, it will be a very long time before they are completely gone. In fact, the banning of OCPs in developing countries happened in the late 20th century. It is also important to note that DDT is still used in malaria-plagued areas such as Africa and Southern Asia, where DDT levels remain much higher than in the United States and Europe.34 As OCPs travel long distances from where they are used through air, water, and migrating food sources such as fish,35 the exposure to DDT would not be confined to only persons living in these DDT-using countries. Therefore, contamination from OCPs and other related chemicals will remain an important issue for research on the causes and mechanisms of neurodegenerative diseases.
Clinical implications
There is one important clinical implication of the presence of neurotoxic OCPs in adipose tissue and redistribution between adipose tissue and brain during weight loss. There are 2 types of weight loss: intentional and unintentional weight loss. While unintentional weight loss is considered as a harmful sign of disease, intentional weight loss is commonly recommended for overweight or obese persons. However, both intentional and unintentional weight loss can release OCPs from adipose tissue to circulation.
Nevertheless, when there is the same amount of weight loss, unintentional weight loss can be more problematic than intentional weight loss. The reason is that health behavior changes such as exercise commonly accompanying intentional weight loss can increase the excretion of OCPs.36 Therefore, the amount of OCPs reaching the brain may be greater in persons with unintentional weight loss than in those with intentional weight loss.
It is important to note that net benefits of intentional weight loss may decrease as people become older. Intentional weight loss in younger obese persons with dysfunctional adipocytes would be beneficial to the brain as well as to the cardiovascular system because the recovery of physiologic function of adipocyte can reduce the chronic release of OCPs from adipose tissue. Even though short-term high release of OCPs during intentional weight loss will occur, the prevention of long-term release of OCPs by mitigating uncontrolled lipolysis may be more important for young persons.
However, benefits of intentional weight loss in older ages might not be as large as in the young if substantial amounts of these chemicals are released from adipose tissue to circulation, with subsequent exposure to brain and other critical organs which are already experiencing age-related functional decline. We can reasonably expect that more chemicals are released from adipose tissue into circulation among elderly people than among younger people as the contamination of adipose tissue with poorly biodegradable lipophilic chemicals accumulates with age.37 Furthermore, the amount of chemicals reaching brain and other critical organs during weight loss also would be increased because the capacity to metabolize and excrete xenobiotics physiologically decreases with aging.38
Unintentional spontaneous weight loss is common among elders, but often ignored.39 As unintentional weight loss can lead to the substantial release of OCPs from adipose tissue, dietary interventions to prevent unintentional weight loss could be helpful to prevent the development and progression of dementia among elderly.
At present, strategies for body weight management in older adults remain controversial as overweight may protect elderly against mortality whereas weight loss may have harmful effects by, for example, promoting sarcopenia and bone loss and increasing susceptibility to infection and other illness.40 The release of OCPs and other chemicals from adipose tissue to circulation can be another potential unappreciated disadvantage of weight loss. Even though mild to moderate weight loss with preservation of muscle mass and strength through physical activity may have beneficial effects on comorbidities, functional performance, and quality of life in obese older adults, maintaining body weight and improving physical fitness and function might be a more appropriate strategy rather than body weight loss itself.
Conclusions
Methodologic issues are often discussed as possible reasons for the puzzling findings that obesity sometimes increases and sometimes decreases the risk of dementia, depending on the age at which it is measured. However, the dynamics of OCPs in relation to weight changes and dysfunctional adipocytes should be factors that may be directly explanatory. This hypothesis should be tested in future experimental and human studies on obesity and dementia.
Glossary
- DDT
dichlorodiphenyltrichloroethane
- OCP
organochlorine pesticide
- POP
persistent organic pollutant
- T2D
type 2 diabetes
Author contributions
Duk Hee Lee: generating the hypothesis and writing the manuscript. Miquel Porta: critical revision of manuscript for intellectual content. Lars Lind: critical revision of manuscript for intellectual content. P. Monica Lind: critical revision of manuscript for intellectual content. David R. Jacobs: critical revision of manuscript for intellectual content.
Study funding
Supported by The Korean Health Technology R&D Project (HI13C0715), funded by the Ministry of Health and Welfare, and the Environmental Health Action Program (2016001370002), funded by the Korea Ministry of Environment of the Republic of Korea.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Pedditizi E, Peters R, Beckett N. The risk of overweight/obesity in mid-life and late life for the development of dementia: a systematic review and meta-analysis of longitudinal studies. Age Ageing 2016;45:14–21. [DOI] [PubMed] [Google Scholar]
- 2.Gustafson D. BMI and dementia: feast or famine for the brain? Lancet Diabetes Endocrinol 2015;3:397–398. [DOI] [PubMed] [Google Scholar]
- 3.Kim KS, Lee YM, Kim SG, et al. Associations of organochlorine pesticides and polychlorinated biphenyls in visceral vs. subcutaneous adipose tissue with type 2 diabetes and insulin resistance. Chemosphere 2014;94:151–157. [DOI] [PubMed] [Google Scholar]
- 4.Lee DH, Porta M, Jacobs DR Jr, Vandenberg LN. Chlorinated persistent organic pollutants, obesity, and type 2 diabetes. Endocr Rev 2014;35:557–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Needham LL, Burse VW, Head SL, et al. Adipose tissue/serum partitioning of chlorinated hydrocarbon pesticides in humans. Chemosphere 1990;20:975–980. [Google Scholar]
- 6.Kim KS, Lee YM, Lee HW, Jacobs DR Jr, Lee DH. Associations between organochlorine pesticides and cognition in U.S. elders: National Health and Nutrition Examination Survey 1999–2002. Environ Int 2015;75:87–92. [DOI] [PubMed] [Google Scholar]
- 7.Lee DH, Lind PM, Jacobs DR Jr, Salihovic S, van Bavel B, Lind L. Association between background exposure to organochlorine pesticides and the risk of cognitive impairment: a prospective study that accounts for weight change. Environ Int 2016;89–90:179–184. [DOI] [PubMed] [Google Scholar]
- 8.Richardson JR, Roy A, Shalat SL, et al. Elevated serum pesticide levels and risk for Alzheimer disease. JAMA Neurol 2014;71:284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim SA, Lee YM, Lee HW, Jacobs DR Jr, Lee DH. Can inconsistent association between hypertension and cognition in elders be explained by levels of organochlorine pesticides? PLoS One 2015;10:e0144205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim SA, Lee YM, Lee HW, Jacobs DR Jr, Lee DH. Greater cognitive decline with aging among elders with high serum concentrations of organochlorine pesticides. PLoS One 2015;10:e0130623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colosio C, Tiramani M, Maroni M. Neurobehavioral effects of pesticides: state of the art. Neurotoxicology 2003;24:577–591. [DOI] [PubMed] [Google Scholar]
- 12.Jain A, Martensson J, Stole E, Auld PA, Meister A. Glutathione deficiency leads to mitochondrial damage in brain. Proc Natl Acad Sci USA 1991;88:1913–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006;443:787–795. [DOI] [PubMed] [Google Scholar]
- 14.Liu Q, Wang Q, Xu C, et al. Organochloride pesticides impaired mitochondrial function in hepatocytes and aggravated disorders of fatty acid metabolism. Sci Rep 2017;7:46339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruzzin J, Petersen R, Meugnier E, et al. Persistent organic pollutant exposure leads to insulin resistance syndrome. Environ Health Perspect 2010;118:465–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ketterer B, Coles B, Meyer DJ. The role of glutathione in detoxication. Environ Health Perspect 1983;49:59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macdonald TL. Chemical mechanisms of halocarbon metabolism. Crit Rev Toxicol 1983;11:85–120. [DOI] [PubMed] [Google Scholar]
- 18.La Merrill M, Emond C, Kim MJ, et al. Toxicological function of adipose tissue: focus on persistent organic pollutants. Environ Health Perspect 2013;121:162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saponaro C, Gaggini M, Carli F, Gastaldelli A. The subtle balance between lipolysis and lipogenesis: a critical point in metabolic homeostasis. Nutrients 2015;7:9453–9474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morigny P, Houssier M, Mouisel E, Langin D. Adipocyte lipolysis and insulin resistance. Biochimie 2016;125:259–266. [DOI] [PubMed] [Google Scholar]
- 21.Jansen A, Lyche JL, Polder A, Aaseth J, Skaug MA. Increased blood levels of persistent organic pollutants (POP) in obese individuals after weight loss: a review. J Toxicol Environ Health B Crit Rev 2017;20:22–37. [DOI] [PubMed] [Google Scholar]
- 22.Lim JS, Son HK, Park SK, Jacobs DR Jr, Lee DH. Inverse associations between long-term weight change and serum concentrations of persistent organic pollutants. Int J Obes 2011;35:744–747. [DOI] [PubMed] [Google Scholar]
- 23.Jandacek RJ, Anderson N, Liu M, Zheng S, Yang Q, Tso P. Effects of yo-yo diet, caloric restriction, and olestra on tissue distribution of hexachlorobenzene. Am J Physiol Gastrointest Liver Physiol 2005;288:G292–G299. [DOI] [PubMed] [Google Scholar]
- 24.Dirinck E, Dirtu AC, Jorens PG, Malarvannan G, Covaci A, Van Gaal LF. Pivotal role for the visceral fat compartment in the release of persistent organic pollutants during weight loss. J Clin Endocrinol Metab 2015;100:4463–4471. [DOI] [PubMed] [Google Scholar]
- 25.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev 2000;21:697–738. [DOI] [PubMed] [Google Scholar]
- 26.Strand BH, Wills AK, Langballe EM, Rosness TA, Engedal K, Bjertness E. Weight change in midlife and risk of mortality from dementia up to 35 Years later. J Gerontol A Biol Sci Med Sci 2017;72:855–860. [DOI] [PubMed] [Google Scholar]
- 27.Sergi G, De Rui M, Coin A, Inelmen EM, Manzato E. Weight loss and Alzheimer's disease: temporal and aetiologic connections. Proc Nutr Soc 2013;72:160–165. [DOI] [PubMed] [Google Scholar]
- 28.Franx BAA, Arnoldussen IAC, Kiliaan AJ, Gustafson DR. Weight loss in patients with dementia: considering the potential impact of pharmacotherapy. Drugs Aging 2017;34:425–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol 2006;5:64–74. [DOI] [PubMed] [Google Scholar]
- 30.Geijselaers SL, Sep SJ, Stehouwer CD, Biessels GJ. Glucose regulation, cognition, and brain MRI in type 2 diabetes: a systematic review. Lancet Diabetes Endocrinol 2015;3:75–89. [DOI] [PubMed] [Google Scholar]
- 31.Jones DS, Greene JA. Is dementia in decline? Historical trends and future trajectories. N Engl J Med 2016;374:507–509. [DOI] [PubMed] [Google Scholar]
- 32.Satizabal CL, Beiser AS, Chouraki V, Chene G, Dufouil C, Seshadri S. Incidence of dementia over three decades in the Framingham heart study. N Engl J Med 2016;374:523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith D. Worldwide trends in DDT levels in human breast milk. Int J Epidemiol 1999;28:179–188. [DOI] [PubMed] [Google Scholar]
- 34.Subramanian A, Ohtake M, Kunisue T, Tanabe S. High levels of organochlorines in mothers' milk from Chennai (Madras) City, India. Chemosphere 2007;68:928–939. [DOI] [PubMed] [Google Scholar]
- 35.Vorkamp K, Riget FF. A review of new and current-use contaminants in the Arctic environment: evidence of long-range transport and indications of bioaccumulation. Chemosphere 2014;111:379–395. [DOI] [PubMed] [Google Scholar]
- 36.Watkins JB III, Crawford ST, Sanders RA. Chronic voluntary exercise may alter hepatobiliary clearance of endogenous and exogenous chemicals in rats. Drug Metab Dispos 1994;22:537–543. [PubMed] [Google Scholar]
- 37.Hue O, Marcotte J, Berrigan F, et al. Plasma concentration of organochlorine compounds is associated with age and not obesity. Chemosphere 2007;67:1463–1467. [DOI] [PubMed] [Google Scholar]
- 38.McLachlan AJ, Pont LG. Drug metabolism in older people: a key consideration in achieving optimal outcomes with medicines. J Gerontol A Biol Sci Med Sci 2012;67:175–180. [DOI] [PubMed] [Google Scholar]
- 39.Gaddey HL, Holder K. Unintentional weight loss in older adults. Am Fam Physician 2014;89:718–722. [PubMed] [Google Scholar]
- 40.Decaria JE, Sharp C, Petrella RJ. Scoping review report: obesity in older adults. Int J Obes 2012;36:1141–1150. [DOI] [PubMed] [Google Scholar]