Abstract
Studies examining geographic variation in care for low back pain often focus on process and outcome measures conditional on patient diagnosis but generally do not take into account a physician’s ability to diagnose the root cause of low back pain. In our case study, we used increased detection of ankylosing spondylitis—a relatively rare inflammatory back disease—as a proxy for diagnostic ability and measured the relationship between ankylosing spondylitis detection, potentially inappropriate low back pain care, and cost. Using 5 years of health insurance claims data, we found significant variation in ankylosing spondylitis detection across metropolitan statistical areas (MSAs), with 8.1% of the variation in detection explained by a region’s racial composition. Furthermore, low back pain patients in MSAs with higher ankylosing spondylitis detection had 7.9% lower use of corticosteroids, 9.0% lower use of opioids, and 8.2% lower pharmacy cost, compared with patients living in low-detection MSAs.
Keywords: back pain, diagnosis, health care costs
Introduction
Over 20 years of research, beginning with the Dartmouth Atlas, has found significant geographic variation in the quality and cost of health care that patients in the United States receive.1-3 Furthermore, this research suggests that areas with higher health care spending do not have better quality, and in some cases, worse quality.4,5 Variation in cost and quality of care extends across highly prevalent conditions and health care services, such as heart attack treatment, cesarean deliveries, and colonoscopies.6-8
To date, most quality of care measures used by payers (e.g., National Committee for Quality Assurance’s Healthcare Effectiveness Data and Information Set) focus on the process of care, patient satisfaction, and, in some cases, patient outcomes; however, there are very few measures related to a physician’s ability to accurately diagnose a patient. Although providing a guideline-based treatment would improve providers’ quality measure scores, patient outcomes will likely suffer if the diagnosis is incorrect.
In this article, we examine whether metropolitan statistical areas (MSAs) where physicians have high rates of detecting a relatively uncommon source of back pain—ankylosing spondylitis (AS)—are more or less likely to provide high-quality, low-cost care to all low back pain patients. We use AS detection—estimated using the demographics-adjusted prevalence observed in claims data—as a proxy for diagnostic ability. Our key outcome measures are use of opioids and steroid injections, 2 treatments often inappropriately used when treating low back pain patients.9,10 Although this article represents a single case study, it can shed light on the question of whether a provider’s ability to identify and accurately diagnose patients is correlated with treatment quality and cost of care, conditional on diagnosis.
Background and Conceptual Framework
Low back pain is one condition with significant geographic variation in treatment patterns, quality of care, and cost. More than half of adults experience low back pain every year, a condition that costs an estimated $86 billion in health care spending and $20 billion in lost productivity annually in the United States.11-13 Unnecessary or non-recommended treatments comparise a large share of these costs. For example, the elimination of unnecessary lumbar imaging is estimated to save more than $300 million annually.14 Furthermore, the rate of opioid prescriptions for low back pain increased 35% in emergency departments (EDs), 38% in primary care clinics, and 75% in specialty clinics every 5 years between 1997 and 2009.15
AS is a chronic rheumatic disease that primarily affects the sacroiliac joints, spine, and peripheral joints.16,17 It is estimated that 0.2% to 0.5% of the US population has AS; however, patient and diagnostic factors make the disease difficult to diagnose.18 Delays in appropriate diagnosis of AS can result in further disease progression, which may lead to decreased physical functioning and quality of life.19
High-quality care for low back pain requires at least 2 components: proper diagnosis of the source of the low back pain and identifying effective treatment conditional on diagnosis.20 To date, most process and outcome measures focus on a patient’s treatment and subsequently assume that the patient population with the disease is known with certainty. In practice, however, different providers have different levels of acuity in detecting the source of low back pain. Previous research has found significant variation in physician approaches for low back pain testing, and these approaches vary across specialties.11,21
The relationship between high-quality diagnostic competency and treatment is especially important as payers move from a fee-for-service model to reimbursement based on measures of value, such as Medicare’s Shared Savings Program for accountable care organizations and its Merit-Based Incentive Payment System (MIPS) for physicians. Payers must consider whether or not these new payment models will be able to reward physicians who are able to both accurately diagnose patients and appropriately treat the patient conditional on diagnosis. Policy makers and payers are usually able to observe—and thus provide monetary incentives to—providers based on treatment choices conditional on diagnosis, but policy makers and payers are generally not able to evaluate provider diagnostic accuracy. If a provider’s diagnostic accuracy is highly correlated with treatment decisions conditional on diagnosis, then rewarding provider treatment decisions may be a sufficient condition for rewarding high-quality physicians within any value-based purchasing program. On the other hand, if the correlation between provider diagnostic ability and treatment quality is weakly or negatively correlated, then providers who most accurately diagnose a disease would be adversely affected financially by any value-based purchasing system.
New Contribution
This study is the first study to our knowledge that measures the relationship between diagnostic ability, quality of care, and cost in low back pain. There have been studies examining whether patient and provider characteristics of a geographic region are correlated with diagnosis rates and medication use, but this is the first study to our knowledge comparing diagnosis rates and inappropriate care directly against one another. Our study extends previous work examining geographic variation in opioid and corticosteroid use,22-24 and more generally the study of geographic variation in process of care measures.13,25,26
In addition, whereas most studies of geographic variation in quality of care focus on highly prevalent diseases, this study aims to measure how a region’s ability to identify relatively uncommon diseases is related to commonly used quality measures for more prevalent diseases. For instance, the Medicare Shared Savings Program includes 33 quality measures for only 5 diseases.27 Thus, many of the value-based purchasing programs currently being implemented by payers largely ignore patients who suffer from less common diseases such as AS.28 This article aims to fill this gap in the literature.
Methods
Data
We used the Truven Health Analytics MarketScan Commercial Claims and Encounters (Commercial) and Medicare Supplemental and Coordination of Benefits (Medicare) administrative claims data sets between July 2007 and June 2013. The MarketScan database includes demographic, enrollment, disease diagnosis, health care utilization, and expenditure information from active employees, early retirees, and Consolidated Omnibus Budget Reconciliation Act (COBRA) continuers. In addition, these data have been widely used in other back pain studies.29-32 In addition, we use 2014 data from the American Community Survey to measure the degree to which there are racial differences across regions.33 Previous research has shown that the prevalence of AS in different regions depends on the prevalence of the genetic factor HLA-B27 in these regions, and this factor is less common among African Americans.34-36
Study Subjects
Our approach identified patients living in the United States with prevalent cases of back pain or AS between 2008 and 2013. We included all individuals aged ≥18 years who had ≥2 claims for a back pain or AS diagnosis (International Classification of Disease, Ninth Revision (ICD-9) code 720.0) during the study period between July 1, 2008, and June 30, 2013. To focus the analysis on chronic rather than acute back pain, patients were only included if their AS or low back pain diagnosis claims were separated by a 90-day period. Low back pain patients were identified using the ICD-9 codes from the National Quality Forum–endorsed low back pain measure37: 721.3, 722.10, 722.32, 722.52, 722.93, 724.02, 724.2, 724.3, 724.5, 724.6, 724.70, 724.71, 724.79, 738.5, 739.3, 739.4, 846.0, 846.1, 846.2, 846.3, 846.8, 846.9, and 847.2. To capture resource utilization and costs over an entire year, patients were required to be enrolled in the data set for at least one continuous 12-month period. Because previous research indicates the prevalence of AS is less than 0.6% in the United States,38 we calculated prevalent cases of chronic low back pain and AS using 5 years of data to increase the precision of our measured detection estimates; patients could appear multiple times in the data set if they were continuously enrolled for multiple 12-month periods. Patients were excluded if they were not continuously enrolled for a 12-month period after an AS or low back pain diagnosis or if they had missing information on their place of residence.
Outcome Variables
We identified 3 types of outcome variables: (1) chronic low back pain or AS measured detection—that is, prevalence as recorded in health insurance claims data; (2) measures of potentially inappropriate or nonrecommended care; and (3) cost of care. Whenever possible, we used measures endorsed by medical organizations, quality measurement agencies, and expert clinicians, such as the Centers for Medicare & Medicaid Services, the American College of Physicians, and the National Committee for Quality Assurance.39-42
We calculated the AS detection metric as the observed prevalence of AS in claims data, where observed prevalence was measured as the number of patients who had ≥1 inpatient or ≥2 outpatient claims with an AS diagnosis within a calendar year.43 To control for differences in patient demographics across MSAs, AS detection by MSA was measured separately for 16 different age-gender categories (e.g., 45- to 54-year-old female, 55- to 64-year-old male). Overall AS detection was a weighted average across these categories where the weights were the share nationally of all individuals in each age-gender category.
Our treatment quality measures were measures of potentially inappropriate care: prescription opioids and steroid injection use. Prescription opioid use was measured as the percentage of back pain patients who filled a prescription for ≥1 opioid drug during a 12-month period. After identifying a list of complete or partial opioid agonists and combination formulations,44 we used Redbook to identify appropriate National Drug Codes (NDCs).45 The steroid injection metric was calculated as the percentage of low back pain patients who were dispensed at least one corticosteroid injection (Current Procedural Terminology codes 62311, 62310, 64479, 64480, 64483, and 64484) during a 12-month period.10,46
Finally, we analyzed the cost of low back pain and AS treatment using 3 metrics: total costs, total costs for disease-modifying antirheumatic drugs (DMARDs), and low back pain- or AS-related medical costs. DMARDs are used to improve disease activity and mobility in patients with AS who do not respond to nonsteroid anti-inflammatory drugs.47 Total costs represented the sum of annual inpatient, outpatient, and pharmacy costs that accrued to payers and patients with low back pain or AS. We calculated DMARD costs as all costs for DMARD prescriptions48; we measured total low back pain- or AS-related medical costs as the sum of inpatient and outpatient costs on claims that had a low back pain or AS diagnosis code. All costs were updated to 2015 USD using the Consumer Price Index, published by the U.S. Bureau of Labor Statistics.49
Geographic Definitions and Other Variables
To measure geographic variations in detection, quality of care, and costs, we constructed each metric at the individual level and then aggregated the metrics by geographic area. We selected MSAs as the geographic unit of observation, as they were the smallest level of geographic granularity available in the MarketScan data. MSAs, which are comprised of individual counties and equivalent entities, represent geographic regions with high population densities and close economic integration. Patients who lived outside of an MSA were assigned to a “rest of state” area, which represented all non-MSA counties in a state. These geographic classifications are used by the Centers for Medicare & Medicare Services in defining its Hospital Wage Index.50
Statistical Analysis
We used generalized linear models (GLMs) to estimate the AS detection, treatments, and health care costs, controlling for differences in patient composition between MSAs. To ensure the analysis was nationally representative, the detection and treatment models were weighted according to the age and gender distribution within each MSA as reported in the 2008-2012 Current Population Survey. When modeling regional variation in use of opioids and corticosteroids, we used a logistic regression controlling for patient age, gender, and year. For the cost models, we included age, gender, year, and the Elixhauser Comorbidity Index as independent variables in each GLM model.51 (See online appendix for first-stage regression model results.)
After calculating the adjusted outcomes by MSA, we measured the relationship between AS measured detection and potentially inappropriate care, as well as costs, using a linear regression model. To facilitate the interpretability of the regression coefficient of AS measured detection on the outcome of interest, we also report the results as the effect of increased AS detection as the product of the coefficient of interest and the difference in AS measured detection between an MSA at the 90th percentile and the 10th percentile. Because all MSA-level outcome variables were calculated from individual patient observations, we used a bootstrapping methodology to conduct a t test to examine whether the regression coefficients of interest were statistically significant. The bootstrap approach resampled 1% of the data with replacements within each stratum and generated 100 bootstrap samples. For each sample, we reestimated the MSA-level outcomes applied in the GLMs described above, and then estimated the coefficient of interest between MSA-level AS measured detection and potentially nonrecommended care or costs. Using the R-squared metric, we also measured the share of regional variation in AS detection across regions that can be explained by an MSA’s share of African American residents.
Results
Out of an initial sample of 141.8 million patient-year observations between July 1, 2008, and June 30, 2013, our final study sample included 2.80 million Medicare-insured patient-year observations (1.14 million unique patients) and 14.4 million commercially insured patient-years (6.59 million unique patients) with a low back pain or AS diagnosis (Table 1). Approximately 70.6% of individuals appeared in the data set for more than 1 year. Within this broader low back pain group, there were 33 031 patient-year observations in the Commercial data set and 4219 patient-year observations in the Medicare data set where the patient was diagnosed with AS.
Table 1.
Consort Diagram.
| Medicare-insured | Commercially insured | Total | |
|---|---|---|---|
| Patient-years (July 1, 2008-June 30, 2013) | 12 743 951 | 129 033 244 | 141 777 195 |
| ≥2 claims for back pain or ankylosing spondylitis evera | 7 816 936 | 48 976 169 | 56 793 105 |
| Meet continuous enrollment during year | 7 352 844 | 44 569 749 | 51 922 593 |
| ≥2 claims for back pain or ankylosing spondylitis during year | 3 487 309 | 19 008 006 | 22 495 315 |
| Age ≥18 at start of period | 3 487 309 | 17 727 842 | 21 215 151 |
| MSA value populated | 2 873 676 | 14 680 968 | 17 554 644 |
| Remove patients in Puerto Rico and Guamb | 2 800 029 | 14 380 897 | 17 180 926 |
| Number with back pain (no ankylosing spondylitis) | 2 795 810 | 14 347 866 | 17 143 676 |
| Number with ankylosing spondylitis | 4219 | 33 031 | 37 250 |
Note. MSA = metropolitan statistical area.
Number of unique patients meeting initial inclusion criteria included 2 439 170 unique Medicare-insured patients and 17 104 801 unique commercially insured patients.
Patients in Puerto Rico and Guam were dropped because these territories are not included in the Current Population Survey. The final analytic file contains 2 800 029 Medicare patient-year observations (1 140 033 unique Medicare-insured patients) and 14 380 897 commercially insured patient-year observations (6 588 563 unique patients).
In our low back pain patient sample, the average age was 51.7 (SD, 16.8), with a higher percentage of females than males (56.4%). The most frequent Elixhauser comorbidities were hypertension (32% of patients), diabetes (12.2%), chronic pulmonary disease (10.8%), and depression (10.6%). Compared with general low back pain patients, patients with AS were younger on average (49.8 vs 51.7), less likely to be female (37.7% vs 56.4%), more likely to use opioids (42.9% vs 37.8%), and less likely to use corticosteroid injections (5.7% vs 10.5%). In addition, AS patients had higher health care spending across all categories; total annual health care spending was more than twice as high, compared with chronic low back pain patients without an AS diagnosis ($21 957 vs $10 371).
AS measured detection in the average MSA was 37 per 100 000 people, whereas chronic low back pain measured detection was 18 233 individuals per 100 000 people. In the average MSA, only 0.21% of patients with low back pain were diagnosed with AS (Table 2). Although measured detection of AS was uniformly low across the country, we observed that it varied across MSAs. Our data were right-skewed in nature, as 74.9% of MSAs had measured detection below national average levels. Furthermore, 100% of MSAs had AS measured detection below the 0.55% national prevalence estimate calculated from the 2009-2010 National Health and Nutrition Examination Survey (NHANES) data.38
Table 2.
Summary Statistics.
| Patient-years |
MSA |
||||||
|---|---|---|---|---|---|---|---|
| Patients with ankylosing spondylitis |
Patients with low back pain but without ankylosing spondylitis |
Patients with low back pain |
|||||
| Mean/% | SD | Mean/% | SD | Mean/% | SD | IQR | |
| Demographics | |||||||
| Age, y | 49.8 | 14.8 | 51.7 | 16.8 | a | a | a |
| Female, % | 37.7 | — | 56.4 | — | a | a | a |
| Potentially nonrecommended care | |||||||
| Patients using opioids, % | 42.9 | — | 37.8 | — | 37.5 | 10.0 | 11.5 |
| Patients using corticosteroid injections, % | 5.7 | — | 10.5 | — | 9.1 | 2.8 | 3.7 |
| Total annual spending (2015 USD) | |||||||
| Inpatient, $ | 4648 | 25 094 | 2725 | 15 558 | 2672 | 660 | 751 |
| Outpatient, $ | 11 026 | 21 285 | 6201 | 13 864 | 6003 | 840 | 1092 |
| Outpatient pharmacy, $ | 6283 | 9460 | 1444 | 3719 | 1399 | 319 | 379 |
| DMARD spending only, $ | 3005 | 10 706 | 120 | 1685 | 126 | 45 | 48 |
| Total, $ | 21 957 | 36 738 | 10 371 | 24 013 | 10 074 | 1418 | 1567 |
| Low back pain- or ankylosing spondylitis–related spending, $ | 4300 | 16 848 | 2197 | 8547 | 2113 | 424 | 515 |
| Measured detectionb | |||||||
| Ankylosing spondylitis (per 100 000) | — | — | — | — | 37 | 23 | 19 |
| Chronic low back pain (per 100 000) | — | — | — | — | 18 233 | 4665 | 498 |
| Number of observations | 37 250 | — | 17 143 676 | 389 | — | — | |
Note. MSA = metropolitan statistical area; IQR = interquartile range; DMARD = disease-modifying antirheumatic drug.
The MSA-level results are adjusted for differences in patient age and gender across MSAs.
Measured detection is annual disease prevalence as measured based on diagnosis codes recorded in health insurance claims data.
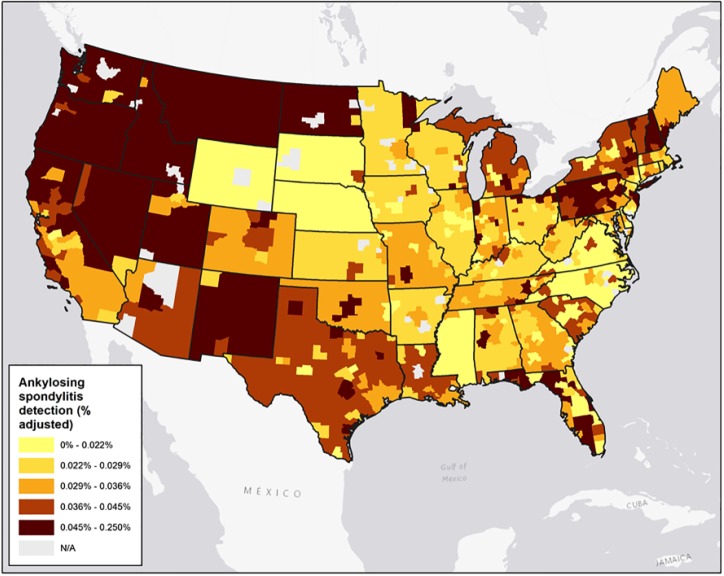
In addition, the magnitude of geographic variation in AS measured detection was higher compared with the geographic variation of chronic low back pain. Measured detection for AS at the 90th percentile MSA was 0.063%, compared with 0.016% at the 10th percentile; the interquartile range (IQR) was 0.019%. The comparable figures for low back pain more generally were 24.4% and 13.2%, with an IQR of 5.0%. Although AS is a less prevalent disease than chronic low back pain, its level of cross-MSA variability is higher, as measured using the coefficient of variation (CV). The CV for AS measured detection (0.631) was almost 2.5 times higher than the CV for measured detection of back pain alone (0.256). Figure 1 highlights this variability in AS measured detection in a map format.
Figure 1.
Ankylosing spondylitis measured detection by metropolitan statistical area (MSA), 2008-2013.
Note. Map of ankylosing spondylitis measured detection at the MSA level.
MSAs with higher rates of AS measured detection delivered more appropriate, cost-effective care to both low back pain and AS patients, as measured by fewer steroid and narcotic users. Table 3 presents the results for ordinary least squares regressions at the MSA-level with AS measured detection as the independent variable in each case. The table also presents the effect of increased AS detection as the product of the coefficient of interest and the difference in AS measured detection between an MSA at the 90th percentile and the 10th percentile (ie, slope coefficient × 0.046%), as well as the percentage change of this level of change in AS detection. Moving from an MSA with AS detection at the 10th percentile to the 90th percentile would decrease steroid use by 7.9% (P = .031) and narcotic use by 9.0% (P = .308) among patients with low back pain or AS; moving from a 25th percentile MSA to a 75th percentile MSA would change these numbers to 3.4% and 3.8%, respectively. Figure 2 shows the relationship between an MSA’s level of AS measured detection and the share of patients using opioids.
Table 3.
Ankylosing Spondylitis Measured Detection and Potentially Nonrecommended Care and Cost, Regression Results.
| Dependent variable | Independent variable | Coefficient | P value | Effect of move from 10th to 90th ankylosing spondylitis detection percentile MSAa | |
|---|---|---|---|---|---|
| Change | % change | ||||
| % of low back pain patients | |||||
| Using steroids | Ankylosing spondylitis measured detection | −16.16 | .031 | −0.75 | −7.9 |
| Constant | 0.098 | .051 | |||
| Using opioids | Ankylosing spondylitis measured detection | −13.23 | .308 | −3.6 | −9.0 |
| Constant | 0.61 | .312 | |||
| Annual per capita spending (2015 USD) | |||||
| Inpatient | Ankylosing spondylitis measured detection | −160559 | .133 | −74 | −2.8 |
| Constant | 2731 | .163 | |||
| Outpatient | Ankylosing spondylitis measured detection | −436991 | .166 | −203 | −3.3 |
| Constant | 6165 | .312 | |||
| Pharmacy (outpatient only) | Ankylosing spondylitis measured detection | −258304 | .003 | −120 | −8.2 |
| Constant | 1494 | .005 | |||
| DMARDs | Ankylosing spondylitis measured detection | 20864 | .056 | 10 | 8.0 |
| Constant | 118 | .037 | |||
| Low back pain- or ankylosing spondylitis–related medical costs | Ankylosing spondylitis measured detection | −142919 | .309 | −66 | −3.1 |
| Constant | 2166 | .295 | |||
| Total | Ankylosing spondylitis measured detection | −857396 | .122 | −398 | −3.9 |
| Constant | 10 391 | .221 | |||
Note. MSA = metropolitan statistical area; DMARDs = disease-modifying antirheumatic drugs.
Ankylosing spondylitis measured detection at the 90th percentile is 0.063% compared with 0.016% at the 10th percentile.
Figure 2.
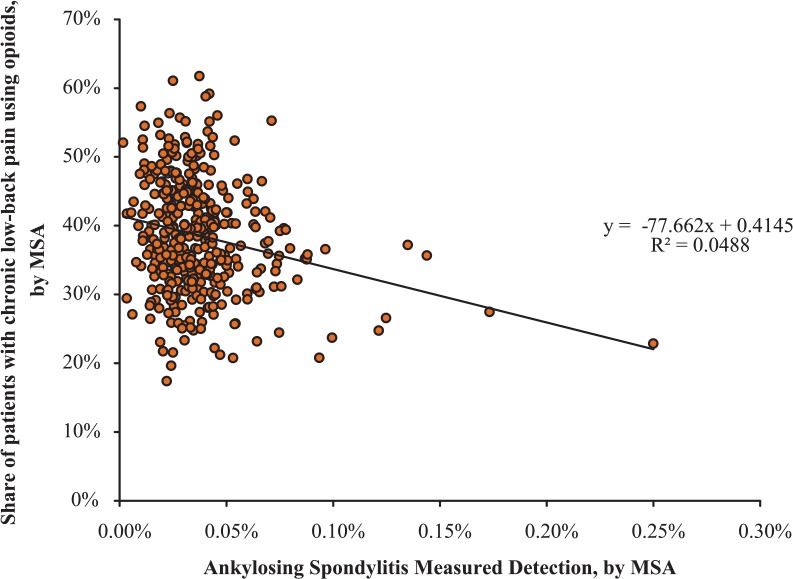
The relationship between ankylosing spondylitis measured detection and opioid use by MSA, 2008-2013.
Note. The x-axis shows ankylosing spondylitis measured detection by MSA, and the y-axis shows the percentage of low back pain patients using opioids, by MSA. The P value is .308. MSA = metropolitan statistical area.
MSAs with better detection of AS not only have lower use of inappropriate or inadvisable treatment among all low back pain patients but also have lower overall pharmacy spending, but higher DMARD use. Total costs were 3.9% (P = .122) lower among AS or low back pain patients living in MSAs at the 90th percentile for AS detection, compared with those at the 10th percentile (or 1.6% higher in MSAs at the 75th percentile compared with those at the 25th percentile of AS detection). Patients living in MSAs with high rates of AS detection (90th percentile) also had 2.8% (P = .133) lower inpatient spending, 3.3% (P = .166) lower outpatient spending, and 8.2% (P = .003) lower total prescription drug spending, compared with MSAs with low rates of AS detection (10th percentile).
Although overall pharmacy spending was lower in MSAs with higher AS measured detection, MSAs at the 90th percentile in AS detection had 8.0% (P = .056) higher DMARD use than MSAs in the 10th percentile in AS detection. While not all results rose to the level of statistical significance, we observed a consistent pattern that higher levels of AS measured detection were associated with lower inpatient, outpatient, pharmacy, and total costs.
We did find that some regions with a large share of African Americans had lower rates of AS detection. The correlation between a region’s AS detection rate and its share of African American residents was −0.286, indicating that a region’s racial composition explains 8.1% of the variation in AS detection.
Discussion
In general, measured AS detection was uniformly low. Average AS detection nationwide was only 0.037%, compared with estimates of 0.55% as measured in 2009-2010 NHANES data.38 As NHANES is a household survey that includes physician and laboratory tests for all participants, our claims-based AS detection measure likely underestimates the true prevalence of AS.
MSAs with higher levels of AS measured detection provided fewer potentially inappropriate services and suggestive evidence that the cost of care was lower when AS measured detection was higher. The observed relationship is due to both a causal and correlational effect. The causal effect occurs because increased AS detection reduces the amount of potentially inappropriate care received by patients with undiagnosed AS. Patients with AS, however, represent a small share of low back pain patients.38 Nevertheless, high levels of AS measured detection may indicate that an MSA is more likely to appropriately diagnose the underlying cause of low back pain, whether or not this cause is AS or another cause of low back pain. Although this hypothesis is speculative, improved low back pain diagnosis may reduce the use of potentially inappropriate care, increase the likelihood the patient receives the correct treatment, and reduce total costs.
We hypothesize that increased AS detection may lead to better health outcomes for patients with the disease, through earlier diagnosis and appropriate treatment options. Because AS can mimic mechanical back pain in its early stages, proper diagnosis can be challenging—particularly in a primary care setting. Accurate diagnosis requires a significant level of clinical knowledge, experience, and suspicion to perform appropriate confirmatory tests at an early stage of the disease.17,19 A diagnosis of AS usually is confirmed by radiography or magnetic resonance imaging (MRI) showing sacroiliitis, in addition to a physical exam.17 As delays in the diagnosis of AS can result in further disease progression and decreased physical functioning, as well as potentially inappropriate health care utilization and higher costs, early diagnosis is clinically important.20
Some possible factors for better AS detection include the availability of specialists (especially radiologists and rheumatologists), and the degree to which evidence-based clinical guidelines and best practices are adopted and implemented by medical groups, health systems, or health plans. Further research in this area could help elucidate these factors and lead to better AS detection more generally.
This study had a number of limitations. First, claims data analyses were unable to capture many dimensions of functional status, such as back pain severity. Furthermore, measured AS detection was dependent on physicians accurately entering diagnosis codes. We cannot rule out that geographic variation in AS measured detection was due to differences across MSAs in coding practices or physician specialty, as patients with back pain commonly see primary care physicians, orthopedic surgeons, and physiatrists in varying degrees. Second, the MarketScan data are a convenience sample. To extrapolate the regression results from the MarketScan sample to the broader US population, we followed past work studying geographic variations using private claims data52 and calculated age-gender stratum weights using 2013 data from the Current Population Survey and applied them to all regression models. Essentially, this approach put more weight on observations in age-gender ranges that were less common in our data, relative to the general population. Third, we found that regions with a larger share of African Americans residents had lower rates of AS detection. This finding, however, is likely to bias our estimates toward finding that higher AS detection is associated with more opioid use and higher cost, when in fact we found the reverse to be true. In the United States, opioid use among African Americans is generally similar53 or lower54 than opioid use among Caucasians. Thus, regions with a lower share of African Americans—and thus higher rates of AS detection—should have relatively higher use of opioid use; in fact, this study found that these regions with high rates of AS detection had lower rates of opioid and corticosteroid use. Similarly, we found that health care costs were generally lower in areas with high rates of AS detection, whereas predicted variation based on racial composition alone would predict the opposite, as total health care spending among African Americans is similar or lower than Caucasians’ health care spending.55 Fourth, the AS detection rate in our data was much lower than estimates from NHANES and other sources, making our results more sensitive to small changes in AS detection in a given region. This study, however, relied on a large, multiyear data set, and this uncertainty is accurately reflected by the bootstrapped standard errors and P values. Finally, this study focused exclusively on low back pain and AS; future work should replicate the analysis using other measures of diagnostic accuracy beyond the detection of AS as well as apply to other disease states.
Conclusion
MSAs with higher AS detection had less use of potentially inappropriate services. Suggestive evidence indicated that areas with increased AS detection also had lower overall costs. The observed improved care and lower costs likely were due to improved early diagnosis of not only AS but also other forms of chronic low back pain. For clinicians, we recommend better integrating radiologists and other specialists earlier in the care of chronic low back pain patients to improve their ability to identify the root causes of low back pain, as well as encourage the adoption and implementation of evidence-based guidelines and best practices by medical groups, delivery systems, and health plans. More broadly, this case study provides one piece of evidence that geographic regions better able to identify and diagnose patients with uncommon disease are also the same geographic regions likely to provide high-quality, low-cost care conditional on diagnosis.
Footnotes
Authors’ Note: Results from this study were presented at the 2015 American College of Rheumatology’s annual meeting.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Jason Shafrin, Jin Joo Shim, and Caroline Huber are employees of Precision Health Economics, which was funded by AbbVie to conduct this study. Jenny Griffith and Arijit Ganguli are AbbVie employees and own AbbVie stock. Dr Wade Aubry is a paid consultant to Precision Health Economics and served as a scientific advisor on the study.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: AbbVie provided financial support for the study and participated in the interpretation of data, review, and approval of the publication.
References
- 1. Newhouse JP, Garber AM. Geographic variation in health care spending in the United States: insights from an Institute of Medicine report. JAMA. 2013;310(12):1227-1228. [DOI] [PubMed] [Google Scholar]
- 2. Wennberg JE, Cooper MM. The Dartmouth Atlas of Health Care. Chicago, IL: American Hospital Publishing; 1996. [PubMed] [Google Scholar]
- 3. Baicker K, Skinner J, Chandra A. Geographic variation in health care and the problem of measuring racial disparities. Perspect Biol Med. 2005;48(1):S42-S53. [PubMed] [Google Scholar]
- 4. MaCurdy T, Bhattacharya J, Perlroth D, et al. Geographic variation in spending, utilization and quality: Medicare and Medicaid beneficiaries. Report prepared by: Acumen LLC, Commissioned by the IOM Committee on Geographic Variation in Health Care Spending and Promotion of High-Value Care. Washington, DC: Institute of Medicine; May 2013. [Google Scholar]
- 5. Hussey PS, Wertheimer S, Mehrotra A. The association between health care quality and cost: a systematic review. Ann Intern Med. 2013;158(1):27-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spatz ES, Beckman AL, Wang Y, Desai NR, Krumholz HM. Geographic variation in trends and disparities in acute myocardial infarction hospitalization and mortality by income levels, 1999-2013. JAMA Cardiol. 2016;1(3):255-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kozhimannil KB, Law MR, Virnig BA. Cesarean delivery rates vary tenfold among US hospitals; reducing variation may address quality and cost issues. Health Aff. 2013;32(3):527-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sheffield KM, Han Y, Kuo Y-F, Riall TS, Goodwin JS. Potentially inappropriate screening colonoscopy in Medicare patients: variation by physician and geographic region. JAMA Intern Med. 2013;173(7):542-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deyo RA, Mirza SK, Turner JA, Martin BI. Overtreating chronic back pain: time to back off? J Am Board Fam Med. 2009;22(1):62-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ivanova JI, Birnbaum HG, Schiller M, Kantor E, Johnstone BM, Swindle RW. Real-world practice patterns, health-care utilization, and costs in patients with low back pain: the long road to guideline-concordant care. Spine J. 2011;11(7):622-632. [DOI] [PubMed] [Google Scholar]
- 11. Mafi JN, McCarthy EP, Davis RB, Landon BE. Worsening trends in the management and treatment of back pain. JAMA Intern Med. 2013;173(17):1573-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gore M, Sadosky A, Stacey BR, Tai K-S, Leslie D. The burden of chronic low back pain: clinical comorbidities, treatment patterns, and health care costs in usual care settings. Spine. 2012;37(11):E668-E677. [DOI] [PubMed] [Google Scholar]
- 13. Goodney PR, Dzebisashvili N, Goodman DC, Bronner KK. Variation in the Care of Surgical Conditions: Spinal Stenosis. A Dartmouth Atlas of Healthcare Series. Lebanon, NH: The Dartmouth Institute of Health Policy & Clinical Practice; 2014. [PubMed] [Google Scholar]
- 14. Srinivas SV, Deyo RA, Berger ZD. Application of “less is more” to low back pain. Arch Intern Med. 2012;172(13):1016-1020. [DOI] [PubMed] [Google Scholar]
- 15. Kao M-CJ, Minh LC, Huang GY, Mitra R, Smuck M. Trends in ambulatory physician opioid prescription in the United States, 1997-2009. PM R. 2014;6(7):575-582.e4. [DOI] [PubMed] [Google Scholar]
- 16. Braun J, Sieper J. Ankylosing spondylitis. Lancet. 2007;369(9570):1379-1390. [DOI] [PubMed] [Google Scholar]
- 17. Goh L, Samanta A. A systematic MEDLINE analysis of therapeutic approaches in ankylosing spondylitis. Rheumatol Int. 2009;29(10):1123-1135. [DOI] [PubMed] [Google Scholar]
- 18. Reveille JD. Epidemiology of spondyloarthritis in North America. Am J Med Sci. 2011;341(4):284-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Clegg DO. Treatment of ankylosing spondylitis. J Rheumatol. 2006;78:24-31. [PubMed] [Google Scholar]
- 20. Deodhar A, Mittal M, Reilly P, et al. Ankylosing spondylitis diagnosis in US patients with back pain: identifying providers involved and factors associated with rheumatology referral delay. Clin Rheumatol. 2016;35(7):1769-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tan A, Zhou J, Kuo Y-F, Goodwin JS. Variation among primary care physicians in the use of imaging for older patients with acute low back pain. J Gen Intern Med. 2016;31(2):156-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Curtis LH, Stoddard J, Radeva JI, et al. Geographic variation in the prescription of schedule II opioid analgesics among outpatients in the United States. Health Serv Res. 2006;41(3, pt 1):837-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zerzan JT, Morden NE, Soumerai S, et al. Trends and geographic variation of opiate medication use in state Medicaid fee-for-service programs, 1996 to 2002. Med Care. 2006;44(11):1005-1010. [DOI] [PubMed] [Google Scholar]
- 24. McDonald DC, Carlson K, Izrael D. Geographic variation in opioid prescribing in the U.S. J Pain. 2012;13(10):988-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ip IK, Raja AS, Seltzer SE, Gawande AA, Joynt KE, Khorasani R. Use of public data to target variation in providers’ use of CT and MR imaging among Medicare beneficiaries. Radiology. 2015;275(3):718-724. [DOI] [PubMed] [Google Scholar]
- 26. Baras JD, Baker LC. Magnetic resonance imaging and low back pain care for Medicare patients. Health Aff. 2009;28(6):w1133-w1140. [DOI] [PubMed] [Google Scholar]
- 27. Centers for Medicare & Medicaid Services. Table: 33 ACO quality measures. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/sharedsavingsprogram/Downloads/ACO-Shared-Savings-Program-Quality-Measures.pdf. Published 2015. Accessed May 20, 2016.
- 28. Malphrus E, McGinnis JM, Blumenthal D. Vital Signs: Core Metrics for Health and Health Care Progress. Washington, DC: National Academies Press; 2015. [PubMed] [Google Scholar]
- 29. Ackerman SJ, Polly DW, Jr, Knight T, Holt T, Cummings J. Management of sacroiliac joint disruption and degenerative sacroiliitis with nonoperative care is medical resource-intensive and costly in a United States commercial payer population. Clinicoecon Outcomes Res. 2014;6:63-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lad SP, Babu R, Baker AA, et al. Complications, reoperation rates, and health-care cost following surgical treatment of lumbar spondylolisthesis. J Bone Joint Surg Am. 2013;95(21):e162. [DOI] [PubMed] [Google Scholar]
- 31. Pivec R, Michael S, Abhishek SC, Paulino CB, Steven FH, Michael AM. Clinical and economic impact of TENS in patients with chronic low back pain: analysis of a nationwide database. Orthopedics. 2013; 36(12):922-928. [DOI] [PubMed] [Google Scholar]
- 32. Ackerman SJ, Polly DW, Jr, Knight T, Schneider K, Holt T, Cummings J., Jr. Comparison of the costs of nonoperative care to minimally invasive surgery for sacroiliac joint disruption and degenerative sacroiliitis in a United States commercial payer population: potential economic implications of a new minimally invasive technology. Clinicoecon Outcomes Res. 2014;6:283-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. FactFinder USCBA. B02001: Race. In: U.S. Census Bureau’s American Community Survey Office, ed. 2014.
- 34. Khan MA. Epidemiology of HLA-B27 and arthritis. Clinical Rheumatology. 1996;15(1):10-12. [DOI] [PubMed] [Google Scholar]
- 35. (NCQA) NCfQA. NQF-Endorsed™ National Voluntary Consensus Standards for Physician-Focused Ambulatory Care: APPENDIX A–NCQA Measure Technical Specifications. http://www.ncqa.org/Portals/0/HEDISQM/NQF_Posting_Appendix.pdf. Published 2008. Accessed December 26, 2014.
- 36. Reveille JD, Weisman MH. The epidemiology of back pain, axial spondyloarthritis and HLA-B27 in the United States. Am J Med Sci. 2013;345(6):431-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Centers for Medicare & Medicaid Services. MRI lumbar spine for low back pain quality measure. https://www.qualitymeasures.ahrq.gov/summaries/summary/49602/imaging-efficiency-percentage-of-mri-of-the-lumbar-spine-studies-with-a-diagnosis-of-low-back-pain-on-the-imaging-claim-and-for-which-the-patient-did-not-have-prior-claimsbased-evidence-of-antecedent-conservative-therapy?q=Tissues. Published 2013. Accessed August 24, 2015.
- 38. National Committee for Quality Assurance NCfQ. Back pain: appropriate use of epidural steroid injections. http://www.ncqa.org/portals/0/programs/recognition/BPRP_FAQ.pdf. Published 2014. Accessed August 24, 2015.
- 39. National Committee for Quality Assurance. Use of imaging studies for low back pain. http://www.ncqa.org/report-cards/health-plans/state-of-health-care-quality/2016-table-of-contents/low-back-pain. Published 2013. Accessed August 24, 2015.
- 40. Chou R, Qaseem A, Snow V, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147(7):478-491. [DOI] [PubMed] [Google Scholar]
- 41. Robinson D, Jr, Hackett M, Wong J, Kimball AB, Cohen R, Bala M. Co-occurrence and comorbidities in patients with immune-mediated inflammatory disorders: an exploration using US healthcare claims data, 2001-2002. Curr Med Res Opin. 2006;22(5):989-1000. [DOI] [PubMed] [Google Scholar]
- 42. Jena AB, Goldman D, Weaver L, Karaca-Mandic P. Opioid prescribing by multiple providers in Medicare: retrospective observational study of insurance claims. BMJ. 2014;348:g1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Micromedex Truven Health Analytics. Red Book. Truven Health Analytics; 2014. [Google Scholar]
- 44. Abbott ZI, Nair KV, Allen RR, Akuthota VR. Utilization characteristics of spinal interventions. Spine J. 2012;12(1):35-43. [DOI] [PubMed] [Google Scholar]
- 45. de Avila Machado MA, Barbosa MM, Almeida AM, et al. Treatment of ankylosing spondylitis with TNF blockers: a meta-analysis. Rheumatol Int. 2013;33(9):2199-2213. [DOI] [PubMed] [Google Scholar]
- 46. Lexi-Comp I. Healthcare Common Procedure Coding System Codes (HCPCS) Database. In: Lexi-Comp I, ed. OH; 2015. [Google Scholar]
- 47. US Department of Labor. Consumer price index. http://www.bls.gov/cpi/.Published. 2015. Accessed August 14, 2015.
- 48. MaCurdy T, Shafrin J, DeLeire T, et al. Geographic Adjustment of Medicare Payments to Physicians: Evaluation of IOM Recommendations. Washington, DC: Acumen LLC; 2012. [Google Scholar]
- 49. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130-1139. [DOI] [PubMed] [Google Scholar]
- 50. Philipson TJ, Seabury SA, Lockwood LM, Goldman DP, Lakdawalla DN. Geographic variation in health care: the role of private markets. Brookings Pap Eco AC. 2010;2010:325-355. [Google Scholar]
- 51. Carey TS, Freburger JK, Holmes GM, et al. Race, care seeking, and utilization for chronic back and neck pain: population perspectives. J Pain. 2010;11(4):343-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chen I, Kurz J, Pasanen M, et al. Racial differences in opioid use for chronic nonmalignant pain. J Gen Intern Med. 2005;20(7):593-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen J, Bustamante AV, Tom SE. Health care spending and utilization by race/ethnicity under the Affordable Care Act’s dependent coverage expansion. Am J Public Health. 2015;105(S3):S499-S507. [DOI] [PMC free article] [PubMed] [Google Scholar]