Abstract
The phase 4 COMPASS-3 study evaluated whether a singular endpoint produces clinically meaningful outcomes in patients with pulmonary arterial hypertension (PAH). The relationship between cardiac magnetic resonance imaging (cMRI)-derived parameters and right heart catheterization (RHC) measurements was also examined. In COMPASS-3 (ClinicalTrials.gov NCT00433329), 100 patients with PAH received bosentan monotherapy for 16 weeks. Patients continued monotherapy if their 6-min walk distance (6MWD) was ≥380 m, or otherwise received add-on sildenafil for an additional 12 weeks. 6MWD, RHC, and cMRI were performed at baseline, week 16, and week 28 (6MWD and cMRI). Baseline median 6MWD was 274 m and 82% of patients had WHO Functional Class III/IV. At week 16, 17% (n = 16) of remaining patients achieved the 6MWD threshold and 78 (83%) did not. In the intention-to-treat population, median 6MWD increased significantly relative to baseline (week 16 = 308 m; week 28 = 327 m; P < 0.001). At week 28, 9/16 (monotherapy) and 15/76 (20%; add-on sildenafil) patients met the target threshold. Baseline cMRI-derived and RHC-derived parameters showed moderate-to-strong correlations (e.g. right to left ventricular end-diastolic ratio [RVEDV:LVEDV] correlated strongly with pulmonary vascular resistance [r = +0.729, P < 0.0001]). cMRI-derived parameters predicted clinical worsening/decline (e.g. week 16 RVEDV:LVDEV [P = 0.0172]). Time to clinical worsening/decline did not differ between patients based on 6MWD threshold achievement. No unexpected safety events were reported. A substantial proportion of patients failed to achieve the goal of 380 m, regardless of treatment. Several cMRI parameters predicted clinical worsening/decline and its non-invasive nature further supports its use in future clinical trials.
Keywords: bosentan, cardiac magnetic resonance imaging, combination therapy, pulmonary arterial hypertension, sildenafil
Introduction
The availability of targeted disease-specific therapies has led to improvements in exercise capacity, World Health Organization (WHO) functional class (FC), and survival in patients with pulmonary arterial hypertension (PAH).1–4 Despite these advances, the prognosis of patients with PAH remains poor, with a five-year survival rate of 55–57%.4,5
The change in 6-min walk distance (6MWD) has been widely utilized as a primary endpoint in clinical trials of PAH to gauge treatment response.5–8 In an observational study of 178 patients receiving PAH-specific therapy, a 6MWD ≥ 380 m was found to correlate with improved survival5 and, until recently, this threshold has been recommended as a therapeutic goal.9,10 As 6MWD can vary with patient age, height, sex, and co-morbid illness,11 the predictive value of specific 6MWD thresholds has been questioned;12 however, one-year survival estimates are consistently higher for patients who score above threshold compared with patients who score below threshold, regardless of specific threshold (i.e. <165, 165–440, and >440 m).13 In addition to 6MWD, disease etiology, cardiac output, and right atrial pressure also predict mortality risk in patients with PAH.14,15
Hemodynamic parameters, measured via right heart catheterization (RHC), are also used to measure treatment response. However, RHC is an invasive procedure, which limits the ability to acquire serial measurements.9,16 In contrast, cardiac magnetic resonance imaging (cMRI) is a non-invasive, high-resolution technique allowing for the visualization and direct measurement of anatomical and functional changes in the right heart (i.e. enhanced volume and pressure measurements compared with echocardiography).9,8,17–19 cMRI-derived parameters, such as right ventricular (RV) volumes and ejection fraction, correlate with traditional measures of functional status, including 6MWD,20–22 and survival.23–27 cMRI is becoming an important tool in the clinical study of PAH as it can provide valuable information in an accurate, reproducible, and non-invasive manner.
COMPASS-3 was an open-label, non-comparative phase IV study that evaluated whether treating a patient to a single prespecified target (6MWD ≥ 380 m) produces clinically meaningful results. The study was also designed to evaluate the utility of cMRI in assessing improved functional capacity in patients with PAH and to explore the correlation between cMRI-derived parameters and traditional assessments of patient clinical status.
Methods
Study design
COMPASS-3 (NCT00433329) was an open-label, exploratory phase 4 study conducted in 23 sites in the United States during 2007–2010. This study was initiated before the publication of recent guidelines which support a treatment approach that involves comprehensive assessment of patient characteristics with the goal of reducing mortality risk (treat-to-outcomes rather than treat-to-target) approach for clinical trials.9 Following a screening period of ≤2 weeks, treatment-naïve patients received oral twice-daily bosentan 62.5 mg for 4 weeks followed by twice-daily bosentan 125 mg (or 62.5 mg, if 125 mg was poorly tolerated) for 12 weeks (Fig. S1). Patients achieving a 6MWD ≥ 380 m at 16 weeks remained on bosentan monotherapy for an additional 12 weeks (125 mg twice daily), while those who did not would go on to receive combination therapy—beginning at week 16—with twice-daily bosentan 125 mg plus sildenafil 20 mg three times daily for an additional 12 weeks. COMPASS-3 conformed to Good Clinical Practice guidelines and Declaration of Helsinki principles. The protocol was approved by the Institutional Review Board/Independent Ethics Committee at each participating site, as described in the online supplement.
Patients
Inclusion criteria included patients aged ≥21 years diagnosed with WHO Group I PAH who were treatment-naïve (i.e. not considered to be candidates for parenteral prostacyclins, per the discretion of the treating physician). PAH was diagnosed by RHC findings of mean pulmonary artery pressure (mPAP) ≥25 mmHg; pulmonary artery wedge pressure (PAWP) or left ventricular end diastolic pressure ≤15 mmHg; and pulmonary vascular resistance (PVR) ≥3 Wood units (WU). Baseline 6MWD entry criterion was 150–360 m. Exclusion criteria are described in the online supplement.
Endpoints
The primary prespecified endpoint was the proportion of patients who achieved a 6MWD ≥ 380 m at 16 weeks and/or at 28 weeks. Hypothesis-generating post-hoc endpoints are described in the online supplement including the change from baseline to weeks 16 and 28 in 6MWD and percent predicted 6MWD, WHO FC, NT-pro-brain natriuretic peptide (NT-pro-BNP), RHC-related parameters (week 16 only), and cMRI-derived parameters. A final follow-up was scheduled at week 52.
Assessments
6MWD was measured per American Thoracic Society guidelines.28 NT-pro-BNP was quantified at a central laboratory (Quintiles Inc.). Hemodynamic evaluations were performed with the patient in the supine position per local standard practice utilizing the internal jugular, subclavian, or femoral vein and a triple (or 4)-lumen, balloon-tipped, thermo-dilution catheter. Cardiac output was measured by either thermo-dilution, measured in triplicates with <10% differences, or the Fick principle, with the same method used for a patient throughout the study.
cMRI was performed using a 1.5-T magnet and software capable of cardiovascular imaging.29 cMRI variables were indexed using baseline body surface area. Bright-blood cine images were acquired using an electrocardiographic-gated steady-state free precession technique.30 Images were sent to a central core laboratory (University of Alabama, Birmingham, AL, USA) for analysis and interpretation. RHC and cMRI were completed within a 48-h period at baseline and week 16. An additional cMRI examination was performed at week 28.
Clinical worsening was defined as hospitalization for worsening in, or complications of, PAH, atrial septostomy, lung transplantation, initiation of parenteral prostanoids, or death between baseline and week 52. Clinical decline was defined as worsening of ≥1 WHO FC plus ≥15% decline in 6MWD between baseline and week 52.
Statistical analyses
Efficacy and safety analyses were performed on the intention-to-treat (ITT) population, which was composed of all patients who received ≥1 dose of study drug. As this was an exploratory study, no formal statistical hypothesis testing was planned; however, P values were generated for illustrative purposes.
For the primary endpoint, patients who did not have a 6MWD result available (regardless of the reason) were considered non-responders and included in the denominator. At week 28, the proportion of patients who achieved a 6MWD ≥ 380 m were summarized for the ITT population and the subgroups of patients receiving bosentan monotherapy or bosentan plus sildenafil combination therapy. The differences in 6MWD and percent predicted 6MWD between the treatment groups at various time-points were compared using the Mann–Whitney test and Hodges–Lehman 95% confidence intervals (CIs). Patient demographics and disease characteristics at baseline were compared post hoc in the monotherapy and combination therapy groups using a mixed model for continuous variables and chi-square test for categorical variables.
P values were calculated for changes from baseline to weeks 16 and 28 for 6MWD, NT-pro-BNP, RHC-related parameters (week 16 only), and cMRI-derived parameters using the t-test for mean values and Wilcoxon rank test for median values. For proportions, 95% CIs were computed from the Clopper–Pearson (Exact) method. For mean values, 95% CIs were computed as the sample mean ± the appropriate quantile of t-distribution × the standard error. For median values, 95% CIs were computed based on a distribution-free method. As part of an exploratory analysis, the correlation between cMRI-derived parameters and traditional patient assessments at baseline and week 16 was estimated using Spearman rank-order correlation coefficients with 95% CIs and associated P values. Parameters examined in correlation analyses are listed in Table S1.
In a preplanned analysis, time to clinical worsening and/or decline was estimated using the Kaplan–Meier method. In a post-hoc analysis, the difference between the monotherapy and combination therapy treatment groups in the time to clinical worsening and/or decline was compared using the Wilcoxon log-rank test. The characteristics of patients who did and did not experience clinical worsening or decline were examined post-hoc. The same statistical tests used to compare the monotherapy and combination therapy treatments groups were employed.
Further comparisons between patients in the monotherapy and combination therapy groups were made using the REVEAL risk score calculator for patients with PAH.14 Developed using patients in the REVEAL registry, the REVEAL risk score calculator predicts patient 12-month survival based on demographic, clinical, and hemodynamic variables. Comparisons between groups were made using an independent t-test with P < 0.05 considered statistically significant.
Predictors of clinical worsening and/or decline were explored post-hoc using univariable and multivariable logistic regression. All parameters with P ≤ 0.10 in the univariable analyses were included in the multivariable analyses. The multivariable models were manually stepwise-reduced to identify groups of non-collinear parameters significantly predictive of clinical worsening and/or decline. All statistical analyses were performed using SAS® version 8.2 or later.
Results
Patient disposition and characteristics
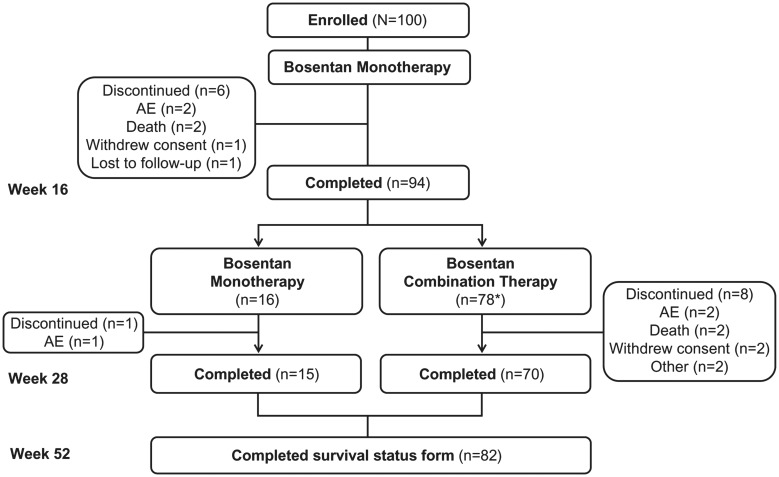
One hundred patients were enrolled and included in the ITT population (Fig. 1). Of these, 94% completed the 16-week monotherapy phase and 85% completed 28 weeks of treatment. Baseline demographics for the ITT population are summarized in Table 1 and functional and biomarker characteristics at baseline are shown in Table 2. Baseline hemodynamics were consistent with advanced PAH (Table 3) and cMRI-derived parameters at baseline confirm the enrollment of a population with adverse RV remodeling (Table 4). cMRI data were excluded in three patients due to the poor quality or inadequate acquisition of results.
Fig. 1.
Patient disposition. *Two patients withdrew consent before dosing. Thus, only 76 patients received combination treatment. AE, adverse event.
Table 1.
Patient demographics and disease characteristics at baseline.
| Patients (n = 100) | |
|---|---|
| Median age (range), years | 57.5 (21–84) |
| Female, n (%) | 82 (82) |
| Median BMI (range), kg/m2* | 29.3 (14.5–47.4) |
| Race, n (%) | |
| White | 81 (81) |
| Black, African American, or African heritage | 18 (18) |
| Other | 1 (1) |
| PAH etiology, n (%) | |
| Idiopathic | 56 (56) |
| Associated | 40 (40) |
| Connective tissue disease | 26 (26) |
| Other† | 13 (13) |
| Congenital heart disease | 1 (1) |
| Familial | 4 (4) |
| Median time since diagnosis (range), years | 0.12 (0–20.92)‡ |
n = 99.
Not specified.
One female patient (aged 58 years) was diagnosed with idiopathic PAH 20.94 years before study start. The next longest time since diagnosis was 4.87 years.
BMI, body mass index; PAH, pulmonary arterial hypertension.
Table 2.
Clinical, functional, and biomarker parameters at baseline, week 16, and week 28.
| Baseline (n = 100) | Week 16 (n = 100) | Week 28 (n = 100) | |
|---|---|---|---|
| Median 6MWD (range), m | n = 100 273.6 (152–360) | n = 94 307.9 (24–517) | n = 84 326.5 (45–640) |
| Median change from baseline, m (range) | – | n = 94 27.6 (−220 – 195) P < 0.001 | n = 84 50.0 (−230 – 430) P < 0.001 |
| Median % predicted 6MWD, % (range)* | n = 99 53.2 (28.8–97.1) | n = 93 58.7 (5.1–125.5) | n = 84 64.8 (10.6–140.4) |
| Median change from baseline, % (range) | – | 4.66 (−48.84 – 45.39) P = 0.002 | 9.54 (−46.92 – 92.39) P < 0.001 |
| WHO FC, n (%) | n = 100 | n = 93 | n = 84 |
| I | 1 (1)† | 6 (6) | 6 (7) |
| II | 17 (17) | 25 (27) | 34 (40) |
| III | 79 (79) | 59 (63) | 42 (50) |
| IV | 3 (3) | 3 (3) | 2 (2) |
| Missing | 0 | 7 | 16 |
| Median NT-pro-BNP, pg/mL (range)‡ | n = 88§ 988.5 (20–52,766) | n = 90 702.0 (35–16,419) | n = 83 566.0 (33–28,276) |
| Median change from baseline, pg/mL (range) | – | n = 80 −73.0 (−14,611 – 10,902) P = 0.055 | n = 74 −73.0 (−14,685 – 5,868) P = 0.008 |
The predicted 6MWD was calculated for men as follows: 7.57 × height (cm) – 5.02 × age (years) – 1.76 × weight (kg) – 309 m. The predicted 6MWD was calculated for women as follows: 2.11 × height (cm) – 5.78 × age (years) – 2.29 × weight (kg) + 667 m.
The patient was male, aged 28 years, and his 6MWD at baseline was 352 m (% predicted = 47%).
The median value is presented due to the skewed distribution of these data.
The vials for 12 samples were broken during transport to or at the central laboratory.
6MWD, 6-minute walk distance; NT-pro-BNP, N-terminal pro-B-type natriuretic peptide; WHO FC, World Health Organization functional class.
Table 3.
RHC-derived hemodynamic parameters.
| Baseline (n = 100) | Week 16 (n = 100) | Mean change from baseline (SD) (n = 100) | P value | |
|---|---|---|---|---|
| Mean mRAP, mmHg (SD) | n = 99 9.7 (5.73) | n = 92 9.3 (6.70) | n = 91 −0.3 (7.03) | 0.733 |
| Mean mPAP, mmHg (SD) | n = 100 46.1 (14.04) | n = 92 41.5 (14.09) | n = 92 −4.3 (9.09) | <0.001 |
| Mean PVR, Wood units (SD) | n = 99 9.8 (5.73) | n = 89 7.4 (4.73) | n = 88 −2.1 (2.94) | <0.001 |
| Mean PVR index, WU/m2 (SD) | n = 98 5.5 (3.43) | n = 88 4.2 (2.86) | n = 87 −1.2 (1.70) | <0.001 |
| Mean SV:PP, (L/min)/(bpm/mmHg) (SD) | n = 95 1.5 (0.74) | n = 90 1.6 (0.78) | n = 85 0.2 (0.93) | 0.096 |
| Mean SVR:PVR (SD) | n = 97 2.7 (1.44) | n = 89 3.6 (3.78) | n = 86 0.8 (3.30) | 0.02 |
| Mean SVO2, % (SD) | n = 85 62.9 (9.02) | n = 78 65.1 (11.12) | n = 75 2.4 (9.54) | 0.03 |
| Mean cardiac output, L/min (SD) | n = 100 4.3 (1.42) | n = 91 4.6 (1.43) | n = 91 0.4 (0.98) | <0.001 |
| Mean cardiac index, L/min/m2 (SD) | n = 99 2.3 (0.69) | n = 90 2.5 (0.67) | n = 90 0.2 (0.54) | <0.001 |
mPAP, mean pulmonary artery pressure; mRAP, mean right artery pressure; PP, pulse pressure; PVR, pulmonary vascular resistance; SD, standard deviation; SV, stroke volume; SVO2, mixed venous oxygen saturation; SVR, systemic vascular resistance.
Table 4.
cMRI-derived parameters.
| Baseline (n = 100) | Week 16 (n = 100) | Mean change from baseline (SD) (n = 100) | P value | |
|---|---|---|---|---|
| Mean RVEDV index, mL/m2* (SD) | n = 95 102.7 (37.27) | n = 87 102.8 (36.44) | n = 83 −0.8 (19.62) | 0.702 |
| Mean RVESV index, mL/m2* (SD) | n = 95 61.5 (35.30)† | n = 87 58.1 (33.97) | n = 83 −4.4 (15.46) | 0.012 |
| Mean RVEF, % (SD) | n = 96 43.4 (14.96) | n = 88 46.4 (15.13) | n = 84 3.1 (8.47) | 0.001 |
| Mean LVEDV index, mL/m2* (SD) | n = 95 60.4 (18.17) | n = 89 63.3 (17.90) | n = 85 3.5 (10.45) | 0.003 |
| Mean LVESV index, mL/m2* (SD) | n = 95 23.7 (10.33) | n = 89 21.8 (9.15) | n = 85 −1.2 (6.41) | 0.085 |
| Mean LVEF, % (SD) | n = 96 60.9 (11.19) | n = 90 65.4 (9.53) | n = 86 4.0 (10.04) | <0.001 |
| Mean RV mass index, g/m2* (SD) | n = 94 28.8 (12.37) | n = 86 30.2 (13.41) | n = 82 1.1 (5.89) | 0.084 |
| Mean RV mass:LV mass ratio (SD) | n = 95 0.7 (0.31) | n = 87 0.7 (0.31) | n = 83 0.0 (0.16) | 0.945 |
| Mean RVEDV:LVEDV ratio (SD) | n = 96 1.9 (0.96) | n = 88 1.8 (0.81) | n = 84 −0.2 (0.37) | <0.001 |
| Mean RVESV:LVESV ratio (SD) | n = 96 3.0 (1.86) | n = 88 2.9 (1.76) | n = 84 −0.2 (1.19) | 0.205 |
| Mean stroke volume index, mL/m2* (SD) | n = 95 36.7 (12.33) | n = 89 41.4 (12.41) | n = 85 4.7 (9.68) | <0.001 |
All data are mean (SD).
Indexed variables were computed using baseline body surface area values.
Value rounded from “0.01.”
LV, left ventricle; LVEDV, left ventricular end diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end systolic volume; RV, right ventricle; RVEDV, right ventricular end diastolic volume; RVEF, right ventricular ejection fraction; RVESV, right ventricular end systolic volume.
6MWD threshold achievement
In the ITT population, 31 patients (mean age = 51.1 years, standard deviation [SD] = 14.2 years) achieved the primary endpoint of a 6MWD ≥ 380 m (n = 16 at 16 weeks and/or n = 15 at 28 weeks) (Table S2). Of the 94 patients who completed 16 weeks of treatment, 16 achieved a 6MWD ≥ 380 m and continued with bosentan monotherapy. Of the 78 patients who did not achieve the 6MWD threshold at week 16, 76 received combination therapy with bosentan plus sildenafil for an additional 12 weeks; two patients withdrew consent before receiving combination therapy. In total, 24/92 (26%) of patients at week 28 achieved the 6MWD target. At all time-points, 6MWD was significantly greater in the cohort of patients who remained on monotherapy vs. combination therapy (see Table S3). At week 28, 9/16 patients who remained on monotherapy maintained a 6MWD ≥ 380 m and 15/76 patients who went on to receive combination therapy achieved the target threshold. When comparing the actual distance walked in relation to the percent predicted 6MWD, patients who remained on monotherapy had significantly higher median percent predicted 6MWD compared with the combination therapy group at week 16 (71% vs. 58%, P < 0.0001) and week 28 (69% vs. 61%, P = 0.0195).
Relative to the monotherapy group, a significantly greater proportion of patients who required combination therapy were women (87% vs. 63%, P = 0.0196) and were WHO FC III or IV (88% vs. 56%, P = 0.0073) (see Table S4). Patients who received combination therapy also had a significantly lower baseline 6MWD; the median difference for the monotherapy and combination therapy subgroups was 64.2 (95% CI = 27.1–89.0, P = 0.0004). No other baseline demographic, laboratory (including NT pro-BNP), or functional parameter (including percent predicted 6MWD) differed between the treatment groups. Patients on combination therapy had significantly lower baseline mean right atrial pressure (mRAP) (8.99 vs. 12.94 mmHg, P = 0.0137), significantly higher baseline cardiac index (2.38 vs. 2.02 L/min/m2, P = 0.0270), significantly higher cardiac output (4.40 vs. 3.66 L/min; P = 0.0487), and significantly higher mixed venous oxygen saturation (SVO2) (64.04% vs. 58.40%; P = 0.0348) relative to patients who remained on monotherapy. No other hemodynamic parameter or cMRI-derived variable at baseline differed between groups.
Changes from baseline
ITT population
In the ITT population, median 6MWD increased significantly from 274 m at baseline to 308 m at week 16 (P < 0.001) and 327 meters at week 28 (P < 0.001) (Table 2). The median percent predicted 6MWD increased from 53.2% at baseline to 64.8% at week 28 (P < 0.001). The proportion of patients in WHO FC III decreased, while the proportions of patients in WHO FC I or II increased (Table 2). Among 84 patients with an assessment at both baseline and week 28, 30 improved by ≥1 WHO FC and two patients deteriorated by ≥1 WHO FC. NT-pro-BNP decreased significantly from a median of 988.5 pg/mL at baseline to 566.0 pg/mL at week 28 (P = 0.008), but not at week 16 (702.0 pg/mL, P = 0.055) (Table 2).
Following 16 weeks in the ITT population, significant decreases in mPAP (−4.3 mm Hg, P < 0.001), PVR (−2.1 WU, P < 0.001), and PVR index (−1.2 WU/m2, P < 0.001) and significant increases in cardiac output (+0.4 L/min, P < 0.001), cardiac index (+0.2 L/min/m2, P < 0.001), SVO2 (+2.4%, P = 0.03), and systemic vascular resistance (SVR):PVR ratio (+0.8, P = 0.02) were seen relative to baseline (Table 3). Between baseline and week 16, there was a significant reduction in right ventricular end systolic volume (RVESV) index (−4.4 mL/m2, P = 0.012), and an increase in right ventricular ejection fraction (RVEF) (+3.1%, P = 0.001) and stroke volume index (+4.7 mL/m2, P < 0.001) (Table 4). Right ventricular end diastolic volume: left ventricular end diastolic volume ratio (RVEDV:LVEDV) decreased significantly (−0.2, P < 0.001) between baseline and week 16, which was associated with a significant improvement in left ventricle filling (LVEDV index = +3.5 mL/m2, P = 0.003) and left ventricular ejection fraction (LVEF = +4.0%, P < 0.001).
Comparisons between monotherapy and combination therapy groups at weeks 16 and 28 are reported in the online supplement.
Correlation of cMRI-derived parameters
Baseline cMRI-derived variables were correlated with RHC-derived parameters acquired at baseline. RVEDV:LVEDV correlated strongly with PVR (r = +0.729, P < 0.0001), mPAP (r = +0.717, P < 0.0001), PVR index (r = +0.704, P < 0.0001), and there was a low but significant correlation with mRAP (r = +0.360, P = 0.0003). In addition, RVEDV:LVEDV correlated inversely and strongly with SVR:PVR ratio (SVR:PVR; r = −0.778, P < 0.0001), and weakly with cardiac index (r = −0.393, P < 0.0001). RVEDV index correlations were moderate with mPAP (r = +0.507, P < 0.0001), PVR (r = +0.473, P < 0.0001), and low with PVR index (r = +0.460, P < 0.0001) and mRAP (r = +0.367, P = 0.0002). RVEDV index was inversely correlated with SVR:PVR (r = −0.581, P < 0.0001) and SVO2 (r = −0.310, P = 0.0049). RVEF was positively correlated with SVR:PVR (r = +0.635, P < 0.0001), inversely correlated with PVR (r = −0.540, P < 0.0001), PVR index (r = −0.499, P < 0.0001), and mPAP (r = −0.553, P < 0.0001), and had low correlations with cardiac output (r = +0.279, P = 0.0056), cardiac index (r = +0.295, P = 0.0035), SVO2 (r = +0.398, P = 0.0002), and mRAP (r = −0.373, P = 0.0002). Correlations between 6MWD and cMRI parameters are reported in the online supplement.
Time to clinical worsening and/or decline
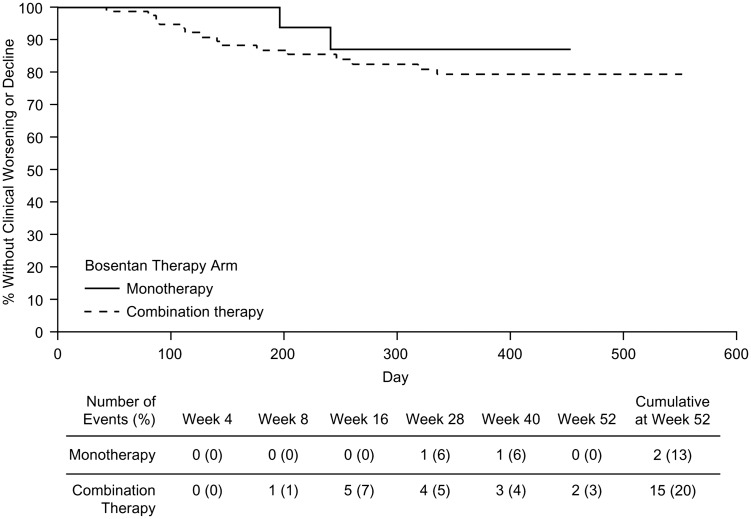
Twenty-two patients (22%) in the ITT population experienced clinical worsening and/or decline, with ten events occurring between baseline and week 16, six between weeks 16 and 28, and six between weeks 28 and 52. Because of the low number of clinical events, it was not possible to derive time-to-event estimates.
By week 52, 13% of patients in the monotherapy group and 20% in the combination therapy group experienced clinical worsening and/or decline (Fig. 2). There was no significant difference between the monotherapy and combination therapy treatment arms in terms of the time to clinical worsening and/or decline (P = 0.475). To examine underlying differences between the monotherapy and combination therapy groups, risk scores were calculated using the REVEAL registry risk score calculator. Mean (SD) risk score between monotherapy and combination therapy groups at baseline (7.2 [1.56] vs. 7.9 [1.25], P = 0.0609) borders statistical significance. When calculated at week 16 (when the decision was made to remain on monotherapy [6MWD ≥ 380 m] or switch to combination therapy [6MWD < 380 m]), patients in the combination therapy group had a significantly higher mean (SD) risk score compared with patients who remained on monotherapy (7.8 [1.81] vs. 6.8 [1.22], P = 0.0053), indicating a high burden of illness in the combination group.
Fig. 2.
Kaplan–Meier plot of time to clinical worsening and/or clinical decline.
Additional results comparing patients who did and did not experience clinical worsening and/or decline are reported in the online supplement.
Predictors of clinical worsening and/or decline
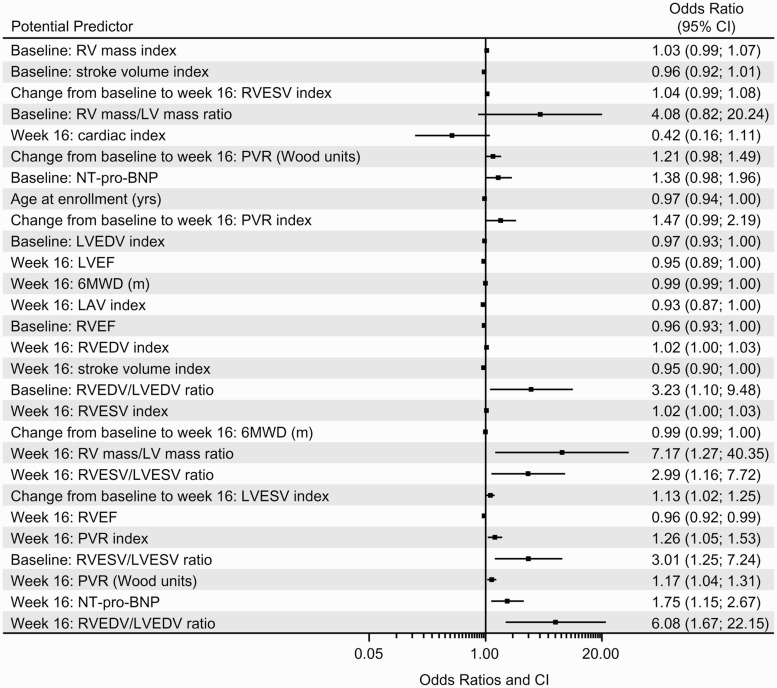
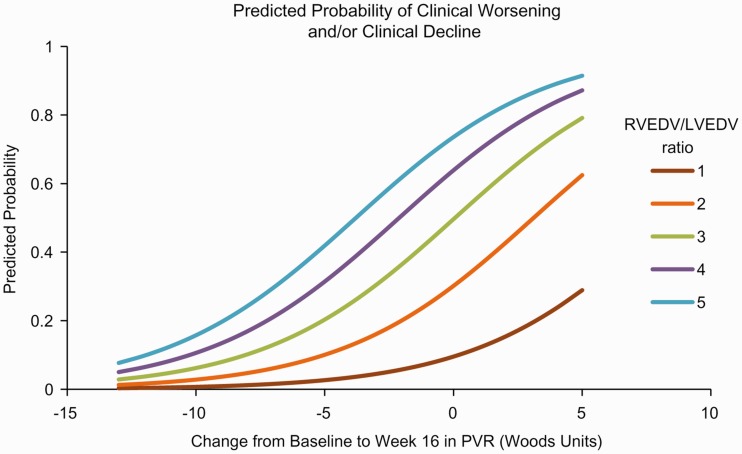
Several parameters were found to be independently predictive of clinical worsening and/or decline in the univariable models. Clinical worsening and/or decline was predicted by baseline measurements of RVEF (P = 0.0360) and RVESV:LVESV (P = 0.0322). Predictors of clinical worsening and/or decline at week 16 included 6MWD (P = 0.0417), NT-pro-BNP (P = 0.0183), PVR (P = 0.0088), PVR index (P = 0.0153), RVEF (P = 0.0156), RVEDV index (P = 0.0348), RVEDV:LVEDV (P = 0.0172), RVESV index (P = 0.0291), right ventricle mass:left ventricle mass (P = 0.0255), left atrial volume index (P = 0.0376), stroke volume index (P = 0.0329). The change from baseline to week 16 in 6MWD (P = 0.0283) and LVESV index (0.0208) were also predictive of clinical worsening and/or decline. A list of all parameters predictive of clinical worsening and/or decline are provided in Table S5. Odds ratios (ORs) determined from univariable analyses are shown in Fig. 3. Three multivariable models (see online supplementary material) were generated before the final model was derived. The final multivariable model investigated the combination of a baseline cMRI-derived parameter with the changes from baseline to week 16 in PVR and 6MWD. Because of the collinearity between baseline cMRI parameters, only one could be included in the multivariable model. The best statistical model given in the equation below included the natural logarithm of baseline RVEDV:LVEDV and the change from baseline to week 16 in PVR in WU (see Table S6), which yielded the following equation for the predicted probability of clinical worsening and/or decline and is illustrated in a competing outcomes plot (Fig. 4).
Fig. 3.
ORs from univariable analyses of baseline parameters for clinical worsening and/or decline. 6MWD, 6-min walk distance; CI, confidence interval; LAV, left atrial volume; LV, left ventricular; LVEDV, left ventricular end diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end systolic volume; NT-pro-BNP, N-terminal pro-B-type natriuretic peptide; PVR, pulmonary vascular resistance; RV, right ventricular; RVEDV, right ventricular end diastolic volume; RVEF, right ventricular ejection fraction; RVESV, right ventricular end systolic volume.
Fig. 4.
Competing outcomes plot.
where is the ratio of right to left ventricular end diastolic volume at baseline, and CFB in PVR is the change from baseline to week 16 in PVR (in WU).
Safety
No unexpected safety events were reported. Safety outcomes are summarized in Table 5 and described in detail in the online supplement.
Table 5.
Safety.
| Baseline to week 16 (n = 100) | Weeks 16–28 |
Weeks 28–52 (n = 100) | Baseline to week 52 (n = 100) | ||
|---|---|---|---|---|---|
| Monotherapy (n = 16) | Combination therapy (n = 76)* | ||||
| Any TEAE | 75 (75) | 6 (38) | 53 (70) | 2 (2) | 89 (89) |
| TEAE by severity | |||||
| Mild | 20 (20) | 4 (25) | 22 (29) | 0 (0) | 23 (23) |
| Moderate | 41 (41) | 1 (6) | 16 (21) | 2 (2) | 41 (41) |
| Severe | 12 (12) | 1 (6) | 11 (14) | 0 (0) | 19 (19) |
| Serious | 2 (2) | 0 (0) | 4 (5) | 0 (0) | 6 (6) |
| TEAE by relationship to study drug | |||||
| Not related | 45 (45) | 5 (31) | 30 (39) | 1 (1) | 43 (43) |
| Related | 30 (30) | 1 (6) | 23 (30) | 1 (1) | 46 (46) |
| Serious TEAE | 18 (18) | 1 (6) | 16 (21) | 0 (0) | 30 (30) |
| Death | 2 (2) | 0 (0) | 1 (1) | 0 (0) | 3 (3) |
Data are number of patients (%).
Eight patients discontinued before week 16 (when determination of monotherapy or combination therapy occurred) and were excluded from week 28 analysis.
TEAE, treatment-emergent adverse event.
Discussion
In the COMPASS-3 study, we evaluated whether 6MWD as a solitary treatment target was clinically meaningful and appropriate for the design of a clinical trial. Overall, 31 patients in the ITT population achieved the primary endpoint. Patients who did not reach 6MWD threshold at week 16 were more likely to be women, at WHO FC III or IV, and have a shorter 6MWD at baseline. Counterintuitively, patients who did not reach 6MWD threshold at week 16 had more preserved hemodynamics at baseline. One potential explanation of better hemodynamics seen in patients who did not reach 6MWD threshold is the MRI data showing less adaptive and more maladaptive RV remodeling. For instance, there was a moderate inverse correlation between RVEDV/LVEDV ratio and 6MWD at baseline (r = −0.541) for patients who experienced clinical worsening and/or decline, but a positive correlation for patients without clinical worsening and/or decline. Thus, RV dilation is linked to worse functional capacity in patients who did poorly clinically (maladaptive remodeling) while in clinically stable patients RV dilation was associated with better functional capacity (adaptive remodeling).
Importantly, achieving 6MWD threshold at either 16 or 28 weeks failed to predict clinical outcome. This observation, combined with aforementioned discordance among individual risk components and outcome within individual PAH patients strongly suggest that clinicians should make use of a wide range of risk factors, as opposed to one or two, when accessing overall risk and treatment response in any individual patient.14,15 These data also exemplify the need to go beyond the use of general risk profiles, as suggested in recent guidelines,9 as patients may exhibit risk features that span across these individual risk profiles. The use of stratified risk equations or calculators, as described in contemporary literature,14 may balance these non-weighted siloes of risk leading to better prediction of outcome for any individual patient.
It is important to note that while up-to-date guidelines were followed when this study was designed in 2007, the current standard of care has since changed. At the time the trial was designed, a treat-to-target approach was recommended,31,32 which was recently updated to emphasize a multivalent treatment approach which reduces patient mortality risk.9 In our analysis, patients in the monotherapy group achieved a greater 6MWD at all time-points compared with patients treated with combination therapy. In addition, the 6MWD between 16 and 28 weeks did not change dramatically in the combination therapy group (299 vs. 309 m) (Table S3). This implies that addition of sildenafil to bosentan did not impact 6MWD, and suggests a lack of overall efficacy on this parameter. While 6MWD may correlate with patient outcome, its use as the solitary endpoint in this clinical trial was not beneficial. Future clinical trials should consider the use of composite risk scores as potential endpoints to produce more clinically meaningful results.
Current guidelines recommend upfront or sequential combination therapy in order to target multiple PAH disease pathways.9 In this study, combination therapy consisted of the recommended (in the respective prescribing information) doses of bosentan and sildenafil. However, both this study and the results from the recently published long-term outcome COMPASS-2 study (which both missed their endpoints) suggests that the combination of sildenafil with bosentan may not be effective.33 In combination, sildenafil efficacy may have been reduced, since bosentan reduces the plasma concentration of sildenafil by approximately 50%.34 More recently, data from the phase 3 AMBITION trial demonstrated the clinical benefit of ambrisentan + tadalafil (another phosphodiesterase type 5 inhibitor) in patients with PAH.35 Additionally, the clinical effectiveness macitentan in the SERAPHIN phase 3 clinical trial was reported in both treatment-naïve patients and in patients receiving background therapy with a PDE5i and/or inhaled prostanoids.36 Further supporting the use of combination therapy, the GRIPHON phase 3 clinical trial evaluated the effectiveness of the oral IP receptor agonist selexipag in both treatment-naïve patients and in patients receiving background therapy.37
Bosentan-based therapy led to improvements in other meaningful parameters, including WHO FC, neurohormone levels, and hemodynamics. There were significant positive changes in RV geometry, function, and LV relationships that resulted in improved parameters of left ventricle filling (LVEDV and PAWP) and correlated with clinical outcome. Correlations between cMRI-derived and RHC-derived measures of cardiac function at baseline also suggest the potential for cMRI to serve as an alternative, or adjunct, to RHC in assessing functional derangements, patient stability, and need for sequential or upfront combination therapy. Similar to transthoracic echocardiography (TTE), cMRI is non-invasive, but offers higher resolution, more precise measurements, and greater reproducibility than TTE.38,39 In clinical practice, tricuspid annular plane systolic excursion, right ventricular fractional area change, systolic velocity, and RV global strain are recommended in follow-up visits for patients with PAH. Although optimal timing is currently unknown, structural changes to the heart can be observed using cMRI or TTE within 4–6 months. While cMRI may not be practical or cost-effective in regular clinical practice, it may provide better data in clinical trials. In addition, these data further confirm previous studies demonstrating the benefits of TTE in clinical trials. In the BREATHE-1 TTE sub-study of 85 patients with PAH, data showed improved TTE variables (e.g. RV systolic function and increase in LV size) in patients treated with bosentan compared with placebo.40
The additional and clinically appropriate relationships between neurohormone levels and 6MWD and measures of RV remodeling by MRI further support the adjunctive role of MRI in accurately characterizing severity of illness in this patient population.
The percentage of patients who experienced clinical worsening and/or decline at week 52 was higher in the combination therapy group compared with the monotherapy group and could reflect that RV remodeling was more adaptive in patients that did not have a clinical worsening event. Additionally, 6MWD has been shown to be prognostic and the monotherapy group had significantly higher 6MWD at baseline compared with the combination therapy group. Further, analysis using the REVEAL risk score calculator showed that at week 16, when patients were divided into groups based on their 6MWD, patients in the combination therapy group had a higher risk score compared to patients in the monotherapy group, reflecting a greater propensity for future events.
Interestingly, time to clinical worsening and/or decline did not differ between patients who did or did not achieve 6MWD threshold at week 16. However, lower RVEF and LVEDV index, RV/LV systolic and diastolic ratios and higher RV mass index at baseline were associated with clinical worsening and/or decline, which extends previous MRI findings related to mortality in patients with PAH.24,27 In addition, the observation that reverse RV remodeling contributed to the prediction of clinical outcome further supports the use of serial MRI as a management tool in PAH. Combining these changes with those seen in hemodynamics or other clinical parameters, such as NT-pro-BNP expression, support the use of a multimodality model in guiding therapeutic choices in these critically ill patients.
Univariable models showed that many cMRI variables were predictive of clinical worsening or decline. However, multivariable analysis showed that many of these cMRI parameters were closely associated and determining individual effects was not statistically feasible. The multivariable model that included RVEDV at baseline and change in PVR from baseline to week 16 best predicted clinical worsening and/or decline. The need to consider multiple endpoints to predict outcome in patients with PAH is not without precedent. In the French PAH registry, sex, baseline 6MWD, and cardiac output were jointly associated with three-year survival.41 Similarly, in the REVEAL registry, numerous parameters, including PVR, PAH etiology, and WHO FC, were collectively predictive of one-year survival.8 The French and REVEAL multivariable models were subsequently validated in prospective cohorts of patients with PAH.14,42 The COMPASS-3 multivariable model requires validation in a larger cohort as it was generated post-hoc in a limited number of patients.
In terms of limitations, COMPASS-3 was a phase 4 open-label study and therefore not a randomized controlled study, and some of the reported analyses were conducted post-hoc. Despite these caveats, the data reported herein represent the most complete set of hemodynamic and cMRI-derived data from patients with PAH published to date. There were 17 (17%) clinical worsening/decline events over the one year of follow-up and this low event rate greatly limits post-hoc comparisons of sequential predictors of worsening. These results may also contain possible bias as the baseline mean 6MWD may be skewed lower due to the inclusion criteria of 6MWD of 150 m. It should be noted that the bioavailability of both sildenafil and bosentan are altered when used in combination.43 In a study of 125 patients with PAH, combinations of sildenafil and bosentan led to a significant decrease in the bioavailability of sildenafil compared to combinations of macitentan and bosentan (P < 0.001) whereas bosentan concentrations were greatly increased in patients when combined with sildenafil. Therefore, the findings of combination therapy in this study may be unique to combinations of sildenafil and bosentan.
In conclusion, using a singular endpoint (6MWD > 380 m) did not serve as a clinically meaningful prognostic indicator and our analyses indicate that a more comprehensive assessment of risk is needed. We detected moderate-to-strong correlations between cMRI-derived and RHC-associated parameters of cardiac function and found cMRI to be both prognostic of clinical outcome and sufficiently sensitive to detect reverse RV remodeling.
Acknowledgments
The authors thank Fiona Brock of Quanticate for her contributions to the statistical analyses, Steven G. Lloyd of the University of Alabama at Birmingham and Birmingham VA Medical Center for his contributions to MRI analysis, and Thomas S. Denney Jr of Auburn University for his contributions to MRI design and experiment analysis. Editing assistance, including figure and table generation, was provided by Penny Baron of BlueMomentum, an Ashfield Company, part of UDG Healthcare plc, and was paid for by Actelion Pharmaceuticals US, Inc. The REVEAL registry is sponsored by Actelion Pharmaceuticals US, Inc.
Declaration of conflicting interests
RLB has grant support from Actelion and serves as a steering committee member for REVEAL. AR has received research support, consulting, and speaking fees from Actelion. He has received consulting and speaking fees from United Therapeutics. He has received research support and speaking fees from Bayer. MAS has grant support from NIH and Aires pharmaceuticals and has served as a consultant to Gilead and United Therapeutics. HG has served as a consultant for Actelion. Dr Gupta’s institution has received research support from Actelion. SM has received research support, consulting, and speaking fees from Actelion pharmaceuticals. AB is a salaried employee of Quanticate International, who were contracted by Actelion Pharmaceuticals US to perform the statistical analyses reported herein. At the time of development of this article, MSZ was a salaried employee and had stock ownership of Actelion Pharmaceuticals US. MHP has served as a consultant and on speakers’ bureaus for Actelion, Bayer, Gilead Sciences, and United Therapeutics. Dr Park’s institution has also received research support from Actelion, Bayer, and United Therapeutics.
Funding
The COMPASS-3 study was funded by Actelion Pharmaceuticals US, Inc. The sponsor hired Quanticate UK to analyze the data, which were collected by the study investigators. The authors had the right to publish these data independent of the sponsor.
References
- 1.D’Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 1991; 115: 343–349. [DOI] [PubMed] [Google Scholar]
- 2.Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med 2004; 351: 1425–1436. [DOI] [PubMed] [Google Scholar]
- 3.Barst RJ, Gibbs JS, Ghofrani HA, et al. Updated evidence-based treatment algorithm in pulmonary arterial hypertension. J Am Coll Cardiol 2009; 54(suppl): S78–S84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benza RL, Miller DP, Barst RJ, et al. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest 2012; 142: 448–456. [DOI] [PubMed] [Google Scholar]
- 5.Sitbon O, Humbert M, Nunes H, et al. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J Am Coll Cardiol 2002; 40: 780–788. [DOI] [PubMed] [Google Scholar]
- 6.Miyamoto S, Nagaya N, Satoh T, et al. Clinical correlates and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension. Comparison with cardiopulmonary exercise testing. Am J Respir Crit Care Med 2000; 161(Pt 1): 487–492. [DOI] [PubMed] [Google Scholar]
- 7.McLaughlin VV, Sitbon O, Badesch DB, et al. Survival with first-line bosentan in patients with primary pulmonary hypertension. Eur Respir J 2005; 25: 244–249. [DOI] [PubMed] [Google Scholar]
- 8.Benza RL, Miller DP, Gomberg-Maitland M, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 2010; 122: 164–172. [DOI] [PubMed] [Google Scholar]
- 9.Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 10.McLaughlin VV, Gaine SP, Howard LS, et al. Treatment goals of pulmonary hypertension. J Am Coll Cardiol 2013; 62(suppl): D73–D81. [DOI] [PubMed] [Google Scholar]
- 11.McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol 2009; 53: 1573–1619. [DOI] [PubMed] [Google Scholar]
- 12.Gaine S, Simonneau G. The need to move from 6-minute walk distance to outcome trials in pulmonary arterial hypertension. Eur Respir Rev 2013; 22: 487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farber HW, Miller DP, McGoon MD, et al. Predicting outcomes in pulmonary arterial hypertension based on the 6-minute walk distance. J Heart Lung Transplant 2015; 34: 362–368. [DOI] [PubMed] [Google Scholar]
- 14.Benza RL, Gomberg-Maitland M, Miller DP, et al. The REVEAL Registry risk score calculator in patients newly diagnosed with pulmonary arterial hypertension. Chest 2012; 141: 354–362. [DOI] [PubMed] [Google Scholar]
- 15.Lee WT, Ling Y, Sheares KK, et al. Predicting survival in pulmonary arterial hypertension in the UK. Eur Resp J 2012; 40: 604–611. [DOI] [PubMed] [Google Scholar]
- 16.Benza R, Biederman R, Murali S, et al. Role of cardiac magnetic resonance imaging in the management of patients with pulmonary arterial hypertension. J Am Coll Cardiol 2008; 52: 1683–1692. [DOI] [PubMed] [Google Scholar]
- 17.McLure LE, Peacock AJ. Cardiac magnetic resonance imaging for the assessment of the heart and pulmonary circulation in pulmonary hypertension. Eur Respir J 2009; 33: 1454–1466. [DOI] [PubMed] [Google Scholar]
- 18.Lang IM, Plank C, Sadushi-Kolici R, et al. Imaging in pulmonary hypertension. JACC Cardiovasc Imaging 2010; 3: 1287–1295. [DOI] [PubMed] [Google Scholar]
- 19.Peacock AJ, Vonk Noordegraaf A. Cardiac magnetic resonance imaging in pulmonary arterial hypertension. Eur Respir Rev 2013; 22: 526–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roeleveld RJ, Vonk-Noordegraaf A, Marcus JT, et al. Effects of epoprostenol on right ventricular hypertrophy and dilatation in pulmonary hypertension. Chest 2004; 125: 572–579. [DOI] [PubMed] [Google Scholar]
- 21.Chin KM, Kingman M, De Lemos JA, et al. Changes in right ventricular structure and function assessed using cardiac magnetic resonance imaging in bosentan-treated patients with pulmonary arterial hypertension. Am J Cardiol 2008; 101: 1669–1672. [DOI] [PubMed] [Google Scholar]
- 22.Peacock AJ, Crawley S, McLure L, et al. Changes in right ventricular function measured by cardiac magnetic resonance imaging in patients receiving pulmonary arterial hypertension-targeted therapy: the EURO-MR study. Circ Cardiovasc Imaging 2014; 7: 107–114. [DOI] [PubMed] [Google Scholar]
- 23.Baggen VJ, Leiner T, Post MC, et al. Cardiac magnetic resonance findings predicting mortality in patients with pulmonary arterial hypertension: a systematic review and meta-analysis. Eur Radiol 2016; 26: 3771–3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Wolferen SA, Marcus JT, Boonstra A, et al. Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension. Eur Heart J 2007; 28: 1250–1257. [DOI] [PubMed] [Google Scholar]
- 25.Gan CT, Lankhaar JW, Westerhof N, et al. Noninvasively assessed pulmonary artery stiffness predicts mortality in pulmonary arterial hypertension. Chest 2007; 132: 1906–1912. [DOI] [PubMed] [Google Scholar]
- 26.Yamada Y, Okuda S, Kataoka M, et al. Prognostic value of cardiac magnetic resonance imaging for idiopathic pulmonary arterial hypertension before initiating intravenous prostacyclin therapy. Circ J 2012; 76: 1737–1743. [DOI] [PubMed] [Google Scholar]
- 27.Van De Veerdonk MC, Kind T, Marcus JT, et al. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol 2011; 58: 2511–2519. [DOI] [PubMed] [Google Scholar]
- 28.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166: 111–117. [DOI] [PubMed] [Google Scholar]
- 29.Marrone G, Mamone G, Luca A, et al. The role of 1.5T cardiac MRI in the diagnosis, prognosis and management of pulmonary arterial hypertension. Int J Cardiovasc Imaging 2010; 26: 665–681. [DOI] [PubMed] [Google Scholar]
- 30.Sievers B, Addo M, Kirchberg S, et al. Impact of the ECG gating method on ventricular volumes and ejection fractions assessed by cardiovascular magnetic resonance imaging. J Cardiovasc Magn Reson 2005; 7: 441–446. [DOI] [PubMed] [Google Scholar]
- 31.Badesch DB, Abman SH, Simonneau G, et al. Medical therapy for pulmonary arterial hypertension: updated ACCP evidence-based clinical practice guidelines. CHEST 2007; 131(6): 1917–1928. [DOI] [PubMed] [Google Scholar]
- 32.Galiè N, Torbicki A, Barst R, et al. Guidelines on diagnosis and treatment of pulmonary arterial hypertension. The Task Force on Diagnosis and Treatment of Pulmonary Arterial Hypertension of the European Society of Cardiology. Eur Heart J 2004; 25(24): 2243–2278. [DOI] [PubMed] [Google Scholar]
- 33.McLaughlin V, Channick RN, Ghofrani HA, et al. Bosentan added to sildenafil therapy in patients with pulmonary arterial hypertension. Eur Respir J 2015; 46: 405–413. [DOI] [PubMed] [Google Scholar]
- 34.Paul GA, Gibbs JS, Boobis AR, et al. Bosentan decreases the plasma concentration of sildenafil when coprescribed in pulmonary hypertension. Br J Clin Pharmacol 2005; 60(1): 107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galiè N, Barberà JA, Frost AE, et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. New Engl J Med 2015; 373(9): 834–844. [DOI] [PubMed] [Google Scholar]
- 36.Pulido T, Adzerikho I, Channick RN, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. New Engl J Med 2013; 369(9): 809–818. [DOI] [PubMed] [Google Scholar]
- 37.Sitbon O, Channick R, Chin KM, et al. Selexipag for the treatment of pulmonary arterial hypertension. New Engl J Med 2015; 373(26): 2522–2533. [DOI] [PubMed] [Google Scholar]
- 38.Anavekar NS, Gerson D, Skali H, et al. Two-dimensional assessment of right ventricular function: an echocardiographic–MRI correlative study. Echocardiography 2007; 24(5): 452–456. [DOI] [PubMed] [Google Scholar]
- 39.Marwick TH, Neubauer S, Petersen SE. Use of cardiac magnetic resonance and echocardiography in population-based studies. Circ Cardiovasc Imaging 2013; 6(4): 590–596. [DOI] [PubMed] [Google Scholar]
- 40.Galiè N, Hinderliter AL, Torbicki A, et al. Effects of the oral endothelin-receptor antagonist bosentan on echocardiographic and doppler measures in patients with pulmonary arterial hypertension. J Am Coll Cardiol 2003; 41(8): 1380–1386. [DOI] [PubMed] [Google Scholar]
- 41.Humbert M, Sitbon O, Chaouat A, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 2010; 122: 156–163. [DOI] [PubMed] [Google Scholar]
- 42.Humbert M, Sitbon O, Yaïci A, et al. Survival in incident and prevalent cohorts of patients with pulmonary arterial hypertension. Eur Respir J 2010; 36: 549–555. [DOI] [PubMed] [Google Scholar]
- 43.Grünig E, Ohnesorge J, Benjamin N, et al. Plasma drug concentrations in patients with pulmonary arterial hypertension on combination treatment. Respiration 2017; 94: 26–37. [DOI] [PubMed] [Google Scholar]