Abstract
The objective of this study is to assess the impact of information on doctors’ attitudes and perceptions toward generics. A cross-sectional survey based on a specially designed 21-item questionnaire was conducted. The survey involved doctors of different specialties working in a public hospital in Greece. The analysis includes descriptive and inferential statistics, reliability and validity tests, as well as structural equation modeling to evaluate the causal model. Statistical analysis was accomplished by using SPSS 20 and Amos 20. A total of 134 questionnaires out of 162 were received, providing a response rate of 82.71%. A number of significant associations were found between information and perceptions about generic medicines with demographic characteristics. It seems that the provision of quality information on generic drugs influences doctors’ attitudes and prescription practices toward generic drugs. This is not a static process but a rather dynamic issue involving information provision policies for strengthening the proper doctors’ attitudes toward generic drugs.
Keywords: drugs, generics, information, medical doctors, survey, Greece
Introduction
Generic medicine policies are rather important for any health care system mainly due to the expenditures associated with pharmaceutical products.1 Although the aforementioned statement is rather evident, not all health care professionals may share the same attitudes across EU countries.2 This may be a result of the distinct drugs financing models, distinct national pharmaceutical reformation policies as well as divergent health care management practices among the different countries.3 An integration of central and southern European pharmaceutical products markets is the “Holy Grail” for National Healthcare Systems (NHS) within the European Union.4 Due to the global economic crisis of 2008, the Greek governments have implemented programs to reduce medication expenses through generics utilization.5 These efforts aimed to influence doctors’ attitudes and perceptions toward generics. This article is studying the impact of the current level of information about generics on doctors’ attitudes toward generics. The survey involved all medical doctors registered at Kavala’s General Public Hospital as well as in the primary health care units of this region in Greece. Out of the 162 registered medical doctors asked to participate in this survey, 134 agreed, completed, and returned the 21-item questionnaire, with a response rate of around 83.7%. Prior to the survey, the hospital scientific committee reviewed and accepted the research protocol. The impact of information on medical doctors’ beliefs toward generic drugs may provide a pathway for strengthening national policies and programs for generics’ utilization.
Theoretical Framework and Hypothesis
An important goal for the Greek NHS is to achieve a reduction of pharmaceutical expenses. Overall, generic drugs increase the competition among pharmaceutical companies and reduce the cost of treatment without jeopardizing quality and safety characteristics.6 The deep economic crisis in Greece in conjunction with the requirements set by the international institutions made the promotion of generic drugs against original drugs a priority.7 The physicians’ perceptions on generic drugs in the era of austerity in Greece have been recently studied by Labiris et al.8 In Greece, brand-generic drug substitution has been extensively promoted by issuing warnings and developing national control mechanisms.9 These include measures for better administrative control through the national e-prescription system that was introduced in 2010 in Greece as well as the inclusion of generics in the e-procurement system for the containment of unnecessary pharmaceutical expenditure. These measures focused on promoting regulations and rules, as well as diminishing misconceptions about generics’ efficiency.10 However, at the same time, the general population has a difficulty accepting specific generic pharmaceutical products, whereas there appears to be a widespread myth among doctors and patients that branded products are better in terms of quality and safety than generic forms.11
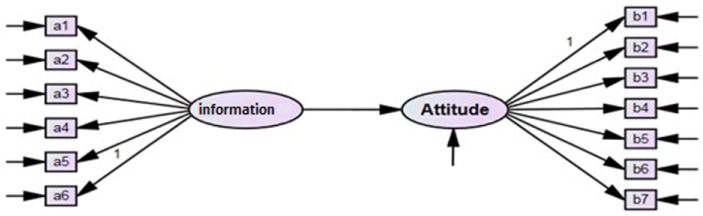
This article aims to evaluate the significance of the role of information in doctors’ attitudes toward generics. For example, informed doctors provide their patients with written instructions that encourage them to purchase and use generic medicines. However, many doctors without access to the scholar medical information still have a negative attitude toward generic drugs and they are not convinced of the bioequivalence between prototype and generic drugs. The research model developed and presented in Figure 1 is based on the studies by Chua et al1 as well as Gevorgyan,12 which involve assessments of both doctors’ information on and their attitudes toward generic medicines. Therefore, the following hypothesis is tested:
Figure 1.
Research model.
Hypothesis 1: Doctors’ information on generic medicines positively affects doctors’ attitude toward generic medicines.
Research Design
Material and Methods
The questionnaire used in our survey was informed by the work of Chua et al11 adopted for Greece and comprised three sections: (1) doctors’ demographics, (2) information about generics, and (3) attitudes toward generic medicines. The questionnaire was initially cross-translated, and qualitatively pilot tested for validity by a group of experts from academia and professionals with research experience. In its final form, the questionnaire together with our research protocol was submitted to the hospital’s scientific committee for review and acceptance. A 5-point Likert scale was used for scale measurement. In our sampling plan, all 162 medical doctors of Kavala’s hospital were included and a total of 134 responses were received within the first month of 2015, indicating a response rate of 83.7%. After data screening, SPSS 20 was used for the descriptive statistics as well as the exploratory factor analysis, whereas structural equation modeling (SEM)13 was developed by Amos 20. Construct validity was assessed through principal component factor analysis whereas construct reliability was assessed using Cronbach’s alpha values.14
Results
Table 1 provides the survey demographics. The responses and significant differences in population subgroups are provided in Table 2. Group differences were assessed using Mann-Whitney and Kruskal-Wallis tests, wherever applicable. The results exhibit that although the majority of the respondents (55.2%) correctly stated that generic drugs are bioequivalent to branded drugs, a quite high percentage of 39.6% were neutral. Furthermore, only the 17.9% of the respondents assumed that a generic medicine must be in the same dose as the brand name medicine and 25.4% had no doubt about it, but still 54.5% of the doctors were neutral. Therefore, the results are indicative of physicians’ misinformation about generic drugs in relation to the issues raised in our survey. Differences have been noted for the statement, “Generic medicines should contain the same dose as the brand name medicines” in regard to doctor’s place of work (U = 451.500, z = −2.019, P = .44), with the doctors in the hospital being better informed than the doctors working in primary health care centers. Also, differences have been identified for the statement, “Generic medicines are less effective compared to brand name medicines” in gender (U = 1823.00, z = −2.097, P = .036), years of practice (U = 6.982, z = 2, P = .030), and work position (U = 1804.500, z = −2.183, P = .029) subgroups. In fact, male doctors, specialists, and doctors with more than 10 years of practice experience expressed a stronger disagreement to the given statement than females. Similar results are reported in another study15 stating for instance that female physicians have more negative perceptions toward generic medicines than male doctors.
Table 1.
Respondents’ Demographic Characteristics.
| Frequency (persons) | Frequency (%) | Cumulative % | |
|---|---|---|---|
| Gender | |||
| Male | 66 | 49.3 | 49.3 |
| Female | 68 | 50.7 | 100 |
| Age | |||
| 24-30 | 31 | 23.1 | 23.1 |
| 31-40 | 48 | 35.8 | 59 |
| 41-50 | 29 | 21.6 | 80.6 |
| 51-64 | 26 | 19.4 | 100.0 |
| Years in practice | |||
| 1-5 | 63 | 47.0 | 47.0 |
| 6-10 | 23 | 17.2 | 64.2 |
| >10 | 48 | 35.8 | 100 |
| Position | |||
| Specialist | 65 | 48.5 | 48.5 |
| Non-specialist | 69 | 51.5 | 100 |
| Responsibility position | |||
| Senior | 26 | 20.1 | 20.1 |
| Non-senior | 117 | 79.9 | 100 |
| Place of work | |||
| Public hospital | 123 | 91.8 | 91.8 |
| Health care centers | 11 | 8.2 | 100 |
Table 2.
Survey Results.
| Items | Strongly agree | Agree | Neutral | Disagree | Strongly disagree | Gendera | Ageb | Years of practiceb | Positiona | Responsibilitya | Place of worka |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Doctors’ information on generic medicines | |||||||||||
| A generic medicine is bioequivalent to brand name medicines | 16 | 58 | 51 | 9 | 0 | .109 | .989 | .819 | .732 | .207 | .223 |
| A generic medicine must be in the same dosage form (eg, tablet, capsule) as the brand name medicine | 26 | 44 | 53 | 7 | 4 | .201 | .637 | .250 | .095 | .235 | .774 |
| A generic medicine must contain the same dose as the brand name medicines | 34 | 24 | 73 | 1 | 2 | .779 | .894 | .657 | .877 | .225 | .044 |
| Generic medicines are less effective compared with brand name medicines (r) | 1 | 12 | 29 | 77 | 15 | .036 | .437 | .030 | .029 | .073 | .924 |
| Generic medicines produce more side effects compared with brand name medicines (r) | 0 | 15 | 36 | 68 | 15 | .040 | .789 | .440 | .606 | .308 | .857 |
| Brand name medicines are required to meet higher safety standards than generic medicines (r) | 1 | 15 | 35 | 70 | 13 | .005 | .921 | .601 | .759 | .899 | .891 |
| Doctors’ attitudes and perceptions toward generics | |||||||||||
| I believe we need a standard guideline for both Doctors and pharmacists on the brand substitution process | 34 | 69 | 20 | 10 | 1 | .954 | .102 | .348 | .640 | .345 | .064 |
| I think the patient should be given enough information about generic medicines to make sure they really understand about the medicines they take | 34 | 78 | 4 | 17 | 1 | .699 | .058 | .622 | .994 | .374 | .960 |
| I believe advertisement by the drug companies will influence my future prescribing pattern | 13 | 66 | 44 | 11 | 0 | .604 | .458 | .384 | .623 | .373 | .839 |
| I need more information on the issues pertaining to the safety and efficacy of generic medicines | 36 | 73 | 19 | 6 | 0 | .817 | .608 | .394 | .650 | .966 | .379 |
| Patient’s socio-economic factor will affect my choice of medicines | 32 | 77 | 15 | 9 | 1 | .832 | .039 | .039 | .089 | .461 | .824 |
| Credibility of the manufactures/suppliers is my concern when prescribing medicines | 31 | 82 | 12 | 8 | 1 | .900 | .661 | .755 | .949 | .733 | .672 |
| Pharmaceutical companies’ product bonuses will influence my choice of medicines | 17 | 39 | 40 | 36 | 2 | .481 | .993 | .377 | .826 | .477 | .896 |
Note. P < .05 is considered significant. Bold values indicate statistical significance. r = reversed item.
Mann-Whitney test was used.
Kruskal-Wallis test was used.
Moreover, men were more positive than women about generics toward the statement, “Generic drugs produce more side effects compared with brand name drugs” (U = 1819.00, z = −2.055, P = .04). Also, men expressed more positive beliefs on generic medicines than the women toward the statement, “Brand name drugs are required to meet higher safety standards than generics” (U = 1664.500, z = −2.818, P = .05).
In Table 2, survey results for the participants’ attitudes toward generics are presented: 76.9% of the respondents were in favor of issuing guidelines on brand substitution for the prescribers and the pharmacists; 83.6% felt that patients should be appropriately provided with trusted information on generic drugs; 59.0% indicated that drug advertising campaigns had a positive impact, and 54.5% reported that they need more information on the safety and efficacy of generics. Finally, 57.5% stated that patients’ socio-economic factors affected the choice of medicines, whereas 84.3% stated that they are influenced by the reliability of manufacturers for prescribing generics. In conclusion, there appears to be a lack of adequate information about generics among doctors. The study revealed that there were significant differences among doctors in different age groups for the statement, “I think patient should be given enough information about generic medicines to make sure they really understand about the medicines they take” (U = 7.501, z = 3, P = .058); the response expressed by the junior doctors, aged 24 to 30 years, under their specialty was more positive than older doctors. That is perhaps because young doctors usually spend more time with patients, to inform them about their condition and options. Finally, there are significant differences among doctors in different age groups (U = 8.386, z = 3, P = .039), and years of practice (U = 6.495, z = 2, P = .039) for the statement, “Patient’s socio-economic factor will affect my choice of medicines.” The respondents aged between 24 and 30 years as well as doctors who had less years of practice, from 1 to 5 years, expressed a stronger agreement with the above statement when compared with older doctors with more experience.
Measurement Analysis and Hypothesis Testing
Two-step approach methodology was adopted based on the suggestions of Hair et al16 and Anderson and Gerbing.12 For the first stage of analysis, explanatory factor analysis was conducted on the data set to examine the construct validity and the unidimensionality of each independent variable. Only Kaiser-Meyer-Olkin (KMO) values greater than 0.7 and factor loadings values greater than 0.6 were accepted and that is a rigorous cut-point. After the deletion of one item (b7) from the second construct as shown in Table 3, the KMO statistics were 0.876 and 0.917 at a significance level of .001 for the 2 independent variables under test. The test of sphericity was also highly significant (χ2 457.14 and 596.20 with 10 degrees of freedom [df], at P < .001). Therefore, it was concluded that a factor analysis of the scale items would be appropriate. Factor loadings ranged from 0.770 to 0.958, a very satisfactory outcome. Therefore, the results indicate that the scales used to measure the independent variables were unidimensional and represented a single concept. Next, confirmatory factor analysis was performed. Item reliability was tested by squared factor loadings (SFLs), whereas contract reliability was assessed through Cronbach coefficient, composite reliability, and average variance extracted (AVE). As Table 3 shows, all used items had SFLs greater than the .50 recommended value. In addition, all the questionnaire scales exhibited Cronbach coefficientsand AVE estimates above the recommended levels of 0.7 and 0.50, respectively. Therefore, these values ensure that each construct is psychometrically sound.17
Table 3.
Explanatory Factor Analysis Results and Confirmatory Factor Analysis Results.
| Factor | Items | Explanatory factor analysis |
Confirmatory factor analysis |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Comp. matrix | Mean | SD | KMO | Bartlett test | Total variance explained | Loadings | Squared factor loadings | Cronbach’s α | Composite reliability | Variance extracted | ||
| Doctors’ information of generic medicines | a1 | 0.779 | 3.60 | 0.785 | 0.876 | 457.14 | 66.288% | 0.73 | 0.54 | 0.898 | 0.90 | 0.60 |
| a2 | 0.782 | 3.60 | 0.958 | 0.74 | 0.55 | |||||||
| a3 | 0.877 | 3.65 | 0.920 | 0.86 | 0.74 | |||||||
| a4 | 0.841 | 3.69 | 0.816 | 0.80 | 0.63 | |||||||
| a5 | 0.826 | 3.62 | 0.830 | 0.78 | 0.61 | |||||||
| a6 | 0.775 | 3.59 | 0.843 | 0.71 | 0.51 | |||||||
| Doctors’ attitude toward generic medicines | b1 | 0.876 | 3.93 | 0.877 | 0.917 | 596.20 | 73.75% | 0.85 | 0.73 | 0.928 | 0.91 | 0.63 |
| b2 | 0.924 | 3.95 | 0.928 | 0.92 | 0.85 | |||||||
| b3 | 0.775 | 3.60 | 0.776 | 0.73 | 0.53 | |||||||
| b4 | 0.850 | 4.04 | 0.770 | 0.81 | 0.65 | |||||||
| b5 | 0.854 | 3.97 | 0.831 | 0.82 | 0.67 | |||||||
| b6 | 0.867 | 4.00 | 0.795 | 0.84 | 0.70 | |||||||
Note. KMO = Kaiser-Meyer-Olkin.
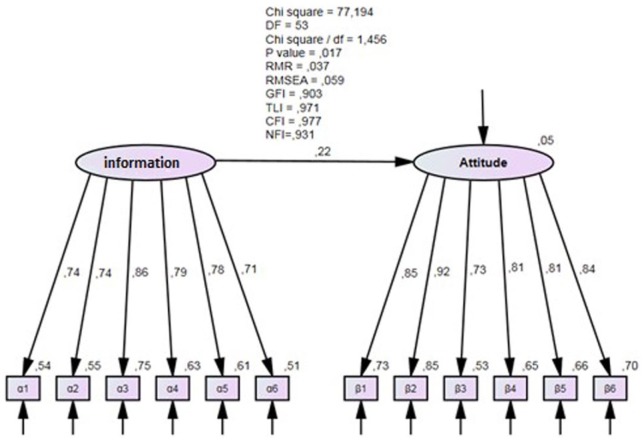
The next step of the analysis was to evaluate the goodness of fit of the structural model. Six common model-fit measures were used to assess the model’s overall goodness of fit: the ratio of chi-square values to degrees of freedom, the comparative fit index (CFI), the normalized fit index (NFI), the root mean square residual (RMR), the root mean square error of approximation (RMSEA), and the goodness-of-fit index (GFI). Generally, good fits are obtained when the CFI and GFI are equal to or greater than 0.90, RMR is equal to or less than 0.05, and the RMSEA is equal to or less than 0.06
Figure 2 presents the measurement and structure model for the goodness-of-fit tests of the hypothetical model. As shown, the adopted fit indices exceed the recommended threshold levels: χ2/df < 3; GFI, NFI, and CFI > 0.90; and RMR < 0.05, indicating that the research model fits the data well. Findings according to path coefficients and their statistical significance in Figure 2 show that the proposed hypothesis is supported. More analytically, the results show that doctors’ information about generic medicines has a statistically significant positive influence on doctors’ attitude toward generic medicine.
Figure 2.
Confirmatory factor analysis of the model.
Note. df = degrees of freedom; RMR = root mean square residual; RMSEA = root mean square error of approximation; GFI = goodness-of-fit index; CFI = comparative fit index; NFI = normalized fit index; TLI = Tucker-Lewis index.
Discussion
Our results suggest that the myth that brand name drugs are “better” than their generic equivalents is still present.18 Perhaps, this explains why the use of generics in Greece is still among the lowest in the European countries. These misconceptions could be altered in order cost–containment programs to succeed.19 Our results by large do not differ to the ones reported in other similar studies: For example, it is reported that Malaysian doctors’ information and knowledge about generics do not diminish misconceptions.20 Sheppard18 also states that doctors consider generics being less secure than branded drugs, whereas in another study21 in Denmark, it is reported that physicians are displeased with the drug substitution process and 58.2% claimed that patients have to be educated and better informed on safety and efficacy of generics, so as to be certain about their high quality. In our study as in others, it was reported that the patient’s socio-economic status was a pivotal issue in doctors’ choice of drugs.22 In France, as it is also reported here, the socio-economic characteristics of patients were the basic reason in doctors’ willingness to prescribe generics.23 It is noticed that successful advertising campaigns launched by pharmaceutical companies are thought by medical doctors to have a positive impact on perceptions toward generics. According to these results, it seems that more should be done in terms of informing all interested parties.
Physicians provide information to patients about generics’ safety, efficacy, and cost. Indeed, if consumers refuse generic’s substitution, they have to pay the price difference between the generic and the more expensive prototype drug.24 Therefore, the establishment of a comprehensive awareness program to improve information about generic products is necessary. In Australia, for example, National Prescribing Service Limited is an independent organization that plays a major role in informing consumers, as well as health care professionals, about generic medicines. Nevertheless, patients often refuse to change their medicines, especially if they are older patients with low level of education or mothers with concerns about their children’s care. Patients suffering from chronic and serious diseases are also reluctant to use generics due to lack of trust in generics’ therapeutic efficacy. Without proper justification, lower prices are thought to be an evidence for poor quality products.25 Information about previous positive experiences as well as the factor of cost saving have an improving impact on patients’ decision to accept generic drugs therapies.26 Information provision and awareness interventions are important for generic medicine acceptance. National Organization for Medicines, Food and Drug Administration, drug representatives, and pharmacists are resources of information about generics.27
Medical doctors have the responsibility to decide whether or not the brand name drug substitution is suggested. Generics are promoted in EU countries by specific policies that are mainly grounded on cost containment, market regulation, and encouragement of efficient use of resources. The reliability of manufacturers is one of the major factors reported by the survey participants. The governments could promote generics by assuring quality/safety and by issuing guidelines on prototype drug substitution.28 However, pharmacists can substitute original medicines with their therapeutic equivalent generic form, unless prohibited by the doctor or if patients raise objections.29 Some physicians mainly in the private health care sector do not support generic substitution due to the lack of incentives and fear of reputation loss. This is also reported in studies disputing bioequivalence of generic medicines.30 These misconceptions should be altered by the central health care authorities in order cost–containment programs to succeed.19 In Greece, policy makers should maintain their financial and nonfinancial incentives to substitute branded drugs.31 Kontodimopoulos et al32 have reported substitution of prototype drugs by generics at a rate of about 26% by 2012, observing the favorable effects of the corresponding reform initiatives in reducing the pharmaceutical expenditures. Furthermore, the procurement procedures of the Health Procurement Committee (EPY) for pharmaceuticals and medical devices in Greece after 2010 allowed request management at a national level, organizing calls for tender and developing a Price List Observatory for price comparisons, greater transparency, and cost control.33
Study Limitations
This is one of the first studies in Greece conducted to systematically evaluate the relation of doctors’ level of information with their attitudes toward generic drugs. As in every research pursuit there are inherent limitations and the results provided should be generalized with caution. Being a pilot study, this survey includes the responses of medical doctors from a specific public hospital in Greece. Further studies of this nature could be useful for unraveling the role of information in shaping the appropriate doctors’ attitudes toward extensive generics adoption and thus reducing unnecessary public spending without improving clinical outcomes.
Conclusions
The findings of the present study indicate that information plays a significant role toward generic medicines. Information provision alters misconceptions about the safety and efficacy of generics and increase doctors’ and patients’ confidence in adhering to generic substitution policies. Health authorities in Greece must further establish generic promotional laws and information policies toward the health care professionals, and hold public campaigns in media about the generics’ quality, to build confidence among doctors, pharmacists, and consumers. Data analysis has shown that there is a significant positive association between information and generic prescription practices. Information-seeking skills would allow doctors to continually retrieve information about pharmaceutical products and research and hence be able to assess drugs more effectively. Furthermore, in a future research more situational (eg, access to scholarly databases, medical specialty, type of hospital) and personal variables (eg, computer and information literacy skills, knowledge of foreign language) could be included, to further explain the role of information in doctors’ perceptions and preferences toward generics.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Chua GN, Hassali MA, Shafie AA, Awaisu A. A survey exploring knowledge and perceptions of general practitioners towards the use of generic medicines in the northern state of Malaysia. Health Policy. 2010;95(2):229-235. [DOI] [PubMed] [Google Scholar]
- 2. Hassali MA, Alresheedy AA, McLachlan A, Ngugen TA, Karamah AL-T, Ibrahim M, Aljadhey H. The experiences of implementing generic medicine policy in eight countries: A review and recommendations for a successful promotion of generic medicine use. Saudi Pharm J. 2014;22(6):491-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vandoros S, Stargart T. Reforms in the Greek pharmaceutical market during the financial crisis. Health Policy. 2013;109:1-6. [DOI] [PubMed] [Google Scholar]
- 4. Dylst P, Vulto A, Simoens S. Overcoming challenges in market access of generic medicines in the European Union. J Generic Med. 2012;9:21-28. [Google Scholar]
- 5. Niakas D. Greek economic crisis and health care reforms: correcting the wrong prescription. Int J Health Serv. 2013;43(4):597-602. [DOI] [PubMed] [Google Scholar]
- 6. Fabiano V, Mameli C, Cattaneo D, et al. Perceptions and patterns of use of generic drugs among Italian family pediatricians: first round results of a web survey. Health Policy. 2012;104:247-252. [DOI] [PubMed] [Google Scholar]
- 7. Ifanti AA, Argyriou AA, Kalofonou FH, Kalofonos HP. Financial crisis and austerity measures in Greece: their impact on health promotion policies and public health care. Health Policy. 2013;113:8-12. [DOI] [PubMed] [Google Scholar]
- 8. Labiris G, Fanariotis M, Kastanioti C, et al. Greek physicians’ perceptions on generic drugs in the era of austerity. Scientifica. 2015;2015:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Geitona M, Zavras D, Hatzikou M, Kyriopoulos J. Generics market in Greece: the pharmaceutical industry’s beliefs. Health Policy. 2006;79(1):35-48. [DOI] [PubMed] [Google Scholar]
- 10. Gourtsoyiannis Y. Health service expenditure and efficiencies in Greece. Health Policy. 2013;111(2):206-207. [DOI] [PubMed] [Google Scholar]
- 11. Shrank WH, Liberman JN, Fischer MA, Girdish C, Brennan TA, Choudhry NK. Physician perceptions about generic drugs. Ann Pharmacother. 2011;45(1):31-38. [DOI] [PubMed] [Google Scholar]
- 12. Gevorgyan T. Physicians’ Prescription Practices and Knowledge About Brand-Name Versus Generic Drugs in Yerevan, Armenia: An Analytical Cross-Sectional Study. Yerevan: College of Health Sciences, American University of Armenia; 2011. [Google Scholar]
- 13. Anderson JC, Gerbing DW. Structural equation modeling in practice: a review and recommended two-step approach. Psychol Bull. 1988;103(3):411-423. [Google Scholar]
- 14. Bollen KA. Structural Equations With Latent Variables. New York, NY: John Wiley; 1989. Wiley Series in Probability and Mathematical Statistics. [Google Scholar]
- 15. Johannesson M, Lundin D. The Impact of Physician Preferences on the Prescription of New Drugs. Stockholm, Sweden: Stockholm School of Economics; 2002. SSE/EFI Working Paper Series in Economics and Finance. [Google Scholar]
- 16. Hair JF, Jr, Anderson RE, Tatham RL, Black WC. Multivariate Data Analysis With Readings. 5th ed. Upper Saddle River, NJ: Prentice-Hall International; 1998. [Google Scholar]
- 17. Nunnally JC, Bernstein IH. Psychometric Theory. 3nd ed. New York, NY: McGraw-Hill; 1994. [Google Scholar]
- 18. Sheppard A. Generic Medicines: Essential Contributors to the Long-Term Health of Society. Brussels, Belgium: IMS Health; 2011. [Google Scholar]
- 19. Quintal C, Mendes P. Underuse of generic medicines in Portugal: an empirical study on the perceptions and attitudes of patients and pharmacists. Health Policy. 2012;104(1):61-68. [DOI] [PubMed] [Google Scholar]
- 20. Kumar R, Hassali MA, Saleem F, et al. Knowledge and perceptions of physicians from private medical centers towards generic medicines: a nationwide survey from Malaysia. J Pharm Policy. 2015;8:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rubak SL, Andersen ML, Mainz J, Olesgaard P, Lauritzen T. How do practitioners evaluate the newly introduced system of substituting prescriptions? Ugeskr Laeger. 2000;162(45):6070-6073. [PubMed] [Google Scholar]
- 22. Figueiras M, Marcelino D, Cortes M. People’s views on the level of agreement of generic medicines for different illness. Pharm World Sci. 2009;30:427-437. [DOI] [PubMed] [Google Scholar]
- 23. Allenet B, Barry H. Opinion and behavior of pharmacists towards substitution of branded drugs by generic drugs: survey of 1000 French community pharmacists. Pharm World Sci. 2003;25(5):197-202. [DOI] [PubMed] [Google Scholar]
- 24. Kobayashi E, Karigome H, Sakurada T, Satoh N, Ueda S. Patients’ attitudes towards generic drugs substitution in Japan. Health Policy. 2011;99(1):60-65. [DOI] [PubMed] [Google Scholar]
- 25. Sharrad AK, Hassali MA. Consumer perception on generic medicines in Basrah, Iraq: preliminary findings from a qualitative study. Res Social Adm Pharm. 2011;7:108-112. [DOI] [PubMed] [Google Scholar]
- 26. Heikkila R, Mantyselka P, Ahonen R. Price, familiarity, and availability determine the choice of drug—a population-based survey five years after generic substitution was introduced in Finland. BMC Clin Pharmacol. 2011;11:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jamshed SQ, Hassali MA, Ibrahim M, Shafi AA, Babar Z. Knowledge, perception and attitude of community pharmacists towards generic medicines in Karachi, Pakistan: a qualitative insight. Top J Pharm Res. 2010;9(4):409-415. [Google Scholar]
- 28. Coster JM. Legislative and regulatory issues affecting generic drug reimbursement. US Pharm. 2012;37(6):22-27. [Google Scholar]
- 29. Omojasola A, Hernandez M, Sansquiry S, Jones L. Perception of generic prescription drugs and utilization of generic drug discount programs. Ethn Dis. 2012;22(4):479-485. [PMC free article] [PubMed] [Google Scholar]
- 30. Chong C, March G, Clark A, Gilbert A, Hassali MA, Bahari MB. A nationwide study on generic medicines substitution practices of Australian community pharmacists and patient’s acceptance. Health Policy. 2011;99(2):139-148. [DOI] [PubMed] [Google Scholar]
- 31. Barros P, Nunes L. 10 Years of Pharmaceutical Policy in Portugal. Lisbon, Portugal: Nova School of Business & Economics; 2011. [Google Scholar]
- 32. Kontodimopoulos N, Kastanioti C, Thireos E, Karanikas H, Polyzos N. The contribution of generic substitution to rationalizing pharmaceutical expenditure in Greek public hospitals under recent economic crisis. J Pharm Health Serv Res. 2013;4(4):211-216 [Google Scholar]
- 33. Kastanioti C, Kontodimopoulos N, Stasinopoulos D, Kapetaneas N, Polyzos N. Public procurement of health technologies in Greece in an era of economic crisis. Health Policy. 2013;109:7-13. [DOI] [PubMed] [Google Scholar]