Abstract
Background
Selective reporting is wasteful, leads to bias in the published record and harms the credibility of science. Studies on potential determinants of selective reporting currently lack a shared taxonomy and a causal framework.
Objective
To develop a taxonomy of determinants of selective reporting in science.
Design
Inductive qualitative content analysis of a random selection of the pertinent literature including empirical research and theoretical reflections.
Methods
Using search terms for bias and selection combined with terms for reporting and publication, we systematically searched the PubMed, Embase, PsycINFO and Web of Science databases up to January 8, 2015. Of the 918 articles identified, we screened a 25 percent random selection. From eligible articles, we extracted phrases that mentioned putative or possible determinants of selective reporting, which we used to create meaningful categories. We stopped when no new categories emerged in the most recently analyzed articles (saturation).
Results
Saturation was reached after analyzing 64 articles. We identified 497 putative determinants, of which 145 (29%) were supported by empirical findings. The determinants represented 12 categories (leaving 3% unspecified): focus on preferred findings (36%), poor or overly flexible research design (22%), high-risk area and its development (8%), dependence upon sponsors (8%), prejudice (7%), lack of resources including time (3%), doubts about reporting being worth the effort (3%), limitations in reporting and editorial practices (3%), academic publication system hurdles (3%), unfavorable geographical and regulatory environment (2%), relationship and collaboration issues (2%), and potential harm (0.4%).
Conclusions
We designed a taxonomy of putative determinants of selective reporting consisting of 12 categories. The taxonomy may help develop theory about causes of selection bias and guide policies to prevent selective reporting.
Introduction
Complete, accurate and timely reporting of all (study protocol-stipulated) outcomes is essential for syntheses of research to be valid and as precise as possible [1, 2]. Complete or unselective reporting refers to both unselective publication of all results of a study as well as unselective or complete reporting within publications on all planned outcomes [3]. In other words, all planned outcomes should be reported on within a reasonable time frame (and the exploratory nature of analyses with any unplanned outcomes should be disclosed).
Selective reporting leads to bias if specific results remain unpublished because the decision to report depends on the nature of the results (e.g., direction or magnitude of the target association). Reporting bias is an important threat to the validity of systematic reviews which clinicians, researchers, policy makers and citizens rely on. Reporting bias is wasteful, distorts the aggregate body of scientific evidence, threatens the credibility of science, but it may also result in suboptimal treatment or even in avoidable harm to, e.g., patients’ health. Therefore, in addition to validity and efficiency reasons, there is an ethical imperative to report all results including those of clinical trials [4, 5].
Journals increasingly require that protocols have been registered before commencing or completing a study to facilitate the detection of selective reporting and increase transparency, and there is some evidence that registration of trials is effective [6, 7]. However, around half of planned outcomes of clinical trials are not reported, and a third to half of registered clinical trials remain unpublished [8–11]. There are numerous reports that suggest that selective publication is a major problem in the clinical domain, but it is also pervasive in basic and translational research [12, 13] and in the social sciences [14]. Selective reporting occurs in various other types of studies and across various designs, e.g., trials with psycho-educational interventions, and quantitative observational and qualitative studies [11, 15, 16].
So-called “protocol-to-publication” and similar studies point to selective reporting of statistically significant results [11, 17, 18]. More generally, “preferred findings” are more often reported. Preferred findings are often statistically significant findings. However, there are exceptions such as in case of studies into adverse effects, or equivalence trials where no difference is preferred. As a consequence, findings preferred by key stakeholders in the research project at issue are likely overrepresented in the scientific literature [19].
Research has suggested that financial conflicts of interest may cause selective reporting (e.g., when studies are sponsored by industry [9, 11, 20, 21]), but non-financial conflicts of interest probably play an important role too. Causes of publication bias, and of reporting bias more generally, may relate to decisions taken by researchers and sponsors, and also decisions by editors [8, 9, 22, 23]. Some have argued that it is human nature to search for positive messages, which suggests that basically, all scientists are at risk [2, 24]. However, certain persons or environments may be at increased risk of selective reporting, such as junior researchers [25] and scientists in more competitive academic environments [26].
Despite numerous studies on selective reporting, there is no accepted taxonomy of its determinants and no explicit causal framework. A recently developed framework of non-publication [22] attempted to answer the questions “what?” (defining dissemination), “who?”(is to blame-actor/stakeholder) and “why?” (stakeholders’ motivations). However, it does not provide a single taxonomy of determinants and its scope was limited to clinical trials.
We aimed to develop a taxonomy of putative determinants of selective reporting. We therefore addressed the questions of “what are possible determinants of selective reporting?,” and “how are putative determinants of selective reporting best grouped based on its content?“.
Methods
Design, protocol and reporting
To develop a taxonomy of putative determinants of selective reporting, we combined principles of systematic reviews [27] with those of inductive qualitative content analysis [28, 29]. Before analyzing full-texts, we piloted search strategies, we piloted abstract and full-text eligibility criteria and reviewed procedures as detailed in the protocol (S1 File). We developed the study protocol based on the preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) guidelines [30,31] as far as applicable. In this article, we report applicable items from the PRISMA [32] guidelines for systematic review and applicable items from guidelines for the reporting of qualitative studies [33, 34].
Eligibility criteria
We included articles that examined or suggested determinants of selective reporting. We searched for articles from any academic discipline reporting on studies employing any type of design based on empirical data, such as intervention studies (with any type of comparator), and observational studies. Additionally, to cover hypotheses on what drives selective reporting, we included non-empirical articles such as editorials presenting opinion, theoretical considerations and anecdotal evidence. To minimize duplication of determinants extracted from articles that were also selected for inclusion in reviews, we excluded reviews. The outcome, selective reporting, comprised non-publication and selective reporting within publications. Our review also covered the possible consequences of selective reporting, including publication bias and other types of reporting bias.
Information sources
We searched PubMed, Embase, PsycINFO and Web of Science to cover a wide range of academic disciplines from inception to January 8th 2015. We limited the search to the English, French, German and Dutch languages.
Search strategy
The search strategy focused on selective reporting to avoid limitation to any preconceived determinants. We used terms for bias and selection combined with terms for reporting and publication (S1 File, Box 1), and we pilot tested the search strategy.
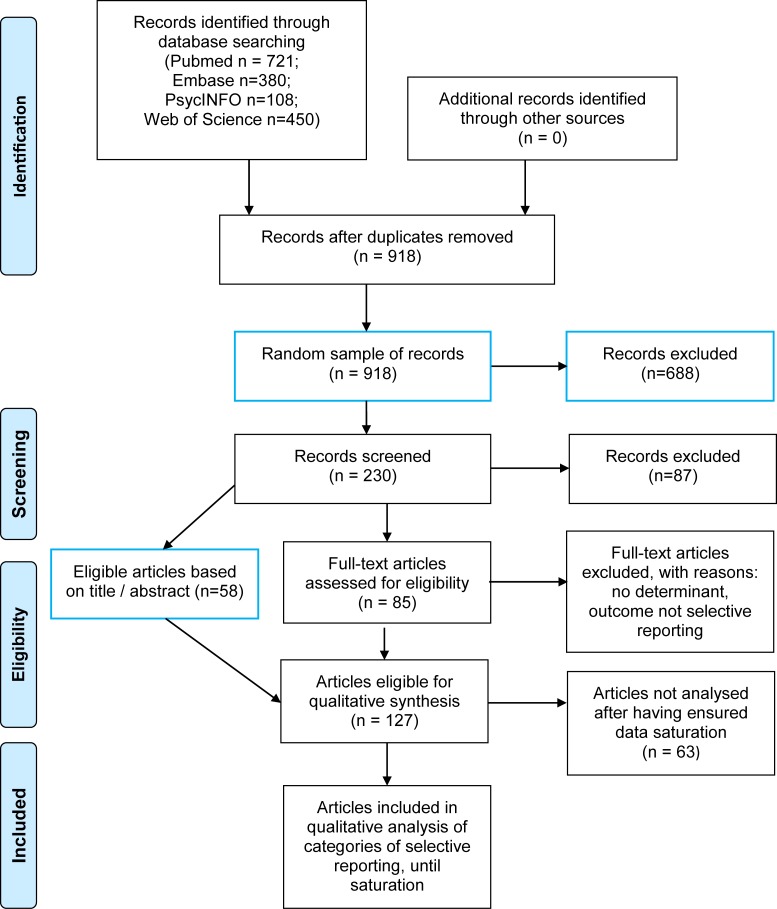
For reasons of feasibility of identifying determinants with qualitative content analysis (S1 File, Box 1), we retained a 25% random sample of the total of 918 hits after deduplication, from all databases searched from inception to January 8th 2015 (Fig 1). We used SPSS version 22’s random allocator function “random sample of cases” to randomly select a quarter comprising 230 hits.
Fig 1. PRISMA flow diagram of identified and analyzed articles.
Selection of articles
Titles and abstracts were screened independently against the inclusion criteria by pairs of two reviewers (JTvdS, CAvdB and MCvS-P). If there was no abstract, we reviewed keywords. We used the first ten percent of abstracts (23) to test a shared understanding of the inclusion criteria and we discussed discrepant interpretations. Of the other abstracts, we calculated inter-rater agreement (percentage) of decisions on retrieval as full-text. We evaluated for eligibility all full-text articles and we calculated inter-rater agreement also for this step.
Data collection process
Full-text data extractions were performed by pairs of researchers (JTvdS, CAvdB, MCvS-P, SFF, and GtR). JTvdS, CAvdB and MCvS-P piloted a standardized full-text data extraction spreadsheet using three empirical and non-empirical articles not included in the random sample. Initially, the reviewers extracted data independently, but after, for each pair of analysists, the analysis of a few articles made it clear that determinants were identified consistently, one reviewer extracted data with verification by another (S1 File, paragraph Data collection process).
Data items
At the level of the article, we abstracted year of publication, academic discipline and study design (S1 File, box 2). More than one determinant per article was possible. We assessed whether evidence of association of a determinant with the outcome was empirical, indicated an actor (stakeholder), referred to a cause or could possibly be interpreted as a cause (we thus explicitly assessed the degree of interpretation [35]). We assessed the type and scope of selective reporting (whether limited to a single medium, e.g., a specific journal) and the nature of the association between a putative determinant and selective reporting (if there was any association, the direction, and strength of association, if applicable).
Analyses
The sample of articles and determinants was summarized using percentages. We used qualitative iterative and inductive content analysis to group determinants by content and form categories of determinants [29]. In particular, the reviewers (pair) extracted putative determinants in the context of the article, and the team subsequently discussed interpretations and categorization. In the content analysis, we coded putative determinants and subsumed them under meaningful categories without imposing any prior model. We considered determinants which were tested even when authors found them unrelated to selective reporting in the particular study, because different studies often vary in finding associations with outcomes. A single dataset with putative determinants was created after discussions on differences of interpretation.
Next, two researchers (JTvdS and CAvdB) independently categorized all determinants into higher-level groupings. This was a non-linear, iterative process as we classified batches of about 50 determinants followed by discussing all classifications of each batch before moving on to the next batch. We avoided overlap in categories [35] by adding descriptions (to serve as definitions) that we developed from the iterative classification of content. In reaching consensus about the categorization we often went back to previous classification work to adapt categories, or to the full-texts to ensure we understood the context. Thus, we constructed a structured list of categories of more specific determinants. An initial classification and any unresolved issues about further classifications were discussed with a third researcher (GtR) to achieve full consensus. Following the principles of content analyses [9], we counted the number of determinants per category for descriptive purposes of which categories were more and which were less prominent in the literature.
Saturation
We analyzed content of articles randomly selected from different decades (< 1980, 1980s, 1990s, 2000–2009, 2010–2015). We concluded analyses when saturation was reached. Saturated data ensure replicability in categories derived from content analyses, which in turn verifies and ensures comprehensiveness [35]. Saturation of categories was determined in two ways. First, during the process of analyzing the batches of determinants we assessed saturation prospectively, during the content analyses, in the usual way for qualitative analyses (i.e., no new categories emerged in the last analyzed articles). After having analyzed about 50 articles, we deemed the newly classified determinants not to be essentially different from those already classified (we could fit them in the categories we had developed), and we assumed saturation. This was confirmed after having analyzed one more batch of determinants representing 10% of articles (13 of 127). Second, retrospectively, after having analyzed the articles, we verified quantitatively how many articles had been analyzed when the first determinant of each category emerged.
Subgroup analyses
We assessed the extent to which various determinants were based on empirical studies or (solely) on opinion (as planned and described in the protocol, S1 File). Further, to avoid inclusion of categories exclusively comprising determinants that were consistently unrelated to outcome, we calculated the proportion of articles that (quantitatively or qualitatively) reported a non-significant or no association, or an unexpected direction of an association between a determinant and selective reporting.
Results
Selection of articles for analyses
We identified 918 unique records, and we included 64 records (articles) in the final analysis (Fig 1). The inter-rater agreement between independent assessors on the need to retrieve the full-text as assessed for 90% of the records was 72% (150/207; agreement about 143 records, doubt about 7, no agreement about 57). The initial agreement about eligibility of full-text assessment was 78% (66/85). All initial disagreements were resolved through discussion.
Description of the dataset: Articles and determinants
Of the 64 articles analyzed, 48 (75%) concerned clinical medicine, and 51 (80%) were published in 2000 or later (Table 1). Almost half of the articles (31) were non-empirical. The empirical studies were mostly observational and quantitative; we found one RCT [36].
Table 1. Characteristics of the 64 analyzed articles.
| Characteristic | % | n |
|---|---|---|
| Year of publication | ||
| < 1980 1980s 1990s 2000–2009 2010–2015 |
2 3 16 44 36 |
1 2 10 28 23 |
| Academic discipline | ||
| Clinical medicine Biomedicine / Life sciences Humanities No specific discipline |
75 9 11 5 |
48 6 7 3 |
| Type of study / study design | ||
| Non-empirical (reflective / theoretical) Observational quantitative, longitudinal* Observational quantitative, cross-sectional* Case description / anecdotal Simulation / modelling† Randomized trial‡ Qualitative Mixed methods (integrated) Review of reviews |
48 27 9 5 5 2 2 2 2 |
31 17 6 3 3 1 1 1 1 |
* Observational quantitative studies included: comparisons of publications (n = 5), comparison of registry records with publications (4), of protocols with publications (4), of submitted with accepted papers (4), of abstracts with publications (4), of public funder database with publications (1) and of industry database with Medline records (1)
† Mathematical simulations of reporting bias, subjective decision-making in peer-review, and the selection process in publication bias, whether purely hypothetical or with use of empirical data
‡ The RCT assessed the effect of blinded peer-review on reviewers’ and editors’ decisions about manuscript acceptance [36]. The determinant was prejudice in the peer-review process, and the outcome was non-publication, considering that the editor’s decision dictates whether the manuscript is published
We extracted 497 determinants from the 64 articles (median 6; range 1 (9 articles) to 22 (3 articles; Table 2; S2 File). Twenty-nine percent (145 determinants) concerned empirical evidence of associations with selective reporting. If an actor (stakeholder) was mentioned (41%, 204 determinants), it was the investigator in about half of cases (110 determinants). In 79% of cases, an association with selective reporting was found or postulated (all in the hypothesized or expected direction).
Table 2. Characteristics of the determinants (n = 497), outcomes and their associations.
| Characteristic | % (n) |
|---|---|
| Evidence of association of determinant with outcome | |
| Empirical Non-empirical (e.g., from viewpoint, or opinion in discussion section, or inference from the literature or theoretical study) |
29 (145) 71 (352) |
| Actor (stakeholder) | |
| involved investigators or authors editors or journals reviewers sponsors or industry government analyst no actor mentioned |
41 (204) 22 (110) 11 (57) 4 (18) 3 (17) 0.2 (1) 0.2 (1) 59 (293) |
| Interpretation of association (hypothesized, whether confirmed or not) in terms of possible causal pathways*† | |
| Describes a cause Allows for a single and clear interpretation of cause Unclear cause or multiple causal interpretations are possible |
22 (111) 20 (98) 58 (288) |
| Type of selective reporting outcome | |
| Non-publication Selective publication in general Selective reporting within publication Reporting bias Other (delayed publication‒which risks e.g., no uptake in reviews) |
59 (292) 16 (78) 14 (70) 11 (53) 1 (4) |
| Scope of selective reporting outcome | |
| Within a single medium (journal or conference) General |
13 (66) 87 (431) |
| Reported association between determinant and outcome† | |
| Present (confirmed) No association |
79 (393) 21 (104) |
Note: the table is based on the pre-planned items for description of 497 determinants abstracted from the 64 articles
* Examples: “The company often owns the study database and controls decisions about publication and release of data” (describes a cause); authors reported “lack of time” as a reason (allows for a single and clear interpretation of cause‒ a cause is implicated (lack of time), but that cause itself begs a more detailed explanation (how does the reporting compete with other duties and why?)); sample size (unclear cause or multiple causal interpretations are possible‒ such as with larger sample size more power, more collaborators, more rigorous design, more quality checks etc.)
†Note that a possible causal interpretation of a determinant under study (a hypothesis) does not necessarily mean that in each case a causal association with the outcome was actually confirmed in the particular study or in the narrative (finding no association was still possible as an empirical result, or a possible causal association could be denied in a comment)
Categories of determinants
Table 3 lists the 12 categories that emerged from coding the extracted determinants, along with descriptions and examples. Focus on preferred findings was the largest category (180 determinants, 36%), which included, for example, significance chasing. This concerned empirical data in 17% (30/180) of cases, and in 93% (168/180) of cases the original authors postulated that a focus on preferred findings was positively associated with selective reporting. By contrast, the second largest category (109 determinants, 22%), poor or flexible research design, was based on empirical findings in half of the cases, and the original authors mentioned a positive association in 57% of cases. The other 10 categories occurred less frequently (8% or less) yet represented distinct concepts. References to the 64 analyzed articles are provided per category, as a supplement (S3 File).
Table 3. Taxonomy of determinants (n = 497) resulting from the inductive qualitative content analyses.
| Determinant classification, category | Description | Examples | % (n in full sample) | % empirical result* (n in category /-per row) | % any relationship† (n in category /per row) |
|---|---|---|---|---|---|
| 1. Focus on preferred findings | A focus on finding results that match preferences, mostly statistically significant or otherwise positive findings, wishful thinking and acting | Significance chasing, finding significant results, larger effect size, suppressing publication of unfavorable results, not being intrigued by null findings | 36 (180/497) |
17 (30/180) |
93 (168/180) |
| 2. Poor or flexible research design | Attributes of study design relating to power and level of evidence provide much leeway in how studies are performed and in interpretation of their results | Not a controlled or blinded study, study protocol unavailable, small sample size | 22 (109/497) |
50 (54/109) |
57 (62/109) |
| 3. High-risk area and its development | Area of research or discipline or specialty including its historical development and competitiveness, the currently dominant paradigms and designs, and career opportunities | Ideological biases in a research field, area with much epidemiological research versus clinical or laboratory research (“hard sciences”), humanities, experimental analytic methods, “hot” fields, publication pressure in the specific field | 8 (39/497) |
31 (12/39) |
72 (28/39) |
| 4. Dependence upon sponsors | Financial conflict of interest resulting in lack of academic freedom | Requirements and influence of funding source with financial interests in study results | 8 (38/497) |
34 (13/38) |
82 (31/38) |
| 5. Prejudice | A conscious or unconscious belief that may be unfounded, and of which one may or may not be aware | Prior belief about efficacy of treatment, author reputation or gender bias in the phase of review | 7 (33/497) |
24 (8/33) |
82 (27/33) |
| 6. Lack of resources, including time | Insufficient manpower or finances | Lack of time resulting from excessive workload, or lack of personnel due to life events | 3 (17/497) |
18 (3/17) |
100 (17/17) |
| 7. Doubts about reporting being worth the effort | Weighing investment of time and means versus likelihood of gain through publication | Anticipating disappointment of yet another rejection or low chances of acceptance of a manuscript, belief that findings are not worth the trouble | 3 (16/497) |
6 (1/16) |
100 (16/16) |
| 8. Limitations in reporting and editorial practices | Constraints and barriers to the practice of reporting relevant detail | Journal space restrictions, author writing skills | 3 (14/497) |
71 (10/14) |
50 (7/14) |
| 9. Academic publication system hurdles | Various hurdles to full reporting related to submission and processing of manuscripts (other than reporting) including those that represent an intellectual conflict of interest | Solicited manuscripts, authors indicating non-preferred reviewers, editor’s rejection rate | 3 (14/497) |
36 (5/14) |
57 (8/14) |
| 10. Unfavorable geographical or regulatory environment‡ | Geographical or regulatory environment that affects how research is being performed | Continents under study included North America, Europe and Asia; few international collaborations; no governmental regulation of commercially sponsored research | 2 (12/497) |
67 (8/12) |
75 (9/12) |
| 11. Relationship and collaboration issues | Intellectual conflict of interest between reporting and maintaining good relationships | Disagreements among co-authors and between authors and sponsors, sponsors prefer to work with investigators who share the sponsor’s position | 2 (8/497) |
13 (1/8) |
100 (8/8) |
| 12. Potential harm | Publishing data can harm individuals | Risk of bioterrorism, or confidentiality restriction | 0.4 (2/497) |
0 (0/2) |
100 (2/2) |
| (13) Not specified | Referring to a stakeholder only | Selective publication not caused by editors | 3 (15/497) |
0 (0/15) |
67 (10/15) |
*Empirical result as described in Table 2, first row
†Any relationship, and in the expected direction if any relationship was being hypothesized, versus no relationship. None of the hypothesized relationships in empirical result were found to be in the opposite direction
‡We aimed to consistently include a direction in all category names. However, the work from which we abstracted the determinants for this category was probably less theory driven (often not providing background or a hypothesis of direction), and more data driven (combining countries in order to attain sufficiently large groups). For example, manuscripts from the US versus all other countries was tested and there were very few manuscripts from other countries. This made it difficult to find a term that clearly describes direction. We therefore used “unfavorable” without further specification
Saturation
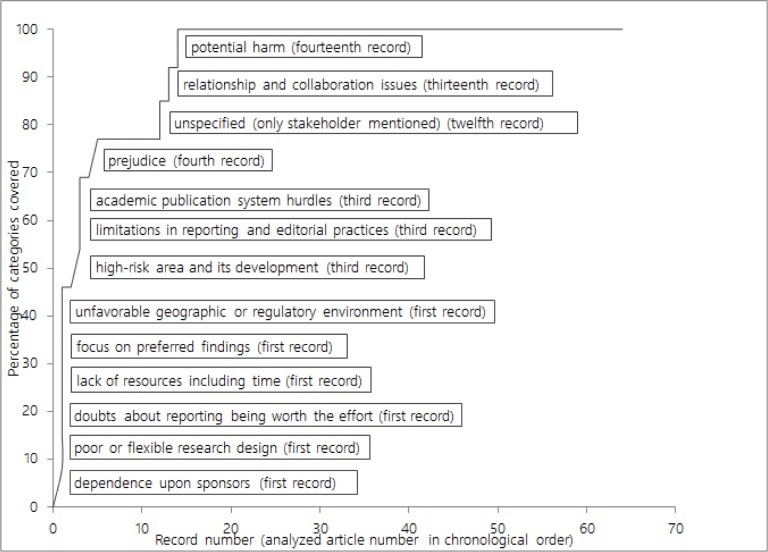
The first determinant in each category usually emerged after analysis of only a few articles (Fig 2). After having extracted 120 determinants (of 497, 24%) from 14 articles, all categories comprised one or more determinants. Assuming that a category emerges upon identification of at least one or more determinants that are dissimilar from other categories, this indicates that saturation could have been reached earlier, with fewer than the set analyzed articles.
Fig 2. Saturation graph.
The horizontal axis displays the articles (records) in chronological order of analysis. The vertical axis displays the percentage of the 12 (plus 1 unspecified) determinant categories containing at least one determinant. The labels describe which determinant category appeared for the first time in which record.
Relationships and similarities between categories in view of possible causality
The descriptions clarified boundaries between categories that were conceptually close, such as high-risk area and its development, and unfavorable geographical and regulatory environment. Both categories represented a wider environment than the research team or institution.
The categories of unfavorable geographical and regulatory environment and academic publication system hurdles were distinct as these included determinants that did not clearly refer to a possible hypothesis regarding a mechanism or cause of selective reporting (an example is provided as a Footnote to Table 3). By including these categories, we were able to classify all determinants, except for 15 that only mentioned a stakeholder (actor) as the source of selective reporting (or the denied source, such as when an actor is believed not to cause selective reporting, in which case we recorded no association between determinant and the outcome of selective reporting).
We found six described instances of interaction between determinants (effect modification). We counted these as classified with the main determinant only. The interactions all clearly described causes. For example, “Outcomes could be deemed post hoc to have little clinical relevance if they fail to show significant findings and may thus be omitted when accommodating space limitations” [37]. In this case, the interaction between focus on preferred findings and limitations in reporting and editorial practices was classified under the first. By contrast, we classified another interaction, between a focus on preferred findings and high-risk area and its development under the latter as the primary category: “Early in the history of a research domain results in either direction are important news but [that] later, when the preponderance of evidence has supported one direction, significant reversals are often more important news than further replications.” [38].
Discussion
We developed a taxonomy of putative determinants of selective reporting in science based on saturated qualitative analyses of a representative sample of the relevant literature. The taxonomy clusters determinants in a meaningful way. It consists of 12 mutually exclusive categories along with descriptions and examples to clarify boundaries and differences between the categories. The taxonomy should give structure and depth to commonly used expressions such as significance chasing (placed in the category focus on preferred findings) and conflict of interest (financial: dependence upon sponsors and relationship or collaboration issues; intellectual: relationship or collaboration issues and academic publication system hurdles).
Two categories, focus on preferred findings and poor or flexible research design, covered over half of the determinants we found. These related mostly to choices of individual researchers or teams. The individual or team level was also referred to in six of the 10 other categories (prejudice, dependence upon sponsors, lack of resources including time, doubts about reporting being worth the effort, limitations in reporting and editorial practices, which refers to individual editors and authors, and relationship and collaboration issues). Four categories referred to the wider environment. These were the categories of academic publication system hurdles and potential harm; the other two included determinants that often lack clear direction or hypothesis (e.g., when a range of disciplines or countries are compared based on distributions): high-risk area and its development, and unfavorable geographic or regulatory environment.
Related conceptual work on selective reporting
The basis of the framework of Bassler et al. [22] comprised 50 highly cited articles published until 2012 identified in Web of Science, and consensus among 10 experts. Our work represents a wider scope of literature. For example, potential harm through bioterrorism was identified through veterinary medicine literature [39]. Also, Web of Science identified less than half of the articles in our sample. We included expert views and aggregate understanding, and determinants that may not have been studied well, yet in a different manner: through analyzing editorials, comments, and the full articles including introduction and discussion sections. We used explicit and transparent inductive qualitative research methods to cover the broad range of putative determinants in the literature. In contrast, Bassler et al. [22] focused on actors and motivations, which complements our work to help understand the multi-causality and multiple system pressures on and rewards for individuals and teams.
Limitations and strengths
The combined quantitative and qualitative approaches including two different ways ‒prospectively and retrospectively‒ to check saturation increased the likelihood of having captured all relevant categories of determinants and served as an internal validation of our approach. However, the data did not suffice to discern patterns of determinants by academic field or strength of evidence. Our work does not cover all possible single determinants. We do believe that the framework with categories likely captures the categories that fit with the large majority of determinants. For example, we did not find lack of a statistician on the team being a putative determinant, but depending on the hypothesis of how it may relate to selective reporting, it may fit, for example, with poor or flexible research design or focus on preferred findings, or lack of resources including time. Further, in our work, discussion to reach consensus was essential because abstracting possible determinants without a prior framework was not a straightforward endeavor, as evidenced by modest initial agreement (72% and 78%) about inclusion of articles in reviewing abstracts and full-text.
Not publishing research outcomes is unethical. Our findings, however, raise questions about possible rare but legitimate reasons to report selectively or to not publish research. Obviously, potential harm can be considered a legitimate reason, when publication may involve misuse by e.g., terrorists, or involves breaking confidentiality restrictions. Fatally flawed research probably should also not be published. However, poor design is preferably prevented in the first place, the academic reviewing system is in place to improve quality and to prevent fatally flawed work to be published or to be included in reviews and meta-analyses [40]. Future guidance may clarify what should be published in such cases.
Future studies
New research, using various methods, should verify the categories we created. The categories and their interrelations may clarify causal pathways and inform theory. (Complex) interventions should probably and most fruitfully address several determinant categories. So far, most empirical work has been performed on poor or flexible research design, but not all findings refer to clear causes and therefore cannot be used to formulate interventions (such as studies examining the association of sample size with selective reporting of positive findings). Future research should also employ qualitative methods to address researchers’ daily decision making and balancing of interests to better understand causal mechanisms and the multiple factors involved.
The taxonomy may also help plan studies on risk profiling (e.g., research domains in which flexible designs are commonly used, or where a particular mission prevails) which in turn may inform efficient policy development on responsible conduct of research. We suggest our work to promote a constructive debate on causes of reporting bias. It is hoped that it will contribute to decrease the mostly deleterious phenomenon of selective reporting in modern science.
Supporting information
(DOC)
(DOCX)
(XLS)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The design of the study and writing of the article was funded by a career award for JTS from the Netherlands Organisation for Scientific Research (NWO; Innovational Research Incentives Scheme: Vidi grant number 917.11.339), and Leiden University Medical Center, Leiden, The Netherlands. CAvdB was supported by a PhD grant from the Dutch Ministry of Health, Welfare and Sports. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects (2013). JAMA. 310(20):2191–4. Epub 2013/10/22. doi: 10.1001/jama.2013.281053 . [DOI] [PubMed] [Google Scholar]
- 2.Dickersin K, Chalmers I. Recognizing, investigating and dealing with incomplete and biased reporting of clinical research: from Francis Bacon to the WHO. J R Soc Med. 2011;104(12):532–8. Epub 2011/12/20. doi: 10.1258/jrsm.2011.11k042 ; PubMed Central PMCID: PMC3241511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The COMPare Trials Project. Goldacre B, Drysdale H, Powell-Smith A, et al. www.COMPare-trials.org, 2016. Website last accessed on 13 April 2017.
- 4.Moorthy VS, Karam G, Vannice KS, Kieny MP. Rationale for WHO's new position calling for prompt reporting and public disclosure of interventional clinical trial results. PLoS Med. 2015;12(4):e1001819 Epub 2015/04/16. doi: 10.1371/journal.pmed.1001819 ; PubMed Central PMCID: PMC4396122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glasziou P, Altman DG, Bossuyt P, Boutron I, Clarke M, Julious S, et al. Reducing waste from incomplete or unusable reports of biomedical research. Lancet. 2014;383(9913):267–76. doi: 10.1016/S0140-6736(13)62228-X . [DOI] [PubMed] [Google Scholar]
- 6.van den Bogert CA, Souverein PC, Brekelmans CT, Janssen SW, Koëter GH, Leufkens HG, et al. Non-publication is common among phase 1, single-center, not prospectively registered, or early terminated clinical drug trials. PLoS One. 2016;11(12):e0167709 doi: 10.1371/journal.pone.0167709 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan AW, Pello A, Kitchen J, Axentiev A, Virtanen JI, Liu A, et al. Association of trial registration with reporting of primary outcomes in protocols and publications. JAMA. 2017. September 11, epublished. doi: 10.1001/jama.2017.13001 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chalmers I, Glasziou P. Avoidable waste in the production and reporting of research evidence. Lancet. 2009;374(9683):86–9. Epub 2009/06/16. doi: 10.1016/S0140-6736(09)60329-9 . [DOI] [PubMed] [Google Scholar]
- 9.Jones CW, Handler L, Crowell KE, Keil LG, Weaver MA, Platts-Mills TF. Non-publication of large randomized clinical trials: cross sectional analysis. BMJ. 2013;347:f6104 Epub 2013/10/31. doi: 10.1136/bmj.f6104 ; PubMed Central PMCID: PMC3812466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross JS, Mulvey GK, Hines EM, Nissen SE, Krumholz HM. Trial publication after registration in ClinicalTrials.Gov: a cross-sectional analysis. PLoS Med. 2009;6(9):e1000144 Epub 2009/11/11. doi: 10.1371/journal.pmed.1000144 ; PubMed Central PMCID: PMC2728480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song F, Parekh S, Hooper L, Loke YK, Ryder J, Sutton AJ, et al. Dissemination and publication of research findings: an updated review of related biases. Health Technol Assess. 2010;14(8):iii, ix-xi, 1–193. Epub 2010/02/26. doi: 10.3310/hta14080 . [DOI] [PubMed] [Google Scholar]
- 12.Macleod MR, Lawson McLean A, Kyriakopoulou A, Serghiou S, de Wilde A, Sherratt N, et al. Risk of Bias in Reports of In Vivo Research: A Focus for Improvement. PLoS Biol. 2015;13(10):e1002273 Epub 2015/10/16. doi: 10.1371/journal.pbio.1002273 ; PubMed Central PMCID: PMC4603955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sena ES, van der Worp HB, Bath PM, Howells DW, Macleod MR. Publication bias in reports of animal stroke studies leads to major overstatement of efficacy. PLoS Biol. 2010;8(3):e1000344 Epub 2010/04/03. doi: 10.1371/journal.pbio.1000344 ; PubMed Central PMCID: PMC2846857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sterling TD. Publication decisions and Their Possible Effects on Inferences Drawn from Tests of Significance—Or Vice Versa. Journal of the American Statistical Association. 1959;54, No. 285 (March 1959):30–4. doi: 10.2307/2282137 [Google Scholar]
- 15.Decullier E, Lheritier V, Chapuis F. Fate of biomedical research protocols and publication bias in France: retrospective cohort study. BMJ. 2005;331(7507):19 Epub 2005/06/22. doi: 10.1136/bmj.38488.385995.8F ; PubMed Central PMCID: PMC558532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petticrew M, Egan M, Thomson H, Hamilton V, Kunkler R, Roberts H. Publication bias in qualitative research: what becomes of qualitative research presented at conferences? J Epidemiol Community Health. 2008;62(6):552–4. Epub 2008/05/15. doi: 10.1136/jech.2006.059394 . [DOI] [PubMed] [Google Scholar]
- 17.Cooper H, DeNeve K, Charlton K. Finding the missing science: The fate of studies submitted for review by a human subjects committee. Psychol Methods. 1997;2(4):447–52. PubMed PMID: WOS:A1997YK36000008. [Google Scholar]
- 18.Hopewell S, Loudon K, Clarke MJ, Oxman AD, Dickersin K. Publication bias in clinical trials due to statistical significance or direction of trial results. Cochrane Database Syst Rev. 2009;(1):MR000006 Epub 2009/01/23. doi: 10.1002/14651858.MR000006.pub3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ioannidis JP. Why most published research findings are false. PLoS Med. 2005;2(8):e124 Epub 2005/08/03. doi: 10.1371/journal.pmed.0020124 ; PubMed Central PMCID: PMC1182327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lundh A, Lexchin J, Mintzes B, Schroll JB, Bero L. Industry sponsorship and research outcome. Cochrane Database Syst Rev. 2017;2:MR000033 Epub 2017/02/17. doi: 10.1002/14651858.MR000033.pub3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGauran N, Wieseler B, Kreis J, Schuler YB, Kolsch H, Kaiser T. Reporting bias in medical research—a narrative review. Trials. 2010;11:37 Epub 2010/04/15. doi: 10.1186/1745-6215-11-37 ; PubMed Central PMCID: PMC2867979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bassler D, Mueller KF, Briel M, Kleijnen J, Marusic A, Wager E, et al. Bias in dissemination of clinical research findings: structured OPEN framework of what, who and why, based on literature review and expert consensus. BMJ Open. 2016;6(1):e010024 Epub 2016/01/24. doi: 10.1136/bmjopen-2015-010024 ; PubMed Central PMCID: PMC4735132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenland S. Accounting for uncertainty about investigator bias: disclosure is informative. J Epidemiol Community Health. 2009;63(8):593–8. Epub 2009/07/15. doi: 10.1136/jech.2008.084913 . [DOI] [PubMed] [Google Scholar]
- 24.Dunkelberg S. Further information on publication bias. Zeitschrift fur Allgemeinmedizin. 2005;81(4):145–6. [Google Scholar]
- 25.Martinson BC, Anderson MS, Crain AL, de Vries R. Scientists' perceptions of organizational justice and self-reported misbehaviors. J Empir Res Hum Res Ethics. 2006;1(1):51–66. Epub 2006/07/01. doi: 10.1525/jer.2006.1.1.51 ; PubMed Central PMCID: PMC1483900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fanelli D. Do pressures to publish increase scientists' bias? An empirical support from US States Data. PLoS One. 2010;5(4):e10271 Epub 2010/04/28. doi: 10.1371/journal.pone.0010271 ; PubMed Central PMCID: PMC2858206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centre for Reviews and Dissemination. Systematic Reviews CRD’s guidance for undertaking reviews in health care. 2009. (third edition) University of York. [Google Scholar]
- 28.Greenhalgh T, Taylor R. Papers that go beyond numbers (qualitative research). BMJ. 1997;315(7110):740–3. Epub 1997/10/07. ; PubMed Central PMCID: PMC2127518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaismoradi M, Turunen H, Bondas T. Content analysis and thematic analysis: Implications for conducting a qualitative descriptive study. Nurs Health Sci. 2013;15(3):398–405. Epub 2013/03/14. doi: 10.1111/nhs.12048 . [DOI] [PubMed] [Google Scholar]
- 30.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1 Epub 2015/01/03. doi: 10.1186/2046-4053-4-1 ; PubMed Central PMCID: PMC4320440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647 Epub 2015/01/04. doi: 10.1136/bmj.g7647 PubMed PMID: 25555855. [DOI] [PubMed] [Google Scholar]
- 32.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097 Epub 2009/07/22. doi: 10.1371/journal.pmed.1000097 ; PubMed Central PMCID: PMC2707599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Brien BC, Harris IB, Beckman TJ, Reed DA, Cook DA. Standards for reporting qualitative research: a synthesis of recommendations. Acad Med. 2014;89(9):1245–51. Epub 2014/07/01. doi: 10.1097/ACM.0000000000000388 . [DOI] [PubMed] [Google Scholar]
- 34.Tong A, Flemming K, McInnes E, Oliver S, Craig J. Enhancing transparency in reporting the synthesis of qualitative research: ENTREQ. BMC Med Res Methodol. 2012;12:181 Epub 2012/11/29. doi: 10.1186/1471-2288-12-181 ; PubMed Central PMCID: PMC3552766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elo S, Kaariainen M, Kanste O, Polkki T, Utriainen K, Kyngas H. Qualitative Content Analysis: A Focus on Trustworthiness. SAGE Open 2014;4(1). Epub 2014/01/09. http://dx.doi.org/10.1177/2158244014522633 [Google Scholar]
- 36.Fisher M, Friedman SB, Strauss B. The effects of blinding on acceptance of research papers by peer review. JAMA. 1994;272(2):143–6. Epub 1994/07/13. . [PubMed] [Google Scholar]
- 37.Chan AW, Altman DG. Identifying outcome reporting bias in randomised trials on PubMed: review of publications and survey of authors. BMJ. 2005;330(7494):753 Epub 2005/02/01. doi: 10.1136/bmj.38356.424606.8F ; PubMed Central PMCID: PMC555875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Preston C, Ashby D, Smyth R. Adjusting for publication bias: modelling the selection process. J Eval Clin Pract. 2004;10(2):313–22. Epub 2004/06/11. doi: 10.1111/j.1365-2753.2003.00457.x . [DOI] [PubMed] [Google Scholar]
- 39.Gyles C. Is there ever good reason to not publish good science? Can Vet J. 2012;53(6):587–8. Epub 2012/12/04. ; PubMed Central PMCID: PMC3354816. [PMC free article] [PubMed] [Google Scholar]
- 40.Ferguson CJ, Brannick MT. Publication bias in psychological science: prevalence, methods for identifying and controlling, and implications for the use of meta-analyses. Psychol Methods. 2012;17(1):120–8. Epub 2011/07/27. doi: 10.1037/a0024445 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOCX)
(XLS)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.