Abstract
Tuberculosis (TB), with the Mycobacterium tuberculosis (Mtb) as the causative agent, remains to be a serious world health problem. Traditional methods used for the study of Mtb in the lungs of TB patients do not provide information about the number and functional status of Mtb, especially if Mtb are located in alveolar macrophages. We have developed a technique to produce ex vivo cultures of cells from different parts of lung tissues surgically removed from patients with pulmonary TB and compared data on the number of cells with Mtb inferred by the proposed technique to the results of bacteriological and histological analyses used for examination of the resected lungs. The ex vivo cultures of cells obtained from the resected lungs of all patients were largely composed of CD14-positive alveolar macrophages, foamy or not, with or without Mtb. Lymphocytes, fibroblasts, neutrophils, and multinucleate Langhans giant cells were also observed. We found alveolar macrophages with Mtb in the ex vivo cultures of cells from the resected lungs of even those TB patients, whose sputum smears and lung tissues did not contain acid-fast Mtb or reveal growing Mtb colonies on dense medium. The detection of alveolar macrophages with Mtb in ex vivo culture as soon as 16–18 h after isolation of cells from the resected lungs of all TB patients suggests that the technique proposed for assessing the level of infection in alveolar macrophages of TB patients has higher sensitivity than do prolonged bacteriological or pathomorphological methods. The proposed technique allowed us to rapidly (in two days after surgery) determine the level of infection with Mtb in the cells of the resected lungs of TB patients and, by the presence or absence of Mtb colonies, including those with cording morphology, the functional status of the TB agent at the time of surgery.
Introduction
Tuberculosis, with the M. tuberculosis as the causative agent, accounts for about 2 million deaths annually and is one of the leading causes of deaths from infectious disease caused by a single agent [1]. According to WHO reports, one-third of the world's human population is infected with Mtb and each infected with this bacterium has a 5–10% risk of developing active TB, which amounts to 8–9 million new cases annually [1, 2]. The pathogenesis of TB depends on the intracellular persistence of Mtb in host macrophages [3, 4]. Macrophages are the cells of the innate immunity system. They are part of the primary immune response, which acts to attack and kill phagocytosed infectious agents, including Mtb. However, in some macrophages Mtb survive and replicate [2–6]. It is considered that the pivotal phase of TB pathogenesis in humans is granulomatous alterations in tissue structure—that of lung tissue in the first place—around foci of infection [2, 7, 8]. A TB granuloma is a highly organized chronic inflammatory structure with a complex cellular composition and many biochemical reactions running in it, which occurs in the form of a local aggregation of mononuclear cells, mostly macrophages, with Mtb in them [8–11]. Additionally, granulomatous TB lesions are observed to contain dendritic cells, lymphocytes, neutrophils, fibroblasts, and multinucleate Langhans giant cells [8, 9, 11, 12]. Granulomas, on the one hand, restrict dissemination of TB infection, while, on the other hand, provide for latency and set the stage for reactivation [13, 14]. The organism of any individual with pulmonary TB displays a broad spectrum of physiologically distinct TB lesions with a wide range of pathological, microbiological, and immunological features [8, 9, 13, 15, 16]. As heterogeneous as they are, many of the TB lesions in the lungs of humans infected with Mtb appear as enclosed structures with caseous necrosis in the center surrounded first by macrophages and finally by peripheral fibrosis [8, 9, 15, 16]. Mycobacteria in the caseous necrotic centers of these granulomas do not normally replicate, because they are stressed by hypoxia, nutrient deprivation and an acid environment [8, 13]. However, as the TB process progresses, caseous necrotic foci in the lungs may liquefy, giving rise to cavitation and allowing caseous matter with Mtb to reach the bronchi [8]. It has been established that Mtb replicating outside and probably within cells occur in large numbers in well-aerated cavity walls [8, 15, 17]. As a result, TB patients cough out sputum from their bronchi and transmit Mtb to people nearby.
Anti-TB chemotherapies are to eliminate Mtb both actively replicating in the cavity walls and present in caseous necrotic centers as scanty, probably dormant bacteria [2, 8, 13, 14]. However, the emergence and propagation of multidrug-resistant (MDR) and extensively drug-resistant (XDR) Mtb have substantially reduced the efficiency of the existing anti-TB chemotherapies and triggered the search for new ways of TB prevention and treatment [1, 18, 19]. One of the effective approaches used in TB treatment is by surgical removal of the affected lung part [2, 13, 17, 20]. Resected lung parts represent a valuable source of information for specifying the diagnosis and the biological features of the TB agent, which is extremely important for assessing the efficiency of preoperative treatment and choosing a postoperative treatment strategy [9, 17, 20]. This is especially important when nothing was known about the properties of the pathogen from respiratory material before surgery [20, 21].
To assess the level of infection by Mtb in the lung tissues of TB patients in clinical settings, the bacteriological method is used, which is by seeding tissue homogenates of resected organs onto special media and counting colony-forming units [2]. However, this method provides only general information about the number of Mtb in the material, telling nothing about how many cells are infected or how many microbes each of those infected cells contains–nor do they tell about the functional status of the TB agent in them. Furthermore, this method is very slow: because of low replication rates of Mtb, it takes 1–2 months after seeding to make final inferences about the infection level in lung tissues and the specific properties of the pathogen. In the human organism, Mtb occurs either in host cells or in necrotic tissue, that is, in the environments that are not like well-aerated liquid or solid nutrient media. Additionally, the TB agent in the human organism is under the control of the immune system permanently and, therefore, the status of Mtb in an organism may be distinct from that observed in vitro.
With histological methods, it is extremely difficult to detect Mtb in alveolar macrophages on tissue sections from resected lung parts, whether by staining for acid-fast Mtb [15–17] or by in situ detection of the transcripts of various Mtb genes [22]. Therefore, histological methods are of little help in assessing infection levels in macrophages in the study organ. Histology of excised lung tissue from TB patients is basically aimed at specifying the diagnosis and the pathomorphological picture of the disease [2, 9, 17, 20].
At present, TB is diagnosed using molecular genetic methods, quite rapid and highly sensitive [20, 23]. However, sole detection of Mtb DNA in the study material does not tell about exact levels of infection by Mtb in the lung macrophages–nor does it tell about the viability or metabolic status of the TB agent. As is known, mycobacterial DNA can persist in human lung tissues for a long time after the pathogen has been destroyed [24, 25].
Because the cells hosting pathogenic Mtb are largely leukocytes, attempts have been made to obtain them from TB patients’ organisms. Ulrichs and the co-workers [9] obtained mononuclear cells from different tissue types in resected lungs using collagenase and Ficoll gradient centrifugation, which allowed these authors to assess only the relative number of macrophages and lymphocytes present in the study piece of lung tissue. Isolation of cells, largely neutrophils, from the bronchoalveolar lavage (BAL) fluid, sputum, and cavity caseum of patients with pulmonary TB allowed the number of cells infected with Mtb to be assessed [26]. In another study [27], Fu and the co-workers did not obtain viable cells from sputa, but caseous matter, which is typical of the sputum coughed out by TB patients; nor could they estimate–not even roughly–the Mtb infection levels in the host cells. Thus, even though the control of the population of alveolar macrophages infected with Mtb in the TB patients’ lungs is important, the issue remains poorly explored. Therefore, to assess the efficiency of the therapy being given, to see when a personalized revision of treatment regimens is needed and to be able to make predictions as to how TB infection will proceed in post-operative TB patients, new techniques are required that enable rapid detection and analysis of cells with Mtb in TB patients [28].
We started our search for ways to analyze Mtb infection levels in macrophages in patients with pulmonary TB by using a mouse model of latent TB infection and ex vivo monolayer cultures of cells that had migrated from individual granulomas obtained from the spleens, lungs, and bone marrow of mice infected with the Bacillus Calmette-Guérin (BCG) vaccine [11, 29]. The BALB/c mice used had, at all times of latent TB infection, differences in the number of granulomas with BCG-containing cells as well as in the number of cells containing BCG mycobacteria in the granulomas. Not a single mouse granuloma–not even the smallest–was found to have all macrophages infected [29]. Granuloma macrophages and dendritic cells were for the first time observed to contain colonies of BCG mycobacteria with cording morphology, which correlates with the virulence of the mycobacteria [29]. A comparison made between BCG mycobacteria in ex vivo cultures of mouse granuloma macrophages and in macrophages infected with the BCG vaccine in vitro in the cell cultures of bone marrow and peritoneal exudate from intact mice revealed that host cells and mycobacteria themselves have different, even opposing, responses to infection in different model systems [30].
Based on our previous work on the isolation of cells from mouse granulomatous inflammatory structures and proceeding with these cells in ex vivo culture, we have developed a technique to isolate alveolar macrophages from the resected lung tissues of patients with pulmonary TB for rapid quantification of Mtb infection levels in macrophages [31]. The results, obtained using the proposed technique, have decision-making potential when questions arise regarding the application and revision of prophylactic and medical treatment measures against pulmonary TB in humans.
Materials and methods
Study design
A technique is reported for obtaining alveolar macrophages from lung parts resected during elective surgery from adults with pulmonary TB with the aim of a rapid assessment of the level of infection with M. tuberculosis in the cells from post-operative patients. Data on the number of alveolar macrophages inferred in two days using the proposed technique were compared to the results of prolonged bacteriological and histological analyses used in routine clinical practice for examination of lung tissue resected from TB in-patients. The participants were selected on a random basis during several months. All the patients’ characteristics, procedures and experiments are presented and discussed in tables, figures, and legends to them.
Patients and ethics statement
Lung tissue specimens were obtained from 21 patients with pulmonary TB at the Department of Thoracic Surgery of the National Medical Research Center of Tuberculosis and Infectious Diseases (Yekaterinburg, Russia) over the period from August 2014 to May 2015. Standard preoperative procedures, including diagnosis of mycobacteria in sputum, chest radiography, and Computed Tomography were performed for all patients (S1 and S2 Tables). All patients included in the present study had been referred for the surgical management of treatment-refractory pulmonary TB. Destroyed regions were removed within the anatomical borders of lung parts from all patients with clinically active TB. All patients were residents of the Ural province of Russia and had received TB treatment supervised by their local clinics. Preoperative M. tuberculosis definition from the diagnostic sputa and chest radiographs were performed on all patients (S1 Table). All procedures involving patients were fully reviewed and approved by the Ethics Committee of the National Medical Research Center of Tuberculosis and Infectious Diseases (27/2014/07/02) and conducted in accordance with the principles expressed in the Helsinki Declaration. Written informed consent was obtained from each patient in this study.
Lung tissue processing
Immediately after surgery, removed lung tissue from each patient was transferred to a biological safety level 3 facility for pathological examination and dissection. In brief, TB lesions were identified macroscopically. For DNA analysis, ~0.5 g of caseous matter from a necrotizing TB lesion was homogenized using a sterile pestle in 1.5-ml tubes with inactivating reagent A (Syntol, Russia) and incubated for 30 min. ~2 g of lung tissue with TB lesions was homogenized and cultured on Lowenstein-Jensen (LJ) medium (Himedia Laboratories, India). Pieces of lung tissues (~10–30 g) obtained from lung parts distant from macroscopic TB lesions and cavities were collected for producing alveolar macrophages for each patient (n = 20), but only the cavity wall of the resected lung was collected for patient 6. The remainders of the lungs were immersed in 10% paraformaldehyde in phosphate-buffered saline (PBS, pH 7.4) for histological analysis of TB lesions.
Isolation and ex vivo culture of alveolar macrophages
Alveolar macrophages were obtained from the specimens of surgically resected lung tissue as described in [31, 32]. In brief, lung tissue specimens were cut into small pieces and incubated in PBS. For separating cell suspension containing alveolar macrophages from closed granulomatous fibrotic tissue, the lung pieces were further rubbed through the metal screen of a sieve with pores 0.5–2.0 mm diameter in PBS. Cells were isolated from the organ homogenate by centrifugation at 400 g for 5 min at room temperature and washed once in PBS. Cell pellets in a complete growth RPMI 1640 medium containing 10% fetal bovine serum, 2 mM glutamine and 50 μg/ml gentamicin (BioloT, St. Petersburg, Russia) were placed to 24-well plates (Orange Scientific, Belgium) with cover glasses in the bottom and cultured in 0.5 ml complete growth medium for 16–18 hours at +37 °C in an atmosphere containing 5% CO2.
Cell staining
At hours 16–18 of ex vivo culture after removal of growth medium with dead cell debris, monolayer cultures of cells on cover slips were washed twice with PBS for removal of nonadherent cells and then fixed with 4% formaldehyde solution in PBS for 10 min at room temperature. After washing with PBS, the viability of cells on the cover slips was, as checked by trypan blue staining, 100%. To visualize acid-fast mycobacteria within host cells, some preparations were washed with PBS and stained by the Ziehl-Neelsen (ZN) method. The cells after ZN staining were further counterstained with Mayer's hematoxylin.
In the experiments using Nile red (Invitrogen, USA, N1142), the cell preparations were incubated with 10 μM of the dye for 15 min at +37 °C in 5% CO2 before fixation. The cell preparations were fixed as described above, washed with PBS, blocked in PBS solution containing 2% BSA, and finally incubated first with rabbit polyclonal primary antibodies to Mycobacteria LAM (Abcam, England, ab20832) diluted 1:200, then with Alexa 488-conjugated goat anti-rabbit IgG secondary antibodies (Thermo Fisher Scientific, USA, A11034) diluted 1:400. Some of the fixed cell preparations were washed with PBS, treated within 2 min in 0.3% Triton-X100 solution, blocked in PBS solution containing 2% BSA, and incubated with rabbit monoclonal primary antibodies to human CD14 (Spring Bioscience, USA, M492) and mouse monoclonal primary antibodies to M. tuberculosis 38kDa (Abcam, England, ab183165) diluted 1:100 and 1:1000, respectively. Fluorescent visualization of CD14 and mycobacterial 38-kDa antigen was enabled using secondary goat polyclonal Alexa 488-conjugated secondary antibodies to rabbit IgG (Thermo Fisher Scientific, USA, A11034) and Alexa 555-conjugated secondary antibodies to mouse IgG (Invitrogen, USA, A21422) diluted 1:400 and 1:500, respectively. The cell preparations were incubated with the appropriate antibodies for 60 min at room temperature. Fluorescent staining was analyzed using the VECTASHIELD® Mounting Medium with DAPI (4´,6-diamidino-2-phenylindole) (Vector Laboratories, USA, H-1200).
Histology
The parts of the lungs from each patient were fixed in 10% paraformaldehyde in PBS overnight and embedded in paraffin using an STP 120–3 spinning tissue processor (Zeiss), sectioned at a 4-μm thickness and stained with hematoxylin and eosin or a mixture of picric acid and fuchsin acid to detect collagen and other types of connective tissue or by the ZN method to visualize acid-fast mycobacteria.
Microscopy
The cytological and histological preparations were examined at the Shared Center for Microscopic Analysis of Biological Objects of the Institute of Cytology and Genetics, SB RAS, using an Axioscop 2 plus microscope (Zeiss) and objectives with various magnifications (Zeiss), and photographed using an AxioCam HRc camera (Zeiss); the images were analyzed using the AxioVision 4.7 microscopy software (Zeiss). Cell preparations stained with fluorescent dyes were examined under an LSM 510 or an LSM 780 laser scanning confocal microscope (Zeiss) using the LSM Image Browser and ZEN 2010 software (Zeiss). All cells were counted separately on each cover slip for each patient in each test.
PCR analysis
After incubation with inactivating reagent A (Syntol, Russia), samples were centrifuged for 5 min at 13,000 rpm. The precipitate was used for DNA extraction with M-Sorb-Tub reagent kits (Syntol, Russia). The DNA obtained was used for PCR with AmpliTub-RV reagent kits (Syntol, Russia). This kit detects specific DNA fragments of the IS6110 gene, which is present in multiple copies in most M. tuberculosis complex strains, and determines the amount of regX, a specific DNA fragment, which is represented by a single copy in the M. tuberculosis complex genome. PCR was performed using a CFX96 analyzer (Bio-Rad, USA).
Bacteriology
Before surgery, the smears of sputum were collected from each patient and stained by the ZN method and Fluorescent Stains Kit for Mycobacteria with auramine and rhodamine (Himedia Laboratories, India). After surgery, decontamination of lung tissue specimens was performed by the standard NaOH-NALC method [33]. After decontamination and homogenization, each specimen was inoculated on LJ medium (Himedia Laboratories, India) for isolation of Mtb. The time to positivity of the LJ medium was 1–2 months. All grown Mtb cultures were identified and confirmed as being in the M. tuberculosis complex using standard procedures.
Statistics
Statistical data processing was performed using MS Excel 2007 (Microsoft). Differences were tested using Student’s t-test and considered statistically significant at P < 0.05.
Results
Isolation of cells from the resected lungs of TB patients
Cells were isolated from small specimens of lung tissue resected during elective surgery from 21 patients with pulmonary TB (Fig 1A and 1B, S1A, S1B, S1E, S1F, S1I, S1J, S1M and S1N Fig). The patients referred to surgery are characterized in S1 Table. Patients, from whom affected lung sections were resected, had different clinical symptoms of TB before surgery; however, according to X-ray, all these individuals had fibrotic and caseotic TB lesions in their lungs. Most of the patients (n = 16) were diagnosed with MDR TB, and patient 6, with XDR TB. Five patients (6÷10) had cavities in their lungs. Based on clinical manifestations, chest radiography, and Computed Tomography (in S2 Table), all the patients were divided into three severity groups with different extents of TB disease: “advanced” (patients 6, 7, and 10), “moderate” (patients 5, 8, 15, 16, and 20), and “minimal” (the other 13 patients). Sputum smears were examined from all the patients before surgery; however, only material from those with cavities was found to contain acid-fast mycobacteria (except for patient 9).
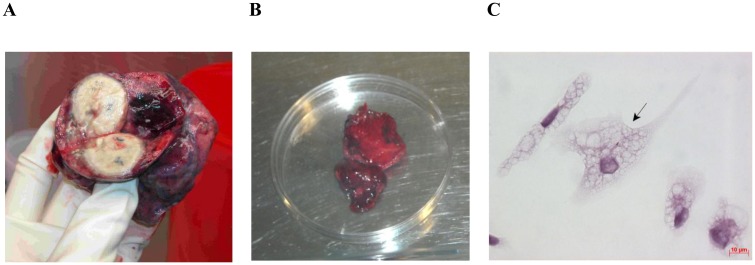
Fig 1. Lung tissue used for ex vivo expansion of alveolar macrophages.
(A) The upper lobe of the left lung surgically removed from patient 8. (B) The tissue specimen of the part of the resected lung (A) distant from macroscopic lesions. The diameter of the Petri dish is 10 cm. (C) Alveolar macrophages produced from the lung tissue specimen (B) and stained by the ZN method after ex vivo culture for 18 hours. The black arrow points to an alveolar macrophage with acid-fast Mtb. The scale bar is 10 μm.
In all the patients, except for patient 6, lung tissue specimens for cell isolation were obtained from sites distal to cavities or other macroscopic TB foci (Fig 1A and 1B, S1E, S1F, S1I, S1J, S1M and S1N Fig). Cells from patient 6 were obtained only from the cavity wall (S1A and S1B Fig). All the lung specimens used for cell isolation had large amounts of fibrotic tissue with small enclosed TB granulomas in it (Fig 1B, S1B, S1F, S1J and S1N Fig). This granulomatous fibrotic tissue with caseous TB lesions was separated from the cell suspension by homogenization of lung tissues and by washing off cells for ex vivo culture (S1C, S1G, S1K and S1O Fig) and then discarded. The pellets obtained by centrifugation of the cell suspension and containing, according to microscopic data, a large number of erythrocytes and small cells with light cytoplasm (probably, lymphocytes) and a much lesser number of larger light cells (probably, macrophages) were seeded to 24-well plates with cover glasses in the bottom separately for each patient.
Thus, as we worked with tissue samples from the resected lungs of TB patients, we separated granulomatous fibrotic tissue with caseous TB lesions from the cells of the surrounding tissue and seeded those cells in ex vivo culture.
Cell composition of ex vivo cultures from TB patients’ lungs
The cell composition of monolayer cultures on cover glasses inferred for each TB patient after ex vivo culture for 16–18 hours and removal of nonadherent cells is presented in S3 Table. The ex vivo cultures of cells obtained from the cavity wall and distant parts of resected lung tissue were largely composed of alveolar macrophages (90–99%), with or without Mtb. All alveolar macrophages had lightly staining vacuolar cytoplasm with various numbers of dense inclusions, which is typical of alveolar macrophages [34], and a large number of cell membrane protrusions (Figs 1C and 2A–2C, S1D, S1H, S1K and S1P Fig). It is noteworthy that no phagosomes with engulfed lymphocytes, erythrocytes, and platelets were observed in alveolar macrophages (Figs 1C and 2A–2C, S1D, S1H, S1K and S1P Fig). Some of the alveolar macrophages obtained from most patients (n = 18) had a large number of denser dark inclusions in the cytoplasm and were what is called smokers’ macrophages (S1H Fig). The presence of smokers’ macrophages in the lung tissue was confirmed by histology of the resected lungs of these patients. CD14, the receptor for lipopolysaccharide in bacterial cell walls and the macrophage/monocyte-specific leukocyte marker, occurred at an increased density in all alveolar macrophages, whether with or without Mtb (Fig 3A–3C).
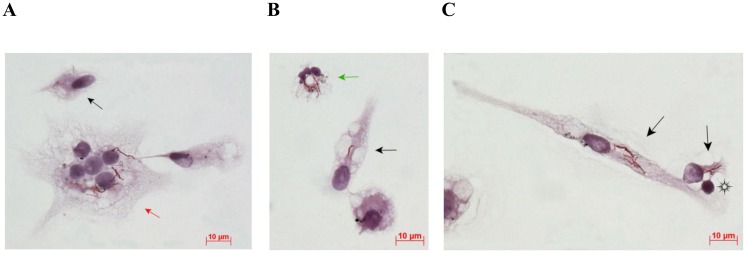
Fig 2. Different cell types obtained from the cavity wall in the resected lung of patient 6 and stained by the ZN method after ex vivo culture for 16 hours.
(A, B) A Langhans giant cell and a neutrophil containing acid-fast Mtb are indicated by the red and green arrows, respectively. (C) A lymphocyte interplaying with alveolar macrophages is indicated by the black snowflake. The other cells (A, B, C) are alveolar macrophages. Those with acid-fast Mtb are indicated by the black arrows, others are uninfected. The scale bars are 10 μm each.
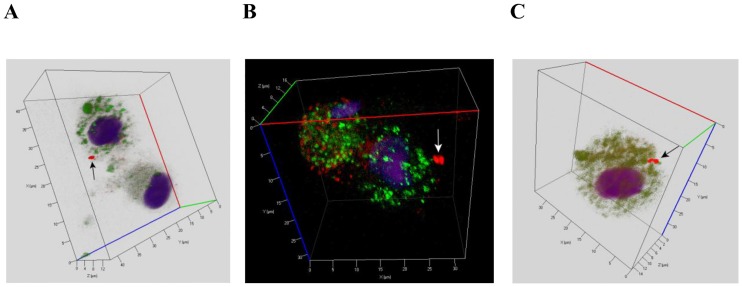
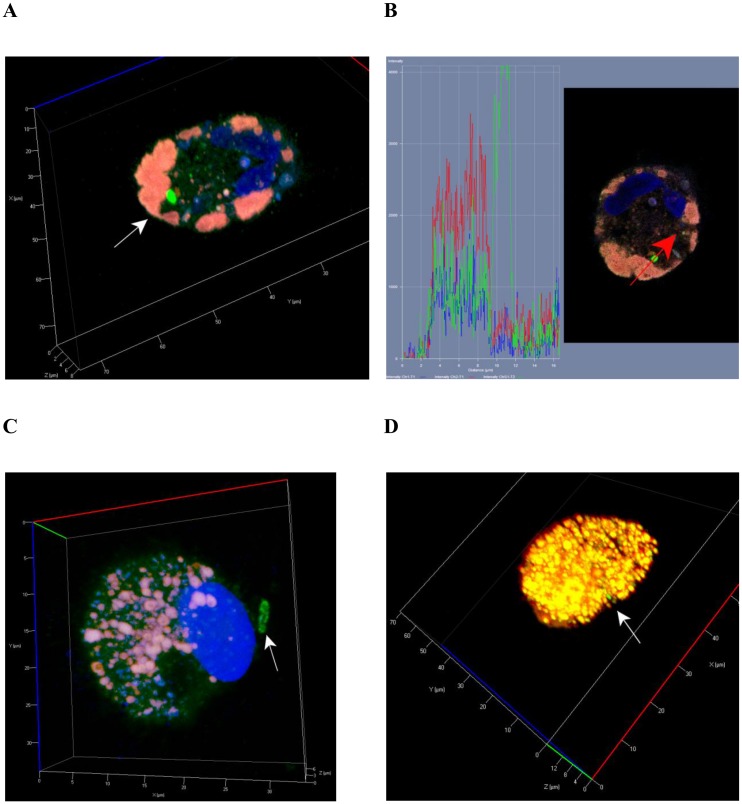
Fig 3. CD14 staining of alveolar macrophages with Mtb performed after ex vivo culture for 18 hours.
(A, B, C) Representative confocal fluorescent 3D images show alveolar macrophages stained by human CD14-specific (green signal) and Mtb Ag38-specific antibodies (red signal). Nuclei are stained by DAPI (blue signal). Alveolar macrophages were obtained from the resected lungs of patients 16 (A, B) and 18 (C). Black or white arrows indicate a single Mtb (A) and mycobacterial colonies (B, C) residing within alveolar macrophages.
In addition to alveolar macrophages, five more cell types were observed: dendritic cells, lymphocytes, fibroblasts, neutrophils, and multinucleate Langhans giant cells. The population sizes of other cell types were much smaller. Lymphocytes were largely located on alveolar macrophages and interacted with them (Fig 2C). Dendritic cells were mostly smaller than macrophages. These cells had an increased number of tiny cell membrane protrusions, and their nuclei and cytoplasm were stained by histochemical dyes very densely. A higher number of polymorphonuclear neutrophils was observed both in the ex vivo cell cultures (about 22% of cells) and in the histological sections of resected lung tissues from only patient 10, which is known to be a predictor of a poor prognosis in TB in humans [35, 36]. Multinucleate Langhans giant cells were found in the ex vivo cultures of cells from a large number of patients (Fig 2A, S2A–S2D Fig). Cells with more than five nuclei in each were attributed to this cell type. Langhans giant cells containing both 5–7 and up to 20–25 nuclei were observed (S2A–S2D Fig). These cells also differed in the number of tobacco smoke particles they contained (S2B and S2D Fig).
The absence of a significant number of fibroblasts in the ex vivo cultures of cells from all the patients examined suggests that the process of cell isolation did not compromise the integrity of fibrotic tissue containing a large number of necrotizing TB granulomas in the lung specimens used for ex vivo expansion of alveolar macrophages (Figs 1B, 4A and 4G, S1A–S1C, S1E–S1G, S1I–S1K and S1M–S1O Fig) or on the histological sections of the lung tissues (Fig 4B–4E). Therefore, neither fibroblasts nor alveolar macrophages can have come from the cells that surround the central part with caseous matter and reside in enclosed necrotizing TB lesions (Fig 4B, 4C and 4E). We propose that alveolar macrophages and other cell types may have come from early, non-fibrotic non-necrotizing TB granulomas, because the ex vivo cultures contained multinucleate Langhans giant cells, which are also the markers of early granulomatous inflammatory lesions without the fibrotic capsule and caseous matter in the lungs of TB patients (Fig 4D).
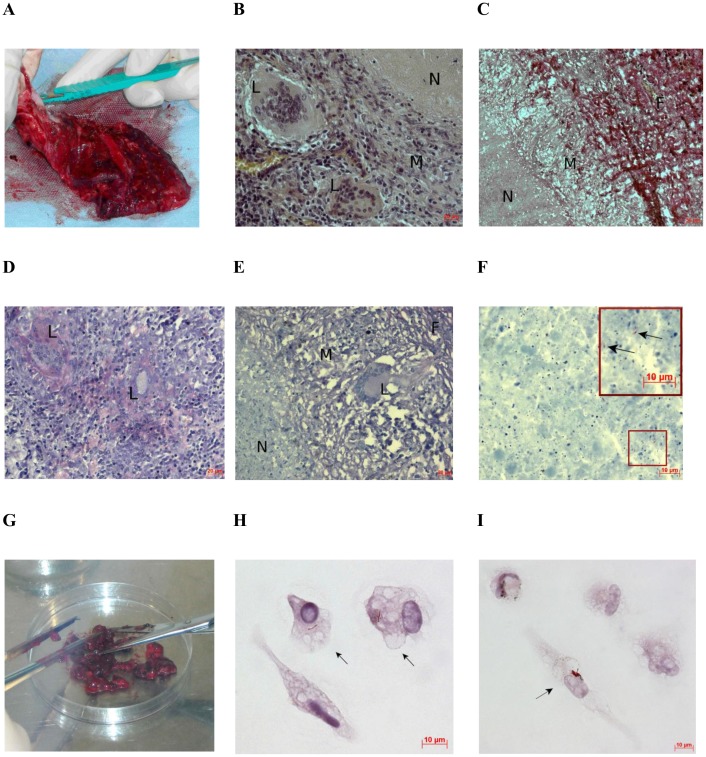
Fig 4. Histological examination of the resected lung from patient 7 shows the absence of cells with Mtb. Ex vivo analysis of alveolar macrophages disagrees.
(A) A surgically removed part of the lung. (B-F) Multiple TB lesions detected by histological analysis of tissue from the resected lung (A): N, caseous necrosis; M, a ring of macrophages; F, fibrous capsule; L, multinucleate Langhans giant cell. The scale bars are (B, D, E) 20 μm each, (C) 50 μm, and (F) 10 μm. (B, C, and E, F) Representative histological images show enclosed necrotizing granulomas stained by hematoxylin/eosin, a mixture of picric acid and fuchsin acid to detect collagen, and after the ZN method, respectively. (D) Multinucleate Langhans giant cells determined in an early non-necrotizing granuloma by ZN staining. (F) Scanty Mtb revealed in the caseous matter. Enlarged view of the part of this image with Mtb indicated by black arrows is shown in the upper right corner. (G) The tissue specimen of the resected lung (A) was cut into small pieces for producing alveolar macrophages. The diameter of the Petri dish is 10 cm. (H, I) Alveolar macrophages stained by the ZN method after ex vivo culture for 18 hours contain Mtb in isolation and as a colony, respectively. The black arrows point to alveolar macrophages with acid-fast Mtb. The scale bars are 10 μm each.
Lipid-rich foamy alveolar macrophages are also important markers of TB inflammation in human lungs [14, 37, 38]. Alveolar macrophages in the ex vivo cell cultures were considered as foamy when more than one-third of the cell surface was stained by Nile red. The ex vivo cultures of cells obtained from each TB patient had different numbers of Nile red-positive alveolar macrophages, with or without Mtb (Fig 5A–5D, S4 Table). The largest lipid-laden vacuoles were detected in foamy alveolar macrophages from the resected lungs of patients 1, 3, 4 (Fig 5A and 5B), and 21.
Fig 5. Foamy alveolar macrophages with Mtb determined in ex vivo cultures.
(A, C, D) Representative confocal fluorescent 3D images show lipid-rich foamy alveolar macrophages stained by Mtb LAM-specific antibodies (green signal) and Nile red (red signal). Nuclei are stained by DAPI (blue signal). Foamy alveolar macrophages obtained from the resected lungs of patients 4 (A, B), 9 (C), and 15 (C). White arrows point to Mtb residing in foamy alveolar macrophages. (B) In the cell profile (A), a foamy alveolar macrophage demonstrates lack of colocalization of Mtb and host lipid bodies (lack of yellow signal). However, the markers studied show colocalization for numerous intracellular lipid-laden vesicles (A, B, C, D).
The ex vivo cultures of cells obtained from the resected lungs of all patients were largely composed of alveolar macrophages; however, the number of these cells was varying even though the lung tissue samples were similar in size (Figs 1B and 4G, S1B, S1F, S1J and S1N Fig, S4 Table). Based on histological assessment of the inflammatory response in the lungs to Mtb infection coupled with analysis of innate immune response cells, the patients were divided into three groups as in S4 Table. The “high-activation” group included three patients (6, 7, and 10) with multiple morphological signs of the TB process and an unfavorable course of disease at that stage of treatment. The lungs of patients 1, 3, 11, 12, 17, 19, and 21 were found to have morphological signs of a weak TB inflammatory process. Thus these patients were included in the “low-activation” group. The rest of the patients (n = 11) were attributed to the “middle-activation” group, because their lungs had morphological signs of TB activity with a risk of progression, should the conditions be unfavorable. The presence of tobacco smokers’ macrophages in the alveolar lumens of the respiratory tissue of the resected lungs had no effect on the form or characteristics of TB lesions, nor had it any on the activation status of TB inflammation in these patients. Note that, in ex vivo culture, an increased number of alveolar macrophages (over 200 thousand) was isolated from distant parts of tissue surgically removed from patients both with high/middle (patients 7, 8 and 14) and low (patient 19) activation status, no matter whether they were tobacco smokers or not (patients 7 and 8 were).
Thus, in ex vivo culture, CD14-positive alveolar macrophages, foamy or not, as well as with or without tobacco smoke particles, were largely obtained from different (cavity walls or distant) tissue samples from the resected lungs of TB patients with different clinical and X-ray findings, extents of TB disease and activation status of TB inflammation.
Alveolar macrophages with Mtb in the resected lungs of TB patients
By histochemical staining after the ZN method, which detects acid-fast mycobacteria (which by definition have undamaged cell walls), and by immunofluorescent staining with antibodies reacting with the major mycobacterial cell wall component glycolipid lipoarabinomannan (LAM) or with Ag38 (a glycolipoprotein involved in the transport of inorganic phosphate by the ABC transporter of the Mtb cell wall and encoded by the pstS-1 gene [39]), we determined cells with Mtb in ex vivo cultures of cells obtained from the lung tissue samples of all investigated TB patients. Note that staining human cells after the ZN method allowed Mtb to be differentiated immediately both in isolation and in colonies of various morphology. Immunofluorescent staining for Mtb, especially those in small colonies, is often required to create confocal 3D imaging of human cells by generating a large number of Z-stacks for an accurate estimate of the number of Mtb in these colonies.
Mtb were largely found in alveolar macrophages (Figs 1C, 2A–2C, 3A–3C, 4H, 4I and 5A–5D, S1D, S1H, S1L and S1P Fig) and rarely in dendritic cells, neutrophils (Fig 2B), and Langhans giant cells (Fig 2A, S2C Fig). No Mtb was found in lymphocytes or fibroblasts. Alveolar macrophages contained individual Mtb (Figs 1C, 3A, 4H and 5A–5D, S1P Fig) or Mtb in colonies (Figs 2A–2C, 3B, 3C, 4I, 6C and 6F, S1D, S1H and S1L Fig), including those with cording morphology (Figs 2C, 4I, 6C and 6F, S1D Fig), when replicating Mtb line up along their longitudinal axes, setting themselves into “braids”. Some Mtb colocalized with lipid bodies in foamy alveolar macrophages, some did not (Fig 5A–5C). At the same time, Mtb in colonies were detected in alveolar macrophages devoid of lipid bodies. Note that the use of the different staining techniques (ZN and LAM/Ag38 immunofluorescent staining) produced similar numbers of alveolar macrophages with Mtb in all cell preparations obtained from the resected lung of each TB patient. Very few individual Mtb or Mtb in colonies were observed outside human cells in the ex vivo cell cultures. Our observation of the morphology of alveolar macrophages and other cells with or without Mtb in all ex vivo cultures suggests that none of those cells was apoptotic or necrotic (Figs 1C, 2A–2C, 3A–3C, 4H, 4I, 5A–5D, 6C and 6F, S1D, S1H, S1L, S1P and S2A–S2D Figs).
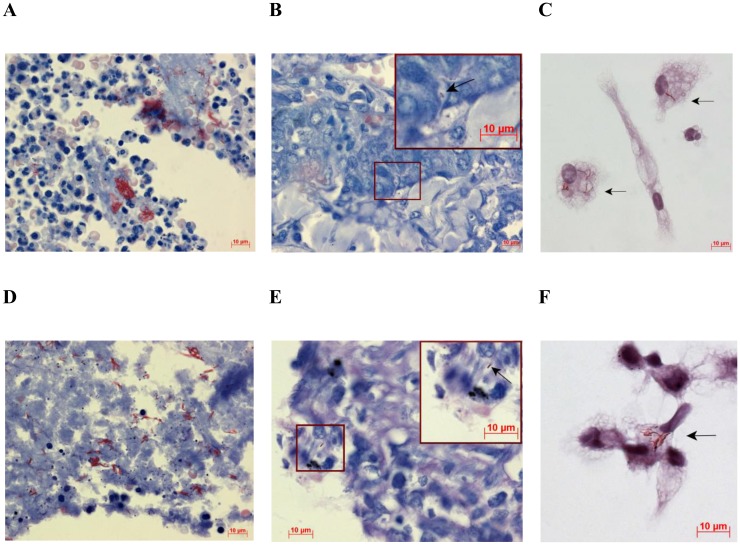
Fig 6. Alveolar macrophages with Mtb in colonies are detected both in cavity walls and in the distant parts of lung tissue.
(A-C and D-F) Lung tissues and alveolar macrophages obtained from patients 6 and 8, respectively. (A, B, D, E) Representative histological images of the cavity walls (A, D) and the distant parts of lung tissue (B, E) stained by the ZN method demonstrate a massive load of replicating Mtb in the cavities and single Mtb within alveolar macrophages in other lung regions, respectively. (B, E) Enlarged views of the parts of these images with alveolar macrophages containing single Mtb (black arrows) are shown in the upper right corners. (C) Alveolar macrophages obtained from the cavity wall and after ex vivo culture for 16 hours contain colonies of replicating Mtb. (F) Alveolar macrophages obtained from the distant part of lung tissue and after ex vivo culture for 18 hours contain replicating Mtb in colonies with cording morphology. Alveolar macrophages (C, F) were stained by the ZN method. The black arrows point to alveolar macrophages with acid-fast Mtb. The scale bars are 10 μm each.
Results of bacteriological and histopathological examination of lung tissue specimens and those of analysis of alveolar macrophages in ex vivo cultures are shown, for comparison, in S4 Table. The patients strongly differed in the number of alveolar macrophages with Mtb in the ex vivo cultures. The largest number of infected alveolar macrophages (up to 38% of the cells examined) was found in the ex vivo culture of cells from the cavity wall of patient 6 (Figs 2A–2C and 6C, S1A–S1D Fig). These data are in a good agreement with histological data, which revealed the presence of a large number of infected cells in the cavity wall from this patient’s lung (Fig 6A). Note that Eum and the co-authors [26] reported that the macrophages with Mtb from BAL and cavity caseum in Korean TB patients, that is, from lung sites close to cavity walls, made up, on average, about 28% and 24%, respectively. However, most of our TB patients, whose cells were isolated from sites distant from macroscopic lesions, had much fewer infected alveolar macrophages. Their number ranged from 0.11% (patient 12) to 6.54% (patient 9). The presence of mycobacterial IS6110 insertions was revealed by PCR in the resected lung specimens from all the patients. However, in many patients, it was not so easy to detect infected alveolar macrophages on the histological sections of the distant parts of lung tissue. In patient 8, a large number of cells with Mtb, including those in colonies with cording morphology, was observed only in the cavity wall (Fig 6D). The tissues distal to this cavity wall were found to have only few macrophages with individual Mtb on the histological sections (Fig 6E). However, the ex vivo culture of cells from patient 8 was found to have about 2% of alveolar macrophages infected with Mtb, which amounts to about 5 thousand infected cells produced from a relatively small lung tissue specimen (Fig 1B). These alveolar macrophages had either individual Mtb (Fig 1C) or Mtb in colonies, some with cording morphology (Fig 6F). The histological sections of lung tissue from patient 7 were not found to have cells with Mtb (Fig 4D and 4E), while the ex vivo culture was found to have about 7 thousand alveolar macrophages with Mtb, including those replicating in colonies (Fig 4H and 4I), which amounts to ~3% of all the macrophages obtained from this relatively small lung tissue specimen (Fig 4G). The histological sections of the resected lung tissue from this patient were found to have individual non-replicating acid-fast Mtb only in the caseous center of fibrotic necrotizing TB granulomas (Fig 4E and 4F), which is typical of enclosed caseous hypoxic lesions in humans [8, 13, 40]. Note that the smears of tissue homogenates of the resected lungs of patients 7 and 8 were found to have Mtb following culture on LJ medium. However, Mtb was found to grow on LJ medium only for patient 8, even though the smear of patient 7 contained more mycobacteria than that of patient 8. Similarly, the smear of patient 9 contained more Mtb than did that of patient 8. However, Mtb failed to grow on LJ medium not only for patient 9, but also for many more others, with or without Mtb in the smears of tissue homogenates. In summary, Mtb grew on LJ medium only for the lung tissue homogenates of patients 6, 8, 10, 20, with Mtb in their smears of tissue homogenates, and patient 11 without Mtb in his smear of tissue homogenate. Noteworthy, a very low number of infected alveolar macrophages was observed in the ex vivo cultures for patients 11 and 20, while no alveolar macrophages with Mtb were found on the histological sections of the distant parts of lung tissue from these and many other patients.
Thus, it can be stated that alveolar macrophages with Mtb, including those replicating in colonies, were detected only in ex vivo culture, but not by bacteriological or histopathological analyses. With our technique, the number of infected cells in the resected lungs of each patient with pulmonary TB was assessed as rapidly as in two days after surgery.
Differences in infection parameters between the groups of TB patients
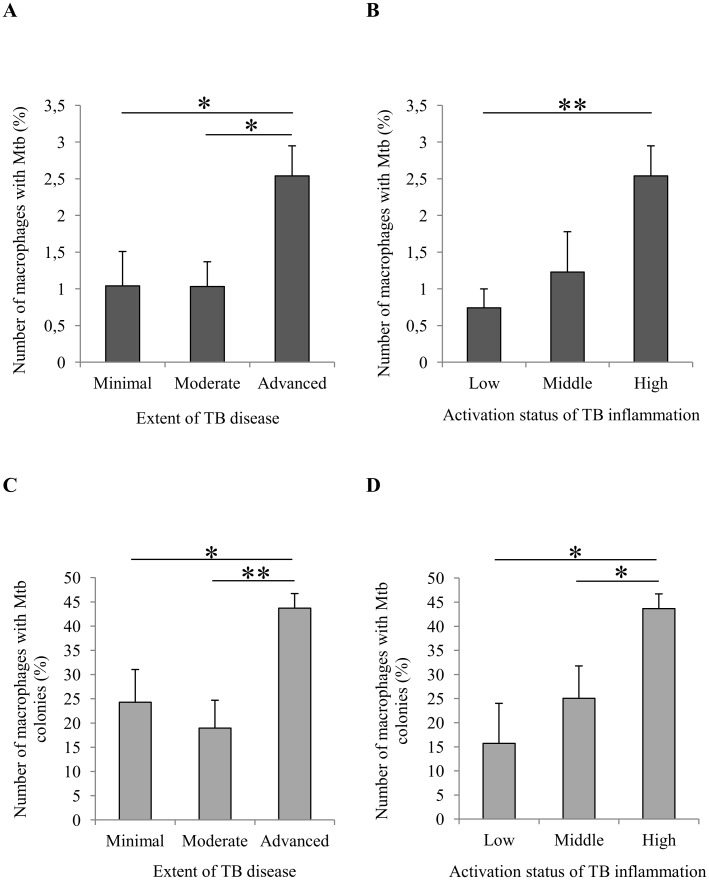
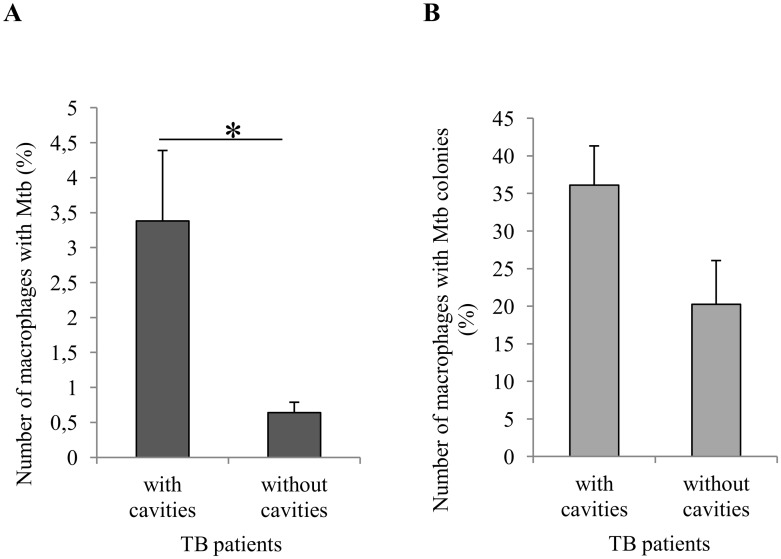
It was found that, after the surgery of patients with pulmonary TB, the number of alveolar macrophages containing either whatever Mtb (individual or as colonies) or replicating Mtb in colonies had changed in the ex vivo cultures of lung tissue from distant parts (S4 Table). A comparison of infection parameters in TB patients with different extents of TB disease as in S1 Table and different activation status of TB inflammation as in S3 Table demonstrated that a considerable increase in the number of alveolar macrophages with Mtb (individual or as colonies) and with replicating microbes occurred only in patients 7 and 10 (“advanced” and “high-activation” groups), who had cavities (Fig 7A–7D). No statistically significant changes in these parameters were found between the “minimal” (n = 13) and “moderate” (n = 5) groups of patients or between the “low-activation” (n = 7) and “middle-activation” (n = 11) groups of patients (Fig 7A–7D). A substantially higher number of infected alveolar macrophages was in the patients with than without cavities, n = 4 and n = 16, respectively (Fig 8A). Just to remind, patients 8 and 9, who had cavities, were "moderate” and “minimal”, respectively, and “middle-activation” both. The difference in the number of alveolar macrophages with replicating Mtb between these groups of patients was not statistically significant (Fig 8B). Smokers with pulmonary TB had a higher number of alveolar macrophages with Mtb as compared with nonsmoking TB patients, n = 17 and n = 3, respectively (S3A Fig). Note that all the patients with cavities were tobacco smokers. At the same time, the ex vivo culture of cells from the resected lung of patient 13 with chronic obstructive pulmonary disease was found to contain only few infected cells from among more than 100 thousand smokers’ macrophages examined in it (S3 Table). No statistically significant difference in the number of alveolar macrophages with replicating Mtb was found between smokers and nonsmokers (S3B Fig). Also, there were no statistically significant differences in the number of foamy alveolar macrophages between all the groups of TB patients (S4A–S4D Fig), including the groups with a different number of Mtb-infected alveolar macrophages in the resected lungs, namely, less or more than 1% out the total number of macrophages examined, n = 13 and n = 7, respectively (S4E Fig).
Fig 7. TB patients with the “advanced” extent of disease and “high-activation" status of TB inflammation have a larger population of alveolar macrophages with Mtb and mycobacterial colonies in the distant parts of lung tissue.
(A, B) The number of alveolar macrophages with Mtb (in isolation or as colonies) expressed as the percentage of the total number of alveolar macrophages analyzed. (C, D) The number of alveolar macrophages with Mtb in colonies expressed as the percentage of the total number of alveolar macrophages with any Mtb. Data are expressed as the means ± SEM. *P < 0.05; **P < 0.01, Student’s t-test.
Fig 8. A significant increase in the number of alveolar macrophages with Mtb in the distant parts of lung tissue in patients with cavities.
(A) The number of alveolar macrophages with Mtb (in isolation or as colonies) expressed as the percentage of the total number of alveolar macrophages analyzed. (B) The number of alveolar macrophages with Mtb in colonies expressed as the percentage of the total number of alveolar macrophages with any Mtb. Data are expressed as the means ± SEM. *P < 0.05, Student’s t-test.
Thus, the highest number of infected alveolar macrophages in ex vivo culture was in the cavity wall and in the tissue samples obtained from the distant parts of the surgically removed lung tissue of TB patients with cavities, no matter how different the infection process in these patients was.
Discussion
M. tuberculosis is spread by airborne transmission and largely invades alveolar macrophages in the lungs [2–6]. The capacity of Mtb to evade the immune response of the host organism and drug treatment makes this bacterium a very successful and widespread human pathogen causing an infectious disease, which is so difficult to cure [1–3]. Today, as TB remains to be a leading global health issue, new technologies are required for analysis of relationships between the pathogen and host cells in TB patients’ lungs for assessing the efficiency of anti-TB chemotherapy, for considering a personalized revision of treatment regimens and for making predictions as to how the tuberculosis infection will proceed in each TB patient [28].
Here, we propose a technique to develop ex vivo cultures of cells from different parts of lung tissues surgically removed from patients with pulmonary TB. This technique allowed us to determine rapidly (in two days after surgery) the level of infection with Mtb in the cells of the resected lungs and, by the presence or absence of Mtb colonies, the functional status of the TB agent at the time of surgery. The ex vivo cultures of cells from cavity wall and distant parts of the resected lungs mostly contained alveolar macrophages, while those of cells from the BAL fluid, sputum and cavity caseum in Korean TB patients, neutrophils [26]. The observed differences in cell composition between these cultures might be both due to some individual features of TB patients and probably because neutrophils are easier to wash away from the lung tissues with the methods the authors used. As a result, although neutrophils and dendritic cells were also present in the ex vivo cell cultures, the main host cells for Mtb in the studied TB patients were CD14-positive alveolar macrophages. Our data are consistent with the opinion [2–6] that the main modulators of TB infection in affected humans are alveolar macrophages, which are long-living cells with a minimum half-life of 4 months [41], rather than the broadly discussed neutrophils [34, 35, 42] or dendritic cells [43, 44].
Interestingly, we did not observe phagosomes with engulfed lymphocytes, erythrocytes, or platelets in alveolar macrophages or other cells in the ex vivo cell cultures. The result is surprising, because the pellets obtained by centrifugation of the lung homogenates and used for ex vivo expansion of alveolar macrophages contained a large number of blood cells too. In our studies of granulomas of mice with latent TB infection we detected many phagosomes with engulfed lymphocytes and platelets at various stages of degradation in mouse granuloma macrophages in the ex vivo culture [29, 30]. Therefore, it is necessary to proceed with studying capabilities for phagocytosis in alveolar macrophages obtained from the resected lung tissues of TB patients.
We have found that the number of infected alveolar macrophages strongly varied across TB patients. Interestingly, an increased number of Mtb-infected alveolar macrophages in the distant parts of the resected lungs correlated, first of all, with the presence of cavities, the walls of which were observed to have a substantial Mtb load both in ex vivo cell cultures and on histological sections. It can therefore be hypothesized that Mtb residing in the cavity walls, where their active replication is promoted by increased access to oxygen, can not only be disseminated outside the organism with sputa coughed out [7, 8, 13, 15], but also reach the lung parts distant from cavities and, probably, other organs, migrating with alveolar macrophages. As was determined using the zebrafish model [45, 46], macrophages can migrate—actively and in a targeted manner—which makes gather at the foci of TB infection and inflammation. Our studies of mouse granuloma macrophages with BCG mycobacteria using an ex vivo model demonstrated that the migratory activity of these cells does not depend on the number of mycobacteria in them [11, 29]. The capacity of alveolar macrophages with Mtb to migrate from cavity walls to distant parts is in part supported by the presence of colonies of Mtb with cording morphology, similar to colonies in the cavity walls in the same patients. Overall, the travel of Mtb-infected cells across TB patients’ lungs requires further research, probably using safe fluorescent labels delivered, say, with aerosols to the cavities before surgery and then testing alveolar macrophages from different parts of the resected lungs for these labels. On the whole, the presence of a large number of neither apoptotic nor necrotic alveolar macrophages with Mtb in the ex vivo cultures of cells from the lungs of TB patients with cavities, no matter to what extent the host cells are infected by Mtb, suggests that these patients require special attention. The use of anti-TB drugs or host-directed therapies that aim to eliminate Mtb in alveolar macrophages residing in cavity walls should prevent Mtb dissemination outside and throughout TB patients’ organisms and thus reduce the risk of extrapulmonary TB.
During isolation of cells from the resected lungs of TB patients, we discarded fibrotic TB foci with caseous matter inside, these foci being the main hallmark of TB inflammation in human lungs after Mtb infection [8, 13]. The absence of extracellular Mtb and fibroblasts in the ex vivo cultures suggests that, however damaging to cells the isolation process may be, the integrity of these TB lesions was not compromised. Thus we failed to obtain extracellular Mtb or alveolar macrophages with Mtb from these fibrotic TB lesions in ex vivo culture. Therefore, fibrotic granulomatous TB lesions are not only reservoirs for Mtb residing within the caseum of necrotic central foci [13, 47], but they also protect the lungs from further Mtb dissemination at a particular stage of infection [4, 48]. Our observation is also supported by a relatively low number of Mtb-infected cells in the ex vivo culture of cells from the resected lungs of most TB patients without cavities. Therefore, the treatment of TB patients as these should be aimed at preventing at preventing encapsulated hypoxic TB lesions with scanty Mtb from evolving into open cavitary lesions heavily loaded with replicating Mtb.
Another hallmark of Mtb infection in the lungs of TB patients is lipid-laden foamy alveolar macrophages, which are thought to promote pathogen survival and replication [14, 37, 38]. However, in our study, the number of foamy alveolar macrophages in the resected lungs strongly varied across TB patients and did not correlate with the particular TB disease characteristics or with the level of infection by Mtb in alveolar macrophages in ex vivo culture. Note that the main conclusions about the effect of lipid-rich macrophages on Mtb infection progression were previously made only by experimenting with in vitro infection of cells with Mtb strain H37Rv and by in vivo studies with animal models [37, 49]. As has been demonstrated (for our contribution, see [30]), in vitro infection of mouse cells and in vivo infection of animals lead to different, even opposing, responses of mycobacteria and macrophages. Also, the hypothesis that foamy alveolar macrophages are directly responsible for the development of cavities in the lungs of TB patients was solely based on the results of histological analysis of some human autopsy specimens and did not consider levels of infection with Mtb in the cells of these lung tissues [50, 51]. Therefore, it is necessary to collect more data about relationships between foamy alveolar macrophages and Mtb in the lungs of TB patients, because adjunctive host-directed therapy proposed for treatment of TB includes the use of statins, which inhibit the biosynthesis of cholesterol in host cells [52, 53].
Thus we found alveolar macrophages with Mtb in the ex vivo cultures of cells from the resected lungs of even those TB patients, whose sputum smears and lung tissue homogenates did not contain acid-fast mycobacteria or reveal growing Mtb colonies on LJ medium. The fact that the lung tissue specimens, which were similar in size, yielded different numbers of alveolar macrophages in ex vivo culture could probably serve as a marker of an inflammatory response to Mtb infection in the lungs of TB patients. However, to confirm this, further research is required. On the whole, we detected a small number of Mtb-infected alveolar macrophages isolated from sites distant from macroscopic lesions in the resected lungs of TB patients. Our data are consistent with the hypothesis that explains the absence of alveolar macrophages with Mtb on the histological sections without cavities by their low number in the lung tissue [54]. The detection of alveolar macrophages with acid-fast Mtb in ex vivo cultures as soon as 16–18 h after isolation of cells from the resected lungs of all TB patients suggests that the technique proposed for assessing the level of infection in alveolar macrophages in the resected lungs of TB patients has higher sensitivity than do bacteriological or pathomorphological methods. The use of our technique demonstrates that Mtb in the alveolar macrophages of TB patients’ lungs remain to be acid-fast, although it was previously believed—because these Mtb were not detected by standard analyses—that, in human lungs, the mycobacterial cell walls undergo substantial changes and Mtb lose acid-fastness [8, 54].
In summary, the observation of a large number of alveolar macrophages with Mtb, including those in colonies, in ex vivo cultures clearly indicates that the efficiency of the anti-TB therapy given some patients (in particular, patients 3 and 6÷10) before surgery was low and that the treatment regimens these patients receive during the post-surgery period should be heavily revised, no matter which TB disease level they have. A small number of alveolar macrophages with non-replicating Mtb detected in the ex vivo cultures of cells from patients 1, 2, 11, 12, 15, 20, and 21 indicates that the anti-TB therapy given them was chosen correctly and applied accurately, and so good outcome should be expected. As far as the other patients, who had been given a relatively efficient anti-TB therapy before surgery and thus had not developed cavities, are concerned, they appear—based on the number of Mtb-infected alveolar macrophages in the ex vivo cultures—to be still in need of close monitoring and better treatment regimens to eliminate replicating Mtb. Thus, the number of Mtb-infected alveolar macrophages in the ex vivo cultures of cells obtained from resected lungs can be used, probably, as a new biomarker of predictive value for assessing treatment efficiency in relation to TB patients before surgery. Furthermore, the ex vivo cell cultures developed are suitable for quick estimation of Mtb virulence (see [31]) and exploring individual points of relationships between alveolar macrophages and Mtb in the lungs of TB patients with different characteristics of TB disease. Alveolar macrophages containing Mtb of different functional status and obtained in large numbers from ex vivo cultures of cells from some TB patients can be used for individualized testing of drugs, both existing and emerging, within the concepts of personalized medicine and adjunctive host-directed therapies [18, 28, 55, 56]. The appropriate choice of treatment regimen, which takes into account the biological properties and functional status of a pathogen in alveolar macrophages in a separate TB patient’s lungs, is believed to prevent exacerbation [2, 8].
Conclusions
The reported technique for developing ex vivo cultures of alveolar macrophages allows the level of infection with Mtb in the host cells in the lungs of post-operative patients with pulmonary TB to be assessed and the functional status of a pathogen to be determined in a matter of unprecedentedly short time. In summary, the proposed method of analysis can be used as the basis for the development of individual strategies in post-operative case treatment and making decisions as to how further prophylactic, epidemiological and medical treatment measures against pulmonary TB should be applied to patients and, if needed, revised.
Supporting information
(A, E, F, M) Lung parts surgically removed from patients 6, 14, 19, and 20, respectively. (B, F, J, N) Tissue specimens with cavity wall (B) and distant parts (F, J, N) from the resected lungs (A, E, F, M), respectively. Petri dishes are each 10 cm in diameter. (C, G, K, O) Cell suspensions containing alveolar macrophages were obtained from the lung specimens (B, F, J, N), respectively, and separated from closed caseous tuberculous lesions in the fibrous capsule staying in the sieves. (D, H, L, P) Alveolar macrophages obtained from cell suspensions (C, G, K, O), respectively, and stained by the ZN method after ex vivo culture for 16–18 hours. The black arrows point to alveolar macrophages with acid-fast Mtb. The scale bars are 10 μm each.
(TIFF)
(A, B, C, D) Langhans giant cells obtained from the lung tissue of patients 2, 7, 8, and 11, respectively, and stained by the ZN method. The black arrow points to a Langhans giant cell with acid-fast Mtb. The scale bars are 10 μm each.
(TIFF)
(A) The number of alveolar macrophages with Mtb (in isolation or as colonies) expressed as the percentage of the total number of alveolar macrophages analyzed. (B) The number of alveolar macrophages with Mtb in colonies expressed as the percentage of the total number of alveolar macrophages with any Mtb. Data are expressed as the means ± SEM. **P < 0.01, Student’s t-test.
(TIFF)
(A, B, C, D, E) The number of foamy alveolar macrophages expressed as the percentage of the total number of alveolar macrophages analyzed. Data are expressed as the means ± SEM. (E) Data on patients differing in the number of alveolar macrophages with Mtb obtained from resected lungs and presented as the percentage of the total number of macrophages examined.
(TIFF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
The authors are thankful to T.E. Aleshina of the Shared Center for Microscopic Analysis of Biological Objects of the Institute of Cytology and Genetics, for technical support.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.World Health Organization. Global Tuberculosis Report 2016.
- 2.Lawn SD, Zumla A. Tuberculosis. Lancet. 2011;378(9785):57–72. doi: 10.1016/S0140-6736(10)62173-3 [DOI] [PubMed] [Google Scholar]
- 3.Cheallaigh CN, de Castro CP, Coleman MM, Hope JC, Harris J. Macrophages and tuberculosis In: Takahashi R, Kai H, editors. Handbook of macrophages. New York: Nova Science Publishers; 2012. pp. 1–41. [Google Scholar]
- 4.Sasindran SJ, Torrelles JB. Mycobacterium tuberculosis infection and inflammation: what is beneficial for the host and for the bacterium? Front Microbiol. 2011;2:2 doi: 10.3389/fmicb.2011.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hmama Z, Pena-Diaz S, Joseph S, Av-Gay Y. Immunoevasion and immunosuppression of the macrophage by Mycobacterium tuberculosis. Immunol Rev. 2015;264(1):220–32. doi: 10.1111/imr.12268 [DOI] [PubMed] [Google Scholar]
- 6.Weiss G, Schaible UE. Macrophage defense mechanisms against intracellular bacteria. Immunol Rev. 2015;264(1):182–203. doi: 10.1111/imr.12266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardona P-J. The key role of exudative lesions and their encapsulation: lessons learned from the pathology of human pulmonary tuberculosis. Front Microbiol. 2015;6(1):1–8. doi: 10.3389/fmicb.2015.00612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lenaerts A, Barry CE III, Dartois V. Heterogeneity in tuberculosis pathology, microenvironments and therapeutic responses. Immunol Rev. 2015;264(1):288–307. doi: 10.1111/imr.12252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ulrichs T, Kosmiadi GA, Jörg S, Pradl L, Titukhina M, Mishenko V, et al. Differential organization of the local immune response in patients with active cavitary tuberculosis or with nonprogressive tuberculoma. J Infect Dis. 2005;192(1):89–97. doi: 10.1086/430621 [DOI] [PubMed] [Google Scholar]
- 10.Espitia C, Rodriguez E, Ramуn-Luing L, Echeverrнa-Valencia G, Vallecillo AJ. Host–pathogen interactions in tuberculosis In: Cardona P-J, editor. Understanding tuberculosis—analyzing the origin of mycobacterium tuberculosis pathogenicity. Vienna: InTech; 2012. pp. 43–76. [Google Scholar]
- 11.Ufimtseva E. Investigation of functional activity of cells in granulomatous inflammatory lesions from mice with latent tuberculous infection in the new ex vivo model. Clin Develop Immunol. 2013;2013:ID371249 doi: 10.1155/2013/371249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srivastava S, Ernst JD, Desvignes L. Beyond macrophages: the diversity of mononuclear cells in tuberculosis. Immunol Rev. 2014;262(1):179–92. doi: 10.1111/imr.12217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barry CE 3rd, Boshoff H, Dartois V, Dick T, Ehrt S, Flynn JA, et al. The spectrum of latent tuberculosis: rethinking the goals of prophylaxis. Nat Rev Microbiol. 2009;7(12):845–55. doi: 10.1038/nrmicro2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russell DG, Cardona P-J, Kim M-J, Allain S, Altare F. Foamy macrophages and the progression of the human TB granuloma. Nat Immunol. 2009;10(9):943–8. doi: 10.1038/ni.1781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaplan G, Post FA, Moreira AL, Wainwright H, Kreiswirth BN, Tanverdi M, et al. Mycobacterium tuberculosis growth at the cavity surface: a microenvironment with failed immunity. Infect Immun. 2003;71(12):7099–108. doi: 10.1128/IAI.71.12.7099-7108.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunter RL, Jagannath C, Actor JK. Pathology of postprimary tuberculosis in humans and mice: Contradiction of long-held beliefs. Tuberculosis. 2007;87(4):267–78. doi: 10.1016/j.tube.2006.11.003 [DOI] [PubMed] [Google Scholar]
- 17.Subbian S, Tsenova L, Kim M-J, Wainwright HC, Visser A, Bandyopadhyay N, et al. Lesion-specific immune response in granulomas of patients with pulmonary tuberculosis: A pilot study. PLOS ONE. 2015;10(7):e0132249 doi: 10.1371/journal.pone.0132249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parida SK, Axelsson-Robertson R, Rao MV, Singh N, Master I, Lutckii A, et al. Totally drug-resistant tuberculosis and adjunct therapies. J Intern Med. 2015;277(4):388–405. doi: 10.1111/joim.12264 [DOI] [PubMed] [Google Scholar]
- 19.Singh A, Budhraj A, Shrivastava A, Satyavana A, Gupta A, Gupta M, et al. Current status of anti-tuberculosis therapy: A patent analysis. Recent Patents Anti-Infect. Drug Discov. 2014;9(1):25–40. [DOI] [PubMed] [Google Scholar]
- 20.Belousova KV, Kravchenko MA, Berdnikov RB, Vakhrusheva DV, Skornyakov SN, Eremeeva NI. Comparative analysis of biological properties of Mycobacterium tuberculosis isolated from surgical and respiratory material. Fundamental Res (Russia). 2014;9:2452–5. [Google Scholar]
- 21.Barry C. More than just bugs in spit. Science. 2015;348(6235):633–4. doi: 10.1126/science.aaa2886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fenhalls G, Stevens L, Moses L, Bezuidenhout J, Betts JC, van Helden P, et al. In situ detection of Mycobacterium tuberculosis transcripts in human lung granulomas reveals differential gene expression in necrotic lesion. Infect Immun. 2002;70(11):6330–8. doi: 10.1128/IAI.70.11.6330-6338.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halse TA, Escuyer VE, Musser KA. Evaluation of a single-tube multiplex real-time PCR for differentiation of members of the Mycobacterium tuberculosis complex in clinical specimens. J Clin Microbiol. 2011;49(7):2562–7. doi: 10.1128/JCM.00467-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bates JH. New diagnostic methods for Tuberculosis In: Friedman LN, editor. Current concepts and treatment. Boca Raton, Fla: CRC Press; 1994. pp. 81–92. [Google Scholar]
- 25.Donoghue HD. Human tuberculosis—an ancient disease, as elucidated by ancient microbial biomolecules. Microb Infect. 2009;11(14–15):1156–62. doi: 10.1016/j.micinf.2009.08.008 [DOI] [PubMed] [Google Scholar]
- 26.Eum S-Y, Kong J-H, Hong M-S, Lee Y-J, Kim J-H, Hwang S-H, et al. Neutrophils are the predominant infected phagocytic cells in the airways of patients. Chest. 2010;137(1):122–8. doi: 10.1378/chest.09-0903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fu LM, Sohaskey CD, Jones B. Sputum expectoration as a useful non-invasive alternative to bronchoalveolar lavage for collecting human alveolar macrophages in tuberculosis research. Sci J Clin Medicine. 2013;2(3):92–7. doi: 10.11648/j.sjcm.20130203.16 [Google Scholar]
- 28.Lienhardt C, Lönnroth K, Menzies D, Balasegaram M, Chakaya J, Cobelens F, et al. Translational research for tuberculosis elimination: priorities, challenges, and actions. PLoS Med. 2016;13(3):e1001965 doi: 10.1371/journal.pmed.1001965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ufimtseva E. Mycobacterium-host cell relationships in granulomatous lesions in a mouse model of latent tuberculous infection. BioMed Res Int. 2015;2015:ID948131 doi: 10.1155/2015/948131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ufimtseva E. Differences between Mycobacterium-host cell relationships in latent tuberculous infection of mice ex vivo and mycobacterial infection of mouse cells in vitro. J Immun Res. 2016;2016:ID4325646 doi: 10.1155/2016/4325646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ufimtseva EG, Eremeeva NI, Kravchenko MI, Skornyakov SN. Method for producing ex vivo alveolar macrophage cultures of the operational material of patients suffering pulmonary tuberculosis and method for estimating virulence of Mycobacterium tuberculosis using the obtained ex vivo alveolar macrophage cultures. RF Patent RU2593725. Bull Izobr. 2016;22:1–30. [Google Scholar]
- 32.Ufimtseva E. Isolation and ex vivo culture of alveolar macrophages from the resected lungs of patients with pulmonary tuberculosis. protocols.io dx.doi.org/10.17504/protocols.io.j4acqse [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erokhin VV, editor. The cultural methods of tuberculosis diagnostics. Moscow, Tver: Triada; 2008. [Google Scholar]
- 34.Schneberger D, Aharonson-Raz K, Singh B. Monocyte and macrophage heterogeneity and Toll-like receptors in the lung. Cell Tissue Res. 2011;343(1):97–106. doi: 10.1007/s00441-010-1032-2 [DOI] [PubMed] [Google Scholar]
- 35.Lowe DM, Redford PS, Wilkinson RJ, O'Garra A, Martineau AR. Neutrophils in tuberculosis: friend or foe? Trends Immunol. 2012;33(1):14–25. doi: 10.1016/j.it.2011.10.003 [DOI] [PubMed] [Google Scholar]
- 36.Lyadova I. Inflammation and immunopathogenesis of tuberculosis progression In: Cardona P-J, editor. Understanding tuberculosis—analyzing the origin of mycobacterium tuberculosis pathogenicity. Vienna: InTech; 2012. pp. 19–42. [Google Scholar]
- 37.Peyron P, Vaubourgeix J, Poquet Y, Levillain F, Botanch C, Bardou F, et al. Foamy macrophages from tuberculous patients’ granulomas constitute a nutrient-rich reservoir for M. tuberculosis persistence. PLoS Pathog. 2008;4(11):e1000204 doi: 10.1371/journal.ppat.1000204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Melo RCN, Dvorak AM. Lipid body–phagosome interaction in macrophages during infectious diseases: host defense or pathogen survival strategy? PLoS Pathog. 2012;8(7): e1002729 doi: 10.1371/journal.ppat.1002729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forrellad MA, Klepp LI, Gioffré A, Sabio y García J, Morbidoni HR, de la Paz Santangelo M, et al. Virulence factors of the Mycobacterium tuberculosis complex. Virulence. 2013;4(1):3–66. doi: 10.4161/viru.22329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arnett E, Krishnan N, Robertson BD, Schlesinger LS. Host pathogen biology for airborne Mycobacterium tuberculosis In: Hickey AJ, Misra A, Fourie PB, editors. Drug delivery systems for tuberculosis prevention and treatment. USA: Wiley; 2016. pp. 11–47. doi: 10.1002/9781118943182.ch2 [Google Scholar]
- 41.Murphy J, Summer R, Wilson AA, Kotton DN, Fine A. The prolonged life-span of alveolar macrophages. Am J Respir Cell Mol Biol. 2008;38(4):380–5. doi: 10.1165/rcmb.2007-0224RC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dallenga T, Schaible UE. Neutrophils in tuberculosis—first line of defence or booster of disease and targets for host-directed therapy? Pathog Dis. 2016;74(3):ftw012 doi: 10.1093/femspd/ftw012 [DOI] [PubMed] [Google Scholar]
- 43.Mortellaro A, Robinson L, Ricciardi-Castagnoli P. Spotlight on mycobacteria and dendritic cells: will novel targets to fight tuberculosis emerge? EMBO Mol Med. 2009;1(1):19–29. doi: 10.1002/emmm.200900008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schreiber HA, Sandor M. The role of dendritic cells in mycobacterium-induced granulomas. Immunol Lett. 2010;130(1):26–31. doi: 10.1016/j.imlet.2009.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davis JM, Ramakrishnan L. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell. 2009;136(1):37–49. doi: 10.1016/j.cell.2008.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berg RD, Levitte S, O’Sullivan MP, O’Leary SM, Cambier CJ, Cameron J, et al. Lysosomal disorders drive susceptibility to tuberculosis by compromising macrophage migration. Cell. 2016;165(1):139–52. doi: 10.1016/j.cell.2016.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramakrishnan L. Revisiting the role of the granuloma in tuberculosis. Nat Rev Immunol. 2012;12(5):352–66. doi: 10.1038/nri3211 [DOI] [PubMed] [Google Scholar]
- 48.Lerner TR, Borel S, Gutierrez MG. The innate immune response in human tuberculosis. Cell Microbiol. 2015;17(9):1277–85. doi: 10.1111/cmi.12480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santucci P, Bouzid F, Smichi N, Poncin I, Kremer L, De Chastellier C, et al. Experimental models of foamy macrophages and approaches for dissecting the mechanisms of lipid accumulation and consumption during dormancy and reactivation of tuberculosis. Front Cell Infect Microbiol. 2016;6:122 doi: 10.3389/fcimb.2016.00122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hunter RL. Tuberculosis as a three-act play: A new paradigm for the pathogenesis of pulmonary tuberculosis. Tuberculosis. 2016;97(1):8–17. doi: 10.1016/j.tube.2015.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown RE, Hunter RL, Hwang S-A. Morphoproteomic-guided host-directed therapy for tuberculosis. Front Immunol. 2017;8:78 doi: 10.3389/fimmu.2017.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parihar SP, Guler R, Khutlang R, Lang DM, Hurdayal R, Mhlanga MM, et al. Statin therapy reduces the Mycobacterium tuberculosis burden in human macrophages and in mice by enhancing autophagy and phagosome maturation. J Infect Dis. 2014;209(5):754–63. doi: 10.1093/infdis/jit550 [DOI] [PubMed] [Google Scholar]
- 53.Ndlovu H, Marakalala MJ. Granulomas and inflammation: host-directed therapies for tuberculosis. Front Immunol. 2016;7:434 doi: 10.3389/fimmu.2016.00434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parrish NM, Dick JD, Bishai WR. Mechanisms of latency in Mycobacterium tuberculosis. Trends Microbiol. 1998;6(3):107–112. [DOI] [PubMed] [Google Scholar]
- 55.Zumla A, Rao M, Parida SK, Keshavjee S, Cassell G, Wallis R, et al. Inflammation and tuberculosis: host-directed therapies. J Intern Med. 2015;277(4):373–87. doi: 10.1111/joim.12256 [DOI] [PubMed] [Google Scholar]
- 56.Cole ST. Inhibiting Mycobacterium tuberculosis within and without. Phil Trans R Soc B. 2016;371(1707):20150506 doi: 10.1098/rstb.2015.0506 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A, E, F, M) Lung parts surgically removed from patients 6, 14, 19, and 20, respectively. (B, F, J, N) Tissue specimens with cavity wall (B) and distant parts (F, J, N) from the resected lungs (A, E, F, M), respectively. Petri dishes are each 10 cm in diameter. (C, G, K, O) Cell suspensions containing alveolar macrophages were obtained from the lung specimens (B, F, J, N), respectively, and separated from closed caseous tuberculous lesions in the fibrous capsule staying in the sieves. (D, H, L, P) Alveolar macrophages obtained from cell suspensions (C, G, K, O), respectively, and stained by the ZN method after ex vivo culture for 16–18 hours. The black arrows point to alveolar macrophages with acid-fast Mtb. The scale bars are 10 μm each.
(TIFF)
(A, B, C, D) Langhans giant cells obtained from the lung tissue of patients 2, 7, 8, and 11, respectively, and stained by the ZN method. The black arrow points to a Langhans giant cell with acid-fast Mtb. The scale bars are 10 μm each.
(TIFF)
(A) The number of alveolar macrophages with Mtb (in isolation or as colonies) expressed as the percentage of the total number of alveolar macrophages analyzed. (B) The number of alveolar macrophages with Mtb in colonies expressed as the percentage of the total number of alveolar macrophages with any Mtb. Data are expressed as the means ± SEM. **P < 0.01, Student’s t-test.
(TIFF)
(A, B, C, D, E) The number of foamy alveolar macrophages expressed as the percentage of the total number of alveolar macrophages analyzed. Data are expressed as the means ± SEM. (E) Data on patients differing in the number of alveolar macrophages with Mtb obtained from resected lungs and presented as the percentage of the total number of macrophages examined.
(TIFF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.