Abstract
The objective of this study was to describe the time required to obtain a negative chlamydia test in pregnant and nonpregnant women following treatment to inform test-of-cure collection and recommend an abstinence period to avoid reinfection. Seventy-two women with Chlamydia trachomatis infection, 36 pregnant and 36 nonpregnant, were enrolled in a prospective cohort study. Women were excluded less than 18 years of age, if they had been treated for chlamydia, reported an allergy to macrolide antibiotics, or if they had Myasthenia Gravis. Women were treated for chlamydia with single-dose therapy and submitted weekly vaginal chlamydia nucleic acid amplification tests (NAATs). Once NAATwere negative, the participants completed the study. Forty-seven women completed the study per protocol. The primary outcome was to determine the time to a negative chlamydia NAAT following treatment, with secondary outcomes of determining the appropriate time to collect a test-of-cure following chlamydia treatment and to recommend an appropriate abstinence period following treatment to avoid reinfection. Results showed that the time to a negative chlamydia NAAT was significantly different between groups (log-rank p = 0.0013). The median number of days to obtain a negative chlamydia NAAT was 8 days (IQR 7–14) in pregnant and 7 days (IQR 6–10) in nonpregnant women (WRST p = 0.04). All participants had a negative chlamydia NAAT by day 29 post-treatment. Following single-dose treatment for chlamydia, both pregnant and nonpregnant women should test negative with NAAT by 30 days post-treatment. Clinicians should collect a test-of-cure in pregnant women no earlier than 1 month. To avoid reinfection, women should avoid condomless intercourse for at least 1 month.
Keywords: Chlamydia trachomatis, test-of-cure, pregnant women
Introduction
To avoid neonatal infection at the time of delivery, the Centers for Disease Control and Prevention (CDC) recommends that pregnant women with Chlamydia trachomatis infection should be retested within 3–4 weeks post-treatment to ensure cure, three months post-treatment to evaluate for reinfection, and in the 3rd trimester.1–3 Universal tests for chlamydia post-treatment in asymptomatic, nonpregnant women are not cost-effective.2,4,5 Therefore, the CDC recommends repeat chlamydia testing three months post-treatment in nonpregnant women at risk for reinfection. Nucleic acid amplification tests (NAATs) are the recommended diagnostic test for diagnosis and repeat testing given their superior sensitivity and specificity when compared to culture for the detection of urogenital chlamydial infections.2,6,7
Current CDC recommendations regarding repeat chlamydia NAATs post-treatment in pregnant women are based on studies in nonpregnant women.8,9 Following treatment with a single dose of azithromycin, Renault et al. collected chlamydia NAATs from self-collected vaginal swabs in nonpregnant women on days 3, 7, 10, and 14 post-treatment. Seventy-nine percent of women had a negative chlamydia NAAT on day 14. Based on the trajectory of negative NAATs collected, they estimated that 100% of women would have a negative NAAT by day 17.8 In a recent study of pregnant women with chlamydial infection, 88% (46/52) had a negative chlamydia NAAT three weeks following single-dose therapy. This study did not address why six women continued to have a positive NAAT despite effective therapy.10
One explanation for a positive chlamydia NAAT three weeks post-treatment may be the presence of noninfectious (dead or inactive) chlamydial organisms detected by NAAT. Following antimicrobial treatment with a single dose of azithromycin or seven days of twice-daily doxycycline, residual nucleic acid from noninfectious chlamydia has been shown to be present in nonpregnant women’s urine for up to three weeks (16–21 days).7,9 Another explanation for the presence of chlamydia within three weeks post-treatment in pregnant women is that elevated levels of progesterone may affect clearance of chlamydia from the genital tract.11,12 A third explanation is failure of single-dose therapy, which has a reported failure rate of 3% (0.4–7.4%).13 Although treatment failure is possible, collection of a chlamydia NAAT within three weeks of treatment may result in a false positive test and unnecessary treatment in some pregnant women.
The primary objective of this study was to determine if there is a difference in time required to obtain a negative chlamydia NAAT following treatment in pregnant and nonpregnant women with a vaginal chlamydial infection. The secondary objective of this study was to estimate the most appropriate time post-treatment to collect a test-of-cure in pregnant women based on when over 90% of women have a negative chlamydia NAAT. The third objective was to approximate the most appropriate time post-treatment when women may safely resume condomless intercourse with their treated partner(s).
Methods
This prospective cohort study was approved by the Institutional Review Board (#19615). In order to simulate clinical practice and evaluate current CDC recommendations, we planned to follow all participants for one month to determine the number of days required to obtain a negative chlamydia NAAT post-treatment.
In order to calculate the sample size, we assumed over 90% of women have a negative chlamydia NAAT by day 17 post-treatment.8 Using P.S.: Power and Sample Size Calculator version 3.0.43, we calculated a sample size for a survival analysis with a log-rank test, assuming that the time to a negative chlamydia NAAT in pregnant women was seven days greater than nonpregnant women.14 Assuming a type I error probability of 0.05, we estimated 33 pregnant and 33 nonpregnant women would be needed to have 80% power to detect a seven-day difference between groups. To account for a 10% dropout rate, we anticipated 72 total participants should be enrolled.
Pregnant and nonpregnant women 18 years of age or older diagnosed with chlamydial infection with a cervical or vaginal APTIMA Combo 2 assay for Chlamydia trachomatis (Hologic, Inc. Bedford, MA) were approached to participate. Women were ineligible if they had been treated for the current chlamydial infection, reported an allergy to macrolide antibiotics, or had myasthenia gravis. Women were recruited from a single academic medical center obstetrics and gynecology clinic in the Southeast. The proportions of ethnicities represented by patients in our practice are: Hispanic (30%) and non-Hispanic (70%: 38% African American, 27% Caucasian, and 5% other). The incidence of chlamydia in this population has been reported to be 6%.15
At the initial visit, participants completed a survey to describe potential characteristics associated with chlamydial infection. Personal information collected from participants included: age, ethnicity, insurance status, highest level of education obtained, current tobacco use, age of sexual debut, and number of lifetime sexual partners. Medical history collected from the participants included: parity, body mass index (BMI), medical allergies, current contraceptive method(s) in nonpregnant women, history of an abnormal cervical cancer screening test, previous diagnosis of sexually transmitted infections (STIs) and/or pelvic inflammatory disease (PID), and HIV status.
Before receiving treatment for a genital chlamydial infection, women enrolled in the study were screened for concurrent vaginal infections, which included bacterial vaginosis (BV), Neisseria gonorrhoeae, and Trichomonas vaginalis. A Gram stain was collected from a vaginal swab and a Nugent score calculated. Nugent scores ≥7 were consistent with BV.16 Prior to May 2013, the diagnosis of Trichomonas was made if there were motile trichomonads seen in culture (InPouch™, Biomed diagnostics, White City, OR). Following May 2013, Trichomonas were diagnosed by NAAT (APTIMA Combo 2 and TV analyte specific reagent, Hologic Inc., Bedford, MA). All Neisseria gonorrhoeae infections were diagnosed with NAAT (APTIMA Combo 2). Patients diagnosed with BV, Neisseria, or Trichomonas were notified by phone and provided treatment. BV was treated with metronidazole 500 mg by mouth twice daily for seven days, Neisseria was treated with ceftriaxone 250 mg intra-muscularly once, and Trichomonas was treated with metronidazole 2000 mg by mouth once.2,17 Expedited partner therapy (EPT) for concurrent STIs was provided.
After completion of the enrollment survey and submission of self-collected vaginal swabs, all participants were directly observed to take azithromycin 1000 mg by mouth for the treatment of chlamydia. Participants were provided a prescription(s) of azithromycin 1000 mg as EPT to be given to their partner(s) with whom they had had sexual contact within 60 days of diagnosis. They were provided seven latex male condoms (Trojan™ ENZ lubricated condoms), one for each day between study visits. They were instructed to provide EPT to their sexual partner(s) and to abstain from condomless intercourse or use condoms with every act of intercourse during the study period.
Following treatment for chlamydia, participants were expected to return for a weekly study visit, defined as seven calendar days from the most recent visit. Study staff called patients in advance to remind them of their appointments. Submission of chlamydia NAAT > 10 calendar days from the most recent visit was defined as a late submission. At follow-up visits, participants provided a self-collected vaginal swab for chlamydia NAAT (APTIMA Combo 2 assay, Hologic, Inc. Bedford, MA). Participants were administered a survey to determine if they had had intercourse in the interval between visits and if they had used condoms with each act of intercourse. Following each study visit, participants were provided condoms. Participants were considered to complete the study once the chlamydia NAAT returned negative. Participants found to have a positive chlamydia NAAT after four weekly tests (≥28 days) were referred for further evaluation for treatment failure or reinfection. Participants’ medical records were monitored up to 90 days post-treatment for chlamydia NAAT results. Subjects were considered lost to follow up if they failed to complete the study by returning to submit chlamydia NAAT within the study period.
Statistical analysis for these data included a survival analysis (log-rank test) and Wilcoxon rank sum tests (WRST) to compare the days required to obtain a negative chlamydia NAAT in pregnant and nonpregnant women. Descriptive variables were compared using Student’s t-test (continuous data), WRST (nonparametric data), and χ2 or Fisher’s exact test (categorical data). Logistic regression was used to determine factors associated with risk of a positive NAAT greater than 21 days post-treatment. All statistical analyses were performed using SAS® 9.4 (Cary, NC).
Results
Seventy-two women, 36 pregnant and 36 nonpregnant, diagnosed with chlamydia infection were recruited from April 2010 to March 2015. Study participants’ descriptive characteristics are provided in Table 1.
Table 1.
Characteristics of women with Chlamydia trachomatis infection.
| N = 72 | |
|---|---|
| Age (years) | 24 ± 5 |
| Parity | 1 (0–2) |
| Gestational age of pregnant participantsa | 16 (11–25) |
| Body mass index (BMI) | 29 8 |
| Public health insurance | 47 (65%) |
| Ethnicity | |
| Non-Hispanic, Black/African American | 60 (83%) |
| Hispanic, non-Black | 1 (1%) |
| Less than a high school education | 47 (65%) |
| Age of sexual debut (years) | 16 (15–17) |
| Number of reported lifetime sexual partners | 5 (3–8) |
| History of abnormal cervical cancer screening | 22 (31%) |
| Current tobacco use | 13 (18%) |
| History of a sexually transmitted infection(s) (STI) | 47 (65%) |
| Bacterial vaginosis at enrollment | 25 (35%) |
| An additional STI at enrollment | 12 (17%) |
| Trichomonas vaginalis | 8 (11%) |
| Neisseria gonorrhoeae | 5 (7%) |
| Reported provision of expedited partner therapy | 42 (58%) |
| Days followed until a negative chlamydia NAAT | 12 (7–20) |
| Late submission of chlamydia NAATb | 25 (35%) |
| Lost to follow upc | 13 (18%) |
Gestational age of pregnant participants is reported as the weeks of gestation during which participants received initial treatment for chlamydial infection.
Late submission of chlamydia NAAT was defined as submission of a NAAT test >10 days following treatment or last study appointment.
Lost to follow up was defined as failure to complete the study by returning to submit chlamydia NAATs until a negative result returned within the study period post-treatment. Means are reported with standard deviation (±) and medians are reported with interquartile ranges (IQR).
Sixty-five percent of women (47/72) reported a previous STI diagnosis; the most commonly reported was chlamydia (31, 43%). Other reported previous STIs included: Trichomonas (12, 17%), Neisseria (9, 13%), genital herpes simplex virus (6, 8%), PID (5, 7%), and external genital warts (1, 1%). No participants reported a history of syphilis or HIV. Seventeen percent (12/72) of participants had a concurrent genital STI at enrollment. Eight participants had Trichomonas, five had Neisseria, and one participant had both Trichomonas and Neisseria. All participants with additional STIs were noted to have negative repeat testing within 90 days of enrollment. Twenty-five participants (35%) had BV at enrollment. Rates of concurrent STIs and BV were similar between groups (Table 2).
Table 2.
A comparison between characteristics of pregnant and nonpregnant women with Chlamydia trachomatis infection.
| Pregnant n = 36 |
Nonpregnant n = 36 |
p-Value | |
|---|---|---|---|
| Age (years) | 23 ± 5 | 25 ± 5 | 0.2 |
| Parity | 1 (0–2) | 1 (0–2) | 0.6 |
| BMI | 29 ± 8 | 29 ± 9 | 0.9 |
| Public health insurance | 28 (78%) | 19 (53%) | 0.05 |
| Non-Hispanic, Black/African American | 31 (86%) | 29 (81%) | 0.8 |
| Less than a high school education | 11 (31%) | 14 (39%) | 0.5 |
| Age of sexual debut (years) | 16 (16–17) | 16 (15–18) | 0.9 |
| Number of reported lifetime sexual partners | 5 (3–8) | 5 (4–8) | 0.5 |
| History of a STI | 28 (78%) | 19 (53%) | 0.03 |
| History of abnormal cervical cancer screening | 13 (36%) | 9 (25%) | 0.3 |
| Current tobacco use | 9 (25%) | 4 (11%) | 0.2 |
| Bacterial vaginosis at enrollment | 10 (29%) | 15 (42%) | 0.3 |
| Another STI at enrollmenta | 5 (14%) | 7 (19%) | 0.2 |
| Reported provision of expedited partner therapy | 20 (56%) | 22 (61%) | 0.6 |
| Days followed until a negative chlamydia NAAT | 14 (7–21) | 10 (7–19) | 0.13 |
| Participants with late submission of chlamydia NAATb | 10 (28%) | 15 (42%) | 0.2 |
| Participants lost to follow upc | 11 (31%) | 3 (6%) | 0.01 |
Additional STIs diagnosed at enrollment were Trichomonas vaginalis and Neisseria gonorrhoeae.
Late submission of chlamydia NAAT was defined as NAAT submission > 10 days from treatment or last NAAT.
Lost to follow up was defined as failure to complete the study by returning to submit chlamydia NAAT until a negative result returned within the study period post-treatment. Means are reported with standard deviation (±) and medians are reported with interquartile ranges (IQR).
All Student’s t-test were interpreted as pooled t-test. Nonparametric continuous variables were compared using Wilcoxon rank sum tests. Categorical variables were compared using χ2 and Fisher’s exact test.
BMI: body mass index; STI: sexually transmitted infection; NAAT: nucleic acid amplification.
Pregnant and nonpregnant participants had similar personal and medical characteristics. Pregnant women were more likely to receive public insurance (78% vs. 53%, p = 0.05) and report previous STIs (78% vs. 53%, p = 0.03) (Table 2). Four nonpregnant women reported using contraception at enrollment: two were taking contraceptive pills, one received injectable contraception, and one had an intrauterine device.
The overall loss to follow-up rate in the study was 18% (13/72), and pregnant women were more likely to be lost to follow up (11, 31% vs. 2, 6%, p = 0.01) (Table 2). The majority of participants lost to follow up did not return for the first study visit post-treatment (8/72, 11%). More pregnant participants were lost to follow up at the first return study visit compared to nonpregnant women, but this difference was not statistically significant (7/36, 19% vs. 1/36, 3%, p = 0.06). Three participants, two pregnant and one nonpregnant, were lost to follow up at the second study visit. One pregnant participant failed to return for the third study visit, and one pregnant participant failed to return for the fourth study visit.
Only one participant had a fourth study visit secondary to a positive NAAT at the third visit. This participant was not pregnant and she subsequently had a negative test 65 days post-treatment. This participant did not follow up for evaluation of a prolonged positive NAAT, and she did not receive further treatment for chlamydial infection during the study period. There were no documented reinfections or treatment failures among study subjects within the study period.
When comparing all pregnant and nonpregnant women in a survival analysis in which participants were censored once a negative chlamydia NAAT was obtained, the time required to obtain a negative chlamydia NAAT following treatment did not differ significantly (p = 0.09). In order to determine a more accurate estimate of the time to negative chlamydia NAAT, we excluded subjects who had late specimen submissions (n = 25). In this analysis, pregnant women were significantly more likely to submit a negative chlamydia NAAT later (p = 0.0013) (Figure 1).
Figure 1.
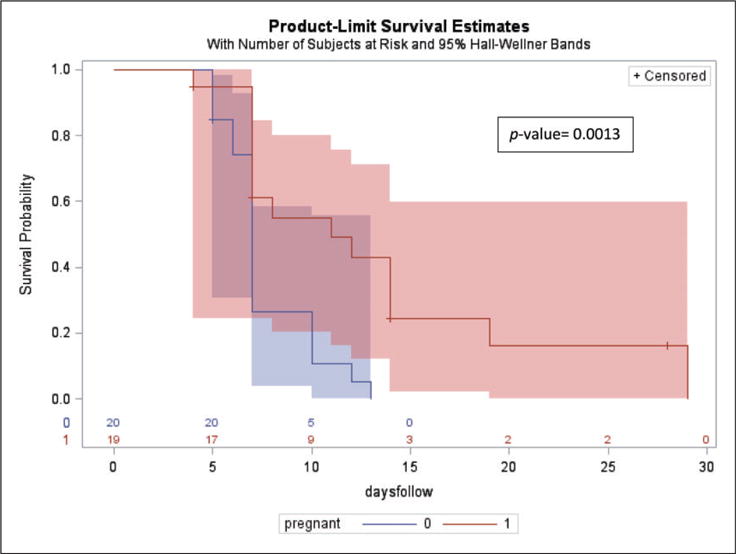
Represents the time (in days) following treatment required for subjects to submit a negative chlamydia NAAT post-treatment. This analysis excludes subjects who submitted late specimens (> 10 days from their subsequent visit). Pregnant subjects are represented by the red line and nonpregnant subjects are represented by the blue line. The shaded blue and red bands surrounding the survival curves (Hall–Wellner bands) represent 95% confidence intervals. Subjects were censored from the analysis following a negative chlamydia NAAT. The median number of days subjects were followed was 7 (IQR 7–12), the total range of days followed was 7–29. Pregnant subjects were followed for a median of 8 days (IQR 7–14 days); the total range was 4–29 days. Nonpregnant subjects were followed for a median of 7 days (IQR 6–10); the total range was 5–13 days.
Among all study participants, variability in the days between study visits was high, and the time to a negative chlamydia NAAT did not follow a normal distribution. The median time to a negative chlamydia NAAT for all of the participants was 12 days (IQR 7–21, range 4–65). There were two outliers, one nonpregnant and one pregnant participant who were followed 65 and 49 days, respectively. When comparing the two groups, the median time to negative chlamydia NAAT following treatment was 14 days (IQR 7–21, range 4–49) in pregnant women and 10 days (IQR 7–19, range 5–65) in nonpregnant women (WRST, p = 0.13).
Among subjects submitting specimens on time, the median number of days subjects were followed was 7 (IQR 7–12, range 7–29). Pregnant subjects were followed for a median of 8 days (IQR 7–14 days, range 4–29 days) compared to nonpregnant subjects, who were followed for a median of 7 days (IQR 6–10, range was 5–13 days) (p = 0.04). By 29 days post-treatment, 100% of subjects who presented on time for specimen submission, had a negative chlamydia NAAT. Figure 2 demonstrates the proportion of the total number of women with on time specimen submission (presenting ≤10 days between visits) who had a positive chlamydia NAAT. By day 20 post-treatment, 94% of pregnant and 96% of nonpregnant participants submitting specimens per study protocol had a negative chlamydia NAAT.
Figure 2.
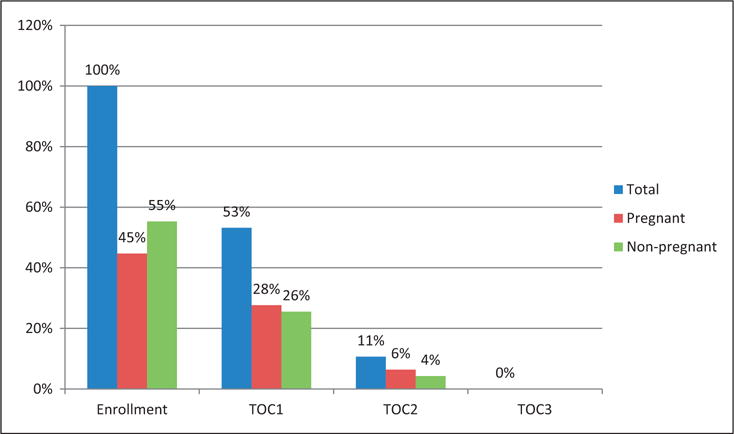
Represents the proportion of subjects (total, pregnant, and nonpregnant) who presented on time for study visits (within 30 days of treatment) and had a positive chlamydia NAAT. Subjects who presented more than 10 days from the preceding visit were excluded from this analysis. At the enrollment visit, 100% of subjects had a positive chlamydia NAAT. TOC1, test-of-cure visit #1, is the first study visit following treatment for chlamydial infection. Subjects presenting on time for TOC1 submitted specimens 4–10 days following treatment. TOC2, test-of-cure visit #2, is the 2nd study visit following treatment. Subjects presenting on time for TOC2 submitted specimens 3–10 days following TOC1. TOC3, test-of-cure visit #3, is the 3rd study visit following treatment. Only one subject presented on time for TOC3, and the chlamydia NAAT was negative.
The only factor associated with a positive chlamydia NAAT 21 days post-treatment was the presence of a concurrent STI at enrollment [OR 5.9 (95% CI: 1.2–28.1, p = 0.03)]. This association was attenuated in multivariable regression when accounting for potential confounding variables: parity, pregnancy, insurer, tobacco use, and EPT provision [OR 5.5 (95% CI: 0.7–40.7, p = 0.1)]. Five subjects, two pregnant and three nonpregnant, had Neisseria infection at enrollment. Due to late specimen submission, three of these participants (two pregnant and one nonpregnant) were excluded from the survival and WRST analysis described above.
All participants were offered EPT to any sexual contact(s) within 60 days of diagnosis. Forty-two (58%) of participants reported providing EPT to their partner(s). The proportions of pregnant and nonpregnant women providing EPT to sexual contacts were similar (20, 56% vs. 22, 61% respectively, p = 0.8). At subsequent study visits, pregnant and nonpregnant participants reported similar rates of intercourse and condom use. The participant reported rates of expedited partner therapy, intercourse and condom use at each study visit are demonstrated in Supplementary Figure 1 (see online supplementary material).
Discussion
In this prospective study of pregnant and nonpregnant women with chlamydial infection, 96% of all study participants had a negative chlamydia NAAT by day 30 post-treatment with single-dose azithromycin. When excluding women who did not follow study protocol for specimen submission, 100% of chlamydia NAAT submitted per protocol were negative by 30 days post-treatment. In our cohort, nonpregnant women were more likely to have a negative chlamydia NAAT before pregnant women. For purpose of investigating the primary outcome, determining if there is a difference in time to negative chlamydia NAAT between pregnant and nonpregnant women, the study was powered to detect a 7-day difference in the time to obtain a negative NAAT, assuming pregnant women would require longer. Despite a higher than anticipated lost to follow up rate and high rate of late specimen submissions, our findings suggest that chlamydia NAAT may be positive longer in pregnant women post-treatment. The difference in time to a negative chlamydia NAAT among pregnant and nonpregnant women may not be clinically relevant, but it may impact the recommended duration of abstinence or condom use post-treatment.
Our secondary outcome of interest was to describe the most appropriate timing for test-of-cure following treatment of chlamydial infection in pregnant women. Ninety-four percent of pregnant women following study protocol had a negative chlamydia NAAT by day 21 post-treatment and 100% by day 29. Our data suggest that test-of-cure in pregnant women should be collected no earlier than 21 days following treatment, potentially no earlier than 30 days post-treatment to avoid false positive results. Our recommendation based on these data is consistent with the 2015 CDC STD Treatment Guidelines, which recommend a test-of-cure no earlier than 3–4 weeks.2,17 Previous studies that informed these guidelines only estimated the predicted time to a negative test-of-cure, based on predictive modeling of nonpregnant women.8 A more recent study, reported that 88% of pregnant women had a negative chlamydia NAAT 21 days following single-dose therapy, which is consistent with our data.10 Our recommended timing for test-of-cure may decrease unnecessary retreatment of noninfectious organisms detected by NAAT before 30 days.
Another secondary aim of this study was to determine the most appropriate time to recommend condomless sex following post-treatment to women treated for chlamydial infection. Our data suggest that over 96% of women had a negative NAAT by day 30. In order to avoid reinfection, women should likely abstain from condomless sex following treatment for chlamydia for at least 1 month. This recommendation differs from the current CDC STD Treatment Guidelines, which recommend abstaining from condomless sex for 7 days following treatment with a single-dose regimen.2 Given the inability to distinguish between live (infectious) chlamydial organisms and dead (noninfectious) organisms detected by NAAT, our recommendation for continued abstinence or consistent condom use for 1 month post-treatment is conservative but likely effective in preventing reinfection.
Women treated for other STI(s) in addition to chlamydia may have a positive NAAT longer post-treatment. In multivariable logistic regression analysis of our participants, women with Neisseria and/or Trichomonas were at risk for a positive chlamydia NAAT > 21 days post-treatment. EPT was available to all participants in the study,18 but only 58% of participants reported providing EPT to their partner(s). In addition, less than 100% of the participants reporting sexual activity reported providing EPT and/or using condoms. In order to prevent reinfection, especially during pregnancy, providers should seek effective strategies for improving compliance with EPT and condom use. Taking into consideration the low rates of EPT and condom use in our study population, providers should assess a woman’s risk for reinfection and consider repeat testing in at risk nonpregnant women 3 months post-treatment.2
The strengths of this study are that it is prospective and all participants who completed the study were followed until a negative chlamydia NAAT was obtained. The participants’ ages and ethnicities are representative of an academic practice in the Southeastern United States. These data may not be generalizable to other regions of the United States or other countries. An unanticipated outcome in this study was the high loss to follow up and late specimen submission rates among participants. The most likely explanation for pregnant participants being loss to follow up is that they elected to receive prenatal care at another hospital. Another unexpected finding was the participants’ difficulty returning for study visits at the scheduled weekly intervals. To account for the variability in follow up, we excluded subjects who submitted specimens >10 days following the subsequent visit. By doing so, we were able to provide a more accurate prediction of negative chlamydia NAAT according to study design. Lastly, these data may also have been more compelling if women submitted a daily chlamydia NAAT, but the cost of daily NAAT for 72 participants exceeded the limited budget. Despite the limitation of specimen collections once per week and a small sample size, our data for compliant participants suggest the anticipated difference in time to a negative chlamydia NAAT.
Despite a small sample size, this study contributes information that is clinically relevant. Our data approximate the time to obtain a negative chlamydia NAAT in women treated with single-dose therapy. Our findings suggest that pregnant women should be retested for chlamydial infection with NAAT no earlier than 21 days post-treatment. Chlamydia NAAT may remain positive longer in pregnant women and women treated for concurrent STI(s) and waiting until 30 days post-treatment to collect a test-of-cure may avoid false positive results that prompt unnecessary retreatment. In order to avoid chlamydial reinfection, women should be counseled to abstain from condomless sex or use condoms with every act of intercourse for at least 1 month post-treatment.
Supplementary Material
Acknowledgments
The authors would like to thank our research assistants, Mackie Talley Steadman and Tamara Pfeffer, for their hard work and dedication.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Department of Obstetrics and Gynecology at the Medical University of South Carolina and by NIH grant UL1 TR001450 (PI Brady).
Footnotes
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Data sharing
Data is available upon request by emailing the principal investigator and author, Gweneth Lazenby, at lazenbgb@musc.edu.
Contributorship statement
All of the listed authors have contributed to this original research. Dr Gweneth Lazenby is the principal investigator and author responsible for manuscript development. Dr Jeff Korte assisted with sample size calculation, study design, and statistical analysis. Dr Sarah Tillman and Florence K Brown assisted with patient recruitment, enrollment, and follow up. Dr David Soper is the senior author and assisted with study design and manuscript development. All authors have provided edits to the manuscript.
References
- 1.Centers for Disease Control and Prevention. 2014 sexually transmitted diseases surveillance: chlamydia. [CDC.gov website]. 17 November 2015. http://www.cdc.gov/std/stats14/chlamydia.htm ((accessed 21 December 2015)
- 2.Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1–137. [PMC free article] [PubMed] [Google Scholar]
- 3.Low N, Stroud K, Lewis DA, et al. Mind your binomials: A guide to microbial nomenclature and spelling in sexually transmitted infections. Sex Transm Infect. 2015;91:154–155. doi: 10.1136/sextrans-2014-051937. [DOI] [PubMed] [Google Scholar]
- 4.Schiotz HAaPAS. Test-of-cure for asymptomatic genital chlamydial infections in women: A cost-benefit analysis. Sex Transm Dis. 1992;19:133–136. [PubMed] [Google Scholar]
- 5.Hoover KW, Tao G, Nye MB, et al. Suboptimal adherence to repeat testing recommendations for men and women with positive Chlamydia tests in the United States, 2008-2010. Clin Infect Dis. 2013;56:51–57. doi: 10.1093/cid/cis771. [DOI] [PubMed] [Google Scholar]
- 6.Chernesky M, Jang D, Gilchrist J, et al. Head-to-head comparison of second-generation nucleic acid amplification tests for detection of Chlamydia trachomatis and Neisseria gonorrhoeae on urine samples from female subjects and self-collected vaginal swabs. J Clin Microbiol. 2014;52:2305–2310. doi: 10.1128/JCM.03552-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papp JR, Schachter J, Gaydos CA, et al. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae–2014. MMWR Recomm Rep. 2014;63:1–19. [PMC free article] [PubMed] [Google Scholar]
- 8.Renault CA, Israelski DM, Levy V, et al. Time to clearance of Chlamydia trachomatis ribosomal RNA in women treated for chlamydial infection. Sex Health. 2011;8:69–73. doi: 10.1071/SH10030. [DOI] [PubMed] [Google Scholar]
- 9.Gaydos CA, Crotchfelt KA, Howell MR, et al. Molecular amplification assays to detect chlamydial infections in urine specimens from high school female students and to monitor the persistence of chlamydial DNA after therapy. J Infect Dis. 1998;177:417–424. doi: 10.1086/514207. [DOI] [PubMed] [Google Scholar]
- 10.Cabeza J, Garcia PJ, Segura E, et al. Feasibility of Chlamydia trachomatis screening and treatment in pregnant women in Lima, Peru: A prospective study in two large urban hospitals. Sex Transm Infect. 2015;91:7–10. doi: 10.1136/sextrans-2014-051531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robinson DP, Klein SL. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm Behav. 2012;62:263–271. doi: 10.1016/j.yhbeh.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaushic C, Zhou F, Murdin AD, et al. Effects of estradiol and progesterone on susceptibility and early immune responses to Chlamydia trachomatis infection in the female reproductive tract. Infect Immun. 2000;68:4207–4216. doi: 10.1128/iai.68.7.4207-4216.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geisler WM, Uniyal A, Lee JY, et al. Azithromycin versus doxycycline for urogenital Chlamydia trachomatis infection. N Engl J Med. 2015;373:2512–2521. doi: 10.1056/NEJMoa1502599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dupont WD, Plummer WD. Power and sample size calculations: A review and computer program. Control Clin Trials. 1990;11:116–128. doi: 10.1016/0197-2456(90)90005-m. [DOI] [PubMed] [Google Scholar]
- 15.Lazenby GB, Soper DE, Nolte FS. Correlation of leukorrhea and Trichomonas vaginalis infection. J Clin Microbiol. 2013;51:2323–2327. doi: 10.1128/JCM.00416-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of Gram stain interpretation. J Clin Microbiol. 1991;29:297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Workowski KA, Berman S. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59:1–110. [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. Legal status of expedited partner therapy. [CDC.gov website]. June 2015. http://www.cdc.gov/std/ept/legal/default.htm (accessed 21 December 2015)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


