Abstract
Objectives
Individuals with bipolar disorder (BPD) exhibit alterations in their phospholipid levels. It is unclear whether these alterations are a secondary consequence of illness state, or if phospholipids and illness risk overlap genetically. If the latter were true, then phospholipids might provide key insights into the pathophysiology of the illness. Therefore, we rank-ordered phospholipid classes by their genetic overlap with BPD risk in order to establish which class might be most informative in terms of increasing our understanding of illness pathophysiology.
Methods
Analyses were conducted in a sample of 558 individuals, unselected for BPD, from 38 extended pedigrees (average family size=14.79, range=2–82). We calculated a coefficient of relatedness for all family members of nine individuals with BPD in the sample (N=185); this coefficient was set to be zero in unrelated individuals (N=373). Then, under an endophenotype ranking value (ERV) approach, this scalar index was tested against 13 serum-based phospholipid concentrations in order to rank-order lipid classes by their respective overlap with BPD risk.
Results
The phosphatidylinositol class was significantly heritable (h2=0.26, P=6.71 × 10−05). It was the top-ranked class, and was significantly associated with BPD risk after correction for multiple testing (β=−1.18, P=2.10 × 10−03, ERV=0.49).
Conclusions
We identified a peripheral biomarker, serum-based phosphatidylinositol, which exhibits a significant association with BPD risk. Therefore, given that phosphatidylinositol and BPD risk share partially common etiology, it seems that this lipid class warrants further investigation, not only in terms of treatment, but also as a promising diagnostic and risk marker.
Keywords: bipolar, family study, genetics, lipidome, phosphatidylinositol
1 INTRODUCTION
Identifying endophenotypes for bipolar disorder (BPD) will garner greater understanding of psychiatric illnesses, including BPD, which in turn will aid in their identification, diagnosis and treatment.1–3 An endophenotype is a biomarker, or measurable characteristic, that is associated with disease.4 Crucially, an endophenotype must share some appreciable portion of its genetic etiology with disease risk.4 This requirement is important because it implies that some portion of the biological processes that underlie the endophenotype overlap with those that are disrupted in disease. Thus, the identification of endophenotypes for psychiatric illnesses should contribute to our understanding of illness pathophysiology.5 Peripheral markers, such as serum-based lipid measurements, hold great promise as endophenotypes for two reasons. First, their underlying biochemical underpinnings are relatively well understood, particularly when compared to, for example, brain-or behavior-based phenotypes. Second, peripheral markers are easily obtainable at comparatively low cost.6 These advantages are particularly appealing for BPD, a psychiatric illness that is ranked as one of the leading causes of disability and premature mortality worldwide,7–9 but whose physiological underpinnings are still largely unknown.10
Lipids and their polyunsaturated fatty acids (PUFAs) constitute basic and essential components of all human cells, in terms of both structure, making up the major component of cell membranes, and function, playing a part in neurotransmission, receptor function, and eicosanoid biosynthesis.11,12 A number of lipidomic alterations have been noted in those with BPD and also major depressive disorder (MDD).13 For example, increases in plasma levels of lipid peroxidation have been noted in euthymic adults with BPD6 while decreases have been noted in adolescent BPD individuals.14 It has been shown that essential PUFAs in red blood cell membranes, including arachadonic and docosahexaenoic acid (DHA), are reduced in BPD individuals in a manic phase15 and in individuals with MDD16, and that the fatty acid composition of phospholipids in serum is altered in those with MDD such that the arachadonic:eicosapentaenoic acid ratios are higher.17,18 Accordingly, a handful of studies suggest that administration of fatty acids may have benefits in the amelioration of mood symptoms.19–21 Brain-based findings, both in vivo and in vitro, indicate significant elevations of phosphatidylcholines in the prefontal cortex,22 significantly reduced choline in the frontal lobe,23 and reduced DHA in the orbitofrontal cortex24 in BPD individuals. In sum, there is evidence for alterations in phospholipids and their fatty acids in BPD and MDD. The direction of those alterations is currently unclear, probably in part because of heterogeneity in methods and patient populations, although a recent and large study on this topic, which utilized plasma-based lipid levels, documented an inverse relationship between phospholipid levels and symptoms of depression.26 The present study is the first to examine the influence of genetic liability for BPD on serum-based phospholipids.
Phosphatidylinositol (PI) (a membrane phospholipid that plays a crucial role in cell physiology and signaling27) and its phosphorylated products phosphoinositides (PtdIns) are particularly interesting in the context of BPD, given that lithium (Li+), the first-line mood stabilizer treatment for BPD,28 acts upon the PI signal transduction pathway.29 While there is converging evidence for inositol phospholipid system dysfunction in BPD,30–38 more work is necessary to clarify this relationship.39 Of course, given the link between phospholipids and the mechanism of action of Li+, it is possible that alterations in lipid levels arise as a secondary consequence of treatment for BPD. This is why establishing such a peripheral marker as an endophenotype of BPD is particularly important, as alterations are demonstrated as a function of genetic proximity to an affected individual, that is, in unaffected relatives who are not exposed to bipolar treatment regimens.5 The implication is that alterations in serum phospholipid levels arise as a consequence of shared etiology, making them and their underlying biochemical mechanisms potentially promising diagnostic and/or treatment targets for BPD.
In the present study, we aimed to1 (1) provide evidence for shared etiology between phospholipid concentrations and BPD risk, and2 (2) determine which phospholipid classes might be the most informative when attempting to isolate potential diagnostic and treatment targets for BPD. We took sum concentrations of 13 phospholipid classes in a sample of 558 Mexican American individuals from 38 randomly ascertained extended pedigrees, and calculated mean-based endophenotype ranking values (ERVs)40 between each phospholipid class and a broad BPD phenotype (incorporating BPD types I and II). We used this broad BPD phenotype to increase the total number of included affected individuals, which in turn reduces the noise associated with any single diagnosis and maximizes power.
2 METHODS
2.1 Participants
Lipidomic and diagnostic data were available in 567 individuals from 38 pedigrees (average family size=14.79, range=2–82). The sample was 64% female and had a mean age of 49.28 years (SD=13.34, range=27–97). The lipidomic data were collected as part of the San Antonio Family Study (SAFS), and diagnostic data were also available in these same individuals as part of assessments conducted in the Genetics of Brain Structure and Function (GOBS) study. GOBS data collection occurred between 2006 and 2016. Of the 567 individuals, nine persons had received a BPD diagnosis (BPD types I (N=4) and II (N=5); see Table 1 for additional diagnostic information). Affected individuals were excluded from the analysis and therefore the analysis was performed in 558 individuals, comprising 185 participants related to an affected individual plus 373 unrelated participants (Table 2).
TABLE 1.
Clinical characteristics of the sample
| Number in affected individuals (N=9) | Number in related individuals (N=185) | Number in unrelated individuals (N=373) | |
|---|---|---|---|
| Any depressive disordera | 9 | 54 | 137 |
| Any anxiety disordera | 5 | 18 | 38 |
| Any alcohol use disordera | 6 | 77 | 113 |
| Any substance use disordera | 1 | 32 | 32 |
| Diabetes medicationb | 0 | 5 | 26 |
| Lipid medicationb | 0 | 1 | 6 |
| Hypertension medicationb | 0 | 8 | 27 |
| Diabetes statusb | 0 | 9 | 45 |
| Heart attackb | 0 | 1 | 2 |
| Heart surgeryb | 0 | 1 | 0 |
| Smokerb | 2 | 58 | 77 |
Collected at the time of Genetics of Brain Structure and Function (GOBS) assessment.
Collected at the time of lipid data collection.
TABLE 2.
Means (and standard deviations) for age and percentage of female individuals by degree of relatedness to an individual with bipolar disorder
| Degree of relatedness | N | Mean age (SD) | % female |
|---|---|---|---|
| – | 9 | 34.65 (4.05) | 67 |
| First | 31 | 45.84 (13.12) | 55 |
| Second | 21 | 56.29 (8.85) | 76 |
| Third | 52 | 46.09 (12.16) | 63 |
| Fourth | 40 | 45.26 (11.83) | 45 |
| Fifth | 33 | 36.50 (7.16) | 58 |
| Sixth | 8 | 42.82 (8.04) | 50 |
| Unrelated | 373 | 51.66 (13.36) | 67 |
All participants were randomly selected from the community with the constraints that they were of Mexican American ancestry, part of a large family, and lived in the San Antonio region. All participants provided written informed consent in compliance with the institutional review board at the University of Texas Science Center of San Antonio.
2.2 Diagnostic assessment
The Mini-International Neuropsychiatric Interview (MINI41), a semi-structured interview, was administered to all participants. Interviews were conducted by masters-and doctorate-level research staff, who had established reliability for diagnosing bipolar disorder (κ≥0.85). Subjects who reported possible pathology were discussed in case conference meetings with licensed psychologists and/or psychiatrists. Consensus diagnoses were determined using available medical records, the MINI, and the interviewer’s narrative.
2.3 Lipid extraction and analysis procedure
The lipid extraction procedure used in this sample has been described in detail elsewhere.42,43 Briefly, lipid extraction in the SAFS is part of an ongoing longitudinal observational investigation comprising four phases of data collection during a 23-year period. The lipidomic data used in the present study were collected during the first phase, between the years 1992 and 1996. The order of the plasma samples was randomized prior to lipid extraction and analysis for each cohort. Quality control plasma samples were included at a ratio of 1:18. Total lipid extraction from a 10-mL aliquot of plasma was performed in a single-phase chloroform:methanol (2:1) extraction.44
Lipid analysis was performed using liquid chromatography–electrospray ionization tandem mass spectrometry using an Agilent 1200 liquid chromatography system (Agilent Technologies, Santa Clara, CA, USA) combined with an Applied Biosystems API 4000 Q/TRAP mass spectrometer with a turbo-ionspray source (350 uC) and the Analyst 1.5 data system.44 We have previously reported the use of precursor ion and neutral loss scans on control plasma extracts to identify the predominant lipid species of the following phospholipid classes: sphingomyelin (SM), phosphatidylcholine (PC), alkylphosphatidylcholine [PC(O)], alkenylphosphatidylcholine [PC(P); plasmalogen], lysophosphatidylcholine (LPC), lysoalkylphosphatidylcholine [LPC(O); lysoplatelet activating factor], phosphatidylethanolamine (PE), phosphatidylinositol (PI), phosphatidylserine (PS), and phosphatidylglycerol (PG).44–46
Multiple reaction monitoring (MRM) experiments were established for the major species of each lipid class identified in plasma. Relative lipid amounts were calculated by relating the peak area of each species to the peak area of the corresponding stable isotope or non-physiological internal standard. Total lipid classes were calculated from the sum of the individual lipid species within each class.43
2.4 Quantitative genetic analysis
All genetic analyses were performed in SOLAR.47 SOLAR implements a maximum likelihood variance decomposition to determine the proportion of variation in a phenotype due to genetic and environmental influences by modeling the covariance amongst family members as a function of genetic proximity. The simplest such decomposition is one where the additive genetic contribution of a trait is indexed by the heritability, or h2. All lipid classes were subject to univariate decomposition analysis to ensure that they were significantly heritable.
2.5 Genetic correlation between BPD and lipid classes: the mean-based endophenotype ranking value (mERV)
The mean-based endophenotype ranking value (mERV) represents an extension of the ERV. The ERV, an effect size ranging between 0 and 1, was developed for the purpose of formally testing endophenotypic status of phenotypes and to rank phenotypes by their standardized genetic covariance with a disease of interest; it is expressed as follows:
where is the heritability of disease risk, is the heritability of the endophenotype, and ρG is the genetic correlation between the two.48 The mERV is an extension of the ERV to be used when the disease of interest is not sufficiently common in the data. For details on the derivation of the mERV, see Glahn et al.40 Briefly, the mERV leverages the many coefficients of relationship that exist in extended-pedigree data. The coefficient of relationship refers to the average number of alleles held in common between individuals; for example, first-degree relatives (e.g. full siblings or parents) share on average 50% of their alleles, second-degree relatives (e.g. grandparents or aunts/uncles) share 25%, third-degree relatives (e.g. great-grandparents or great-aunts/-uncles) share 12.5%, and so on. Thus, it is possible, given an individual with a disease, to index all other pedigree members by their degree of relatedness to that individual. This scalar can then be used to perform a fixed-effect single-degree-of-freedom test, within the univariate variance components analysis outlined above, providing an estimate of the standardized genetic covariance between the potential endophenotype and illness risk. The mERV can then be used in the same way as the ERV to rank potential endophenotypes by their degree of standardized genetic overlap with illness risk. In the present paper, the mERV was applied to BPD and all available lipid classes.
We Bonferroni-corrected α (=0.05) by the effective number of traits using the method outlined by Cheverud;49 correcting for the total number of traits would be overly conservative, given the extent to which they are all correlated with one another.50 Applying this method to the pairwise genetic correlations between the 13 phospholipid classes reveals that we have 10.36 effective traits, thus α=0.05/10.36=4.82 × 10−03.
We tested the potential influence of confounding variables, in particular BMI and major depressive disorder (MDD), on all lipid classes. We tested the potential genetic overlap, using a bivariate polygenic model, between the lipids and potential psychiatric and metabolic confounds. We then included those covariates with a significant genetic overlap with the lipid class, using a liberal threshold of P<.10 in order to increase confidence that important covariates were included in the univariate polygenic model described above in addition to the BPD coefficient of relationship (which was fixed in the model). We tested the following variables that were collected at the time of blood sampling as part of the SAFS assessment51: body mass index (BMI); diabetes status; ever had a heart attack; smoking status; and hypertension status. In addition, we investigated the following variables from the GOBS assessment taken from the MINI41: any depressive disorder; any anxiety disorder; any alcohol use disorder; any substance use disorder.
3 RESULTS
3.1 Family profiles
According to our consensus diagnoses, nine individuals met the criteria of our broad BPD phenotype, four individuals met the criteria for BPD I and five met the criteria for BPD II. Table 2 shows the mean age and percent female of each category of relatedness to an affected individual, as well as the unrelated group. No two affected BPD individuals fell within the same pedigree, but these individuals were related to 185 individuals (Table 2).
3.2 Indexing genetic relatedness between BPD liability and all lipid classes
The heritabilities of all lipid classes are shown in Table 3, inspection of which indicates that each class is significantly heritable. Also shown in Table 3 are the β estimates from the mERV analysis along with the P-values, of which one withstood a multiple testing correction, that for the PI class (β=−1.18, P=2.10 × 10−03, ERV=0.49); this class was deemed to be significantly heritable (h2=0.26, SE=0.08, P=6.71 × 10−05).
TABLE 3.
Heritability and degree of bipolar relatedness for all lipid classes
| Lipid class | h2 (P-value) | β (P-value) | ERV | Clinical covariates included* |
|---|---|---|---|---|
| PI | 0.26 (6.71×10−05) | −1.18 (2.10−03) | 0.49 | Any alcohol use disorder; diabetes status |
| LPE | 0.19 (7.82×10−03) | −0.83 (0.03) | 0.33 | None |
| LPC | 0.32 (3.10×10−11) | −0.70 (0.06) | 0.27 | None |
| PC(O) | 0.40 (4.34×10−15) | 0.54 (0.08) | 0.20 | Any anxiety disorder; any depressive disorder |
| PE | 0.41 (8.69×10−14) | −0.43 (0.24) | 0.17 | Diabetes status |
| PC(P) | 0.32 (1.58×10−04) | 0.48 (0.21) | 0.19 | Any anxiety disorder |
| PG | 0.38 (1.20×10−14) | −0.24 (0.12) | 0.18 | Diabetes status; any alcohol use disorder |
| LPC(O) | 0.52 (1.84×10−10) | 0.57 (0.70) | 0.18 | Diabetes status; BMI |
| PC | 0.28 (3.00×10−11) | −0.24 (0.17) | 0.10 | Any alcohol use disorder; diabetes status; BMI |
| PS | 0.34 (1.01×10−14) | 0.21 (0.54) | 0.08 | Any substance use disorder; hypertension; smoker; BMI |
| SM | 0.38 (1.17×10−14) | 0.19 (0.94) | 0.08 | Smoker; any alcohol use disorder; any substance use disorder; any anxiety disorder |
| PE(O) | 0.38 (2.50×10−06) | 0.16 (0.71) | 0.06 | Any depressive disorder |
| PE(P) | 0.45 (6.96×10−09) | 0.16 (0.70) | 0.06 | Any anxiety disorder; any depressive disorder |
Bold indicates class surviving multiple testing correction.
BMI, body mass index; ERV, endophenotype ranking value; PI, phosphatidylcholine; LPE, lysophosphatidylethanolamine; LPC, lysophosphatidylcholine; PC(O), alkylphosphatidylcholine; PE, phosphatidylethanolamine; PC(P), alkenylphosphatidylcholine; PG, phosphatidylglycerol; LPC(O), lysoalkylphosphatidylcholine; PC, phosphatidylcholine; PS, phosphatidylserine; SM, sphingomyelin; PE(O), alkylphosphatidylethanolamine; PE(P), alkenylphosphatidylethanolamine.
Threshold of P<.1.
3.3 Investigating the influence of potential confounds: metabolic and psychiatric
Of the metabolic variables, only diabetes status (ρg=0.35, SE=0.15, P=.03) was significantly associated with PI levels. None of the other metabolic covariates (including BMI, hypertension, heart attack, and smoking status) were significantly associated with PI. Of the psychiatric diagnoses, only alcohol use disorders was significantly associated with PI levels (ρg=−0.58, SE=0.28, P=.04). None of the other psychiatric diagnoses (including depression, anxiety and substance abuse) were significantly associated with PI. Thus, aside from diabetes status and alcohol use disorders, given the lack of significant genetic overlap between the other metabolic and psychiatric phenotypes, we assumed that we need not covary for them when investigating the genetic overlap between BPD risk and PI. Most notably, neither BMI nor major depression demonstrate a shared etiology with serum levels of PI in the present sample, and accordingly neither is likely to be a confounding factor in the association between BPD risk and PI that is present in the sample.
After controlling for diabetes status (in addition to age, age2, sex, and their interactions), first-degree relatives of affected individuals exhibited lower levels of PI than unaffected, unrelated controls (Cohen’s d=−0.53), while second-to sixth-degree relatives exhibited levels intermediate between those of first-degree relatives (Cohen’s d=0.46) and controls (Cohen’s d=−0.52). The levels of PI in cases, unaffected relatives and unaffected unrelated individuals are shown in Figure 1. In general, it appears that the PI levels vary as a function of genetic proximity to an affected individual. It is important to note that cases were not included in the analyses outlined above, and thus their seemingly anomalous PI levels should not be of concern; firstly there are only nine cases and secondly their PI levels are subject to confounding factors such as mood-stabilizing medication.
FIGURE 1.
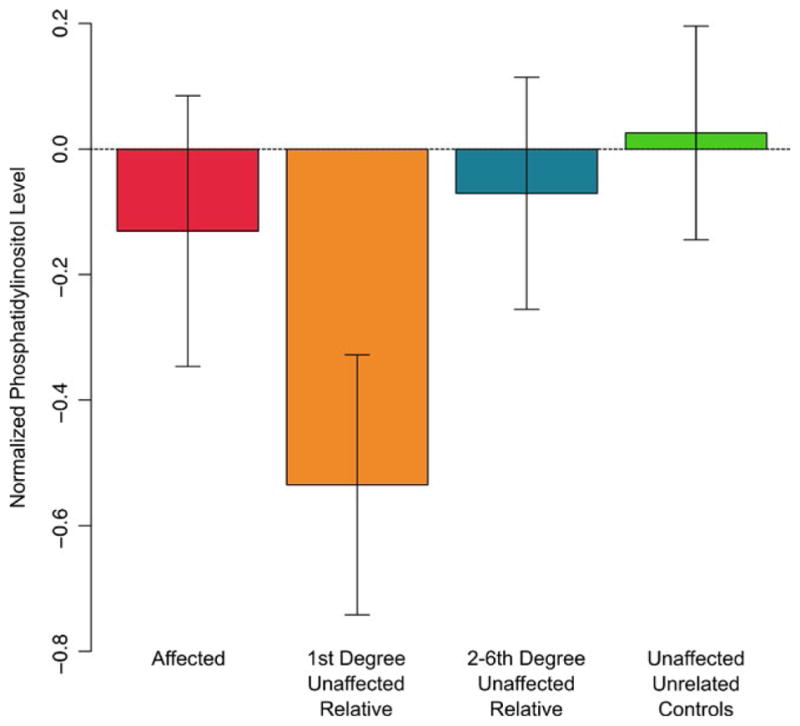
Average phosphatidylinositol levels (inverse Guassian normalized) between groups after controlling for age, age2, sex, any alcohol use disorder and diabetes status. [Colour figure can be viewed at wileyonlinelibrary.com]
4 DISCUSSION
In the present study, we investigated the relative genetic overlap between BPD risk and 13 phospholipid classes; this was in an effort to rank the phospholipids according to which might be most informative when attempting to disentangle the etiology of BPD. To our knowledge, this is the first study to investigate possible genetic overlap between BPD risk and serum phospholipid levels. The existence of significant genetic overlap between BPD risk and phospholipid levels, and more specifically between illness risk and PI, strongly suggests that PI is not merely a secondary manifestation of either illness state or treatment but rather an endophenotypic marker of the illness with the potential for aiding early detection and diagnosis, as well as enhanced treatment regimens.
Phosphatidylinositols are membrane phospholipids found mostly on the inner leaflet of the cell and are characterized by an inositol ring, or head group, extending into the cytoplasm.52 Despite their relatively low abundance compared to other membrane lipids, it is the metabolism of this phospholipid class which gives rise to second messengers which are major contributors to the myriad aspects of cellular regulation that make up the PI signal transduction pathway.53,54 PI is implicated in well-characterized signal transduction pathways, alterations in the molecular components of which, in particular Phosphatidylinositol 4,5-bisphosphate (PIP2) and protein kinase C levels, have been previously associated with BPD.31,37,55–65 The present study, to our knowledge, is the first to suggest a shared etiology between serum PI levels and risk for BPD. PI is a particularly interesting candidate endophenotype for BPD given that lithium (Li+), a mood-stabilizing drug and treatment of choice for BPD,66 is thought to act upon the PI signaling pathway. The inositol-depletion hypothesis posits that lithium acts by preventing the production of PI via inhibition of inositol monophosphatase, thereby limiting turnover of inositol in the cell.29,66
The direction of the relationship between BPD risk and PI in the present study was negative, meaning that heightened risk for BPD was associated with low levels of the phospholipid. This is the only study, to our knowledge, to assess phospholipid levels in serum in relation to BPD risk. However, Demirkan and colleagues also showed a negative correlation between plasma-based phospholipids (phosphatidylcholine and sphingomyelin) and symptoms of depression and anxiety.26 Therefore, while our results are seemingly in keeping with the previous literature, they are not necessarily what the inositol-depletion hypothesis might predict, where Li+ theoretically works to decrease high levels of inositol in BPD subjects. Of course, the present study, like that of Demirkan and colleagues,26 relies on peripheral indices of lipid levels. This allows us only to speculate on the ways in which these findings might be interpreted in the brain. There is surprisingly little information in the literature regarding either the direct origin of PI in circulation or the relationship between levels in the periphery and in the brain. Serum PI is likely hepatic in origin, as the majority of circulating lipids in lipoproteins are generated from the liver. The level of PIs is dependent on the availability of myo-inositol, which in turn is synthesized from glucose. The enzymes responsible for all these steps are found in liver cells as well as other organs. There is evidence to suggest that lipids and their fatty acids are shuttled to the brain from the liver, where they play crucial roles in neuro-development, -inflammation and -protection.67–69
Future work might attempt to determine the levels of phospholipids in the brain and even the relationship between phospholipid levels in the brain and in the periphery. One such line of research might utilize phosphorous-31 magnetic resonance spectroscopy (31P MRS), an imaging method that allows non-invasive measurement of biological compounds (e.g. phospholipids) in vivo. In BPD, this technique has been used to demonstrate significantly reduced choline, indicating altered phospholipid metabolism, in the frontal lobe.23 The phosphomonester (PME) signal in 31P MRS reflects the level of phosphocholine and phosphoethanolamine in addition to choline and myo-inositol; 70 there is evidence to suggest that the PME signal increases during manic states and decreases during depressed states.33,71–73 Thus, in addition to the unknown relationship between phospholipid levels in the periphery and brain, it is possible that a second level of complexity exists where levels in both are affected by BPD illness phase. Evidence from post-mortem studies for phospholipid alterations in the brain in BPD is mixed; in some studies, alterations in phospholipids and/or their fatty acids have not been observed in BPD subjects versus controls,74–76 while in other studies such alterations have been observed.24,77 There is little consistency in terms of the focal brain region across these studies, which may explain the inconsistencies in the results. In addition, it is possible that lipidomic abnormalities in relation to affective disorders may be characterized differently in other ethnic populations. For example, non-Hispanic populations exhibit altered lipidomic profiles and associated risk for myocardial infarcation relative to Hispanics.78 Thus, overall, it is important that the generalizability of the findings of the present study should be further tested in future research.
In the present study, there was a gap in time between the collection of the lipidomic data and the occurrence of the psychiatric assessments. There is relatively little known about the longitudinal variability of serum phospholipid levels. There is evidence to suggest that phospholipids vary as a function of age,43 and as a consequence we residualized the phospholipid traits for age (in fact for age, age2, sex, and their interactions) at the time of data collection. We consider this a potential strength of the study as it suggests that variation in PI reflects an early etiological step in the development of BPD. We cannot investigate this in detail in the present sample, with the limited number of affected cases available, but it is possible that the present results hint that alterations in PI reflect an “at-risk” condition for BPD. Certainly, longitudinal studies of other peripheral markers, in this case markers of inflammation, support an etiological role of inflammation in risk for MDD.79–81
Two potential criticisms might be leveled at the present study regarding the affected individuals. The first criticism is that no two affected individuals with BPD occur in the same family, thus negating the idea that genetic factors underlie the illness. However, the heritability of BPD is not in question, having been established by numerous family-based studies and large genome wide association studies previously.1 Also, it is not improbable that no two affected individuals would be part of the same family. Rather, what we would expect is that an individual with an affected relative would have an increased risk for developing BPD compared to an individual without an affected relative. This risk may not necessarily be represented phenotypically as the full manifestation of the disorder within the relative’s lifetime (or indeed by the time of assessment), but may influence the expression of phenotypes related to the disorder. The second criticism is that only nine affected individuals were included in the present study, but importantly these individuals originated from extended pedigrees. Therefore, because the question under investigation in this study was one of genetic liability, this sample, comprising multiple extended pedigrees encapsulating many degrees of relatedness, provides the statistical power needed to adequately test hypotheses about putative pleiotropy between phospholipids and BPD risk. Indeed, an advantage of large, extended pedigrees such as this (where family sizes varied between 2 and 82 individuals) is that many unaffected relatives, encapsulating many degrees of relatedness, are available for a small number of cases.40 That being said, in subsequent work where we attempt to fine-tune our hypotheses regarding PI and its role in BPD, we may need greater numbers of probands.
The ERV is the product of three terms: the square root of the heritability of the endophenotype, the square root of the heritability of the disease, and the absolute value of the genetic correlation between the two. Similarly, the power of the ERV is a function of all three of these components, in the same way that the power of a genetic correlation is. While the heritability of the endophenotypes (i.e. the phospholipid class) can be directly estimated in the present sample, the heritability of BPD cannot, given that we have nine affected individuals. For a single endophenotype, the heritability of BPD is not identifiable (in the statistical sense) with this method. However, in principle with enough endophenotypes, the heritability of BPD may be estimable from the method due to the constraints on the parameter spaces of both the heritability and the genetic correlation, but it would generally be difficult to resolve. The total ERV, which our inference of genetic correlation/pleiotropy is based upon, is well estimated in the design. One of the substantial benefits of this method of calculating the ERV is that it does not require affected relatives of cases and thus is very useful for studying genetic determinants (shared via endophenotypes) of low-frequency diseases.
In summary, the findings presented here highlight PI as having a significant genetic overlap with BPD risk. While it has been previously demonstrated that those with BPD exhibit altered levels of phospholipids, this is the first study to highlight a shared etiology between BPD and phospholipids. It is unlikely that the association between PI and BPD risk in the present study arose as an artifact of lithium treatment, as affected individuals were excluded from all genetic analyses. Rather, the serum level of this lipid appears to vary in unaffected individuals as a function of genetic relatedness to a BPD individual and therefore the present study highlights the potential utility of serum-level measurements of PI as an indicator of illness risk. Moreover, this study suggests that the well-characterized PI signaling pathway may be an interesting avenue of research for BPD, potentially providing testable hypotheses for research aiming to improve diagnostic markers and/or treatment targets for BPD.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Barnett JH, Smoller JW. The genetics of bipolar disorder. Neuroscience. 2009;164:331–343. doi: 10.1016/j.neuroscience.2009.03.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frey BN, Andreazza AC, Houenou J, et al. Biomarkers in bipolar disorder: a positional paper from the International Society for Bipolar Disorders Biomarkers Task Force. Aust N Z J Psychiatry. 2013;47:321–332. doi: 10.1177/0004867413478217. [DOI] [PubMed] [Google Scholar]
- 3.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 4.Beauchaine TP. The role of biomarkers and endophenotypes in prevention and treatment of psychopathological disorders. Biomark Med. 2009;3:1–3. doi: 10.2217/17520363.3.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glahn DC, Knowles EE, McKay DR, et al. Arguments for the sake of endophenotypes: examining common misconceptions about the use of endophenotypes in psychiatric genetics. Am J Med Genet B Neuropsychiatr Genet. 2014;165:122–130. doi: 10.1002/ajmg.b.32221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Versace A, Andreazza AC, Young LT, et al. Elevated serum measures of lipid peroxidation and abnormal prefrontal white matter in euthymic bipolar adults: toward peripheral biomarkers of bipolar disorder. Mol Psychiatry. 2014;19:200–208. doi: 10.1038/mp.2012.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murray CJL, Lopez AD. The Global Burden of Disease: A Comprehensive Assessment of Mortality and Disability from Disease, Injuries, and Risk Factors in 1990 and Projected to 2020. Cambridge, Mass: Harvard University Press; 1996. [Google Scholar]
- 8.Baldessarini RJ, Tondo L. Suicide risk and treatments for patients with bipolar disorder. JAMA. 2003;290:1517–1519. doi: 10.1001/jama.290.11.1517. [DOI] [PubMed] [Google Scholar]
- 9.Baldessarini RJ, Pompili M, Tondo L. Suicide in bipolar disorder: risks and management. CNS Spectr. 2006;11:465–471. doi: 10.1017/s1092852900014681. [DOI] [PubMed] [Google Scholar]
- 10.Manji HK, Quiroz JA, Payne JL, et al. The underlying neurobiology of bipolar disorder. World Psychiatry. 2003;2:136–146. [PMC free article] [PubMed] [Google Scholar]
- 11.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown HA, Murphy RC. Working towards an exegesis for lipids in biology. Nat Chem Biol. 2009;5:602–606. doi: 10.1038/nchembio0909-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horrobin DF, Bennett CN. Depression and bipolar disorder: relationships to impaired fatty acid and phospholipid metabolism and to diabetes, cardiovascular disease, immunological abnormalities, cancer, ageing and osteoporosis. Possible candidate genes. Prostaglandins Leukot Essent Fatty Acids. 1999;60:217–234. doi: 10.1054/plef.1999.0037. [DOI] [PubMed] [Google Scholar]
- 14.Scola G, McNamara RK, Croarkin PE, et al. Lipid peroxidation biomarkers in adolescents with or at high-risk for bipolar disorder. J Affect Disord. 2016;192:176–183. doi: 10.1016/j.jad.2015.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiu CC, Huang SY, Su KP, et al. Polyunsaturated fatty acid deficit in patients with bipolar mania. Eur Neuropsychopharmacol. 2003;13:99–103. doi: 10.1016/s0924-977x(02)00130-x. [DOI] [PubMed] [Google Scholar]
- 16.Peet M, Murphy B, Shay J, Horrobin D. Depletion of omega-3 fatty acid levels in red blood cell membranes of depressive patients. Biol Psychiatry. 1998;43:315–319. doi: 10.1016/s0006-3223(97)00206-0. [DOI] [PubMed] [Google Scholar]
- 17.Adams PB, Lawson S, Sanigorski A, Sinclair AJ. Arachidonic acid to eicosapentaenoic acid ratio in blood correlates positively with clinical symptoms of depression. Lipids. 1996;31(Suppl):S157–S161. doi: 10.1007/BF02637069. [DOI] [PubMed] [Google Scholar]
- 18.Maes M, Smith R, Christophe A, Cosyns P, Desnyder R, Meltzer H. Fatty acid composition in major depression: decreased omega 3 fractions in cholesteryl esters and increased C20: 4 omega 6/C20:5 omega 3 ratio in cholesteryl esters and phospholipids. J Affect Disord. 1996;38:35–46. doi: 10.1016/0165-0327(95)00092-5. [DOI] [PubMed] [Google Scholar]
- 19.Stoll AL, Severus WE, Freeman MP, et al. Omega 3 fatty acids in bipolar disorder: a preliminary double-blind, placebo-controlled trial. Arch Gen Psychiatry. 1999;56:407–412. doi: 10.1001/archpsyc.56.5.407. [DOI] [PubMed] [Google Scholar]
- 20.Grosso G, Pajak A, Marventano S, et al. Role of omega-3 fatty acids in the treatment of depressive disorders: a comprehensive meta-analysis of randomized clinical trials. PLoS ONE. 2014;9:e96905. doi: 10.1371/journal.pone.0096905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frangou S, Lewis M, McCrone P. Efficacy of ethyl-eicosapentaenoic acid in bipolar depression: randomised double-blind placebo-controlled study. Br J Psychiatry. 2006;188:46–50. doi: 10.1192/bjp.188.1.46. [DOI] [PubMed] [Google Scholar]
- 22.Schwarz E, Prabakaran S, Whitfield P, et al. High throughput lipidomic profiling of schizophrenia and bipolar disorder brain tissue reveals alterations of free fatty acids, phosphatidylcholines, and ceramides. J Proteome Res. 2008;7:4266–4277. doi: 10.1021/pr800188y. [DOI] [PubMed] [Google Scholar]
- 23.Cecil KM, DelBello MP, Morey R, Strakowski SM. Frontal lobe differences in bipolar disorder as determined by proton MR spectroscopy. Bipolar Disord. 2002;4:357–365. doi: 10.1034/j.1399-5618.2002.02235.x. [DOI] [PubMed] [Google Scholar]
- 24.McNamara RK, Jandacek R, Rider T, et al. Deficits in docosahexaenoic acid and associated elevations in the metabolism of arachidonic acid and saturated fatty acids in the postmortem orbitofrontal cortex of patients with bipolar disorder. Psychiatry Res. 2008;160:285–299. doi: 10.1016/j.psychres.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soares JC, Mallinger AG, Dippold CS, Frank E, Kupfer DJ. Platelet membrane phospholipids in euthymic bipolar disorder patients: are they affected by lithium treatment? Biol Psychiatry. 1999;45:453–457. doi: 10.1016/s0006-3223(98)00048-1. [DOI] [PubMed] [Google Scholar]
- 26.Demirkan A, Isaacs A, Ugocsai P, et al. Plasma phosphatidylcholine and sphingomyelin concentrations are associated with depression and anxiety symptoms in a Dutch family-based lipidomics study. J Psychiatr Res. 2013;47:357–362. doi: 10.1016/j.jpsychires.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- 28.Fountoulakis KN, Vieta E, Sanchez-Moreno J, Kaprinis SG, Goikolea JM, Kaprinis GS. Treatment guidelines for bipolar disorder: a critical review. J Affect Disord. 2005;86:1–10. doi: 10.1016/j.jad.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Lenox RH, Wang L. Molecular basis of lithium action: integration of lithium-responsive signaling and gene expression networks. Mol Psychiatry. 2003;8:135–144. doi: 10.1038/sj.mp.4001306. [DOI] [PubMed] [Google Scholar]
- 30.Jope RS, Song L, Li PP, et al. The phosphoinositide signal transduction system is impaired in bipolar affective disorder brain. J Neurochem. 1996;66:2402–2409. doi: 10.1046/j.1471-4159.1996.66062402.x. [DOI] [PubMed] [Google Scholar]
- 31.Soares JC, Mallinger AG. Intracellular phosphatidylinositol pathway abnormalities in bipolar disorder patients. Psychopharmacol Bull. 1997;33:685–691. [PubMed] [Google Scholar]
- 32.Baum AE, Akula N, Cabanero M, et al. A genome-wide association study implicates diacylglycerol kinase eta (DGKH) and several other genes in the etiology of bipolar disorder. Mol Psychiatry. 2008;13:197–207. doi: 10.1038/sj.mp.4002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kato T, Shioiri T, Takahashi S, Inubushi T. Measurement of brain phosphoinositide metabolism in bipolar patients using in vivo 31P-MRS. J Affect Disord. 1991;22:185–190. doi: 10.1016/0165-0327(91)90064-y. [DOI] [PubMed] [Google Scholar]
- 34.Silverstone PH, McGrath BM, Kim H. Bipolar disorder and myo-inositol: a review of the magnetic resonance spectroscopy findings. Bipolar Disord. 2005;7:1–10. doi: 10.1111/j.1399-5618.2004.00174.x. [DOI] [PubMed] [Google Scholar]
- 35.Atagun MI, Sikoglu EM, Can SS, et al. Investigation of Heschl’s gyrus and planum temporale in patients with schizophrenia and bipolar disorder: a proton magnetic resonance spectroscopy study. Schizophr Res. 2015;161:202–209. doi: 10.1016/j.schres.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manji HK, Lenox RH. Signaling: cellular insights into the pathophysiology of bipolar disorder. Biol Psychiatry. 2000;48:518–530. doi: 10.1016/s0006-3223(00)00929-x. [DOI] [PubMed] [Google Scholar]
- 37.Hahn CG, Friedman E. Abnormalities in protein kinase C signaling and the pathophysiology of bipolar disorder. Bipolar Disord. 1999;1:81–86. doi: 10.1034/j.1399-5618.1999.010204.x. [DOI] [PubMed] [Google Scholar]
- 38.McNamara RK, Ostrander M, Abplanalp W, Richtand NM, Benoit SC, Clegg DJ. Modulation of phosphoinositide-protein kinase C signal transduction by omega-3 fatty acids: implications for the pathophysiology and treatment of recurrent neuropsychiatric illness. Prostaglandins Leukot Essent Fatty Acids. 2006;75:237–257. doi: 10.1016/j.plefa.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 39.Harwood AJ. Lithium and bipolar mood disorder: the inositol-depletion hypothesis revisited. Mol Psychiatry. 2005;10:117–126. doi: 10.1038/sj.mp.4001618. [DOI] [PubMed] [Google Scholar]
- 40.Glahn DC, Williams JT, McKay DR, et al. Discovering schizophrenia endophenotypes in randomly ascertained pedigrees. Biol Psychiatry. 2015;77:75–83. doi: 10.1016/j.biopsych.2014.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- 42.Meikle PJ, Wong G, Barlow CK, et al. Plasma lipid profiling shows similar associations with prediabetes and type 2 diabetes. PLoS ONE. 2013;8:e74341. doi: 10.1371/journal.pone.0074341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weir JM, Wong G, Barlow CK, et al. Plasma lipid profiling in a large population-based cohort. J Lipid Res. 2013;54:2898–2908. doi: 10.1194/jlr.P035808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meikle PJ, Wong G, Tsorotes D, et al. Plasma lipidomic analysis of stable and unstable coronary artery disease. Arterioscler Thromb Vasc Biol. 2011;31:2723–2732. doi: 10.1161/ATVBAHA.111.234096. [DOI] [PubMed] [Google Scholar]
- 45.Borg ML, Andrews ZB, Duh EJ, Zechner R, Meikle PJ, Watt MJ. Pigment epithelium-derived factor regulates lipid metabolism via adipose triglyceride lipase. Diabetes. 2011;60:1458–1466. doi: 10.2337/db10-0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boslem E, MacIntosh G, Preston AM, et al. A lipidomic screen of palmitate-treated MIN6 beta-cells links sphingolipid metabolites with endoplasmic reticulum (ER) stress and impaired protein trafficking. Biochem J. 2011;435:267–276. doi: 10.1042/BJ20101867. [DOI] [PubMed] [Google Scholar]
- 47.Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Glahn DC, Curran JE, Winkler AM, et al. High dimensional endophenotype ranking in the search for major depression risk genes. Biol Psychiatry. 2012;71:6–14. doi: 10.1016/j.biopsych.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheverud JM. A simple correction for multiple comparisons in interval mapping genome scans. Heredity (Edinb) 2001;87:52–58. doi: 10.1046/j.1365-2540.2001.00901.x. [DOI] [PubMed] [Google Scholar]
- 50.Knowles EE, Carless MA, de Almeida MA, et al. Genome-wide significant localization for working and spatial memory: Identifying genes for psychosis using models of cognition. Am J Med Genet B Neuropsychiatr Genet. 2014;165:84–95. doi: 10.1002/ajmg.b.32211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mitchell BD, Almasy LA, Rainwater DL, et al. Diabetes and hypertension in Mexican American families: relation to cardiovascular risk. Am J Epidemiol. 1999;149:1047–1056. doi: 10.1093/oxfordjournals.aje.a009750. [DOI] [PubMed] [Google Scholar]
- 52.Falkenburger BH, Jensen JB, Dickson EJ, Suh BC, Hille B. Phosphoinositides: lipid regulators of membrane proteins. J Physiol. 2010;588:3179–3185. doi: 10.1113/jphysiol.2010.192153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Delage E, Puyaubert J, Zachowski A, Ruelland E. Signal transduction pathways involving phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate: convergences and divergences among eukaryotic kingdoms. Prog Lipid Res. 2013;52:1–14. doi: 10.1016/j.plipres.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 54.Balla T. Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiol Rev. 2013;93:1019–1137. doi: 10.1152/physrev.00028.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mitchell PB, Manji HK, Chen G, et al. High levels of Gs alpha in platelets of euthymic patients with bipolar affective disorder. Am J Psychiatry. 1997;154:218–223. doi: 10.1176/ajp.154.2.218. [DOI] [PubMed] [Google Scholar]
- 56.Wang HY, Markowitz P, Levinson D, Undie AS, Friedman E. Increased membrane-associated protein kinase C activity and translocation in blood platelets from bipolar affective disorder patients. J Psychiatr Res. 1999;33:171–179. doi: 10.1016/s0022-3956(98)90057-7. [DOI] [PubMed] [Google Scholar]
- 57.Perez J, Tardito D, Mori S, Racagni G, Smeraldi E, Zanardi R. Abnormalities of cyclic adenosine monophosphate signaling in platelets from untreated patients with bipolar disorder. Arch Gen Psychiatry. 1999;56:248–253. doi: 10.1001/archpsyc.56.3.248. [DOI] [PubMed] [Google Scholar]
- 58.Tardito D, Mori S, Racagni G, Smeraldi E, Zanardi R, Perez J. Protein kinase A activity in platelets from patients with bipolar disorder. J Affect Disord. 2003;76:249–253. doi: 10.1016/s0165-0327(02)00065-4. [DOI] [PubMed] [Google Scholar]
- 59.Ikenaga EH, Talib LL, Ferreira AS, Machado-Vieira R, Forlenza OV, Gattaz WF. Reduced activities of phospholipases A2 in platelets of drug-naive bipolar disorder patients. Bipolar Disord. 2015;17:97–101. doi: 10.1111/bdi.12229. [DOI] [PubMed] [Google Scholar]
- 60.Soares JC, Chen G, Dippold CS, et al. Concurrent measures of protein kinase C and phosphoinositides in lithium-treated bipolar patients and healthy individuals: a preliminary study. Psychiatry Res. 2000;95:109–118. doi: 10.1016/s0165-1781(00)00175-x. [DOI] [PubMed] [Google Scholar]
- 61.Soares JC, Mallinger AG. Abnormal phosphatidylinositol (PI)-signalling in bipolar disorder. Biol Psychiatry. 1996;39:461–464. doi: 10.1016/0006-3223(95)00527-7. [DOI] [PubMed] [Google Scholar]
- 62.Friedman E, Hoau-Yan-Wang, Levinson D, Connell TA, Singh H. Altered platelet protein kinase C activity in bipolar affective disorder, manic episode. Biol Psychiatry. 1993;33:520–525. doi: 10.1016/0006-3223(93)90006-y. [DOI] [PubMed] [Google Scholar]
- 63.Brown AS, Mallinger AG, Renbaum LC. Elevated platelet membrane phosphatidylinositol-4,5-bisphosphate in bipolar mania. Am J Psychiatry. 1993;150:1252–1254. doi: 10.1176/ajp.150.8.1252. [DOI] [PubMed] [Google Scholar]
- 64.Karege F, Schwald M, El Kouaissi R. Drug-induced decrease of protein kinase a activity reveals alteration in BDNF expression of bipolar affective disorder. Neuropsychopharmacology. 2004;29:805–812. doi: 10.1038/sj.npp.1300384. [DOI] [PubMed] [Google Scholar]
- 65.Pandey GN, Dwivedi Y, SridharaRao J, Ren X, Janicak PG, Sharma R. Protein kinase C and phospholipase C activity and expression of their specific isozymes is decreased and expression of MARCKS is increased in platelets of bipolar but not in unipolar patients. Neuropsychopharmacology. 2002;26:216–228. doi: 10.1016/S0893-133X(01)00327-X. [DOI] [PubMed] [Google Scholar]
- 66.Malhi GS, Tanious M, Das P, Coulston CM, Berk M. Potential mechanisms of action of lithium in bipolar disorder. Current understanding. CNS Drugs. 2013;27:135–153. doi: 10.1007/s40263-013-0039-0. [DOI] [PubMed] [Google Scholar]
- 67.Farooqui AA, Horrocks LA, Farooqui T. Modulation of inflammation in brain: a matter of fat. J Neurochem. 2007;101:577–599. doi: 10.1111/j.1471-4159.2006.04371.x. [DOI] [PubMed] [Google Scholar]
- 68.Tanaka K, Farooqui AA, Siddiqi NJ, Alhomida AS, Ong WY. Effects of docosahexaenoic Acid on neurotransmission. Biomol Ther (Seoul) 2012;20:152–157. doi: 10.4062/biomolther.2012.20.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Heneka MT, Carson MJ, El Khoury J, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim JE, Lyoo IK, Renshaw PF. Neurochemical and Metabolic Imaging in Bipolar disorder. In: Strakowski SM, editor. The Bipolar Brain: Integrating Neuroimaging and Genetics. Oxford University Press; 2012. pp. 79–102. [Google Scholar]
- 71.Kato T, Takahashi S, Shioiri T, Murashita J, Hamakawa H, Inubushi T. Reduction of brain phosphocreatine in bipolar II disorder detected by phosphorus-31 magnetic resonance spectroscopy. J Affect Disord. 1994;31:125–133. doi: 10.1016/0165-0327(94)90116-3. [DOI] [PubMed] [Google Scholar]
- 72.Kato T, Takahashi S, Shioiri T, Inubushi T. Alterations in brain phosphorous metabolism in bipolar disorder detected by in vivo 31P and 7Li magnetic resonance spectroscopy. J Affect Disord. 1993;27:53–59. doi: 10.1016/0165-0327(93)90097-4. [DOI] [PubMed] [Google Scholar]
- 73.Yildiz A, Sachs GS, Dorer DJ, Renshaw PF. 31P Nuclear magnetic resonance spectroscopy findings in bipolar illness: a meta-analysis. Psychiatry Res. 2001;106:181–191. doi: 10.1016/s0925-4927(01)00082-8. [DOI] [PubMed] [Google Scholar]
- 74.Igarashi M, Ma K, Gao F, et al. Brain lipid concentrations in bipolar disorder. J Psychiatr Res. 2010;44:177–182. doi: 10.1016/j.jpsychires.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hamazaki K, Choi KH, Kim HY. Phospholipid profile in the postmortem hippocampus of patients with schizophrenia and bipolar disorder: no changes in docosahexaenoic acid species. J Psychiatr Res. 2010;44:688–693. doi: 10.1016/j.jpsychires.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hamazaki K, Maekawa M, Toyota T, Dean B, Hamazaki T, Yoshikawa T. Fatty acid composition of the postmortem corpus callosum of patients with schizophrenia, bipolar disorder, or major depressive disorder. Eur Psychiatry. 2016;39:51–56. doi: 10.1016/j.eurpsy.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 77.McNamara RK, Rider T, Jandacek R, Tso P. Abnormal fatty acid pattern in the superior temporal gyrus distinguishes bipolar disorder from major depression and schizophrenia and resembles multiple sclerosis. Psychiatry Res. 2014;215:560–567. doi: 10.1016/j.psychres.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Willey JZ, Rodriguez CJ, Carlino RF, et al. Race-ethnic differences in the association between lipid profile components and risk of myocardial infarction: the Northern Manhattan Study. Am Heart J. 2011;161:886–892. doi: 10.1016/j.ahj.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gimeno D, Kivimaki M, Brunner EJ, et al. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychol Med. 2009;39:413–423. doi: 10.1017/S0033291708003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pasco JA, Nicholson GC, Williams LJ, et al. Association of high-sensitivity C-reactive protein with de novo major depression. Br J Psychiatry. 2010;197:372–377. doi: 10.1192/bjp.bp.109.076430. [DOI] [PubMed] [Google Scholar]
- 81.Zunszain PA, Hepgul N, Pariante CM. Inflammation and depression. Curr Top Behav Neurosci. 2013;14:135–151. doi: 10.1007/7854_2012_211. [DOI] [PubMed] [Google Scholar]


