Abstract
Osteoporosis is an enormous and growing public health problem. Once considered an inevitable consequence of ageing, it is now eminently preventable and treatable. Ironically, despite tremendous therapeutic advances, there is an increasing treatment gap for patients at high fracture risk. In this Series paper, we trace the evolution of drug therapy for osteoporosis, which began in the 1940s with the demonstration by Fuller Albright that treatment with oestrogen could reverse the negative calcium balance that developed in women after menopause or oophorectomy. We note a watershed in osteoporosis drug discovery around the year 2000, when the approach to developing novel therapeutics shifted from one driven by discoveries in animal studies and clinical observations (eg, oestrogen, calcitonin, and teriparatide) or opportunistic repurposing of existing compounds (eg, bisphosphonates) to one driven by advances in fundamental bone biology (eg, denosumab) coupled with clues from patients with rare bone diseases (eg, romosozumab, odanacatib). Despite these remarkable advances, concerns about rare side-effects of anti-resorptive drugs, particularly bisphosphonates, and the absence of clear evidence in support of their long-term efficacy is leading many patients who could benefit from drug therapy to not take these drugs. As such, there remains an important clinical need to develop ways to enhance patient acceptance and compliance with these effective drugs, and to continue to develop new drugs that do not cause these side-effects and have prolonged anabolic effects on bone. Such changes could lead to a true reversal of this potentially devastating disease of ageing.
Introduction
The past 30 years have witnessed remarkable developments in our understanding of the pathogenesis of osteoporosis and the availability of new drugs to treat the disease. In the late 1980s, a doctor could offer a postmenopausal woman little else but oestrogen replacement and perhaps calcitonin, along with calcium and vitamin D supplementation, as a treatment for osteoporosis. In 2017, the options include not only oestrogen and calcitonin, but also a selective oestrogen-receptor modulator (SERM; raloxifene),1 four bisphosphonates (alendronate, risedronate, ibandronate, and zoledronic acid),2 a human monoclonal antibody to the receptor activator of nuclear factor-κB (NF-κB) ligand (RANKL; denosumab),3 and the parathyroid hormone analogue teriparatide.4 On the horizon are two new drugs: a parathyroid hormone-related peptide analogue (abaloparatide)5 and a humanised monoclonal antibody to sclerostin (romosozumab).6 A third candidate drug, the cathepsin K inhibitor odanacatib, had significant anti-fracture efficacy, but the pivotal phase 3 clinical trial was terminated because of an unforeseen increased risk of stroke.7 Despite this remarkable progress in drug development for the prevention and treatment of fractures, major challenges to implementing appropriate treatment remain, even for patients who unequivocally need therapy. For example, among 22 598 patients in an American commercial health plan who had a hip fracture, use of bisphosphonates decreased from only 15% in 2004 to 3% in 2013.8
In this Series paper, we briefly review the history of drug development for osteoporosis, noting an important shift in discovery approaches around the year 2000. We then focus on drugs that are on the horizon and present ongoing challenges to ensuring that patients who are at increased fracture risk receive appropriate treatment. Finally, we review the case that despite the tremendous progress to date, compelling reasons exist for continued drug discovery and development for the prevention of fractures.
Overview of existing therapies
An overview of existing drugs for the prevention or treatment of osteoporosis and their development pathways are shown in table 1.
Table 1. Drugs for the prevention or treatment of osteoporosis and their drug development pathway.
| Drug names | Drug development pathway | Approval status* | |
|---|---|---|---|
| Anti-resorptive | |||
| Oestrogen (oral, transdermal) | Oestrogen | Clinical observations | Approved |
| Selective oestrogen-receptor modulators | Raloxifene, bazedoxifene plus oestrogen | Medicinal chemistry | Approved |
| Calcitonin (human, salmon) | ·· | Physiological studies | Approved |
| Bisphosphonates | Alendronate, isedronate, ibandronate, zoledronic acid | Opportunistic discovery of bone-protective effect of existing chemical compounds | Approved |
| RANKL antibody | Denosumab | Understanding of fundamental bone biology drives development of a novel therapeutic | Approved |
|
| |||
| Anabolic | |||
|
| |||
| Parathyroid hormone or parathyroid hormone-related peptide analogues | Teriparatide, abaloparatide | Clinical observations lead first to animal studies and then to human studies | Approved |
|
| |||
| Mixed | |||
|
| |||
| Sclerostin antibody | Romosozumab | Characterisation of rare bone diseases and fundamental bone biology drives development of a novel therapeutic | Pending approval |
| Cathepsin K inhibitor | Odanacatib | Characterisation of rare bone diseases and fundamental bone biology drives development of a novel therapeutic | Terminated |
RANKL=receptor activator of nuclear factor κB ligand.
All drugs with approved status, except for abaloparatide, have been approved in most countries, including the USA, Europe, and Australia; abaloparatide has only been approved in the USA.
Oestrogen
Seminal observations by Fuller Albright9 in the 1940s established the key role for oestrogen in regulating bone metabolism in women, and oestrogen was subsequently found to also have an important role in the male skeleton.10 Oestrogen treatment is effective in both the prevention11 and treatment12 of osteoporosis. The definitive study of the efficacy and side-effects of oestrogen was the Women's Health Initiative (WHI), in which 16 608 postmenopausal women aged 50–79 years were randomly assigned to receive conjugated equine oestrogen (0.625 mg/day) and medroxyprogesterone acetate (2.5 mg/day, to prevent uterine hyperplasia) or placebo for an average of 5.6 years. In addition to increasing bone mineral density (BMD) at multiple sites, oestrogen treatment reduced the risk of fracture by 24% (hazard ratio [HR] 0.76, 95% CI 0.69–0.83).13 However, because of the well documented side-effects of oestrogen that were revealed in the WHI study, including an increase in cardiovascular events and breast cancer risk,14 oestrogen is now used primarily for short-term prevention of menopausal hotflashes. Transdermal oestrogen (0.05–0.10 mg/day) combined with a progestin (in women with an intact uterus) is an alternative regimen for osteoporosis prevention and treatment,12 but whether transdermal oestrogen causes similar adverse events as oral conjugated oestrogen remains unclear. Ultra-low dose oestrogen (0.014 mg/day) has also been shown to prevent reductions in BMD in postmenopausal women without causing uterine hyperplasia15 and has been approved for osteoporosis prevention by the US Food and Drug Administration (FDA). However, whether this ultra-low dose prevents fractures or is associated with the other adverse events noted in the WHI study is not known. Thus, oestrogen-based therapies might not be viable long-term treatments for osteoporosis.
SERMs
SERMs were developed from oestrogen using medicinal chemistry approaches with the goal of having oestrogenlike (agonistic) effects in some tissues (eg, bone) with neutral or antagonistic effects in other tissues (eg, uterus and breast).1 The prototypic drug, raloxifene, is FDA-approved for the prevention and treatment of osteoporosis. It has been shown to reduce the risk of vertebral, but not non-vertebral, fractures.16 Although it reduces breast cancer risk,17 raloxifene increases the incidence of hotflashes and venous thromboembolism.16 The combination of another SERM, bazedoxifene, with conjugated oestrogens, reduces hotflashes and preserves BMD in postmenopausal women.18 This combination drug is now FDA-approved for the prevention of hotflashes and osteoporosis, although whether the long-term adverse effects of this combination therapy are similar to those observed for conjugated oestrogens in the WHI study remains an open question.
Calcitonin
Calcitonin was developed for the prevention and treatment of osteoporosis on the basis of discoveries in animal and human studies.19 Today, both human and salmon forms of calcitonin, which have relatively similar efficacy, are approved worldwide for use in patients with osteoporosis. However, given the limited efficacy of calcitonin in fracture prevention relative to other available agents and concerns about an increased risk of cancer with long-term calcitonin use,20,21 it is now rarely used for osteoporosis prevention or treatment.
Bisphosphonates
Bisphosphonates are the most widely used drugs for the prevention and treatment of osteoporosis, with four options available at present (table 1). The history of their development is of interest, since their discovery as effective drugs for osteoporosis was largely opportunistic.22 Bisphosphonates are chemically stable analogues of pyrophosphate compounds, which are widely found in nature. Naturally occurring pyrophosphate circulates in the human body as an endogenous water softener. The early use of bisphosphonates was mainly as corrosion inhibitors and as complexing agents in the textile, fertiliser, and oil industries; they were subsequently found to inhibit calcification and inhibit bone resorption. The mechanism underlying their anti-osteoclast effects was delineated decades after their clinical use had been initiated; it involves inhibition of the enzyme farnesyl pyrophosphate synthase, which generates isoprenoid lipids that are used for the post-translational modifications of small guanosine triphosphate (GTP)-binding proteins required for osteoclast viability and function.23 Bisphosphonate treatment is associated with 40–70% reductions in vertebral fractures and 40–50% reductions in hip fractures. Thus, these are extremely effective drugs for the treatment of osteoporosis, but the main concerns limiting their use are the rare side-effects, such as atypical femur fractures24 and osteonecrosis of the jaw,25 and unproven efficacy after 5 years of treatment.26
Teriparatide
The development of teriparatide, a truncated form of parathyroid hormone, can also be traced back to Fuller Albright,27 who made the clinical observation that chronic parathyroid hormone excess caused marked bone loss due to increased bone resorption, but it was also associated with an increase in bone formation. Findings from studies with animals and human beings subsequently showed that, by contrast with continuous exposure, intermittent exposure of bone to parathyroid hormone increased bone formation with smaller increases in bone resorption, resulting in a net anabolic effect.28 These studies eventually led to a pivotal clinical trial,4 in which teriparatide treatment at the FDA-approved dose of 20 μg/day was found to reduce the risk of vertebral fractures by 65% (risk ratio [RR] 0.35, 95% CI 0.22–0.55) and reduce the risk of non-vertebral fractures by 53% (0.47, 0.25–0.88). However, because of an increase in the risk of osteosarcoma in growing rodents treated with high doses of teriparatide,29 the FDA limited the treatment duration with teriparatide to 24 months. Reassuringly, subsequent postmarketing surveillance of patients treated with teriparatide has not found an increase in osteosarcoma risk above that expected in the population.30 Despite intensive investigation, the underlying mechanisms for the anabolic skeletal effects of intermittent parathyroid hormone treatment versus the catabolic effects of continuous parathyroid hormone exposure remain elusive. Abaloparatide, a parathyroid hormone-related peptide analogue, might offer some advantages compared with teriparatide, and has recently been approved by the FDA.31
Denosumab
The year 2000 can be viewed as a watershed year around which the fundamental process of drug discovery for osteoporosis changed, with the first example being denosumab. Before this time, drug development for osteoporosis grew out of clinical observations related to oestrogen and parathyroid hormone (teriparatide, abaloparatide), medicinal chemistry modifications to oestrogen (SERMs raloxifene and bazedoxifene), therapeutic extensions of physiological studies (calcitonin), or purely opportunistic discovery (bisphosphonates). The development of denosumab as a novel therapeutic, however, was driven by advances in fundamental bone biology. Subsequent drug discoveries (romosozumab, odanacatib) followed a similar research and development framework, which also relied on insights gained from studying bone biology in patients with rare bone diseases. As such, the fundamental process of drug discovery for osteoporosis has changed in the post-2000 era.
The origin of denosumab can be traced to a fetal rat intestine cDNA-sequencing project at Amgen that was unrelated to bone physiology. A novel cDNA with homology to the TNF-receptor superfamily had been isolated,32 and transgenic mice overexpressing this cDNA clone had marked increases in bone mass, hence the name osteoprotegerin (“to protect bone” in Latin). Subsequently, mice with targeted ablation of osteoprotegerin were found to have severe osteoporosis and arterial calcifcations.33 Further intensive studies in bone biology, including expression cloning using osteoprotegerin as a probe, led to the identification of its ligand, the receptor activator of NF-κB ligand (RANKL), and the understanding that with macrophage colony-stimulating factor (M-CSF), RANKL was both necessary and sufficient for osteoclast development.34 These findings ultimately led to the development of denosumab, a human monoclonal antibody to RANKL, as a novel anti-resorptive drug for osteoporosis. Results of the FREEDOM trial35 showed that treatment with denosumab was associated with a 68% reduction in vertebral fractures (RR 0.32, 95% CI 0.26–0.41) and a 40% reduction in hip fractures (HR 0.60, 95% CI 0.37–0.97).
Strontium ranelate
Strontium is included in this discussion for completeness but is not approved in the USA. Although it has been in use in Europe and elsewhere for some time, safety concerns led the European Medicines Agency to restrict its use in 2013 to treatment of severe osteoporosis.36 Specifically, the use of strontium has been associated with an increase in the risk of myocardial infarction, thromboembolic events, and rare but serious skin reactions (DRESS syndrome). In a phase 3 clinical trial in postmenopausal women,37 strontium was associated with a 41% reduction in vertebral fractures (RR 0.59, 95% CI 0.48–0.73) but no reduction in non-vertebral fractures. Strontium is associated with increased concentrations of serum markers of bone formation and reduced concentrations of bone resorption markers, although these effects have not been confirmed by bone histomorphometry.37 The production of strontium has been permanently discontinued and only limited stock will be available after August, 2017.38
New drugs on the horizon
Shift in drug discovery approaches
Since the development of denosumab, new drugs for osteoporosis treatment have been discovered by careful analysis of rare bone diseases and bone cell biology, particularly subcellular assessment of the osteoclast—underlining the importance of translational research.39 Thus, the two human bone phenotypes of sclerosteosis40 and van Buchem's disease,41 both characterised by increased bone mass and intrinsic resistance to fractures as a result of functional loss of the Wnt inhibitor sclerostin, served as so-called experiments of nature to explore the clinical efficacy of sclerostin inhibition. Other Wnt inhibitors, including dickkopf-1 (DKK-1), are also being targeted in drug development pathways. These developments capitalised on the profound understanding of osteoblast biology, Wnt signalling, genetics, and developmental biology and on advances in biotechnology to generate monoclonal antibodies.42 Deconstruction of osteoclast biology was catalysed by the discovery of M-CSF and RANKL, the two crucial factors in osteoclast differentiation and activation.3 Subsequently, studies of transcription factors and key components of osteoclast structure and function led to the identification of novel targets, including c-Src kinase, αvβ3 integrin, and, the protease cathepsin K (the lysosomal enzyme required for degradation of collagen).43 Cathepsin K received prime attention because of the skeletal phenotype of pycnodysostosis, a genetic disorder that disrupts the cathepsin K gene and is associated with decreased osteoclast resorptive activity without impaired osteoblast function.44 Careful clinical characterisation and analysis of cathepsin K-deficient mice corroborated the hypothesis that the protease activity of cathepsin K could be specifically inhibited.45
Two new drugs are now on the horizon in addition to the now discontinued cathepsin K inhibitor odanacatib (table 2).
Table 2. New osteoporosis drugs and their effects in clinical phase 3 trials.
| Development status | Clinical trial | Dose and delivery | Major action | Vertebral fracture reduction | Adverse effects | |
|---|---|---|---|---|---|---|
| Abaloparatide (parathyroid hormone-related peptide analogue) | Approved (USA) | ACTIVE5 | 80 μg/day; subcutaneous | Osteoanabolic | 86% | Injection-site reaction, hypercalcaemia, dizziness, orthostatic hypotension, headache, arthralgia |
| Romosozumab (sclerostin antibody) | Pending approval (USA, Europe) | FRAME6 | 210 mg/month; subcutaneous | Osteoanabolic; antiresorptive | 73% | Injection-site reaction, osteonecrosis of the jaw, atypical femoral fracture |
| Odanacatib (cathepsin K inhibitor) | Terminated | LOFT46 | 50 mg/day; oral | Antiresorptive | 54% | Morphea-like skin lesions, atypical femoral fracture, stroke |
Parathyroid hormone-related peptide analogues
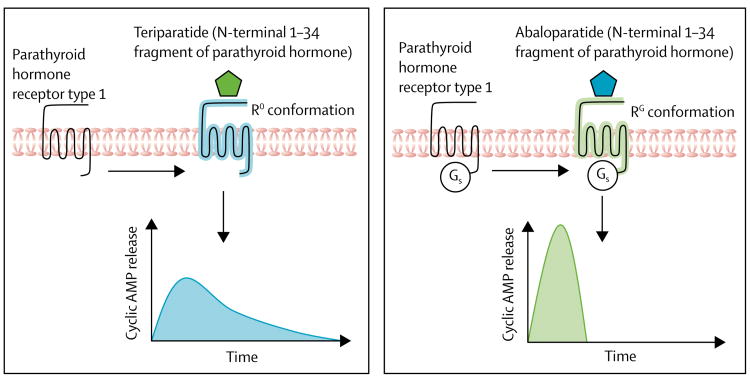
Intermittent activation of the parathyroid receptor type 1 by short pulses of full-length parathyroid hormone (an 84 aminoacid peptide) or fragments thereof (eg, the N-terminal 1–34 fragment, teriparatide) exerts anabolic effects on bone, although the underlying cellular mechanisms are not fully understood.47 Despite the clear advantage of parathyroid hormone as a bone-forming agent, its clinical administration has limitations, including concurrent stimulation of bone resorption, which limits the build-up of new bone; less potency at cortical bone; and hypercalcaemia.5 Two conformations of parathyroid receptor type 1 exist (figure 1): the R0 conformation, which results in prolonged cyclic adenosine monophosphate (cAMP) signalling responses in cells and prolonged hypercalcaemia in animals, and the RG conformation, with a more transient cAMP response. In subsequent comparative studies of teriparatide and abaloparatide (the 1–34 N-terminal fragment of parathyroid hormone-related peptide, known as a mediator of humoral hypercalcaemia of malignancy), was found to favour the RG conformation more than teriparatide did.48 This result provided the rationale to develop abaloparatide with the prospects of stimulating bone formation with less concomitant bone resorption and less hypercalcaemia.48
Figure 1. Differential effects of teriparatide (parathyroid hormone 1–34) versus abaloparatide (parathyroid hormone-related peptide 1–34) on parathyroid hormone receptor 1 signalling.
(A) Teriparatide activates parathyroid hormone receptor 1 towards the R0 conformation, which results in intracellular release of the second messenger cyclic AMP. (B) By contrast, abaloparatide activates the receptor towards the RG conformation with a more transient cyclic AMP increase. AMP=adenosine monophosphate.
The effects of abaloparatide (20, 40, or 80 μg/day) versus placebo or teriparatide (20 μg/day) for 24 weeks on BMD at the lumbar spine, total hip, and femoral neck has been tested in a phase 2 randomised controlled trial49 involving 222 postmenopausal women with osteoporosis. At the lumbar spine, treatment with abaloparatide increased BMD by 2.9% (20 μg), 5.2% (40 μg), and 6.7% (80 μg), whereas teriparatide increased BMD by 5.5%. At the femoral neck, abaloparatide increased BMD by 2.7% (20 μg), 2.2% (40 μg), and 3.1% (80 μg), whereas teriparatide increased BMD by 1.1%. These data indicate a more potent effect of abaloparatide than teriparatide on the spine, an area rich in trabecular bone, and a robust positive effect of abaloparatide on the hip, which is rich in cortical bone, wherease teriparatide had only marginal effects at this site.49
In the phase 3 ACTIVE trial,5 2463 postmenopausal women (mean age 69 years) were randomly assigned to receive either placebo, abaloparatide (80 μg/day), or open-label teriparatide (20 μg/day) for 18 months. The primary endpoint was reduction of new morphometric vertebral fractures. Abaloparatide reduced the incidence of new vertebral fractures by 86% (HR 0.14, 95% CI 0.05-0.39) and reduced the risk of non-vertebral fractures by 43% (0.57, 0.32-1.00) compared with placebo, whereas teriparatide reduced the incidence of vertebral and non-vertebral fractures by 80% (0.20; 0.08 to 0.47) and 28% (0.72, 0.42-1.22), respectively.5 In a head-to-head comparison, abaloparatide was superior to teriparatide in reducing the incidence of major osteoporotic fractures (fractures of the upper arm, wrist, hip, or clinical spine) by 65% (0.45, 0.21-0.95), but differences in non-vertebral fractures or clinical fractures were not significant. The incidence of hypercalcaemia, a prespecified safety endpoint, was lower with abaloparatide (3.4%) than with teriparatide (6.4%). Other common adverse effects with abaloparatide were similar to those with teriparatide, which included dizziness, orthostatic hypotension, headache, and arthralgia. On the basis of these findings, the FDA approved abaloparatide as a treatment for osteoporosis in women for a maximum duration of 24 months in April, 2017. However, abaloparatide does carry a label warning of a osteosarcoma risk similar to that of teriparatide, which is based on findings from preclinical studies in rats.31 In addition to the injectable form, a transdermal abaloparatide patch is currently being tested in a randomised, double-blind, placebo-controlled, phase 2 study in postmenopausal women with osteoporosis (NCT01674621).
Sclerostin inhibitors
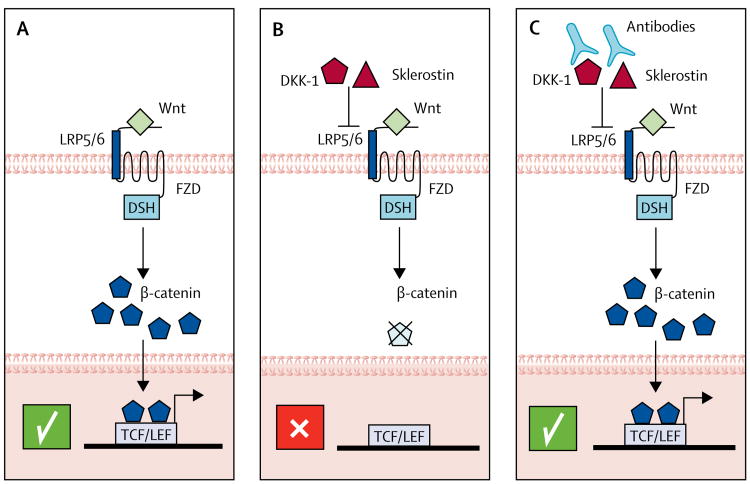
In view of the increased bone mass and strength in the absence of functional sclerostin, as observed in rodents and human beings affected by sclerosteosis and van Buchem disease, neutralisation of sclerostin emerged as a novel therapeutic approach. An overview of the Wnt signalling pathway, the role of Wnt signalling inhibitors in limiting this pathway, and the use of specific antibodies to these inhibitors to activate Wnt signalling in bone are presented in figure 2.50,51 Preclinical data corroborated the proof-of-concept that absence of sclerostin or inhibition with monoclonal antibodies enhanced bone strength and conferred resistance to bone fractures.52 Thus, several sclerostin antibodies, including romosozumab (AMG-785)53 and BPS804,54 are being clinically evaluated, the evaluation of romosozumab being most advanced (table 2). In a phase 2 study of romosozumab versus placebo, alendronate, and teriparatide in 419 postmenopausal women,55 romosozumab for 12 months increased BMD at the lumbar spine and the hip. The 11.3% increase in BMD at the lumbar spine and the 4.1% increase in BMD at the total hip with romosozumab was greater than with any other treatment. Romosozumab increased bone formation markers and reduced bone resorption markers, both of which were reversible after discontinuation. A common side-effect associated with romosozumab was mild local injection-site reactions.
Figure 2. Overview of the Wnt signalling pathway—effects of ligands, inhibitors, and targeted therapies.
(A) After binding of Wnt to the LRP5/6 receptor, β-catenin translocates into the nucleus, binds to TCF/LEF transcription factors, and stimulates transcription of osteoblast genes, which results in enhanced bone formation. (B) The endogenous Wnt inhibitors sclerostin and DKK-1 interfere with Wnt signal transduction, resulting in less β-catenin translocation into the nucleus. Osteoblastic functions and bone formation is reduced. (C) Antibodies against sclerostin or DKK-1, or both, neutralise the Wnt inhibitors, thus restoring the scenario of unopposed Wnt signalling as depicted in panel A, which leads to enhanced osteoblastic bone formation. LRP5=low-density lipoprotein receptor-related protein 5. FZD=frizzled. DSH=dishevelled. DKK-1=dickkopf-related protein 1.
In the phase 3 trial FRAME,6 the effects of romosozumab on fracture reduction (210 mg once per month) compared with placebo for 12 months followed by denosumab (60 mg twice per year) for an additional 12 months were assessed in 7180 women with postmenopausal osteoporosis, based on a T score of −2.5 to –3.5 at the total hip or femoral neck. After 12 months, patients receiving romosozumab had a 73% reduced risk of new vertebral fractures (RR 0.27, 95% CI 0.16–0.47); after an additional 12 months of denosumab treatment for both groups, those patients who had received romosozumab had a 75% reduced risk of new vertebral fractures (0.25, 0.16–0.40).6 However, the effect of romosozumab on non-vertebral fractures, which accounted for most (>85%) clinical fractures, was not significantly reduced at 12 months or 24 months, so the potential effects of romosozumab on non-vertebral fracture risk must be studied further. Additionally, most of the anabolic effect of romosozumab (based on bone formation markers) was evident within the first 3 months of treatment, so the possibility that intermittent, short-term (eg, 3 months) treatment with romosozumab might be just as effective as 12 months of continuous treatment warrants further study. Two cases of osteonecrosis of the jaw and one case of atypical femur fracture were seen in the romosozumab group. All other adverse events were similar between the two groups.
Ongoing clinical trials with romosozumab include a phase 3 comparison with alendronate for fracture prevention in postmenopausal osteoporosis (ARCH; NCT01631214), a phase 3 comparison with teriparatide for changes in BMD in postmenopausal osteoporosis (STRUCTURE; NCT01796301), and a phase 2 trial for changes in BMD in men with osteoporosis (BRIDGE; NCT02186171). Data released from the ARCH study56 have shown that treatment with romosozumab is associated with more cardiovascular events than with alendronate, which requires further investigation.
Targeting other Wnt inhibitors
In addition to sclerostin, other inhibitors of Wnt signalling, such as DKK-1 and soluble frizzled, have redundant functions.57 Increased serum DKK-1 concentrations were linked to osteolytic bone lesions in myeloma bone disease or metastatic breast cancer, so DKK-1 antibodies are being explored for treatment of malignant bone diseases. Of note, sclerostin inhibition results in increased DKK-1 concentrations, which limits the anabolic effects of sclerostin blockade.58 Use of bispecific antibodies against sclerostin and DKK-1 in one approach to circumvent this problem; these antibodies are superior to blocking antibodies against individual Wnt inhibitors in rodent models with regards to bone mass and fracture healing.58 However, antibodies against DKK-1 alone or against both DKK-1 and sclerostin have not been clinically tested in patients with osteoporosis.
Cathepsin K inhibitors
Cathepsin K is secreted by mature osteoclasts to degrade bone matrix proteins, including type 1 collagen.59,60 Conceptually, selective inhibition of cathepsin K in osteoclasts reduces their resorptive activity but leaves them alive, allowing paracrine signalling to the osteoblasts.59 This selective inhibition was thought to result in intact osteoblast function, unlike other anti-resorptive drugs that concurrently reduce osteoclast and osteoblast activity.
In clinical trials, the oral cathepsin K inhibitor odanacatib, at 50 mg once a week, continuously increased spine BMD in postmenopausal women (n=399) by inhibiting bone resorption and only transiently decreased bone formation markers.61 On the basis of these promising results, a phase 3 study (LOFT)46 was initiated in 16 071 women with postmenopausal osteoporosis who received either odanacatib (50 mg/week) or placebo, with event-driven reduction of new vertebral fractures as the primary endpoint. The study was stopped early after an interim analysis indicated efficacy, with a reduction in new morphometric vertebral fractures. Compared with placebo, treatment with odanacatib reduced the relative risk of new and worsening morphometric vertebral fractures by 54% and reduced the risk of clinical vertebral fractures by 72%, clinical hip fractures by 47%, and clinical non-vertebral fractures by 23% (all p<0.001). Adverse effects that were more common with odanacatib than with placebo included adjudicated morphea-like skin lesions, atypical femoral fractures, and strokes. The final results of the LOFT trial have not been published; however, in view of the HR for strokes of 1.37 (95% CI 1.10–1.71), Merck announced in September, 2016, that it will discontinue the development of odanacatib.7
The growing gap in treatment options
Despite the increasing number of effective drugs to treat osteoporosis, discouraging evidence suggests that many patients who should receive pharmacological treatment are either not being offered these drugs or, when prescribed, are not taking them.62 This is true even in patients recovering from hip fracture, for whom there is universal agreement of the importance of pharmacological therapy.63 Although many reasons exist for this gap in osteoporosis treatment, perhaps the two most important reasons are fear of rare side-effects and concerns regarding long-term efficacy.
Fear of rare side-effects
As highlighted by Gina Kolata in a New York Times article,64 patient concerns with side-effects, particularly atypical femur fractures, are an important contributor to the lack of appropriate treatment for osteoporosis. Although these side-effects have only been clearly associated with bisphosphonates, patient perceptions about these risks are extending to all osteoporosis drugs, which is particularly concerning because atypical femur fractures are extremely rare. So although the relative risk of atypical femur fractures in patients taking bisphosphonates is increased, the absolute risk ranges from 3.2–50 cases per 100 000 person-years.24 When used in patients who are at high risk of fracture, these drugs are estimated to prevent 80–5000 fragility fractures for each atypical femur fracture possibly induced by treatment.65 Several steps can be taken to address this problem,66 such as improved patient and doctor education regarding both the risk-benefit ratio of these drugs and the prodromal symptoms (eg, groin or hip pain) of atypical femur fractures; potential use of dual x-ray energy absorptiometry to monitor patients on therapy specifically for features of atypical femur fractures;67 identification of high-risk patients using femur geometrical characteristics and other risk factors for atypical femur fractures;68 and the development of pharmacogenomic markers identifying patients at increased risk of atypical femur fractures.
A second rare side-effect of bisphosphonate use is osteonecrosis of the jaw, which was initially described in the setting of high-dose bisphosphonate use in patients with metastatic cancer.25 This side-effect is extremely rare in patients treated at doses recommended for osteoporosis, with an estimated incidence of 0.001–0.01%.25 Again, better education of patients, doctors, and dental practioners, along with maintenance of good oral hygiene and dental health, are key to overcoming this barrier to treatment.
Concerns regarding long-term efficacy
As highlighted by a position statement26 from the FDA, data regarding the anti-fracture efficacy of bisphosphonates after 5 years of use is scarce and perhaps conflicting. This assessment, combined with the observation that the risk of the rare side-effects of atypical femur fractures and osteonecrosis of the jaw increases with duration of therapy,24,25 has led to legitimate concerns about the long-term (>5 years) treatment of patients with bisphosphonates or other anti-resorptive agents, such as denosumab. However, data from the Fracture Intervention Trial Long-term Extension (FLEX)69 showed that postmenopausal women with low hip T scores (−2.0 to −2.5) who continued treatment with alendronate for 10 years had fewer clinical vertebral fractures than women receiving placebo after 5 years had. Similarly, in the HORIZON extension study of zoledronic acid,70 women with T scores less than −2.5 had fewer morphometric vertebral fractures after six annual infusions than women who received only 3 years of treatment had. On the basis of these studies, current recommendations are to treat patients who warrant therapy with a bisphosphonate for 5 years and then reassess, basing subsequent treatment on the level of fracture risk and potentially considering a so-called drug holiday for a variable period of time, albeit in the absence of data showing the efficacy of this approach.71 Long-term treatment (up to 10 years) with denosumab in an open-label extension of the FREEDOM trial has been shown to have a persistent benefit by reducing non-vertebral fractures.72
The case for new drug development
Classic anti-resorptive drugs such as bisphosphonates and denosumab are associated with the risk of osteonecrosis of the jaw and atypical femur fractures. Although the anti-fracture benefit by far outweighs their risk, public perception of these complications has contributed to reduced treatment rates. The cathepsin K inhibitor odanacatib was unique because it left osteoclasts viable, allowing intercellular communication and perhaps reducing the risk of osteonecrosis of the jaw, but its development programme has been terminated due to safety concerns, specifically an increased risk of stroke. Nonetheless, knowledge stemming from its use provided proof-of-concept that osteoclastic enzymes can be powerfully inhibited, with an effect on clinical outcomes. Future directions might employ small molecules and small interfering RNA-based therapies against newly identified osteoclastic enzymes.
Any potent anti-resorptive drug, including bisphosphonates, denosumab, and odanacatib, might impair intrinsic repair mechanisms, thereby increasing risk of atypical femur fractures in susceptible individuals with anatomic and biomechanic predisposition. Further research into the pathogenesis and individual predisposition of atypical femur fractures is thus needed.
Most drugs, with the exception of bisphosphonates and strontium ranelate, had no sustained effect on bone metabolism or even an overshooting response when they were discontinued. Thus, a strategy of combining or alternating treatment, or sequential regimens, is needed. As evident from the DATA Switch study73 and the FRAME study,6 a sequence of anabolic treatment with teriparatide or romosozumab immediately followed by denosumab might yield the most potent skeletal effect. Since discontinuation of anabolic drugs at any time leads to rapid bone loss and increased fractures, a continued treatment strategy is warranted. Cyclic, rather than continuous treatment regimens, combined with and tailored to a better understanding of bone-cell communication with these interventions could help maximise anti-fracture efficacy and minimise adverse skeletal effects. Also, precision medicine that takes the genetic and epigenetic background and individual fracture risk into account might help to tailor therapy. Such approaches are facilitated by the decreasing cost of whole-genome sequencing and could soon be offered, for example, to guide therapy for young adults with otherwise unexplained fractures.
As evident from the developmental process of recent osteoporosis drugs, new pharmacological approaches will probably be based on mechanisms of rare diseases and fundamental bone biology. Specifically, there is a need for new drugs that lack the rare side-effects of osteonecrosis of the jaw and atypical femur fractures; have proven efficacy for more than 5 years of treatment; have more sustained effects on bone formation (anabolic effects of all available, or soon to be available, anabolic drugs [teriparatide, abaloparatide, romosozumab] wane after 6–9 months of treatment for reasons that remain unclear); stimulate mechanosensing and capitalise on the emerging knowledge of osteocyte biology (ie, by mimicking biomechanical impact and exercise); have an improved effect on cortical bone and, thus, improved effect on reducing the risk of hip fractures; enhance muscle growth and subsequently bone strength (antibody-based blockade of the activin receptor by bimagrumab74 is being assessed in a phase 2 study [NCT02152761] in women and men older than 60 years who have osteoporosis after a hip fracture); improve fracture healing, possibly by targeting the various stages of fracture healing in a phase-adapted manner; and have a combined effect against falls and fractures.
Conclusion
The evolution of drug development for osteoporosis, from clinical observations or opportunistic discoveries to the more recent framework of fundamental bone biology driving novel therapeutics, is truly remarkable. It represents perhaps one of the best examples of private and public investments in discovery science facilitating the development of drugs that have the potential to benefit patients. With the available anti-resorptive drugs and the expanding list of anabolic options for the treatment of osteoporosis, the burden of fractures in the population could potentially become markedly reduced. However, concerns regarding side-effects and long-term efficacy are contributing to substantial under-treatment of patients, even of those patients who clearly warrant drug therapy. This problem can only be addressed by strengthened approaches to enhance doctors' and patients' understanding of the benefits versus risks of drug treatment for osteoporosis. The treatment gap could also potentially be narrowed by increased awareness, among both patients and doctors, of newly developed drugs that do not have these side-effects and are perhaps even more effective at reversing bone loss. As such, translational efforts across the spectrum, from basic drug discovery and development, to clinical trials of new drugs, to the dissemination and implementation of existing therapies, are essential if the burden of preventable fractures in the population is to be reduced.
Search strategy and selection criteria.
We searched PubMed for articles published between Jan 1, 1990, to April 30, 2017, with the terms “osteoporosis”, “bone loss”, and “fractures” in combination with the terms “randomised-controlled trials”, “bisphosphonates”, “denosumab”, “raloxifene”, “parathyroid hormone”, “strontium ranelate”, “sclerostin”, “abaloparatide”, or “cathepsin K”. Peer-reviewed full articles resulting from this search strategy and key references cited in those articles were reviewed. Only articles published in English were included.
Acknowledgments
This work was supported by NIH grants P01 AG004875, AG048792, AR027065, and AR068275 (SK) and by Deutsche Forschungsgemeinschaft grants Forschergruppe-1586 SKELMET, SFB-655, and Transregio-67 (LCH). We thank Franziska Lademann (Dresden Technical University, Germany) for her assistance with the figures.
Footnotes
Declaration of interests: SK declares no competing interests. LCH has received personal fees from Alexion (advisory board, lectures), Amgen (advisory board, lectures, clinical studies, research support), Eli Lilly (lectures), UCB (lectures), Radius (advisory board), and Merck (advisory board), unrelated to the present work.
This is the first in a Series of two papers about osteoporosis
Contributor Information
Sundeep Khosla, Robert and Arlene Kogod Center on Aging and Endocrine Research Unit, Mayo Clinic College of Medicine and Science, Mayo Clinic, Rochester, MN, USA.
Lorenz C Hofbauer, Division of Endocrinology, Diabetes, and Bone Diseases and Centre for Healthy Aging, Carl Gustav Carus University Hospital, Dresden Technical University, Dresden, Germany.
References
- 1.Riggs BL, Hartmann LC. Selective estrogen-receptor modulators—mechanisms of action and application to clinical practice. N Engl J Med. 2003;348:618–29. doi: 10.1056/NEJMra022219. [DOI] [PubMed] [Google Scholar]
- 2.Khosla S, Bilezikian JP, Dempster DW, et al. Benefits and risks of bisphosphonate therapy for osteoporosis. J Clin Endocrinol Metab. 2012;97:2272–82. doi: 10.1210/jc.2012-1027. [DOI] [PubMed] [Google Scholar]
- 3.Kearns AE, Khosla S, Kostenuik PJ. Receptor activator of nuclear factor κB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr Rev. 2008;29:155–92. doi: 10.1210/er.2007-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–41. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 5.Miller PD, Hattersley G, Riis BJ, et al. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: a randomized clinical trial. JAMA. 2016;316:722–33. doi: 10.1001/jama.2016.11136. [DOI] [PubMed] [Google Scholar]
- 6.Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375:1532–43. doi: 10.1056/NEJMoa1607948. [DOI] [PubMed] [Google Scholar]
- 7.Mullard A. Merck & Co. drops osteoporosis drug odanacatib. Nat Rev Drug Discov. 2016;15:445–46. doi: 10.1038/nrd.2016.207. [DOI] [PubMed] [Google Scholar]
- 8.Kim SC, Kim DH, Mogun H, et al. Impact of the US Food and Drug Administration's safety-related announcements on the use of bisphosphonates after hip fracture. J Bone Miner Res. 2016;31:1535–40. doi: 10.1002/jbmr.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albright F. Post-menopausal osteoporosis. Trans Assoc Am Physicians. 1940;55:298–305. [Google Scholar]
- 10.Khosla S, Amin S, Orwoll E. Osteoporosis in men. Endocr Rev. 2008;29:441–64. doi: 10.1210/er.2008-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindsay R, Aitkin JM, Anderson JB, Hart DM, MacDonald EB, Clarke AC. Long-term prevention of postmenopausal osteoporosis by oestrogen. Lancet. 1976;i:1038–40. doi: 10.1016/s0140-6736(76)92217-0. [DOI] [PubMed] [Google Scholar]
- 12.Lufkin EG, Wahner HW, O'Fallon WM, et al. Treatment of postmenopausal osteoporosis with transdermal estrogen. Ann Intern Med. 1992;117:1–9. doi: 10.7326/0003-4819-117-1-1. [DOI] [PubMed] [Google Scholar]
- 13.Cauley JA, Robbins J, Chen Z, et al. Effect of estrogen plus progestin on risk of fracture and bone mineral density: the Women's Health Initiative randomized trial. JAMA. 2006;290:1729–38. doi: 10.1001/jama.290.13.1729. [DOI] [PubMed] [Google Scholar]
- 14.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 15.Ettinger B, Ensrud KE, Wallace R, et al. Effects of ultralow-dose transdermal estradiol on bone mineral density: a randomized clinical trial. Obstet Gynecol. 2004;104:443–51. doi: 10.1097/01.AOG.0000137833.43248.79. [DOI] [PubMed] [Google Scholar]
- 16.Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene results from a 3-year randomized clinical trial. JAMA. 1999;282:637–45. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- 17.Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727–41. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 18.Lindsay R, Gallagher C, Kagan R, Pickar JH, Constantine G. Efficacy of tissue-selective estrogen complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92:1045–52. doi: 10.1016/j.fertnstert.2009.02.093. [DOI] [PubMed] [Google Scholar]
- 19.Austin LA, Heath H., III Calcitonin: physiology and pathophysiology. N Engl J Med. 1981;304:269–78. doi: 10.1056/NEJM198101293040505. [DOI] [PubMed] [Google Scholar]
- 20.Chesnut CH, 3rd, Silverman S, Andriano K, et al. for the PROOF Study Group. A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the Prevent Recurrence Of Osteoporotic Fractures study. Am J Med. 2000;109:267–76. doi: 10.1016/s0002-9343(00)00490-3. [DOI] [PubMed] [Google Scholar]
- 21.Novartis. Drug regulatory affairs. FDA Joint Reproductive Health Drugs and Drug Safety and Risk Management Advisory Committee Meeting on the benefit/risk of salmon calcitonin for the treatment of postmenopausal osteoporosis. [accessed June 22, 2017];2013 Jan 29; https://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/reproductivehealthdrugsadvisorycommittee/ucm341781.pdf.
- 22.Russell RG. Bisphosphonates: the first 40 years. Bone. 2011;49:2–19. doi: 10.1016/j.bone.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 23.Russell RGG, Watts NB, Ebetino FH, Rogers MJ. Mechanisms of action of bisphosphonates: similarities and differences and their potential influences on clinical efficacy. Osteoporos Int. 2008;19:733–59. doi: 10.1007/s00198-007-0540-8. [DOI] [PubMed] [Google Scholar]
- 24.Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone Mineral Research. J Bone Miner Res. 2014;29:1–23. doi: 10.1002/jbmr.1998. [DOI] [PubMed] [Google Scholar]
- 25.Khosla S, Burr D, Cauley J, et al. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479–91. doi: 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- 26.Whitaker M, Guo J, Kehoe T, Benson G. Bisphosphonates for osteoporosis—where do we go from here? N Engl J Med. 2012;366:2048–51. doi: 10.1056/NEJMp1202619. [DOI] [PubMed] [Google Scholar]
- 27.Albright F, Aub JC, Bauer W. Hyperparathyroidism. A common and polymorphic condition as illustrated by seventeen proved cases from one clinic. JAMA. 1934;102:1276–87. [Google Scholar]
- 28.Reeve J, Williams D, Hesp R, et al. Anabolic Effect of low doses of a fragment of human parathyroid hormone on the skeleton in postmenopausal osteoporosis. Lancet. 1976;1:1035–38. doi: 10.1016/s0140-6736(76)92216-9. [DOI] [PubMed] [Google Scholar]
- 29.Vahle JL, Long GG, Sandusky G, Westmore M, Linda Y, Sato M. Bone neoplasms in F344 rats given teriparatide [rhPTH(1-34)] are dependent on duration of treatment and dose. Toxicol Pathol. 2004;32:426–38. doi: 10.1080/01926230490462138. [DOI] [PubMed] [Google Scholar]
- 30.Andrews EB, Gilsenan AW, Midkiff K, et al. The US postmarketing surveillance study of adult osteosarcoma and teriparatide: study design and findings from the last 7 years. J Bone Miner Res. 2012;27:2429–37. doi: 10.1002/jbmr.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.US Food and Drug Administration. Drugs@FDA: FDA approved drug products. [accessed May 9, 2017];Abaloparatide. 2017 https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=208743.
- 32.Simonet WS, Lacey DL, Dunstan CR, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–19. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 33.Bucay N, Sarosi I, Dunstan CR, et al. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–68. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lacey DL, Timms E, Tan HL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–76. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 35.Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–65. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 36.European Medicines Agency. Recommendation to restrict the use of Protelos/Osseor (strontium ranelate) [accessed May 9, 2017];2013 Apr 26; http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2013/04/news_detail_001774.jsp&mid=WC0b01ac058004d5c1.
- 37.Meunier PJ, Roux C, Seeman E, et al. The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med. 2004;350:459–68. doi: 10.1056/NEJMoa022436. [DOI] [PubMed] [Google Scholar]
- 38.Servier. Protos discontinuation. [accessed May 16, 2017]; http://www.servier.com.au/products/protosdiscontinuation.
- 39.Lacey DL, Boyle WJ, Simonet WS, et al. Bench to bedside: elucidation of the OPG-RANK-RANKL pathway and the development of denosumab. Nat Rev Drug Discov. 2012;11:401–19. doi: 10.1038/nrd3705. [DOI] [PubMed] [Google Scholar]
- 40.Hamersma H, Gardner J, Beighton P. The natural history of sclerostosis. Clin Genet. 2003;63:192–97. doi: 10.1034/j.1399-0004.2003.00036.x. [DOI] [PubMed] [Google Scholar]
- 41.van Lierop AH, Hamdy NA, van Egmond ME, Bakker E, Dikkers FG, Papapoulos S. Van Buchem disease: clinical, biochemical, and densitometric features of patients and disease carriers. J Bone Miner Res. 2013;28:848–54. doi: 10.1002/jbmr.1794. [DOI] [PubMed] [Google Scholar]
- 42.Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19:179–92. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- 43.Teitelbaum SL. Osteoclasts: what do they do and how do they do it? Am J Pathol. 2007;170:427–35. doi: 10.2353/ajpath.2007.060834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gelb BD, Shi GP, Chapman HA, Desnick RJ. Pycnodysostosis, a lysosomal storage disease caused by cathepsin K deficiency. Science. 1996;273:1236–38. doi: 10.1126/science.273.5279.1236. [DOI] [PubMed] [Google Scholar]
- 45.Gowen M, Lazner F, Dodds R, et al. Cathepsin K knockout mice develop osteopetrosis due to a deficit in matrix degradation but not demineralization. J Bone Miner Res. 1999;14:1654–63. doi: 10.1359/jbmr.1999.14.10.1654. [DOI] [PubMed] [Google Scholar]
- 46.Bone HG, Dempster DW, Eisman JA, et al. Odanacatib for the treatment of postmenopausal osteoporosis: development history and design and participant characteristics of LOFT, the Long-Term Odanacatib Fracture Trial. Osteoporos Int. 2015;26:699–712. doi: 10.1007/s00198-014-2944-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Canalis E, Giustina A, Bilezikian JP. Mechanisms of anabolic therapies for osteoporosis. N Engl J Med. 2007;357:905–16. doi: 10.1056/NEJMra067395. [DOI] [PubMed] [Google Scholar]
- 48.Hattersley G, Dean T, Corbin BA, Bahar H, Gardella TJ. Binding selectivity of abaloparatide for PTH-type-1-receptor conformations and effects on downstream signaling. Endocrinology. 2016;157:141–49. doi: 10.1210/en.2015-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leder BZ, O'Dea LS, Zanchetta JR, et al. Effects of abaloparatide, a human parathyroid hormone-related peptide analog, on bone mineral density in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 2015;100:697–706. doi: 10.1210/jc.2014-3718. [DOI] [PubMed] [Google Scholar]
- 50.Baron R, Hesse E. Update on bone anabolics in osteoporosis treatment: rationale, current status, and perspectives. J Clin Endocrinol Metab. 2012;97:311–25. doi: 10.1210/jc.2011-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baron R, Rawadi G. Targeting the Wnt/β-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology. 2007;148:2635–43. doi: 10.1210/en.2007-0270. [DOI] [PubMed] [Google Scholar]
- 52.Li X, Ominsky MS, Niu QT, et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res. 2008;23:860–69. doi: 10.1359/jbmr.080216. [DOI] [PubMed] [Google Scholar]
- 53.Lim SY, Bolster MB. Profile of romosozumab and its potential in the management of osteoporosis. Drug Des Devel Ther. 2017;11:1221–31. doi: 10.2147/DDDT.S127568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lewiecki EM. Role of sclerostin in bone and cartilage and its potential as a therapeutic target in bone diseases. Ther Adv Musculoskelet Dis. 2014;6:48–57. doi: 10.1177/1759720X13510479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McClung MR, Grauer A, Boonen S, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370:412–20. doi: 10.1056/NEJMoa1305224. [DOI] [PubMed] [Google Scholar]
- 56.Amgen. Amgen and UCB announce top-line phase 3 data from active-comparator study of EVENITY (romosozumab) in postmenopausal women with osteoporosis. [accessed June 22, 2017];2017 May 21; www.amgen.com/media/news-releases/2017/05/amgen-and-ucbannounce-topline-phase-3-data-from-activecomparator-study-ofevenity-romosozumab-in-postmenopausal-women-with-osteoporosis/
- 57.Kahn M. Can we safely target the WNT pathway? Nat Rev Drug Discov. 2014;13:513–32. doi: 10.1038/nrd4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Florio M, Gunasekaran K, Stolina M, et al. A bispecific antibody targeting sclerostin and DKK-1 promotes bone mass accrual and fracture repair. Nat Commun. 2016;7:11505. doi: 10.1038/ncomms11505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khosla S. Odanacatib: location and timing are everying. J Bone Miner Res. 2012;27:506–08. doi: 10.1002/jbmr.1541. [DOI] [PubMed] [Google Scholar]
- 60.Costa AG, Cusano NE, Silva BC, Cremers S, Bilezikian JP. Cathepsin K: its skeletal actions and role as a therapeutic target in osteoporosis. Nat Rev Rheumatol. 2011;7:447–56. doi: 10.1038/nrrheum.2011.77. [DOI] [PubMed] [Google Scholar]
- 61.Langdah lB, Binkley N, Bone H, et al. Odanacatib in the treatment of postmenopausal women with low bone mineral density: five years of continued therapy in a phase 2 study. J Bone Miner Res. 2012;27:2251–58. doi: 10.1002/jbmr.1695. [DOI] [PubMed] [Google Scholar]
- 62.Khosla S, Shane E. A crisis in the treatment of osteoporosis. J Bone Miner Res. 2016;31:1485–87. doi: 10.1002/jbmr.2888. [DOI] [PubMed] [Google Scholar]
- 63.Eisman JA, Bogoch ER, Dell R, et al. for the ASBMR Task Force on Secondary Fracture Prevention. Making the first fracture the last fracture: ASBMR Task Force on Secondary Fracture Prevention. J Bone Miner Res. 2012;27:2039–46. doi: 10.1002/jbmr.1698. [DOI] [PubMed] [Google Scholar]
- 64.Kolata G. [accessed April 16, 2017];New York Times. 2016 Jun 1; http://www.nytimes.com/2016/06/02/health/osteoporosis-drugs-bones.html?_r=0.
- 65.Black DM, Rosen CJ. Clinical practice. Postmenopausal osteoporosis. N Engl J Med. 2016;374:254–62. doi: 10.1056/NEJMcp1513724. [DOI] [PubMed] [Google Scholar]
- 66.Khosla S, Cauley JA, Compston J, et al. Addressing the crisis in the treatment of osteoporosis: a path forward. J Bone Miner Res. 2017;32:424–30. doi: 10.1002/jbmr.3074. [DOI] [PubMed] [Google Scholar]
- 67.McKenna MJ, van der Kamp S, Heffernan E, Hurson C. Incomplete atypical femoral fractures: assessing the diagnostic utility of DXA by extending femur length. J Clin Densitom. 2013;16:579–83. doi: 10.1016/j.jocd.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 68.Mahjoub Z, Jean S, Leclerc JT, et al. Incidence and characteristics of atypical femoral fractures: clinical and geometrical data. J Bone Miner Res. 2013;31:767–76. doi: 10.1002/jbmr.2748. [DOI] [PubMed] [Google Scholar]
- 69.Black DM, Schwartz AV, Ensrud KE, et al. Effects of continuing or stopping alendronate after 5 years of treatment The Fracture Intervention Trial Long-Term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927–38. doi: 10.1001/jama.296.24.2927. [DOI] [PubMed] [Google Scholar]
- 70.Black DM, Reid IR, Boonen S, et al. The Effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-Pivotal Fracture Trial (PFT) J Bone Miner Res. 2012;27:243–54. doi: 10.1002/jbmr.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Adler RA, El-Hajj Fuleihan G, Bauer DC, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a Task Force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2016;31:16–35. doi: 10.1002/jbmr.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5:513–23. doi: 10.1016/S2213-8587(17)30138-9. [DOI] [PubMed] [Google Scholar]
- 73.Leder BZ, Tsai JN, Uihlein AV, et al. Denosumab and teriparatide transitions in postmenopausal osteoporosis (the DATA-Switch study): extension of a randomised controlled trial. Lancet. 2015;386:1147–55. doi: 10.1016/S0140-6736(15)61120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tankó LB, Goldhahn J, Varela A, et al. Does activin receptor blockade by bimagrumab (BYM338) pose detrimental effects on bone healing in a rat fibula osteotomy model? Calcif Tissue Int. 2016;99:310–21. doi: 10.1007/s00223-016-0148-0. [DOI] [PubMed] [Google Scholar]