Abstract
Normative changes in cognitive function are expected with increasing age. Research on the relationship between normative cognitive decline and moderate-to-vigorous physical activity (MVPA) and sedentary behavior (SED) needs further investigation in Hispanic/Latinos adults. We assessed the cross-sectional association between accelerometer assessed MVPA and SED with cognitive function in 7,478 adults aged 45–74 years from the Hispanic Community Health Study/Study of Latinos. At baseline, cognitive tests included two executive function tests (Digit Symbol Substitution Test (DSST), a test of language (Word Fluency), and a test of memory (Spanish English Verbal Learning Test). Multiple regression models were used to examine associations of time spent in MVPA and SED with cognitive function by age groups, adjusted for age, education, sex, acculturation, and field center. Mean time spent in sedentary behaviors was 12.3 hours/day in females and 11.9 hours/day in males (75% and 77% of accelerometer wear time, respectively). Higher SED, but not MVPA, was associated with lower DSST raw scores (β −0.03 with each 10-min increment in SED; P < 0.05), indicating lower performance in executive function in all age groups. No associations were observed for MVPA and SED with tests of language or memory tests. Our findings suggest a distinct association of SED but not MVPA on executive functioning in middle-aged and older Latino adults. Longitudinal studies are needed to more conclusively determine causal links.
Keywords: physical activity, sedentary behavior, Latinos, cognitive function, Hispanics, cognition
Introduction
With increasing age adults experience normative cognitive decline—cognitive aging, at a rate of 0.04–0.05 SD units/year (Alwin and Hofer, 2011; Castora-Binkley et al., 2015; Hayden et al., 2011; Royall et al., 2005). One possible source of variation in cognitive performance with increasing age is race and ethnicity (Sachs-Ericsson and Blazer, 2005). Although cognition and age-related cognitive decline have been studied extensively, there is currently no consensus regarding their relationship with race/ethnicity(Castora-Binkley et al., 2013; Ho et al., 2011; Masel et al., 2010; Yaffe et al., 2009). In particular, studies evaluating racial/ethnic differences in cognitive function have shown that non-Hispanic Blacks ( NHB) decline at faster rates compared with non-Hispanic Whites (NHW) (Castora-Binkley et al., 2013; Masel and Peek, 2009; Park et al., 2003; Yaffe et al., 2009; Zsembik and Peek, 2001). However, while several studies have reported no association between Hispanic ethnicity and the rate of cognitive decline, (Castora-Binkley et al., 2013; Karlamangla et al., 2009; Masel and Peek, 2009) others have suggested that Hispanics experience a faster decline in cognitive function in old age (Alley et al., 2007). A better understanding of the patterns of cognitive decline among older Hispanics is important because while the number of older adults in the United States is rapidly growing among all racial/ethnic groups, it is expected to grow faster among Hispanics compared with same-age NHW (Pew Research Center, 2015).
Research has consistently shown that black race is a risk factor for poorer cognitive function when compared to whites, however, it is still unclear as to whether these disparities extend equally to all racial ethnic groups. Perhaps, other factors besides race or ethnicity, such as behavioral factors (i.e physical activity, sedentary time) could account for the reported racial and ethnic disparities. Physical activity can have a positive effect on a wide range of cognitive functions, although the effect appears to be both general and specific in nature. The general effect of increasing physical activity varies across cognitive processes, and the specific effect appears more related to executive control processes (i.e. working memory, multi-tasking) (Hillman et al., 2008). For example, in a study by Vásquez et al. 2015, Hispanics and non-Hispanic blacks who participate in vigorous physical activity (either intermittently or consistently) had better general cognitive function compared with non-active participants after 10 years of follow up (Vasquez et al., 2015). Still, there is insufficient information as to whether a similar consistent pattern of association exists in Hispanics.
Evidence suggests that increasing time spent in sedentary behaviors is distinctly associated with poor health. Sedentary behavior refers to activities that resulting in low levels of energy expenditure (<1.5 metabolic equivalents) while in a sitting or reclining posture while awake (Tremblay, 2012). Recent population-based estimates of accelerometer-derived sedentary behaviour have reported that U.S. adults spend, on average, 55% of their waking hours sedentary (Matthews et al., 2008). Research has also shown racial, ethnic, and age variation with higher sitting time for Mexican American and Hispanics/Latinos females, and non-Hispanic Black males, even after adjusting for education (Sisson et al., 2012).
Finally, there are documented sex differences in specific cognitive abilities reported from childhood through adulthood (McCarrey et al., 2016). On average, men outperform women on spatial tasks, while women outperform men on some tests of verbal ability differences (Hebert et al., 2000; Mortensen et al., 2014). However, the effect of sex, age,and race/ethnicity continue to be less well studied in the relationship between physical activity behaviors and cognition across the life span. Specifically, identifying these relationships among Hispanics/Latinos is of public health importance given documented racial/ ethnic disparities in cognition and this fast-growing segment of the U.S. population. Moreover, few studies have used accelerometry-based data to study the association of physical activity and sedentary behavior on cognition in minority populations such as Hispanics/Latinos. The purpose of this study is to describe the cross-sectional association between physical activity and sedentary behavior and specific domains of cognitive function (executive function and memory), and how this varies by sex and age in a sample of US Hispanic/Latino middle aged and older adults in the US.
Methods
Study design and population
The Hispanic Community Health Study/Study of Latinos (HCHS/SOL) is a cohort study of 16,415 community-dwelling Hispanic/Latino adults of diverse backgrounds (Central American, Cuban, Dominican, Mexican, Puerto Rican, and South American) living in four US cities (Miami, Fl, Bronx, NY, Chicago, IL, and San Diego, CA). Participants were recruited using a 2-stage area probability sampling design, as described previously (Lavange, Kalsbeek et al. 2010; Sorlie, Aviles-Santa et al. 2010). Participants were ages 18–74 years at screening, able to travel to the local study field center, and had no plans to move out of the study area; 41.7% of screened individuals were enrolled.
The HCHS/SOL baseline examination was conducted in 2008–2011 by bilingual interviewers in either English or Spanish and included a comprehensive clinical evaluation, accelerometry, and questionnaires covering a variety of sociodemographic, medical, environmental and lifestyle topics. A neurocognitive battery was administered by study staff to those age 45 years and older (n=9,714). The current analysis excluded participants with incomplete neurocognitive data (n=373), those who did not wear the accelerometer (n=847), those and those who attempted accelerometry but who were not adherent to the accelerometer protocol (n=1,006; at least 3 days with 10 hours of wear time each). These exclusions yielded an analytic sample of n=7,478 (77% of participants age 45 years and older). Institutional review boards at each participating institution approved the study and all participants gave written informed consent.
Assessment of sedentary behavior and physical activity
All participants were asked to wear an accelerometer (Actical model 198-0200–03; Respironics Co. Inc., Bend, Oregon) for seven days with removal only for swimming, showering, and sleeping (Evenson et al., 2015). The Actical was positioned above the right iliac crest and programmed to measure accelerations in “counts” in 1-minute epochs. The Actical has been previously shown to be reliable (r=1.0) (Choi et al., 2011; Esliger et al., 2007; Esliger and Tremblay, 2006; Welk et al., 2004). Non-wear time was determined using the Choi algorithm (Choi et al., 2011; Vasquez et al., 2016). Accelerometer data were summarized as the number of minutes per day spent sedentary (<100 counts/minute) and in moderate-vigorous physical activity (MVPA; >=1535 counts/minute), according to established cut-points (Colley et al., 2011; Wong et al., 2011).
Assessment of cognitive function
The HCHS/SOL neurocognitive battery included 1) Brief Spanish English Verbal Learning Test (B-SEVLT); 2) Word Fluency (WF) test; and 3) Digit Symbol Substitution Test (DSST), as described previously (Gonzalez et al., 2015). The B-SEVLT is a measure of episodic verbal learning and memory that is psychometrically equivalent in English and Spanish versions and consists of three 15-word learning trials, a fourth interference trial, and a fifth recall trial of the initial word set (Gonzalez et al., 2001). Two aspects of the B-SEVLT were analyzed here: “learning” defined as the sum of the first three trials, and “delayed recall” representing the retention of previously learned material and defined as the number of words correctly recited in the fifth trial. The Brief Spanish English Verbal Learning Test—is reported to have a test-retest reliability coefficient of 0.65 and 0.72 for the internal consistency intraclass correlation (Campo and Morales, 2004). In the fluency test, participants were asked to produce as many words as possible beginning with the letters F, then A, in consecutive 1-minute trials (Spreen O and Strauss, 1998). This test has a test-retest reliability correlation of 0.79 and a construct validity of 0.66 (Cohen and Stanczak, 2000). In the DSST of the Wechsler Adult Intelligence Scale, (Lezak MMD, 1993) scores represent the number of digit-symbol pairs transcribed in 90 seconds using a key that pairs each of the digits 1–9 with unique symbols. The DSST has high test-retest reliability with correlation coefficients ranging from 0.82 and 0.88 (Matarazzo and Herman, 1984). All tests were administered by trained personnel who were certified for proficiency by licensed neuropsychologists with regular, periodic recertification. From the raw cognitive test scores, we created a global cognitive function index using an average of the four indices ([individual value – mean value] /SD).
Other covariates
Hispanic/Latino background was assessed by asking, “Which of the following best describes your Hispanic/Latino heritage?” Other self-reported sociodemographic characteristics considered were age, years of education, sex, annual household income, highest level of education, employment status, and place of birth. The Physical Component Score (PCS) of the SF-12 Health Survey Version 2.0 (QualityMetric Inc., Lincoln, RI) was included as an aggregate measure of self-reported physical health status to account for prevalent diagnosed and undiagnosed conditions that may limit physical activity and also be related to cognitive functioning. The test-retest reliability of the self-reported physical health survey was reported to be 0.89 (Ware et al., 1996). Body mass index (BMI) was calculated as weight in kilograms divided by height squared (normal 18.5< BMI < 25; overweight, 25 - < 30; obese, ≥30).
In addition, an index of comorbid conditions was derived to control for potential physical limitation as a confounding factor (Sorlie et al., 2010; Vasquez et al., 2016). Each of the following conditions counted for one point in the index: Diabetes was defined according to the American Diabetes Association definition as either fasting glucose >=126mg/dL, 2-h post oral glucose tolerance test >=200mg/dL, glycated hemoglobin A1c >=6.5%, or anti-diabetes medication use (as determined through scanning of medications used in the past 4 weeks). Cardiovascular disease was defined as a composite of CHD (myocardial infarction, coronary insufficiency, and angina), cerebrovascular events (including ischemic stroke, hemorrhagic stroke, and transient ischemic attack), peripheral artery disease (intermittent claudication), and heart failure. Obstructive lung disease was based on spirometry ratio (FEV1/FVC) < 0.70.
Self-reported history of cancer was measured with responses to the question “Has a doctor ever said that you have cancer or a malignant tumor?”
Statistical analysis
Because individuals in the HCHS/SOL cohort were selected with unequal probabilities (Lavange et al., 2010) all analyses were conducted using sampling weights that were non-response adjusted, trimmed, and calibrated by age, sex, and Hispanic/Latino group to the characteristics of each field center’s target population from the 2010 US Census. We further accounted for by missing or incomplete accelerometer data (Evenson et al., 2014) using inverse probability weighting (Seaman and White 2013) as described previously (Arredondo et al., 2016). Error terms account for the cluster stratified sample using Taylor series linearization methods. All tests of significance were two-sided at a significance level of 5%. Analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC) and SUDAAN release 11.0.1 (RTI International, Research Triangle Park, NC).
The association between sedentary behavior, MVPA, and cognitive function was examined by first constructing a series of multivariable linear regression models with each cognitive measure and the cognitive function index modeled separately as dependent variables. Initial models adjusted for age, sex, years of education, and field center. Subsequent models were further adjusted for additional variables defined a priori as potential confounders including Hispanic/Latino background, employment status, annual household income, and a comorbidities index. Next, to avoid assuming a linear relationship between exposures, we modeled sedentary behavior as a four-level variable (with quartile cut-points), and MVPA as binary (meeting or not meeting accelerometer guidelines). Finally, to evaluate some of the differences reported in the literature, we explored heterogeneity in the effect across levels of age and sex by constructing fully adjusted models with interaction terms between age and sex and MVPA and sedentary behaviors (i.e. age*sedentary behavior; sex*sedentary behavior) as well as main effects and each of these covariates.
Results
Sample Characteristics - Participants of HCHS/SOL study communities were 62% female, with approximately 38% having less than a high school level of education (Table 1). The majority of the sample was born outside the mainland (US) (90%) including those born in Puerto Rico. Mean time spent in sedentary behaviors was 12.3 hours/day in females and 11.9 hours/day in males (75% and 77% of accelerometer wear time, respectively). The estimated proportion obtaining >=150 minutes/week of MVPA or >=75 minutes/week of vigorous activity, or a combination of the two, from the accelerometer was 23.3 % (95% CI, 21.5–25.3) for females and 42.5 % for males (95% CI, 39.8–45.3). Time spent in sedentary behavior was higher with older age ranging from a mean 11.7 hours/day among those age 45–54 years to 12.9 hours/day for those age 65–75 years, and the proportion meeting accelerometer data MVPA cutpoints was subsequently lower with age (see supplement Table A.1). We conducted analyses to evaluate the difference in cognitive scores by cross-classified quartiles of sedentary behaviors and moderate-vigorous physical activity and found no meaningful changes in our results (data not shown).
Table 1.
Characteristics of the study population, overall and by sex.
| Overall weighted % | Women weighted % | Men weighted % | |
|---|---|---|---|
| N | 7478 | 4670 | 2808 |
| Age in years | |||
| 45–54 | 48% | 46% | 51% |
| 55–64 | 32% | 33% | 30% |
| 65–75 | 20% | 21% | 19% |
| Household Income | |||
| Less than $30,000 | 64% | 66% | 61% |
| $30,000 or more | 30% | 26% | 34% |
| Not reported | 6% | 8% | 4% |
| Educational attainment | |||
| < High school diploma/GED | 38% | 39% | 37% |
| At most high school diploma/GED | 22% | 20% | 24% |
| Beyond high school education | 40% | 40% | 40% |
| BMI Classification | |||
| Underweight (BMI<18.5) | 0% | 1% | 0% |
| Normal weight (18.5≤BMI<25) | 17% | 15% | 18% |
| Overweight (25≤BMI<30) | 41% | 37% | 45% |
| Obese (BMI≥30) | 42% | 47% | 36% |
| Cardiovascular disease | 9% | 7% | 12% |
| Diabetes | 27% | 28% | 27% |
| Hypertension | 47% | 49% | 45% |
| Obstructive lung disease | 12% | 9% | 15% |
| History of cancer | 7% | 8% | 5% |
| Cognitive outcome measures, mean ( Confidence Interval) | |||
| Cognitive Function overall score | 0.00 (−0.15, 0.15) | 0.47 (0.29, 0.66) | −0.57 (−0.77, −0.37) |
| Word Fluency | 18.5 (18.2, 18.8) | 18.6 (18.2, 19.0) | 18.3 (17.9, 18.7) |
| Digit Symbol Substitution Test | 34.1 (33.5, 34.7) | 34.9 (34.2, 35.5) | 33.2 (32.4, 34.0) |
| Spanish English Verbal Learning Test | 22.6 (22.4, 22.9) | 23.7 (23.4, 24.0) | 21.4 (21.1, 21.7) |
| Sum 3 trials | |||
| Spanish English Verbal Learning Test free recall | 8.2 (8.1, 8.3) | 8.6 (8.5, 8.8) | 7.6 (7.4, 7.7) |
| Cognitive outcome measures, mean | 7478 | 4670 | 2808 |
| Continuous accelerometer measures, median (IQR)* | |||
| Accelerometer wear time, hour/day | 15.1 (13.5, 18.1) | 14.9 (13.4, 17.7) | 15.4 (13.6, 18.4) |
| Mean counts per minute of wear time | 123 (80, 185) | 107 (73, 158) | 147 (94, 225) |
| Sedentary behavior, hour/day | 12.3 (11.2, 13.3) | 12.4 (11.4, 13.3) | 12.1 (10.8, 13.3) |
| Moderate-vigorous physical activity, min/day | 10.9 (3.5, 25.5) | 7.6 (2.1, 19.2) | 16.2 (6.3, 33.5) |
Summarized as the average daily value of each variable on adherent days
BMI: body mass index; IQR: interquartile range;
Main Study Analyses
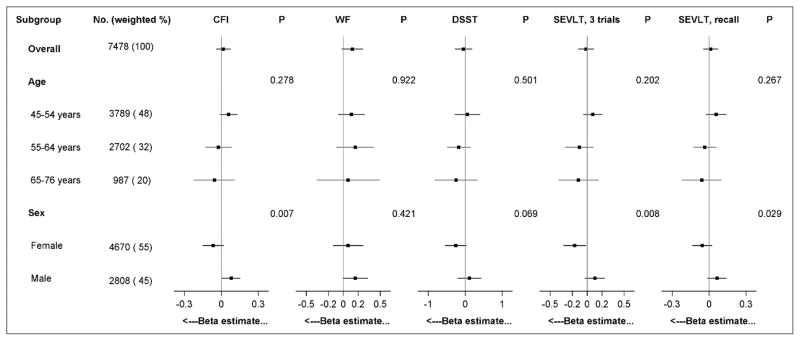
Figure 1 shows adjusted interactions between sex and sedentary behavior for the association with cognitive function index and both scores from the SEVLT (P<0.05 for each dependent variable). Among women, associations were in the expected direction with each 10-minute increase in sedentary behavior correlated with a 0.17 point reduction in the SEVLT 3 trials (P=0.031). In men, however, each 10-minute higher sedentary behavior was correlated with a beneficial 0.08-point increase in the cognitive function index (P=0.038). There was also a null relationship between MVPA and each cognitive measure consistent across sex and age groups (see supplement Figure A.1).
Figure 1. Group-specific estimates for the association between sedentary behavior and cognitive measures among Hispanic/Latino adults.
Betas (95% CI) are plotted by background group and represent the mean change in dependent variable for each 60-minute/day increase in sedentary behavior, derived from separate linear regression models for each interaction tested; P-values test for heterogeneity in the association between sedentary behavior and each outcome across subgroups; final models presented are adjusted for age, sex, years of education, Hispanic/Latino background, field center, employment status, annual household income, and self-reported physical health. Abbreviations: CFI, cognitive function index; WF, word fluency; DSST, Digit Symbol Substitution Test, SEVLT.
In table 2 most cognitive function results were generally null, although there was a nominally significant association between higher SED (but not MVPA) and slightly lower DSST raw scores (β −0.23 with each 10-min increment in SED; P=0.05). We did not observe associations of MVPA and SED with the other two cognitive tests nor with the global cognition index (P > 0.05). The statistically significant association of SED and DSST scores was attenuated and not statistically significant after adjustment of Hispanic/Latino background, socioeconomic status, and self-rated health. In addition, there were no statistically significant associations between MVPA and the global cognitive function index.
Table 2.
Adjusted regression coefficients (betas) assessing the relationship among objectively measured PA and sedentary behavior with cognitive function
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| β (SE) | p-value | β (SE) | p-value | |
| Sedentary behavior (each 60 min/day increase) | ||||
| Cognitive function overall score | −0.037 (0.031) | 0.23 | 0.017 (0.031) | 0.582 |
| Word Fluency | 0.038 (0.071) | 0.60 | 0.122 (0.074) | 0.099 |
| Digit Symbol Substitution Test | −0.231 (0.116) | 0.05 | −0.046 (0.120) | 0.699 |
| SEVLT Sum 3 Trials | −0.083 (0.055) | 0.13 | −0.014 (0.054) | 0.799 |
| SEVLT Free Recall | −0.020 (0.031) | 0.53 | 0.012 (0.031) | 0.699 |
| MVPA (each 10 min/day increase) | ||||
| Cognitive function overall score | −0.006 (0.018) | 0.71 | −0.023 (0.015) | 0.13 |
| Word Fluency | −0.005 (0.042) | 0.90 | −0.030 (0.040) | 0.45 |
| Digit Symbol Substitution Test | 0.008 (0.056) | 0.89 | −0.030 (0.052) | 0.57 |
| B-SEVLT (Sum 3 Trials) | −0.004 (0.027) | 0.88 | −0.031 (0.026) | 0.23 |
| SEVLT Free Recall | −0.014 (0.018) | 0.44 | −0.026 (0.017) | 0.12 |
B-SEVLT : Brief Spanish English Verbal Learning Test; SEVLT: Spanish English Verbal Learning Test; MVPA: moderate to vigorous physical activity.
Model 1. Adjusted for age, sex, years of education, and field center
Model 2. Fully adjusted models include age, sex, years of education, Hispanic/Latino background, employment status, annual household income, birth within the US 50 states, aggregate physical health status and field center
Discussion
In this large community based study with a standardized accelerometer-based measure of physical activity, we did not find any evidence among Hispanics/Latinos that an active lifestyle may be associated with improved cognitive function. We did observe significant associations between sedentary behavior and lower performance in executive functioning, which were attenuated after adjusting for potential confounders. Our findings agree with other studies that suggest possible sex differences with lower cognitive performance in women than in men on tests of executive function (Digit Symbol Substitution Test (DSST), Word Fluency), and memory (Spanish English Verbal Learning Test). We observed that women had a fairly consistent detrimental effect of high time spent in sedentary behavior while men did not. The average rate of cognitive decline in a normal, community-dwelling population is 0.04 SD to 0.05 SD/annually (Hayden et al., 2011; Royall et al., 2005). In contextualizing our findings, it is helpful to note the observed 6.5-point difference in the DSST score when comparing women age 55–64 years to women age greater than or equal to 65 years in this population. This approximately 0.65 lower score associated with each one year in age difference is greater in magnitude than the nonsignificant association we observed among women with a 60-minute increment in sedentary behavior (beta, −0.23; SE, 0.15). This comparison suggests that the association between a full hour of additional sedentary behavior with DSST even among women is not only not statistically significant, but also that any association for which we may have lacked the power to detect is of small magnitude that may not be clinically significant.
Sleep, which also may be related to cognitive decline, has a complex bidirectional relationship with sedentary and exercise habits during waking hours. Prior studies from HCHS/SOL suggest that obstructive sleep apnea, short sleep duration and long sleep duration may influence cognitive test performance (Ramos et al 2016, 2015). These potential effects of sleep appeared to be stronger among women as compared men in HCHS/SOL, paralleling the differences by sex subgroup in our present report (Ramos et al 2016 (Ramos et al., 2015). Among older women, the association between cognitive function was restricted to the DSST, a measure of executive function with no relationships observed in men. However, none of these studies adjust for the effect of MVPA and SED. A possible explanation for our discrepant findings can be that women in our sample may have other unmeasured exposures which may influence their cognitive performance in the presence of sedentary behavior. Dissimilar to our findings, a cross-sectional study by Rosano et al 2005 reported higher executive function scores for older women than men (Faulkner et al., 2006; Rosano et al., 2005).
The results of this cross-sectional study were largely null, as seen in prior studies (Barnes et al., 2008; Kerr et al., 2013), and there are several reasons why some of our findings may conflict with prior evidence supporting a protective effect of physical activity on cognitive function among non-Hispanic whites. Some of the possible explanations could be the different MVPA patterns of participation reported by Hispanic/Latinos in the different age groups analyzed in this study, and the use of accelerometry (an objective measure) to ascertain MVPA and SED instead of the traditional self-report ascertainment. In a study conducted by Kerr et al 2013, the relationship between cognitive functioning and the time spent in objectively measured physical activity was evaluated and their findings suggest that low levels of PA were not related to cognitive score. However, it is important to note that when looking at physical activity the use of standard cutpoints are for a general adult population. The importance of this is that the cutpoints used of our study may undercount the number of minutes that the sample is likely doing, particularly for those who are older and have lower fitness. This may be one reason why we did not find associations with MVPA. Thus, it may be that specific cutpoints are needed for older adults in order to see and association if one exist (Hooker et al., 2011).
Our large study failed to confirm previous studies that have documented associations between physical activity and improved cognitive function, possibly leading to delaying of cognition-impairing diseases (Chen et al., 2009; Gillum and Obisesan, 2010). Nonetheless, our study findings, linking sedentary behavior to sex specific cognitive function, have public health relevance as it suggests the need for interventions to specifically incorporate sedentary behavior reduction along with efforts to increase physical activity among middle age and older adults. The primary limitation of this study is its cross-sectional nature. The direction of the associations observed in this study cannot be completely ascertained given the cross-sectional design. However, despite the cross-sectional nature of the study design, there is novelty in the assessment of these associations in a diverse Hispanic/Latino population, which has not been previously done. Although, our data suggested a beneficial association of sedentary behavior in men's cognitive function, but not in women, this was one of several subgroup analyses and may have been due to chance. Therefore, this should be interpreted as a hypothesis generating observation that require replication in future research. Another limitation is that we were unable to determine dementia based on screening. However, given the age of our sample we estimate that the prevalence of dementia in this sample would be quite low. Our study strengths include the large sample of US Hispanics/Latinos representing different background groups, which makes the findings more generalizable to community-dwelling Hispanics/Latinos. Another strength is the objective evaluation of physical activity and sedentary behavior through a standardized accelerometry protocol with high adherence. However, we understand that although the Actical provides an objective measurement which can help reduce self-reporting bias, limitations of accelerometer derived measures should be acknowledged. Specifically, the measures of sedentary time and MVPA used in this study reflect spatial displacement in multiple dimensions derived from accelerometers. Given accelerometer positioning, it is plausible that some measurement bias may be introduced if time spent standing still was incorrectly measured as sitting due to the inability of the Actical accelerometer to differentiate different postures. In addition, similar to Qibin et al 2015, our accelerometer protocol specified a 1-minute epoch length, but the use of a shorter epoch length may lead to different results (Qi et al., 2015). Despite these potential issues, error associated with accelerometer measurement is likely to be non-differential, therefore resulting in conservative estimates that are biased towards the null.
Conclusion
We found a small associations between time spent in sedentary behavior (but not MVPA) and slightly lower DSST raw scores among women, but an opposite effect in men. Understanding the length of time Hispanics/Latinos spend physically active and in sedentary behaviors as well as determining the predictors of sedentary behavior is important to inform the development of effective public health strategies that can lead to better cognitive function among Hispanics/ Latinos.
Supplementary Material
Highlights.
We observed sex differences for Digit Symbol Substitution Test function.
Moderate/vigorous physical activity was not associated with cognition among Latinos.
Distinct association of sedentary behavior on executive function among Latinos.
Acknowledgments
The authors thank the staff and participants of HCHS/SOL for their important contributions. A complete list of staff and investigators has been provided by Sorlie P., et al. in Ann Epidemiol. 2010 Aug; 20: 642–649 and is also available on the study website http://www.cscc.unc.edu/hchs/
Funding
The Hispanic Community Health Study/Study of Latinos was carried out as a collaborative study supported by contracts from the National Heart, Lung, and Blood Institute (NHLBI) to the University of North Carolina (N01-HC65233), University of Miami (N01-HC65234), Albert Einstein College of Medicine (N01-HC65235), Northwestern University (N01-HC65236), and San Diego State University (N01-HC65237). The following Institutes/Centers/Offices contribute to the HCHS/SOL through a transfer of funds to the NHLBI: National Center on Minority Health and Health Disparities, the National Institute of Deafness and Other Communications Disorders, the National Institute of Dental and Craniofacial Research, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Neurological Disorders and Stroke, and the Office of Dietary Supplements.
Abbreviations
- BMI
Body Mass Index
- B-SEVLT
Brief Spanish English Verbal Learning Test
- CHD
Coronary Heart Disease
- DSST
Digit Symbol Substitution Test
- FEV1
Forced Expiratory Volume 1
- FVC
Forced Vital Capacity
- HCHS/SOL
Hispanic Community Health Study/Study of Latinos
- MVPA
Moderate-to-Vigorous Physical Activity
- PCS
Physical Component Score
- SD
Standard Deviation
- SED
Sedentary Behavior
- WF
Word Fluency
Footnotes
Conflict of interest:
Drs. Gonzalez and Tarraf report no conflicts of interest. They are supported by NIH/NIA (AG48642). Drs. Gellman, Lamar, and Kaplan also receive support from AG48642. All other authors reported no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alley D, Suthers K, Crimmins E. Education and Cognitive Decline in Older Americans: Results From the AHEAD Sample. Res Aging. 2007;29:73–94. doi: 10.1177/0164027506294245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alwin DF, Hofer SM. Health and cognition in aging research. J Gerontol B Psychol Sci Soc Sci. 2011;66(Suppl 1):i9–16. doi: 10.1093/geronb/gbr051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arredondo EM, Sotres-Alvarez D, Stoutenberg M, Davis SM, Crespo NC, Carnethon MR, Castaneda SF, Isasi CR, Espinoza RA, et al. Physical Activity Levels in U.S. Latino/Hispanic Adults: Results From the Hispanic Community Health Study/Study of Latinos. Am J Prev Med. 2016;50:500–8. doi: 10.1016/j.amepre.2015.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DE, Blackwell T, Stone KL, Goldman SE, Hillier T, Yaffe K. Cognition in older women: the importance of daytime movement. J Am Geriatr Soc. 2008;56:1658–64. doi: 10.1111/j.1532-5415.2008.01841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo P, Morales M. Normative data and reliability for a Spanish version of the verbal Selective Reminding Test. Archives of clinical neuropsychology : the official journal of the National Academy of Neuropsychologists. 2004;19:421–35. doi: 10.1016/S0887-6177(03)00075-1. [DOI] [PubMed] [Google Scholar]
- Castora-Binkley M, Peronto CL, Edwards JD, Small BJ. A Longitudinal Analysis of the Influence of Race on Cognitive Performance. J Gerontol B Psychol Sci Soc Sci. 2013 doi: 10.1093/geronb/gbt112. [DOI] [PubMed] [Google Scholar]
- Castora-Binkley M, Peronto CL, Edwards JD, Small BJ. A longitudinal analysis of the influence of race on cognitive performance. J Gerontol B Psychol Sci Soc Sci. 2015;70:512–8. doi: 10.1093/geronb/gbt112. [DOI] [PubMed] [Google Scholar]
- Chen JH, Lin KP, Chen YC. Risk factors for dementia. J Formos Med Assoc. 2009;108:754–64. doi: 10.1016/S0929-6646(09)60402-2. [DOI] [PubMed] [Google Scholar]
- Choi L, Liu Z, Matthews CE, Buchowski MS. Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc. 2011;43:357–64. doi: 10.1249/MSS.0b013e3181ed61a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MJ, Stanczak DE. On the reliability, validity, and cognitive structure of the Thurstone Word Fluency Test. Archives of clinical neuropsychology : the official journal of the National Academy of Neuropsychologists. 2000;15:267–79. [PubMed] [Google Scholar]
- Colley RC, Garriguet D, Janssen I, Craig CL, Clarke J, Tremblay MS. Physical activity of Canadian adults: accelerometer results from the 2007 to 2009 Canadian Health Measures Survey. Health Rep. 2011;22:7–14. [PubMed] [Google Scholar]
- Esliger DW, Probert A, Connor Gorber S, Bryan S, Laviolette M, Tremblay MS. Validity of the Actical accelerometer step-count function. Med Sci Sports Exerc. 2007;39:1200–4. doi: 10.1249/mss.0b013e3804ec4e9. [DOI] [PubMed] [Google Scholar]
- Esliger DW, Tremblay MS. Technical reliability assessment of three accelerometer models in a mechanical setup. Med Sci Sports Exerc. 2006;38:2173–81. doi: 10.1249/01.mss.0000239394.55461.08. [DOI] [PubMed] [Google Scholar]
- Evenson K, Sotres-Alvarez D, Deng Y, Marshall S, Isasi C, Esliger D, Davis S. Accelerometer Adherence and Performance in a Cohort Study of US Hispanic Adults. Med Sci Sports Exerc. 2014 doi: 10.1249/MSS.0000000000000478. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenson KR, Sotres-Alvarez D, Deng YU, Marshall SJ, Isasi CR, Esliger DW, Davis S. Accelerometer adherence and performance in a cohort study of US Hispanic adults. Med Sci Sports Exerc. 2015;47:725–34. doi: 10.1249/MSS.0000000000000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner KA, Redfern MS, Rosano C, Landsittel DP, Studenski SA, Cauley JA, Zmuda JM, Simonsick EM, Kritchevsky SB, et al. Reciprocal influence of concurrent walking and cognitive testing on performance in older adults. Gait & posture. 2006;24:182–9. doi: 10.1016/j.gaitpost.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Gillum RF, Obisesan TO. Physical activity, cognitive function, and mortality in a US national cohort. Ann Epidemiol. 2010;20:251–7. doi: 10.1016/j.annepidem.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez HM, Mungas D, Reed BR, Marshall S, Haan MN. A new verbal learning and memory test for English- and Spanish-speaking older people. Journal of the International Neuropsychological Society : JINS. 2001;7:544–55. doi: 10.1017/s1355617701755026. [DOI] [PubMed] [Google Scholar]
- Gonzalez HM, Tarraf W, Gouskova N, Gallo LC, Penedo FJ, Davis SM, Lipton RB, Arguelles W, Choca JP, et al. Neurocognitive function among middle-aged and older Hispanic/Latinos: results from the Hispanic Community Health Study/Study of Latinos. Arch Clin Neuropsychol. 2015;30:68–77. doi: 10.1093/arclin/acu066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden KM, Reed BR, Manly JJ, Tommet D, Pietrzak RH, Chelune GJ, Yang FM, Revell AJ, Bennett DA, et al. Cognitive decline in the elderly: an analysis of population heterogeneity. Age and ageing. 2011;40:684–9. doi: 10.1093/ageing/afr101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert LE, Wilson RS, Gilley DW, Beckett LA, Scherr PA, Bennett DA, Evans DA. Decline of language among women and men with Alzheimer's disease. J Gerontol B Psychol Sci Soc Sci. 2000;55:P354–60. doi: 10.1093/geronb/55.6.p354. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nature reviews Neuroscience. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- Ho AJ, Raji CA, Becker JT, Lopez OL, Kuller LH, Hua X, Dinov ID, Stein JL, Rosano C, et al. The effects of physical activity, education, and body mass index on the aging brain. Hum Brain Mapp. 2011;32:1371–82. doi: 10.1002/hbm.21113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker SP, Feeney A, Hutto B, Pfeiffer KA, McIver K, Heil DP, Vena JE, Lamonte MJ, Blair SN. Validation of the actical activity monitor in middle-aged and older adults. Journal of physical activity & health. 2011;8:372–81. doi: 10.1123/jpah.8.3.372. [DOI] [PubMed] [Google Scholar]
- Karlamangla AS, Miller-Martinez D, Aneshensel CS, Seeman TE, Wight RG, Chodosh J. Trajectories of cognitive function in late life in the United States: demographic and socioeconomic predictors. Am J Epidemiol. 2009;170:331–42. doi: 10.1093/aje/kwp154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr J, Marshall SJ, Patterson RE, Marinac CR, Natarajan L, Rosenberg D, Wasilenko K, Crist K. Objectively measured physical activity is related to cognitive function in older adults. J Am Geriatr Soc. 2013;61:1927–31. doi: 10.1111/jgs.12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavange LM, Kalsbeek WD, Sorlie PD, Aviles-Santa LM, Kaplan RC, Barnhart J, Liu K, Giachello A, Lee DJ, et al. Sample design and cohort selection in the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20:642–9. doi: 10.1016/j.annepidem.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MMD. Neuropsychological Assessment. 2. Oxford University Press; New York: 1993. [Google Scholar]
- Masel MC, Peek MK. Ethnic differences in cognitive function over time. Ann Epidemiol. 2009;19:778–83. doi: 10.1016/j.annepidem.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masel MC, Raji M, Peek MK. Education and physical activity mediate the relationship between ethnicity and cognitive function in late middle-aged adults. Ethn Health. 2010;15:283–302. doi: 10.1080/13557851003681273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matarazzo JD, Herman DO. Base rate data for the WAIS-R: test-retest stability and VIQ-PIQ differences. Journal of clinical neuropsychology. 1984;6:351–66. doi: 10.1080/01688638408401227. [DOI] [PubMed] [Google Scholar]
- Matthews CE, Chen KY, Freedson PS, Buchowski MS, Beech BM, Pate RR, Troiano RP. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am J Epidemiol. 2008;167:875–81. doi: 10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarrey AC, An Y, Kitner-Triolo MH, Ferrucci L, Resnick SM. Sex differences in cognitive trajectories in clinically normal older adults. Psychol Aging. 2016;31:166–75. doi: 10.1037/pag0000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen EL, Flensborg-Madsen T, Molbo D, Fagerlund B, Christensen U, Lund R, Osler M, Avlund K. The relationship between cognitive ability and demographic factors in late midlife. J Aging Health. 2014;26:37–53. doi: 10.1177/0898264313508780. [DOI] [PubMed] [Google Scholar]
- Park HL, O'Connell JE, Thomson RG. A systematic review of cognitive decline in the general elderly population. Int J Geriatr Psychiatry. 2003;18:1121–34. doi: 10.1002/gps.1023. [DOI] [PubMed] [Google Scholar]
- Pew Research Center. US Population Projections: 2005–2050. 2015. [Google Scholar]
- Qi Q, Strizich G, Merchant G, Sotres-Alvarez D, Buelna C, Castaneda SF, Gallo LC, Cai J, Gellman MD, et al. Objectively Measured Sedentary Time and Cardiometabolic Biomarkers in US Hispanic/Latino Adults: The Hispanic Community Health Study/Study of Latinos (HCHS/SOL) Circulation. 2015;132:1560–9. doi: 10.1161/CIRCULATIONAHA.115.016938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos AR, Tarraf W, Rundek T, Redline S, Wohlgemuth WK, Loredo JS, Sacco RL, Lee DJ, Arens R, et al. Obstructive sleep apnea and neurocognitive function in a Hispanic/Latino population. Neurology. 2015;84:391–8. doi: 10.1212/WNL.0000000000001181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano C, Simonsick EM, Harris TB, Kritchevsky SB, Brach J, Visser M, Yaffe K, Newman AB. Association between physical and cognitive function in healthy elderly: the health, aging and body composition study. Neuroepidemiology. 2005;24:8–14. doi: 10.1159/000081043. [DOI] [PubMed] [Google Scholar]
- Royall DR, Palmer R, Chiodo LK, Polk MJ. Normal rates of cognitive change in successful aging: the freedom house study. Journal of the International Neuropsychological Society : JINS. 2005;11:899–909. doi: 10.1017/s135561770505109x. [DOI] [PubMed] [Google Scholar]
- Sachs-Ericsson N, Blazer DG. Racial differences in cognitive decline in a sample of community-dwelling older adults: the mediating role of education and literacy. Am J Geriatr Psychiatry. 2005;13:968–75. doi: 10.1176/appi.ajgp.13.11.968. [DOI] [PubMed] [Google Scholar]
- Sisson SB, Camhi SM, Tudor-Locke C, Johnson WD, Katzmarzyk PT. Characteristics of step-defined physical activity categories in U.S. adults. Am J Health Promot. 2012;26:152–9. doi: 10.4278/ajhp.100326-QUAN-95. [DOI] [PubMed] [Google Scholar]
- Sorlie PD, Aviles-Santa LM, Wassertheil-Smoller S, Kaplan RC, Daviglus ML, Giachello AL, Schneiderman N, Raij L, Talavera G, et al. Design and implementation of the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20:629–41. doi: 10.1016/j.annepidem.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 2. Oxford University Press; New York: 1998. [Google Scholar]
- Tremblay A. Letter to the Editor: Standardized use of the terms “sedentary” and “sedentary behaviours”. Appl Physiol Nutr Metab. 2012;37:540–42. doi: 10.1139/h2012-024. [DOI] [PubMed] [Google Scholar]
- Vasquez E, Botoseneanu A, Bennett JM, Shaw BA. Racial/Ethnic Differences in Trajectories of Cognitive Function in Older Adults: Role of Education, Smoking, and Physical Activity. J Aging Health. 2015 doi: 10.1177/0898264315620589. [DOI] [PubMed] [Google Scholar]
- Vasquez E, Strizich G, Gallo L, Marshall SJ, Merchant GC, Murillo R, Penedo FJ, Salazar C, Sotres-Alvarez D, et al. The Role of Stress in Understanding Differences in Sedentary Behavior in Hispanic/Latino Adults: Results From the Hispanic Community Health Study/Study of Latinos Sociocultural Ancillary Study. Journal of physical activity & health. 2016;13:310–7. doi: 10.1123/jpah.2014-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Welk GJ, Schaben JA, Morrow JR., Jr Reliability of accelerometry-based activity monitors: a generalizability study. Med Sci Sports Exerc. 2004;36:1637–45. [PubMed] [Google Scholar]
- Wong SL, Colley R, Connor Gorber S, Tremblay M. Actical accelerometer sedentary activity thresholds for adults. Journal of physical activity & health. 2011;8:587–91. doi: 10.1123/jpah.8.4.587. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Fiocco AJ, Lindquist K, Vittinghoff E, Simonsick EM, Newman AB, Satterfield S, Rosano C, Rubin SM, et al. Predictors of maintaining cognitive function in older adults: the Health ABC study. Neurology. 2009;72:2029–35. doi: 10.1212/WNL.0b013e3181a92c36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsembik BA, Peek MK. Race differences in cognitive functioning among older adults. J Gerontol B Psychol Sci Soc Sci. 2001;56:S266–74. doi: 10.1093/geronb/56.5.s266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.