Abstract
Head and neck irradiation (IR) during cancer treatment causes by-stander effects on the salivary glands leading to irreversible loss of saliva secretion. The mechanism underlying loss of fluid secretion is not understood and no adequate therapy is currently available. Delivery of an adenoviral vector encoding human aquaporin-1 (hAQP1) into the salivary glands of human subjects and animal models with radiation-induced salivary hypofunction leads to significant recovery of saliva secretion and symptomatic relief in subjects. To elucidate the mechanism underlying loss of salivary secretion and the basis for AdhAQP1-dependent recovery of salivary gland function we assessed submandibular gland function in control mice and mice 2 and 8 months after treatment with a single 15-Gy dose of IR (delivered to the salivary gland region). Salivary secretion and neurotransmitter-stimulated changes in acinar cell volume, an in vitro read-out for fluid secretion, were monitored. Consistent with the sustained 60% loss of fluid secretion following IR, a carbachol (CCh)-induced decrease in acinar cell volume from the glands of mice post IR was transient and attenuated as compared with that in cells from non-IR age-matched mice. The hAQP1 expression in non-IR mice induced no significant effect on salivary fluid secretion or CCh-stimulated cell volume changes, except in acinar cells from 8-month group where the initial rate of cell shrinkage was increased. Importantly, the expression of hAQP1 in the glands of mice post IR induced recovery of salivary fluid secretion and a volume decrease in acinar cells to levels similar to those in cells from non-IR mice. The initial rates of CCh-stimulated cell volume reduction in acinar cells from hAQP1-expressing glands post IR were similar to those from control cells. Altogether, the data suggest that expression of hAQP1 increases the water permeability of acinar cells, which underlies the recovery of fluid secretion in the salivary glands functionally compromised post IR.
INTRODUCTION
More than half a million patients worldwide, and about 50 000 in the USA, undergo radiation treatment for head and neck cancers each year.1 A debilitating consequence of this treatment is an irreversible loss of salivary flow due to diminished function of the salivary glands, leading to xerostomia, a dry mouth in patients.2,3 Saliva is critical for the protection and maintenance of oral health with lubricatory, antimicrobial, reparative and remineralizing properties.4 A reduction in salivary secretion leads to dysphagia, mucositis, as well as an increase in oral infections, including rampant dental caries.3,5 The available treatments are primarily palliative in nature. A major drawback in developing a successful treatment strategy for this condition has been the lack of clear understanding of the mechanism by which irradiation (IR) induces a decrease in salivary gland function. The studies in several animal models, including mini-pig, rats and mice,6–11 have reported that following a single dose of IR there is a slow loss of salivary gland tissue3 due to likely damage of progenitor cell population within the gland.12 In addition, the effects of IR on neuronal cells as well as vasculature have also been suggested to contribute to the loss of salivary gland function and regenerative capacity.13,14 Irrespective of the animal model used, it appears that at earlier time points after IR despite lack of overt loss or disruption of salivary gland tissue, stimulated salivary gland fluid secretion is severely depressed. The mouse model is very useful for studying the early effects of IR since the onset of fibrosis is relatively slow and thus the salivary gland is relatively intact up to 6 months after IR.3,6 Both single-dose and fractionated IR procedures for salivary glands have been well established in the mouse.10,15
Recent studies have assessed treatments that prevent loss of function due to IR in mice. For example, the protection of salivary gland function was seen by MnSOD-plasmid liposome gene therapy16,17 or pretreatment with nitric oxide synthase inhibitors; reactive oxygen species scavengers, like resveratrol; hyperbaric oxygen therapy; anti-oxidants like, quercetin; and use of IR technologies that allow parts of the salivary glands that contain critical cell populations to be spared.7–9,11,18 As well as protection afforded by TK1B (Tousled-like kinase 1B) gene therapy.19 Alternatively, other strategies aim towards recovery of function or regeneration of tissue after IR, including stem cell transplantation methods.20,21 We have developed a gene therapy strategy in which adenoviral vector-encoding human aquaporin-1 (hAQP1) cDNA (AdhAQP1) was delivered, by retrograde instillation via the main excretory duct, to salivary glands of animals displaying persistent loss of salivary gland function due to IR.22 Expression of hAQP1 in salivary glands at 2 months post IR was effective in increasing salivary fluid secretion of small and large animal models, including rats23 and miniature pigs.24 Further, a phase I clinical trial recently was conducted in subjects who had undergone IR and exhibited symptoms of dry mouth.25 In that study, AdhAQP1 was infused into single parotid glands via the main excretory ducts. Five out of the eleven treated subjects displayed an improvement in salivary gland function as demonstrated by both subjective and objective criteria.25 Thus, the hAQP1 gene therapy strategy appears to be promising for the recovery of salivary gland function post IR. However, a key question that remains to be addressed is how exogenous hAQP1 expression leads to an increase in fluid secretion from the salivary glands.
The primary site of fluid secretion in salivary glands is the acinar cell, which secretes water and electrolytes in response to neurotransmitter stimulation.26 The process of secretion involves a cascade of events, which trigger an increase in cytosolic [Ca2+] and activation of ion fluxes that generate the required osmotic gradient to drive water movement. Fluid secretion from acinar cells is accompanied by a decrease in cell volume,26,27 the extent of which correlates well with salivary gland secretion in the mouse.6 In the present study, we have assessed agonist-stimulated acinar cell volume changes in mice 2 and 8 months post IR to determine the effects of IR on acinar cell physiology that can account for IR-induced loss of fluid secretion. Further, we have examined AdhAQP1-induced modification of cellular function that underlies the recovery of fluid secretion from irradiated murine salivary glands. We used a single radiation dose of 15 Gy, which has been shown to reproducibly induce persistent decrease (about 60%) in salivary fluid secretion without any distress or deleterious effects to the health of the animal; that is, mucositis or weight loss.10 Herein, AdhAQP1 was delivered to the submandibular glands of mice, 2- and 8-month-old post -IR as well as aged-matched non-IR controls, which resulted in the exogenous expression of hAQP1 locally within the gland. In addition to salivary flow measurements, cluster preparations of the salivary glands were used to measure agonist-stimulated acinar cell volume decreases. Our results reveal that acinar cells from IR mice display a significant attenuation of the cell volume change following agonist stimulation, which can account for the decrease in the amount of fluid secreted from the salivary glands of irradiated mice. Importantly, the cells from glands receiving AdhAQP1 display expression of the hAQP1 water channel in acinar cell plasma membranes together with an increase in the rate and extent of cell shrinkage in response to cholinergic stimulation. These findings demonstrate an increased water permeability in transduced acinar cells, which enhances agonist-stimulated cell shrinkage. Altogether, these data provide a mechanism to account for the recovery of salivary flow following the delivery of AdhAQP1 to the salivary glands post IR treatment.
RESULTS AND DISCUSSION
Effect of IR on fluid secretion and morphology of mouse submandibular glands
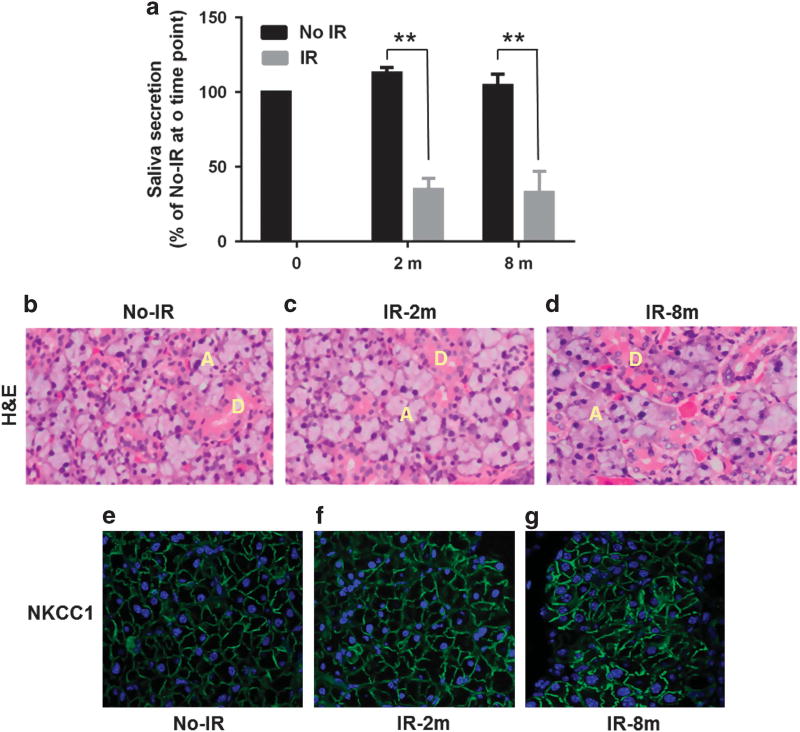
The mice were irradiated using previously described and well-characterized protocols6 (details are in the ‘Materials and methods’ section). The mice were irradiated when they were 8 weeks old and functional assessments were made at 2 months and 8 months post IR. Age-matched mice (4 and 10 months old, respectively) that did not receive IR were used as controls. There was a >60% decrease in salivary flow from mice at both 2 and 8 months post IR compared with their age-matched non-irradiated mice (Figure 1a). Importantly, despite the loss of salivary gland function, hematoxylin and eosin staining demonstrated that the salivary glands obtained from the mice 2 and 8 months post IR had, in general, a normal histologic appearance (Figures 1b–d). Further, examination of NKCC1, an ion transporter localized in the basolateral membrane of acinar cells that is critical for fluid secretion from salivary acinar cells, exhibited a similar pattern and level of immunofluorescence signal in the control and irradiated glands (Figures 1f–h). Altogether, these data suggest that loss of saliva flow following IR is not due to overt tissue damage, a finding similar to that previously reported.6,28
Figure 1.
Effect of IR on fluid secretion and morphology of mouse submandibular glands. (a) Salivary flow in irradiated (IR; 2 and 8 months post IR) and control non-irradiated (No-IR) aged-matched mice is displayed relative to that in control 8-week-old mice (that is, age before IR). Asterisks indicate values that are significantly different from their control No IR (**P < 0.01, n = 8 per group). (b–d) H&E staining of glands from control mice (before IR) and mice 2 months and 8 months post IR, ducts (D) and acini (A) are indicated. (e–g) NKCC1 (Na-K-Cl cotransporter isoform 1) expression in salivary gland sections from the same set of tissues displayed in b–d. Green signal represents localization of NKCC1 in the basolateral region of the acinar cells. H&E, hematoxylin and eosin.
IR treatment attenuated agonist-stimulated decrease in the volume of salivary gland acinar cells
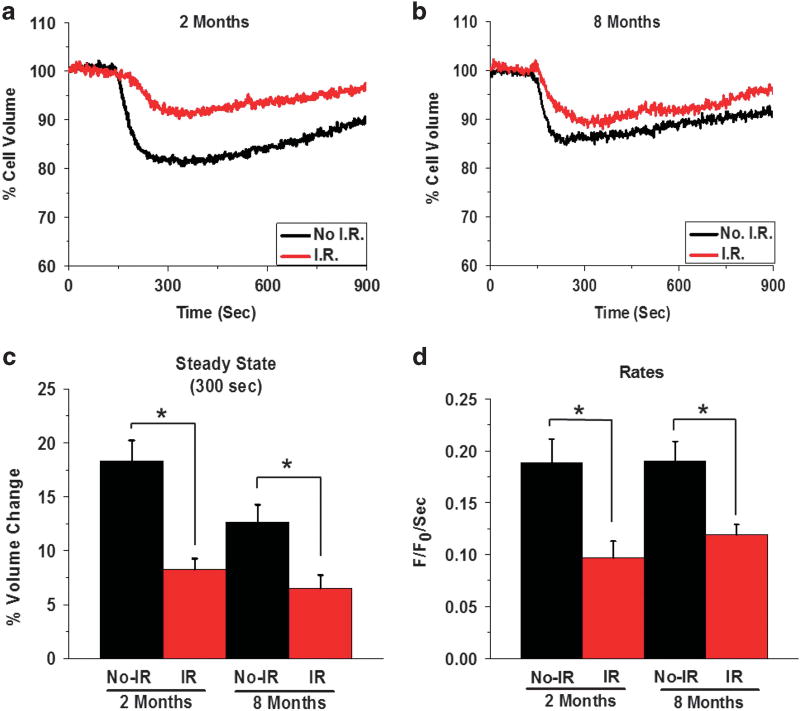
Agonist-stimulated fluid secretion from salivary gland acinar cells is accompanied by a reduction in cell volume which is followed by a recovery in cell volume by a process termed regulatory volume increase. The initial loss of cell volume is due to loss of water from the cells.6,26,29 We examined cell volume changes caused by the muscarinic agonist, CCh (1 µm) in acinar cells obtained from the glands of mice 2 and 8 months post IR as well as from age-matched non-irradiated (No-IR) control mice. The data presented in Figures 2a and b show average traces obtained from experiments carried out with eight animals for No-IR and seven animals for IR groups, and five animals for No-IR and nine animals for IR groups at 2 and 8 months post IR, respectively. Both submandibular glands from each mouse were combined for lobule preparations and at least three to four samples were assayed from each preparation (with >10 regions of interest measured per sample). CCh induced a decrease (18%) in volume of acinar cells from control mice (No-IR), which was followed by a slow recovery of the cell volume (see Figures 2a and c; the regulatory volume increase). The steady state decrease in cell volume was significantly less (12%) in cells from the older mice although there was no apparent difference in the recovery of cell volume when compared with cells from the younger mice. Importantly, acinar cells from the glands of mice post IR displayed a significant reduction in the rate and extent of cell volume decrease compared with cells from age-matched non-IR controls. The cells from 2-month IR group displayed 8% decrease in volume as compared with 18% in control cells. The initial rate of volume decrease was 0.09 ± 0.01 F/F0 per second in cells from IR mice vs 0.18 ± 0.02 F/F0 per second in cells from non-IR mice, respectively (P < 0.05). In the case of acini from 8-month post-IR mice CCh induced 6.5% decrease in volume compared with 12% in control cells with initial rates of 0.11 ± 0.01 F/F0 per second vs 0.18 ± 0.019 F/F0 per second, respectively (P < 0.05). These results demonstrate that the process of agonist-stimulated cell volume decrease is disrupted in acinar cells of salivary glands as a consequence of IR, consistent with our previous findings.6 Further studies are required to understand the specific cellular mechanisms that are altered by IR to induce these changes in acinar cells.
Figure 2.
Irradiation causes attenuation of agonist-stimulated acinar cell volume decreases. (a and b) Average traces displaying cell volume changes in acinar cells from age-matched No-IR mice and IR mice, 2 and 8 months post IR. (c and d) Average data with statistical evaluation showing maximum extent of volume decrease after 300 s of stimulation, steady-state cell volume (c), as well as initial rates of volume change (d) in the four sets of cells. The data shown are mean ± s.e.m. for at least 25 regions of interest (ROIs), representative of three to four samples each from four mice in control non-IR as well as IR groups. Asterisks indicate values significantly different from their controls (*P < 0.05).
Delivery of AdhAQP1 to salivary glands increases salivary flow in IR-mice
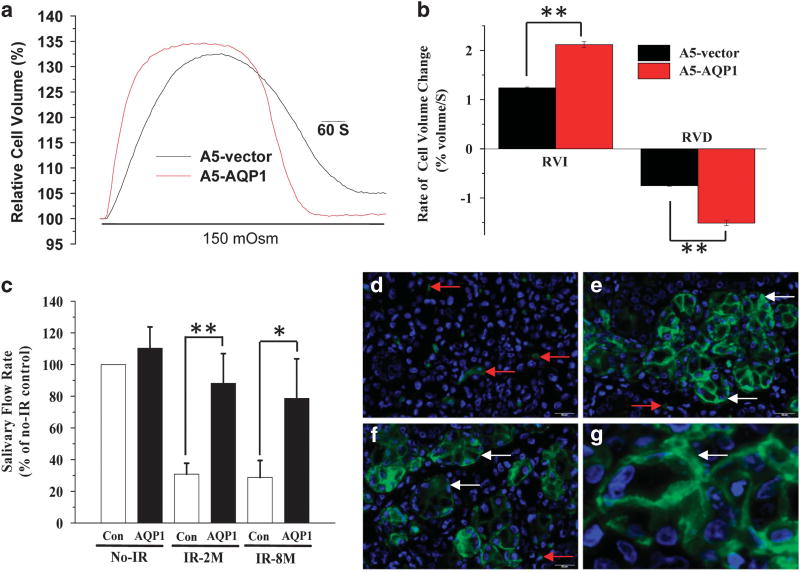
The functional consequence of AQP1 expression on water permeability was first assessed by transducing the rat submandibular ductal cell line, A5, with AdhAQP1. The cell volume response to hypotonic stress, which includes passive swelling followed by regulated volume decrease, was used as a read-out of plasma membrane water permeability. Figure 3a shows changes in calcein fluorescence in control and AdhAQP1 transduced cells. The swelling as well as shrinkage phases of the response were accelerated in the latter set of cells, demonstrating an increase in the water permeability due to AQP1 expression (Figures 3a and b). This finding agrees with previously reported data showing that the rate of water movement across an epithelial monolayer is increased following adenoviral-mediated AQP1 expression.30
Figure 3.
Adenoviral vector-mediated expression of hAQP1 enhances cellular water permeabilty and induces recovery of salivary fluid secretion in A5 cells and IR mice. (a and b) Cell volume responses in A5 cells following exposure to hypotonic medium: (a) time course of cell swelling followed by regulatory volume decrease (RVD) shown in control cells (labeled vector; treated with Ad-control) and cells treated with AdhAQP1. Average data with statistical evaluations are shown in b. Asterisks indicate values that are significantly different from their respective control group (**P < 0.001). (c) Salivary flow rate in No-IR mice and IR-mice 2 and 8 months post radiation. Function in each group was measured before and after AdhAQP1 delivery into the salivary glands and is expressed relative to that in the control No-IR group without vector treatment. Asterisks indicate values that are significantly different from their respective control group (*P < 0.05, n = 8 per group; **P < 0.001, n = 8 per group). The data shown are mean values ± s.e.m. (n = 8 mice). (d–g) hAQP1expression (green signal, red arrow indicates murine AQP1-positive endothelial cells, and white arrow indicates hAQP1 transgene-positive signal) after transduction with AdhAQP1 in the salivary glands (e–g) with an enlarged high image shown in g. Endogenous AQP1 signal in control gland without the AdhAQP1 treatment (d). As expected, only endothelial cells are positive for AQP1 in these glands. RVI, regulatory volume increase.
AdhAQP1 delivery into the submandibular glands of IR mice induced about a threefold increase in saliva flow rate, both at 2 and 8 months after IR (Figure 3c). AQP1, which is not endogenously expressed in the salivary gland epithelial cells, was detected in the acinar cells of glands that received AdhAQP1 (Figures 3e–g), but not in glands that received the AdControl vector. (Figure 3d). The transgenic hAQP1 was detected in the plasma membrane of acinar cells (in basolateral and apical regions) in glands from 2 month and 8 month post-IR mice, Figures 3e and f, respectively. In control mice (Figure 3d), hAQP1 is localized strictly in endothelial cells, as previously described31 (see representative red arrows in Figures 3d–f). An enlarged image shows AQP1 expression in an AdhAQP1-treated submandibular gland 2 months post IR (Figure 3g). Herein, we have not studied or discussed the expression of hAQP1 in duct cells, as the focus of this study was, and the functional assays were performed, only with acinar cells.
Exogenous expression of hAQP1 in salivary glands induced recovery of cell volume responses in acinar cells of IR-mice
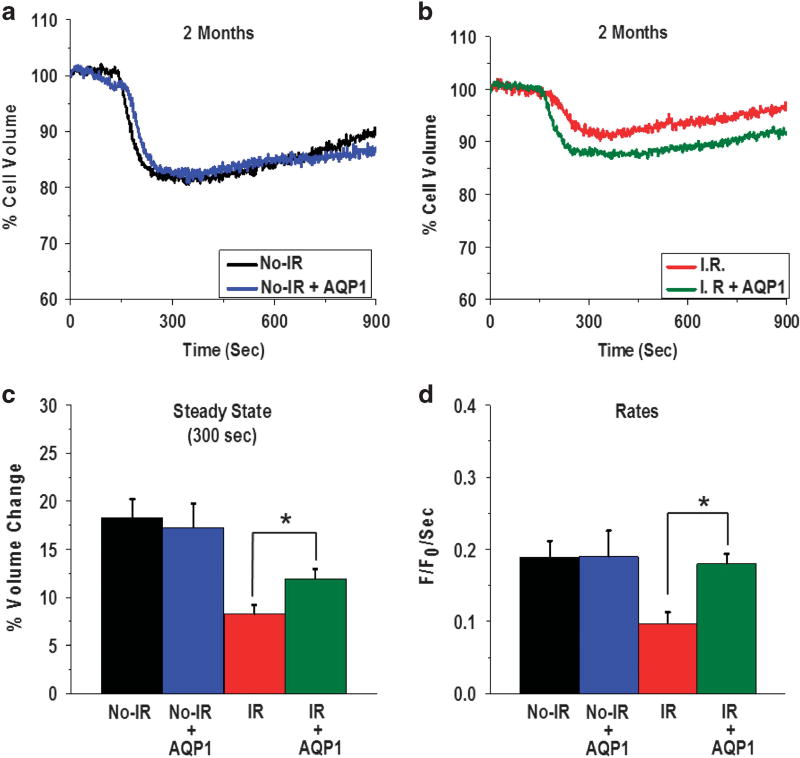
The salivary glands were removed from mice that received AdhAQP1 (age-matched control non-IR and 2 months post-IR mice) and used for acinar lobule preparation. CCh stimulation induced a 17% volume decrease in the acinar cells from control No-IR mice that received AdhAQP1. The volume change was not significantly different from the volume change (18%) observed in cells from No-IR mice that did not receive the vector (Figure 4a). The initial rates of this volume decrease in the two cell sets were also not different from each other (0.18 ± 0.03 F/F0 per second in mice with AdhAQP1 vs 0.18 ± 0.02 F/F0 per second in cells from mice without AdhAQP1; Figure 4d).
Figure 4.
hAQP1 expression in the acinar cells of salivary glands of mice 2 months post IR increases CCh-stimulated cell volume decreases. Average traces displaying cell volume change in No-IR (a) and 2 months post-IR groups (b) with and without AdhAQP1 treatment of mice. (c and d) Relative volume change and initial rates of volume change, respectively, calculated from the data shown in a and b. Asterisks indicate values that are significantly different from value indicated (*P < 0.05). The data shown are average traces from 30 ROIs and represent the data obtained from five mice in each group. ROI, region of interest.
In contrast, AdhAQP1 expression in the glands of mice 2 months post IR increased both the steady-state amplitude and the rate of the acinar cell volume decrease, as compared with that found in cells from IR mice that did not receive AdhAQP1 (Figures 4b–d). Steady-state volume after CCh stimulation was 88.06 ± 0.97% and 91.74 ± 0.99% of the initial volume in cells from IR mice with and without AdhAQP1, respectively. This represents a cell volume decrease of 12 vs 9% (P < 0.05; Figure 4c). In addition, initial rates of the cell volume decrease increased significantly from 0.09 ± 0.01 F/F0 per second no-vector IR cells to 0.17 ± 0.01 F/F0 per second in cells treated with AdhAQP1 (P < 0.05, Figure 4d). Together, these data demonstrate that exogenous expression of transgenic hAQP1 in acinar cells from IR mice induces an increase in water movement from acinar cells. This is most likely a result of an increase in the water permeability of the cells and can account for the increase in pilocarpine-stimulated saliva secretion measured in these mice. As (i) saliva secretion was measured over a 15-min time frame and (ii) the rate of secretion was highest soon after stimulation and declines with time over the 15-min period, the increase in initial rate of acinar cell volume reduction is highly likely to have a significant impact on the measured saliva volume in the animal.
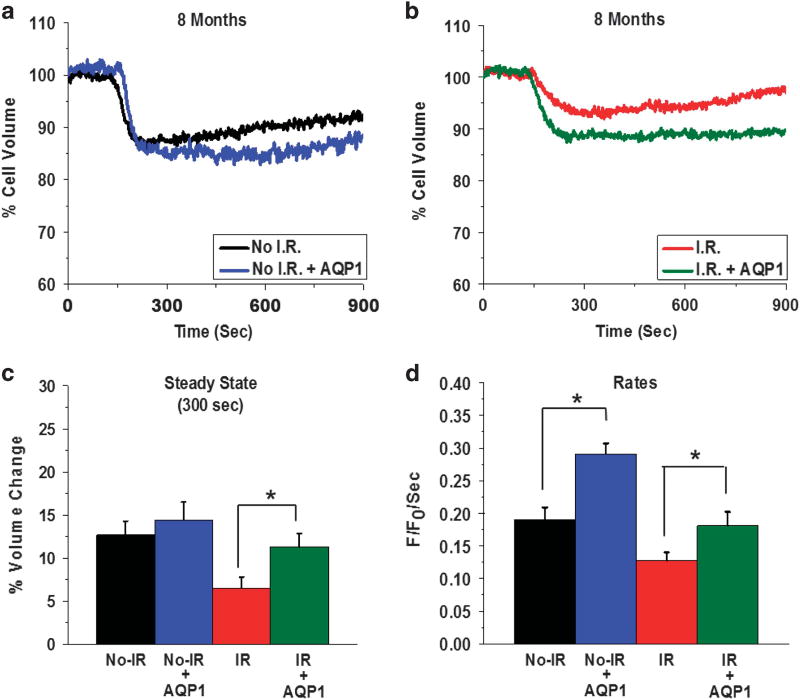
In addition, as salivary dysfunction persists and is irreversible, we also examined the effect of AdhAQP1 treatment in acinar cells from glands at 8 months post IR. Similar effects of the AdhAQP1 were detected in these mice. Average traces (Figures 5a and b) show CCh-stimulated volume changes in cells from the control No-IR group with and without AdhAQP1 treatment, as well as with cells from the IR groups. CCh stimulation decreased cell volume to a steady-state level of 87.35 ± 1.6% of the resting volume (that is, 12% decrease) in cells from No-IR mice without AdhAQP1 treatment. This was similar to steady-state volume 85.57 ± 2.0% of initial volume (that is, 14% decrease) seen following CCh stimulation of cells from AdhAQP1-treated No-IR mice (Figure 5c). In the case of IR mice, the cells from mice that received AdhAQP1 displayed a steady-state volume of 88.72 ± 1.57% of resting volume (11% decrease) compared with cells from IR mice that did not receive AdhAQP1; 93.52 ± 1.2% (that is, 6% volume decrease). Thus, the extent of cell volume decrease was enhanced, by AdhAQP1 delivery in mice 8 months post IR, by 1.8-fold (a significant change) as compared with mice not receiving AdhAQP1, (P < 0.05).
Figure 5.
hAQP1 expression in the acinar cells of salivary glands of mice 8 months post IR increases CCh-stimulated cell volume decreases. Average traces displaying cell volume change in No-IR (a) and 8 months post-IR groups (b) with and without AdhAQP1 treatment of mice. (c and d) Relative volume change and initial rates of volume change, respectively, calculated from the data shown in a and b. Asterisks indicate values that are significantly different from value indicated (*P < 0.05). The data shown are average traces from 38 ROIs and are representative of data obtained from five mice in each group. ROI, region of interest.
The initial rates of cell volume decrease were significantly increased in cells from IR mice receiving AdhAQP1, as well as cells from No-IR mice that received the AdhAQP1. In the No-IR group, the rate of cell volume decrease was 0.18 ± 1.2 F/F0 per second, while in No-IR mice treated with AdhAQP1 it was 0.29 ± 0.04 F/F0 per second. In cells from IR mice, the rate of volume change was 0.12 ± 0.012 F/F0 per second compared with cells from IR mice that received AdhAQP1, 0.18 F/F0 per second ± 0.02 (both cells sets showed significant change with the AdhAQP1 treatment, (P < 0.05, Figure 5d)). Further studies, however, are required to clarify why AdhAQP1 appears to increase function in cells from the older non-irradiated mice, compared with that in the age-matched younger mice. Most importantly, we show that acinar cells from irradiated mice display a significant recovery in CCh-stimulated cell volume reduction that can explain the recovery of salivary secretion in 10-month-old mice, that is, 8 months post IR.
Together, the results presented herein reveal that exogenous hAQP1 expression in murine acinar cells after gene transfer increases the water permeability of these cells, which then serves to enhance the rate and extent of cell volume reduction induced by the secretagogue CCh. The reduction in cell volume is tightly coupled to, and thus denotes, the secretion of water from acinar cells.29 Our findings demonstrate that the enhancement of agonist-stimulated reduction in acinar cell volume underlies the recovery of fluid secretion measured in IR mice after AdhAQP1 delivery into the gland. We show that IR glands display normal morphology despite loss of function at the ages studied. It is well established that agonist stimulation of acinar cells leads to generation of an osmotic gradient that drives water from the cell. Previous studies reported by Delporte et al.30 demonstrate that hAQP1 expression in polarized epithelial cells increased the initial rate of water secretion depending on the osmotic gradient exerted on the cell. The hAQP1-induced changes in acinar cell volume that we have described above are in complete agreement with this previous finding. Although we do not have a detailed understanding as to why fluid secretion is decreased in acinar cells post IR, our data indicate that attenuation of cell volume reduction in response to agonist stimulation is a key underlying factor in this salivary gland dysfunction. Further studies, however, are required to determine the exact mechanism that accounts for IR-induced persistent attenuation of agonist-stimulated acinar cell volume reduction. Irrespective of the mechanism, our data demonstrate that AdhAQP1-mediated expression of hAQP1 in salivary gland acinar cells of IR mice leads to the recovery of fluid secretory function at the cellular level, which then leads to enhanced salivary secretion in vivo. These findings indicate that agonist stimulation of cells from IR-mice causes generation of a sufficient osmotic gradient to support salivary secretion, as AdhAQP1-transduced salivary glands in the IR mice regain normal levels of salivary secretion. At a cellular level, this is reflected in the recovery of the initial rate of cell volume reduction.
In summary, based on the present findings, we propose that vector-mediated hAQP1 expression in acinar cells of IR mice enhances the water permeability of the cells, thus leading to the recovery of salivary gland fluid secretion. Interestingly, we have recently reported that the cytomegalovirus promoter in the AdhAQP1 vector used in the clinical trial and herein was likely not methylated32 in human salivary gland cells and thus can be functional for prolonged time periods versus when expressed in mouse cells. Our findings help to understand the beneficial observations made in that recent clinical trial conducted in which AdhAQP1 was delivered to salivary glands of individuals who had IR-induced salivary hypofunction after head-and-neck IR treatment.25
MATERIALS AND METHODS
Construction of recombinant vectors
The vectors, AdhAQP1 and Ad Control, used in this study were prepared exactly as described previously.23,33
Experimental animals and irradiation
C3HHENMTU (C3H) mice at the required age were purchased from the National Cancer Institute Animal Production Facility. The mice were 8 weeks of age at the time of experimentation and weighed between 20–25 g. All the experiments were carried out under the aegis of a protocol approved by the NIDCR and NCI Animal Care Committees and were in compliance with the Guide for the Care and Use of Laboratory Animal Resource (1996), National Research Council. Salivary gland IR was accomplished by placing each animal into a specially built Lucite jig such that the animal could be immobilized without the use of anesthetics.10,13 In addition, the jig was fitted with a Lucite cone that surrounded the head and prevented head movement during the radiation exposure.10,13 Lead shields were designed to cover the jigs with the mice with a small aperture in the lead shield that allowed radiation to the salivary gland area of the immobilized animal. A single radiation dose of 15 Gy was delivered to the animal by a Therapax DXT300 X-ray irradiator (Pantak, Inc., East Haven, CT, USA) using 2.0 mm Al filtration (300-kilovoltage peak) at a dose rate of 1.9 Gy min−1. Immediately after irradiation, the animals were removed from the Lucite jig and housed (five animals per cage) in a climate and a light/dark-controlled environment and allowed free access to dough diet (soft food). The protocol we have used here to assess IR effects on salivary gland function was previously developed in collaboration with Dr James Mitchell of the Radiation Biology Branch in NCI.10 The effects of radiation on saliva secretion in mouse models have already been well described.6–10 The 15-Gy single dose was established by earlier work from our laboratory, including a radiation dose response curve (from 0, 5, 12.5, 15, 17.5 and 20 Gy) that was performed. We found a significant reproducible reduction in saliva output from 12.5 through 20 Gy.10 At this dose, saliva secretion was fully protected by treating the animal with reactive oxygen species scavengers, like Tempol, whereas at higher doses, Tempol was not as effective.34 Importantly, under these conditions there were no other oral complications, such as mucositis, that were seen at doses ≤ 15 Gy. Furthermore, the irradiated animals did not undergo weight loss or display any neurological or other untoward consequences up to 8 months after IR. In addition, this same single dose of 15 Gy was used in mice to test successful gene therapy-based, and other, interventions.13,35
In vivo viral vector delivery and saliva collection
Two months or 8 months post IR, No-IR control or irradiated C3H mice were anesthetized with ketamine (60 mg kg−1) and xylazine (8 mg kg−1) intramuscularly. Vectors were delivered to both submandibular glands by intraoral cannulation.33 Complexes first were formed using 1 × 1012 molecules of pDsRed2-N1 plasmid DNA (as a positive transduction indicator, Clontech Laboratories, Inc., Mountain View, CA, USA) per gland, 0.5 mm polyethyleneimine, and 5 × 1010 particles of Ad-control (as control vector) or AdhAQP1 in a volume of 50 µl. This complex was administered by retrograde ductal instillation. The salivary secretion was measured 7 days post transduction. For whole saliva collection, anesthetized mice were treated with 0.5 mg kg−1 of pilocarpine solution subcutaneously following which the saliva was collected as described10,13 The submandibular glands were removed, fixed and used for hematoxylin and eosin, or immunofluorescence, staining. For cell volume measurements, C3H mice were killed in a carbon monoxide chamber and the submandibular glands were removed and placed in cold Tyrode’s solution (in mm):130 NaCl, 5.4 KCl, 1 CaCl2, 10 HEPES, 1 MgCl2, 10 glucose, 1% bovine serum albumin: pH 7.4.
Preparation of mouse cell lobules
The submandibular gland in cold Tyrode’s solution were thoroughly gassed with 95% O2 and 5% CO2. The glands were finely minced (approximately 100 µm in size) and loaded with 1 µm calcein (Molecular Probes, Invitrogen, Eugene, OR, USA) for 30 min and then washed in dye-free Tyrode’s solution for an additional 30 min.6 The loading and washing were done in the dark at room temperature on a rocker.
Cell volume measurements
The calcein-loaded lobules were placed in a perfusion chamber on cover slips that were coated with Cell Tak (from BD Bioscience, Bedford, MA, USA). The clusters were placed in an extracellular solution that included: 130 NaCl, 5.4 KCl, 1 CaCl2, 10 HEPES, 1 MgCl2, 10 glucose, pH 7.4. The cover slip was then placed on a large rectangular open bath chamber. The clusters were imaged using a Leica SP2 confocal mounted on a DM IRE2 inverted microscope. The regions of interest were chosen to measure the fluorescence intensity, a × 20 (0.4 NA) dry objective was used and the images were taken every 2 s. The fluorescence intensity was determined as a function of time and expressed relative to the initial fluorescence. Calcein was excited with the 488-nm line of an argon ion laser. The emitted fluorescence was measured at a wavelength of 510 nm. The clusters were stimulated with 1 µm Cch, an acetylcholine analog (Sigma, St. Louis, MO, USA). All the experiments were performed at 37 °C.
Immunofluorescence
The submandibular glands were fixed in 10% formalin and embedded in paraffin and sectioned (5 µm). After de-paraffinization and rehydration, antigen retrieval was carried out with 1 mm ethylenediaminetetraacetic acid (pH 8) and 0.05% Tween 20 in a microwave oven for 10 min. The sections were blocked with 20% goat serum in 5% bovine serum albumin for 1 h and then incubated with required primary antibodies; monoclonal anti-aquaporin-1 antibody, rabbit IgG (Abcam Inc., Cambridge, MA, USA) or polyclonal anti-NKCC1 antibody in 5% bovine serum albumin in phosphate-buffered saline for 1 h at room temperature or overnight, and then washed with phosphate-buffered saline. The sections were incubated with secondary antibodies, Alexa Fluor488 donkey antigoat immunoglobulin (Ig) G (H+L) for 1 h, washed with phosphate-buffered saline, and mounted with Prolong Gold antifade reagent with DAPI (4′,6-diamidino-2-phenylindole; Invitrogen).
Cell culture and measurement of regulated volume decrease
The A5 cell line was derived from rat submandibular gland36 and grown in McCoy’s 5A medium (Invitrogen) with 10% fetal bovine serum (Invitrogen,), 100 U ml−1 penicillin G (Invitrogen), 100 µg ml−1 streptomycin (Invitrogen). The cells were incubated at 37 °C in a humidified 5% CO2 atmosphere. The A5 cells were transduced using complex of 2 × 1010 molecules of pDsRed2-N1 plasmid DNA/5 × 105 cells, 0.1 mm polyethyleneimine and 5 × 107 particles of Ad-control or Adh-AQP1in a volume of 20 µl. Seven days post transduction, these A5 cells were used for the measurements of volume changes. For this, the cells were loaded with the fluorescent probe calcein (1 µm with 10 min loading) and excited at 488 nm. The emitted fluorescence was measured at 510 nm.37 The A5 cells were exposed to a hypotonic shock (HTS; from 300 to 150 mOsm by omitting NaCl from normal external solution). The changes in fluorescence intensity of calcein were calculated as percentage of regulated volume decrease and the peak time (300 s) was defined as the time to reach maximum cell volume decrease.
Statistical analysis
The data are presented as means ± s.e. Origin 9 (Origin Lab, Northampton, MA) was used for data analysis and display. The significant differences between individual groups were tested using an analysis of variance.
Acknowledgments
We thank Dr James B Mitchell, Chief of the Radiation Oncology Branch in NCI, NIH, for his longstanding collaboration with our group, as well as for allowing us to use their IR facilities for these studies.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Jensen SB, Pedersen AM, Vissink A, Andersen E, Brown CG, Davies AN, et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: prevalence, severity and impact on quality of life. Support Care Cancer. 2010;18:1039–1060. doi: 10.1007/s00520-010-0827-8. [DOI] [PubMed] [Google Scholar]
- 3.Vissink A, Mitchell JB, Baum BJ, Limesand KH, Jensen SB, Fox PC, et al. Clinical management of salivary gland hypofunction and xerostomia in head-and-neck cancer patients: successes and barriers. Int J Radiat Oncol Biol Phys. 2010;78:983–991. doi: 10.1016/j.ijrobp.2010.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amerongen AV, Veerman EC. Saliva—the defender of the oral cavity. Oral Dis. 2002;8:12–22. doi: 10.1034/j.1601-0825.2002.1o816.x. [DOI] [PubMed] [Google Scholar]
- 5.Jensen SB, Pedersen AM, Vissink A, Andersen E, Brown CG, Davies AN, et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: management strategies and economic impact. Support Care Cancer. 2010;18:1061–1079. doi: 10.1007/s00520-010-0837-6. [DOI] [PubMed] [Google Scholar]
- 6.Liu X, Cotrim A, Teos L, Zheng C, Swaim W, Mitchell J, et al. Loss of TRPM2 function protects against irradiation-induced salivary gland dysfunction. Nat Commun. 2013;4:1515. doi: 10.1038/ncomms2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spiegelberg L, Braks JA, Djasim UM, Farrell E, van der Wal KG, Wolvius EB. Effects of hyperbaric oxygen therapy on the viability of irradiated soft head and neck tissues in mice. Oral Dis. 2014;20:e111–e119. doi: 10.1111/odi.12162. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi A, Inoue H, Mishima K, Ide F, Nakayama R, Hasaka A, et al. Evaluation of the effects of quercetin on damaged salivary secretion. PLoS One. 2015;10:e0116008. doi: 10.1371/journal.pone.0116008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takeda I, Kizu Y, Yoshitaka O, Saito I, Yamane GY. Possible role of nitric oxide in radiation-induced salivary gland dysfunction. Radiat Res. 2003;159:465–470. doi: 10.1667/0033-7587(2003)159[0465:pronoi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 10.Vitolo JM, Cotrim AP, Sowers AL, Russo A, Wellner RB, Pillemer SR, et al. The stable nitroxide tempol facilitates salivary gland protection during head and neck irradiation in a mouse model. Clin Cancer Res. 2004;10:1807–1812. doi: 10.1158/1078-0432.ccr-03-0194. [DOI] [PubMed] [Google Scholar]
- 11.Xu L, Yang X, Cai J, Ma J, Cheng H, Zhao K, et al. Resveratrol attenuates radiation-induced salivary gland dysfunction in mice. Laryngoscope. 2013;123:E23–E29. doi: 10.1002/lary.24276. [DOI] [PubMed] [Google Scholar]
- 12.Nanduri LS, Maimets M, Pringle SA, van der Zwaag M, van Os RP, Coppes RP. Regeneration of irradiated salivary glands with stem cell marker expressing cells. Radiother Oncol. 2011;99:367–372. doi: 10.1016/j.radonc.2011.05.085. [DOI] [PubMed] [Google Scholar]
- 13.Cotrim AP, Sowers A, Mitchell JB, Baum BJ. Prevention of irradiation-induced salivary hypofunction by microvessel protection in mouse salivary glands. Mol Ther. 2007;15:2101–2106. doi: 10.1038/sj.mt.6300296. [DOI] [PubMed] [Google Scholar]
- 14.Knox SM, Lombaert IM, Haddox CL, Abrams SR, Cotrim A, Wilson AJ, et al. Parasympathetic stimulation improves epithelial organ regeneration. Nat Commun. 2013;4:1494. doi: 10.1038/ncomms2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cotrim AP, Yoshikawa M, Sunshine AN, Zheng C, Sowers AL, Thetford AD, et al. Pharmacological protection from radiation +/− cisplatin-induced oral mucositis. Int J Radiat Oncol. 2012;83:1284–1290. doi: 10.1016/j.ijrobp.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Epperly MW, Wegner R, Kanai AJ, Kagan V, Greenberger EE, Nie S, et al. Effects of MnSOD-plasmid liposome gene therapy on antioxidant levels in irradiated murine oral cavity orthotopic tumors. Radiat Res. 2007;167:289–297. doi: 10.1667/RR0761.1. [DOI] [PubMed] [Google Scholar]
- 17.Guo H, Seixas-Silva JA, Jr, Epperly MW, Gretton JE, Shin DM, Bar-Sagi D, et al. Prevention of radiation-induced oral cavity mucositis by plasmid/liposome delivery of the human manganese superoxide dismutase (SOD2) transgene. Radiat Res. 2003;159:361–370. doi: 10.1667/0033-7587(2003)159[0361:porioc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 18.van Luijk P, Pringle S, Deasy JO, Moiseenko VV, Faber H, Hovan A, et al. Sparing the region of the salivary gland containing stem cells preserves saliva production after radiotherapy for head and neck cancer. Sci Transl Med. 2015;7:305ra147. doi: 10.1126/scitranslmed.aac4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Timiri Shanmugam PS, Dayton RD, Palaniyandi S, Abreo F, Caldito G, Klein RL, et al. Recombinant AAV9-TLK1B administration ameliorates fractionated radiation-induced xerostomia. Hum Gene Ther. 2013;24:604–612. doi: 10.1089/hum.2012.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lombaert IM, Brunsting JF, Wierenga PK, Faber H, Stokman MA, Kok T, et al. Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS One. 2008;3:e2063. doi: 10.1371/journal.pone.0002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nanduri LS, Lombaert IM, van der Zwaag M, Faber H, Brunsting JF, van Os RP, et al. Salisphere derived c-Kit+ cell transplantation restores tissue homeostasis in irradiated salivary gland. Radiother Oncol. 2013;108:458–463. doi: 10.1016/j.radonc.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 22.Baum BJ, Wellner RB, Zheng C. Gene transfer to salivary glands. Int Rev Cytol. 2002;213:93–146. doi: 10.1016/s0074-7696(02)13013-0. [DOI] [PubMed] [Google Scholar]
- 23.Delporte C, O'Connell BC, He X, Lancaster HE, O'Connell AC, Agre P, et al. Increased fluid secretion after adenoviral-mediated transfer of the aquaporin-1 cDNA to irradiated rat salivary glands. Proc Natl Acad Sci USA. 1997;94:3268–3273. doi: 10.1073/pnas.94.7.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shan Z, Li J, Zheng C, Liu X, Fan Z, Zhang C, et al. Increased fluid secretion after adenoviral-mediated transfer of the human aquaporin-1 cDNA to irradiated miniature pig parotid glands. Mol Ther. 2005;11:444–451. doi: 10.1016/j.ymthe.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Baum BJ, Alevizos I, Zheng C, Cotrim AP, Liu S, McCullagh L, et al. Early responses to adenoviral-mediated transfer of the aquaporin-1 cDNA for radiation-induced salivary hypofunction. Proc Natl Acad Sci USA. 2012;109:19403–19407. doi: 10.1073/pnas.1210662109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melvin JE, Yule D, Shuttleworth T, Begenisich T. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol. 2005;67:445–469. doi: 10.1146/annurev.physiol.67.041703.084745. [DOI] [PubMed] [Google Scholar]
- 27.Ambudkar IS. Calcium signalling in salivary gland physiology and dysfunction. J Physiol. 2015 doi: 10.1113/JP271143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Connell AC, Redman RS, Evans RL, Ambudkar IS. Radiation-induced progressive decrease in fluid secretion in rat submandibular glands is related to decreased acinar volume and not impaired calcium signaling. Radiat Res. 1999;151:150–158. [PubMed] [Google Scholar]
- 29.Teos LY, Zhang Y, Cotrim AP, Swaim W, Won JH, Ambrus J, et al. IP3R deficit underlies loss of salivary fluid secretion in Sjogren's Syndrome. Sci Rep. 2015;5:13953. doi: 10.1038/srep13953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delporte C, Hoque AT, Kulakusky JA, Braddon VR, Goldsmith CM, Wellner RB, et al. Relationship between adenovirus-mediated aquaporin 1 expression and fluid movement across epithelial cells. Biochem Biophys Res Commun. 1998;246:584–588. doi: 10.1006/bbrc.1998.8668. [DOI] [PubMed] [Google Scholar]
- 31.Li J, Nielsen S, Dai Y, Lazowski KW, Christensen EI, Tabak LA, et al. Examination of rat salivary glands for the presence of the aquaporin CHIP. Pflugers Arch. 1994;428:455–460. doi: 10.1007/BF00374565. [DOI] [PubMed] [Google Scholar]
- 32.Zheng C, Baum BJ, Liu X, Goldsmith CM, Perez P, Jang SI, et al. Persistence of hAQP1 expression in human salivary gland cells following AdhAQP1 transduction is associated with a lack of methylation of hCMV promoter. Gene Ther. 2015;22:758–766. doi: 10.1038/gt.2015.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng CY, Baum BJ. Evaluation of viral and mammalian promoters for use in gene delivery to salivary glands. Mol Ther. 2005;12:528–536. doi: 10.1016/j.ymthe.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Cotrim AP, Hyodo F, Matsumoto K, Sowers AL, Cook JA, Baum BJ, et al. Differential radiation protection of salivary glands versus tumor by Tempol with accompanying tissue assessment of Tempol by magnetic resonance imaging. Clin Cancer Res. 2007;13:4928–4933. doi: 10.1158/1078-0432.CCR-07-0662. [DOI] [PubMed] [Google Scholar]
- 35.Zheng C, Cotrim AP, Rowzee A, Swaim W, Sowers A, Mitchell JB, et al. Prevention of radiation-induced salivary hypofunction following hKGF gene delivery to murine submandibular glands. Clin Cancer Res. 2011;17:2842–2851. doi: 10.1158/1078-0432.CCR-10-2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown AM, Rusnock EJ, Sciubba JJ, Baum BJ. Establishment and characterization of an epithelial cell line from the rat submandibular gland. J Oral Pathol Med. 1989;18:206–213. doi: 10.1111/j.1600-0714.1989.tb00764.x. [DOI] [PubMed] [Google Scholar]
- 37.Liu X, Bandyopadhyay BC, Nakamoto T, Singh B, Liedtke W, Melvin JE, et al. A role for AQP5 in activation of TRPV4 by hypotonicity: concerted involvement of AQP5 and TRPV4 in regulation of cell volume recovery. J Biol Chem. 2006;281:15485–15495. doi: 10.1074/jbc.M600549200. [DOI] [PubMed] [Google Scholar]