Abstract
BACKGROUND
Ebola virus has been detected in the semen of men after their recovery from Ebola virus disease (EVD). We report the presence of Ebola virus RNA in semen in a cohort of survivors of EVD in Sierra Leone.
METHODS
We enrolled a convenience sample of 220 adult male survivors of EVD in Sierra Leone, at various times after discharge from an Ebola treatment unit (ETU), in two phases (100 participants were in phase 1, and 120 in phase 2). Semen specimens obtained at baseline were tested by means of a quantitative reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assay with the use of the target sequences of NP and VP40 (in phase 1) or NP and GP (in phase 2). This study did not evaluate directly the risk of sexual transmission of EVD.
RESULTS
Of 210 participants who provided an initial semen specimen for analysis, 57 (27%) had positive results on quantitative RT-PCR. Ebola virus RNA was detected in the semen of all 7 men with a specimen obtained within 3 months after ETU discharge, in 26 of 42 (62%) with a specimen obtained at 4 to 6 months, in 15 of 60 (25%) with a specimen obtained at 7 to 9 months, in 4 of 26 (15%) with a specimen obtained at 10 to 12 months, in 4 of 38 (11%) with a specimen obtained at 13 to 15 months, in 1 of 25 (4%) with a specimen obtained at 16 to 18 months, and in no men with a specimen obtained at 19 months or later. Among the 46 participants with a positive result in phase 1, the median baseline cycle-threshold values (higher values indicate lower RNA values) for the NP and VP40 targets were lower within 3 months after ETU discharge (32.4 and 31.3, respectively; in 7 men) than at 4 to 6 months (34.3 and 33.1; in 25), at 7 to 9 months (37.4 and 36.6; in 13), and at 10 to 12 months (37.7 and 36.9; in 1). In phase 2, a total of 11 participants had positive results for NP and GP targets (samples obtained at 4.1 to 15.7 months after ETU discharge); cycle-threshold values ranged from 32.7 to 38.0 for NP and from 31.1 to 37.7 for GP.
CONCLUSIONS
These data showed the long-term presence of Ebola virus RNA in semen and declining persistence with increasing time after ETU discharge. (Funded by the World Health Organization and others.)
On March 17, 2016, The World Health Organization (WHO), the Chinese Center for Disease Control and Prevention (China CDC), and the Centers for Disease Control and Prevention (CDC) joined the government of Sierra Leone in marking the end of the most recent flare-up of Ebola virus disease (EVD) in the country; and in June 2016, the WHO declared the end of Ebola virus (EBOV) transmission in the Republic of Guinea and in Liberia.1 Unprecedented in its magnitude, this epidemic was responsible for the deaths of at least 3590 people in Sierra Leone.1
The main mode of transmission of EBOV is direct contact with the blood or body fluids of a person with EVD or from the body of a person who died from EVD.2,3 In addition, EBOV can persist in the body fluids of survivors of EVD during convalescence,4,5 and this persistence could result in transmission of the virus. The potential for the persistence of EBOV, particularly in the semen of male survivors, arouses concern about the risk of sexual transmission.6
Previously, EVD survivors had been advised to practice sexual abstinence or to use a condom during sexual activity for 3 months after recovery. These recommendations were based on EBOV-detection results from semen specimens that were obtained from eight survivors of EVD or Marburg virus disease in previous epidemics,5,7–11 in which the longest period that infectious virus was detected in semen after symptom onset was 82 days.5,7
In March 2015, a woman in Liberia received a diagnosis of EVD, and her only potential exposure that could be ascertained was sexual contact with a male survivor of EVD. Further investigation showed EBOV RNA in the survivor’s semen 199 days after the onset of his symptoms, and the genetic sequence matched the sequence in the sample obtained from the case patient.12 Although no infectious virus was detected in this semen specimen, the possibility that infectious EBOV could persist in the semen of survivors approximately 6 months after symptom onset prompted the WHO and the CDC to revise their guidelines regarding the length of time that survivors should practice safer sex (in particular, with the use of condoms) or sexual abstinence.13,14
A study that was published in August 2016 described 429 survivors of EVD who were enrolled in the National Semen Testing Program in Liberia.15 The authors found that the longest interval between discharge from an Ebola treatment unit and a positive result on reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assay was 565 days (18.5 months). Furthermore, a report from Guinea presented data on a male survivor in whom EBOV was detected by means of PCR in semen 531 days (17.4 months) after symptom onset, and sequencing data provided evidence of sexual transmission approximately 470 days (15.4 months) after symptom onset.16 Cases that have been linked to sexual contact with survivors of EVD from the West African outbreak of EVD have not been systematically documented, and fewer than 20 cases in total have been reported (Knust B, CDC; Formenty P, WHO: personal communication).
The Sierra Leone Ministry of Health and Sanitation, in collaboration with the Sierra Leone Ministry of Defense; the Sierra Leone Ministry of Social Welfare, Gender, and Children’s Affairs; the WHO; the CDC; and later the China CDC initiated a cohort study investigating the duration of virus persistence in the body fluids of survivors of EVD in Sierra Leone. This article describes the participants’ characteristics at entry in the cohort of male survivors of EVD whose semen was tested by means of RT-PCR; it is an update of a preliminary report, which is available with the full text of this article at NEJM.org.
METHODS
STUDY DESIGN, CONDUCT, AND OVERSIGHT
This was an observational cohort study of a convenience sample of 220 male survivors of EVD in Sierra Leone.17 The study was designed by the Sierra Leone Ministry of Health and Sanitation; the Sierra Leone Ministry of Social Welfare, Gender, and Children’s Affairs; the WHO; and the CDC. The Sierra Leone Ministry of Defense, the Sierra Leone Ministry of Health and Sanitation, the WHO, the CDC, and the China CDC gathered the data. Semen specimens were analyzed by the CDC during phase 1 and by the China CDC during phase 2 (phases 1 and 2 are defined below). Data analysis was performed and supervised by the WHO, the CDC, and the China CDC. Manuscript planning and drafting were overseen and performed by the Sierra Leone Ministry of Health and Sanitation, the WHO, the CDC, and the China CDC. The overall coordination of the study was ensured by WHO, in collaboration with the Sierra Leone Ministry of Health and Sanitation and the Sierra Leone Ministry of Defense. All the research activities were performed in accordance with all the applicable laws, regulations, and policies related to the protection of human participants and animals. The research protocol was reviewed and approved by the Sierra Leone Ethical Review Board and the WHO Ethical Review Committee. Participants received a compensation for each visit to the study site. A complete list of the members of the Sierra Leone Ebola Virus Persistence Study Group is provided in the Appendix, available with the full text of this article at NEJM.org.
STUDY POPULATION, SAMPLING, AND ELIGIBILITY CRITERIA
The 220 male survivors of EVD who were included in the cohort were identified at informational events that were held in conjunction with local associations for survivors of EVD.17 Men who indicated their interest in participation were offered enrollment in the study. Recruitment was conducted in two phases. Phase 1 enrolled 100 male participants (who had only semen tested) who had been recruited from the Western Area District in the capital of Freetown, and phase 2 enrolled 120 male participants (who had semen and other body fluids tested; only the results of the semen testing are reported in this article) who had been recruited from the Western Area District and from Lungi (Port Loko District).
Participants were eligible for inclusion if they were men, 18 years of age or older, and could provide an official EVD survivor certificate that had been issued by the Sierra Leone Ministry of Health and Sanitation. Such certificates were provided to persons with laboratory-confirmed cases of EVD when they were discharged from an Ebola treatment unit. Entry in the study began after an informed-consent form was signed; enrollment was followed by a standardized questionnaire and specimen collection.
DATA COLLECTION AND COUNSELING
At the time of enrollment, all the participants were administered a standardized questionnaire by a member of the study team in order to gather information about their sociodemographic characteristics, EVD episode, self-reported health status, and sexual behavior. The date of discharge from an Ebola treatment unit was ascertained from the participants’ EVD survivor certificates.
Participants received pre–Ebola-test counseling at the time of enrollment and post–Ebola-test counseling 2 weeks later, when they received their individual RT-PCR results. The counseling included information about the test performed, the meaning of the results, and education about sexual risk-reduction practices, including appropriate condom use and disposal.18 Participants were referred to a clinic for survivors of EVD if needed, as determined by the trained medical study staff, or requested.
SPECIMEN COLLECTION AND LABORATORY ANALYSES
Only semen specimens were obtained and tested for EBOV persistence in phase 1, and specimens of semen and other body fluids were obtained and tested for EBOV persistence in phase 2. At enrollment, participants were asked to provide a semen specimen in a private room and were provided instructions to ensure that proper infection-control procedures were followed. Trained counselors also offered participants a voluntary, confidential rapid test for the human immunodeficiency virus (HIV) in accordance with the national testing algorithm. However, this article focuses only on the semen testing.
Semen specimens were refrigerated (at 5 to 8°C) for no longer than 3 days. The semen specimens that had been obtained from phase 1 participants were transported to and tested at the CDC field laboratory in Bo District, Sierra Leone, and the semen specimens that had been obtained from phase 2 participants were transported to and tested at the China CDC Jui Laboratory in Freetown.
In phase 1, quantitative RT-PCR assays were performed that targeted EBOV NP and VP40 gene targets and the human β2-microglobulin (B2M) gene (Thermo Fisher Scientific), as described previously.19–21 A specimen was considered to be positive if the NP and VP40 gene targets were both detected within 40 cycles of replication, and the results were considered to be indeterminate if one of the NP or VP40 gene targets was detected but not both.
In phase 2, a double-channel quantitative RT-PCR detection kit was used to detect EBOV NP and GP genes22 and B2M. A specimen was considered to be positive if either or both of the NP or GP gene targets were detected within 38 cycles of replication. There was no indeterminate result. For the semen samples in both phase 1 and phase 2, the specimen was considered to be negative if both EBOV gene targets were not detected and the findings with respect to B2M status were positive. Amplification of B2M served as an extraction control and RNA quality control.
The cycle-threshold value for each gene target is reported as the number of replication cycles that had occurred when the target was first detected. Cycle-threshold values have an inverse association with RNA quantity, such that lower cycle-threshold values indicate higher quantities of RNA in given specimens.23
STATISTICAL ANALYSIS
For the analysis of EBOV persistence in semen, baseline data from phase 1 and phase 2 were pooled into one sample of 220 male survivors of EVD. The descriptive analysis that we present here focuses on two aspects: the sociodemographic data of all the participants, according to the site of origin (from Freetown, an urban area; and from Lungi, a semiurban area); and the number of participants who had a positive, indeterminate, or negative result on quantitative RT-PCR at enrollment, according to the number of days between the date of discharge from an Ebola treatment unit and the date that the semen specimen was obtained. When the duration between discharge and the date of the specimen collection was reported in months, a 30-day interval per month applied. We report the median cycle-threshold values, according to months after discharge, with the range (minimum and maximum) of values that was observed for the NP and VP40 gene targets (phase 1) and for the NP and GP gene targets (phase 2). For other quantitative variables, means with standard deviations and medians with interquartile ranges are reported. Data analysis was performed with the use of SAS/STAT software (SAS Institute).24
RESULTS
STUDY PARTICIPANTS
A total of 220 male survivors of EVD were enrolled: 100 participants were enrolled in the Free-town urban site during phase 1 (May 27, 2015, through July 7, 2015), and 120 participants (60 in the Freetown urban site and 60 in the semi-urban Lungi site) during phase 2 (November 11, 2015, through May 12, 2016).
The sociodemographic characteristics of the study participants are presented in Table 1, according to study site and phase. There were key differences between the sites. As compared with the participants from the Freetown urban site, participants from the Lungi semiurban site were slightly older, were more likely to have received no formal education, were more often engaged in a long-term relationship or married, reported that there were more people in their household, and reported that more household members had been infected with EBOV. Among 195 men (89%) who agreed to be tested for HIV, 1 was found to be HIV-positive.
Table 1.
Characteristics of Study Participants at Baseline, According to Study Site and Phase.*
| Characteristic | Freetown Site | Lungi Site | Total | |
|---|---|---|---|---|
| Phase 1 (N = 100) | Phase 2 (N = 60) | Phase 2 (N = 60) | Phases 1 and 2 (N = 220) | |
| Recruitment period | May 27, 2015–July 7, 2015 | Nov. 11, 2015–Feb. 17, 2016 | Feb. 3, 2016–May 12, 2016 | May 27, 2015–May 12, 2016 |
|
| ||||
| Age | ||||
|
| ||||
| Mean — yr | 29.7±8.4 | 31.3±8.3 | 34.8±11.5 | 31.5±9.5 |
|
| ||||
| Median (interquartile range) — yr | 27 (24–34) | 30 (25–36) | 34 (26–43) | 29 (25–36) |
|
| ||||
| Distribution — no./total no. (%) | ||||
|
| ||||
| ≤25 yr | 36/99 (36) | 18/60 (30) | 13/60 (22) | 67/219 (31) |
|
| ||||
| 26–35 yr | 44/99 (44) | 25/60 (42) | 22/60 (37) | 91/219 (42) |
|
| ||||
| >35 yr | 19/99 (19) | 17/60 (28) | 25/60 (42) | 61/219 (28) |
|
| ||||
| Highest level of education — no. (%)† | ||||
|
| ||||
| No education | 14 (14) | 10 (17) | 19 (32) | 43 (20) |
|
| ||||
| Primary education | 49 (49) | 13 (22) | 8 (13) | 70 (32) |
|
| ||||
| Secondary education | 37 (37) | 37 (62) | 33 (55) | 107 (49) |
|
| ||||
| Marital status — no. (%) | ||||
|
| ||||
| Married or in a long-term relationship | 51 (51) | 31 (52) | 40 (67) | 122 (55) |
|
| ||||
| Single, divorced, widowed, or separated | 49 (49) | 29 (48) | 20 (33) | 98 (45) |
|
| ||||
| Current household size, including self — no./total no. (%) | ||||
|
| ||||
| ≤4 persons | 32/100 (32) | 17/57 (30) | 7/59 (12) | 56/216 (26) |
|
| ||||
| 5–8 persons | 30/100 (30) | 35/57 (61) | 8/59 (14) | 73/216 (34) |
|
| ||||
| 9–12 persons | 27/100 (27) | 4/57 (7) | 12/59 (20) | 43/216 (20) |
|
| ||||
| >12 persons | 11/100 (11) | 1/57 (2) | 32/59 (54) | 44/216 (20) |
|
| ||||
| No. of household members with EVD, excluding self — no. (%) | ||||
|
| ||||
| 0 | 36 (36) | 16 (27) | 6 (10) | 58 (26) |
|
| ||||
| 1 or 2 | 34 (34) | 16 (27) | 11 (18) | 61 (28) |
|
| ||||
| 3 or 4 | 15 (15) | 13 (22) | 14 (23) | 42 (19) |
|
| ||||
| ≥5 | 15 (15) | 15 (25) | 29 (48) | 59 (27) |
|
| ||||
| Duration between ETU discharge and semen-specimen collection | ||||
|
| ||||
| Mean — mo | 5.9±1.9 | 11.5±3.2 | 15.6±2.9 | 10.0±4.9 |
|
| ||||
| Median (interquartile range) — mo | 6.2 (4.5–7.1) | 11.3 (9.0–14.0) | 15.5 (13.7–17.5) | 8.6 (6.2–14.2) |
|
| ||||
| Range — mo | 1.3–11.2 | 4.1–19.3 | 8.5–22.3 | 1.3–22.3 |
|
| ||||
| No semen specimen — no. | 2 | 5 | 3 | 10 |
|
| ||||
| Result of Ebola RT-PCR semen testing at baseline — no./total no. (%) | ||||
|
| ||||
| Positive | 46/98 (47) | 8/55 (15) | 3/57 (5) | 57/210 (27) |
|
| ||||
| Indeterminate‡ | 13/98 (13) | 0/55 | 0/57 | 13/210 (6) |
|
| ||||
| Negative | 39/98 (40) | 47/55 (85) | 54/57 (95) | 140/210 (67) |
Plus–minus values are means ±SD. The Freetown site is in an urban area, and the Lungi site in a semiurban area. Percentages may not total 100 because of rounding. ETU denotes Ebola treatment unit, EVD Ebola virus disease, and RT-PCR reverse transcriptase–polymerase chain reaction.
Primary education was defined as 1 to 8 years of school, and secondary education as more than 8 years of school.
An indeterminate result indicates that one of the gene targets was detected and one was not detected; this finding applies only to the assay from the Centers for Disease Control and Prevention that was used during phase 1.
Overall, the mean (±SD) duration between the date of discharge from an Ebola treatment unit and the baseline visit was 10.0±4.9 months. This duration was longer among the participants in phase 2 of the study (11.5±3.2 months in Freetown and 15.6±2.9 months in Lungi) than among those in phase 1 (5.9±1.9 months in Freetown) because of delayed recruitment that started after the epidemic had ended (Table 1).
DETECTION OF EBOLA VIRUS RNA IN SEMEN
Of the 210 participants who provided a semen specimen for analysis at study entry, 57 (27%) had positive results on quantitative RT-PCR (Table 1); 46 were participants from phase 1 of the study and 11 were from phase 2. Overall, EBOV RNA was detected in semen in all 7 men from whom a specimen was obtained within 3 months after discharge from an Ebola treatment unit, in 26 of 42 (62%) from whom a specimen was obtained 4 to 6 months after discharge, in 15 of 60 (25%) from whom a specimen was obtained 7 to 9 months after discharge, in 4 of 26 (15%) from whom a specimen was obtained 10 to 12 months after discharge, in 4 of 38 (11%) from whom a specimen was obtained 13 to 15 months after discharge, and in 1 of 25 (4%) from whom a specimen was obtained 16 to 18 months after discharge; all 12 semen specimens that were obtained 19 months or more after discharge were negative (Fig. 1). In addition, the results of 4 semen specimens obtained at 4 to 6 months after discharge from an Ebola treatment unit, 7 obtained at 7 to 9 months after discharge, and 2 obtained at 10 to 12 months after discharge were indeterminate (only one target positive) when the NP and VP40 targets were assessed. The proportion of men with semen specimens that tested negative by means of quantitative RT-PCR increased with the duration between the date of discharge from an Ebola treatment unit and the date that the specimen was obtained.
Figure 1. Results on Reverse Transcriptase–Polymerase Chain Reaction in Semen Specimens Obtained at Baseline from Survivors of Ebola Virus Disease, According to Time after Discharge from an Ebola Treatment Unit (ETU).
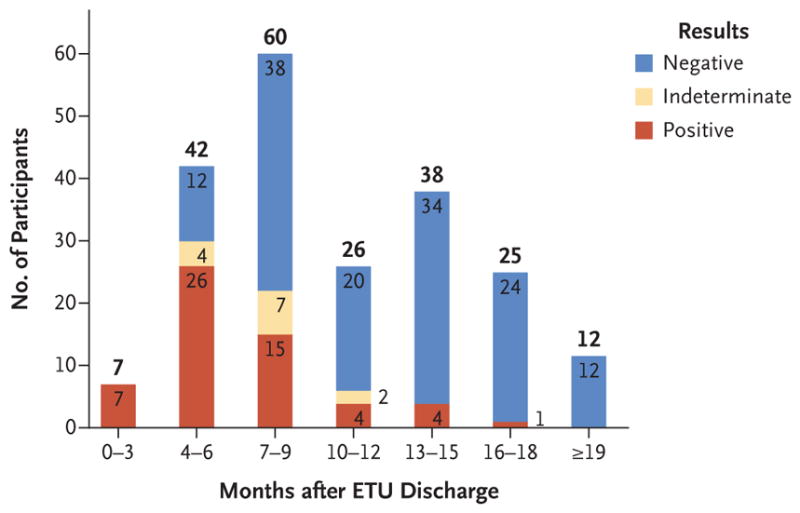
An indeterminate result indicates that one of the gene targets was detected and one was not detected; this finding applies only to the assay from the Centers for Disease Control and Prevention that was used during phase 1.
In phase 1, the median cycle-threshold values of the detected NP and VP40 target genes in semen specimens increased over time after discharge from an Ebola treatment unit. For specimens obtained within 3 months after discharge, the values were 32.4 with the NP gene target and 31.3 with the VP40 gene target; for those obtained at 4 to 6 months, the values were 34.3 and 33.1, respectively; and for those obtained at 7 to 9 months, the values were 37.4 and 36.6, respectively (Table 2). For a single baseline specimen that was obtained at 10 to 12 months, the cycle-threshold values were 37.7 for NP and 36.9 for VP40. A total of 11 participants in phase 2 tested positive for NP and GP target genes (these participants were recruited at a later stage after discharge from an Ebola treatment unit), and the cycle-threshold values ranged from 32.7 to 38.0 for the NP target gene and from 31.1 to 37.7 for the GP target gene; the numbers were too small for us to investigate trends over time.
Table 2.
Proportion of Positive Findings on Quantitative Reverse-Transcriptase–Polymerase-Chain-Reaction (RT-PCR) Assay and Cycle-Threshold Values in the Semen of Survivors of EVD, According to Time after ETU Discharge.
| Time after ETU Discharge | Phase 1 | Phase 2 | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Positive Result | Cycle-Threshold Value* | Positive Result | Cycle-Threshold Value† | |||
| NP target | VP40 target | NP target | GP target | |||
| no./total no. (%) | median (range) | no./total no. (%) | median (range) | |||
| 0–3 Mo | 7/7 (100) | 32.4 (20.9–36.0) | 31.3 (20.2–34.9) | — | — | — |
|
| ||||||
| 4–6 Mo | 25/39 (64) | 34.3 (26.3–38.6) | 33.1 (26.1–39.7) | 1/3 (33) | 35.8 | 34.8 |
|
| ||||||
| 7–9 Mo | 13/48 (28) | 37.4 (28.5–38.8) | 36.6 (28.2–38.3) | 2/12 (17) | 35.0–35.1 | 32.9–34.4 |
|
| ||||||
| 10–12 Mo | 1/4 (25) | 37.7 | 36.9 | 3/22 (14) | 38.0‡ | 37.1 (36.0–37.5) |
|
| ||||||
| 13–15 Mo | — | — | — | 4/38 (11) | 35.3 (32.7–37.4) | 34.4 (31.1–36.7) |
|
| ||||||
| 16–18 Mo | — | — | — | 1/25 (4) | 37.9 | 37.7 |
|
| ||||||
| ≥19 Mo | — | — | — | 0/12 | — | — |
Cycle-threshold values were assessed in participants who had positive results; if only one participant had a positive result, that value is shown. The value was determined by means of the assay from the Centers for Disease Control and Prevention, which was a quantitative RT-PCR assay that used Ebola virus–specific gene targets (NP and VP40) and the human β2-microglobulin (B2M) gene, as described previously.19–21 The findings were considered to be positive if the VP40 and the NP gene targets were both detected within 40 cycles of replication. Higher cycle-threshold values indicate lower RNA levels. The findings were considered to be negative if both Ebola virus gene targets were not detected and the findings regarding B2M status were positive. The findings were ruled to be indeterminate if one of the VP40 or NP gene targets was detected but not both. The results for one participant from whom a specimen was obtained at 10 months were indeterminate.
Cycle-threshold values were assessed in participants who had positive results. For phase 2, the median of the cycle-threshold value is not presented if data were available for fewer than three participants; in that case, the range is presented if there were two observations, and a single value is presented when there was just one observation. The value was determined by means of the assay from the Chinese Center for Disease Control and Prevention, which was a double-channel quantitative RT-PCR detection kit that used Ebola virus NP and GP and B2M.22 The findings were considered to be positive if either the NP or GP gene target was detected within 38 cycles of replication. The findings were considered to be negative if both Ebola virus gene targets were not detected and the findings regarding B2M status were positive.
A cycle-threshold value of 38.0 indicates a negative result. A single value is reported here because values were missing for the other two participants.
The longest time that was reported between discharge from an Ebola treatment unit and the initial time that the semen specimen was obtained in a man who tested positive was 470 days (15.7 months). Conversely, the shortest time after discharge that a participant had a negative result on an initial semen specimen was 100 days (3.3 months). Indeterminate results were encountered in 13 initial specimens that were obtained in the range of 144 to 335 days (4.8 to 11.2 months) after discharge from an Ebola treatment unit.
DISCUSSION
We conducted a cross-sectional analysis involving 220 male survivors of EVD who had enrolled in a prospective observational cohort for the investigation of EBOV persistence in semen. Participants were recruited in two different phases and at different study sites: Freetown in the Western Area District, and Lungi in the Port Loko District.
Sampling was not random, and further analysis was conducted to understand how the group of participants that was recruited in this study is representative of the overall population of survivors of EVD in Sierra Leone. The participants in phase 1 were recruited between 4 and 6 months earlier than those in phase 2. The differences between the participants in phase 1 and those in phase 2 are therefore biased by this difference in the number of months after discharge at the time of enrollment.
In the preliminary report, the semen RT-PCR test results at baseline were provided with respect to time since symptom onset, whereas in the current analysis we used time after discharge from an Ebola treatment unit. This change was made because discrepancies were found in the symptom-onset date for the cohort during data cleaning, and the team opted to use the discharge date instead in order to ensure validity. Although this change affected the distribution and the longest duration to a negative result at study entry, the date of discharge was retained as a reference for the analysis. Therefore, the exact number of days of RNA persistence in semen cannot be directly compared between the two reports.
All seven participants who provided a semen specimen during the first 3 months after discharge from an Ebola treatment unit had positive results on quantitative RT-PCR. This finding is consistent with those of previous studies involving male survivors of the Ebola and Marburg virus diseases. The percentage of male participants with positive results declined with the increased time between the date of discharge and the date of enrollment in the study. Because the longitudinal analysis of EBOV RNA shedding in semen over time is ongoing, we do not yet know how long this detection will continue. Follow-up analysis is ongoing to elucidate the dynamic of the clearance of EBOV in semen at different points in time.
The quantitative RT-PCR assays that were used in this study to test semen specimens are the same that were used to test blood specimens obtained from patients with suspected EVD. The assays are highly sensitive,19,22 and the detection of viral RNA does not necessarily indicate that infectious virus is present in blood or semen.21,25,26
We found that the median cycle-threshold values for the EBOV gene targets increased (indicating lower RNA values) when the analysis was performed with samples that had been obtained from participants who had a longer duration between the date of discharge from an Ebola treatment unit and the date of entry in the study. The cycle-threshold values that were obtained for EBOV target genes have been shown to correlate with viral load in blood,23 with an increasing cycle-threshold value indicating a decrease in the viral load. However, the detection of viral RNA does not necessarily indicate that infectious virus is present. A limited study that examined the relationship between cycle-threshold values and virus isolation did not detect infectious virus in blood specimens obtained from patients with EVD when cycle-threshold values were greater than 35.5 with the NP gene target.25 Similarly, in semen obtained from survivors of EVD that was tested in the United States, the highest cycle-threshold value for the NP target gene that yielded a virus isolate was 30; a total of 12 specimens with a cycle-threshold value greater than 30 did not yield any virus isolates.27 In this study cohort, we found that men provided specimens that were positive on quantitative RT-PCR several months after the discharge date and that the cycle-threshold values increased with time.
The potential contribution of sexual transmission to the scale of the epidemic is largely unknown, and we do not yet have sufficient information to assess the risk of transmission by means of sexual intercourse, oral sex, or other sex acts from men with viable virus in their semen. However, the unprecedented number of more than 16,000 survivors of EVD across Sierra Leone, Guinea, and Liberia, roughly half of whom are male, creates the potential for transmission and the initiation of new chains of transmission, even months after the outbreak has ended. Even though only rare cases of EVD have been linked to sexual transmission, research is needed to investigate whether infectious virus may be present in vaginal fluid or other body fluids after recovery, and the testing of additional body fluids in both male and female survivors is planned. It is also important to note that the three affected countries have a very low rate of HIV infection and other sexually transmitted infections, with a prevalence of HIV infection among adults of 1.3% in Sierra Leone,28 1.6% in Guinea,29 and 1.1% in Liberia30; these rates may also have influenced risks of EBOV sexual transmission.
Understanding the duration of Ebola virus shedding in survivors of EVD, and preventing further transmission, was essential for ultimately controlling the Ebola epidemic in West Africa. On the basis of the preliminary report of this study, the WHO, the CDC, and other partners engaged with the Ministries of Health of the three affected countries to establish and implement national semen-testing programs and preventive behavioral counseling. These efforts were essential to help survivors of EVD who participated in the initiatives, and they may have mitigated the risks of sexual transmission. Such programs helped men and women to understand their individual risk and to take appropriate measures to protect their sexual partners, specifically with regard to condom use and disposal. Such programs could also provide links to care and counseling programs for survivors. At the beginning of the epidemic and throughout the period that it lasted, survivors of EVD were stigmatized. There were instances when survivors were denied access to their homes after being discharged from the Ebola treatment unit. After the epidemic, the level of stigmatization of survivors has decreased.31,32 Currently, most survivors of EVD have been reintegrated into their communities, but health care access for survivors of EVD remains a concern.33,34 Because the implementation of semen-testing programs has been limited, outreach activities are needed to provide education regarding recommendations and risks to survivor communities and sexual partners of survivors in a way that does not further stigmatize the community of survivors of EVD. Due respect and continuing efforts that have strong sustainable support from within the local communities are crucial in mitigating negative effects in terms of further stigma attached to survivors.
Supplementary Material
Acknowledgments
Supported by the WHO, the CDC, the China CDC, the Paul G. Allen Family Foundation, the Sierra Leone Ministry of Health and Sanitation, and the Joint United Nations Program on HIV/AIDS. The WHO acknowledges the financial contribution of the WHO Ebola Response Program, the Paul G. Allen Family Foundation, and the UNDP (United Nations Development Program)–UNFPA (United Nations Population Fund)–UNICEF–WHO–World Bank Special Program of Research, Development and Research Training in Human Reproduction (HRP), a cosponsored program executed by the WHO in support of the Sierra Leone Ebola Virus Persistence Study.
APPENDIX
The authors’ full names and academic degrees are as follows: Gibrilla F. Deen, M.D., Nathalie Broutet, M.D., Ph.D., Wenbo Xu, M.D., Barbara Knust, D.V.M., Foday R. Sesay, M.D., Suzanna L.R. McDonald, Ph.D., Elizabeth Ervin, M.P.H., Jaclyn E. Marrinan, M.Sc., Philippe Gaillard, M.D., Ph.D., Ndema Habib, Ph.D., Hongtu Liu, Ph.D., William Liu, Ph.D., Anna E. Thorson, M.D., Ph.D., Francis Yamba, M.B., Ch.B., Thomas A. Massaquoi, M.D., Faustin James, M.B., Ch.B., Archchun Ariyarajah, M.Sc., Christine Ross, M.D., Kyle Bernstein, Ph.D., Antoine Coursier, M.A., John Klena, Ph.D., Marylin Carino, M.P.H., Alie H. Wurie, M.D., Yong Zhang, M.D., Ph.D., Marion S. Dumbuya, R.N., Neetu Abad, Ph.D., Baimba Idriss, M.D., Teodora Wi, M.D., Sarah D. Bennett, M.D., Tina Davies, M.S., Faiqa K. Ebrahim, M.D., Elissa Meites, M.D., Dhamari Naidoo, Ph.D., Samuel J. Smith, M.D., Patricia Ongpin, M.A., Tasneem Malik, R.N., Anshu Banerjee, Ph.D., Bobbie R. Erickson, M.P.H., Yongjian Liu, Ph.D., Yang Liu, M.D., Ke Xu, Ph.D., Aaron Brault, Ph.D., Kara N. Durski, M.P.H., Jörn Winter, Ph.D., Tara Sealy, M.P.H., Stuart T. Nichol, Ph.D., Margaret Lamunu, M.D., James Bangura, M.D., Sihem Landoulsi, M.Sc., Amara Jambai, M.D., Oliver Morgan, Ph.D., Guizhen Wu, M.D., Mifang Liang, M.D., Qiudong Su, M.D., Yu Lan, M.Sc., Yanzhe Hao, Ph.D., Pierre Formenty, D.V.M., Ute Ströher, Ph.D., and Foday Sahr, M.D.
The authors’ affiliations are as follows: the Sierra Leone Ministry of Health and Sanitation (G.F.D., F.Y., F.J., A.H.W., S.J.S., J.B., A.J.), the Sierra Leone Ministry of Defense (F.R.S., T.A.M., M.S.D., B.I., F.S.), and the Sierra Leone Ministry of Social Welfare, Gender, and Children’s Affairs (T.D.) — all in Freetown, Sierra Leone; World Health Organization (N.B., S.L.R.M., J.E.M., P.G., N.H., A.E.T., A.A., A.C., M.C., T.W., F.K.E., D.N., A. Banerjee, K.N.D., M. Lamunu, S.L., P.F.) and the Joint United Nations Program on HIV/AIDS (P.O.), Geneva; Chinese Center for Disease Control and Prevention (W.X., H.L., W.L., Y.Z., Yongjian Liu, Yang Liu, K.X., G.W., M. Liang, Q.S., Y.H.) and Beijing Institute of Microbiology and Epidemiology (Y. Lan), Beijing; and the Centers for Disease Control and Prevention, Atlanta (B.K., E.E., C.R., K.B., J.K., N.A., S.D.B., E.M., T.M., B.R.E., A. Brault, J.W., T.S., S.T.N., O.M., U.S.).
Footnotes
The authors’ full names, academic degrees, and affiliations are listed in the Appendix.
A preliminary version of this article was published on October 14, 2015, at NEJM.org.
The views expressed in this article are those of the authors and do not necessarily represent the official positions of the Sierra Leone Ministry of Health and Sanitation, the World Health Organization (WHO), the Centers for Disease Control and Prevention (CDC), or the Chinese Center for Disease Control and Prevention (China CDC).
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.World Health Organization. Ebola situation report. 2016 Jun 10; ( http://apps.who.int/iris/bitstream/10665/208883/1/ebolasitrep_10Jun2016_eng.pdf)
- 2.Dowell SF, Mukunu R, Ksiazek TG, Khan AS, Rollin PE, Peters CJ. Transmission of Ebola hemorrhagic fever: a study of risk factors in family members, Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179(Suppl 1):S87–S91. doi: 10.1086/514284. [DOI] [PubMed] [Google Scholar]
- 3.Dietz PM, Jambai A, Paweska JT, Yoti Z, Ksiazek TG. Epidemiology and risk factors for Ebola virus disease in Sierra Leone — 23 May 2014 to 31 January 2015. Clin Infect Dis. 2015;61:1648–54. doi: 10.1093/cid/civ568. [DOI] [PubMed] [Google Scholar]
- 4.Kreuels B, Addo MM, Schmiedel S. Severe Ebola virus infection complicated by gram-negative septicemia. N Engl J Med. 2015;372:1377. doi: 10.1056/NEJMc1500455. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez LL, De Roo A, Guimard Y, et al. Persistence and genetic stability of Ebola virus during the outbreak in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179(Suppl 1):S170–S176. doi: 10.1086/514291. [DOI] [PubMed] [Google Scholar]
- 6.Rogstad KE, Tunbridge A. Ebola virus as a sexually transmitted infection. Curr Opin Infect Dis. 2015;28:83–5. doi: 10.1097/QCO.0000000000000135. [DOI] [PubMed] [Google Scholar]
- 7.Rowe AK, Bertolli J, Khan AS, et al. Clinical, virologic, and immunologic follow-up of convalescent Ebola hemorrhagic fever patients and their household contacts, Kikwit, Democratic Republic of the Congo. J Infect Dis. 1999;179(Suppl 1):S28–S35. doi: 10.1086/514318. [DOI] [PubMed] [Google Scholar]
- 8.Bausch DG, Towner JS, Dowell SF, et al. Assessment of the risk of Ebola virus transmission from bodily fluids and fomites. J Infect Dis. 2007;196(Suppl 2):S142–S147. doi: 10.1086/520545. [DOI] [PubMed] [Google Scholar]
- 9.Emond RT, Evans B, Bowen ET, Lloyd G. A case of Ebola virus infection. Br Med J. 1977;2:541–4. doi: 10.1136/bmj.2.6086.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martini GA, Schmidt HA. Spermato-genic transmission of the “Marburg virus” (causes of “Marburg simian disease”) Klin Wochenschr. 1968;46:398–400. doi: 10.1007/BF01734141. (In German.) [DOI] [PubMed] [Google Scholar]
- 11.Thorson A, Formenty P, Lofthouse C, Broutet N. Systematic review of the literature on viral persistence and sexual transmission from recovered Ebola survivors: evidence and recommendations. BMJ Open. 2016;6(1):e008859. doi: 10.1136/bmjopen-2015-008859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christie A, Davies-Wayne GJ, Cordier-Lassalle T, et al. Possible sexual transmission of Ebola virus — Liberia, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:479–81. [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Interim advice on the sexual transmission of the Ebola virus disease. 2016 Jan 21; ( http://www.who.int/reproductivehealth/topics/rtis/ebola-virus-semen/en/)
- 14.Centers for Disease Control and Prevention. Ebola (Ebola virus disease) — transmission. 2015 ( https://www.cdc.gov/vhf/ebola/transmission/index.html)
- 15.Soka MJ, Choi MJ, Baller A, et al. Prevention of sexual transmission of Ebola in Liberia through a national semen testing and counselling programme for survivors: an analysis of Ebola virus RNA results and behavioural data. Lancet Glob Health. 2016;4(10):e736–e743. doi: 10.1016/S2214-109X(16)30175-9. [DOI] [PubMed] [Google Scholar]
- 16.Diallo B, Sissoko D, Loman NJ, et al. Resurgence of Ebola virus disease in Guinea linked to a survivor with virus persistence in seminal fluid for more than 500 days. Clin Infect Dis. 2016;63:1353–6. doi: 10.1093/cid/ciw601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deen GF, McDonald SLR, Marrinan JE, et al. Implementation of a study to examine the persistence of Ebola virus in the body fluids of Ebola virus disease survivors in Sierra Leone: Methodology and lessons learned. PLoS Negl Trop Dis. 2017;11(9):e0005723. doi: 10.1371/journal.pntd.0005723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abad N, Malik T, Ariyarajah A, et al. Development of risk reduction behavioral counseling for Ebola virus disease survivors enrolled in the Sierra Leone Ebola Virus Persistence Study, 2015–2016. PLoS Negl Trop Dis. 2017;11(9):e0005827. doi: 10.1371/journal.pntd.0005827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. Ebola virus VP40 real-time RT-PCR assay. 2014 ( https://www.fda.gov/MedicalDevices/Safety/EmergencySituations/ucm161496.htm#ebola)
- 20.Centers for Disease Control and Prevention. Ebola virus NP real-time RT-PCR assay. 2014 ( https://www.fda.gov/downloads/medicaldevices/safety/emergencysituations/ucm436307.pdf)
- 21.Erickson BR, Sealy TK, Flietstra T, et al. Ebola virus disease diagnostics, Sierra Leone: analysis of real-time reverse transcription-polymerase chain reaction values for clinical blood and oral swab specimens. J Infect Dis. 2016;214(Suppl 3):S258–S262. doi: 10.1093/infdis/jiw296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Q, Zhang Y, Wang HY, et al. Detection and analysis of Ebola virus in Sierra Leone-China Friendship Biosafety Laboratory from March 11 to April 20, 2015. Biomed Environ Sci. 2016;29:443–7. doi: 10.3967/bes2016.057. [DOI] [PubMed] [Google Scholar]
- 23.Towner JS, Rollin PE, Bausch DG, et al. Rapid diagnosis of Ebola hemorrhagic fever by reverse transcription-PCR in an outbreak setting and assessment of patient viral load as a predictor of outcome. J Virol. 2004;78:4330–41. doi: 10.1128/JVI.78.8.4330-4341.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The SAS system for Windows, release 9.4. Cary, NC: SAS Institute; 2014. [Google Scholar]
- 25.Spengler JR, McElroy AK, Harmon JR, Ströher U, Nichol ST, Spiropoulou CF. Relationship between Ebola virus real-time quantitative polymerase chain reaction-based threshold cycle value and virus isolation from human plasma. J Infect Dis. 2015;212(Suppl 2):S346–S349. doi: 10.1093/infdis/jiv187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sissoko D, Duraffour S, Kerber R, et al. Persistence and clearance of Ebola virus RNA from seminal fluid of Ebola virus disease survivors: a longitudinal analysis and modelling study. Lancet Glob Health. 2017;5(1):e80–e88. doi: 10.1016/S2214-109X(16)30243-1. [DOI] [PubMed] [Google Scholar]
- 27.Uyeki TM, Erickson BR, Brown S, et al. Ebola virus persistence in semen of male survivors. Clin Infect Dis. 2016;62:1552–5. doi: 10.1093/cid/ciw202. [DOI] [PubMed] [Google Scholar]
- 28.UNAIDS. Sierra Leone: HIV and AIDS estimates. 2015 ( http://www.unaids.org/en/regionscountries/countries/sierraleone)
- 29.UNAIDS. Guinea: HIV and AIDS estimates. 2015 ( http://www.unaids.org/en/regionscountries/countries/guinea)
- 30.UNAIDS. Liberia: HIV and AIDS estimates. 2015 ( http://www.unaids.org/en/regionscountries/countries/liberia)
- 31.Lee-Kwan SH, DeLuca N, Adams M, et al. Support services for survivors of Ebola virus disease — Sierra Leone, 2014. MMWR Morb Mortal Wkly Rep. 2014;63:1205–6. [PMC free article] [PubMed] [Google Scholar]
- 32.Rabelo I, Lee V, Fallah MP, et al. Psychological distress among Ebola survivors discharged from an Ebola treatment unit in Monrovia, Liberia — a qualitative study. Front Public Health. 2016;4:142. doi: 10.3389/fpubh.2016.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Bortel T, Basnayake A, Wurie F, et al. Psychosocial effects of an Ebola outbreak at individual, community and international levels. Bull World Health Organ. 2016;94:210–4. doi: 10.2471/BLT.15.158543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Medecins Sans Frontieres. Surviving Ebola, then helping others fight it. 2014 ( http://www.doctorswithoutborders.org/article/surviving-ebola-then-helping-others-fight-it)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


