Background
Research in the field of pig-to-primate xenotransplantation has made significant advances during the past two years, with life-supporting pig kidneys in nonhuman primates (NHPs) surviving for many months or even more than a year (1–3, and Tector AJ, personal communication). The results have been so encouraging that clinical trials are in the early stages of planning (4,5). There are some data that suggest that genetically-engineered pig grafts transplanted into NHPs selected for no or low levels of anti-pig antibody, particularly IgG, survive longer than grafts transplanted into NHPs with higher levels of antibody.
In prospective clinical trials, if the initial patients are selected on the basis of no or low detectable levels of anti-pig antibody, will this be sufficient to ensure consistent prolonged graft survival or will additional genetic modifications, e.g., expression of human complement- or coagulation-regulatory proteins, be essential? Data from selected studies in which anti-pig antibody levels were known are here reviewed.
Evidence that selection of NHPs with no or low levels of anti-pig antibodies is associated with prolonged pig organ graft survival has slowly accumulated since the first studies of organ transplantation from pigs that did not express the important galactose-α1,3-galactose (Gal) antigens were reported in 2005 (6–8). The baboon recipients were selected for low anti-nonGal antibody levels, i.e., antibodies directed to the remaining pig antigens that were not Gal (nonGal antigens) (9,10) (Table 1). Survival of heterotopic (non-life-supporting) heart grafts extended to 6 months (6,7) and of life-supporting kidney grafts to a little less than 3 months (8). The discrepancy in graft survival between heart and kidney may not be simply related to whether or not the organ was life-supporting, as molecular differences may contribute to the poorer outcome of pig kidney grafts in NHPs (11). However, whether orthotopic (life-supporting) heart xenograft survival will match heterotopic (non-life-supporting) heart xenograft survival has yet to be determined.
Table 1.
Graft or recipient survival after genetically-engineered pig heart or kidney transplantation in NHPs
| Year (Ref) | Recipient (n) | Anti-pig Ab | Pig Organ | Survival (days, unless stated) |
|---|---|---|---|---|
| 2005 (6KK) | Baboon (8) | Low | GTKO heart | >16,>23,>56,59,67,78,110,179d (median 63) |
| 2005 (8KY) | Baboon (11) | Low | GTKO kidney | 4,13,16,18,26,31,33,56,68,81,83d (median 31) |
| 2009 (13ME) | Baboon (9) | Variable | GTKO heart | <1,1,6,6,7,12,12,35,56d (median 7) |
| 2009 (13ME) | Baboon (3) | Variable | GTKO kidney | 2,5,12d (median 5) |
| 2010 (14CL) | Baboon (6) | Variable | GTKO/hCD46 kidney | 2,4,9,10,10,16d (median 9.5) |
| 2015b (15HI) | Baboon (7) | Variable | GTKO/hCD46/CD55 heart | 15,18,23,33d (median 20.5) |
| 2015b (15HI) | Baboon (7) | Variable | GTKO/hCD46/TBM heart | 52,99,130d (median 99) |
| 2015a (2HI) | Baboon (1) | High IgM/low IgG | Multiple GE kidney | 136d |
| 2015 (1LH) | Rhesus (2) | Low | GTKO/hCD55 kidney | >126, >133 (>6,>10m)1 |
| 2015 (1LH) | Rhesus (1) | High | GTKO/hCD55 kidney | 6d |
| 2017 3(HI) | Baboon (2) | Low | GTKO/CD46/TBM kidney | 12,12d |
| 2017 (3HI) | Baboon (2) | Low | Multiple GE kidney | >7m, >8m |
Final outcome unpublished (Adams A, personal communication)
GE = genetically engineered; TBM - thrombomodulin
In baboons that did not develop complications associated with the immunosuppressive regimen, e.g., infection, all heterotopic heart grafts developed thrombotic microangiopathy (Figure 1) (12) and the recipient developed features of a consumptive coagulopathy (though this has not been reported in all studies [discussed in 13]). This was attributed to vascular endothelial activation by a low level of anti-pig antibody combined with incompatibility of coagulation factors between pig and baboon. As there was no evidence for an elicited (T cell-mediated) antibody response, this activation was presumably associated with a continuing low level of production of natural (preformed) antibody, possibly in association with such factors as complement deposition and innate immune cell activity. These encouraging results could not be repeated when the selection of the recipient baboons was not based on low antibody levels (Table 1) (14,15).
Figure 1. Severe thrombotic microangiopathy in a GTKO pig heart that functioned for almost 6 months after transplantation into an immunosuppressed baboon.

(Reproduced with permission from Tseng Y-L, et al. Transplantation 2005;80:1493–1500)
The hypothesis that low antibody levels are important gained considerable support from studies in the GTKO/CD55 pig-to-rhesus monkey kidney transplantation model (1). Using an identical immunosuppressive regimen (based on costimulation blockade), a kidney transplanted into a monkey with a high level of anti-nonGal antibody functioned for only 6 days, whereas two with low antibody levels functioned for 6 and 10 months, respectively (Table 1). However, grafts were eventually lost through delayed antibody-mediated rejection and coagulation dysfunction (Adams A, personal communication), again suggesting that very gradual antibody binding to the graft vascular endothelium (combined with coagulation incompatibilities between pig and NHP) may have been detrimental.
A low level of antibody (or even no detectable antibody) may therefore be insufficient to ensure truly long-term graft survival, and may not even be sufficient for short-term survival. This is exemplified by a recent study in four baboons (all selected for low antibody levels, and all treated with an identical experimental protocol), in which the expression of different specific human complement- and coagulation-regulatory proteins on the pig kidney grafts resulted in a remarkable difference in graft survival from 12 days (n=2) to >7 months (n=2) (Table 1) (3). (The poor results when kidneys from GTKO/CD46/hThrombomodulin pigs were transplanted, which were in contrast to excellent results when identical hearts were transplanted (16), is likely related to the much greater expression of hThrombomodulin in the heart than in the kidneys in these pigs [Ayares D, personal communication].) Expression of an effective human coagulation-regulatory protein was therefore essential in obtaining prolonged graft survival.
Furthermore, the expression of human complement- +/− coagulation-regulatory proteins can result in relatively prolonged graft survival even when the recipient NHPs have not been selected for low antibody levels (Table 1) (2,17,18), with survival extending to >2 years (16).
These observations supported earlier studies in which the expression of a human complement-regulatory protein on GTKO pigs extended early graft survival in non-selected baboons (19,20). It should also be remembered that the original studies of pig organ transplantation in NHPs carried out by White, Cozzi, and their colleagues, used pigs in which no pig antigens had been deleted (i.e., wild-type pigs), but which expressed a human complement-regulatory protein, CD55 (21). When the pig was transgenic for a human complement-regulatory protein, extensive studies in NHPs indicated that heart and kidney graft survival was significantly extended when compared with that of wild-type pig organs despite the fact that no attempt was made to reduce the levels of anti-pig antibody (reviewed in 22 and 23).
Recent progress
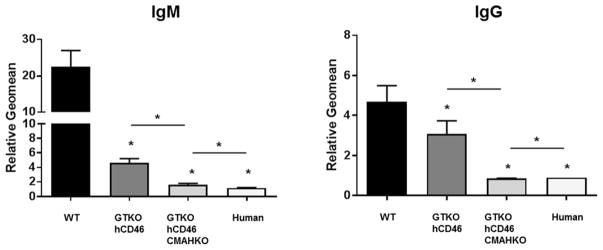
In the early pig-to-NHP organ transplantation studies, the only known pig antigen against which humans and NHPs had natural antibodies was Gal (24), and so it was only possible to delete expression of this single antigen. It has long been known that pigs (but not humans) express N-glycolylneuraminic acid (Neu5Gc) (25), and therefore humans make anti-Neu5Gc antibodies (26). This is irrelevant to studies in NHPs as all Old World NHPs express Neu5Gc, as do pigs (reviewed in 27). Human serum IgM and IgG binding to cells from ‘double-KO’ pigs, i.e., pigs that express neither Gal nor Neu5Gc (GTKO/CMAHKO pigs), is significantly less than to cells from GTKO pigs (Figure 2) (28).
Figure 2. Human serum IgM (left) and IgG (right) binding to aortic endothelial cells (AECs).
AECs were cultured from a wild-type (WT) pig, an α1,3-galactosyltransferase gene-knockout (GTKO) pig expressing the human complement-regulatory protein, CD46, and a GTKO/CD46 pig that did not express NeuGc (GTKO/CD46/CMAHKO). Human IgM and IgG binding to GTKO/CD46/CMAHKO pig cells is almost at the level of binding to human aortic endothelial cells. (* = p<0.05) (Modified from Lee W, et al. Xenotransplantation 2016;23:137–150)
More recently, a third pig antigen target has been identified, Sda, the product of β1,4N-acetylgalactosaminyltransferase-2 (β4GalNT2) (29). When using target cells from pigs in which expression of all three antigens had been deleted (‘triple-KO’ [TKO] pigs) (30,31), a relatively large number of humans (approximately one-third of the subjects tested) were identified who appeared to have no antibody binding to these cells (32). It can be argued, therefore, that such patients would do well after receiving a graft from a TKO pig, just as patients with no pre-existing donor-specific allo-antibodies do well after organ allotransplantation (as long as an elicited antibody response is prevented).
Preventing a donor-specific antibody response, however, has proved difficult even following allotransplantation. Many patients undergoing kidney allotransplantation, who had no detectable donor-specific antibodies at the time of the transplant, develop de novo antibodies after transplantation. It seems just as likely, or even more so, that such de novo antibodies will develop against a pig xenograft.
Several studies suggest that there is no cross-reactivity between those antibodies directed to human MHC (anti-HLA antibodies) and those directed to swine MHC (anti-SLA antibodies) (33–36), though others indicate that this is not always so (32, and reviewed in 33). Indeed, Oostingh et al (37) provided data to suggest that almost half of patients with a panel reactive antibody level of >64% had antibodies that cross-reacted with SLA. Although the data reported by Oostingh et al have not been confirmed by several other groups, donor MHC may represent a problem with regard to both pre-existing and de novo antibodies.
In those humans in whom a low level of anti-TKO pig antibodies was identified, there was evidence that some of these antibodies may be directed to MHC swine leukocyte class I antigens (SLA-I) (32). The discrepancies in the results from different studies may be related to (i) the SLA phenotype of the specific pig from which the cells were derived, and/or (ii) the number of human sera tested, which was far greater in the studies by Martens and his colleagues (32). Genetic engineering techniques will likely allow the specific SLA targets to be deleted in these pigs, allowing the patient to receive a pig graft against which he/she has no antibodies.
In addition to the above (and possibly other) protein-directed antibodies, some human anti-TKO pig antibodies may be directed to hitherto unidentified pig glycans, although the level of these antibodies is low. When these antigen targets are identified, this will allow their knockout but, in view of the very low binding of antibodies in most humans to TKO pig cells, this effort is likely to be associated with diminishing impact, and may indeed not be cost-effective. Furthermore, some of these glycans may have important functions in pigs and therefore knockout may possibly have a deleterious effect. Until then, the effect of antibody binding to the vascular endothelium of the pig graft may be minimized by the transplantation of organs from TKO pigs expressing human complement- and/or coagulation-regulatory proteins, such as the complement regulators CD46, CD55, and CD59, and/or the coagulation regulators thrombomodulin, endothelial protein C receptor, tissue factor pathway inhibitor, and CD39 (2,3,17). The theory is that the presence of the human (transgenic) proteins inhibits the effect of even low levels of antibody binding to the graft vascular endothelium, perhaps preventing activation of the endothelium by complement or coagulation factors, and protecting the endothelium from the effects of complement and/or dysfunctional coagulation/thrombosis.
Furthermore, Lin et al demonstrated in vitro that exposure of human platelets and monocytes to porcine aortic endothelial cells (in the absence of serum antibodies) can -induce procoagulant tissue factor expression on the platelets and monocytes (38). In the GTKO pig-to-baboon kidney transplant model, Lin et al also demonstrated that activation of platelets and monocytes to express tissue factor was associated with the initiation and onset of consumptive coagulopathy, in the absence of an elicited antibody response, and with minimal antibody deposition in the graft (as demonstrated by immunohistochemistry) (15). Such a mechanism could potentially lead to the development of a thrombotic microangiopathy that would appear to be independent of serum antibody binding. Whether this activation of tissue factor occurs if the porcine vascular endothelial cells are from a TKO pig is as yet unknown, but in this respect it is notable that the upregulation of tissue factor expression on platelets was similar whether the platelets were exposed to wild-type, GTKO, or GTKO/CD46 pig cells (39). Unless the mechanism of the direct procoagulant effect of platelets and monocytes on vascular endothelial cells (38) can be elucidated and prevented, protection of an organ by expression of one or more coagulation-regulatory proteins may be very important.
In addition to antibody, complement, and platelets, cells of the innate immune system, e.g., neutrophils, natural killer cells, macrophages, may be playing a significant role. At present, their respective roles remain unclear, although natural killer cells may be adding to the inflammatory response to a pig xenograft. In addition, innate immune cells may be promoting/augmenting the adaptive immune response.
Conclusions
What can be concluded from the data available to date? Current studies suggest that the major prerequisites for pig xenograft survival are (i) a low level of (or, preferably, no) pre-existing natural anti-pig antibody, (ii) prevention of an elicited antibody response (secondary to efficient inhibition of a T cell response), and possibly (iii) prevention of an inflammatory response (40–42). To achieve these goals will require a combination of genetic engineering of the pig and effective immunosuppressive and anti-inflammatory regimens.
An important point that has to be addressed is whether the current methods of measuring anti-pig antibody actually detect all antibody. Different methods, e.g., different target cells, can generate variable results, even using the same serum sample (43, and Zhang Z, et al, Xenotransplantation, in press). For a clinical trial, if the absence of antibody is considered essential, this may need to be confirmed using various different assays.
However, there is every prospect that, if immunosuppressive therapy that successfully prevents a T cell-mediated, elicited antibody response is administered, some patients with no or low levels of antibody will do well after receiving a TKO pig organ graft. Others may require grafts from TKO pigs in which some SLA antigens have also been deleted (or replaced by HLA). Still others with higher levels of anti-pig antibody (particularly of IgM, making the graft more susceptible to complement-mediated hyperacute rejection) are likely to require the transplantation of TKO grafts that are additionally protected by the expression of one or more human complement- and/or coagulation-regulatory proteins (or by expression of other transgenes with specific functions).
It should also be noted that a further benefit of these genetic engineering approaches appears to be that deletion of an antigenic target, e.g., Gal, or the expression of a human complement-regulatory protein, e.g., CD46, both result in some reduction in the T cell response to the graft (44,45), which in turn can mitigate T cell-dependent B cell activation and antibody production.
It could reasonably be argued that, if there is no evidence that expression of these human (transgenic) proteins is detrimental to the pig or to the recipient, e.g., by acting as receptors for certain viruses and microorganisms, such as measles (46), then their expression in all pig grafts will provide another level of protection that may prevent or at least delay the development of chronic rejection (e.g., graft atherosclerosis), which remains a significant problem today in patients with long-term allografts. Expression of a human complement-regulatory protein in the pig organ may be particularly important in patients with high levels of anti-pig IgM associated with T cell-independent B cell proliferation/activation (even if they have no anti-pig IgG). Furthermore, not all complement activation is antibody-mediated, and so the expression of a human complement-regulatory protein might be protective against activation of the alternative complement pathway, e.g., associated with ischemia-reperfusion, to which all xenografts will be exposed.
The expression of a human coagulation-regulatory protein might prevent any procoagulant effect from the expression of tissue factor on the donor vascular endothelial cells or on the recipient’s platelets and innate immune cells (after exposure to pig vascular endothelial cells), as reported by Lin (15,38), and this may prolong graft survival. However, if the mechanism by which human platelets, and possibly innate immune cells, activate pig vascular endothelial cells in the absence of serum antibody can be determined, the expression of a human coagulation-regulatory protein may prove less necessary.
Eventually, using genetic engineering techniques, it seems likely that it will be possible to provide a graft (that does not express any antigens against which the patient has pre-existing antibodies) for any patient in need of an organ transplant (even patients who are highly-sensitized to HLA). Long-term graft survival in successfully-immunosuppressed patients would therefore be anticipated, and perhaps further increased by the expression of selected human transgenes. In turn, these genetic manipulations, particularly those that lead to ‘compatibility’ of the pig and recipient MHC, will facilitate achieving the ultimate goal, namely the induction of tolerance to pig organs.
The recipient of the graft will need to receive an effective immunosuppressive regimen. It appears that, by increasing the genetic manipulation of the pig organ, conventional immunosuppressive therapy may be sufficient to prevent a T cell response (and thus a T cell-dependent elicited antibody response) (Iwase H, et al, unpublished data). A low level of immunosuppressive therapy would appear essential unless T cell tolerance can be induced by such methods as (i) hematopoietic cell chimerism, or (ii) donor-specific thymus transplantation (47).
Acknowledgments
Work on xenotransplantation at UAB is supported in part by NIH grant #U19 AI090959/08.
Abbreviations
- CMAHKO
cytidine monophosphate-N-acetylneuraminic acid hydroxylase gene-knockout
- Gal
galactose-α1,3-galactose
- GTKO
α1,3-galactosyltransferase gene-knockout
- Neu5Gc
N-glycolylneuraminic acid
- NHP
nonhuman primate
- TKO
triple-knockout (pigs that do not express the 3 known pig antigens, Gal, NeuGc, or Sda)
References
- 1.Higginbotham L, Mathews D, Breeden CA, et al. Pre-transplant antibody screening and anti-CD154 costimulation blockade promote long-term xenograft survival in a pig-to-primate kidney transplant model. Xenotransplantation. 2015;22:221–230. doi: 10.1111/xen.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iwase H, Liu H, Wijkstrom M, et al. Pig kidney graft survival in a baboon for 136 days: longest life-supporting organ graft survival to date. Xenotransplantation. 2015;22:302–309. doi: 10.1111/xen.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iwase H, Hara H, Ezzelarab M, et al. Immunological and physiologic observations in baboons with life-supporting genetically-engineered pig kidney grafts. Xenotransplantation. 2017;24 doi: 10.1111/xen.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper DKC, Wijkstrom M, Hariharan S, et al. Selection of patients for initial clinical trials of solid organ xenotransplantation. Transplantation. 2016 Dec 1; doi: 10.1097/TP.0000000000001582. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper DKC, Pierson RN, III, Hering BJ, et al. Regulation of clinical xenotransplantation – time for a reappraisal? Transplantation. 2017 doi: 10.1097/TP.0000000000001683. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuwaki K, Tseng YL, Dor FJ, et al. Heart transplantation in baboons using α1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005;11:29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 7.Tseng YL, Kuwaki K, Dor FJ, et al. α 1,3-galactosyltransferase gene-knockout pig heart transplantation in baboons with survival approaching six months. Transplantation. 2005;80:1493–1500. doi: 10.1097/01.tp.0000181397.41143.fa. [DOI] [PubMed] [Google Scholar]
- 8.Yamada K, Yazawa K, Shimizu A, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of α1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11:32–34. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- 9.Ezzelarab M, Hara H, Busch J, et al. Antibodies directed to pig non-Gal antigens in naïve and sensitized baboons. Xenotransplantation. 2006;13:400–407. doi: 10.1111/j.1399-3089.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- 10.Rood PP, Hara H, Busch JL, et al. Incidence and cytotoxicity of antibodies in cynomolgus monkeys directed to nonGal antigens, and their relevance for experimental models. Transpl Int. 2006;19:158–165. doi: 10.1111/j.1432-2277.2005.00252.x. [DOI] [PubMed] [Google Scholar]
- 11.Knosalla C, Yazawa K, Behdad A, et al. Renal and cardiac endothelial heterogeneity impact acute vascular rejection in pig-to-baboon xenotransplantation. Am J Transplant. 2009;9:1006–1016. doi: 10.1111/j.1600-6143.2009.02602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Houser SL, Kuwaki K, Knosalla C, et al. Thrombotic microangiopathy and graft arteriopathy in pig hearts following transplantation into baboons. Xenotransplantation. 2004;11:416–425. doi: 10.1111/j.1399-3089.2004.00155.x. [DOI] [PubMed] [Google Scholar]
- 13.Byrne GW, Azimzadeh AM, Ezzelarab M, et al. Histopathologic insights into the mechanism of anti-non-Gal antibody-mediated pig cardiac xenograft rejection. Xenotransplantation. 2013;20:292–307. doi: 10.1111/xen.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ezzelarab M, Garcia B, Azimzadeh A, et al. The innate immune response and activation of coagulation in α1,3-galactosyltransferase gene-knockout xenograft recipients. Transplantation. 2009;87:805–812. doi: 10.1097/TP.0b013e318199c34f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin CC, Ezzelarab M, Shapiro R, et al. Recipient tissue factor expression is associated with consumptive coagulopathy in pig-to-primate kidney xenotransplantation. Am J Transplant. 2010;10:1556–1568. doi: 10.1111/j.1600-6143.2010.03147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohiuddin MM, Singh AK, Corcoran PC, et al. Chimeric 2C10R4 anti-CD40 antibody therapy is critical for long-term survival of GTKO.hCD46.hTBM pig-to-primate cardiac xenograft. Nat Commun. 2016;7:11138. doi: 10.1038/ncomms11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwase H, Ekser B, Satyananda V, et al. Pig-to-baboon heterotopic heart transplantation – exploratory preliminary experience with pigs transgenic for human thrombomodulin and comparison of three costimulation blockade-based regimens. Xenotransplantation. 2015;22:211–220. doi: 10.1111/xen.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohiuddin MM, Singh AK, Corcoran PC, et al. One-year heterotopic cardiac xenograft survival in a pig to baboon model. Am J Transplant. 2014;14:488–489. doi: 10.1111/ajt.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGregor CG, Ricci D, Miyagi N, et al. Human CD55 expression blocks hyperacute rejection and restricts complement activation in Gal knockout cardiac xenografts. Transplantation. 2012;93:686–692. doi: 10.1097/TP.0b013e3182472850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Azimzadeh AM, Kelishadi SS, Ezzelarab MB, et al. Early graft failure of GalTKO pig organs in baboons is reduced by expression of a human complement pathway-regulatory protein. Xenotransplantation. 2015;22:310–316. doi: 10.1111/xen.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cozzi E, White DJ. The generation of transgenic pigs as potential organ donors for humans. Nat Med. 1995;1:964–966. doi: 10.1038/nm0995-964. [DOI] [PubMed] [Google Scholar]
- 22.Lambrigts D, Sachs DH, Cooper DKC. Discordant organ xenotransplantation in primates: world experience and current status. Transplantation. 1998;66:547–561. doi: 10.1097/00007890-199809150-00001. [DOI] [PubMed] [Google Scholar]
- 23.Cooper DKC, Satyananda V, Ekser B, et al. Progress in pig-to-nonhuman primate transplantation models (1998–2013): a comprehensive review of the literature. Xenotransplantation. 2014;21:397–419. doi: 10.1111/xen.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Good AH, Cooper DKC, Malcolm, et al. Identification of carbohydrate structures that bind human antiporcine antibodies: implications for discordant xenografting in man. Transplant Proc. 1992;24:559–562. [PubMed] [Google Scholar]
- 25.Bouhours D, Pourcel C, Bouhours JE. Simultaneous expression by porcine aorta endothelial cells of glycosphingolipids bearing the major epitope for human xenoreactive antibodies (Gal alpha1-3Gal), blood group H determinant and N-glycolylneuraminic acid. Glycoconj J. 1996;13:947–953. doi: 10.1007/BF01053190. [DOI] [PubMed] [Google Scholar]
- 26.Zhu A, Hurst R. Anti-N-glycolylneuraminic acid antibodies identified in healthy human serum. Xenotransplantation. 2002;9:376–381. doi: 10.1034/j.1399-3089.2002.02138.x. [DOI] [PubMed] [Google Scholar]
- 27.Padler-Karavani V, Varki A. Potential impact of the non-human sialic acid n-glycolylneuraminic acid on transplant rejection risk. Xenotransplantation. 2011;18:1–5. doi: 10.1111/j.1399-3089.2011.00622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee W, Hara H, Ezzelarab MB, et al. Initial in vitro studies on tissues and cells from GTKO/hCD46/NeuGcKO pigs. Xenotransplantation. 2016;23:137–150. doi: 10.1111/xen.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Byrne GW, Du Z, Stalboerger P, Kogelberg H, McGregor CG. Cloning and expression of porcine beta1, 4 n-acetylgalactosaminyl transferase encoding a new xenoreactive antigen. Xenotransplantation. 2014;21:543–554. doi: 10.1111/xen.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Estrada JL, Martens G, Li P, et al. Evaluation of human and non-human primate antibody binding to pig cells lacking GGTA1/CMAH/beta4GALNT2 genes. Xenotransplantation. 2015;22:194–202. doi: 10.1111/xen.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li P, Estrada JL, Burlak C, et al. Efficient generation of genetically distinct pigs in a single pregnancy using multiplexed single-guide rna and carbohydrate selection. Xenotransplantation. 2015;22:20–31. doi: 10.1111/xen.12131. [DOI] [PubMed] [Google Scholar]
- 32.Martens GR, Reyes LM, Butler JR, et al. Humoral reactivity of renal transplant-waitlisted patients to cells from GGTA1/CMAH/B4GalNT2, and SLA class I knockout pigs. Transplantation. 2017 Apr;101(4):e86–e92. doi: 10.1097/TP.0000000000001646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooper DKC, Tseng Y-L, Saidman SL. Allo- and xeno-antibody cross-reactivity in transplantation. Transplantation. 2004;77:1–5. doi: 10.1097/01.TP.0000105116.74032.63. [DOI] [PubMed] [Google Scholar]
- 34.Hara H, Ezzelarab M, Rood PP, et al. Allosensitized humans are at no greater risk of humoral rejection of GT-KO pig organs than other humans. Xenotransplantation. 2006;13:357–365. doi: 10.1111/j.1399-3089.2006.00319.x. [DOI] [PubMed] [Google Scholar]
- 35.Wong BS, Yamada K, Okumi M, et al. Allosensitization does not increase the risk of xenoreactivity to α1,3-Galactosyltransferase gene-knockout miniature swine in patients on transplantation waiting lists. Transplantation. 2006;82:314–319. doi: 10.1097/01.tp.0000228907.12073.0b. [DOI] [PubMed] [Google Scholar]
- 36.Albritton A, Leonard DA, Leto Barone A, et al. Lack of cross-sensitization between -1,3-galactosyltransferase knockout porcine and allogeneic skin grafts permits serial grafting. Transplantation. 2014;97:1209–1215. doi: 10.1097/TP.0000000000000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oostingh GJ, Davies HF, Tang KC, Bradley JA, Taylor CJ. Sensitisation to swine leukocyte antigens in patients with broadly reactive HLA specific antibodies. Am J Transplant. 2002;2:267–273. doi: 10.1034/j.1600-6143.2002.20312.x. [DOI] [PubMed] [Google Scholar]
- 38.Lin CC, Chen D, McVey JH, Cooper DKC, Dorling A. Expression of tissue factor and initiation of clotting by human platelets and monocytes after incubation with porcine endothelial cells. Transplantation. 2008;86:702–709. doi: 10.1097/TP.0b013e31818410a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ezzelarab MB, Liu YW, Lin CC, et al. Role of P-selectin and P-selectin glycoprotein ligand-1 interaction in the induction of tissue factor expression on human platelets after incubation with porcine aortic endothelial cells. Xenotransplantation. 2014;21:16–24. doi: 10.1111/xen.12068. [DOI] [PubMed] [Google Scholar]
- 40.Ezzelarab MB, Ekser B, Azimzadeh A, et al. Systemic inflammation in xenograft recipients precedes activation of coagulation. Xenotransplantation. 2015;22:32–47. doi: 10.1111/xen.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iwase H, Ekser B, Zhou H, et al. Further evidence for sustained systemic inflammation in xenograft recipients (SIXR) Xenotransplantation. 2015;22:399–405. doi: 10.1111/xen.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iwase H, Liu H, Li T, et al. Therapeutic regulation of systemic inflammation in xenograft recipients. Xenotransplantation. 2017;24 doi: 10.1111/xen.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Azimzadeh AM, Kelishadi SS, Ezzelarab MB, et al. Early graft failure of GalTKO pig organs in baboons is reduced by expression of a human complement pathway-regulatory protein. Xenotransplantation. 2015;22:310–6. doi: 10.1111/xen.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilhite T, Ezzelarab C, Hara H, et al. The effect of Gal expression on pig cells on the human T-cell xenoresponse. Xenotransplantation. 2012;19:56–63. doi: 10.1111/j.1399-3089.2011.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ezzelarab MB, Ayares D, Cooper DKC. Transgenic expression of human CD46: does it reduce the primate T-cell response to pig endothelial cells? Xenotransplantation. 2015;22:487–489. doi: 10.1111/xen.12209. [DOI] [PubMed] [Google Scholar]
- 46.Nuutila J, Jalava-Karvinen P, Hohenthal U, et al. Use of complement regulators, CD35, CD46, CD55, and CD59, on leukocytes as markers for diagnosis of viral and bacterial infections. Hum Immunol. 2013;74:522–530. doi: 10.1016/j.humimm.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 47.Griesemer A, Yamada K, Sykes M. Xenotransplantation: immunological hurdles and progress toward tolerance. Immunol Rev. 2014;258:241–258. doi: 10.1111/imr.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]